Introduction
Mesenchymal stem cells (MSCs) are pluripotent stem
cells, which are formed during the early development of the
mesoderm. With a high capacity for self-renewal and the potential
for multi-directional differentiation, these cells are present in
several tissues and can also be cultured in vitro (1). Under defined specific culture
conditions, these MSCs can be induced to differentiate into
neuronal cells, osteoblasts, chondrocytes, muscle cells and fat
cells. Therefore, MCSs hold great promise for use in cell
replacement therapy and tissue engineering (1). Baksh et al (2) found that human umbilical
cord-derived MSCs (hUCMSCs) have a strong proliferative and
differentiation potential. As hUMSCs are obtained using
non-invasive procedures, thus avoiding ethical restrictions, they
are considered an ideal source of human-derived MSCs for use in
clinical studies and research.
Gangliosides, a class of glycosphingolipids
containing sialic acid, are present in the cell membrane of various
cell types in vertebrates and are particularly present in high
levels in the gray matter of the brain. Gangliosides are associated
with nerve cell differentiation and the length of neurites, as well
as the formation of synapses (3).
Monosialoteterahexosyl ganglioside (GM1) is a major ganglioside
species in mammals, and it has been shown that the application of
exogenous GM1 promotes cell regeneration in the nervous system and
the formation of synapses (4). In
the present study, we demonstrate that GM1 is effective in inducing
the differentiation of hUMSCs into neuron-like cells in
vitro. We further investigated the related mechanisms and
observed that the expression of several markers of neuronal cells
was induced by GM1 in the hUMSCs within a time span of 6 h.
Materials and methods
Materials
Human umbilical cords obtained from women with
full-term pregnancies whose babies were delivered by cesarean
section were provided by the Obstetrics-Gynecology Department of
the Second Affiliated Hospital of Hebei Medical University,
Shijiazhuang, China. This study was approved by the Ethics
Committee of the Second Hospital of Hebei Medical University. The
single sialic acid four hexose ganglion glucoside ester sodium
injection was obtained from Qilu Pharmaceuticals (Jinan, China);
L-DMEM/F-12, H-DMEM/F-12 and fetal bovine serum (FBS) were obtained
from Gibco (Carlsbad, CA, USA); penicillin-streptomycin was from
Beijing Solarbio Science and Technology (Beijing, China) and
paraformaldehyde was obtained from the Tianjin Institute of
Chemical Preparations (Tianjin, China). Antibody against human
epidermal growth factor EGF (huEGF) was purchased from Beijing
Jiamei North Biological Technology (Beijing, China); FITC-CD19
(561295), FITC-CD34 (Rs-0646III), PE-CD11b (BYK-11127R), PE-CD73
(ANT-414), PE-CD90 (ab92574), PE-CD45 (PRO-482), PE-CD105 (CYT-424)
antibodies and antibody to glial fibrillary acidic protein (GFAP;
DIA-0302) were obtained from Becton-Dickinson (San Jose, CA, USA);
antibody to neurofilament protein (NF-H; MAB1615) was from Cell
Signaling Technology (Beverly, MA, USA); antibody to microtubule
binding protein-2 (MAP-2; AB5622) was from Millipore (Billerica,
MA, USA) and the PS immunohistochemistry kit was from Beijing
Zhongshan Golden Bridge Biotechnology, Co. (Beijing, China).
Methods
Isolation and culture of hUMSCs
Following delivery, the collected umbilical cords
were placed in high glucose Dulbecco's Modified Eagle's Medium
(H-DMEM)/F12 culture medium under aseptic conditions, stored at 4°C
and then transported to a cell culture room within 2 h for
processing as follows: the umbilical cords were rinsed thoroughly
with D-Hank's medium, and the umbilical artery and umbilical vein
were removed after withdrawing the blood sample. The tissue was cut
into 1-mm3 sections, digested with 0.2% collagenase II
and then placed into a culture flask containing 2 ng/ml EGF, 20%
FBS, 25 mM L-glutamic acid and 100 U/ml penicillin-streptomycin
mixture in H-DMEM/F12. The flask was placed into an incubator at
37°C with 5% CO2 and saturated humidity to obtain
primary cells. When the cells achieved 80–90% confluency, they were
passaged 1:3 following digestion with trypsin
0.25%-ethylenediaminetetraacetic acid (EDTA) 0.2 g/l into single
cells. The culture medium used during passaging was H-DMEM/F12
containing 100 U/ml penicillin streptomycin/mixture and 10%
FBS.
Analysis of the cellular phenotype of
hUMSCs
Single cell suspensions of hUMSCs in the logarithmic
growth phase were prepared and then placed into 10 tubes at
1×106/tube in phosphate-buffered saline (PBS). Mouse
anti-human monoclonal antibodies to CD11-PE, CD45-PE, CD73-PE,
CD90-PE, CD105-PE, HLA-DR-PE (P8950; Biotechnology LP and
Sigma-Aldrich Co., St. Louis, MO, USA), CD19-FITC and CD34-FITC
were added separately (each 5 µl) into 8 tubes. Anti-mouse
IgG1-PE and anti-mouse IgG1-FITC antibody (each 7 µl) were
added to the other 2 tubes as isotype controls. The cells and
antibodies in all tubes were mixed thoroughly and incubated at 4°C
for 30 min. The cells were then rinsed in PBS and resuspended in
400 µl of PBS prior to detection by flow cytometry.
Differentiation of hUMSCs into
neuron-like cells
The hUMSCs of the third passage in the logarithmic
growth phase were rinsed twice with PBS and digested with trypsin
0.25%-EDTA 0.2 g/l. After terminating the digestion with FBS, the
cells were centrifuged at 1,500 rpm at 37°C, resuspended in culture
medium and seeded at 1×105/ml into 5 polylysine-coated
6-well cell culture plates. The plates were divided randomly into 5
groups (groups A–E). When the cells achieved 70–80% confluency, GM1
induction solution was added to groups A–D at concentrations of 50,
100, 150 and 200 µg/ml, respectively. Only low glucose DMEM
(L-DMEM) was added to group E (control). All of the treated plates
were incubated for 6 h at 37°C with 5% CO2 and saturated
humidity, and the morphological changes in the cells were observed
using an inverted phase contrast microscope (serial no. G02B21/00;
Olympus Optical Company, Ltd., Tokyo, Japan) every hour during this
incubation period.
Identification of differentiated
cells
Following the induction of differentiation for 6 h,
the expression levels of the neuronal-specific proteins, MAP-2,
NF-H and GFAP, were detected by immunohistochemistry as follows:
the culture medium was discarded, and the cells were rinsed gently
once with PBS prior to fixing with 4% paraformaldehyde for 20 min
at room temperature. After this step and all subsequent steps, the
cells were rinsed with PBS (3×5 min) unless otherwise stated. The
cell membranes were then disrupted with PBS containing 0.5% Triton
X-100 for 15 min at room temperature away from light. Following
incubation with 3% H2O2 at room temperature
for 5 min, normal goat serum was added for blocking at room
temperature for 15 min. The blocking solution was removed, and
primary antibodies to MAP-2, GFAP and NF-H were then added (each
diluted 1:200) in PBS. Following incubation at 4°C overnight,
biotinylated secondary antibody (15180A01; Zhongshan Golden Bridge
Biotechnology, Co.) was added to the cells followed by further
incubation at room temperature for 15 min, followed by the addition
of horseradish peroxidase-conjugated streptavidin at room
temperature for 15 min. Color development was performed by adding
freshly prepared diaminobenzidine (DAB; Zhongshan Golden Bridge
Biotechnology, Co.) for 1 min. After counterstaining with
hematoxylin, the cells were rinsed repeatedly with water.
Following the induction of differentiation for 6 h,
the expression levels of the neuroal-specific proteins, MAP-2, NF-H
and GFAP, were also detected by immunofluorescence staining as
follows: the medium from the culture plates was discarded, and the
cells were rinsed once gently with PBS and fixed with 4%
paraformaldehyde for 20 min at room temperature. The cells were
then rinsed with PBS (3×5 min) unless otherwise stated. The cell
membranes were disrupted with PBS containing 0.5% Triton X-100 for
15 min at room temperature away from light. PBS with 2% BSA was
used for blocking at room temperature for 1 h. Primary antibodies
to MAP-2, GFAP and NF-H, each diluted at 1:200, were then added
followed by incubation at 4°C overnight, followed by the addition
of the secondary antibody at 37°C for 0.5 h in the dark. After the
secondary antibody was removed, 4′,6-diamidino-2-phenylindole
(DAPI; Roche Diagnostics GmbH, Roche Applied Science, Mannheim,
Germany) was added to stain the nucleus at room temperature for 15
min. After the final rinse with PBS (3×5 min), the samples were
examined under a fluorescence microscope (serial no. G02B21/00;
Olympus Optical Company, Ltd.).
Determination of neuron-like cell
ratios
A total of 10 non-overlapping fields in each group
were selected under an inverted microscope, and the total number of
cells and neuron-like cells was determined. The proportion of
neuron-like cells in each group was analyzed using an χ2
test and the results are presented as the means ± SEM.
Statistical analysis
SPSS 19.0 software was used to perform statistical
analysis of the experimental data with a completely randomized
design by analysis of variance among groups and the
Student-Newman-Keuls (SNK) method (Q-test). A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
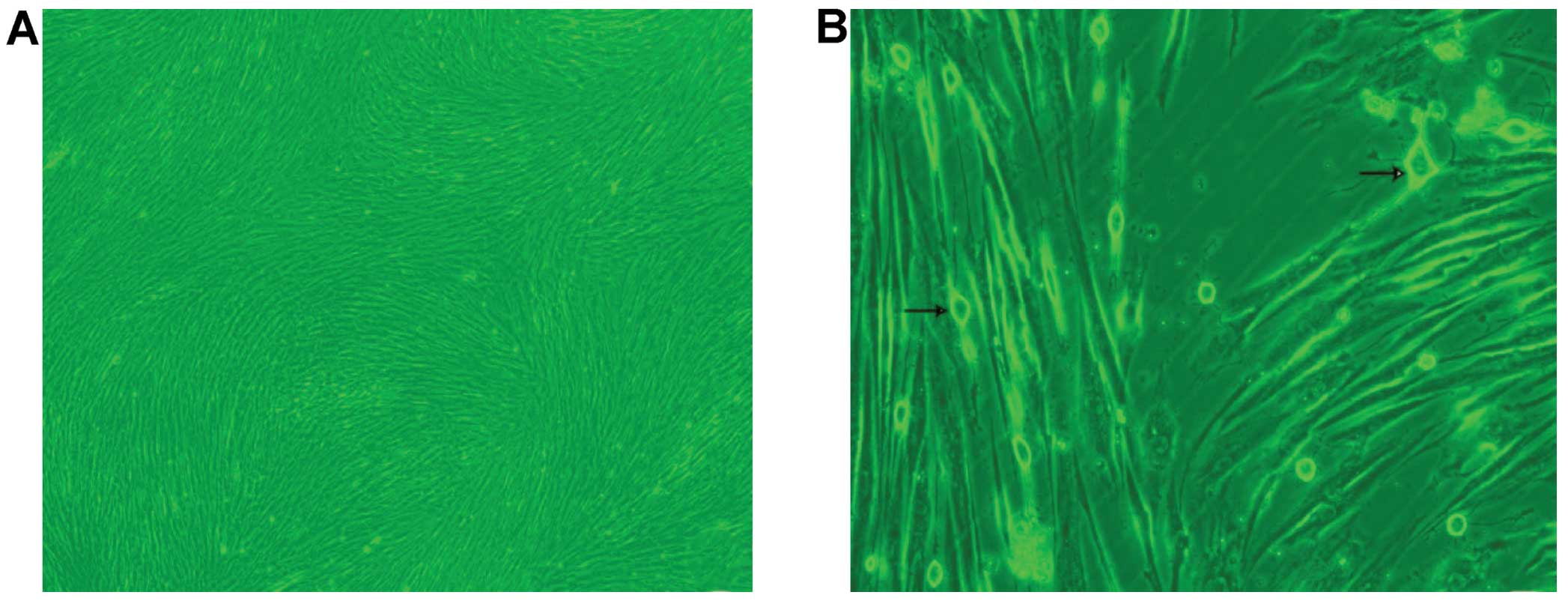
Growth and morphological changes of
hUMSCs
At 12 after passaging the primary cultured cells
(1:1), the majority of the cells became adherent, but were not
outstretched and were triangular or diamond-shaped. The cell
culture medium was replaced to remove the non-adherent cells after
24 h. The adherent cells proliferated rapidly and became
significantly larger, appearing uniformly as long fusiform shapes
after 48 in culture. On the 7th day, the cells reached 80–90%
confluency in a radial or spiral pattern with no overlap. The
majority of the cells had adhered to the plates after 24 h and
achieved 80–90% confluency on the 7th day, arranged in a radial or
spiral pattern (Fig. 1A).
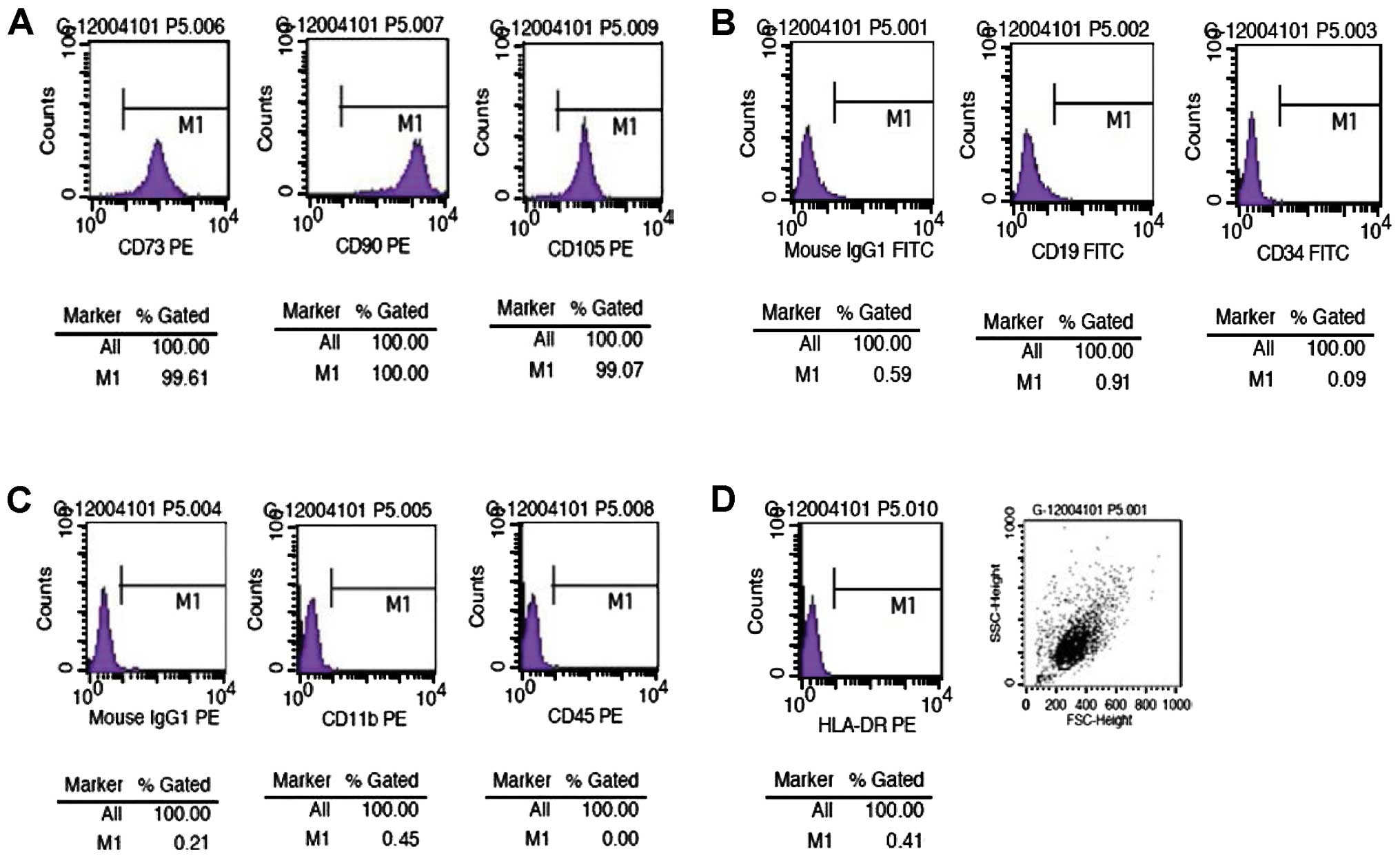
Cellular phenotype of the hUMSCs
The examination of the cellular phenotype of the
hUMSCs at passage 2, 5 and 6 by flow cytometry revealed that the
cells co-expressed CD105, CD90 and CD73, but not CD11b, CD34, CD19,
CD45 or histocompatibility antigen HLA-DR (MHC-II) (Fig. 2).
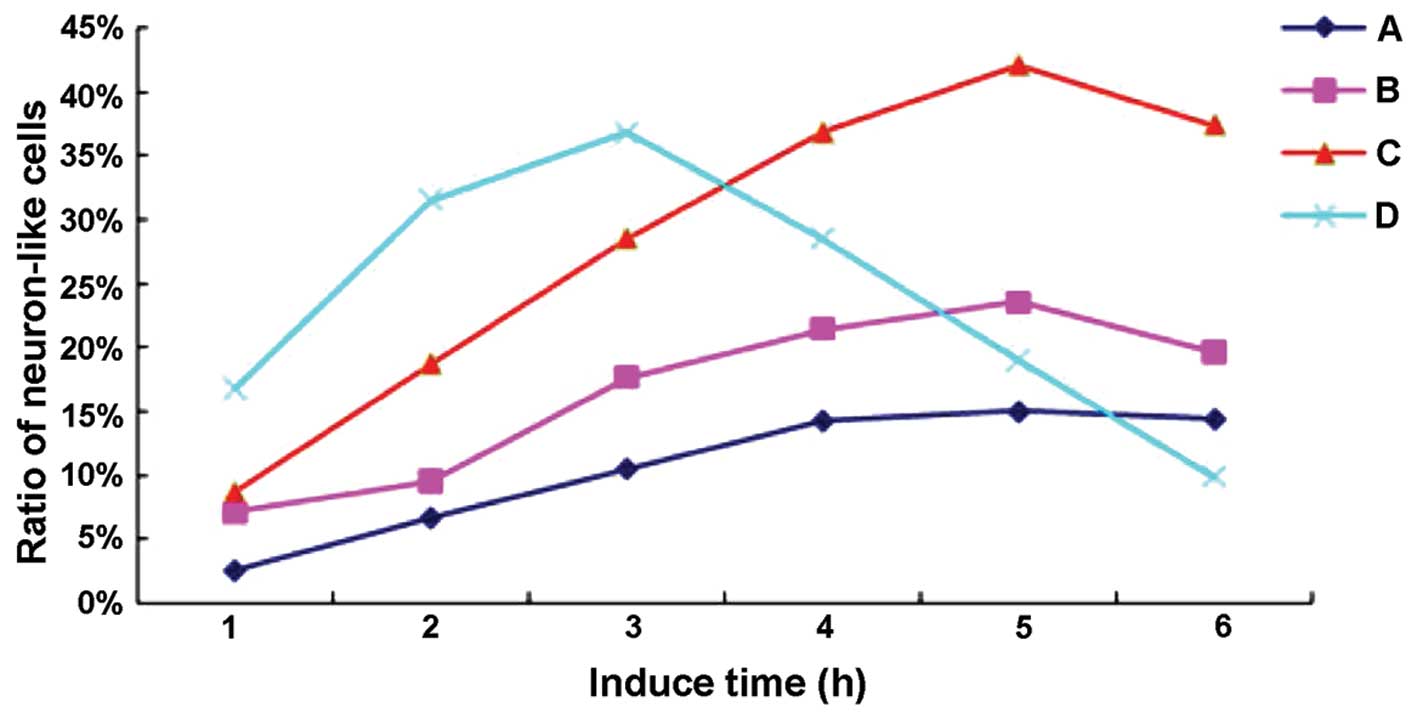
Morphological and quantitative changes of
neuron-like cells
Following induction with GM1 for 1 h, some of the
cell bodies in each experimental group contracted into elliptical
shapes and then extended protuberances from two poles, which
appeared like spindles. Group D had the most neuron-like cells,
while group A had the least. Following the induction of
differentiation for 3 h, the neuron-like cells with longer
protuberances gradually increased in all the experimental groups,
but group D still had the most. Following the induction of
differentiation for 4 h, the number of neuron-like cells in groups
A–C continued to increase and the cells appeared as bipolar or
multipolar cells (Fig. 1B), while
the number of neuron-like cells in group D began to decline.
Following the induction of differentiation for 5 h, the number of
neuron-like cells did not increase further in groups A and B, and
some of the cells even became detached. However, group C had the
most neuron-like cells, although some cells became detached as
well. Group D had an abundant number of detached neuron-like cells.
Following the induction of differentiation for 6 h, no neuron-like
cells were observed in groups A–C, and only a few of them were
observed in group D. No obvious changes in the number of
neuron-like cells were observed before and after sham-induction in
the controls (group E). Fig. 3
illustrates the changes in the proportion of neuron-like cells in
each experimental group over the induction period.
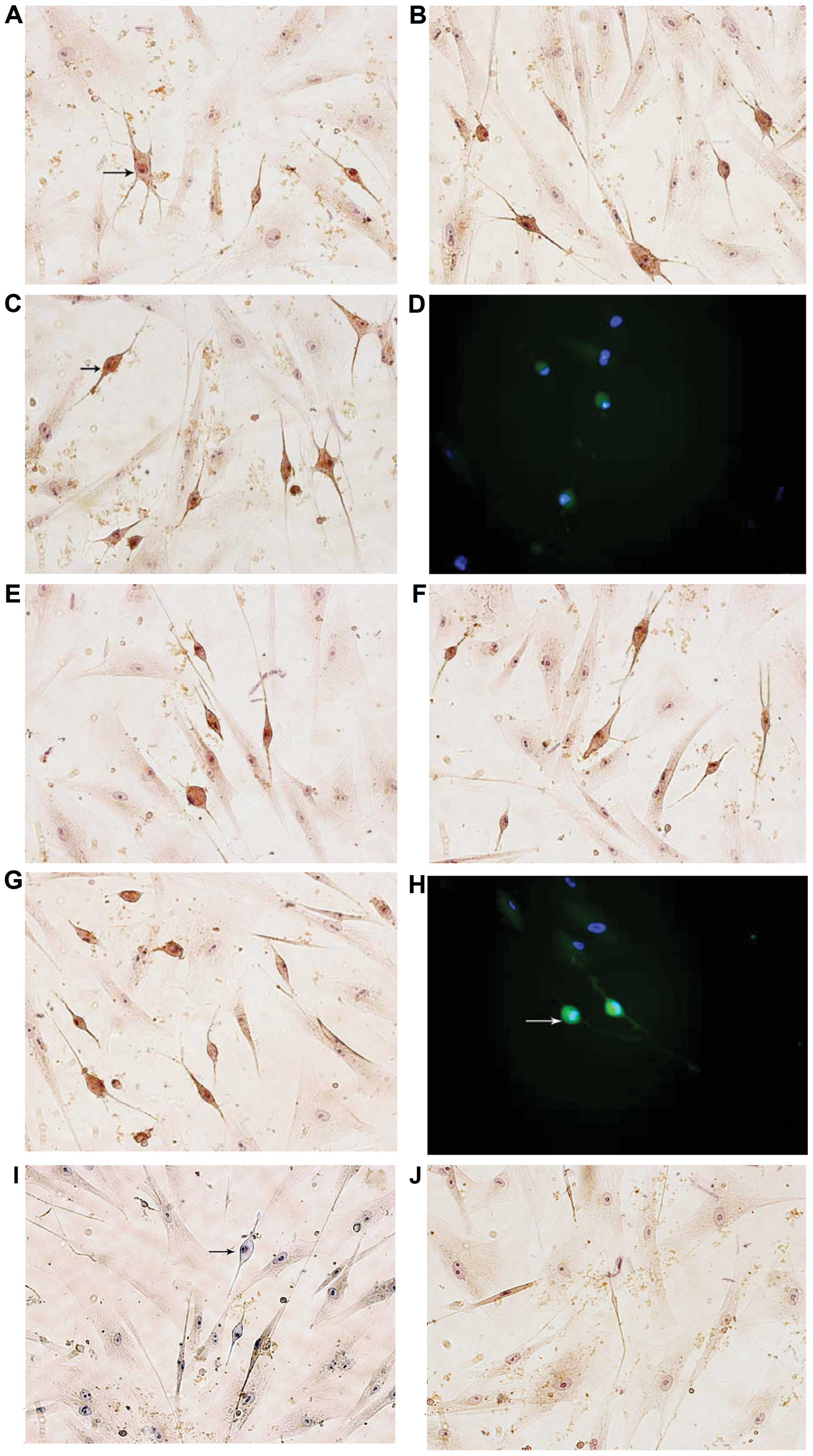
Immunocytochemical detection of
neuron-specific markers
The immunocytochemical staining of NF-H and MAP-2 in
the majority of neuron-like cells was positive, while GFAP was
negative in the hMSCs treated for 6 h with various concentrations
of GM1 (Fig. 4I). By contrast, no
cells were positively stained for these markers in the control
group, and immunocytochemical staining was negative when PBS was
used instead of the primary antibody (Fig. 4J).
Discussion
MSCs are adult stem cells derived from the mesoderm
and can be isolated from tissues such as bone marrow, fat,
placenta, umbilical cord blood and the umbilical cord. They have
the potential of self-renewal, as well as high proliferative and
multi-directional differentiation potential. Compared to stem cells
derived from bone marrow, placenta and other tissues, hUMSCs have
greater practical advantages. First, the umbilical cord as a source
of stem cells has no associated ethical controversy and can be
readily obtained at a low cost. Second, hUMSCs will not cause
teratomas and have tumor suppressor properties. Since hUMSCs also
have advantageous properties, such as low immunogenicity, migratory
ability and genetic stability, as well as immune regulation, stroma
support and paracrine functions, they hold great potential for use
in clinical treatments (5).
Studies on the transplantation of MSCs for the treatment of nervous
system diseases have achieved encouraging results (6,7).
However, animal experiments have shown that only a small proportion
of MSCs transplanted into damaged nerve tissue can differentiate
into neuron-like cells (8,9).
In vitro experiments have also demonstrated that although
the injured spinal cord tissue fluid can induce bone marrow-derived
stem cells (BMSCs) to differentiate into neuron-like cells, the
differentiation rate is not high (10,11). Therefore, finding drugs which can
induce or promote the differentiation of MSCs into neuronal cells
is important. This study confirmed that ganglioside GM1 induced
hUMSCs to differentiate into neuron-like cells in vitro,
characterized by the expression of the neuron-specific proteins,
MAP-2 and NF-H, but not that of the astrocyte marker, GFAP.
Gangliosides are glycosphingolipids that contain
sialic acid and are formed by hydrophobic ceramide and hydrophilic
single sialic acid oligosaccharide chains. GM1 has been confirmed
to have effects on cell-cell recognition and transmembrane signal
transmission and adhesion. It can also regulate polypeptide growth
factors to influence cell proliferation and promote cell maturation
(12). Exogenous gangliosides can
cross the blood brain barrier and promote nerve pullulation, axonal
regeneration and synapse formation (13). They can also regulate and enhance
the role of neurotrophic factors (14), resist apoptosis after nerve injury
(15) and promote the
proliferation of neural stem cells (16).
Zhao et al (17) demonstrated that sub-totipotent
stem cells still retain sub-totipotent genes after the embryo has
developed into an adult, but they gradually lose some of the
original stem cell phenotype. If the tissue-specific gene
expression program of such cells is activated in an appropriate
microenvironment, they can differentiate into various histiocytes.
As hUMSCs are sub-totipotent stem cells, GM1 may provide a
microenvironment to activate the specific expression programs of
nerve cells and thereby induce them to differentiate into neural
cells. At least three mechanisms of activation are possible. First,
GM1 may exert its effects on transmembrane ion flow in the hUMSCs.
Ca2+ is a second messenger in cells, and changes in its
concentration inside and outside of cells and its flow across the
membrane, which are regulated by the Ca2+-ATP enzyme,
may cause different biological consequences. Cui et al
(18) indicated that exogenous
GM3 has bidirectional regulatory effects on Ca2+-ATP
enzyme activity, e.g., inhibition at low density and activation at
high density. Liu et al (19) pointed out that TMP as a
Ca2+ chelator may inhibit intracellular Ca2+
signaling to upregulate the gene expression of NSE and Nurrl,
thereby accelerating the differentiation of hUMSCs into nerve
cells. Thus, GM1 (especially at 150 µg/ml) may inhibit the
Ca2+-ATP enzyme at the cell membrane of hUMSCs, thereby
reducing the concentration of intracellular Ca2+ and
affect the signal transduction, ultimately inducing expression of
neuron-specific genes and promoting differentiation into nerve
cells. The second possible explanation for the mechanism of action
by GM1 may be through its effects on nerve growth factor (NGF). GM1
can promote the generation of NGF (20), and the high concentration of
neurotrophic factors may simulate a microenvironment during
embryonic developmental stages of neurogenesis. A neurotrophic
factor enriched environment can increase the expression of MsC
membrane proteins TrkA, TrkB and TkrC, which are neurotrophic
factor receptors. The binding of neurotrophin with its receptor
would initiate altered gene expression (21) and promote MSC differentiation into
nerve cells. Finally, the impact of GM1 on hUMSCs may be on the
length of neurites by activating an ectopic protein kinase on the
surface of the cell membrane. This ganglioside sensitive protein
kinase may serve as a transfersome to transmit signals from the
outside of the cell to the inside, thus regulating the growth and
length of the neurite.
In conclusion, gangliosides have been widely used as
neuroprotective drugs and have played a positive role in clinic
treatments. In this study, GM1 was shown to facilitate the
development of neuron-like cells from primary hUMSCs in
vitro. However, whether GM1 can induce hUMSCs transplanted
in vivo to differentiate into neural cells effectively will
require further study.
Acknowledgments
Project supported by the Topic Outstanding Youth
Science Foundation of Natural Science Fund of Hebei (no.
C2009001547), the Natural Science Fund of Hebei (no. H2013206399),
the Medical Science Research Key Project of Hebei (no.
20130240).
References
|
1
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ning N and Chen NH: Progress in the
research of ganglioside's biological activities. Sheng Li Ke Xue
Jin Zhan. 40:24–30. 2009.In Chinese. PubMed/NCBI
|
|
4
|
Zhang Q and Zuo PP: Advances in the study
of neuroprotective mechanisms of ganglioside GM1. Chin Pharmacol
Bull. 20:1329–1333. 2004.
|
|
5
|
Wang Y, Zhang JL, Hang XB, et al:
Umbilical cord mesenchymal stem cell research present situation and
the clinical treatment. Chongqing Med. 42:2161–2163. 2013.
|
|
6
|
Yu JX, Chen F, Sun J, Wang JM, Zhao QJ,
Ren XJ, Ma FX, Yang SG, Han ZB and Han ZC: Umbilical cord
mesenchymal stem cell transplantation for treatment of experimental
autoimmune myasthenia gravis in rats. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 19:744–748. 2011.In Chinese. PubMed/NCBI
|
|
7
|
Yang HQ, Wang YF, Li DS, et al:
Application of umbilical cord mesenchymal stem cell transplantation
in the treatment of two cases of hereditary spastic paraplegia.
Chin J Tissue Eng Res. 15:167–170. 2011.In Chinese.
|
|
8
|
Zhao ZM, Zhang QJ, Han ZC, et al:
Improving functional outcome following bone marrow mesenchymal stem
cells transplantation to injured spinal cord in rats. Chin J
Neurosurg. 19:582003.
|
|
9
|
Lee JB, Kuroda S, Shichinohe H, Yano S,
Kobayashi H, Hida K and Iwasaki Y: A pre-clinical assessment model
of rat autogeneic bone marrow stromal cell transplantation into the
central nervous system. Brain Res Brain Res Protoc. 14:37–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YA, Wang RS, Zhang C, et al: The
inducting differentiation with the spinal cord extracts on rat bone
mesenchymal stem cells in vitro. J Apoplexy Nerv Dis.
20:5362003.
|
|
11
|
Mei XF, Qin SJ, Fan GY, et al: Adult rat
bone marrow stromal cells differentiate into neurons by the
extracts of injured spinal cords. Chin J Clin Anat. 23:264–267.
2005.
|
|
12
|
Dawson TM, Hung K, Dawson VL, Steiner JP
and Snyder SH: Neuroprotective effects of gangliosides may involve
inhibition of nitric oxide synthase. Ann Neurol. 37:115–118. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schengrund CL and Mummert CM: Exogenous
gangliosides. How do they cross the blood-brain barrier and how do
they inhibit cell proliferation. Ann NY Acad Sci. 845:278–284.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levi-Montalcini R: The nerve growth factor
35 years later. Science. 237:1154–1162. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuo Y, Liao WH, Wu BM, Wang H and Chen Z:
The anti-apoptosis effect of Ganglioside (GM1) after the spinal
cord injury. Chin J Spine Spinal Cord. 13:536–538. 2003.In
Chinese.
|
|
16
|
Man Y, Li HW, Yang B, et al: Effects of
different dose of ganglioside on proliferation and differentiation
of nerve stem cells. Chin J Clin Rehabil. 8:4634–4635. 2004.In
Chinese.
|
|
17
|
Zhao CH, Fang BJ, Han Q, et al: Study
about biological property of pluripotent stem cells and
transplantation application. J Chin Microcircul. 8:3452004.
|
|
18
|
Cui W, Liu YK, Zhang XY, et al: The impact
of ectogenic ganglioside GM3 on Ca2+-ATP enzyme and
Ca2+ concentration in red blood cell cytoplasm. Acta
Academica Med Shanghai. 21:385–387. 1994.
|
|
19
|
Liu YY, Zhao XX, Zhao HB, Ge BF, Liu XY
and Chen KM: Tetramethylpyrazine induces the differentiation of
mouse bone marrow-derived mesenchymal stem cells into nerve cells
mediated by Ca2+ signaling. Gansu Nong Ye Da Xue Xue
Bao. 45:1–5. 2010.In Chinese.
|
|
20
|
Liberini P, Pioro EP, Maysinger D, Ervin
FR and Cuello AC: Long-term protective effects of human recombinant
nerve growth factor and monosialoganglioside GM1 treatment on
primate nucleus basalis cholinergic neurons after neocortical
infarction. Neuroscience. 53:625–637. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan Y, Yang SY, Han ZC, et al:
Amplification and differentiation towards neuron - like cells of
human umbilical cord derived mesenchymal stem cells. Chin J
Neuromed. 5:230–236. 2006.
|