Introduction
Sepsis or systemic inflammatory response syndrome
(SIRS) is caused by infection (1)
and is a type of complication that is commonly observed in trauma,
major surgery, burns and other clinical diseases (2), which can lead to septic shock and
multiple organ dysfunction syndrome (MODS), one of the leading
causes of mortality in patients (3). Several studies have shown that
immunosuppression is implicated in sepsis (4), which is mainly attributed to the
accelerating apoptosis of lymphocytes (mainly including B
lymphocytes and T-helper cells) and dendritic cells (5). Hotchkiss et al (4) found that lymphocyte apoptosis is
associated with the severity of sepsis. Apoptosis of great a number
of lymphocytes in sepsis renders the body in an immunosuppressive
state, which cannot effectively regulate the specific immune
response against a pathogenic infection, resulting in multiple
organ failure and mortality (4).
Tumor necrosis factor (TNF)-α-inducible protein
8-like 2 (TIPE2), a newly identified protein, is essential for
maintaining immune homeostasis (6). TIPE2 shares considerable sequence
homology with members of the TNF-1-inducible protein 8 (TNFAIP8)
family, which is thought to regulate cellular and immune
homeostasis. TNFAIP8, the first identified member of this family,
is able to enhance cell survival and inhibit apoptosis (7,8).
TIPE2 is a recently identified negative regulator of innate and
adaptive immunity and is preferentially expressed in lymphoid
tissues (9).
Rhodiola rosea, a rhodiola plant of the
Crassulaceae family, is a perennial herb or shrub plant that
is widely distributed in China. Rhodiola rosea, a common
Tibetan medicine, is mainly used in the treatment of conditions
including hot phlegm cough, haemoptysis, injuries, burns,
leucorrhoea and diabetes (10).
Modern pharmacological studies have shown that Rhodiola
rosea has the following effects: Anti-ageing, anti-fatigue,
anti-oxidant, anti-tumor and anti-viral effects, as well as
enhancement of learning memory, resistance to microwave radiation,
enhancement of immunity, protection of the viscera, enhancement of
the physique, improvement of haematopoietic function, resistance to
fatigue, lowering of blood sugar and prevention of altitude
sickness (11,12). Rhodiola rosea is highly
effective in the treatment of diabetes (12), ischaemic heart disease (13) and free radical injury during
cerebral ischaemia-reperfusion (14,15). Salidroside is one of the major
active components of Rhodiola rosea (16). In recent years, in vitro
experiments as well as animal experiments have indicated that
Rhodiola rosea can enhance cellular immunity and humoral
immune function (17).
The present study aimed to determine whether
Rhodiola rosea extract is able to enhance the expression of
TIPE2, reduce thymus T-lymphocyte apoptosis and improve immunity.
By observing changes in thymus T lymphocytes, TIPE2 and immune
cells in mice with sepsis which were pre-treated with Rhodiola
rosea extract, the present study aimed to elucidate the immune
regulatory mechanisms of Rhodiola rosea.
Materials and methods
Preparation of plant extract
An ethanolic extract of the Rhodiola rosea
root was used in the present study. The Rhodiola rosea root
used for the extraction was obtained from Baoxing Biotechnologies
Co., Ltd. (Guangzhou, China) and was authenticated by a plant
taxonomist. The plant root (50 g) was first dried and ground to a
coarse plant powder, which was then extracted by boiling with 500
ml of 70% ethanol twice for 2 h each. The extract was concentrated
under reduced pressure, precipitated with ethanol and finally spray
dried using a WD800ASL microwave extraction apparatus (Galanz Co.,
Shunde, China) to yield a reddish-brown powder. The yield of the
Rhodiola rosea extract was ~3–5% (w/w).
Mice
Thirty-two nine-week-old male BALB/c mice weighing
22–30 g were purchased from the Experimental Animal Center of
Kunming Medical University (Kunming, China) and were acclimated to
laboratory conditions for one week prior to the experiment. All
experiments were approved and conducted in accordance with the
guidelines of the Animal Care Committee of Kunming Medical
University (Kunming, China). The experimental procedures were
approved by the Ethics Committee of the Institute of Yunan
University of Traditional Chinese Medicine (TCM; Kunming, China).
The initial body weight of the mice showed no significant
difference among the four groups in the present study. They were
maintained in a well-ventilated, controlled room at 20°C with a
12-h light/dark cycle with access to water and food ad
libitum.
Generation of TIPE2-deficient mice
Next, 2.2-kb and 5.0-kb TIPE2 genomic fragments were
amplified using polymerase chain reaction (PCR) and cloned into the
XhoI/NheI and NotI/SalI sites of the
pOSDUPDE vector (a gift from Dr X.P. Zhong, Department of
Pediatrics, Duke University, Durham, NC, USA), respectively. As has
been previously described (18),
PCR was conducted with Lightcycler (Roche Diagnostics, Mannheim,
Germany). All reactions were performed using a SYBR Green PCR mix
(Takara Biotechnology, Otsu, Japan), according to the following
thermal profile: denaturation at 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 30 sec. The
primer sequences were obtained from Sangon Biological Engineering
Co., Ltd. (Shanghai, China) and were as follows: TIPE2 forward,
5′-GGAACATCCAAGGCAAGACTG-3′ and reverse,
5′-AGCACCTCACTGCTTGTCTCATC-3′. Gene-specific amplifications were
demonstrated with melting curve and gel-migration analyses. Gels
were stained using SYBR Gold Nucleic Acid Gel Stain (Invitrogen
Life Technologies, Carlsbad, CA, USA) in 1X TBE (dilution,
1:16,000) buffer for 40 min and bands were visualized by UV
transillumination on a Bio-Rad Gel Doc 2000 (Bio-Rad,
Ivry-sur-Seine, France). As has been previously described (19), wild-type (WT) C57BL/6 mice, which
carry a TIPE2 gene-null mutation, were generated by backcrossing
TIPE2−/− mice to WT C57BL/6 mice for 12 generations. TL1
embryonic stem (ES) cells from 129S6/SvEvTac mice (Shanghai
Laboratory Animal Center of the Chinese Academy of Science,
Shanghai, China) were transfected with the targeting vector and
subjected to positive and negative selection using G418 (Guangzhou
Huawei Chemical Co., Ltd, Guangzhou, China) and ganciclovir (Jena
Biosciences, Jena, Germany), respectively (20). Two ES cell clones, in which the
TIPE2 gene (including exons 1 and 2) was replaced by the
neomycin resistance gene cassette, were identified using Southern
blot analyses. For the southern blot analysis, 7–10 µg of
isolated DNA were digested with EcoRI and NruI and
separated on a 0.8% agarose/Tris acetate EDTA (TAE) gel. Following
DNA transfer, the membranes were hybridized using the
TIPE2-specific genomic probe StB12.3 (21). The mutant ES cells were injected
into four-day-old C57BL/6J mouse blastocysts (Shanghai Laboratory
Animal Center of the Chinese Academy of Science). The resulting
chimeric male offspring were crossed with 129S6/SvEvTac females for
germ-line transmission. Unless otherwise indicated, all mice used
in the present study were of the 129S6/SvEvTac background. Age- and
gender-matched littermates were used as controls. The mice were
housed in the Experimental Animal Center of Kunming Medical
University under pathogen-free conditions. All procedures used were
pre-approved by the Institutional Animal Care and Use
Committee.
Reagents
Flow cytometry was performed by using a FACSCalibur
flow cytometer from Becton-Dickinson (Franklin Lakes, NJ, USA). The
SHIMADZU analytical balance (type, AUW120D) was obtained from
Shanghai Ruifang Instrument Co., Ltd. (Shanghai, China). The ALPU
cryogenic refrigerator (type, DW-60L398) was obtained from Bio-Rad
Laboratories Inc. (Hercules, CA, USA). The TRIzol kit and fetal
calf serum (FCS) were purchased from Invitrogen Life Technologies.
The reverse transcription (RT) reaction kit was obtained from
Takara Biotechnology. TRIzol and the electrophoresis reagents were
purchased from ProMag Co., Ltd. (Ningbo, China). The PCR
amplification reagent kit and DNA ladder marker were obtained from
Sangon Biological Engineering Co., Ltd.. β-actin was obtained from
Santa Cruz Biotechnology Inc. (Dallas, TX, USA); TNF-α, interleukin
(IL)-6, IL-10, interferon (IFN)γ and IL-12 ELIS) kits were obtained
from the Science and Technology Development Center of the People’s
Liberation Army General Hospital (Beijing, China). Rabbit
anti-mouse Fas was from Cell Signaling Technology (Beverly, MA,
USA), and fas ligand (FasL, 1:200) and B-cell lymphoma 2 (Bcl-2,
1:200) polyclonal antibodies were obtained from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Fluorescently labeled
antibodies, including a CD3-peridinin chlorophyll, CD4-fluorescein
isothiocyanate (FITC) and CD8-allophycocyanin cocktail (all 1:200)
were purchased from BD Biosciences. RPMI-1640 medium and EDTA
disodium salt were obtained from Sigma-Aldrich (St Louis, MO,
USA).
Animal model of sepsis
Sepsis was induced by caecal ligation and puncture
(CLP), as previously described (22). Briefly, the mice were
anaesthetised by isoflurane inhalation, and a 2-cm ventral midline
abdominal incision was performed. The cecum was then exposed,
ligated immediately distal to the ileocecal valve to avoid
intestinal obstruction, punctured twice with an 18-guage needle,
and returned to the abdominal cavity. The incision was then closed
in layers. Sham-operated animals underwent the same procedure, with
the exception that the cecum was neither ligated nor punctured. The
animals were resuscitated with 3 ml/100 g body weight normal
saline, which was administered subcutaneously immediately after
surgery.
Groupings and treatments in vivo
According to a random number table, 80 mice were
randomly divided into four groups: Normal control group,
TIPE2-deficient group, sham-operated group (sham group), sepsis
model group (model group) and Rhodiola rosea extract
treatment group (treatment group), with 20 mice per group. The
model group and treatment group were induced by CLP. Animals in the
treatment group were administered intraperitoneal injections of
Rhodiola rosea extract (at 50 mg/kg body weight) 8 h prior
to surgery, while the normal control, sham and control groups were
given the same volume of normal saline.
Sample preparation
After the animals in each group were anaesthetised
with ether 24 h following CLP, the right internal carotid artery
was isolated. Blood was extracted (5 ml) and the blood was
centrifuged (10,000 × g for 5 min) to collect the supernatant. The
blood was dispensed into two sterile tubes, sealed with sealing
glue and placed into the freezer at −20°C for examination. The
thymus was compressed in Teflon tissue homogenizers (BILON-08;
Shanghai Bilon Instrument Manufacturing Co. Ltd., Shanghai,China),
and the resulting single-cell suspensions were pelleted at 300 xg,
subjected to hypotonic shock for red cell removal, washed in
RPMI-1640 containing 10% fetal bovine serum (FBS) and quantified.
Macrophages were removed from the cell suspensions by plastic
adherence (23) using a Kenker 24
cell adherent culture plate (Longchuan Jiaxing Bio Technology Co.,
Ltd., Jiaxing, China) in pre-warmed RPMI-1640 containing 5% FCS at
37°C for 1 h in a CO2 incubator. The T lymphocytes were
purified on nylon wool columns [Nylon Fiber Column T (L-Type); Wako
Pure Chemical Industries, Ltd., Wako, Japan] according to the
method of Julius et al (24). The thymus tissues were removed for
histopathological and immunofluorescence analyses.
RT-quantitative (q)PCR
T cells were homogenized in TRIzol™ reagent using a
Mixer 301 (Invitrogen Life Technologies). Total RNA was extracted
according to the manufacturer’s instructions. Total RNA (1
µg) was incubated with 200 units Moloney Murine Leukemia
Virus reverse transcriptase in buffer containing 50 mmol/l Tris HCl
(pH 8.3) (Shanghai Jiang Lai Biological Technology Co., Ltd.,
Shanghai, China), 75 mmol/l KCl (Shanghai Jiang Lai Biological
Technology Co., Ltd.), 3 mmol/l MgCl2 (Sangon Biological
Engineering Co., Ltd.), 20 units RNase inhibitor (Sangon Biological
Engineering Co., Ltd.), 1 µmol/l poly(dT) oligomer (Becton
Dickinson) and 0.5 mmol/l each dNTP (Becton Dickinson) in a final
volume of 20 µl. The reaction mixture was incubated at 42°C
for 1 h and then at 94°C for 5 min to inactivate the enzyme. A
total of 80 µl diethyl pyrocarbonate treated water was added
to the reaction mixture prior to storage at 70°C. Real-time PCR was
performed as previously described (12). Briefly, RNA samples were treated
with DNase and subjected to quantitative PCR, which was performed
with the ABI Prism 7000 sequence detection system (Applied
Biosystems, Fisher Termo Scientific, Waltham, MA, USA) using
SYBR-Green I dye (Gibco-BRL, Invitrogen Life Technologies), and the
threshold cycle numbers were obtained using ABI Prism 7000 SDS
software version 1.0 (Applied Biosystems). Conditions for
amplification were 1 cycle at 94°C for 5 min followed by 40 cycles
of 94°C for 30 sec, 58°C for 30 sec and 72°C for 45 sec. The primer
sequences (Sangon Biological Engineering Co., Ltd.) used in the
present study are shown in Table
I. β-actin was used as an endogenous control. The DNA products
of the RT-PCR reactions were separated by 4% SDS-PAGE in the same
buffer. The polyacrylamide gels were dried and scanned using the
ImageQuant densitometer (LI-COR Biosciences, Lincoln, NE, USA) and
the comparative Ct (2−ΔΔCt) method for relative
expression was performed (25).
The PCR products were subjected to melting curve analysis, and the
standard curve was used to confirm the correct amplification.
 | Table IPrimer sequences used for using
reverse transcription polymerase chain reaction to validate the
microarray analysis. |
Table I
Primer sequences used for using
reverse transcription polymerase chain reaction to validate the
microarray analysis.
| Gene | Primer
sequence | Product (bp) |
|---|
| TIPE2 | F:
5′-GGGAACATCCAAGGCAAG-3′
R: 5′-AGCTCATCTAGCACCTCACT-3′ | 195 |
| Fas | F:
5′-GACCCAGAATACCAAGTGCAAGTG-3′
R: 5′-CTTGCCCTCCTTGATGTTATTTTC-3′ | 400 |
| FasL | F:
5′-CGTGAGTTCACCAACCAAAGC-3′
R: 5′-CCCAGTTTCGTTGATCACAAG-3′ | 219 |
| Bcl-2 | F:
5′-TTCTCCTTCCAGCCTGAGAGCAA-3′
R: 5′-ATGACCCCACCGAACTCAAAG-3′ | 317 |
| β-actin | F:
5′-GACTACCTCATGAAGATCCTCACC-3′
R: 5′-TCTCCTTAATGTCACGCACGATT-3′ | 190 |
Western blot analysis
T lymphocytes were washed twice with ice-cold
phosphate-buffered saline (PBS) and lysed in lysis buffer [25 mM
Tris-HCl pH 7.5 (Shanghai Jiang Lai Biological Technology Co.,
Ltd.), 150 mM NaCl (Shanghai Jiang Lai Biological Technology Co.,
Ltd.), 1% Nonidet P-40 (Sangon Biological Engineering Co., Ltd.), 5
mM sodium pyrophosphate (Xinxiang Huaxing Chemical Co., Ltd.,
Xinxiang, China), 1 mM sodium orthovanadate (Selleck Chemicals,
Houston, TX, USA), 10 mM sodium fluoride (Shanghai Kunxin Chemical
Technology Co., Ltd., Zibo, China), 10% glycerol (Solarbio Science
& Technology, Beijing, China), 1 mM phenylmethylsulfonyl
fluoride (Chemical Reagent Co., Ltd., Shanghai, China), 25
µg/ml leupeptin (Shanghai Hufeng Chemical Co., Ltd.,
Shanghai, China), 25 µg/ml aprotinin (Shanghai Hufeng
Chemical Co., Ltd.) and 2 µg/ml pepstatin (Shanghai Hufeng
Chemical Co., Ltd.)] and incubated for 30 min on ice. The proteins
were separated using 4–12% gradient SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. The primary antibodies used
included rabbit anti-TIPE2 monoclonal antibody (1:200), rabbit
anti-Fas monoclonal antibody (1:200), rabbit anti-FasL monoclonal
antibody (1:200), mouse anti-Bcl-2 monoclonal antibody (1:200) and
were purchased from Wuhan Boster Biological Technology, Ltd. The
secondary antibodies used were horseradish peroxidase (HRP)-linked
goat anti-rabbit immunoglobulin G (IgG) (1:4,000 dilution; Amersham
Pharmacia Biotech, Piscataway, NJ, USA) and sheep anti-mouse
IgG-HRP (1:8,000 dilution; Amersham Pharmacia Biotech). The blots
were visualized by enhanced chemiluminescence (ECL) using a
Pierce-ECL western blotting substrate (Pierce Biotechnology Inc.,
Rockford, IL, USA) and a Johnson enhanced chemiluminescence
immunoassay analyzer (Shanghai Qian Jin Industrial Co., Ltd.,
Shanghai, China). Immunoreactive proteins were visualized using
horseradish peroxidase-conjugated secondary antibodies and
chemiluminescence (Millipore, Billerica, MA, USA). The relative
expression intensity of the proteins was determined as the target
band grey value normalized to the β-actin band grey value. The
experiment was repeated three times.
Flow cytometric detection of the
apoptotic rate of thymic T cells
Thymus T lymphocytes were fixed with 70% ethanol and
treated with RNase (Heino Chemical Co., Ltd., Zhuhai, China). Next,
the nuclei were stained with propidium iodide (PI; Abcam,
Cambridge, MA, USA) and FITC-Annexin V (BD Pharmingen, San Diego,
CA, USA). The DNA content was measured using a FACSCalibur flow
cytometer and CellQuest software (Becton Dickinson). Ten thousand
cells were quantified in all of the assays. Apoptotic cells were
quantified as the percentage of cells stained with Annexin V.
Flow cytometric T-lymphocyte
quantification and terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL)
For flow cytometric analysis, the thymus T
lymphocytes were fixed in 70% ethanol overnight at 4°C. Thymus T
lymphocytes were washed in PBS with 0.1% bovine serum albumin. The
cells were incubated with 1 U ml of RNase A (DNase free; BD
Pharmingen) and 10 µg ml-1 of PI overnight at room
temperature in the dark. The cells were analyzed using a
FACSCalibur flow cytometer, and CellQuest software (Becton
Dickinson) was used to determine the relative DNA content based on
the presence of red fluorescence. TUNEL analysis was performed to
determine the apoptotic rate in the thymus according to the
manufacturer’s instructions (Roche Applied Science, Basel,
Switzerland). Six micrographs were randomly selected and the
numbers of healthy or apoptotic thymocytes were counted. The
percentage of apoptotic cells was defined as the percentage of the
percentage of TUNEL-positive cells.
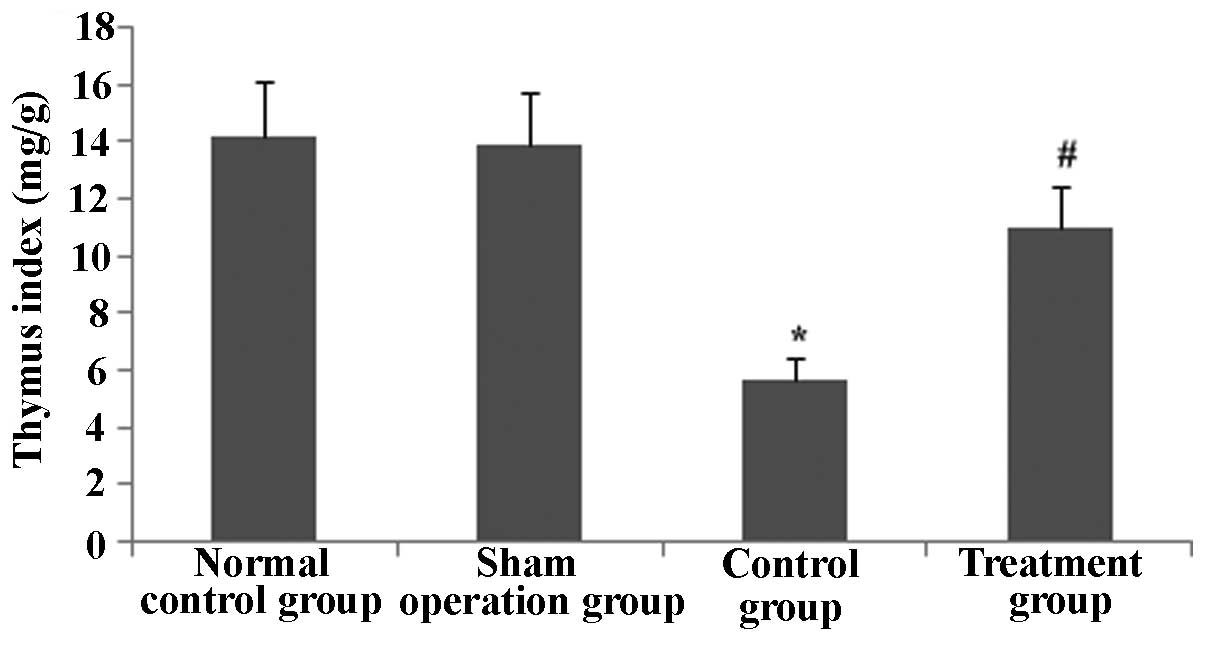
Thymus index assay
Mice were sacrificed by cervical dislocation and the
thymus was removed. The surface of the thymus was dried of blood
using a filter paper and weighed using a one hundred thousandth
electronic balance. The thymus index was calculated as follows:
Thymus index = thymus weight/body weight ×100%.
Determination of thymus T-lymphocyte
sub-sets (CD3+, CD4+ and
CD8+)
Thymus T lymphocyte suspensions in cold PEB buffer
[PBS supplemented with 2 mM EDTA and 0.5% BSA (JRH Biosciences,
Lenexa, KS, USA)] were incubated with supermagnetic microbeads
(Microbead™ M530; Dongguan Sanhe Chemical Co., Ltd., Dongguan,
China) conjugated to anti-mouse CD3, anti-mouse CD4 or anti-mouse
CD8 monoclonal antibodies at 4°C for 15 min. The cells were washed
twice and loaded onto magnetic separation columns [Nylon Fiber
Column T (L-Type); Dako Cytomation, Carpinteria, CA, USA). The
columns were washed three times with cold PEB buffer, and the
CD3+, CD4+ or CD8+ T cells were
then eluted. After purification, the cells were consistently
>95% viable, as assessed using trypan blue exclusion (Takara
Biotechnology). Fluorescence-assisted cell sorting analysis was
performed using a Becton Dickinson LSR analyse (Becton Dickinson)
and anti-mouse FITC-CD3 (BD Biosciences), anti-mouse FITC-CD4
(Sigma-Aldrich) and anti-mouse FITC-CD8 (BD Biosciences).
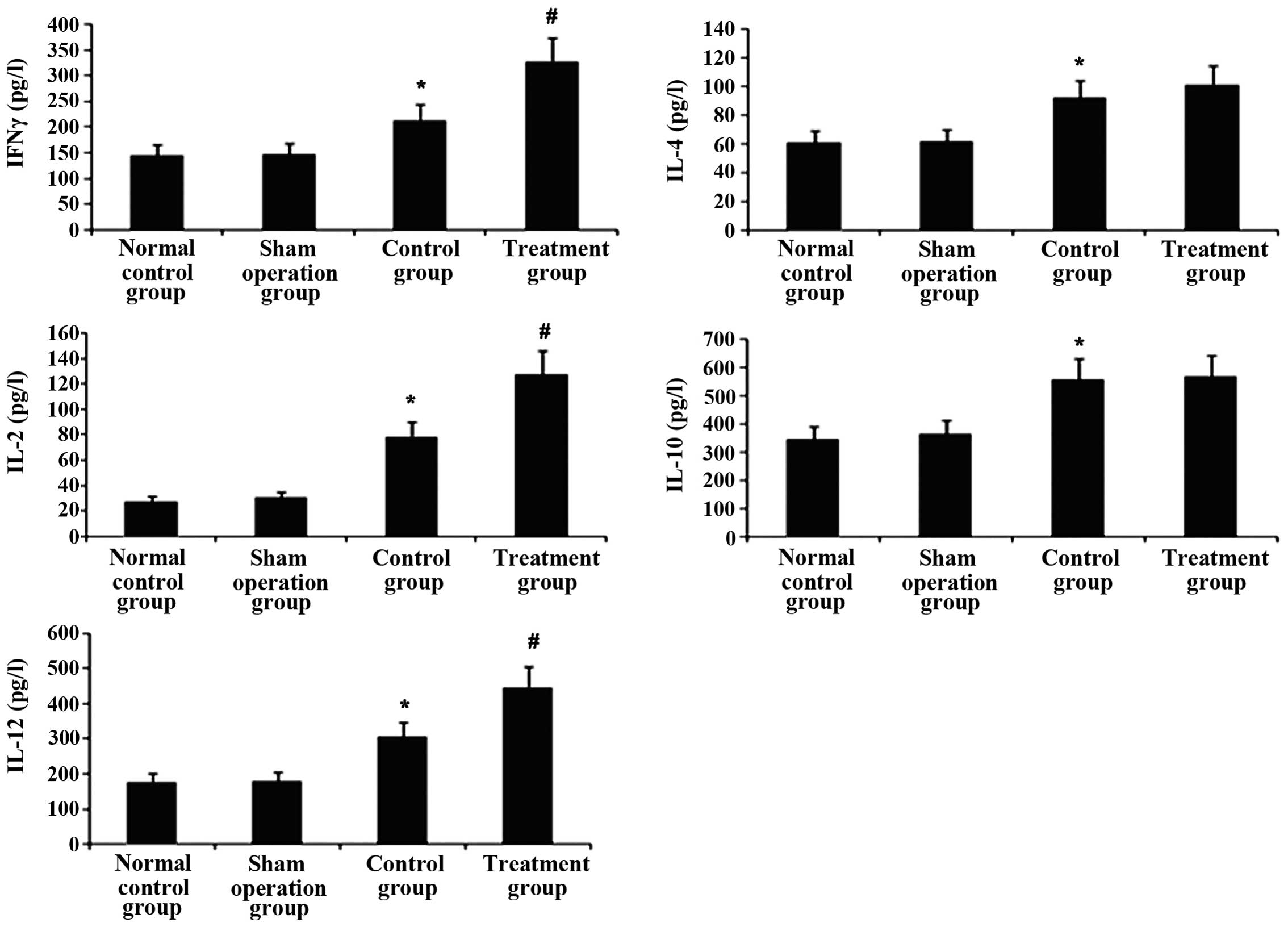
Th1 and Th2 cytokine assays
Th1 cytokines (IL-2, IL-12 and IFNγ) and Th2
cytokine (IL-4 and IL-10) were determined using commercially
available ELISA kits (Pierce Biotechnology Inc.), according to the
manufacturer’s instructions.
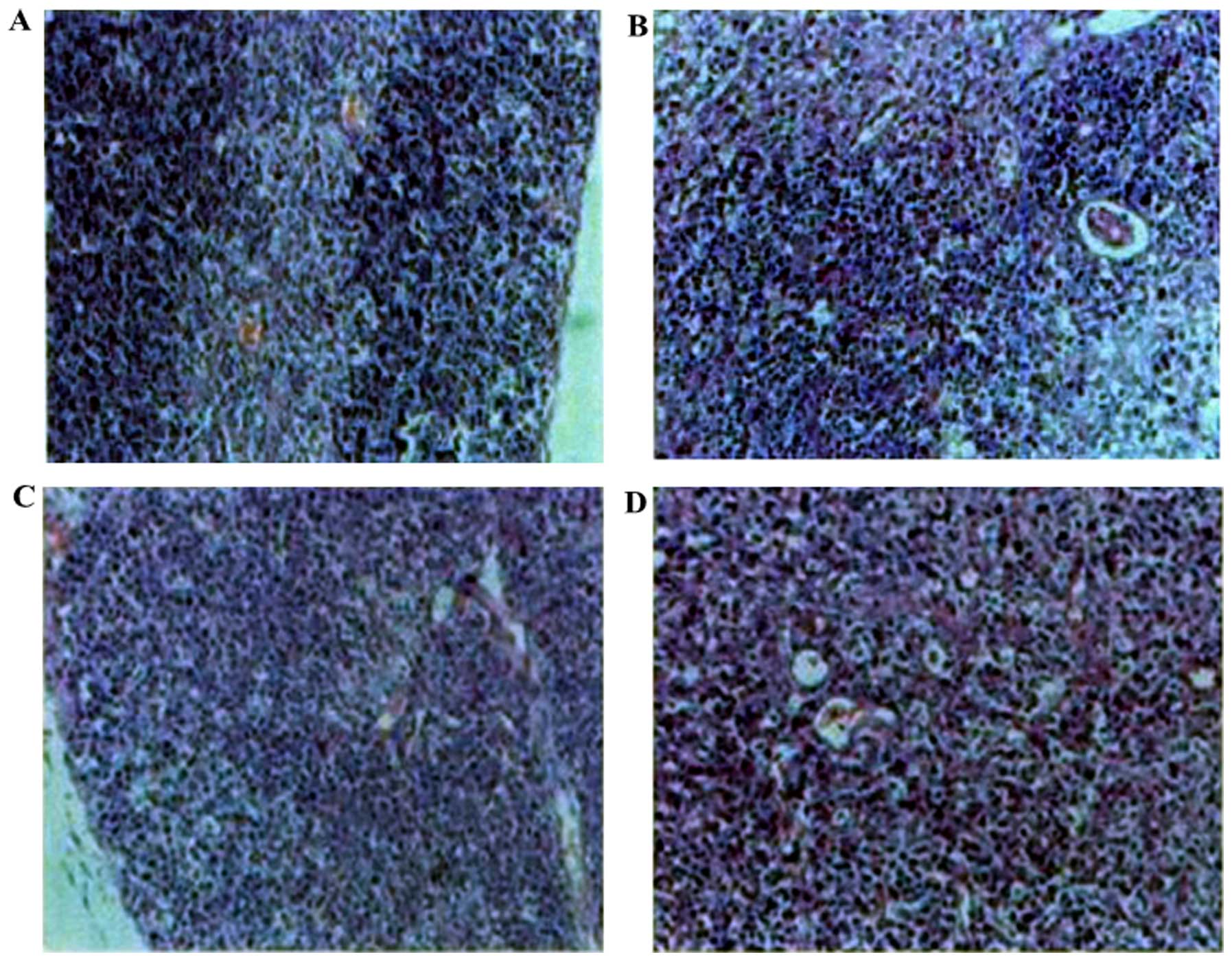
Histopathological examination
Thymus tissue was fixed in 10% formalin for 24 h
followed by dehydration. The thymus was embedded in paraffin wax,
sectioned into 5-µm slices and stained with Mayer’s
hematoxylin and eosin (Merck Millipore, Darmstadt, Germany).
Micrographs of the lung sections were captured with a CX21 light
microscope (Olympus, Tokyo, Japan).
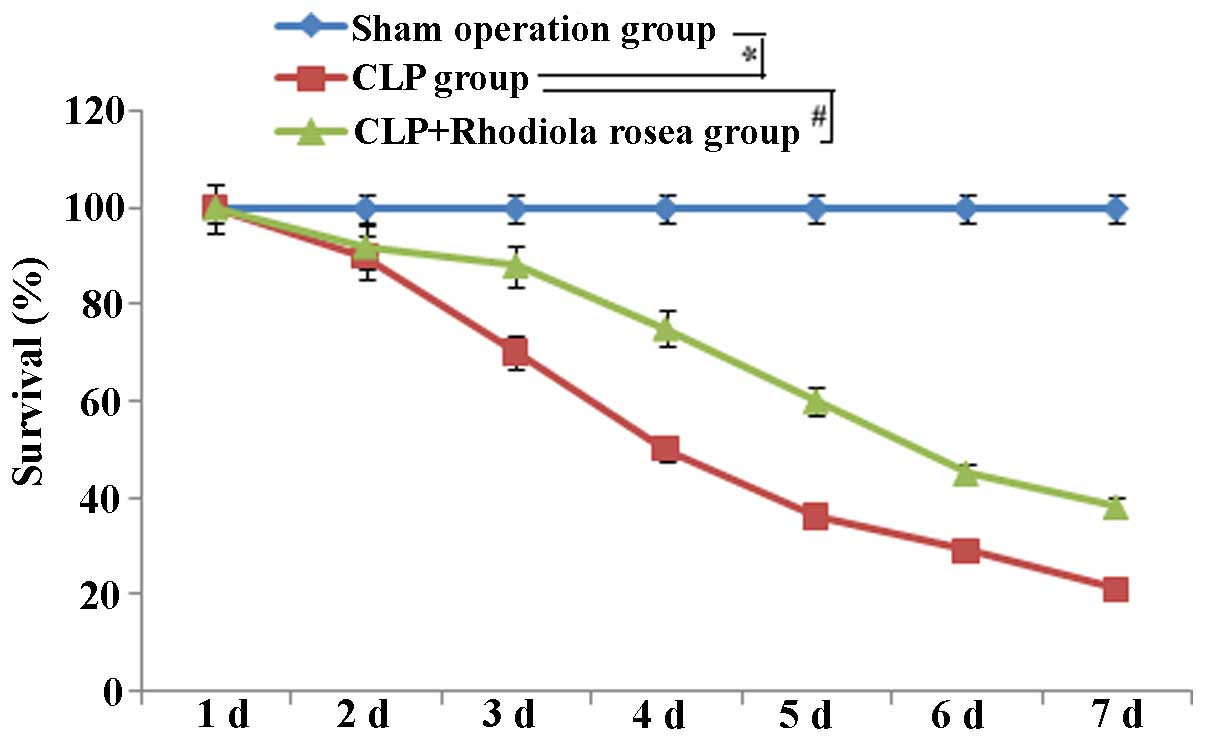
Survival curves
Another 45 mice were divided into the sham operation
group, CLP group and CLP plus Rhodiola rosea extract group
(n=15 per group) to determine the survival rate. The treatments
were identical to those of the previous experiments (3). Observation was commenced from the
start of Rhodiola rosea extract treatment, while the
endpoint was set at 120 h after Rhodiola rosea extract
treatment.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. For comparisons among multiple groups, one-way or two-way
analysis of variance followed by Bonferroni’s post-hoc test was
used to determine significant differences. Differences between two
groups were tested using Student’s t-test. P<0.05 was considered
to indicate a statistically significant difference between values.
All statistical analyses were carried out using SPSS version 13.0
for Windows (SPSS Inc., Chicago, IL, USA).
Results
Downregulation of TIPE2 decreases Fas and
FasL protein expression and apoptosis, while increasing Bcl-2 in
thymus T-lymphocytes of septic mice
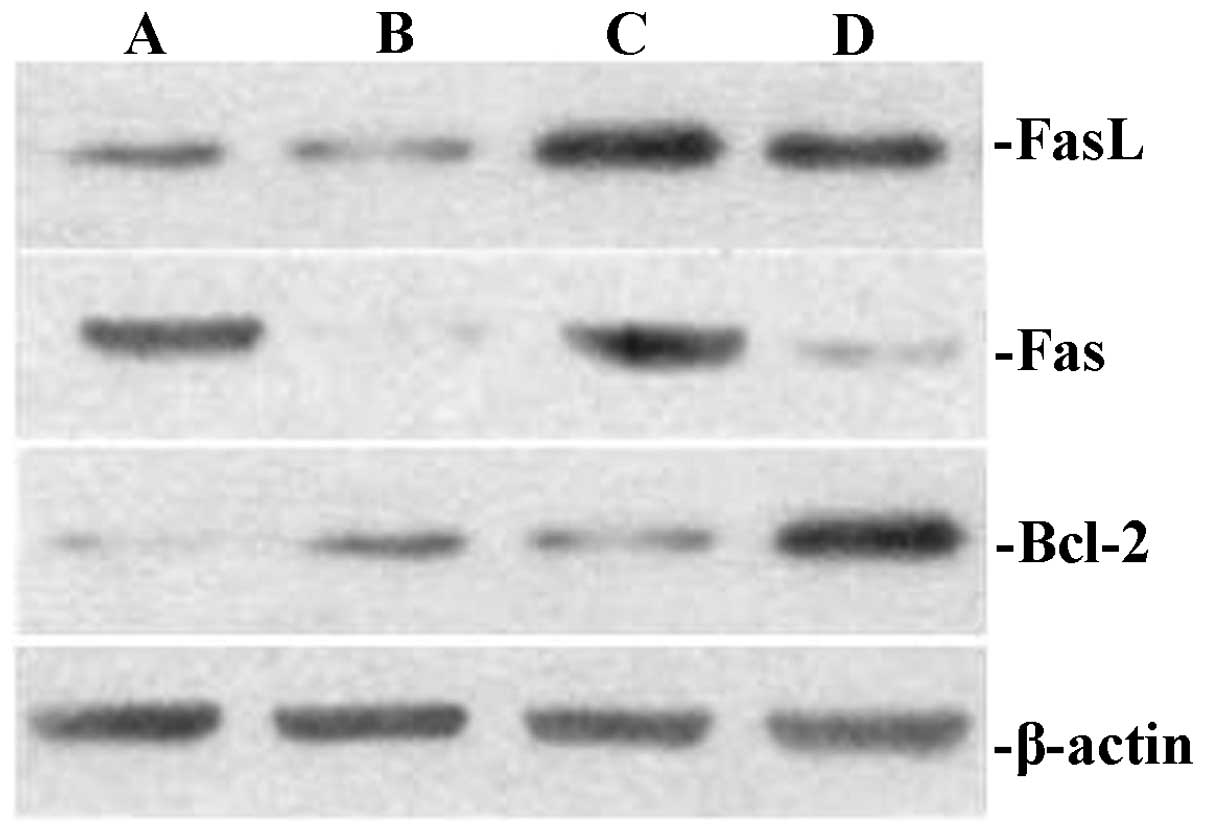
The present study investigated the effects of TIPE2
on Bcl-2, Fas and FasL expression in thymic T cells in septic mice.
After 12 h, the CLP model was established in TIPE2-deficient mice
and WT mice. Bcl-2, FaS and FasL protein expression in thymic T
cells were determined using western blot analysis. The thymus
T-lymphocyte apoptosis rate was assessed using flow cytometric
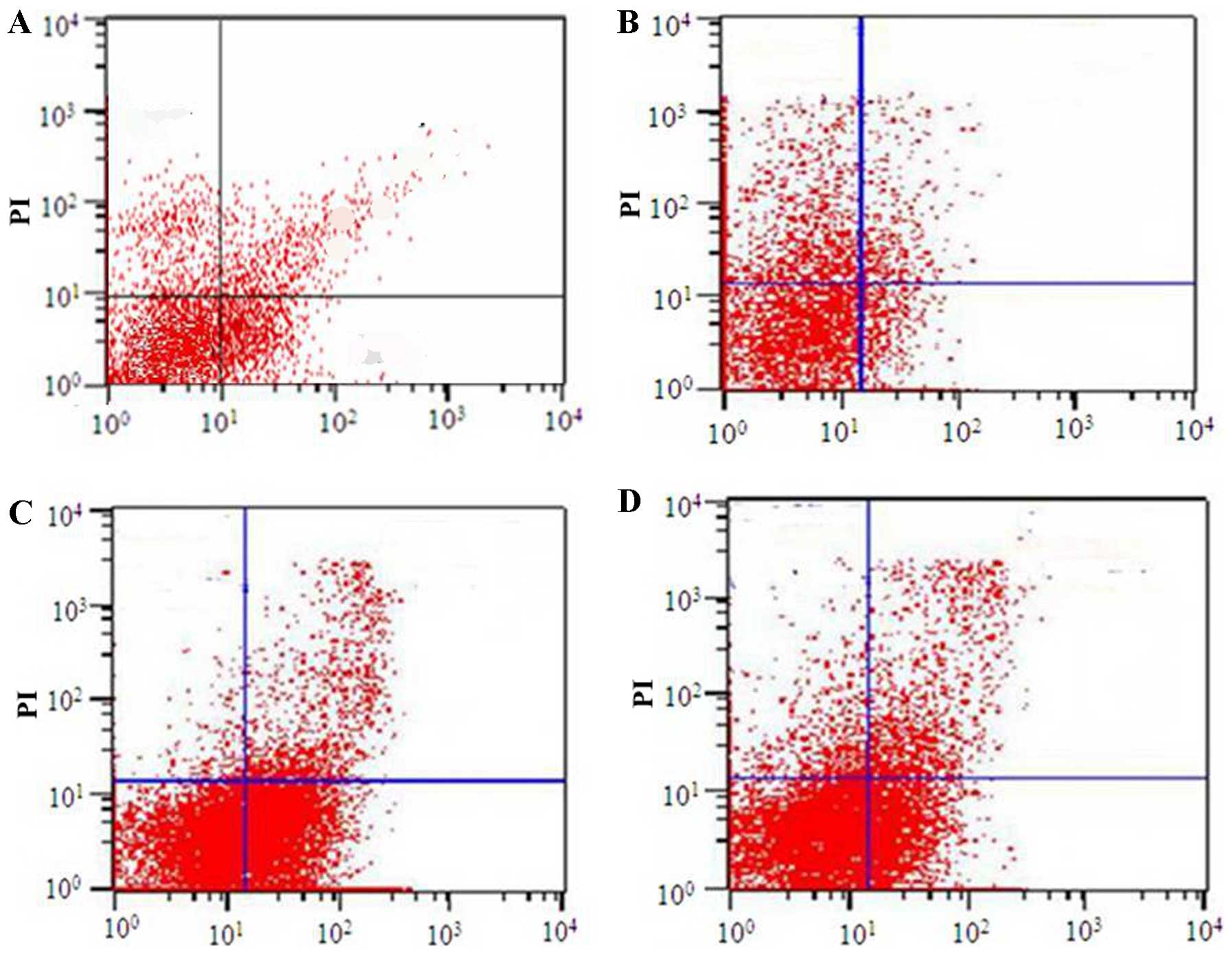
analysis. As shown in Figs.
1Figure 2Figure 3–4, Fas and FasL protein expression and
T-lymphocyte apoptosis were markedly increased in TIPE2-deficient
mice compared with those in WT mice (P<0.05). In addition, Bcl-2
protein expression was significantly decreased in TIPE2-deficient
mice compared with those in WT mice (P<0.05).
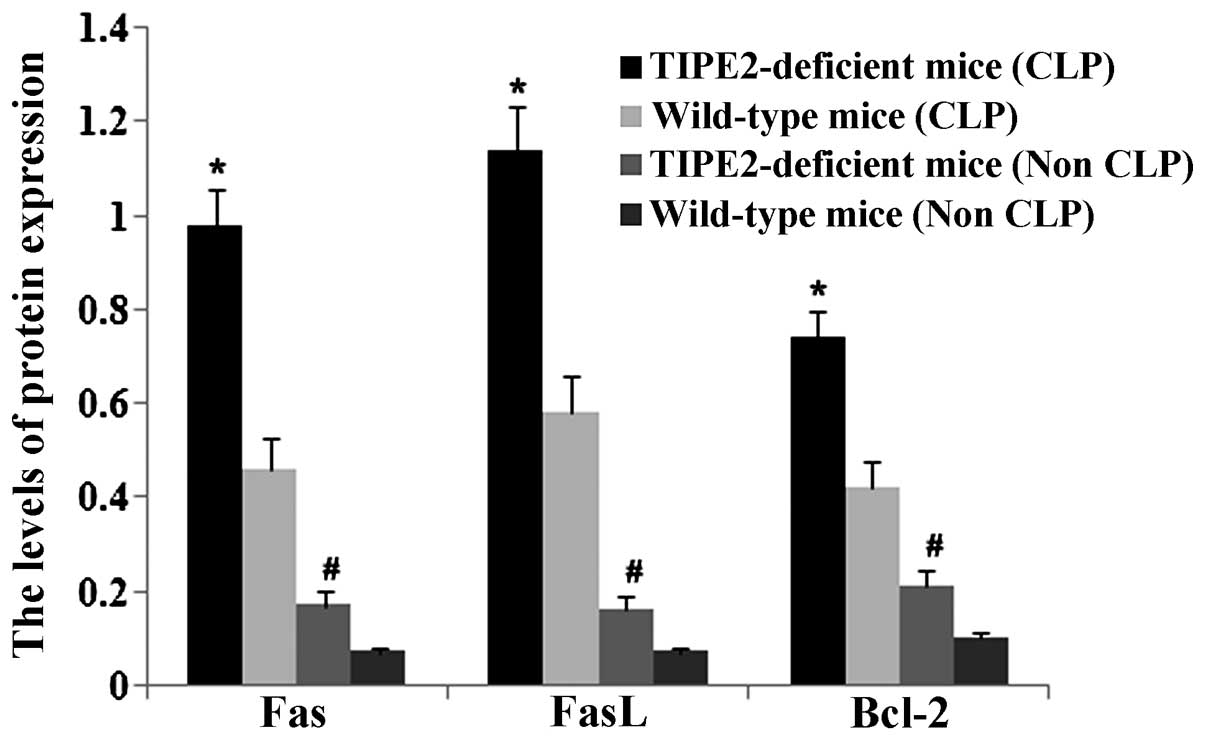
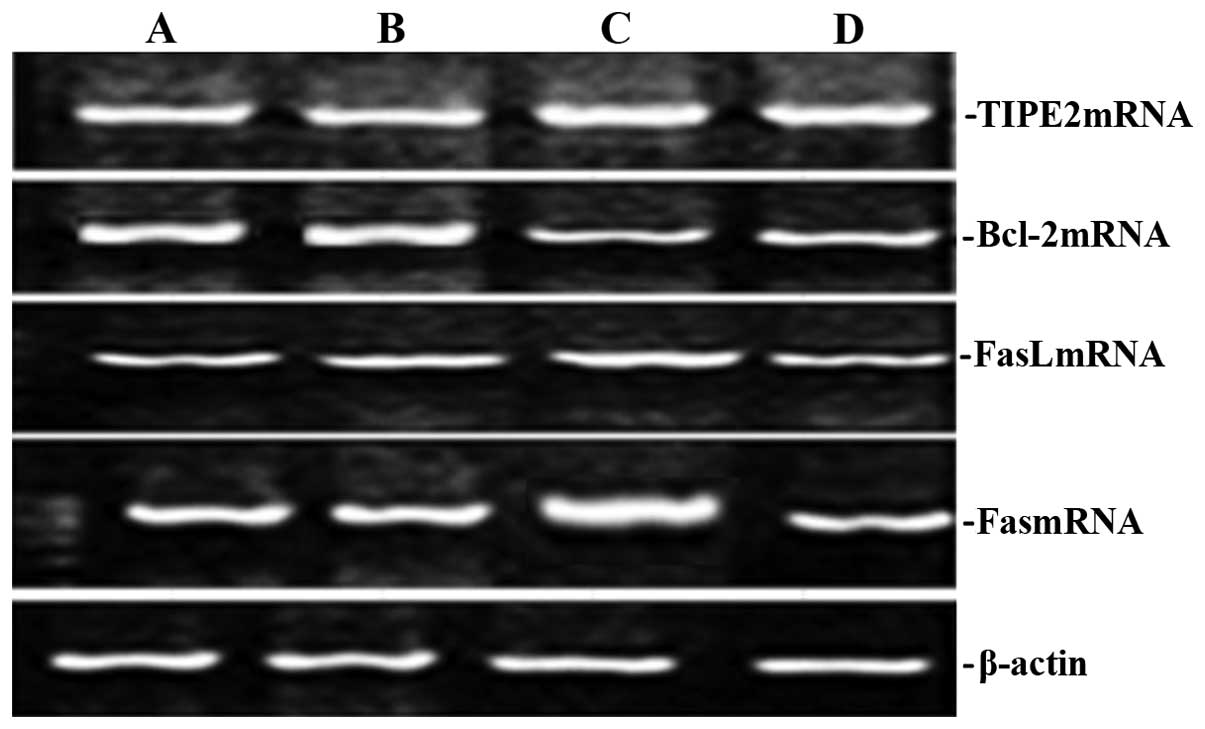
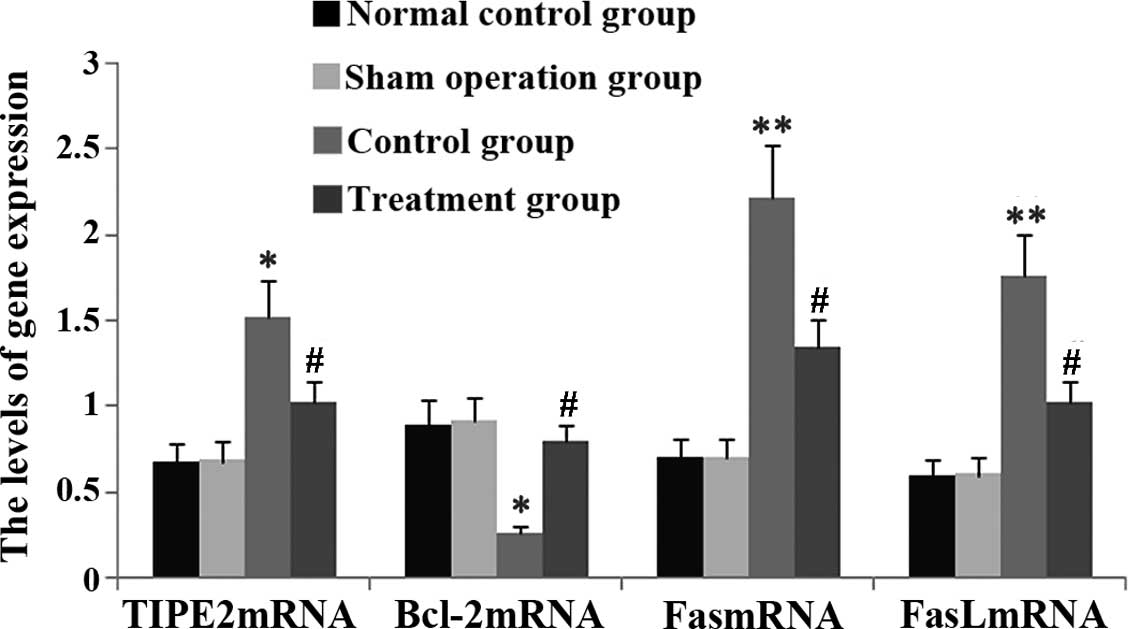
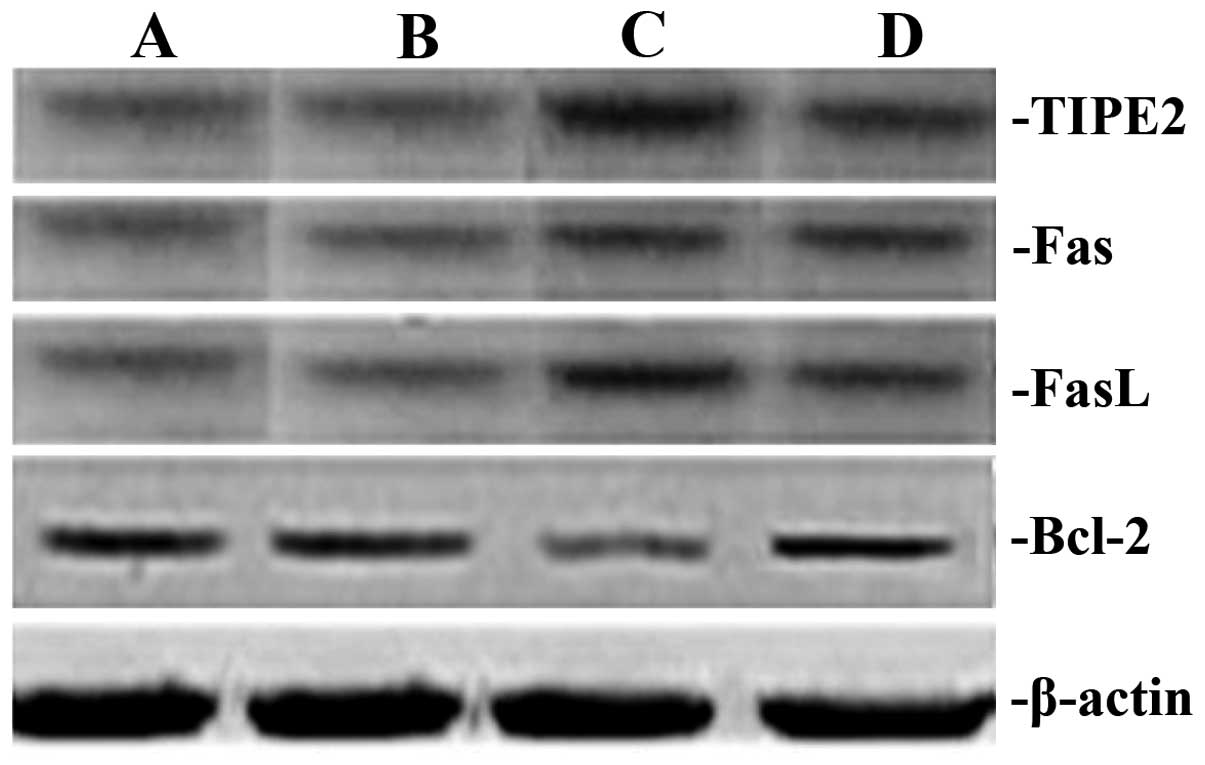
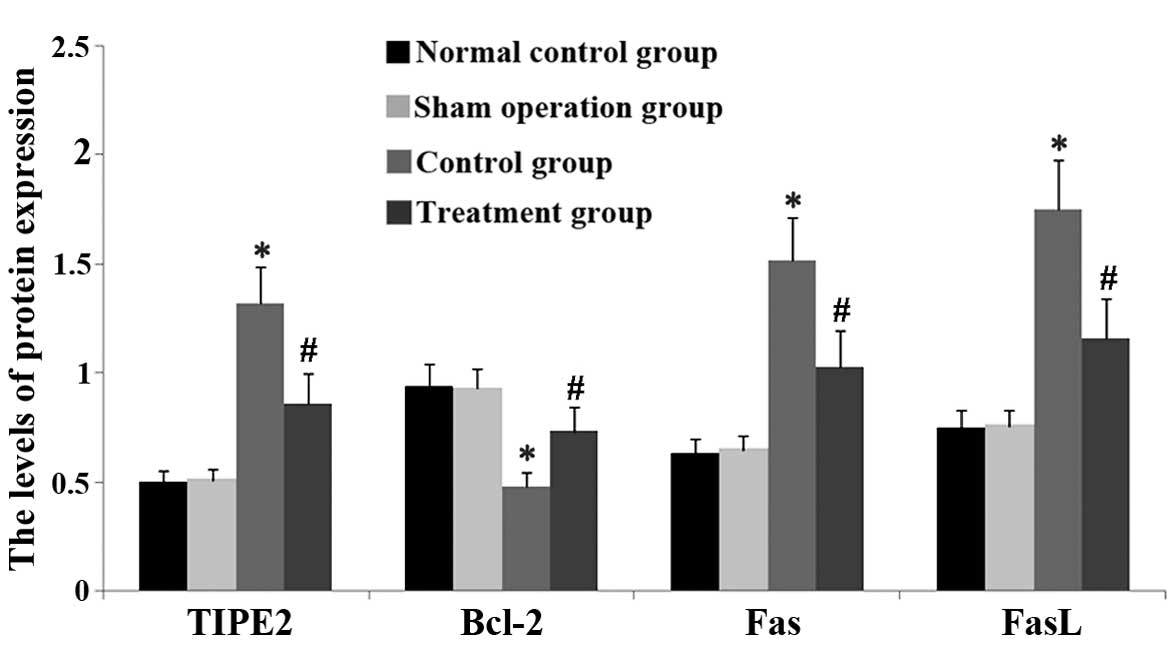
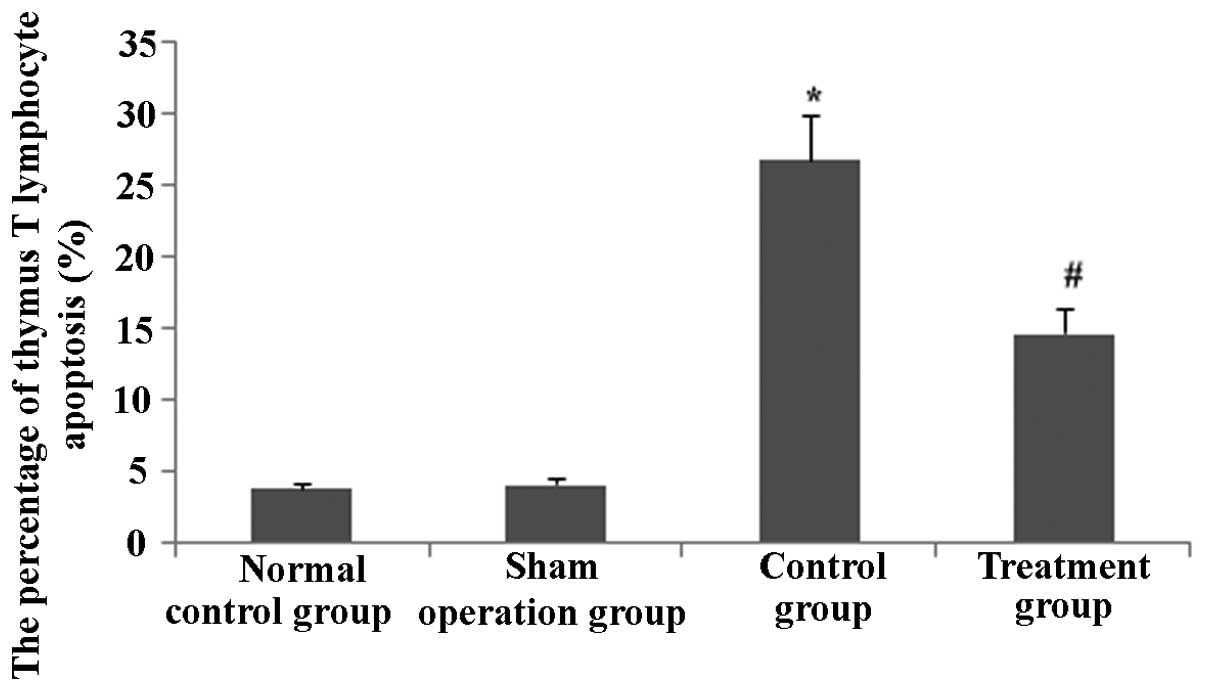
Rhodiola rosea extract attenuates
CLP-induced increases in TIPE2, FaS and FasL expression as well as
decreases in Bcl-2 expression
To assess the potential effect of Rhodiola
rosea on the expression of TIPE2, Bcl-2, FaS and FasL in
CLP-induced mice, their protein and mRNA levels were determined
using RT-qPCR and western blot analyses, respectively, in thymic T
cells of mice at 24 h after CLP challenge, which was performed 8 h
after pre-treatment with Rhodiola rosea extract. As shown in
Figs. 5Figure 6Figure 7–8, the expression of TIPE2, FaS and FasL
was markedly enhanced, while the expression of Bcl-2 was
significantly decreased at 24 h after CLP challenge as compared
with those in the control groups (all P<0.05). Of note,
pre-treatment with Rhodiola rosea extract decreased the
expression of TIPE2, FaS and FasL, and increased the expression of
Bcl-2 as compared with that in the CLP group (P<0.05), therefore
attenuating the CLP-induced changes.
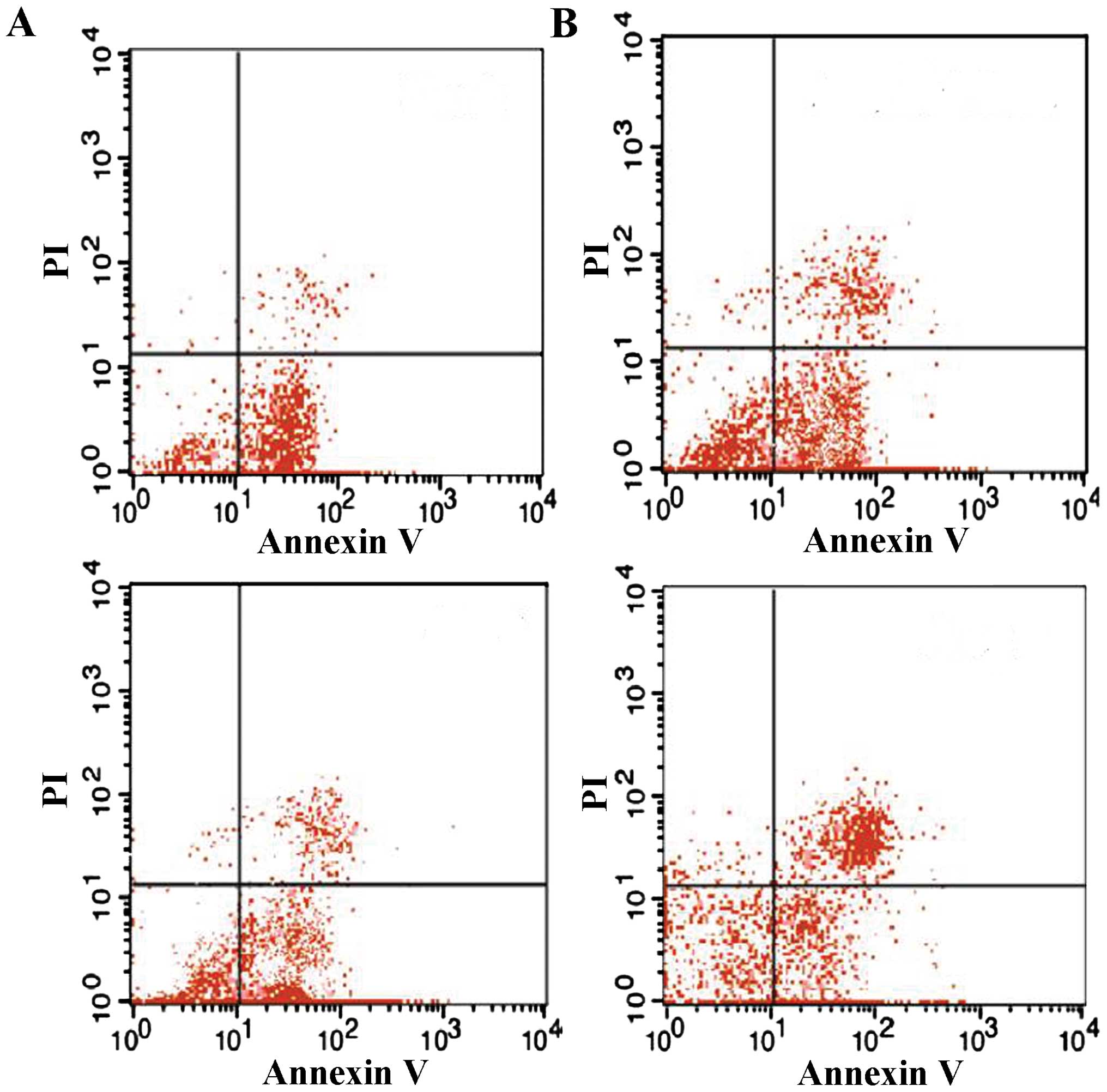
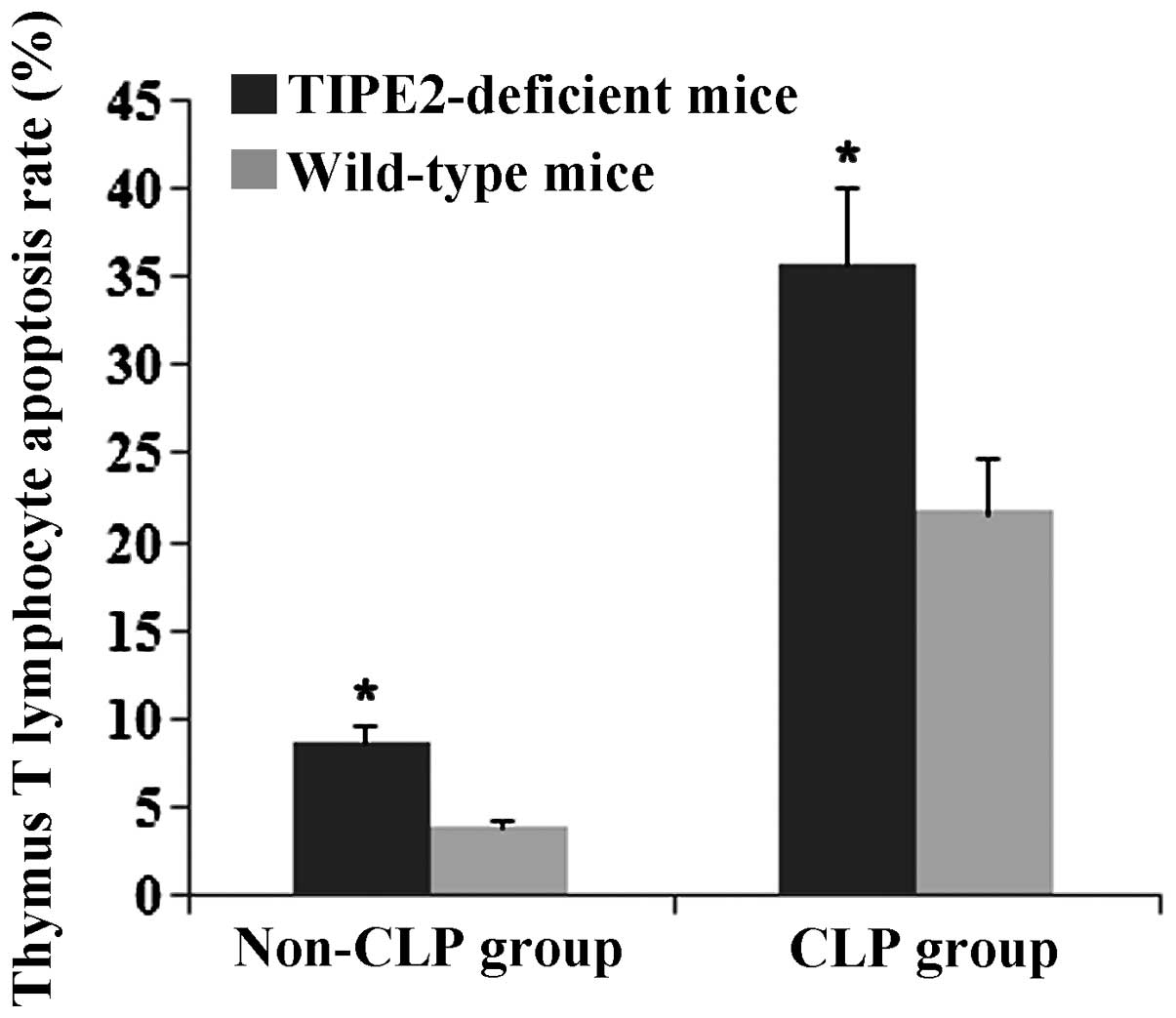
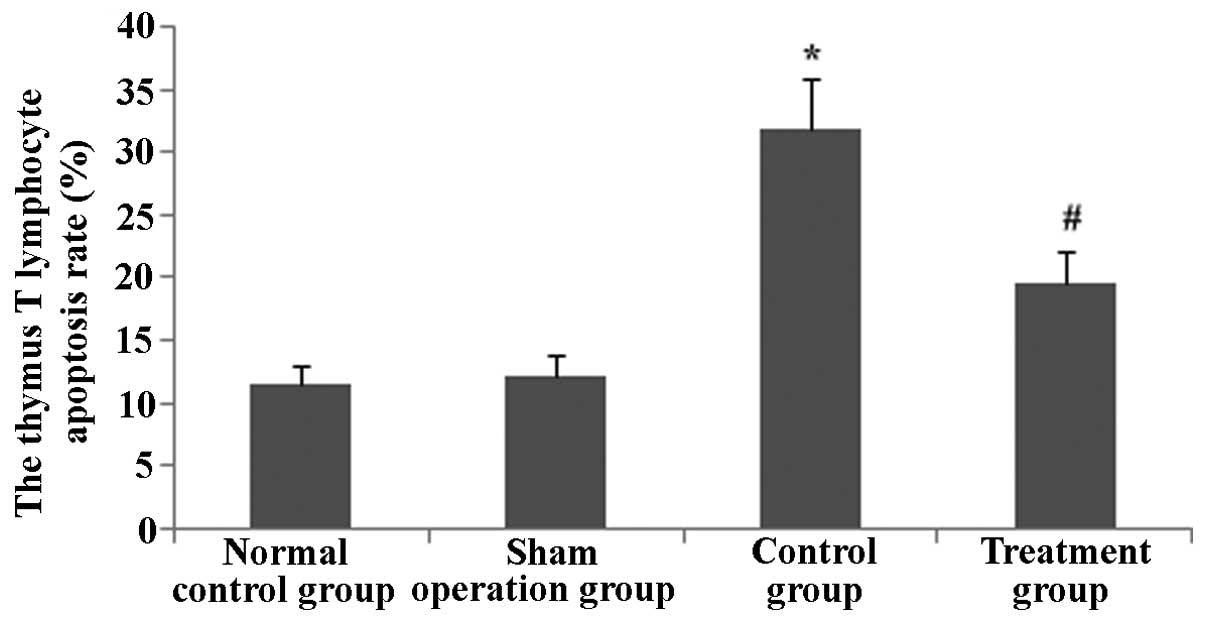
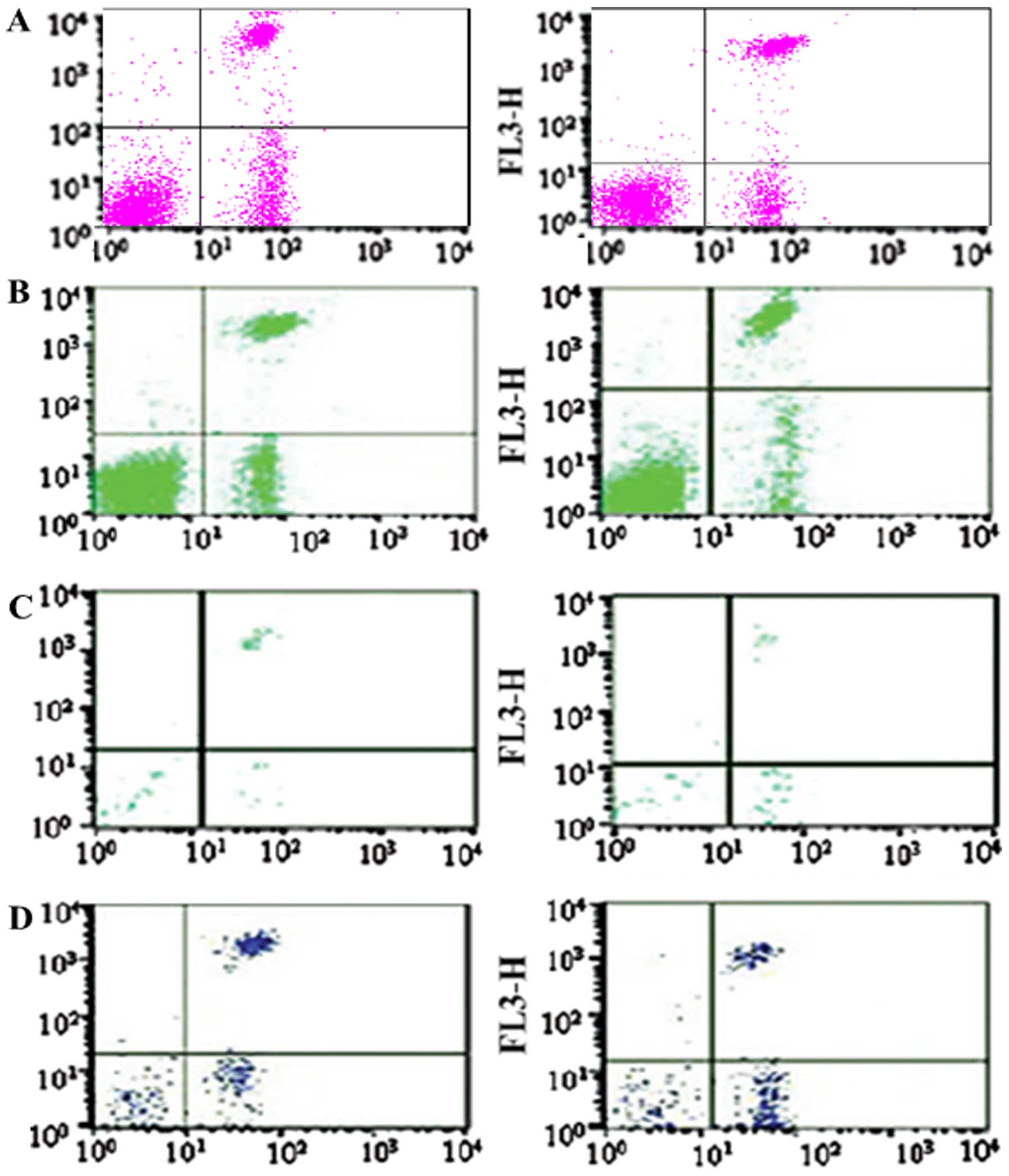
Rhodiola rosea extract reduces the
apoptotic rate of thymus T lymphocytes in septic mice
To determine the effect of Rhodiola rosea
extract on thymus T-lymphocyte apoptosis in septic mice, flow
cytometry and TUNEL assays were performed. After the groups of mice
were induced with CLP, the apoptotic rate of thymus lymphocytes was
markedly enhanced. Of note, in the group pre-treated with
Rhodiola rosea extract, the apoptotic rate of thymus
lymphocytes was markedly decreased as compared with that in the CLP
group (all P<0.05). Thus, Rhodiola rosea extract markedly
decreased the apoptotic rate of thymus lymphocytes (Figs. 9Figure 10Figure 11–12).
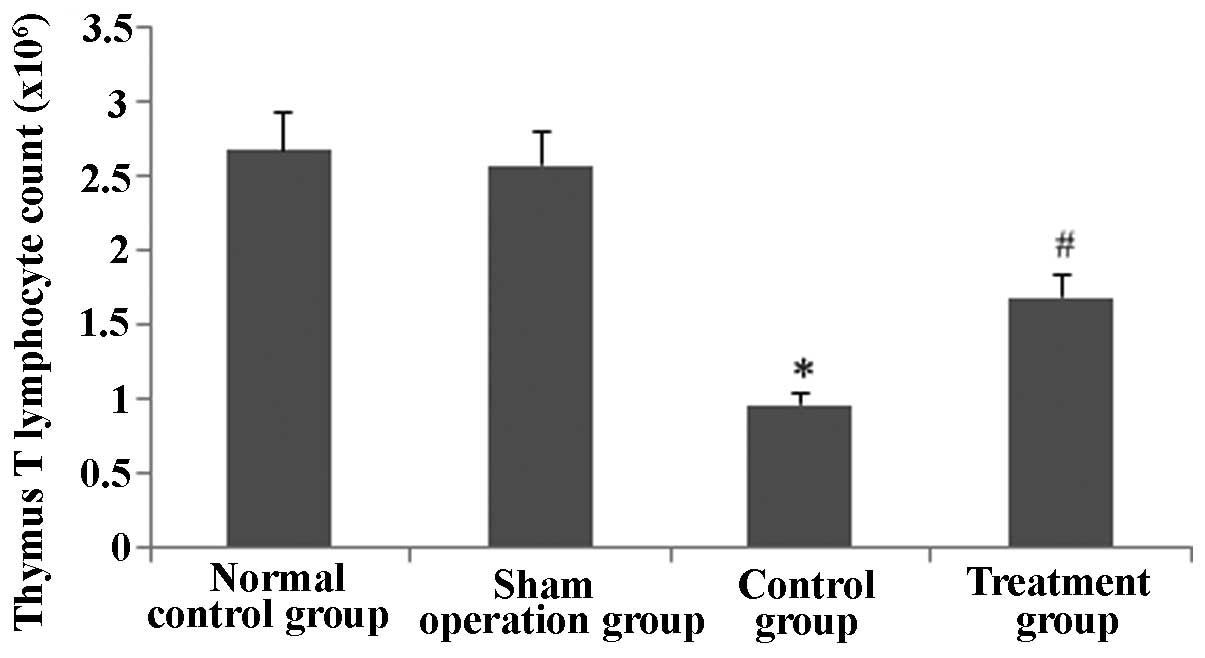
Rhodiola rosea extract attenuates
decreases in the thymus T-lymphocyte count in septic mice
As sepsis stimulated thymus T lymphocyte apoptosis,
the thymus T-lymphocyte count was determined. As shown in Figs. 13 and 14, the thymus T-lymphocyte counts were
markedly decreased in CLP-induced mice at 24 h (P<0.05);
however, in the group pre-treated with Rhodiola rosea
extract, the thymus T lymphocyte count was significantly increased
(P<0.05).
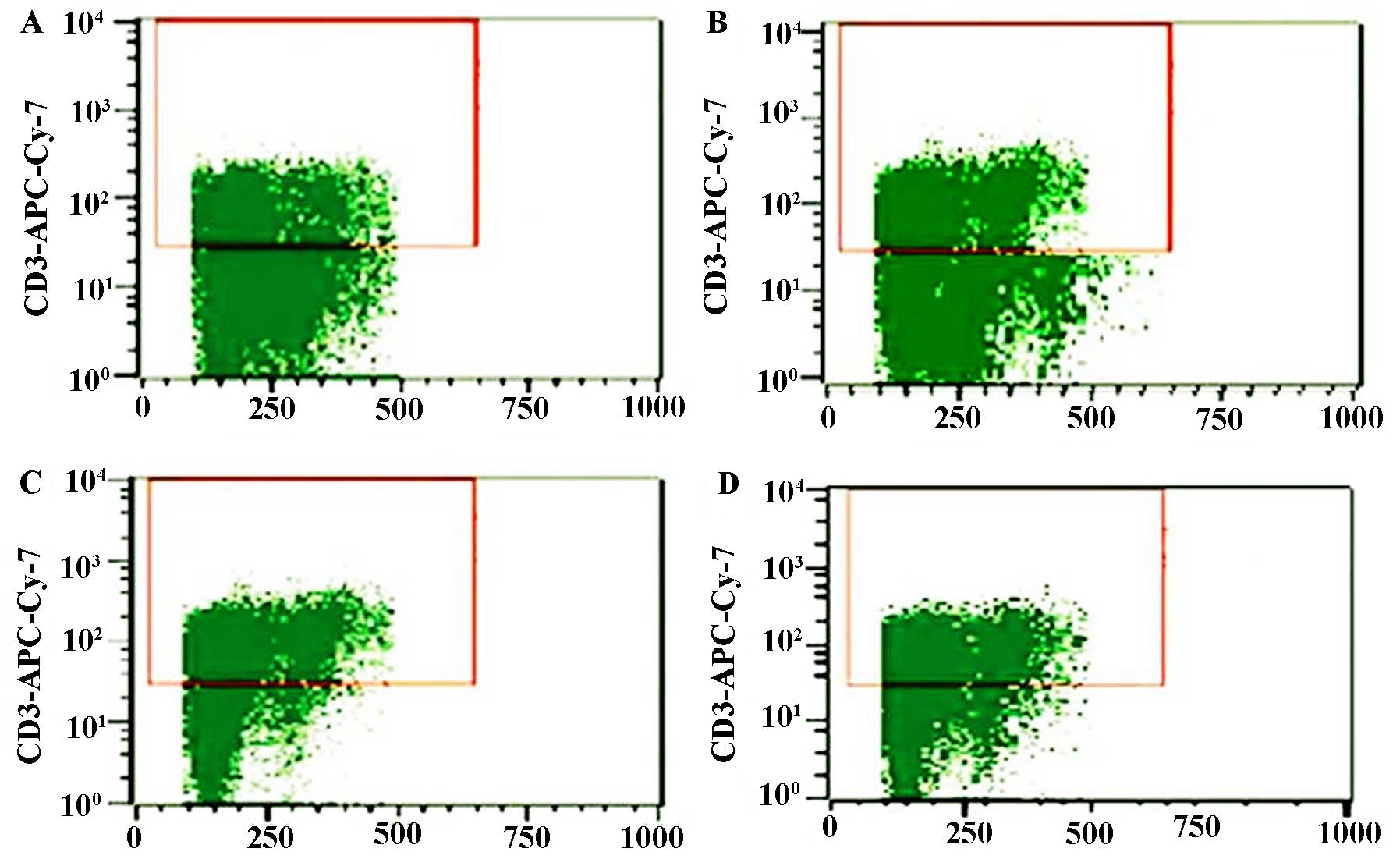
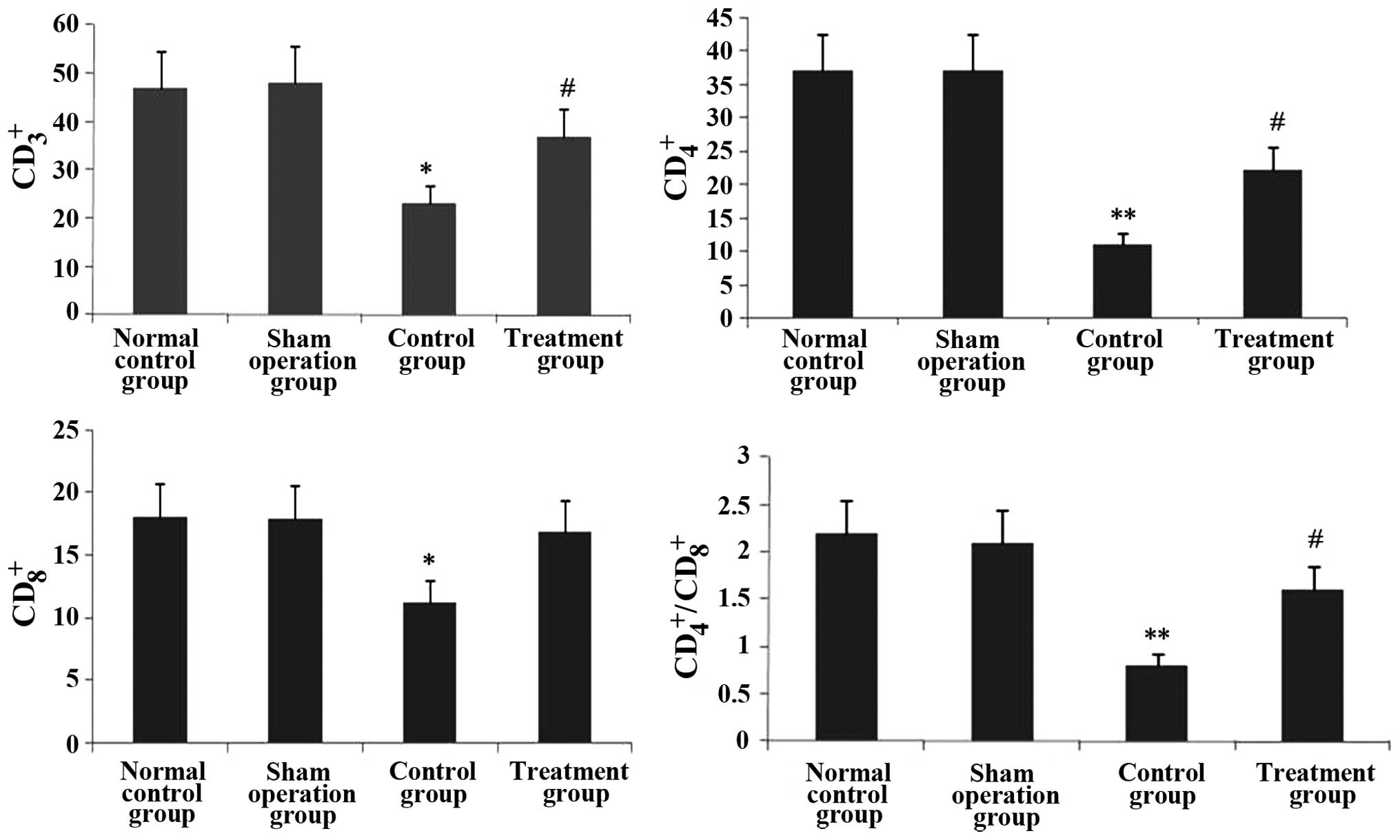
Rhodiola rosea extract rescues the
decreases in CD3+, CD4+, CD8+ and
CD4+/CD8+ T-lymphocyte sub-sets in septic
mice
The effect of Rhodiola rosea extract on
thymus T-lymphocyte sub-sets in septic mice was evaluated. As shown
in Figs. 15 and 16, the CD3+,
CD4+, CD8+ and
CD4+/CD8+ T-lymphocyte sub-sets were markedly
decreased in septic mice (all P<0.05). However, pre-treatment
with Rhodiola rosea extract significantly increased the
CD3+, CD4+, CD8+ and
CD4+/CD8+ T-lymphocyte sub-sets (all
P<0.05).
Rhodiola rosea extract enhances increases
of Th1 cytokines in the plasma of septic mice, while not affecting
increases in Th2 cytokines
To study the effects of Rhodiola rosea
extract on Th1/Th2 cytokines, levels of Th1 cytokines (IFNγ, IL-2
and IL-12) and Th2 cytokines (IL-4 and IL-10) in plasma were
measured using ELISA. As shown in Fig. 17, Th1 and Th2 cytokines were
markedly enhanced at 24 h after CLP challenge. Of note,
pre-treatment with Rhodiola rosea extract led to further
increases in Th1 cytokines (IFNγ, IL-2 and IL-12; all P<0.05),
while Th2 cytokine levels were not significantly altered compared
with those in the model (sepsis) group. These results indicated
that treatment with Rhodiola rosea extract attenuated the
host’s immunity.
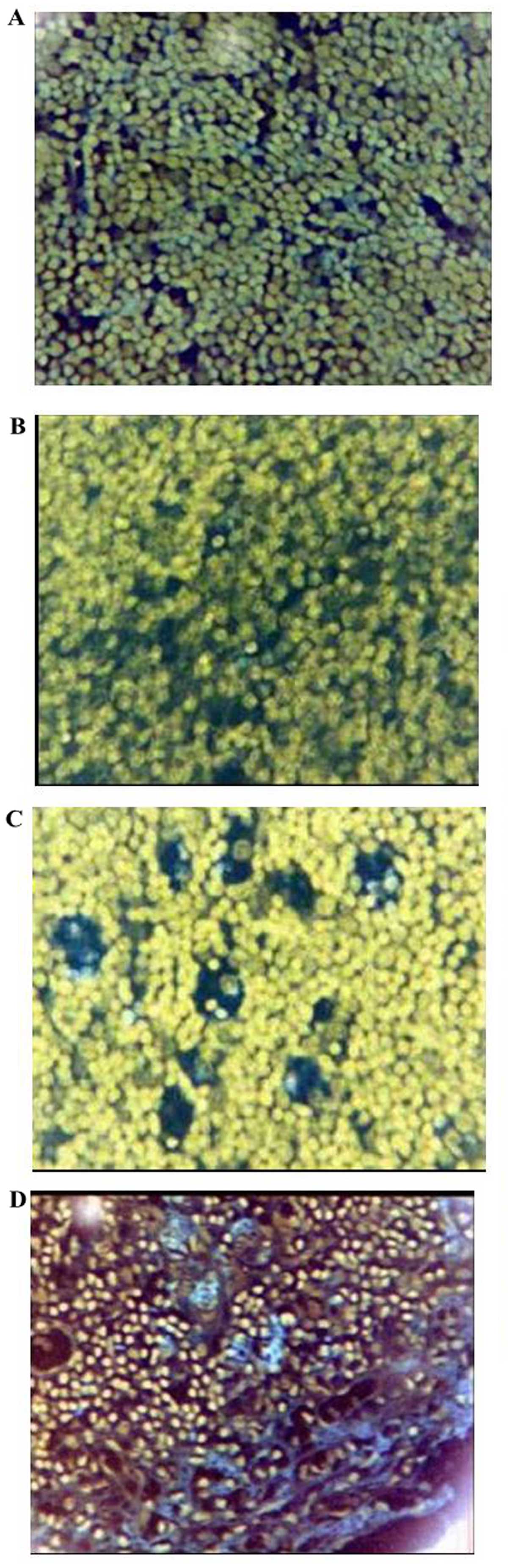
Histopathological changes of the
thymus
To evaluate the effects of Rhodiola rosea
extract on the histopathological changes in the thymus of mice with
CLP-induced sepsis, histological assessment was performed of thymus
tissue collected 24 h after the administration of CLP with or
without treatment. As shown in Fig.
18, the thymus lobular structure disappeared and the cortical
and medullary lymphocytes were significantly reduced after the CLP
operation. After administration of Rhodiola rosea extract,
the thymus exhibited lobular structures, and cortical and medullary
lymphocytes were significantly increased. The thymus index was also
determined. As shown in Fig. 19,
the thymus index was markedly lowered at 24 h after CLP
(P<0.05), while pre-treatment with Rhodiola rosea extract
markedly increased the thymus index (P<0.05). Thus, Rhodiola
rosea had a protective effect on the thymus.
Rhodiola rosea extract increases the
survival of septic mice
The survival of mice was markedly decreased in mice
receiving CLP compared to that of mice in the control group. The
decreased survival induced by CLP was significantly attenuated by
pre-treatment with Rhodiola rosea extract as compared to
that in the untreated CLP-induced group (Fig. 20).
Discussion
Several studies have shown that immune disorders are
involved throughout the entire occurrence and developmental process
of sepsis, which ultimately leads to organ failure and subsequent
mortality (26). The regulation
of the body’s immunity has long been a focus of biomedical research
(27). Sepsis is one of the
leading causes of mortality of clinical patients (28). The occurrence and development of
sepsis are caused by an imbalance of pro-inflammatory and
anti-inflammatory mechanisms (29). When the disease develops to a
certain degree, the body’s immune function is inhibited to various
degrees, which eventually leads to a highly lethal immune disorder
that is characterized by immune cell apoptosis and severe paralysis
(30). The large decrease in the
number and function of immune cells through apoptosis is one of the
major causes of the high mortality in patients with severe sepsis
(31,32). Apoptosis of cells in central
lymphoid organs (spleen) and peripheral circulating lymphocytes in
patients with severe sepsis was shown to be significantly
increased, particularly in patients with septic shock, and
eventually leads to paralysis which is dominated by a specific
immune dysfunction (33). Thus,
the reduction of lymphocyte apoptosis is currently one important
and challenging aspect of sepsis treatment and an important measure
to decrease the mortality rate of patients with sepsis (34). The present study revealed that
mice with sepsis demonstrated an increase in thymus T-lymphocyte
apoptosis as well as a decrease in the thymus T-lymphocyte count.
Furthermore, CD+, CD4+ and
CD4+/CD8+ T-lymphocyte sub-sets were
decreased and the secretion of IL-2 and IFN-γ was decreased,
leading to immune suppression in the host.
TNFAIP8 has an important regulatory role in cell
apoptosis and signal transduction, as well as tumor occurrence,
development and invasion (35).
TIPE2, a newly discovered protein of the of TNFAIP8 family, has
drawn increasing attention (36).
TIPE2 was initially discovered by Sun et al (37) at the University of Pennsylvania in
an experimental autoimmune encephalomyelitis model and is an
essential protein that is important in maintaining immune
homeostasis. Sun et al (37) found that TIPE2 was associated with
caspase-8 and regulated the nuclear factor (NF)-κB pathway via
apoptotic enzymes. There is evidence indicating that TIPE2 can
inhibit the activation of activator protein-1 and NF-κB, while
cells lacking TIPE2 genes demonstrate high reactivity in response
to Toll-like receptor and T-cell receptor signaling (38). In addition, in a sepsis-induced
mouse model treated with low doses of endotoxin, septic shock
occurred in TIPE2-knockout mice (39). However, further analysis has
indicated that the downregulation of TIPE2 genes induces persistent
lymphocyte activation, enhancement of Fas expression, promotion of
lymphocyte apoptosis and increased production of IL-4, IL-6, IL-12
and IFN-γ (37). TIPE2 also
functions to regulate cell death, and the expression of inactive
TIPE2 genes can inhibit Fas-mediated apoptosis and antigen
receptor-induced cell death (40). Moreover, the numbers of T
lymphocytes, B lymphocytes and dendritic cells in TIPE2-knockout
septic mice were significantly increased (41). Thus, TIPE2, as a newly discovered
protein, has an important role in immune homeostasis. The results
of the present showed that with an enhancement in TIPE2 expression,
the expression of apoptosis-promoting proteins, including Fas, FasL
and Bcl-2, also increased T-lymphocyte apoptosis and the
T-lymphocyte count, and significantly decreased the
CD3+, CD4+ and
CD4+/CD8+ T-lymphocyte sub-sets as well as
immunity. However, these changes were reversed in animals
pre-treated with Rhodiola.
In human sepsis, two immunologically distinct stages
are observed: An early pro-inflammatory phase and a late,
compensatory anti-inflammatory phase (42). CLP-induced sepsis increases
lymphocyte apoptosis, which has been shown to cause
immunosuppression during the late phase of human sepsis (43). Immunological cytokines are
functionally categorised into two groups: One group with Th1-like
properties and a second group with Th2-like properties. Activated
CD4+ T cells are programmed to secrete cytokines with
inflammatory properties, including the Th1-type cytokines IFN-γ,
IL-2 and IL-12, or cytokines with anti-inflammatory properties,
including the Th2-type cytokines IL-4 and IL-10 (44). In addition to attenuating the
abrupt release of pro-inflammatory cytokines, including TNF-α,
IL-1β and IL-6, treatment with chlorogenic acid enhanced serum Th1
cytokines during the late phase of sepsis without affecting Th2
cytokine release (45). It has
been suggested that increases in Th1 cytokines are beneficial in
the late stage of sepsis when immunosuppression predominates and
can cause mortality (46).
Decreased Th1 function has been previously reported in patients
with peritonitis, and defective T-cell proliferation and cytokine
secretion correlate with mortality. In the present study, CLP
facilitated the secretion of Th1 cytokines and Th2 cytokines. Of
note, pre-treatment with Rhodiola rosea further increased
the secretion of Th1 cytokines while not affecting Th2 cytokine
levels, and therefore improved the host’s immunity.
The thymus is an important immunoregulatory organ
and has an important role in SIRS and MODS. The results of the
present study indicated that the apoptotic rate of thymus T
lymphocytes increased, the lobular structure of the thymus
disappeared, that cortical and medullary lymphocytes were
significantly reduced, and that the thymus volume and thymus index
were deceased 24 h after CLP. However, in the group pre-treated
with Rhodiola rosea extract, the apoptotic rate of thymus T
lymphocytes decreased, the thymus exhibited lobular structures,
cortical and medullary lymphocytes were significantly increased,
and the thymus volume and thymus index were increased.
Furthermore, the results of the present study showed
that the survival of the mice was higher in the treated group
compared with that in the model group. Furthermore, the
pathological changes were milder in the treated group compared with
those in the model group, and the apoptotic rate was significantly
decreased (P<0.05), which demonstrated that Rhodiola
rosea extract has a protective effect on the thymus by
preventing apoptosis. The apoptotic rate was dependent on the TIPE2
status of T cells from the thymus and was decreased in septic mice
pre-treated with Rhodiola rosea; furthermore, changes in Th1
and Th2 cytokines were also in parallel with changes in TIPE2
expression, which suggested that Rhodiola rosea extract can
alleviate thymus injury by lowering TIPE2 expression, increasing
the secretion of Th1 cytokines, inhibiting thymus T-lymphocyte
apoptosis, and increasing the CD3+, CD4+,
CD8+ and CD4+/CD8+ T-lymphocyte
sub-sets, ameliorating the host’s immunity and increasing the
survival of mice.
In recent years, studies have shown that Rhodiola
rosea can strongly enhance cellular immunity and humoral immune
function in mice, which has become a research hotspot (47). Previous studies have indicated
that the extract of Rhodiola rosea can inhibit tumor growth
in S-180 tumor-bearing mice, potentially via the enhancement of the
body’s immune function, thereby inducing the secretion of cytotoxic
proteins by T cells, resulting in increases in antibody-secreting B
cells and antibodies, thus inhibiting the growth of tumors
(48,49). Previous studies have shown that
within the dose range of 500–2,000 mg/kg, Rhodiola rosea can
enhance the relative quality of the immune response, foot pad
thickness, thymus weight and spleen antibody production in BALB/C
mice (50). Intragastric
administration of the stem and leaf extract of Rhodiola
rosea at 250–500 mg/kg to mice for seven days increased the
quality of the immune response in normal mice and significantly
enhanced phagocytosis by the reticuloendothelial system (51). Reproduction of these experimental
conditions in a rat model with low immune function induced by
cyclophosphamide, which was treated with rhodiola polysaccharides
from Tibetan Rhodiola rosea demonstrated that rhodiola
polysaccharides had no significant effect on white blood cells in
peripheral blood or the thymus quality, although it lowered the
peripheral blood haemoglobin content in normal mice and improved
the blood haemoglobin content in immunodeficient mice (52). In addition, Rhodiola rosea
promoted the differentiation of spleen lymphocytes and the activity
of natural killer cells, thus reversing the abovementioned changes
in immunosuppressed mice (51).
Rhodiola can also strengthen the function of the mononuclear
phagocyte system, thereby improving the body’s immune defence
(53). In accordance with the
results of a previous study (54), the present study indicated that
the total glycosides of rhodiola can suppress TIPE2 expression in
mice with sepsis, lower the expression of the apoptosis-promoting
proteins Fas and FasL, decrease T-lymphocyte apoptosis, increase
the number of thymus T lymphocytes and the CD3+,
CD4+ and CD4+/CD8+ T-lymphocyte
sub-sets, increase the levels of Th1 cytokines, including IFNγ,
IL-2 and IL-12, and enhance the host’s immunity.
In conclusion, the present study showed that the
total glycosides of rhodiola were able to suppress the
sepsis-induced overexpression of TIPE2 in mice, inhibit the
upregulated expression of the apoptosis-promoting proteins Fas and
FasL, increase the decreased expression of the apoptosis-inhibiting
protein Bcl-2, decrease the enhanced T-lymphocyte apoptosis,
increase the decreased numbers of thymus T lymphocytes and
CD3+, CD4+ and
CD4+/CD8+ T-lymphocyte sub-sets, further
enhance the Th1 cytokines IFNγ, IL-2 and IL-12, and enhance the
host’s immunity. These findings provided a theoretical basis for
the application of rhodiola in the treatment of sepsis.
Acknowledgments
The authors would like to thank Professor Mei-Xian
Su (Department of Emergency, The Second Affiliated Hospital of
Kunming Medical University, Kunming, China) for providing helpful
comments on the present study and Mr. Xu Liu (Department of
Emergency, The First Affiliated Hospital of Kunming Medical
University, Kunming, China) for providing excellent technical
assistance.
Abbreviations:
|
TIPE2
|
TNF-α-inducible protein 8-like 2
|
|
CLP
|
caecal ligation and puncture
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
FasL
|
Fas ligand
|
|
Fas
|
Fas receptor
|
|
Th1
|
T helper type 1 cells
|
|
Th2
|
T helper type 2 cells
|
|
IL-2
|
interleukin-2
|
|
IL-10
|
interleukin-10
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IFNγ
|
interferon gamma
|
References
|
1
|
Cepinskas G and Wilson JX: Inflammatory
response in micro-vascular endothelium in sepsis: role of oxidants.
J Clin Biochem Nutr. 42:175–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotchkiss RS, Coopersmith CM, McDunn JE
and Ferguson TA: The sepsis seesaw: tilting toward
immunosuppression. Nat Med. 15:496–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng H, Guo L, Song Z, Gao H, Wang D, Fu
W, Han J, Li Z, Huang B and Li XA: Caveolin-1 protects against
sepsis by modulating inflammatory response, alleviating bacterial
burden, and suppressing thymocyte apoptosis. J Biol Chem.
285:25154–25160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hotchkiss RS, Tinsley KW and Karl IE: Role
of apoptotic cell death in sepsis. Scand J Infect Dis. 35:585–592.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotchkiss RS, Osmon SB, Chang KC, Wagner
TH, Coopersmith CM and Karl IE: Accelerated lymphocyte death in
sepsis occurs by both the death receptor and mitochondrial
pathways. J Immunol. 174:5110–5118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Wei X, Liu L, Liu S, Wang Z,
Zhang B, Fan B, Yang F, Huang S, Jiang F, Chen YH and Yi F: TIPE2,
a novel regulator of immunity, protects against experimental
stroke. J Biol Chem. 287:32546–32555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar
|
|
8
|
Zhang S, Zhang Y, Wei X, Zhen J, Wang Z,
Li M, Miao W, Ding H, Du P, Zhang W, He M and Yi F: Expression and
regulation of a novel identified TNFAIP8 family is associated with
diabetic nephropathy. Biochim Biophys Acta. 1802:1078–1086. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu MW, Su MX, Wang YH and Qian CY: Effect
of Melilotus extract on lung injury via the upregulation of tumor
necrosis factor-α-induced protein-8-like 2 in septic mice. Mol Med
Rep. 11:1675–1684. 2015.
|
|
10
|
Guo Y, Zhao Y, Zheng C, Meng Y and Yang Y:
Synthesis, biological activity of salidroside and its analogues.
Chem Pharm Bull (Tokyo). 58:1627–1629. 2010. View Article : Google Scholar
|
|
11
|
Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman
M and Zhu YC: Antioxidants in Chinese herbal medicines: a
biochemical perspective. Nat Prod Rep. 21:478–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F, Tang H, Xiao F, Gong J, Peng Y and
Meng X: Protective effect of salidroside from Rhodiolae Radix on
diabetes-induced oxidative stress in mice. Molecules. 16:9912–9924.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong H, Xin H, Wu LX and Zhu YZ:
Salidroside attenuates apoptosis in ischemic cardiomyocytes: a
mechanism through a mitochondria-dependent pathway. J Pharmacol.
14:399–408. 2010.
|
|
14
|
Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan
IA and Oh S: Anti-inflammatory and neuroprotective effects of
constituents isolated from Rhodiola rosea. Evid Based Complement
Alternat Med. 2013:5140492013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spanakis M, Vizirianakis IS, Batzias G and
Niopas I: Pharmacokinetic interaction between losartan and Rhodiola
rosea in rabbits. Pharmacology. 91:112–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan S, He J, Guo W, Wei J, Lu J and Deng
X: Adjuvant effects of salidroside from Rhodiola rosea L. on the
immune responses to ovalbumin in mice. Immunopharmacol
Immunotoxicol. 33:738–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu L, Yuan J and Zhang S: Rejuvenating
activity of salidroside (SDS): dietary intake of SDS enhances the
immune response of aged rats. Age. 35:637–646. 2013. View Article : Google Scholar :
|
|
18
|
Taylor M, Cieslak M, Rees GS, Oojageer A,
Leith C, Bristow C, Tawn EJ, Winther JF and Boice JD Jr: Comparison
of germ line minisatellite mutation detection at the CEB1 locus by
Southern blotting and PCR amplification. Mutagenesis. 25:343–349.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Fayngerts S, Wang P, Sun H,
Johnson DS, Ruan Q, Guo W and Chen YH: TIPE2 protein serves as a
negative regulator of phagocytosis and oxidative burst during
infection. Proc Natl Acad Sci USA. 109:15413–15418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tavernier G, Wolfrum K, Demeester J, De
Smedt SC, Adjaye J and Rejman J: Activation of
pluripotency-associated genes in mouse embryonic fibroblasts by
non-viral transfection with in vitro-derived mRNAs encoding Oct4,
Sox2, Klf4 and cMyc. Biomaterials. 33:412–417. 2012. View Article : Google Scholar
|
|
21
|
Tassone F, Pan R, Amiri K, Taylor AK and
Hagerman PJ: A rapid polymerase chain reaction-based screening
method for identification of all expanded alleles of the fragile X
(FMR1) gene in newborn and high-risk populations. J Mol Diagn.
10:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wichterman KA, Baue AE and Chaudry IH:
Sepsis and septic shock - a review of laboratory models and a
proposal. J Surg Res. 29:189–201. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Felzmann T, Witt V, Wimmer D, Ressmann G,
Wagner D, Paul P, Hüttner K and Fritsch G: Monocyte enrichment from
leukapharesis products for the generation of DCs by plastic
adherence, or by positive or negative selection. Cytotherapy.
5:391–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Julius MH, Simpson E and Herzenberg LA: A
rapid method for the isolation of functional thymus-derived murine
lymphocytes. Eur J Immunol. 3:645–649. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Celik S, Erdogan S and Tuzcu M: Caffeic
acid phenethyl ester (CAPE) exhibits significant potential as an
antidiabetic and liver-protective agent in streptozotocin-induced
diabetic rats. Pharmacol Res. 60:270–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osuchowski MF, Welch K, Siddiqui J and
Remick DG: Circulating cytokine/inhibitor profiles reshape the
understanding of the SIRS/CARS continuum in sepsis and predict
mortality. J Immunol. 177:1967–1974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JS, Kim SJ and Lee SM: Genipin
attenuates sepsis-induced immunosuppression through inhibition of T
lymphocyte apoptosis. Int Immunopharmacol. 27:15–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalil AC and Opal SM: Sepsis in the
severely immunocompromised patient. Curr Infect Dis Rep.
17:4872015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oberholzer A, Oberholzer C and Moldawer
LL: Sepsis syndromes: understanding the role of innate and acquired
immunity. Shock. 16:83–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao D, Zhang D, Xiang D, Liu QI, Liu Y,
Lv L and Xing X: Effects of fentanyl, midazolam and their
combination on immune function and mortality in mice withsepsis.
Exp Ther Med. 9:1494–1500. 2015.PubMed/NCBI
|
|
31
|
Muenzer JT, Davis CG, Chang K, Schmidt RE,
Dunne WM, Coopersmith CM and Hotchkiss RS: Characterization and
modulation of the immunosuppressive phase of sepsis. Infect Immun.
78:1582–1592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parker SJ and Watkins PE: Experimental
models of gram-negative sepsis. Br J Surg. 88:22–30. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wesche DE, Lomas-Neira JL, Perl M, Chung
CS and Ayala A: Leukocyte apoptosis and its significance in sepsis
and shock. J Leukoc Biol. 78:325–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wijnands KA, Castermans TM, Hommen MP,
Meesters DM and Poeze M: Arginine and citrulline and the immune
response in sepsis. Nutrients. 7:1426–1463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laliberté B, Wilson AM, Nafisi H, Mao H,
Zhou YY, Daigle M and Albert PR: TNFAIP8:a new effector for
Galpha(i) coupling to reduce cell death and induce cell
transformation. J Cell Physiol. 225:865–874. 2010. View Article : Google Scholar
|
|
36
|
Li D, Song L, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 33:422–427. 2009.
View Article : Google Scholar
|
|
37
|
Sun H, Gong S, Carmody RJ, Hilliard A, LI
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma
C, Chen YH and Zhang L: The unique expression profile of human
TIPE2 suggests new functions beyond its role in immune regulation.
Mol Immunol. 48:1209–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jadali Z, Amiri MM and Ravanbakhsh M:
Apoptosis of peripheral blood mononuclear cells in patients with
sepsis. Indian J Pathol Microbiol. 53:646–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brudecki L, Ferguson DA, McCall CE and El
Gazzar M: Myeloid-derived suppressor cells evolve during sepsis and
can enhance or attenuate the systemic inflammatory response. Infect
Immun. 80:2026–2034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Courtine E, Cagnard N, Mazzolini J, Antona
M, Pène F, Fitting C, Jacques S, Rousseau C, Niedergang F,
Gerondakis S, et al: Combined loss of cRel/p50 subunits of NF-κB
leads to impaired innate host response in sepsis. Innate Immun.
18:753–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chauhan PS, Satti NK, Sharma P, Sharma VK,
Suri KA and Bani S: Differential effects of chlorogenic acid on
various immunological parameters relevant to rheumatoid arthritis.
Phytother Res. 26:1156–1165. 2012. View Article : Google Scholar
|
|
46
|
Muralinath M, Kuehn MJ, Roland KL and
Curtiss R: Immunization with Salmonella enterica serovar
Typhimurium-derived outer membrane vesicles delivering the
pneumococcal protein PspA confers protection against challenge with
Streptococcus pneumoniae. Infect Immun. 79:887–894. 2011.
View Article : Google Scholar :
|
|
47
|
Udintsev SN and Shakhov VP: The role of
humoral factors of regenerating liver in the development of
experimental tumors and the effect of Rhodiola rosea extract on
this process. Neoplasma. 38:323–331. 1991.PubMed/NCBI
|
|
48
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: the anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Loo WT, Jin LJ, Chow LW, Cheung MN and
Wang M: Rhodiola algida improves chemotherapy-induced oral
mucositis in breast cancer patients. Expert Opin Investig Drugs.
19:S91–S100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Skopńska-Rózewska E, Wójcik R, Siwicki AK,
Sommer E, Wasiutyński A, Furmanowa M, Malinowski M and Mazurkiewicz
M: The effect of Rhodiola quadrifida extracts on cellular immunity
in mice and rats. Pol J Vet Sci. 11:105–111. 2008.
|
|
51
|
Diwaker D, Mishra KP, Ganju L and Singh
SB: Rhodiola inhibits dengue virus multiplication by inducing
innate immune response genes RIG-I, MDA5 and ISG in human
monocytes. Arch Virol. 159:1975–1986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li HX, Sze SC, Tong Y and Ng TB:
Production of Th1- and Th2-dependent cytokines induced by the
Chinese medicine herb, Rhodiola algida, on human peripheral blood
monocytes. J Ethnopharmacol. 123:257–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen SP, Huang Liu R, Lu TM, Wei JC, Wu
TC, Tsai WY, Tsai CH and Yang CC: Complementary usage of Rhodiola
crenulata (L.) in chronic obstructive pulmonary disease patients:
The effects on cytokines and T cells. Phytother Res. 29:518–525.
2015. View Article : Google Scholar
|
|
54
|
Hu B, Zou Y, Liu S, Wang J, Zhu J, Li J,
Bo L and Deng X: Salidroside attenuates concanavalin A-induced
hepatitis via modulating cytokines secretion and lymphocyte
migration in mice. Mediators Inflamm. 2014:3140812014. View Article : Google Scholar : PubMed/NCBI
|