Introduction
Apelin was first identified as an endogenous ligand
for the orphan G protein-coupled receptor APJ from bovine stomach
extracts in 1998 (1). Apelin/APJ
are widely expressed in various tissues, including the heart, lung,
liver, brain and bone (2). They
have multiple biological activities, including regulation of food
intake, blood pressure and endoplasmic reticulum (ER) stress
(3–6). Several studies have shown that
apelin was abundantly expressed in malignant carcinoma tissues,
where it promoted the occurrence, proliferation and metastasis of
cancer cells. Li et al found that apelin-13 increased
vascular smooth muscle cell proliferation by upregulating the
expression of cyclin D1, which was involved in an ERK-dependent
activation of the Jagged-1/Notch3 signaling pathway (7). Apelin is associated with tumor cell
proliferation, invasion and tube formation in vitro
(8–10). Apelin-13 could induce lung
adenocarcinoma cell proliferation and autophagy via
ERK1/2 pathway (9).
Apelin may be associated with angiogenesis and tumorigenesis.
Recent studies demonstrated that a high level of apelin mRNA is
expressed in a human breast carcinoma cell line (11). However, the mechanism of apelin-13
in breast cancer remains unknown.
The cell cycle is one of the most important
activities in cell life processes; the G1 phase
activation is the critical process. Cyclin D1 is responsible for
cell cycle progression in the transition from
G0/G1 to S phase. The overexpression of
cyclin D1 was identified in lung and breast cancer. Cyclin D1 may
have an important role in the generation and development of human
breast cancer. Extracellular matrix metalloproteinases (MMPs) are a
family of Zn2+-dependent enzymes. MMP-1 (collagenase I)
specifically degrades collagen I, a major component of the
extracellular matrix (ECM). In a previous study, overexpression of
MMP-1 was associated with advanced stages of breast cancer and may
be a predictive marker for invasive disease (12). In the present study, the
correlation of apelin-13, cyclin D1 and MMP-1 in MCF-7 cells was
evaluated.
The oncogene nuclear receptor coactivator amplified
in breast cancer 1 (AIB1) is a transcriptional coactivator that is
overexpressed in breast cancer. AIB1 has been identified as
amplified in 2–10% of human breast cancer tumors and overexpressed
in 30–60% (13–17). The correlation between AIB1 and
proliferation has also been shown for prostate (18), esophageal squamous cell (19) and urothelial cancer (20). AIB1 can interact with other
signaling pathways (21–23). Therefore, the association between
AIB1 and apelin-13 was investigated, as well as their effect on
proliferation and invasion with or without PD98059 treatment.
The present study reported that apelin-13 stimulated
the proliferation of MCF-7 and promoted the expression of AIB1 and
cyclin D1. The ERK1/2 inhibitor PD98059 attenuated the
expression of cyclin D1, AIB1 and MMP-1 and prevented
apelin-13-induced MCF-7 proliferation and invasion. The study
provided evidence that the effects of apelin-13 on MCF-7
proliferation and invasion were partly mediated by the
ERK/AIB1/cyclin D1/MMP-1 signaling pathways.
Materials and methods
Cell culture
MCF-7 human breast adenocarcinoma cells (ATCC,
Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS),
100 units penicillin and 100 µg streptomycin (all from
Gibco-BRL, Gaithersburg, MD, USA). The cells were maintained in the
growing medium in a humidified 5% CO2 atmosphere at
37°C.
MTT assay
MCF-7 cells were cultured in 96-well plates
(5×104 cells) and were synchronized for 24 h in DMEM
containing 0.1% FBS. After treatment with (0.01, 0.1 and 10
µM) apelin-13 or pre-treatment with PD98059 (Sigma, St.
Louis, MO, USA) for 1 h followed by the addition of 10 µM
apelin-13 for 24 h, the cells were incubated with MTT solution
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 4 h at 37°C.
Non-reduced MTT was removed by aspiration and the formazan crystals
were dissolved in dimethyl sulfoxide (150 µl/well) for 30
min at 37°C. The formazan was spectroscopically quantified at 490
nm using a Bio-Rad Microplate Reader (Bio-Rad, Hercules, CA,
USA).
Cell proliferation assay
MCF-7 cells in 3 ml of DMEM with 10% FBS were plated
in a 6-well plate at a density of 1×106 cells/well and
incubated at 37°C with 5% CO2. After 24 h, the cells
were treated with apelin-13 (0.01, 0.1 and 10 µM) in
serum-free medium and incubated for 12 h under similar conditions.
BrdU (Amresco LLC, Solon, OH, USA) was added to the culture medium
1 h before the end of the 24-h incubation period, cells were
collected and each cell sample was subsequently analyzed by flow
cytometry.
Tumor invasion assay
The invasion of MCF-7 cells was measured using
Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA). After
12 h of serum depletion, MCF-7 cells were detached and resuspended
with DMEM containing 0.1% bovine serum albumin (BSA) and added to
the upper compartment with 2×105 cells per each
Transwell insert. The lower compartment of each well contained 0.5
ml of DMEM with 0.1% BSA with apelin-13 (0.01, 0.1 and 10
µM) or pre-treatment with PD98059 for 1 h followed by the
addition of 10 µM apelin-13. After 6 h of incubation, the
upper compartment was fixed and stained with 0.1% crystal violet
(Zhuo Kang Biological Technology Co., Ltd., Shanghai, China) for 10
min. The number of invasion cells in 10 fields per filter was
counted using a microscope (magnification, ×100). Crystal violet
was eluted with 33% acetic acid, and the eluent optical density
value was measured at 570 nm using the Bio-Rad Microplate
Reader.
RNA extraction
Total RNA from MCF-7 cells was extracted with TRIzol
reagent (Invitrogen Japan K.K., Tokyo, Japan) following the
manufacturer's instructions, and subsequently frozen at −20°C in
RNase-free water [Takara Biotechnology (Dalian) Co., Ltd.,
Liaoning, China]. Single-stranded cDNA was reverse transcribed from
total RNA (500 ng) using a First Strand cDNA Synthesis kit for
RT-PCR [Takara Biotechnology (Dalian) Co., Ltd.] and a random
primer. Reverse transcription conditions were 1 cycle of 30°C for
10 min, 45°C for 30 min, 95°C for 5 min, and finally 5°C for 5
min.
Reverse transcription polymerase chain
reaction (RT-PCR)
cDNA (1 µg) was reverse-transcribed according
to the manufacturer's instructions [Takara Biotechnology (Dalian)
Co., Ltd.]. The sequences for the sense and antisense primers
respectively were: Cyclin D1, 5′-GATGCCAACCTCCTCAACGAC-3′ and
5′-CTCCTCGCACTTCTGTTCCTC-3′ (171 bp); MMP-1,
5′-CCTTCTACCCGGAAGTTGAG-3′ and 5′-TCCGTGTAGCACATTCTGTC-3′ (158 bp);
AIB1, 5′-CCAGCTGCACACTCTTCTCA-3′ and 5′-TAAGAAAACCCTGCTGGGGC-3′
(488 bp); and GAPDH, 5′-TCGGAGTCAACGGATTTGGTCGTA-3′ and
5′-TGGCATGGACTGTGGTCATGAGTC-3′ (525 bp). The cyclin D1 and MMP-1
amplifi-cation program consisted of 5 min for initial denaturation
at 95°C followed by 35 cycles at 94°C for 30 sec, 60°C for 30 sec,
72°C for 30 sec and 7 min for the final extension at 72°C. The AIB1
and GAPDH amplification program consisted of 5 min for initial
denaturation at 95°C followed by 35 cycles at 94°C for 30 sec, 60°C
for 30 sec, 72°C for 40 sec and 7 min for the final extension at
72°C. The PCR products underwent electrophoresis in a 1.5% (w/v)
agarose gel (Biowest, Barcelona, Spain), with a thickness ~0.5 cm.
The gel imaging was scanned and the optical density of the
electrophoretic bands was determined using BandScan 5.0 software
(Glyko, Novato, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
MCF-7 cells were plated at a density of
2×105 cells/well in 96-well plates. The concentrations
of MMP-1 and cyclin D1 were quantified by ELISA kits (Invitrogen
Life Technologies). The colorimetric reaction was measured using a
Microplate Reader (Bio-Rad) at 450 nm wavelength.
Western blotting
To assay the levels of protein expression and
phosphorylation of p44/42 ERK1/2 and β-actin, MCF-7
cells were cultured in 6-well plates and treated with 0.01, 0.1 and
10 µM of recombinant apelin-13 or pre-treatment with PD98059
for 1 h followed by the addition of 10 µM apelin-13 for 12
h. For immunoblotting, cells were washed twice with ice-cold
phosphate-buffered saline and subsequently lysed in
radioimmunoprecipitation assay buffer with a cocktail of protease
inhibitors on ice for 15 min. Following centrifugation at 13,000 ×
g for 20 min at 4°C, the quantity of protein in the supernatants
was detected using the bicinchoninic acid Protein Assay kit
(Beijing Solarbio Science & Technology Co., Ltd., Shanghai,
China). The protein samples were separated by SDS-PAGE and were
transferred onto PVDF membranes (Millipore, Billerica, MA, USA).
Following blocking with 5% non-fat milk for 1 h, the membranes were
incubated with primary antibodies specific for phospho-p44/42
ERK1/2 (1:2,000; #4370) and β-actin (1:2,000; #4970)
(all from Cell Signaling Technology, Inc., Danvers, MA, USA)
overnight at 4°C. Blots underwent three 8 min washes with
Tris-buffered saline with 0.1% Tween-20 and were incubated with
secondary antibodies IgG (1:8,000; #14708; Cell Signaling
Technology, Inc.) for 1 h. The membranes were washed as above and
the bands were scanned by the Tanon 5500 fully automatic digital
gel image analysis system (Tanon Science & Technology Co.,
Ltd., Shanghai, China).
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD). The mean and SD of each dataset were calculated
using the SigmaPlot 10.0 software (Systat Software, San Jose, CA,
USA). The differences between groups were assessed using the t-test
to determine P-values (P<0.05 was considered to indicate a clear
difference, and P<0.01 was considered to indicate a
statistically significant difference).
Results
Apelin-13 promotes the proliferation of
MCF-7 cells and cyclin D1 expression
Previous studies have suggested that apelin
stimulates cell proliferation and invasion in several cell lines,
including endothelial, lung and oral squamous cells (7,9,10).
The present study examined the effects of apelin-13 on MCF-7
proliferation and invasion. Using the MTT and flow cytometry assay,
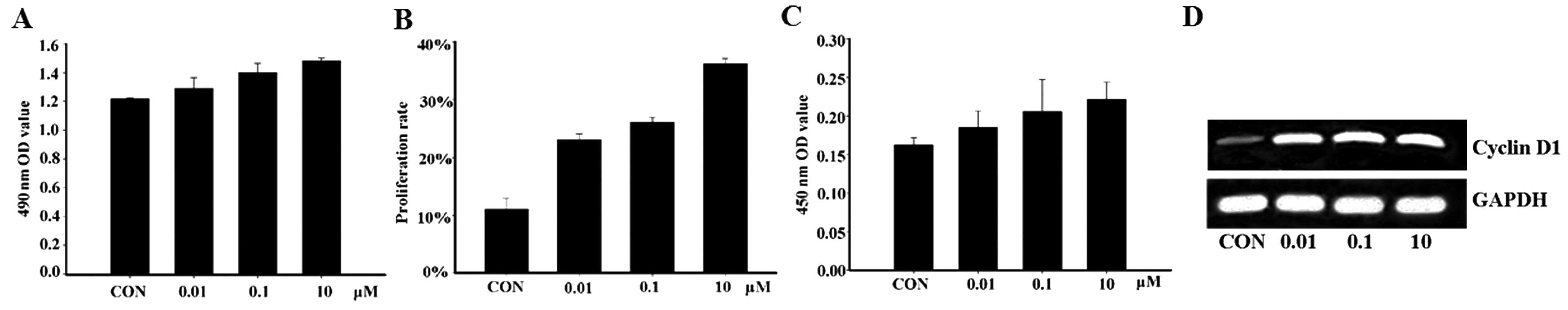
the treatment of MCF-7 with apelin-13 was demonstrated to
significantly increase cell proliferation (Fig. 1A and B). In order to explore the
effect of apelin-13 on cyclins, the expression level of cyclin D1
was detected by ELISA and RT-PCR analysis. The results showed that
the protein and mRNA expression levels of cyclin D1 were
significantly increased after MCF-7 cells were incubated with
apelin-13 for 12 h, suggesting that apelin-13 affects the cell
cycle through increasing cyclin D1 expression, and subsequently
induces MCF-7 cell proliferation (Fig. 1C and D).
Apelin-13 promotes the invasion of MCF-7
cells and MMP-1 expression
Degradation of the stromal connective tissue and
basement membrane components are critical elements in tumor
invasion. This is particularly true regarding the interstitial
collagens, which were degraded by MMPs. MMP-1 is a member of a
family of enzymes that can degrade the majority of ECM
macromolecules, particularly types I and III. Therefore, whether
apelin-13 could promote MCF-7 cell invasion and MMP-1 expression
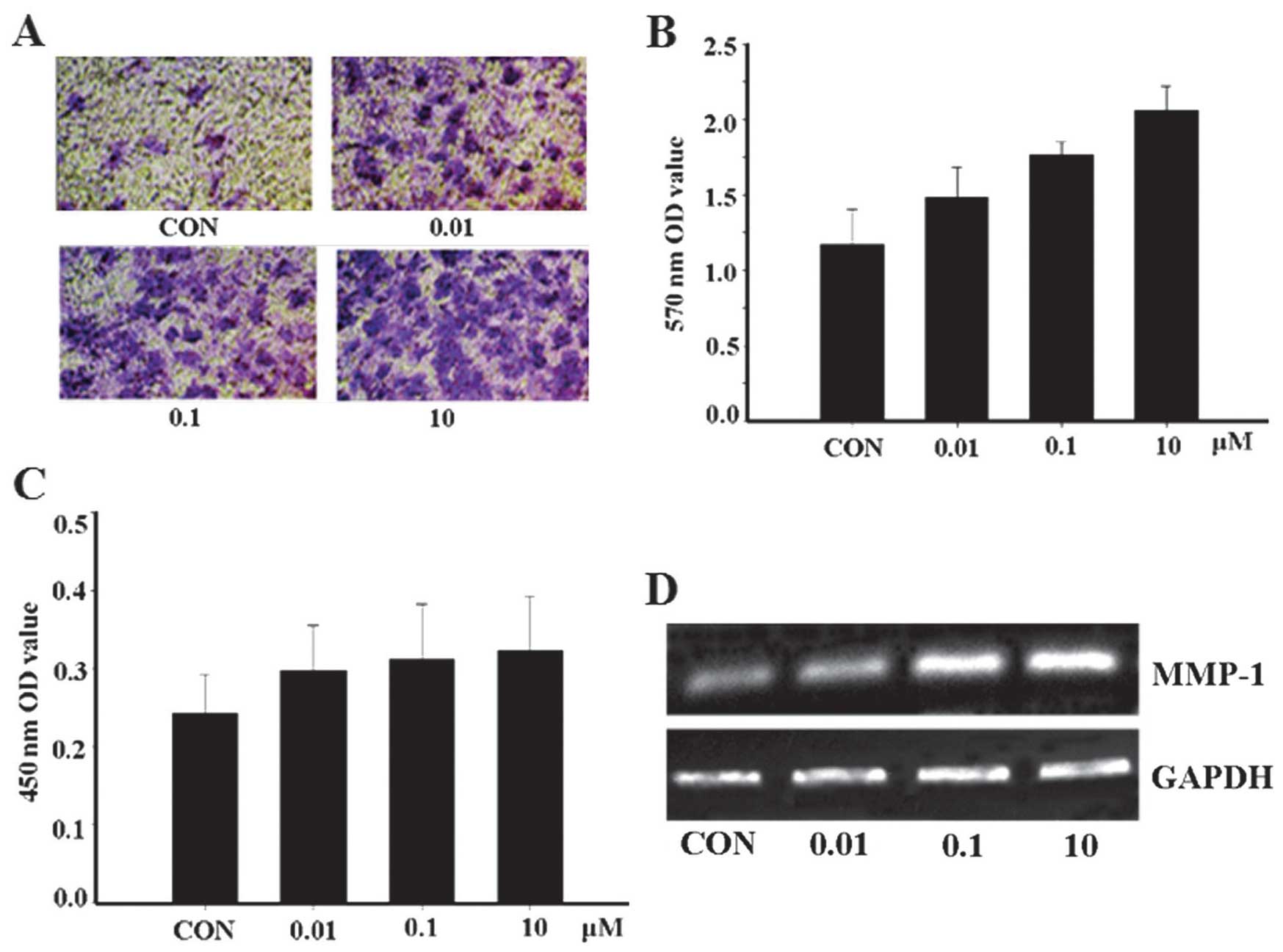
was investigated. As hypothesized, the results revealed that the
quantity of apelin-13-treated cells passing through the basement
membrane into the lower compartment was significantly greater
compared with that of the control group (Fig. 2A and B). This finding suggests
that apelin-13 can promote the invasion of MCF-7 cells.
Subsequently, the expression of MMP-1 was examined and the
expression of the proteins was closely correlated with apelin-13
(Fig. 2C and D). The results
showed that MMP-1 may be critically involved in the metastasis of
tumors.
Apelin-13 activates the phosphorylation
of ERK1/2 in MCF-7 cells
To evaluate the potential signaling pathways
involved in apelin-13-induced MCF-7 cell proliferation and
invasion, the role of apelin-13 on ERK1/2 was examined
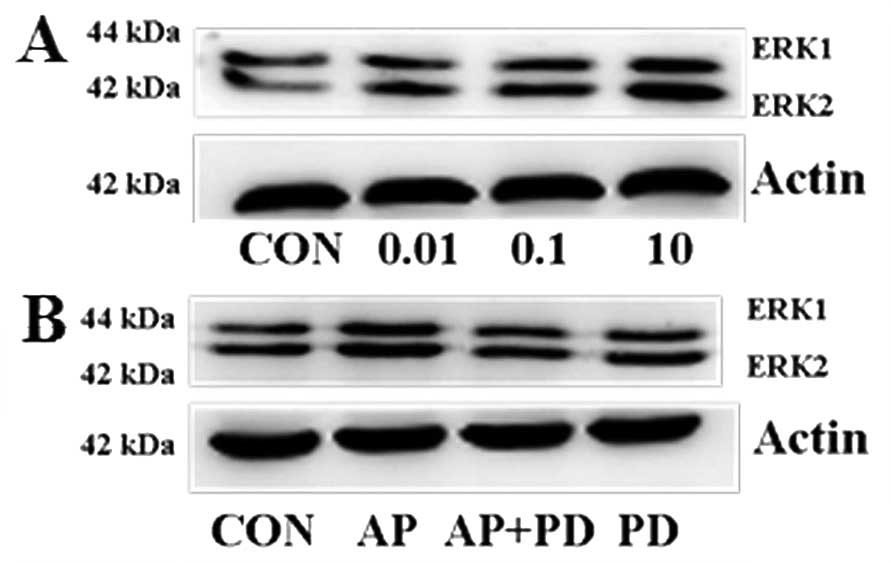
by western blotting. As is shown in Fig. 3A, dose-dependent effects of
apelin-13 on ERK1/2 activation were determined.
ERK1/2 reached maximal phosphorylation upon treatment
with apelin-13 at 10 µM. PD98059 (an inhibitor of
ERK1/2) significantly inhibited the overexpression of
apelin-13-induced ERK1/2 phosphorylation (Fig. 3B).
PD98059 inhibits apelin-13-induced MCF-7
cell proliferation
To further evaluate whether ERK1/2 has
any effect on apelin-13-induced MCF-7 cell proliferation, the
protein and mRNA expression levels of cyclin D1 were detected. MTT
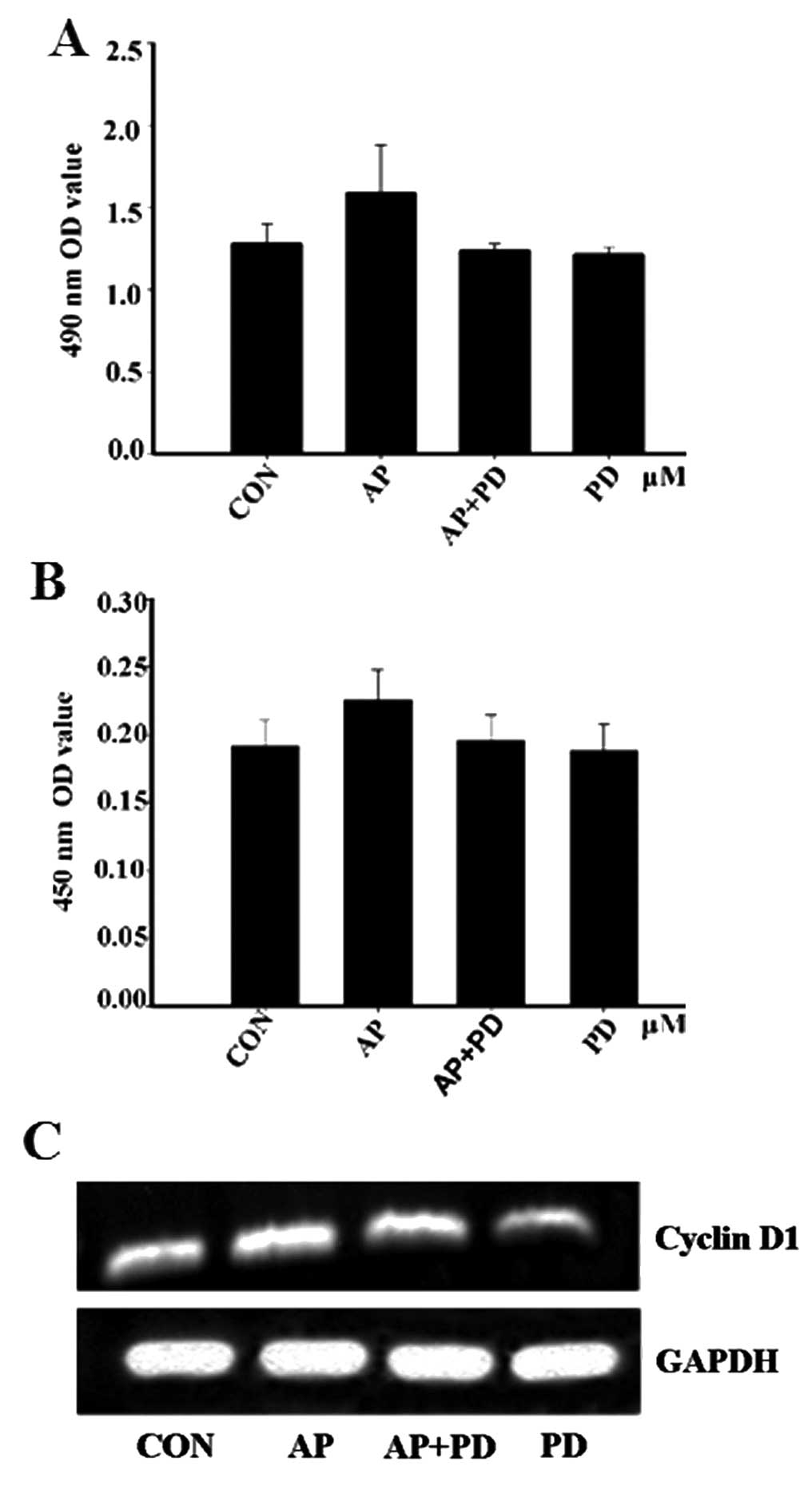
analysis indicated that pre-treatment with PD98059 prevented
apelin-13-induced MCF-7 proliferation (Fig. 4A). The data were confirmed by
ELISA and RT-PCR. The levels of cyclin D1 expression were decreased
following pre-incubation with PD98059 for 1 h (Fig. 4B and C). These results suggested
that apelin-13 influenced MCF-7 cell proliferation, possibly via
ERK1/2 signaling cascades.
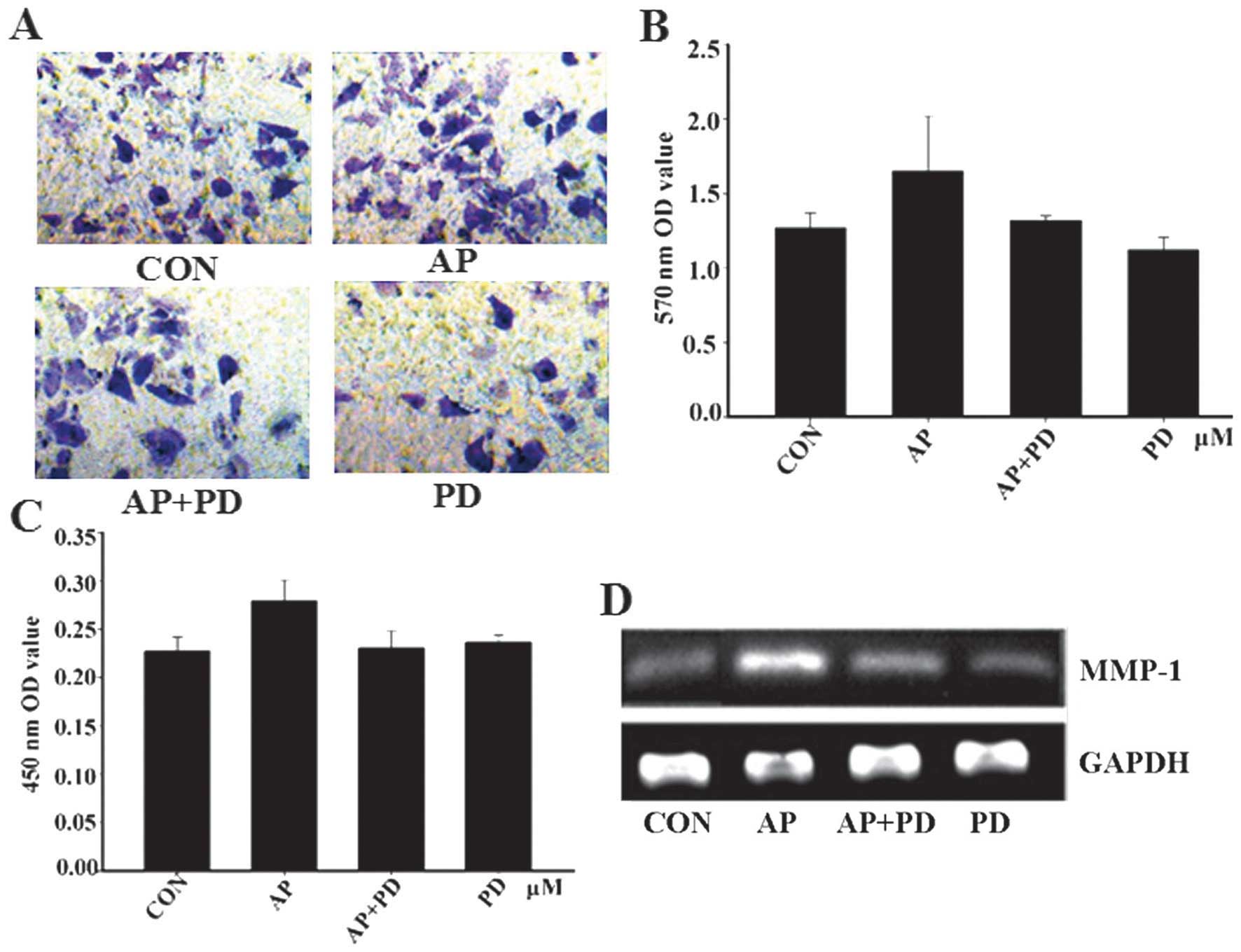
PD98059 inhibits apelin-13-induced MCF-7
cell invasion
To address the functional significance of
apelin-mediated MCF-7 cell invasion in the context of
ERK1/2 activation, the effect of PD98059 upon MCF-7 cell
invasion and MMP-1 was examined. MCF-7 cell invasion and the
expression of MMP-1 were reduced by PD98059 (Fig. 5). These results suggested that
apelin-13-induced invasion of MCF-7 cells and ERK1/2 may
be involved in the signaling pathway.
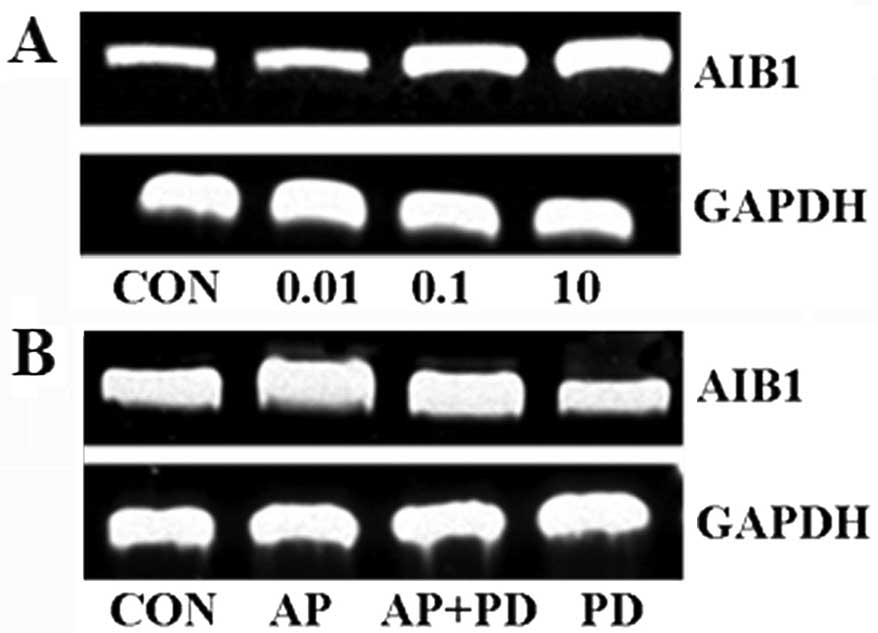
Apelin-13 promotes AIB1 mRNA expression
via ERK1/2
AIB1 amplification and overexpression are associated
with proliferation, invasion and poor prognosis in breast cancer.
Therefore, AIB1 mRNA expression was detected by RT-PCR. Apelin-13
could promote the endogenous levels of AIB1 mRNA in a
concentration-dependent manner. Additionally, the molecular
mechanism of AIB1 overexpression was observed following treatment
with the apelin-13. The result showed that PD98059 inhibited AIB1
expression. Together, these data suggested that apelin-13 promoted
AIB1 expression via the ERK1/2 signal pathway (Fig. 6).
Discussion
In the present study, the regulation and signaling
of apelin on breast cancer cells were investigated. A previous
finding identified the role for AIB1 in breast cancer and described
a new mechanism of ERα/AIB1 gene regulation, which could regulate
cyclin D1 genes depending on the promoter context (24). The coactivator protein AIB1 has
previously been associated with the initiation of breast cancer.
The MMPs are indicated in the basic processes of tumor progression,
such as degradation of basement membrane and ECM, stimulation of
cellular proliferation and invasion. In the present study, several
methods by which apelin-13 regulated cell proliferation, invasion
and the underlying mechanisms and signaling pathways were
demonstrated as follows: i) MCF-7 cell proliferation was documented
by the MTT and flow cytometry assays; ii) MCF-7 cell invasion was
measured by the Transwell assay; iii) cyclin D1, MMP-1 and AIB1
were transcriptionally upregulated by apelin-13, induced in a
dose-dependent manner; iv) apelin-13 enhanced the phosphorylation
of ERK1/2 in a dose-dependent manner; and v) cyclin D1,
MMP-1 and AIB1 were transcriptionally inhibited by PD98059. These
results showed that apelin-13 treatment promoted human MCF-7
proliferation and invasion in a dose-dependent manner, which was
consistent with our previously reported results (Fig. 1A and B; Fig. 2A and B). To further explore the
role of apelin-13-induced proliferation and invasion of MCF-7
cells, the expression of cyclin D1 and MMP-1 were detected using
ELISA and RT-PCR assays. The results revealed that the expression
levels were upregulated in a concentration-dependent manner
(Fig. 1C and D; Fig. 2C and D). Finally, the downstream
pathways that contribute to proliferating MCF-7 cells were
investigated. The apelin/APJ system has been shown to induce
various signaling pathways, including the JNK, P38 MAPK and
ERK1/2 pathways. The present results showed that
apelin-13 promoted the expression of ERK1/2
phosphorylation and PD98059 (an ERK1/2 inhibitor)
inhibited MCF-7 cell proliferation and invasion induced by
apelin-13 (Figs. 3Figure 4–5). These data also revealed that the
activation of the ERK1/2 pathway could regulate cyclin
D1, MMP-1 and ERK1/2 expression. PD98059 was able to
repress cyclin D1 and MMP-1 expression (Fig. 4B and C; Fig. 5C and D). These results indicated
that apelin-13 induced MCF-7 cell proliferation and invasion via
the ERK signaling pathway. Additionally, AIB1 has an important role
that is relevant to breast cancer cell survival and proliferation.
Activation of the ERK1/2 pathway upon growth factor or
hormone stimulation led to the subsequent transactivation of AIB1
in MCF-7 breast cancer cells (25). ERK1/2 signaling was
affected in mice with reduced AIB1 levels during Neu-induced
tumorigenesis (26). Therefore,
the effects of apelin-13 and PD98059 on AIB1 mRNA expression were
analyzed. The results suggested that AIB1 expression was promoted
by apelin-13 and reduced following pre-treatment with PD98059
(Fig. 6). These data suggested
that AIB1 may have an important role in tumor maintenance by
regulating cyclin D1 and MMP-1 in breast cancer cells, which may be
due to the activation of the ERK cascade and thereby resulting in
activation of the cyclin D1 promoter.
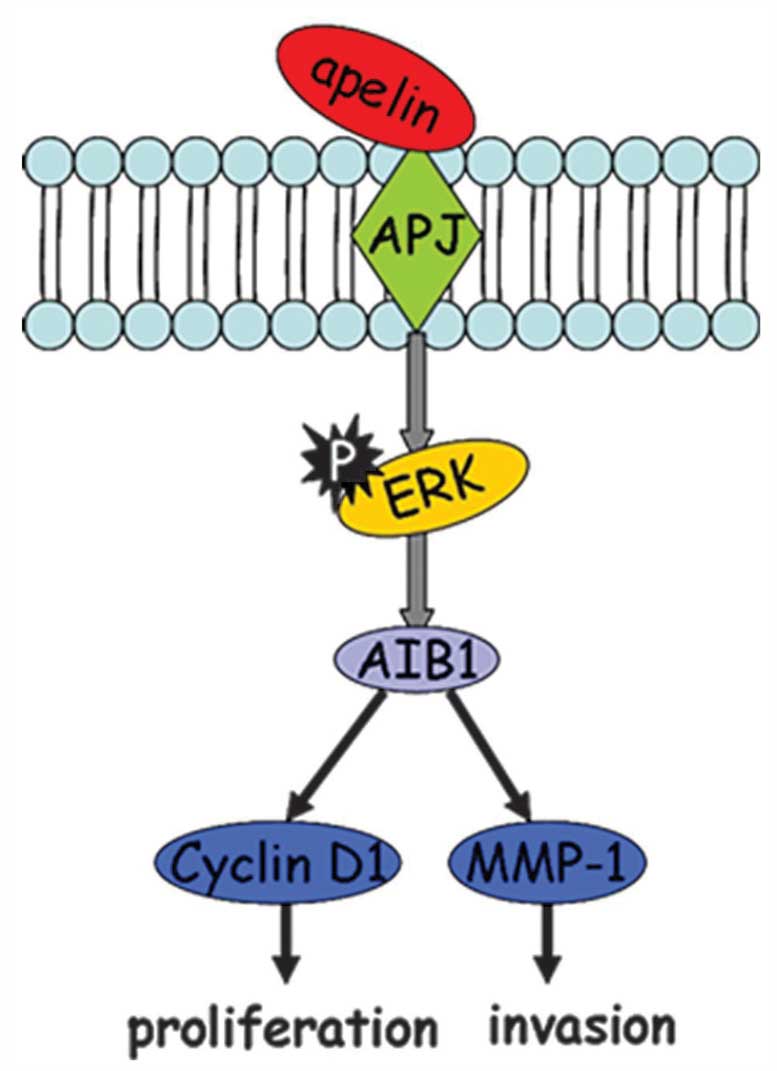
In conclusion, as shown in Fig. 7, apelin-13-induced MCF-7
proliferation and invasion was mediated through the
ERK1/2/AIB1 signaling pathway, further leading to
transcriptional activation of downstream target proteins cyclin D1
and MMP-1. This novel finding not only revealed the mechanism of
apelin-13-induced MCF-7 cell proliferation, but also provided
certain potential targets for future treatment of breast
cancer.
Acknowledgments
The present study was supported by the Natural
Science Fund of Jilin (grant no. 201215001) and the Jilin Province
Science and Technology Research Projects (grant no.
20140204059YY).
References
|
1
|
Tatemoto K, Hosoya M, Habata Y, et al:
Isolation and characterization of a novel endogenous peptide ligand
for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Carroll AM, Selby TL, Palkovits M and
Lolait SJ: Distribution of mRNA encoding B78/apj, the rat homologue
of the human APJ receptor, and its endogenous ligand apelin in
brain and peripheral tissues. Biochim Biophys Acta. 1492:72–80.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sunter D, Hewson AK and Dickson SL:
Intracerebroventricular injection of apelin-13 reduces food intake
in the rat. Neurosci Lett. 353:1–4. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishida J, Hashimoto T, Hashimoto Y, et al:
Regulatory roles for APJ, a seven-transmembrane receptor related to
angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem.
279:26274–26279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akcılar R, Turgut S, Caner V, Akcılar A,
Ayada C, Elmas L and Özcan TO: Apelin effects on blood pressure and
RAS in DOCA-salt-induced hypertensive rats. Clin Exp Hypertens.
35:550–557. 2013. View Article : Google Scholar
|
|
6
|
Jeong K, Oh Y, Kim S-J, Kim H, Park KC,
Kim SS, Ha J, Kang I and Choe W: Apelin is transcriptionally
regulated by ER stress-induced ATF4 expression via a p38
MAPK-dependent pathway. Apoptosis. 19:1399–1410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Li L, Xie F, Zhang Z, Guo Y, Tang G,
Lv D, Lu Q, Chen L and Li J: Jagged-1/Notch3 signaling transduction
pathway is involved in apelin-13-induced vascular smooth muscle
cells proliferation. Acta Biochim Biophys Sin (Shanghai).
45:875–881. 2013. View Article : Google Scholar
|
|
8
|
Masri B, Morin N, Cornu M, Knibiehler B
and Audigier Y: Apelin (65–77) activates p70 S6 kinase and is
mitogenic for umbilical endothelial cells. FASEB J. 18:1909–1911.
2004.PubMed/NCBI
|
|
9
|
Yang L, Su T, Lv D, Xie F, Liu W, Cao J,
Sheikh IA, Qin X, Li L and Chen L: ERK1/2 mediates lung
adenocarcinoma cell proliferation and autophagy induced by
apelin-13. Acta Biochim Biophys Sin (Shanghai). 46:100–111. 2014.
View Article : Google Scholar
|
|
10
|
Heo K, Kim YH, Sung HJ, et al:
Hypoxia-induced up-regulation of apelin is associated with a poor
prognosis in oral squamous cell carcinoma patients. Oral Oncol.
48:500–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Greeley GH Jr and Qiu S:
Immunohistochemical localization of apelin in human normal breast
and breast carcinoma. J Mol Histol. 39:121–124. 2008. View Article : Google Scholar
|
|
12
|
Poola I, DeWitty RL, Marshalleck JJ,
Bhatnagar R, Abraham J and Leffall LD: Identification of MMP-1 as a
putative breast cancer predictive marker by global gene expression
analysis. Nat Med. 11:481–483. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan XY, Xu J, Anzick SL, Zhang H, Trent
JM and Meltzer PS: Hybrid selection of transcribed sequences from
microdissected DNA: Isolation of genes within amplified region at
20q11-q13.2 in breast cancer. Cancer Res. 56:3446–3450.
1996.PubMed/NCBI
|
|
14
|
Anzick SL, Kononen J, Walker RL, Azorsa
DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM and
Meltzer PS: AIB1, a steroid receptor coactivator amplified in
breast and ovarian cancer. Science. 277:965–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bautista S, Vallès H, Walker RL, Anzick S,
Zeillinger R, Meltzer P and Theillet C: In breast cancer,
amplification of the steroid receptor coactivator gene AIB1 is
correlated with estrogen and progesterone receptor positivity. Clin
Cancer Res. 4:2925–2929. 1998.PubMed/NCBI
|
|
16
|
Bouras T, Southey MC and Venter DJ:
Overexpression of the steroid receptor coactivator AIB1 in breast
cancer correlates with the absence of estrogen and progesterone
receptors and positivity for p53 and HER2/neu. Cancer Res.
61:903–907. 2001.PubMed/NCBI
|
|
17
|
List HJ, Reiter R, Singh B, Wellstein A
and Riegel AT: Expression of the nuclear coactivator AIB1 in normal
and malignant breast tissue. Breast Cancer Res Treat. 68:21–28.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou HJ, Yan J, Luo W, Ayala G, Lin SH,
Erdem H, Ittmann M, Tsai SY and Tsai MJ: SRC-3 is required for
prostate cancer cell proliferation and survival. Cancer Res.
65:7976–7983. 2005.PubMed/NCBI
|
|
19
|
Xu FP, Xie D, Wen JM, Wu HX, Liu YD, Bi J,
Lv ZL, Zeng YX and Guan XY: SRC-3/AIB1 protein and gene
amplification levels in human esophageal squamous cell carcinomas.
Cancer Lett. 245:69–74. 2007. View Article : Google Scholar
|
|
20
|
Luo JH, Xie D, Liu MZ, Chen W, Liu YD, Wu
GQ, Kung HF, Zeng YX and Guan XY: Protein expression and
amplification of AIB1 in human urothelial carcinoma of the bladder
and over-expression of AIB1 is a new independent prognostic marker
of patient survival. Int J Cancer. 122:2554–2561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louie MC, Zou JX, Rabinovich A and Chen
HW: ACTR/AIB1 functions as an E2F1 coactivator to promote breast
cancer cell proliferation and antiestrogen resistance. Mol Cell
Biol. 24:5157–5171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torres-Arzayus MI, Font de Mora J, Yuan J,
Vazquez F, Bronson R, Rue M, Sellers WR and Brown M: High tumor
incidence and activation of the PI3K/AKT pathway in transgenic mice
define AIB1 as an oncogene. Cancer Cell. 6:263–274. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tilli MT, Reiter R, Oh AS, Henke RT,
McDonnell K, Gallicano GI, Furth PA and Riegel AT: Overexpression
of an N-terminally truncated isoform of the nuclear receptor
coactivator amplified in breast cancer 1 leads to altered
proliferation of mammary epithelial cells in transgenic mice. Mol
Endocrinol. 19:644–656. 2005. View Article : Google Scholar
|
|
24
|
O'Hara J, Vareslija D, McBryan J, et al:
AIB1:ERα transcriptional activity is selectively enhanced in
aromatase inhibitor-resistant breast cancer cells. Clin Cancer Res.
18:3305–3315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Font de Mora J and Brown M: AIB1 is a
conduit for kinase-mediated growth factor signaling to the estrogen
receptor. Mol Cell Biol. 20:5041–5047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fereshteh MP, Tilli MT, Kim SE, Xu J,
O'Malley BW, Wellstein A, Furth PA and Riegel AT: The nuclear
receptor coactivator amplified in breast cancer-1 is required for
Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis
in mice. Cancer Res. 68:3697–3706. 2008. View Article : Google Scholar : PubMed/NCBI
|