Introduction
The hair follicle is a skin organ that grows and
undergoes cyclic morphological changes during its lifetime
(1). It consists of complex
structures, such as the outer root sheath (ORS) and the inner root
sheath (IRS), the matrix, and the mesenchymal portions that include
the connective tissue sheath (CTS) and dermal papilla (DP)
(2). The DP plays a crucial role
in hair formation, growth and cycling and can induce follicle
neogenesis and encapsulation in overlying epithelial cells
(3).
Human placental extract (HPE) contains a variety of
growth factors, cytokines and other physiologically active
substances (4). The many
biological functions of HPE are a matter of increasing interest.
Several studies using animal models have provided evidence
demonstrating the corticotropin-releasing factor (CRF)-like action
of HPE, its effects on the expression of interleukin-8 (a known
mediator of inflammation) (5),
its improvement of liver function (6,7),
anti-platelet aggregation activity (8) and its ability to be suppressed by
gluco-corticoids (9). In the
present study, we focused on the biological effects of HPE on human
dermal papilla cells (hDPCs). In particular, we aimed to determine
whether HPE is effective in promoting the hair-inductive capacity
of hDPCs in culture in order to develop novel treatment strategies
for hair loss.
Minoxidil (MXD) has been approved by the US Food and
Drug Administration (FDA) for the treatment of hair loss in males
(10). However, drugs containing
MXD have limited therapeutic applications due to their
unsatisfactory results and side-effects, such as the resumption of
hair loss after discontinuing the use of the product (11). To complement these drugs, some
researchers have attempted to use a combination of agents, such as
5-aminolevulinic acid and iron ion (12), herbal extracts and platelet-rich
plasma (13), and Trifolium
pratense extract and a biomimetic peptide (14).
Wnt/β-catenin signaling promotes the development of
new hair follicles and is required for the initiation of hair
morphogenesis (15). Within the
established hair follicle, Wnt cascade signals play a key role in
the activation of bulge stem cells to progress toward hair
formation, and these signals are mediated by β-catenin and lymphoid
enhancer-binding factor 1 (Lef1) (16). In the absence of a Wnt signal,
glycogen synthase kinase-3β (GSK-3β) phosphorylates β-catenin, as
well as adenomatous polyposis coli (APC) and axin, leading to the
degradation and ubiquitination of cytosolic β-catenin. However, in
the presence of Wnt signaling, the phosphorylation of β-catenin by
GSK-3β is suppressed, and β-catenin is dephosphorylated and
stabilized, and then enters the nucleus to interact with the T-cell
factor (Tcf)/LEF transcription factors to regulate the
transcription of target genes (17). GSK-3β is a cytoplasmic
serine/threonine protein kinase which is known to play central
roles in a variety of biological processes (18). Importantly, the direct inhibition
of the function of GSK-3β results in the cellular accumulation of
β-catenin (19). For instance,
valproic acid (VPA), which activates the Wnt/β-catenin pathway, has
been shown to promote hair re-growth in vitro and in
vivo (20).
In the present study, to elucidate the mechanisms
underlying the regulation of hDPC proliferation, we focused on the
β-catenin signaling pathway. Our results demonstrated that
β-catenin mediated the proliferation of hDPCs through the
inhibition of GSK-3β by phosphorylation (Ser9). Our
findings demonstrated that HPE promoted hair growth and may thus
have potential for use as an alternative therapeutic option in the
treatment of hair loss.
Materials and methods
Isolation and culture of hDPCs
hDPCs were purchased from Cefobio (Seoul, Korea) as
primary cells and were grown in Dulbecco's modified Eagle's medium
(DMEM; Invitrogen/Gibco-BRL, Grand Island, NY, USA) supplemented
with 5% fetal bovine serum (FBS; Invitrogen/Gibco-BRL) and 1%
penicillin in a humidified environment. Human follicular DPCs in
the third or fourth passage were used.
Isolation of nuclear and cytoplasmic
proteins
Nuclear and cytoplasmic proteins were isolated using
the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit
following the detailed instructions provided by the manufacturer
(Pierce Biotechnology, Rockford, IL, USA). Protease inhibitor
tablets (Roche Diagnostics, Basel, Switzerland) and the phosphatase
inhibitors, 10 mM sodium pyrophosphate and 100 µM sodium
orthovanadate, were added to cytoplasmic extraction reagent I
(CERI; Pierce Biotechnology) and nuclear extraction reagent (NER;
Pierce Biotechnology) prior to use.
Western blot analysis
hDPCs were prepared using lysis buffer [50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25%
deoxycholic acid] containing a protease inhibitor cocktail (Roche
Molecular Biochemicals, Indianapolis, IN, USA). Protein was
quantified using a Bio-Rad DC protein assay kit II (Bio-Rad
Laboratories, Hercules, CA, USA), separated on 10% sodium dodecyl
sulfate SDS-PAGE gel, and electrotransferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA). After blocking with 5%
non-fat milk in Tris-buffered saline containing 0.5% Tween-20
(Sigma-Aldrich, St. Louis, MO, USA), the blots were probed with
antibodies against β-catenin (Cat. no. 610154; BD Transduction
Laboratories, Lexington, KY, USA) and p-AKT (Cat. no. 3787), AKT
(Cat. no. 9272), dephosphorylated β-catenin (Cat. no. 8814),
p-GSK-3β (Cat. no. 9322), GSK-3β (Cat. no. 9315), p-ERK (Cat. no.
9101), ERK (Cat. no. 4695) and actin (Cat. no. 4968; all from Cell
Signaling Technology, Danvers, MA, USA) and then exposed to
horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit
secondary antibodies. GAPDH (Cat. no. 5174) and lamin B1 (Cat. no.
9087; both from Cell Signaling Technology) were taken as cytoplasm
and nuclear extract control. Protein expression was detected using
an enhanced chemiluminescence (ECL) system (Amersham Pharmacia,
Piscataway, NJ, USA). Images of the blotted membranes were obtained
using a Lumino Image Analyzer (LAS-1000; Fujifilm, Tokyo, Japan).
The protein levels were compared to a loading control (actin or
non-phosphorylated protein).
5-Bromo-2-deoxyuridine (BrdU)
incorporation assay
BrdU incorporation was quantified using the BrdU
Cell Proliferation Assay kit (Cell Signaling Technology) according
to the manufacturer's instructions. hDPCs were seeded at a density
of 3×103 cells/wells in a 96-well plate. Briefly, the
cells were examined for BrdU incorporation at 48 h. For the pathway
determination, the following inhibitors were used: LY294002 (a
specific inhibitor of phosphatiylinositol 3-kinase (PI3K), 440206;
Calbiochem, San Diego, CA, USA), BIO (a specific inhibitor of
GSK-3β, B1686; Sigma-Aldrich), PD98059 (a specific inhibitor of
extracellular signal-regulated kinase (ERK) 1/2), 513000;
Calbiochem) for 30 min and then were treated with the MXD or HPE
plus MXD. At the designated time points, BrdU (10 µM) was
added to each well. After 4 h, the medium was replaced with
fixing/denaturing solution, and the cells were incubated with
anti-BrdU antibody for 1 h. The incorporated BrdU was determined by
measuring the HRP-conjugated antibody to BrdU and
3,3′,5,5′-tetrameth-ylbenzidine (TMB) substrates. The absorbance
was read at 450 nm using a SpectraMax 340 microplate reader
(Molecular Devices, Sunnyvale, CA, USA). The results are expressed
as the percentage inhibition of BrdU incorporation compared to the
untreated control. Values are expressed as the means ± SD of 3
separate experiments, each performed in triplicate.
Alkaline phosphatase (ALP) activity
ALP activity was observed using an ALP Live Stain
kit (Invitrogen Life Technologies, Carlsbad CA, USA) according to
the manufacturer's instructions. Briefly, the cells were washed
twice in fresh medium, and an appropriate amount of 1X ALP Live
Stain solution was then directly applied to the cell culture, after
which the cells were incubated at 37°C for 30 min. The cells were
then rinsed 3–4 times with fresh medium and observed under an
Olympus BX51 fluorescence microscope (Olympus, Melville, NY, USA)
using a standard FITC filter.
Immunocytochemistry
The DPCs at 1.0×104 cells/500 µl
per chamber were seeded into a chamber slide, serum-starved for 24
h, and then treated with HPE, MXD or HPE plus MXD for 48 h.
Following treatment with 4% paraformaldehyde for 10 min and 0.1%
Triton X-100 for 5 min, the cultured hDPCs were incubated with
anti-β-catenin antibody (1:500, Cat. no. 610154; BD Transduction
Laboratories) at 4°C overnight and then with FITC-labeled goat
anti-mouse IgG (1:1,000, Cat. no. NB720-F; Novus Biologicals,
Littleton, CO, USA). A 4′6-diamidino-2-phenylindole (DAPI) mounting
medium kit (Golden Bridge International, Inc., Mukilteo, WA, USA)
was used to counterstain the nuclei. The immunostained cells were
mounted in medium containing DAPI and visualized using an Olympus
FLUOVIEW FV10i confocal microscope (Olympus Optical Co., Ltd.,
Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was purified using the RNeasy Plus Mini
kit (Qiagen, Valencia, CA, USA) following the manufacturer's
instructions. A total of 1 µg of DNase-treated total RNA was
used for first-strand cDNA. This reaction was performed using a
random primer and Moloney murine leukemia virus (M-MLV) reverse
transcriptase (Invitrogen Life Technologies). The primers used for
RT-PCR were as follows: Sonic hedgehog (SHH) sense, 5′-ACC
GAG GGC TGG GAC GAA GA-3′ and antisense, 5′-ATT TGG CCG CCA CCG AGT
T-3′; transforming growth factor (TGF)−β1 sense,
5′-AAA TTG AGG GCT TTC GCC TTA-3′ and antisense, 5′-GAA CCC GTT GAT
GTC CAC TTG-3′; TGF-β2 sense, 5′-TCC AAA GAT TTA ACA TCT CCA
ACC-3′ and antisense, 5′-CAT GCT CCA GCA CAG AAG TTC G-3′;
ALP sense, 5′-TGG AGC TTC AGA AGC TCA ACA CCA-3′ and
antisense, 5′-ATC TCG TTG TCT GAC TAC CAG TCC-3′; and
versican sense, 5′-GAC GAC TGT CTT GGT GG-3′ and antisense,
5′-ATA TCC AAA CAA GCC TG-3′. The PCR cycling conditions were as
follows: 30–35 cycles at 94°C for 2–10 min, 94°C for 30 sec to 3
min, 50–58°C for 30 sec to 1 min, 72°C for 30 sec to 1 min, and
72°C for 4–7 min. The PCR products were run on 0.75–1.5% agarose
gels. Images of the gels were analyzed using Quantity One software
(Bio-Rad Laboratories).
Isolation and culture of rat vibrissa
follicles
All procedures involving animals were conducted in
accordance with the guidelines of the Institutional Animal Care and
Use Committee of Chung-Ang University in Korea (institutional
review board no. 14–0058). The isolation of rat vibrissa follicles
was performed as previously described (21). Briefly, rat vibrissa follicles
were harvested from 23-day-old male Wistar rats. The rats were
sacrificed using carbon dioxide (CO2). Subsequently,
both the left and right mystacial pads were removed from the rats
and placed in a 1:1 (v/v) solution of Earle's balanced salts
solution (EBSS; Sigma-Aldrich). Anagen vibrissa follicles were then
dissected using a stereomicroscope (Olympus) from the posterior
sections of the mystacial pads, with considerable care being taken
to remove the surrounding connective tissue without damaging the
vibrissa follicle. Using this method, we were able to isolate
<30 follicles from each animal. The isolated follicles were then
placed in separate wells in 12-well plates that contained 1 ml
Williams' medium E supplemented with 2 mM L-glutamine (both from
Gibco-BRL), 10 µg/ml insulin (Sigma-Aldrich), 50 nM
hydrocortisone (Sigma-Aldrich), 100 unit/ml penicillin and 100
µg/ml streptomycin at 37°C and cultivated in an atmosphere
comprised of 5% CO2 and 95% air. The isolated follicles
were then treated with the vehicle (Williams' medium E) as a
control and 20% HPE. The hydrolysate of HPE (Laennec; GCJBP Corp.,
Yongin, Korea) was provided by GCJBP. MXD (Sigma-Aldrich) was used
as a positive control in the culture systems. The culture medium
was changed every 3 days, and photographs of the cultured rat
vibrissae follicles were acquired using a stereomicroscope for 3
weeks. The lengths of the hair follicles were measured using a DP
controller (Olympus).
Immunohistochemical staining
Cryosections (7 µm) were cut from the rat
vibrissae follicles embedded in Tissue-Tek (Miles Diagnostic
Division, Elkhart, IN, USA) and frozen in isopentane cooled by
liquid nitrogen. Prior to staining, the cryosections were fixed in
acetone for 10 min at −20°C. Some sections were stained for
immunohistochemical markers using monoclonal antibodies against
anti-β-catenin (1:500, Cat. no. 610154; BD Transduction
Laboratories), anti-TGF-β1 (1:100, Cat. no. ab92486; Abcam,
Cambridge, MA, USA) and anti-TGF-β2 (1:30, Cat. no. ab66045;
Abcam). Immunohistochemical analysis was performed using a
high-temperature antigen unmasking technique. Briefly, the sections
were heated in an unmasking solution (citrate buffer, pH 6.0),
washed, and then incubated with primary monoclonal antibodies at
room temperature for 1 h. This procedure was followed by incubation
with secondary antibodies (EnVision Detection kit K5007; Dako,
Glostrup, Denmark). Reaction products were developed using
diaminobenzidine solution as a chromogen. After the chromogen
reactions, the sections were rinsed and counterstained with
hematoxylin. After rinsing, the sections were dehydrated and
covered with permount (Thermo Fisher Scientific, Inc., Fair Lawn,
NJ, USA). Counterstained sections were then examined by light
microscopy using an Olympus BX40 microscope (Olympus America Inc.,
Melville, NY, USA) in order to assess histological changes. Two
independent, blinded pathologists evaluated each section. Each
pathologist assigned every section a score according to the
following scale: 0, negative control; +, moderately increased
staining; ++, considerably increased staining, based on the
percentages of stained cells in each category.
Statistical analysis
Statistical analyses of the data were performed
using the Student's t-test or multivariate analysis of variance
(ANOVA). The results are expressed as the means ± standard
deviation of at least 3 independent experiments, and a P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of HPE and MXD on the
proliferation of cultured hDPCs
To evaluate the effects of HPE and MXD on DPCs, we
examined BrdU and ALP activation. The cells were treated with
various concentrations of HPE (5, 10 and 20%), MXD (0.1, 0.5 and 1
µM), or HPE plus MXD for 48 h. The BrdU incorporation assay
confirmed that hDPC proliferation was increased following treatment
with HPE and MXD, with a significant increase observed following
treatment with 20% HPE and 20% HPE plus 0.5 µM MXD (Fig. 1A). To directly examine the effects
of HPE on cell proliferation, we measured ALP expression in the
hDPCs, as ALP is a well-established DP marker (22). As shown in Fig. 1B, there was a marked increase in
ALP expression at 72 h of culture, when the cells were treated with
a combination of 10% HPE and 0.5 µM MXD compared to
treatment with 10% HPE alone. These results suggest that HPE and
MXD effectively influence ALP expression. We then investigated the
changes in the expression of DP signature genes by performing
RT-PCR on hDPCs treated with 5 and 10% HPE, 0.5 µM MXD, or
HPE plus MXD for 30 min or 24 h. Notably, the SHH and
versican expression levels were increased in a
dose-dependent manner when the cells were treated with HPE
(Fig. 1C). These results suggest
that HPE, which upregulates the gene expression of SHH and
versican in DPCs, targets proliferation effectors in hDPCs.
Based on the collective results, we hypothesized that combined
treatment with HPE and MXD significantly increased the
proliferation of hDPCs.
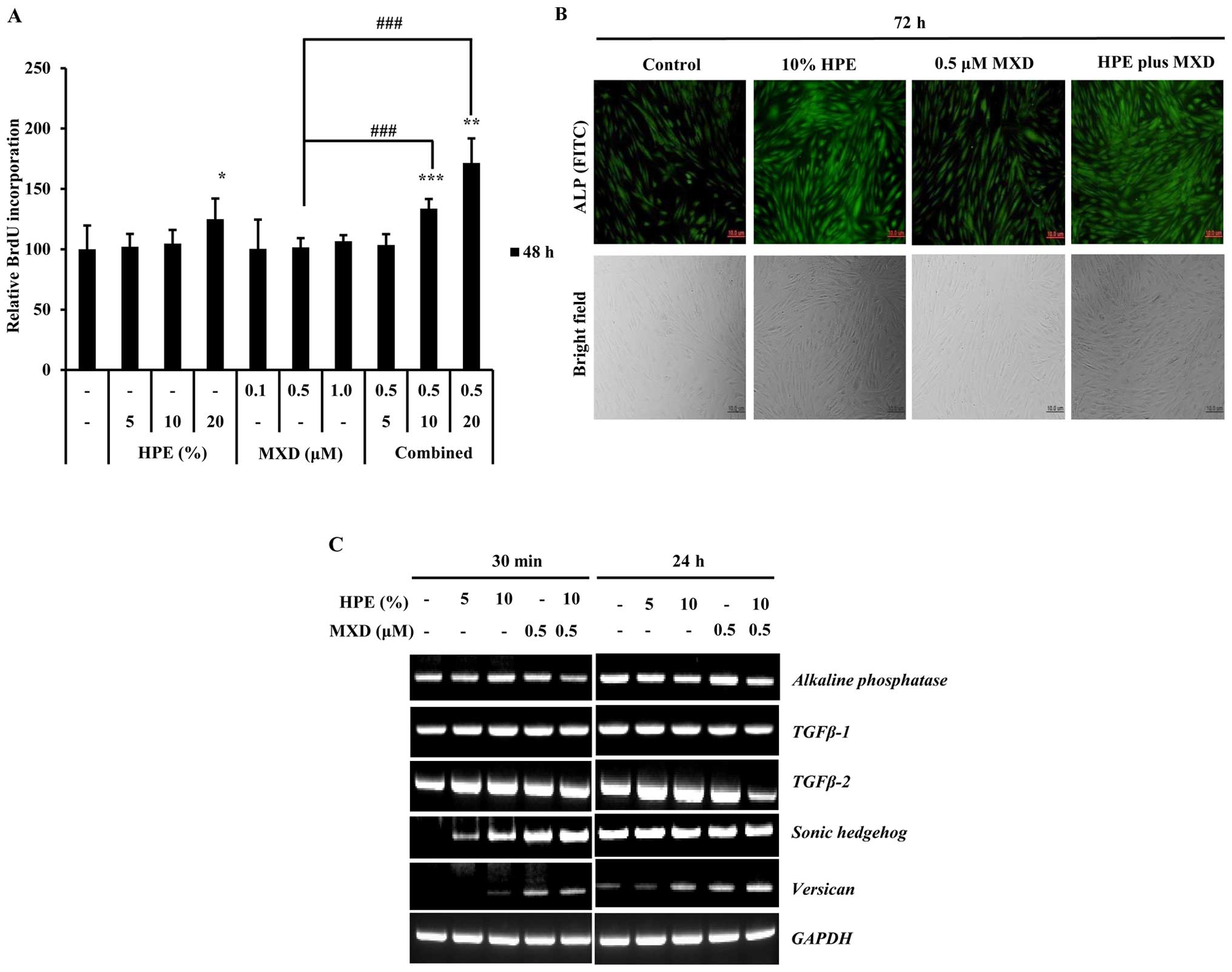 | Figure 1Human placental extract (HPE)
promotes the proliferation of human dermal papilla cells (hDPCs).
BrdU incorporation assay was used to determinate cellular
proliferation following treatment with HPE and minoxidil (MXD). (A)
Effects of HPE and MXD on DNA synthesis by DPCs. DNA synthesis was
evaluated by measuring the incorporation of BrdU after the DPCs
were incubated with various concentrations of HPE (5, 10 or 20%)
and MXD (0.1, 0.5 and 1 µM) in serum-free medium for 24 h.
Each value represents the percentage of the value of the control.
Results represent the means ± SD of 3 independent experiments, n=3.
(B) Effects of HPE and MXD on the activation of alkaline
phosphatase (ALP) activity in hDPCs. Morphology and ALP activity of
dermal papilla (DP) cells following treatment with HPE and MXD.
Cell morphology (bottom panels) was examined under a bright-field
microscope. FITC (green) staining indicates ALP-expressing cells.
Scale bar, 10 µm. (C) RT-PCR of transforming growth factor β
(TGF-β)1, TGF-β-2, alkaline phosphatase (ALP),
Sonic hedgehog (SHH), verisican and GAPDH
expression using total RNA prepared from HPE- and MXD-treated
hDPCs. *P<0.05, **P<0.01,
***P<0.001, compared with control;
###P<0.001, compared to treatment with 0.5 µM
MXD alone. |
HPE activates the β-catenin/GSK-3β
pathway in hDPCs
The cells were treated with various concentrations
of HPE (0, 1, 2, 5, 10 and 20%) for 48 h, and the expression of
β-catenin, and GSK-3β was measured by western blot analysis.
Treatment with HPE increased the total level of β-catenin protein
in a dose-dependent manner. In particular, the levels of
dephospho-β-catenin, an active form of β-catenin protein, were
elevated. The p-GSK-3β (Ser9) levels began to markedly
increase following treatment with 10% HPE, whereas the total GSK-3β
protein levels did not increase (Fig.
2A and B). GSK-3β is inactivated by phosphorylation at
Ser9; thus, these results suggested that the increase in
the β-catenin protein levels in the hDPCs induced by HPE was due to
the decreased destruction caused by GSK-3β inactivation. These
results suggest that treatment with 10% HPE induces cell
proliferation through the activation of β-catenin and the
inactivation of GSK-3β in hDPCs. As shown in Fig. 2C, the whole cell or tissue lysates
were separated into nuclear and cytoplasmic fractions, and western
blot analysis was performed with anti-β-catenin antibody. We found
that treatment with HPE induced the nuclear translocation of
β-catenin, since an increasing amount of β-catenin was detected in
the nuclear fraction 48 h after the cells were treated with HPE in
a dose-dependent manner. These results suggest that HPE induces
β-catenin and p-GSK-3β (Ser9) activation in hDPCs.
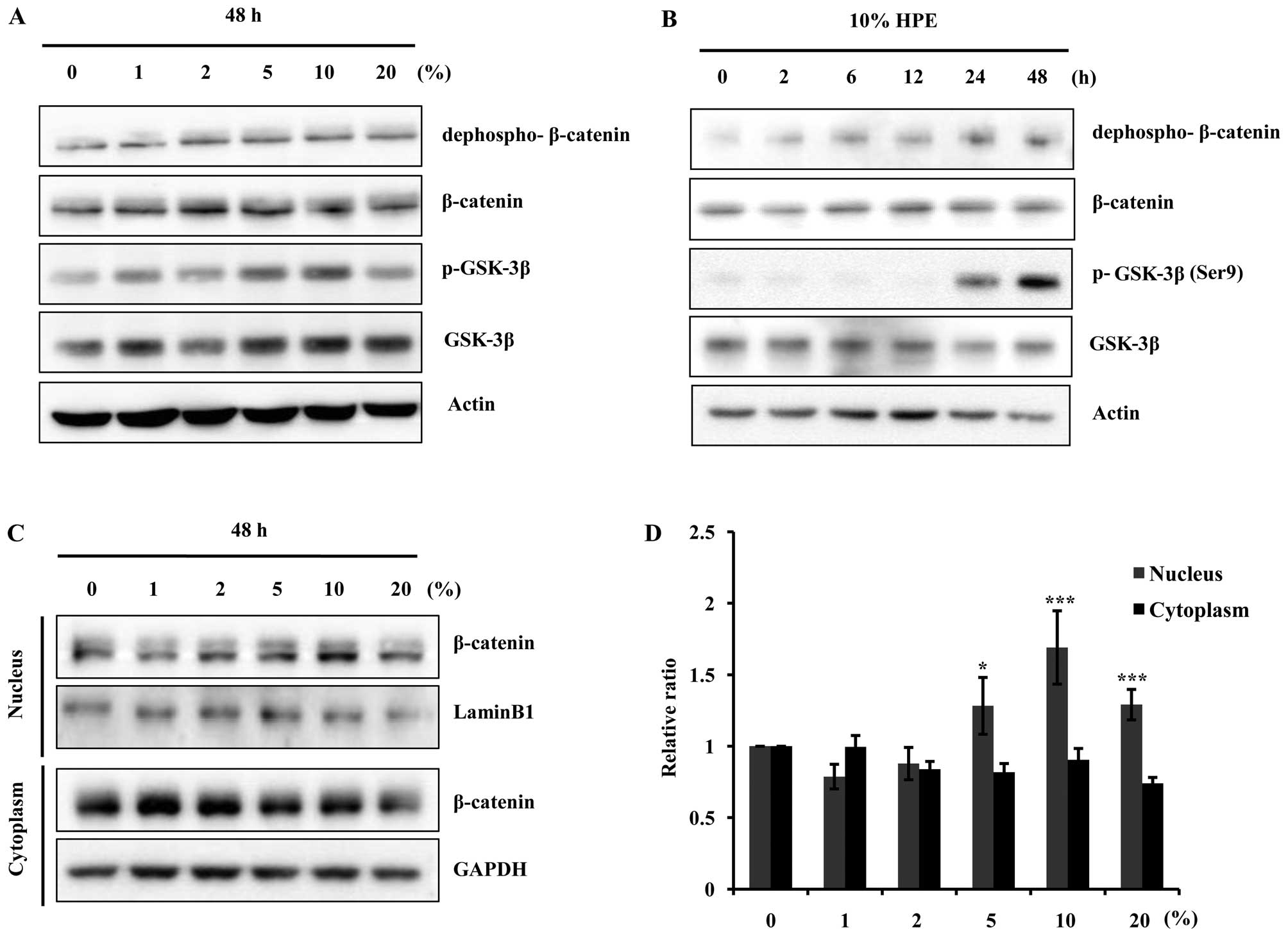 | Figure 2Human placental extract (HPE)
stimulates β-catenin signaling pathway through glycogen synthase
kinase-3β (GSK-3β) inhibition and nuclear β-catenin accumulation.
Western blot analysis was performed to demonstrate the dose- and
time-dependent increase in β-catenin expression in the cells
harvested after HPE treatment. (A) Cells were treated with HPE (0,
1, 2, 5, 10 and 20%) for 48 h. Cell lysates were prepared and
subjected to western blot analysis for dephospho-β-catenin,
β-catenin, phosphorylated (p)-GSK-3β and GSK-3β. (B) Western blot
analysis for dephospho-β-catenin, β-catenin, p-GSK-3β, and GSK-3β
in human dermal papilla cells (hDPCs) treated with 10% HPE for 0,
2, 6, 12 and 24 h. (C) Western blot analysis of cytoplasmic,
nuclear and total β-catenin levels in hDPCs. Cells were treated
with HPE (0, 1, 2, 5, 10 and 20%) for 48 h. (D) The relative ratio
of cytoplasmic, nuclear and total β-catenin proteins was determined
by calculating the ratio of each protein in each of the irradiation
treatment groups relative to the corresponding band intensity in
the normal group. *P<0.05, ***P<0.001,
compared with control. |
HPE and MXD effectively promote the
activation of GSK-3β and the nuclear translocation of β-catenin in
hDPCs
MXD induces the nuclear accumulation of β-catenin
and increases the phosphorylation of GSK-3β (23). Thus, we examined the effects of
HPE and MXD on proteins related to cell growth in the hDPCs by
western blot analysis. Treatment with MXD (0.5 µM) plus HPE
(10%) significantly increased the protein expression of GSK-3β and
β-catenin compared with the cells treated with HPE or MXD alone for
12 h. The protein expression was increased more significantly
following treatment with 0.5 µM MXD in combination with 10%
HPE compared to treatment with MXD alone (Fig. 3A and B). Moreover, we observed
that treatment with HPE plus MXD effectively promoted β-catenin and
p-GSK-3β (Ser9) activation in a time-dependent manner
(Fig. 3C and D). These results
suggest that treatment with a combination of HPE and MXD is more
effective than treatment with HPE or MXD alone.
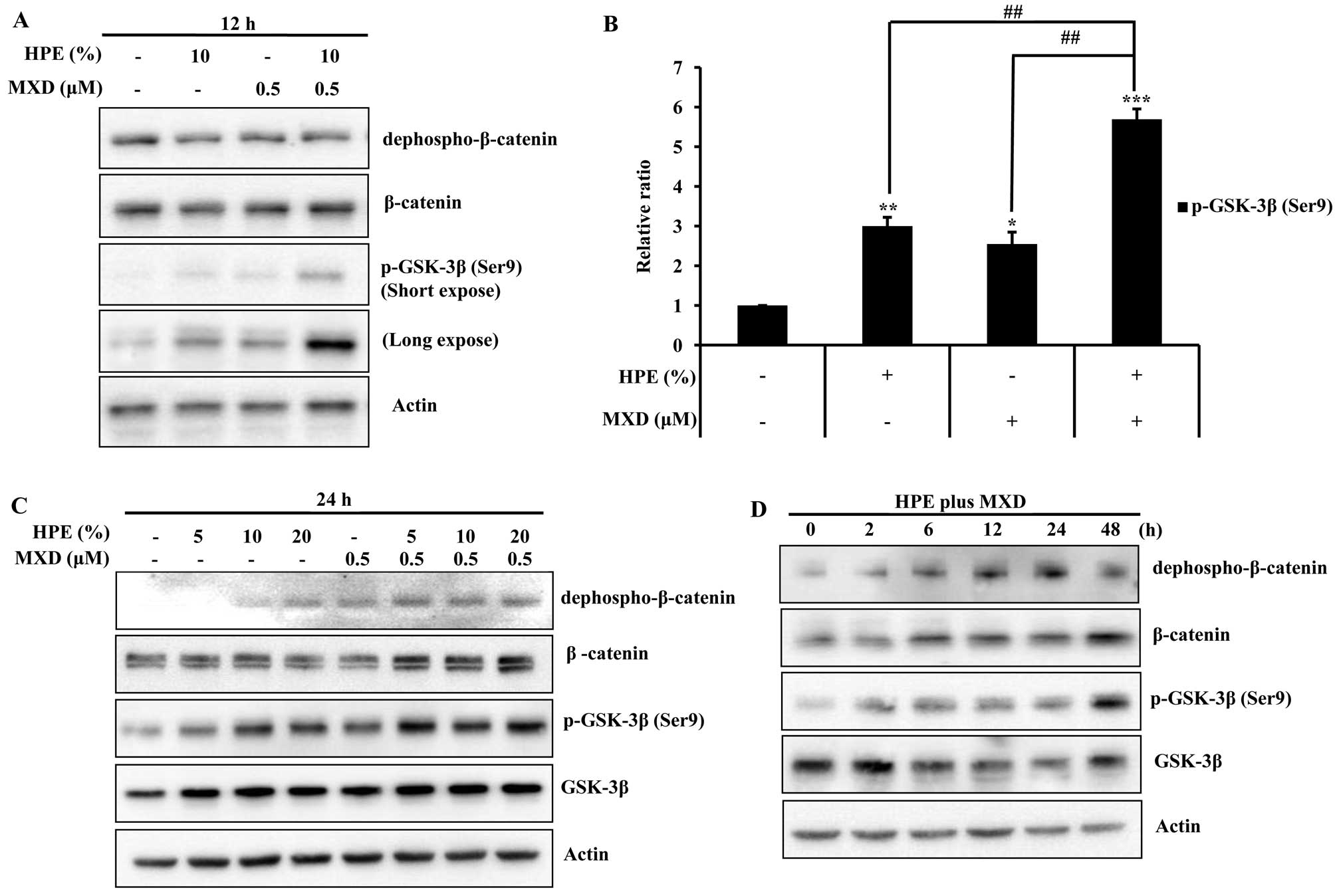 | Figure 3Effects of human placental extract
(HPE) and minoxidil (MXD) on the phosphorylation of glycogen
synthase kinase-3β (GSK-3β) in human dermal papilla cells (hDPCs).
(A) Cells were treated with 10% HPE, 0.5 µM MXD, or HPE plus
MXD for 12 h. Cell lysates were prepared and subjected to western
blot analysis for dephospho-β-catenin, β-catenin and phosphorylated
(p)-GSK-3β. (B) The relative ratios of each protein were determined
by calculating the ratio of p-GSK-3β in the cells treated with HPE,
MXD or HPE plus MXD relative to the corresponding band intensity in
the control. (C) Cells were treated with HPE (5, 10 and 20%), MXD
(0.5 µM), or HPE plus MXD for 24 h. Cell lysates were
subjected to western blot analysis using the indicated antibodies.
(D) Cells were treated with HPE plus MXD for 0, 2, 6, 12, 24 or 48
h. Western blot analysis was performed to detect
dephospho-β-catenin, β-catenin, and p-GSK-3β. Data shown are
representative of 3 independent experiments. Values are shown as
relative ratios. *P<0.05, **P<0.01,
***P<0.001, compared with
control;##P<0.01, compared to treatment with HPE plus
MXD. |
To elucidate the mechanisms underlying the induction
of β-catenin activation in the cells treated with HPE and HPE plus
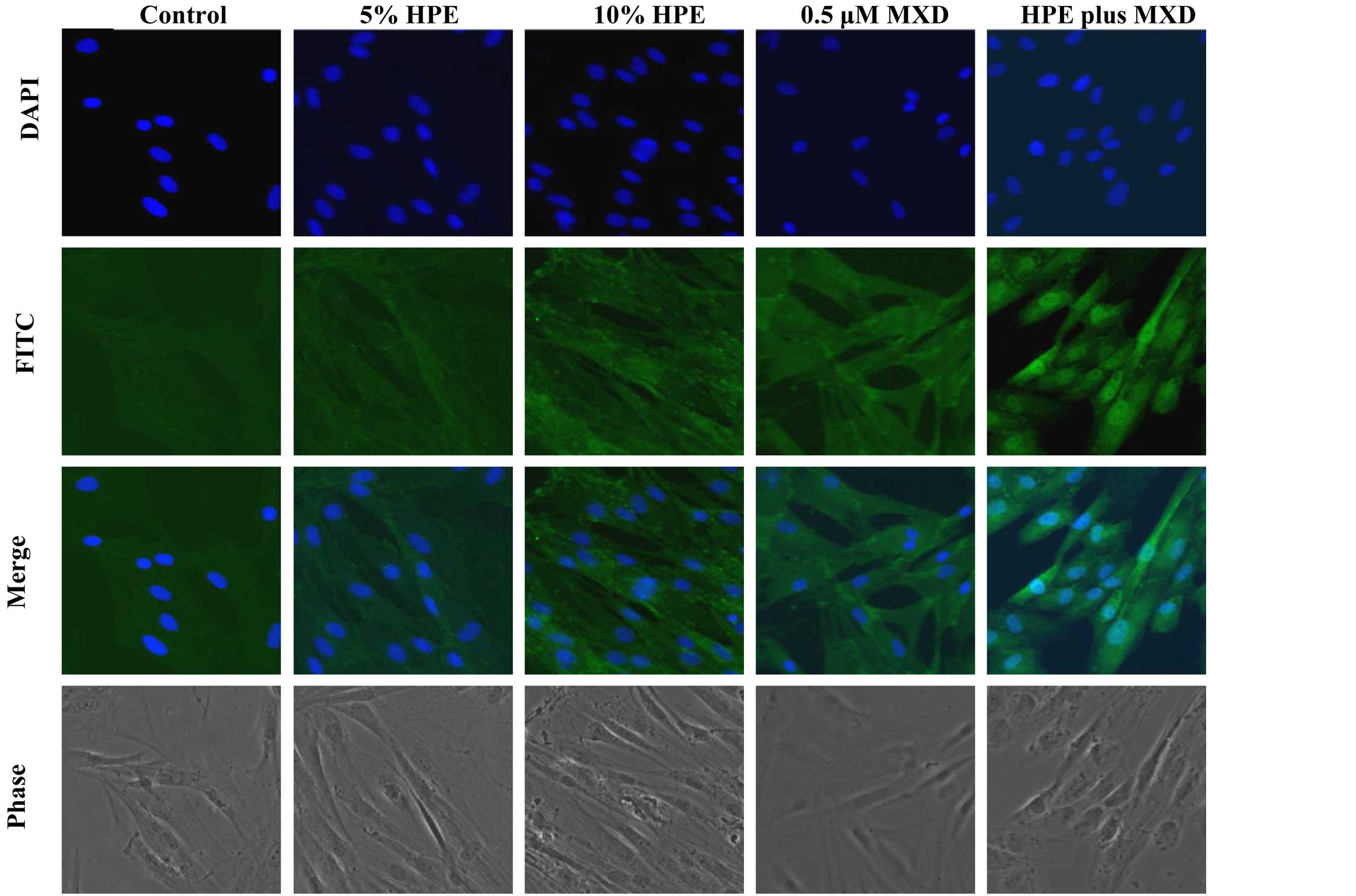
MXD, we performed immunocytochemistry on the hDPCs. The
localization of β-catenin was examined after treating the hDPCs
with HPE, MXD or HPE plus MXD for 24 h. β-catenin was mainly
associated with the cell membrane and was weakly detected in the
nuclei. In the HPE- or MXD-treated cells, the increased expression
of β-catenin was noted. Treatment with HPE plus MXD induced the
nuclear translocation of β-catenin in the hDPCs (Fig. 4). These results suggest that
treatment with HPE plus MXD causes the significant induction of
GSK-3β phosphorylation at the Ser9 residue. GSK-3β
inactivation was synchronized with the accumulation of β-catenin
after 48 h. Thus, the combination of HPE and MXD may be important
for the crosstalk involved in β-catenin/GSK-3β signaling.
Phosphorylation of GSK-3β
(Ser9) is critically involved in the HPE- and
MXD-induced increase in the levels of growth-related proteins in
hDPCs
Growth factor signaling has previously been
implicated in the regulation of the GSK-3β/β-catenin axis in hair
morphogenesis and growth signaling (20). Recently, it was shown that
Wnt/β-catenin signaling is involved in regulating the expression of
extracellular-signal-regulated kinase (ERK) and
phosphatidylinositol-3-kinase (PI3K)/AKT as well as in the
proliferation of hDPCs (24,25). In this study, to elucidate the
molecular mechanisms involved in the HPE-associated acceleration of
MXD-induced hair growth in hDPCs, we examined the role of the ERK,
PI3K/AKT and GSK-3β signaling pathway in the proliferation of these
cells using the specific mitogen-activated protein kinase (MAPK)
inhibitors, PD98059 (20 µM, for ERK1/2), LY294002 (20
µM, for PI3K) and 6-bromoindirubin-3′-oxime (BIO; 1.5
µM, for GSK-3β). As shown in Fig. 5A, DNA synthesis at 48 h increased
by 1.3-fold in the cells treated with 0.5 µM MXD in
combination with 10% HPE compared to those treated with HPE alone.
Treatment with HPE significantly influenced proliferation through
the induction of the GSK-3β pathway. We also observed a similar
reduction in the GSK-3β protein expression levels at 48 h by
western blot analysis (Fig. 3D).
The effects of pre-treatment with each inhibitor were examined by
western blot analysis in order to determine the concentrations
required to significantly decrease HPE-induced dephospho-β-catenin
and p-GSK-3β in these cells (Fig.
5B). Consistently, PD98059 and LY294002, but not BIO blocked
the HPE-induced activation of dephospho-β-catenin. Soma et
al (16), reported that
Wnt/β-catenin activation results in the maintenance of the
hair-inducing ability of hDPCs. Taken together, these results
indicate that pGSK-3β activation plays a pivotal role in the HPE
stimulation of MXD-induced hair growth.
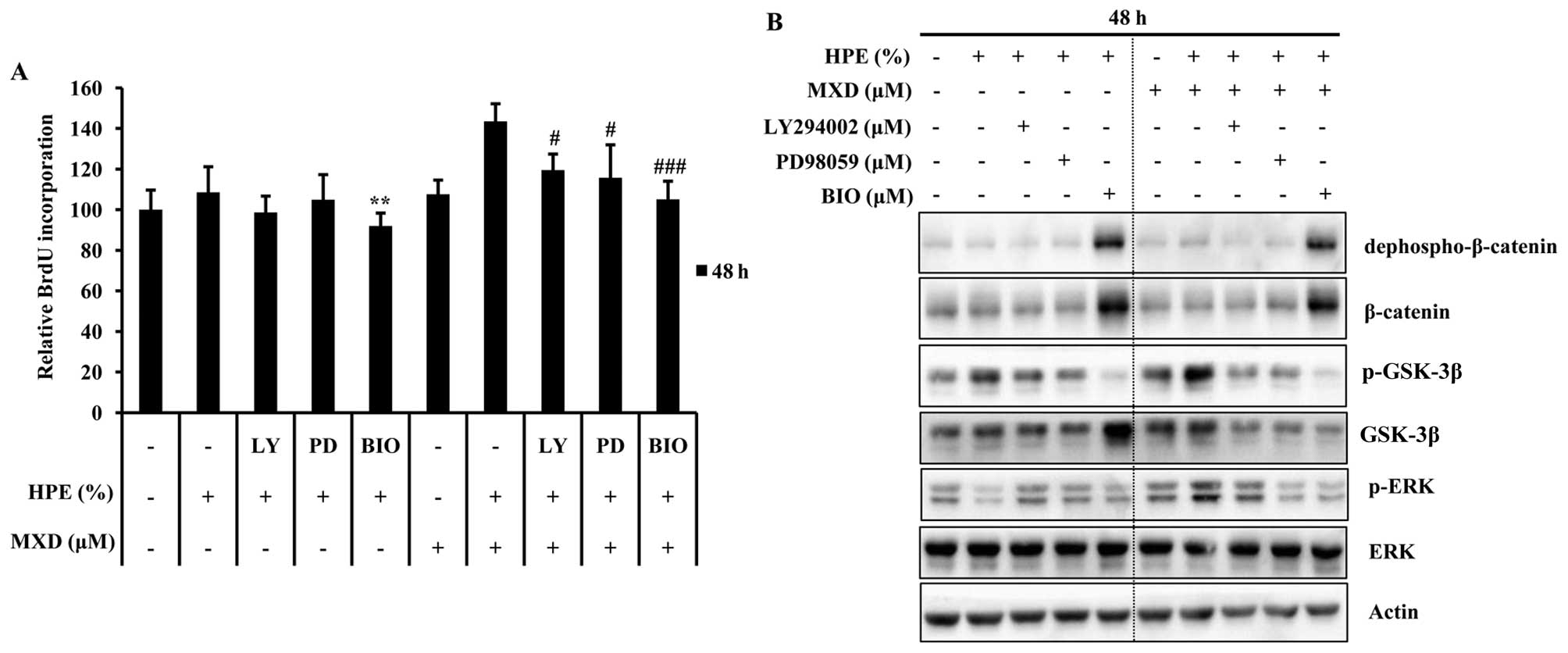 | Figure 5Human placental extract (HPE) and
minoxidil (MXD) induce a β-catenin cascade and regulate hair
growth-related protein expression in human dermal papilla cells
(hDPCs). Effects of phosphatidylinositol 3-kinase (PI3K),
extracellular signal regulated kinase (ERK), and glycogen synthase
kinase-3β (GSK-3β) inhibitor on HPE-induced proliferation of hDPCs.
(A) hDPC cells were treated with 20 µM of ERK inhibitor
(PD98059), 20 µM of PI3K inhibitor (LY294002), or 1.5
µM of GSK-3β inhibitor (BIO) for 30 min and then were
treated as described in the Materials and mehtods with the
indicated concentrations of 10% HPE, 0.5 µM MXD or HPE plus
MXD. The proliferation of all of the cells was measured at 48 h by
colorimetric-based assays using BrdU incorporation into hDPCs, and
(B) the cell lysates were prepared and subjected to western blot
analysis for dephospho-β-catenin, β-catenin, and phospho
(p)-GSK-3β, total GSK-3β, actin. Data are presented as means ± SD.
**P<0.01, compared to treatment with 10% HPE,
#P<0.05, ###P<0.001 compared to
treatment with HPE plus MXD. LY, LY 294002; PD, PD98059. |
HPE and MXD induce hair fiber elongation
and delay catagen progression in rat vibrissa follicles
To determine whether HPE stimulates MXD-induced hair
fiber elongation, we examined the effects of HPE and MXD using an
organ culture of the rat vibrissa follicle. The rat vibrissae
follicles were treated with 20% HPE, 10 µM MXD or 10% HPE
plus 5 µM MXD for 21 days (Fig. 6). The hair fiber lengths of the
vibrissa follicles treated with 5 µM MXD plus 10% HPE
(123.14±9.23) were similar to the lengths of the follicles treated
with 10 µM MXD (119.78±8.33; Fig. 6A and B). As a result, no synergy
was observed following treatment with 5 µM MXD plus 10% HPE
compared to treatment with 10 µM MXD alone. Thus, it can be
concluded that a lower concentration is required in combination
treatment than in individual treatment in order to produce a
similar effect. We then investigated the mechanisms through which
HPE stimulates MXD-induced hair fiber elongation by measuring the
expression levels of β-catenin, TGF-β1 and TGF-β2 in the cultured
rat vibrissae follicles. After 21 days, the cultured vibrissa
follicles, which were expected to be in the anagen-catagen
transition phase, were positive for TGF-β2, whereas the vibrissae
follicles in the bulb region treated with 20% HPE for 21 days were
negative for TGF-β2. These results support the conclusion that
treatment with HPE decreases the expression of TGF-β2, thereby
preventing apoptosis in the bulb region (Fig. 6C). Collectively, these data
demonstrate that HPE in combination with a low concentration of MXD
exerts a positive effect on hair growth and delays catagen
progression.
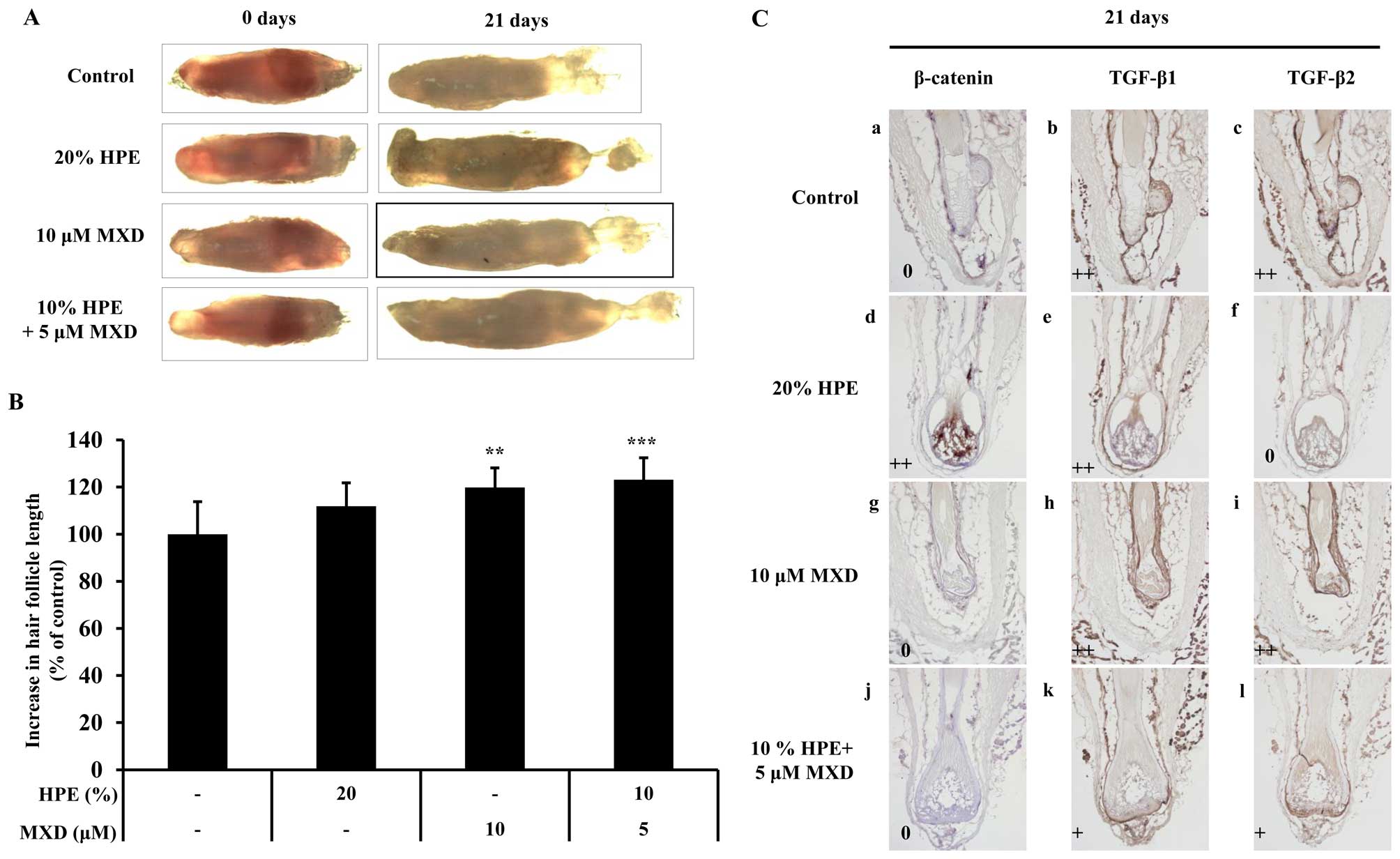 | Figure 6The elongation effect of human
placental extract (HPE) on the hair fiber length of rat vibrissa
follicles. Individual vibrissae follicles from Wistar rats were
microdissected and cultured in William's E medium at 37°C under 5%
CO2. (A) Vibrissa follicles were then treated with 20%
HPE, 10 µM minoxidil (MXD) or 10% HPE plus 5 µM MXD
for 21 days. The differences in the lengths of the vibrissa
follicles compared to those of the control group on 21 days are
shown. (B) Data are presented as the percentage of the length of
the treated follicles based on the mean length of the control
follicles ± SE. **P<0.01, ***P<0.001
compared with control. (C) Optimal cutting temperature (OCT)
compound-embedded sections of vibrissae follicles from the normal
(a, b and c), 20% HPE (d, e and f), 10 µM MXD (g, h and i),
and 10% HPE + 5 µM MXD (j, k and l) groups were
immunostained with anti-β-catenin (a, d, g and j),
anti-transforming growth factor β (TGF-β)1 (b, e, h and k),
anti-TGF-β2 (c, f, i and l) antibodies. Sections of the vibrissa
follicles were then stained with anti-β-catenin, anti-TGF-β1, and
anti-TGF-β2 antibodies after 21 days. Sections were counterstained
with hematoxylin for the visualization of nuclei. The brown dots
signify positively stained cells. Images shown are representative
examples from each group. The IHC intensity score represents the
average of the scores in each IHC staining intensity category (++,
pronounced findings; +, moderate findings;0, no/scant findings
identified in each core. Original magnification, ×200. |
Discussion
A steadily increasing number of products have been
claimed to be useful for treating hair loss (26). However, pharmaceutical hair loss
management still suffers from a lack of research. FDA-approved hair
loss drugs, such as dihydrotes-tosterone-suppressing 5α-reductase
inhibitor (finasteride) and antihypertensive potassium channel
opener (MXD), have been evaluated in clinical trials (10,27). However, given the widely
underestimated psychological burden of hair loss on affected
patients and the limited, transient sexual side-effects and
somewhat unpredictable efficacy of finasteride (28) and MXD (11–29,30) in hair loss management, more
pharmacological treatment options are needed. In the search for
alternatives to oral finasteride and topical MXD for the treatment
of hair loss, HPE, a potent hair growth modulator, needs to be
investigated as a promising candidate in in vitro and in
vivo studies.
In the present study, we demonstrated that HPE
increased the proliferation of hDPCs in a dose-dependent manner and
in combination with MXD by examining the activation of BrdU and
ALP, which play roles in DPC proliferation and in hair induction
and growth (Fig. 1A and B). Our
results indicated that combined treatment with HPE and MXD
significantly increased the proliferation of hDPCs.
Versican belongs to the hyalectan family and is
characterized by its ability to bind hyaluronan (31). The overall consensus is that
versican, together with hyaluronan, forms a pericellular matrix
that modulates cell proliferation, adhesion and migration (32). It has been reported that versican
affects apoptosis by associating with the functions of TGF-β
(33) and regulation by the
β-catenin signaling pathway (34). In the present study, we noted that
HPE and MXD significantly upregulated the gene expression levels
SHH and versican in the hDPCs in an indirect manner
(Fig. 1C). Further studies are
required to focus on determining the role of versican and TGF-β in
hDPC proliferation.
In the present study, we evaluated the possibility
that HPE stimulates the hair growth-promoting effect induced by MXD
by lowering the required efficacious dosage in hDPC cells. In
addition, we noted that HPE and MXD effectively induced GSK-3β
phosphorylation at the Ser9 residue, and GSK-3β
inactivation was synchronized with the accumulation of β-catenin
(Figs. 2 and 3). The results suggest that HPE
stimulates the MXD-induced phosphorylation of GSK-3β at
Ser9 in hDPCs. The observation that HPE effectively
promotes MXD-induced proliferation suggests that GSK-3β regulates
MXD-induced proliferation through the ERK, PI3K/AKT and GSK-3β
pathways via BrdU incorporation (Fig.
5). Therefore, our results suggest that treatment of cells with
HPE and MXD provides the signals necessary for p-GSK-3β
(Ser9) activation in hDPCs. As previously described,
alternative inhibitors of GSK-3β, lithium chloride (LiCl) or
beryllium chloride (BeCl2), stimulate hair re-growth and the hair
cycle to the anagen phase, but abnormally increase the thickness of
the epidermis (35). Further
investigations are required to focus on determining whether HPE,
the potential hair re-growth agent, targets the GSK-3β signaling
pathway in a pre-clinical study.
TGF-β2 is involved in the promotion of the hair
placode, contributes to anagen induction, and is highly expressed
in hDPCs (36). In the present
study, we observed that HPE was a potential suppressor of TGF-β2.
Furthermore, we demonstrated that HPE was effective in inducing
hair elongation in rat vibrissa hair follicle, and that treatment
with HPE led to a delay in catagen progression (Fig. 6). The suppression of TGF-β2 is
expected to provide a novel and efficient tool for hair cycle
regulation.
The data of the present study demonstrated that
treatment with HPE alone and HPE plus MXD resulted in a greater
increase in proliferation, GSK-3β phosphorylation, and the nuclear
translocation of β-catenin compared to treatment with HPE or MXD
alone. These observations suggest that HPE and MXD have a potent
additive effect and indicate that this combination may prove to be
an effective therapeutic option for the treatment of alopecia.
However, additional investigations are required to validate the
results presented in our study. In conclusion, the present study
demonstrates that HPE combined with a low concentration of MXD
exerts additional and more positive effects on hair growth. This
may result in the more practical dosing of MXD in the management of
hair loss.
Acknowledgments
The present study was supported by a National
Research Foundation of Korea grant funded by the Korean government
(2011–0008687).
References
|
1
|
Paus R and Cotsarelis G: The biology of
hair follicles. N Engl J Med. 341:491–497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hardy MH: The secret life of the hair
follicle. Trends Genet. 8:55–61. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stenn KS and Paus R: Controls of hair
follicle cycling. Physiol Rev. 81:449–494. 2001.PubMed/NCBI
|
|
4
|
Nair B and Elmore AR; Cosmetic Ingredient
Review Expert panel: Final report on the safety assessment of human
placental protein, hydrolyzed human placental protein, human
placental enzymes, human placental lipids, human umbilical extract,
placental protein, hydrolyzed placental protein, placental enzymes,
placental lipids, and umbilical extract. Int J Toxicol. 21(Suppl
1): 81–91. 2002. View Article : Google Scholar
|
|
5
|
Shibasaki T, Odagiri E, Shizume K and Ling
N: Corticotropin-releasing factor-like activity in human placental
extracts. J Clin Endocrinol Metab. 55:384–386. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung J, Lee HJ, Lee JM, Na KH, Hwang SG
and Kim GJ: Placenta extract promote liver regeneration in
CCl4-injured liver rat model. Int Immunopharmacol. 11:976–984.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu KX, Kato Y, Kaku T and Sugiyama Y:
Human placental extract stimulates liver regeneration in rats. Biol
Pharm Bull. 21:44–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sur TK, Biswas TK, Ali L and Mukherjee B:
Anti-inflammatory and anti-platelet aggregation activity of human
placental extract. Acta Pharmacol Sin. 24:187–192. 2003.PubMed/NCBI
|
|
9
|
Rosen T, Krikun G, Ma Y, Wang EY, Lockwood
CJ and Guller S: Chronic antagonism of nuclear factor-kappaB
activity in cytoblasts by dexamethasone: a potential mechanismfor
antiinflammatory action of glucocorticoids in human placenta. J
Clin Endocrinol Metab. 83:3647–3652. 1998.PubMed/NCBI
|
|
10
|
Topical minoxidil approved by FDA. Clin
Pharm. 7:8588621988.
|
|
11
|
Rossi A, Cantisani C, Melis L, Iorio A,
Scali E and Calvieri S: Minoxidil use in dermatology, side effects
and recent patents. Recent Pat Inflamm Allergy Drug Discov.
6:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morokuma Y, Yamazaki M, Maeda T, Yoshino
I, Ishizuka M, Tanaka T, Ito Y and Tsuboi R: Hair growth
stimulatory effect by a combination of 5-aminolevulinic acid and
iron ion. Int J Dermatol. 47:1298–1303. 2008. View Article : Google Scholar
|
|
13
|
Rastegar H, Ahmadi Ashtiani H, Aghaei M,
Ehsani A and Barikbin B: Combination of herbal extracts and
platelet-rich plasma induced dermal papilla cell proliferation:
involvement of ERK and Akt pathways. J Cosmet Dermatol. 12:116–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loing E, Lachance R, Ollier V and Hocquaux
M: A new strategy to modulate alopecia using a combination of two
specific and unique ingredients. J Cosmet Sci. 64:45–58.
2013.PubMed/NCBI
|
|
15
|
Chu EY, Hens J, Andl T, Kairo A, Yamaguchi
TP, Brisken C, Glick A, Wysolmerski JJ and Millar SE: Canonical WNT
signaling promotes mammary placode development and is essential for
initiation of mammary gland morphogenesis. Development.
131:4819–4829. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soma T, Fujiwara S, Shirakata Y, Hashimoto
K and Kishimoto J: Hair-inducing ability of human dermal papilla
cells cultured under Wnt/β-catenin signalling activation. Exp
Dermatol. 21:307–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woodgett JR: Judging a protein by more
than its name: GSK-3. Sci STKE. 2001:re122001.PubMed/NCBI
|
|
19
|
Li L, Yuan H, Weaver CD, Mao J, Farr GH
III, Sussman DJ, Jonkers J, Kimelman D and Wu D: Axin and Frat1
interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated
regulation of LEF-1. EMBO J. 18:4233–4240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jo SJ, Choi SJ, Yoon SY, Lee JY, Park WS,
Park PJ, Kim KH, Eun HC and Kwon O: Valproic acid promotes human
hair growth in in vitro culture model. J Dermatol Sci. 72:16–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Philpott MP and Kealey T: Cyclical changes
in rat vibrissa follicles maintained In vitro. J Invest Dermatol.
115:1152–1155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Handjiski BK, Eichmüller S, Hofmann U,
Czarnetzki BM and Paus R: Alkaline phosphatase activity and
localization during the murine hair cycle. Br J Dermatol.
131:303–310. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwack MH, Kang BM, Kim MK, Kim JC and Sung
YK: Minoxidil activates β-catenin pathway in human dermal papilla
cells: a possible explanation for its anagen prolongation effect. J
Dermatol Sci. 62:154–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang KA, Hwang YL, Lee MH, Kim NR, Roh
SS, Lee Y, Kim CD, Lee JH and Choi KC: Adenosine stimulates growth
of dermal papilla and lengthens the anagen phase by increasing the
cysteine level via fibroblast growth factors 2 and 7 in an organ
culture of mouse vibrissae hair follicles. Int J Mol Med.
29:195–201. 2012.
|
|
25
|
Yoon SY, Yoon JS, Jo SJ, Shin CY, Shin JY,
Kim JI, Kwon O and Kim KH: A role of placental growth factor in
hair growth. J Dermatol Sci. 74:125–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdullah F and Rashid RM: Alopecia:
Botanical approaches in review. J Drugs Dermatol. 9:537–541.
2010.PubMed/NCBI
|
|
27
|
Frankel S: Study of the food and drug
administration files on Propecia: dosages, side effects, and
recommendations. Arch Dermatol. 135:257–258. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Irwig MS and Kolukula S: Persistent sexual
side effects of finas-teride for male pattern hair loss. J Sex Med.
8:1747–1753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trüeb RM: Chemotherapy-induced hair loss.
Skin Therapy Lett. 15:5–7. 2010.PubMed/NCBI
|
|
30
|
Lourith N and Kanlayavattanakul M: Hair
loss and herbs for treatment. J Cosmet Dermatol. 12:210–222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahmani M, Wong BW, Ang L, Cheung CC,
Carthy JM, Walinski H and McManus BM: Versican: signaling to
transcriptional control pathways. Can J Physiol Pharmacol.
84:77–92. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
du Cros DL, LeBaron RG and Couchman JR:
Association of versican with dermal matrices and its potential role
in hair follicle development and cycling. J Invest Dermatol.
105:426–431. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wight TN: Arterial remodeling in vascular
disease: a key role for hyaluronan and versican. Front Biosci.
13:4933–4937. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Li Y, Wang Y, Wu J, Yang G, Yang
T, Gao Y and Lu Y: Versican gene: regulation by the β-catenin
signaling pathway plays a significant role in dermal papilla cell
aggregative growth. J Dermatol Sci. 68:157–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SH, Yoon J, Shin SH, Zahoor M, Kim HJ,
Park PJ, Park WS, Min S, Kim HY and Choi KY: Valproic acid induces
hair regeneration in murine model and activates alkaline
phosphatase activity in human dermal papilla cells. PLoS One.
7:e341522012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hibino T and Nishiyama T: Role of
TGF-beta2 in the human hair cycle. J Dermatol Sci. 35:9–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|