Introduction
Hypertrophic scarring/hypertrophic scars (HS) is a
frequent complication following burns and trauma wounds (1). Patients with HS often complain of
pain, pruritus and loss of joint mobility (2). Although the pathological mechanisms
underpinning HS formation remain elusive, it has been demonstrated
that HS formation results from the dysregulation of the wound
healing process (3). For example,
exaggerated inflammation (4), the
overabundant production of collagen (5), excessive contraction at the wound
site (6), the reduced apoptosis
of fibroblasts (7) or delayed
re-epithelialization (8) during
wound healing has been reported to lead to the formation of HS.
Transforming growth factor-β1 (TGF-β1) has been
widely reported to play an essential role in wound healing and HS
formation (9). In particular,
there is evidence indicating that TGF-β1 mediates fibroblast
proliferation, collagen production, extracellular matrix (ECM)
deposition and myofibroblast differentiation in the wound healing
process (10). Moreover, it has
been reported that the enhanced expression of TGF-β1 stimulates
collagen synthesis in fibroblasts by activating the Smad2/3
signalling pathways, resulting in the overabundant accumulation of
collagen (11). In addition, the
number of myofibroblasts, which decreases at the end of the normal
wound healing process, continues to be found at high levels in HS
tissues (12). The overexpression
of TGF-β1 has been shown to contribute to the persistence of
myofibroblasts, since TGF-β1 stimulates normal dermal fibroblasts
to differentiate into myofibroblasts by upregulating α-smooth
muscle actin (α-SMA) expression (13). Therefore, TGF-β1 is a key target
for the development of novel therapeutic strategies for HS
(14).
Shikonin (SHI,
C16H16O5), an active component
extracted from the Chinese herb, Radix Arnebiae, has been
widely demonstrated to possess various biological activities, such
as anti-inflammatory, anti-bacterial, anti-angiogenic and
anti-tumorigenic properties (15). Importantly, in our preliminary
studies, we found that SHI attenuated the expression of TGF-β1 in
keratinocyte/fibroblast co-culture conditioned medium, indicating
the potential use of SHI as a treatment for HS [Fan et al
(unpublished data)]. In this study, we aimed to investigate the
effects of SHI on TGF-β1-stimulated hypertrophic scar-derived human
skin fibroblasts (HSFs) and examined the underlying mechanisms.
Materials and methods
Preparation of TGF-β1 and SHI
TGF-β1 (Merck, Sydney, NSW, Australia) was dissolved
in 4 mM hydrogen chloride (HCl) containing 0.1% bovine serum
albumin (BSA) (both from Sigma-Aldrich, Castle Hill, NSWales,
Australia) and stored at −20°C until use. SHI powder was produced
by the National Institute for the Control of Pharmaceutical and
Biological Products (Beijing, China). SHI was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich) as a stock solution and stored at
−20°C until use.
Cell culture
The HSFs isolated from 3 diffident patients were
purchased from Cell Research Corp. (Singapore), with ethical
approval obtained from the Queensland University of Technology
(1300000063). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Life Technologies, Victoria, Australia)
containing 10% fetal calf serum (FCS; HyClone, Smithfield, QLD,
Australia), 1% v/v penicillin/streptomycin solution and 1% v/v 2 mM
L-glutamine (both from Life Technologies) at 37°C in an incubator
with 5% CO2. The cell culture medium was replaced every
2–3 days.
Cell viability and proliferation
The effects of SHI on the viability and
proliferation of the TGF-β1-stimulated HSFs were determined using
alamarBlue® and CyQUANT® assays, as
previously described (16).
Briefly, the HSFs (6×104 cells/well) were seeded into
12-well cell plates for 24 h. Serial dilutions of SHI in medium
containing 5 ng/ml TGF-β1 were applied to the plates at final
concentrations of 0, 0.5 and 1 µg/ml. Treatments excluding
TGF-β1 and SHI were also included as controls. After 72 h of
incubation, alamarBlue (0.1 µM; Sigma-Aldrich) was added to
each well and the plates were incubated at 37°C for a further 1 h.
The fluorescence in the samples from the wells was measured at λex
570-10 nm and λem 590 nm using a POLARstar OPTIMA Microplate Reader
(BMG Labtech, Ortenberg, Germany). To measure cell proliferation,
the CyQUANT reagents (Life Technologies) were applied as per the
manufacturer's instructions and cell proliferation was measured at
λex 485P and λem 520P using a POLARstar OPTIMA Microplate Reader
(BMG Labtech).
Measurement of total collagen
production
The total amount of collagen present in the HSF
cultures was examined by Sirius red staining, as previously
described (17). The HSFs were
treated as described above. After 72 h of treatment, the cells were
stained with Sirius red (Sigma-Aldrich) and incubated at 37°C for
90 min. The plates were then washed with tap water and air-dried
overnight. The Sirius red stain was dissolved in 0.1 M sodium
hydroxide (NaOH; Sigma-Aldrich) and the absorbance was read at 540
nm using a 96-well microplate reader (Bio-Rad, Hercules, CA,
USA).
Cell contraction assay
The effects of SHI on TGF-β1-stimulated HSF
contraction were determined using a Cell Contraction assay kit
(Jomar Bioscience, Adelaide South Australia), as per the
manufacturer's instructions. Briefly, the HSFs were resuspended in
medium containing various concentrations of SHI (0, 0.5 and 1
µg/ml) at 2×106 cells/ml. Subsequently, a 0.1 ml
cell suspension mixed with 0.4 ml collagen solution provided by the
assay kit was seeded into each well in a 24-well plate at 37°C for
1 h. Following collagen polymerization, 1 ml DMEM was added on top
of the collagen gels. The gels were incubated for 2 days, and were
then detached from the plate. TGF-β1 (5 ng/ml) was added on the top
of each gel and images of each gel were captured using a Nikon
SMZ800 microscope (Nikon, Tokyo, Japan). The diameter of each gel
was measured using a ruler at 0, 12, 24 and 48 h.
Reverse trascription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to investigate gene expression in
the HSFs treated with TGF-β1 and SHI. The genes of interest and
their respective primers are listed in Table I. The HSFs were treated as
described above for 24 and 48 h. Total RNA was then extracted from
the cells using the Qiagen RNeasy Mini kit (Qiagen, Limburg, The
Netherlands), as per the manufacturer's instructions. The RNA
concentration was measured using a NanoDrop® ND-1000
Spectrophotometer (NanoDrop, Wilmington, DE, USA). First-strand
cDNA synthesis was performed using Superscript™ III Reverse
Transcriptase (Life Technologies). Briefly, the RNA sample (100 ng)
from each group was mixed with 1 µl random hexamers, 1
µl deoxyribonucleotide triphosphate (dNTP) and DEPC-treated
water to yield a final volume at 10 µl. The mixture was
incubated at 65°C for 5 min. Each sample was then further mixed
with 2 µl 10X RT buffer, 4 µl magnesium chloride
(MgCl2), 2 µl dithiothreitol (DTT), 1 µl
RNaseOUT and 1 µl SuperScript RT and incubated at 25°C for
10 min, 50°C for 50 min followed by 5 min at 85°C. Each sample was
finally mixed with 1 µl RNase H and incubated at 37°C for 20
min. RT-qPCR was performed using the SYBR-Green reagent and
analysed using the ΔΔCt method on an ABI 7500 Thermal Cycler (both
from Life Technologies). The reaction for each group contained 10
µl SYBR-Green reagent, 1 µM forward and reverse
primers and 100 ng cDNA samples. Amplification was initiated at
95°C for 10 min followed by 40 cycles at 95°C for 15 sec and
finally terminated at 60°C for 1 min. The cycle threshold (Ct)
value of the gene of interest was obtained and first normalised to
the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) (ΔCt) and then further converted to the control (ΔΔCt). The
fold-change of each target gene was calculated using the 2(−ΔΔCt)
method.
 | Table IPrimers used in RT-qPCR. |
Table I
Primers used in RT-qPCR.
| Protein name | Corresponding gene
name | Primer
sequences |
|---|
| Collagen I | COL1A1 | F:
5′-ACGAAGACATCCCACCAATC-3′ |
| R:
5′-AGATCACGTCATCGCACAAC-3′ |
| Collagen III | COL3A1 | F:
5′-GCCTCCCGGAAGTCAAGGAGAAAG-3′ |
| R:
5′-CTTTAGGACCGGGGAAGCCCATG-3′ |
| αSMA | αSMA | F:
5′-CTGCTGAGCGTGAGATTGTC-3′ |
| R:
5′-CTCAAGGGAGGATGAGGATG-3′ |
| Smad2 | SMAD2 | F:
5′-GGCGAATCGGCGGGG-3′ |
| R:
5′-CCTCTTGTATCGAACCTCCCG-3′ |
| GAPDH | GAPDH | F:
5′-TCTTTTGCGTCGCCAGCCGAG-3′ |
| R:
5′-TGACCAGGCGCCCAATACGAC-3′ |
Identification of activated signalling
pathways
The signalling pathways activated in the HSFs
treated with SHI and stimulated with TGF-β1 were determined by
western blot analysis. The HSFs were treated as described above for
30 and 60 min. Whole cell lysates were then collected in lysis
buffer (RIPA buffer; Sigma-Aldrich) containing protease inhibitor
cocktail (Roche Applied Science, Indianapolis, IN, USA), 2 mM
sodium vanadate and 10 mM sodium fluoride (both from
Sigma-Aldrich). The protein concentrations were measured using the
bicinchoninic acid assay (BCA; Thermo Fisher Scientific, Waltham,
MA, USA). Equal amounts of protein (10 µg) from each cell
lysate were prepared and separated using 12% sodium dodecyl
sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto nitrocellulose membranes (Bio-Rad). The membranes
were incubated with primary antibodies overnight at 4°C in Odyssey
blocking buffer (LI-COR Biosciences, Lincoln, NE, USA). Primary
antibodies included extracellular signal-regulated kinase (ERK;
#9102, rabbit anti-human), phosphorylated ERK (p-ERK, #9106, mouse
anti-human) and Smad2/3 (#3102, rabbit anti-human) from Genesearch
(Arundel, QLD, Australia); phosphorylated Smad2/3 (p-Smad2/3,
#sc-11769-R, rabbit anti-human) from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA; α-SMA (#ab5694, rabbit anti-human) from
Abcam (Cambridge, UK) and GAPDH (#G8795, mouse anti-human) from
Sigma-Aldrich. Secondary antibodies conjugated with Alexa Fluor 680
or 800 (Life Technologies) were then applied as species
appropriate. Images were captured and analysed using the Odyssey
Infrared Imaging system and software (LI-COR Biosciences).
Statistical analysis
All experiments were performed in triplicate, with
each treatment tested individually 3 times in each assay using
cells from 3 different patients. The data are expressed as the
percentage of the control group. One-way ANOVA and Tukey's post-hoc
test were applied and a value of p<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of SHI on HSF viability and
proliferation
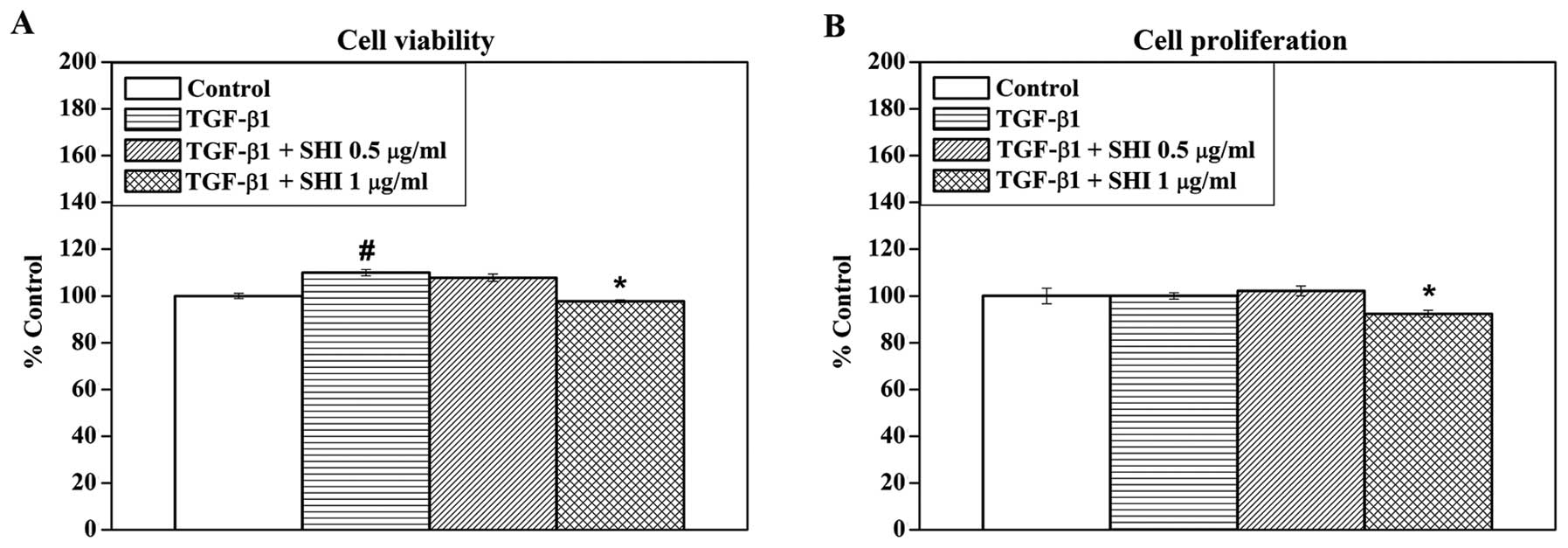
To determine the effects of SHI on the viability and
proliferation of TGF-β1- stimulated HSFs, the HSFs were treated
with TGF-β1 and various concentrations of SHI (0, 0.5 and 1
µg/ml). TGF-β1 alone significantly increased the viability
of the HSFs by 9.9±1.3% compared to the control (no treatment;
p<0.05; Fig. 1A). However, no
effect of TGF-β1 alone on HSF proliferation was observed (Fig. 1B). In addition, SHI at 0.5
µg/ml had no significant effect on the viability and
proliferation of the TGF-β1-stimulated HSFs, while SHI at 1
µg/ml decreased the viability and proliferation of the
TGF-β1-stimulated HSFs compared to the control (p<0.05) by
2.3±0.6 and 7.7±1.5%, respectively (Fig. 1). These data indicate that SHI
reduces the TGF-β1-induced increase in HSF viability in a
dose-dependent manner.
SHI attenuates TGF-β1-induced collagen
production in HSFs
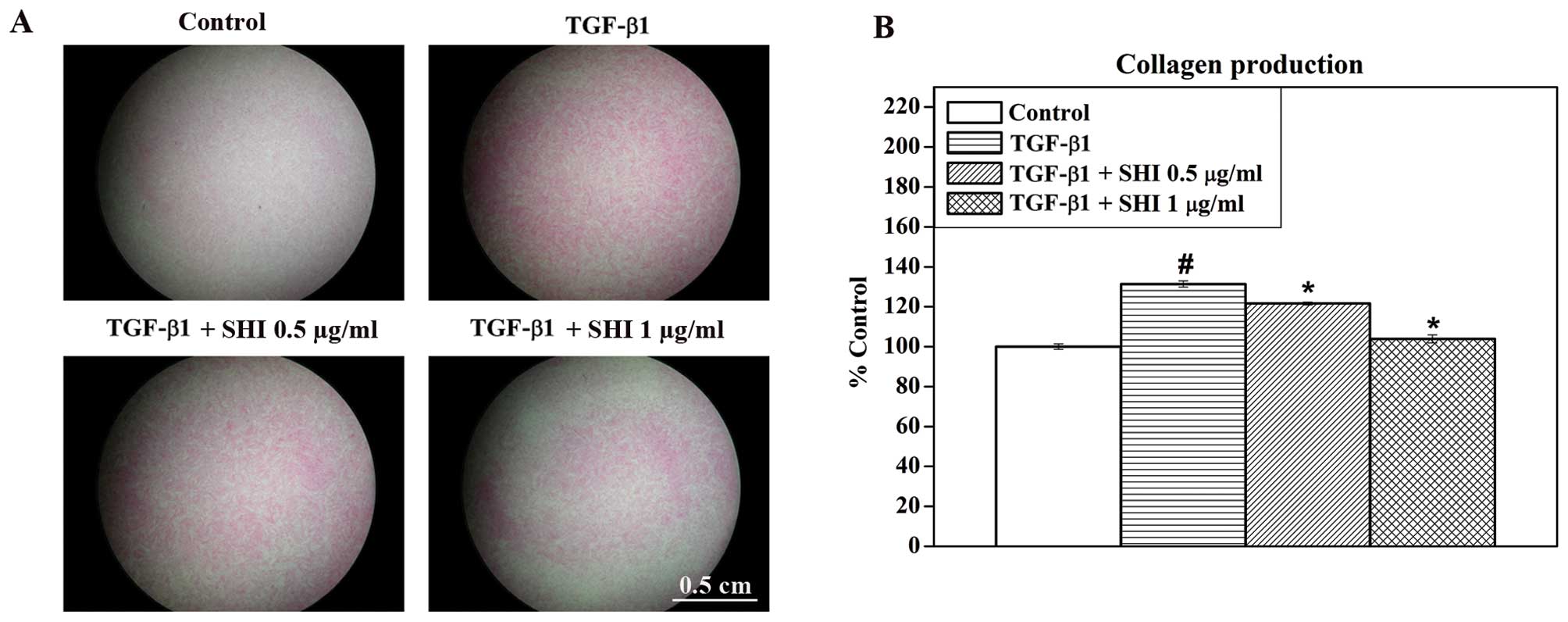
To determine the effects of SHI on collagen
production in the TGF-β1-stimulated HSFs, total collagen present in
the HSFs was stained with Sirius red (Fig. 2A) and was then further analysed
quantitatively (Fig. 2B). A
greater amount of collagen (red staining) was observed in the HSFs
treated with TGF-β1 alone compared to the the control group (no
treatment; Fig. 2A). However,
decreased amounts of collagen were detected in the
TGF-β1-stimulated HSFs treated with SHI at 0.5 and 1 µg/ml
(Fig. 2A). Consistent with the
results from Sirius red staining, the quantitative data indicated
that that TGF-β1 alone significantly increased the total amount of
collagen by 31.4±1.5% compared to the control group (p<0.05;
Fig. 2B). However, treatment with
SHI at 0.5 and 1 µg/ml attenuated collagen production in the
TGF-β1-stimulated HSFs by 9.7±0.7 and 27.4±1.9%, respectively,
compared to the group treated with TGF-β1 alone (p<0.05;
Fig. 2B). These results
demonstrate that SHI inhibits the TGF-β1-induced increase in
collagen production in HSFs in a dose-dependent manner.
TGF-β1-induced contraction of HSFs is
inhibited by SHI
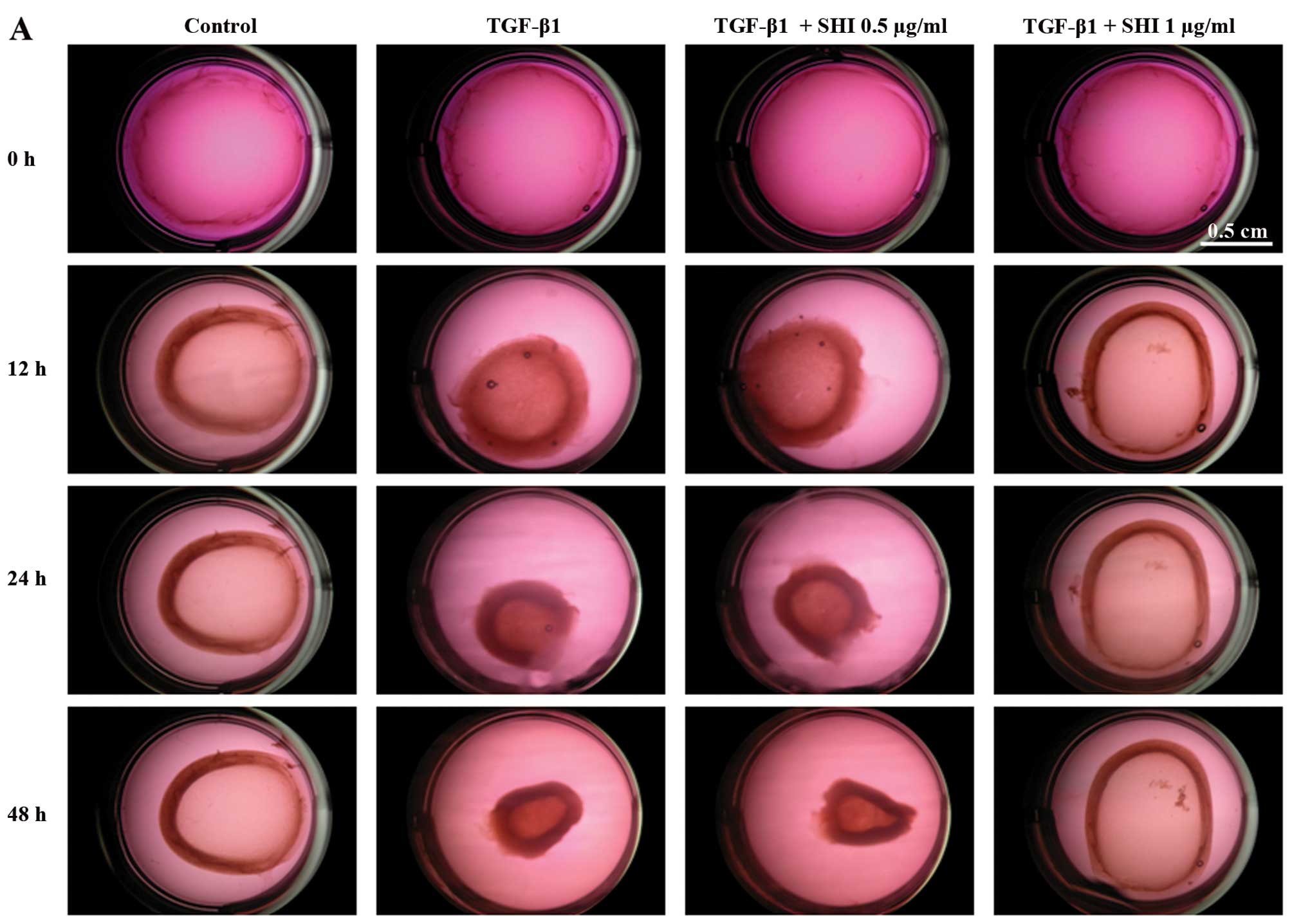
The effects of SHI on HSF-mediated gel contraction
were evaluated using a collagen gel contraction assay. Treatment
with TGF-β1 alone significantly enhanced the contraction of the
gels containing the HSFs by 7.7±2.2, 28.4±1.4 and 40.3±5.0% at 12,
24 and 48 h, respectively, compared to the control group (no
treatment; p<0.05; Fig. 3). In
addition, the contraction of the gels containing HSFs treated with
TGF-β1 and 0.5 µg/ml SHI was 14.1±1.3, 31.3±2.3 and
37.5±2.4% greater than that of the control group at 12, 24 and 48
h, respectively (p<0.05; Fig.
3). However, the HSFs treated with TGF-β1 and 1 µg/ml
SHI showed no significant differences in gel contraction compared
to the control group (p<0.05; Fig.
3). Taken together, these results suggest that SHI attenuates
the TGF-β1-induced contraction of gels containing HSFs in a
dose-dependent manner.
Effects of SHI on gene expression in
TGF-β1-stimulated HSFs
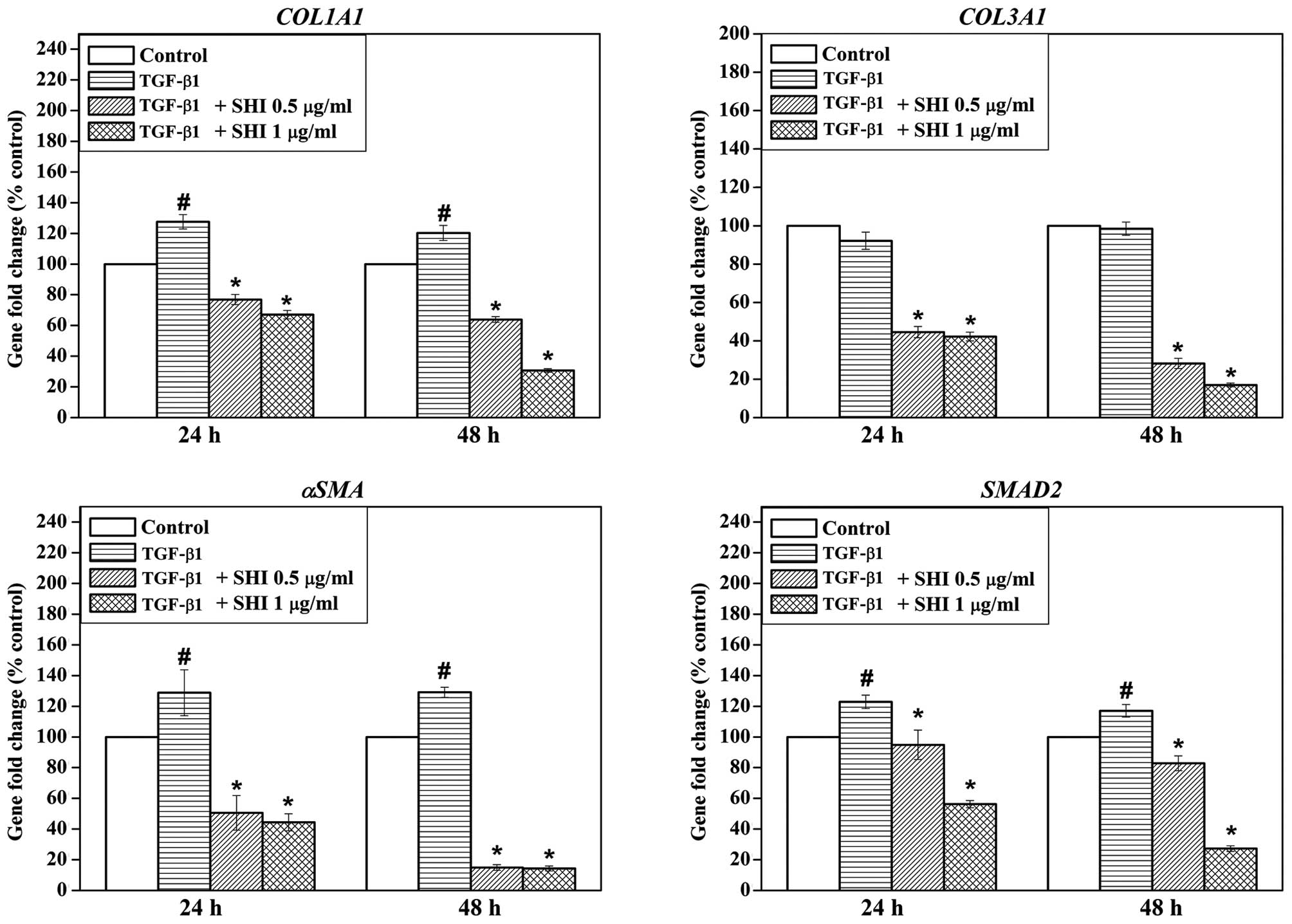
The gene expression of collagen, type I, alpha 1
(COL1A1), collagen, type III, alpha 1 (COL3A1), α-SMA
and SMAD2 in the HSFs following treatment with TGF-β1 and
SHI was examined by RT-qPCR. Treatment with TGF-β1 alone
significantly upregulated COL1A1, α-SMA and
SMAD2 gene expression in the HSFs at 24 and 48 h compared to
the control group (no treatment; p<0.05; Fig. 4). No effect of TGF-β1 on the
expression of COL3A1 in the HSFs was observed (Fig. 4). However, treatment with SHI at
either 0.5 or 1 µg/ml significantly decreased the expression
of COL1A1, COL3A1, α-SMA and SMAD2 in
the HSFs compared to treatment with TGF-β1 alone (Fig. 4), indicating that SHI suppresses
the TGF-β1-induced changes in gene expression in the HSFs.
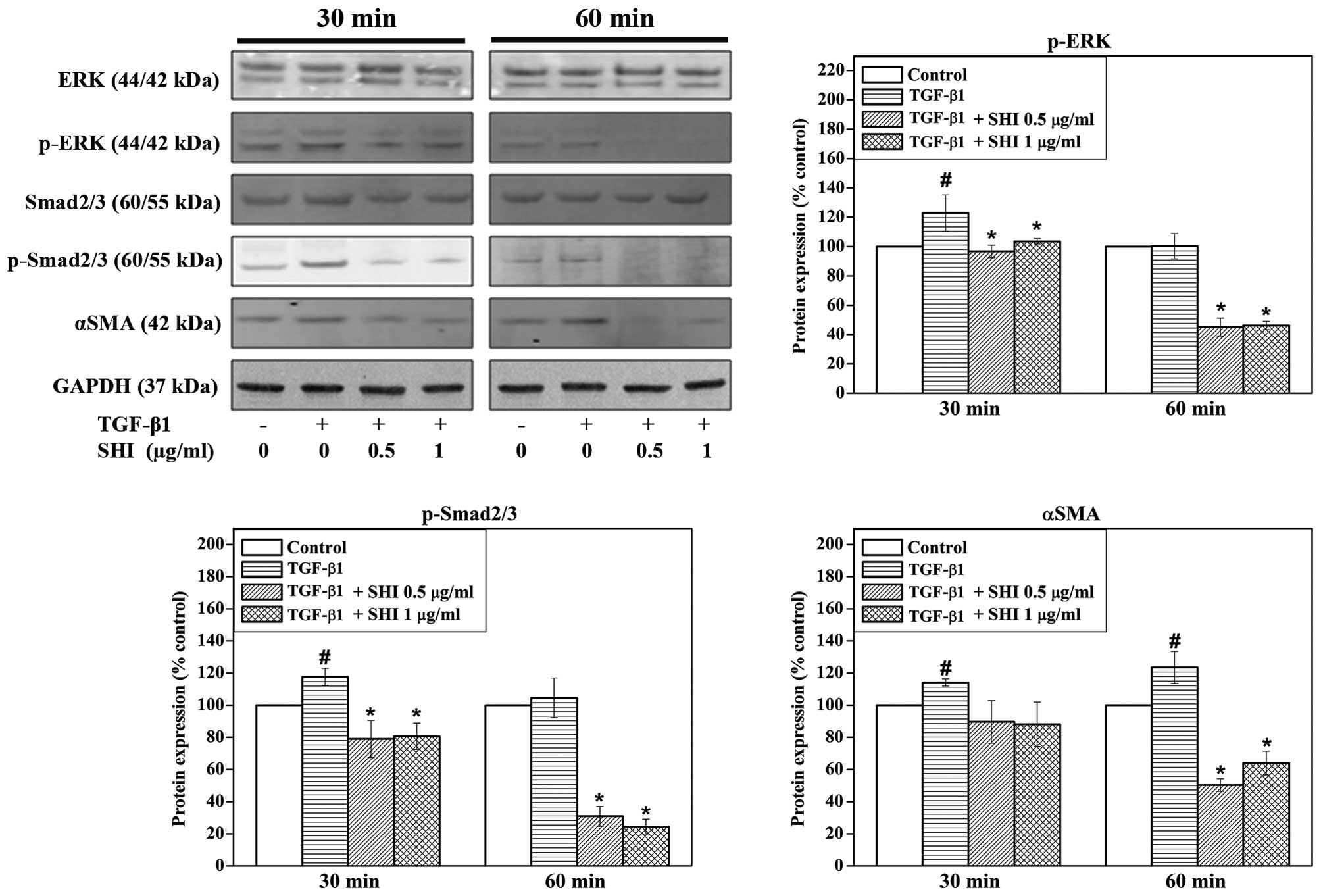
SHI-induced activation of signalling
pathways in HSFs
To elucidate the underlying mechanisms of
SHI-induced decrease in collagen production in the
TGF-β1-stimulated HSFs, the expression of ERK, p-ERK, Smad2/3,
p-Smad2/3 and α-SMA was determined by western blot analysis. No
effects of TGF-β1 and SHI on the expression of ERK and Smad2/3 were
detected in the HSFs (Fig. 5).
However, the expression of p-ERK and p-Smad2/3 was significantly
upregulated in the HSFs following treatment with TGF-β1 alone for
30 min, while the protein expression of these signalling molecules
was decreased in the HSFs treated with both TGF-β1 and SHI (either
0.5 or 1 µg/ml) for 30 and 60 min (Fig. 5). In addition, the expression of
α-SMA increased in the HSFs treated with TGF-β1 alone for 30 and 60
min, whereas the TGF-β1-induced upregulation of α-SMA expression
was inhibited when the HSFs were also treated with SHI (either 0.5
or 1 µg/ml) for 60 min.
Discussion
TGF-β1 is a multifunctional cytokine regulating cell
proliferation, collagen production, cell differentiation and ECM
degradation during wound healing processes (10). Enhanced levels of TGF-β1 in
cutaneous wound healing often leads to the formation of HS
(11). Based on the data of our
previous studies (unpublished data), in this study, we investigated
the effects of SHI on TGF-β1-stimulated HSFs and found that SHI
reduced the TGF-β1-induced collagen production in HSFs, as well as
cell contraction, indicating the potential therapeutic value of SHI
as a novel treatment strategy for HS.
During the early stages of wound healing,
fibroblasts migrate to the wound lesion and produce collagen,
assisting the replacement of the blood clot with new granulation
tissue (18). Excessive collagen
is degraded by proteases, such as collagenases, at the final stage
of wound healing (19). TGF-β1
has been demonstrated to play an essential role in the production
of collagen by regulating the Smad2/3 signalling pathways (11). Following the binding of TGF-β1 to
its receptor, TβR-I, the phosphorylation of Smad2/3 protein occurs
intracellularly (20). p-Smad2/3
then heteromultimerises with Smad4, resulting in its transfer into
the nucleus and the triggering of the expression of target genes,
such as COL1A1 (21). In
accordance with the results of previous studies (unpublished data),
our data suggest that TGF-β1 increases total collagen production,
phosphorylates Smad2/3 and upregulates COL1A1 gene
expression in HSFs. However, these changes induced by TGF-β1 in the
HSFs can be significantly attenuated when SHI treatment is
concomitantly applied, indicating that SHI attenuates
TGF-β1-induced collagen production in HSFs.
TGF-β1-induced Smad2/3 signalling has been reported
to be associated with the activation of mitogen-activated protein
kinases (MAPKs), including ERK, c-Jun N-terminal kinases (JNK) and
mitogen-activated protein kinase 14 (also known as p38α) (22). The linker region of Smads, in
particular, plays an essential role in mediating Smad function
(23). Studies have indicated
that ERK phosphorylates the linker region of Smad2/3, thereby
regulating the function of Smad2/3 in fibroblasts (24,25). Additionally, ERK inhibitors
significantly block the phosphorylation of the Smad linker region
(26). Furthermore, the
activation of ERK has been observed to enhance the duration of
Smad2/3 transcriptional activity (24). In order to provide insight into
the underlying mechanisms responsible for the SHI-induced decrease
in collagen production in the TGF-β1-stimulated HSFs, we examined
the expression of MAPKs following treatment with SHI. As shown by
our results, the expression of phosphorylated JNK (p-JNK) and p38
(p-p38) was not evident in the HSFs following treatment with TGF-β1
and SHI (data not shown). However, treatment with SHI significantly
attenuated the TGF-β1-induced phosphorylation of ERK (p-ERK) in the
HSFs (Fig. 5). These data suggest
that SHI reduces the TGF-β1-induced collagen production in HSFs
through the ERK/Smad signalling pathway.
During the formation of granulation tissue, some
dermal fibroblasts change their phenotype to become myofibroblasts,
which are responsible for wound contraction (27). Contraction is essential for the
wound healing process as it reduces the wound size, thereby
enabling wound closure. However, excessive wound contraction leads
to the formation of HS (28).
Myofibroblasts, cells characterised by the presence of α-SMA
(29), have been demonstrated to
play a key role in HS formation (30). Indeed, an increased expression of
αSMA has been widely reported in HS tissues (31). TGF-β1 has been shown to upregulate
the expression of αSMA, as TGF-β1 stimulates normal fibroblasts to
differentiate into myofibroblasts (13). In this study, we also provide
evidence that TGF-β1 improves the ability of HSFs to contract gels
and that it enhances the expression of αSMA in HSFs. Moreover, SHI
attenuates the TGF-β1-induced contraction of HSFs and αSMA
upregulation in HSFs in a dose-dependent manner.
Taken together, the data reported herein indicate
that TGF-β1 plays a key role in cellular processes crucial to wound
healing and HS formation. Increased levels of TGF-β1 lead to the
formation of HS by stimulating collagen production and wound
contraction. Furthermore, to the best of our knowledge, this study
demonstrates for the first time that SHI reduces TGF-β1-induced
collagen production through the ERK/Smad signalling pathway and
attenuates the TGF-β1-induced contraction of gels containing HSFs
by downregulating αSMA expression. This evidence supports the
potential use of SHI as a novel treatment strategy for HS.
Acknowledgments
The authors would like to acknowledge the support of
the Australian Government's Cooperative Research Centres Program
and the Queensland University of Technology (QUT) Tissue Repair and
Regeneration Program. We also acknowledge the PhD scholarship from
QUT and the Wound Management Innovation Cooperative Research
Centre.
Abbreviations:
|
αSMA
|
α-smooth muscle actin
|
|
COL1A1
|
collagen, type I, alpha 1
|
|
COL3A1
|
collagen, type III, alpha 1
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
ECM
|
extracellular matrix
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FCS
|
fetal calf serum
|
|
HS
|
hypertrophic scarring
|
|
HSFs
|
hypertrophic scar- derived human skin
fibroblasts
|
|
MAPK
|
mitogen-activated protein kinase
|
|
RT-qPCR
|
reverse transcriptase-quantitative
polymerase chain reaction
|
|
SDS-PAGE
|
sodium dodecyl sulphate polyacrylamide
gel electrophoresis
|
|
SMAD2
|
SMAD family member 2
|
|
SHI
|
shikonin
|
|
TGF-β1
|
trans forming growth factor-β1
|
References
|
1
|
Aarabi S, Longaker MT and Gurtner GC:
Hypertrophic scar formation following burns and trauma: New
approaches to treatment. PLoS Med. 4:e2342007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao Z, Zhang M, Liu Y and Ren L:
Botulinum toxin type a inhibits connective tissue growth factor
expression in fibroblasts derived from hypertrophic scar. Aesthetic
Plast Surg. 35:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Z, Li S, Cao C, Wu J, Ma B and Tran V:
shRNA targeting SFRP2 promotes the apoptosis of hypertrophic scar
fibroblast. Mol Cell Biochem. 352:25–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu XJ, Xu MJ, Fan ST, Wu Z, Li J, Yang
XM, Wang YH, Xu J and Zhang ZG: Xiamenmycin attenuates hypertrophic
scars by suppressing local inflammation and the effects of
mechanical stress. J Invest Dermatol. 133:1351–1360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang D, Shen KH and Wang HG: Pressure
therapy upregulates matrix metalloproteinase expression and
downregulates collagen expression in hypertrophic scar tissue. Chin
Med J (Engl). 126:3321–3324. 2013.
|
|
6
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic scarring and keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar :
|
|
7
|
Liu BH, Chen L, Li SR, Wang ZX and Cheng
WG: Smac/DIABLO regulates the apoptosis of hypertrophic scar
fibroblasts. Int J Mol Med. 32:615–622. 2013.PubMed/NCBI
|
|
8
|
Simon F, Bergeron D, Larochelle S,
Lopez-Vallé CA, Genest H, Armour A and Moulin VJ: Enhanced
secretion of TIMP-1 by human hypertrophic scar keratinocytes could
contribute to fibrosis. Burns. 38:421–427. 2012. View Article : Google Scholar
|
|
9
|
Yin L, Zhao X, Ji S, He C, Wang G, Tang C,
Gu S and Yin C: The use of gene activated matrix to mediate
effective SMAD2 gene silencing against hypertrophic scar.
Biomaterials. 35:2488–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang
Y, Yu C and Jin Y: Mesenchymal stem cells prevent hypertrophic scar
formation via inflammatory regulation when undergoing apoptosis. J
Invest Dermatol. 134:2648–2657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Ghahary A, Shen Q, Scott PG, Roy K
and Tredget EE: Hypertrophic scar tissues and fibroblasts produce
more transforming growth factor-beta1 mRNA and protein than normal
skin and cells. Wound Repair Regen. 8:128–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ehrlich HP, Desmoulière A, Diegelmann RF,
Cohen IK, Compton CC, Garner WL, Kapanci Y and Gabbiani G:
Morphological and immunochemical differences between keloid and
hypertrophic scar. Am J Pathol. 145:105–113. 1994.PubMed/NCBI
|
|
13
|
Varga J and Abraham D: Systemic sclerosis:
A prototypic multi-system fibrotic disorder. J Clin Invest.
117:557–567. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colwell AS, Phan TT, Kong W, Longaker MT
and Lorenz PH: Hypertrophic scar fibroblasts have increased
connective tissue growth factor expression after transforming
growth factor-beta stimulation. Plast Reconstr Surg. 116:1387–1392.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones LJ, Gray M, Yue ST, Haugland RP and
Singer VL: Sensitive determination of cell number using the CyQUANT
cell proliferation assay. J Immunol Methods. 254:85–98. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malkusch W, Rehn B and Bruch J: Advantages
of Sirius Red staining for quantitative morphometric collagen
measurements in lungs. Exp Lung Res. 21:67–77. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urioste SS, Arndt KA and Dover JS: Keloids
and hypertrophic scars: Review and treatment strategies. Semin
Cutan Med Surg. 18:159–171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piek E, Moustakas A, Kurisaki A, Heldin CH
and ten Dijke P: TGF-(beta) type I receptor/ALK-5 and Smad proteins
mediate epithelial to mesenchymal transdifferentiation in NMuMG
breast epithelial cells. J Cell Sci. 112:4557–4568. 1999.PubMed/NCBI
|
|
21
|
Wrana JL and Attisano L: The Smad pathway.
Cytokine Growth Factor Rev. 11:5–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massague J: Integration of Smad and MAPK
pathways: A link and a linker revisited. Genes Dev. 17:2993–2997.
2003. View Article : Google Scholar
|
|
23
|
Inman GJ: Linking Smads and
transcriptional activation. Biochem J. 386:e1–e3. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hough C, Radu M and Doré JJ: Tgf-beta
induced Erk phosphorylation of smad linker region regulates smad
signaling. PLoS One. 7:e425132012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darby IA and Hewitson TD: Fibroblast
differentiation in wound healing and fibrosis. Int Rev Cytol.
257:143–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X,
Li YY and Xu WS: Recombinant human decorin inhibits TGF-β1-induced
contraction of collagen lattice by hypertrophic scar fibroblasts.
Burns. 35:527–537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Franz M, Spiegel K, Umbreit C, Richter P,
Codina-Canet C, Berndt A, Altendorf-Hofmann A, Koscielny S, Hyckel
P, Kosmehl H, et al: Expression of Snail is associated with
myofibroblast phenotype development in oral squamous cell
carcinoma. Histochem Cell Biol. 131:651–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ibrahim MM, Bond J, Bergeron A, Miller KJ,
Ehanire T, Quiles C, Lorden ER, Medina MA, Fisher M, Klitzman B, et
al: A novel immune competent murine hypertrophic scar contracture
model: A tool to elucidate disease mechanism and develop new
therapies. Wound Repair Regen. 22:755–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Dodd C, Shankowsky HA, Scott PG
and Tredget EE; Wound Healing Research Group: Deep dermal
fibroblasts contribute to hypertrophic scarring. Lab Invest.
88:1278–1290. 2008. View Article : Google Scholar : PubMed/NCBI
|