Introduction
Parkinson's disease (PD) is a neurodegenerative
disorder characterized by progressive and selective degeneration of
dopamine (DA) neurons in the substantia nigra pars compacta (SNpc),
a region that controls movement (1,2).
The initial symptoms of PD include basal tremor, muscular rigidity,
bradykinesia, cognitive impairment, postural abnormalities and
instability (3). The cause of PD
remains undefined. However, a number of environmental, immune
(4), and genetic (5) cues have been associated with the
onset of this disease. Accumulative evidence has revealed many
biochemical processes and molecular mechanisms that are involved in
mediating neuronal cell death in PD. These processes and mechanisms
include oxidative stress, apoptosis, inflammation, mitochondrial
dysfunction (6,7) and ubiquitin-proteasome system
dysfunction (8).
1-Methyl-4-phenylpyridinium (MPP+), the
active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(MPTP), is a neurotoxin that selectively destroys nigrostriatal DA
neurons in vivo as shown in studies using rodents and
non-human primates (9).
MPP+ induces apoptotic cell death by releasing
cytochrome c, leading to the opening of the mitochondrial
permeability transition pore (MTP) and subsequently activating
caspases (10–13). Oxidative stress is also involved
in dopaminergic neuronal cytotoxicity by the observation that
infusion of MPP+ into the brain increases hydroxyl
radicals and the formations of lipid peroxides in the striatum
(14).
Although PD symptoms can be effectively treated by
DA replacement therapy, the current treatments are not successful
in altering the progression of the disease (15). Additionally, long-term treatment
with a DA agonist or levodopa leads to severe motor deficits, such
as motor fluctuation and dyskinesia, and non-motor adverse
reactions, such as cardiac arrhythmia, DA dysregulation syndrome,
abdominal discomfort, PD dementia, and sleep disorders (1). Therefore, the optimal strategy is to
identify a drug with neuroprotective traits that exhibits few or no
adverse side effects.
Piperine
(1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]-(E,E)-piperidine
1-piperonylpiperidine) is a pungent nitrogenous alkaloid present in
black pepper (Piper nigrum), long pepper (Piper
longum) and other Piper species fruits (family Piperaceae)
(16). It has anti-inflammatory
(17), antioxidant (18), antipyretic, gastroprotective and
antidiarrheal properties in rodents (19,20). Pharmacological studies have
reported that piperine possesses anticancer and antioxidative
properties (21,22). In some countries of Asia, Piper
longum L. has also been applied in folk medicine to ameliorate
asthma, intestinal disorder, and poor peripheral blood circulation
(23). Piperine possesses
powerful antidepressant (24)
properties and protects against cognitive impairment in animal
models of Alzheimer's disease (25). Piperine has also been reported to
inhibit MPP+-induced mitochondrial dysfunction and cell
death in PC12 cells (26).
However, whether piperine exerts neuroprotective effects against
the MPTP-induced mouse model of PD remains to be reported.
In the present study, we hypothesized that piperine,
consistent with its antioxidant property, exerted anti-parkinsonian
effects by attenuating neuronal oxidative stress, apoptosis, and
inflammation. For this purpose, we assessed the ability of piperine
to protect against MPTP-induced motor and cognitive impairments in
the rotarod and Morris water maze (MWM) tests as well as
MPTP-induced reductions of dopaminergic neurons in SNpc. To
determine the mechanism of the observed effects, we assayed lipid
peroxidation by measuring activity of the oxidative stress marker
malondialdehyde (MDA) and the antioxidant enzyme superoxide
dismutase (SOD). Microglial activation, the pro-inflammatory
cytokine interleukin-1β (IL-1β), and expression of the
pro-apoptotic protein Bax and the anti-apoptotic peptide Bcl-2 were
also assessed.
Materials and methods
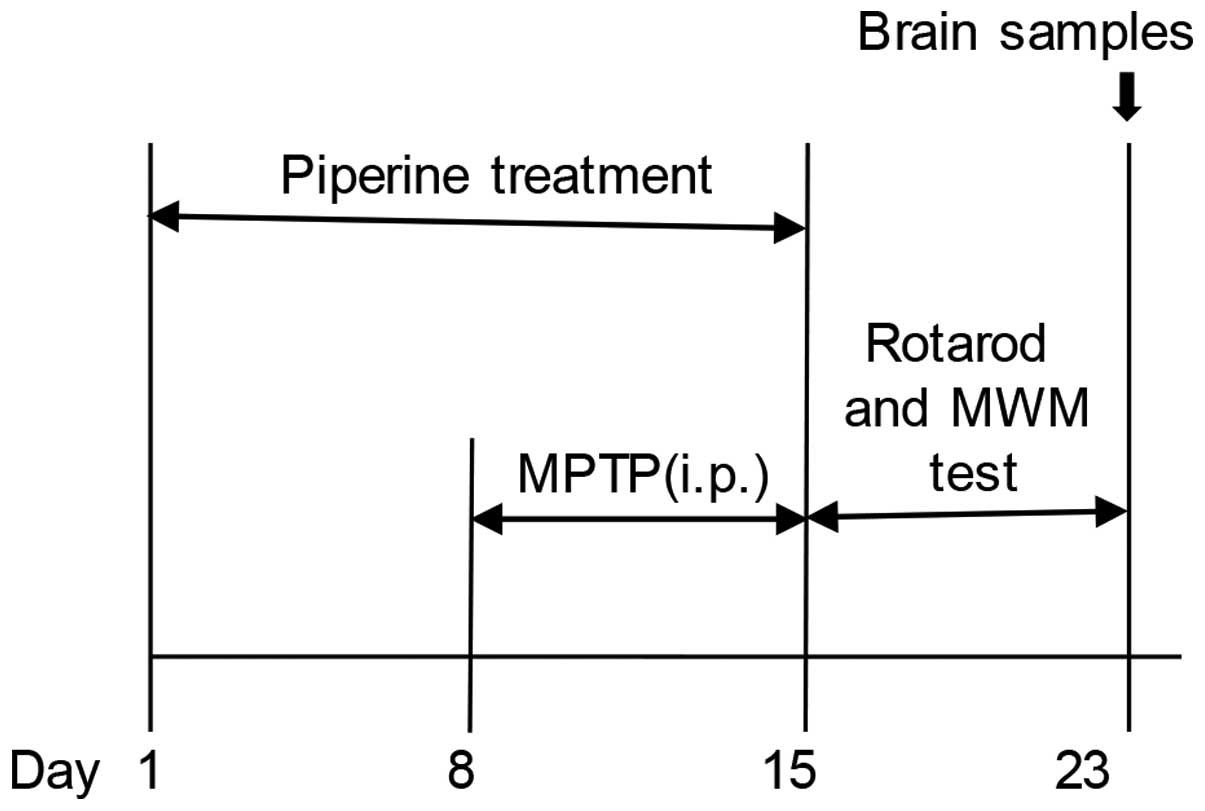
Experimental animals and treatment
Male C57BL/6 mice weighing 18–20 g were obtained
from JXJ Experimental Animal Co., Ltd., Shanghai, China
(2010002601739) and kept in a room maintained on a 12 h light/dark
cycle and temperature of 20–22°C with food and water available
ad libitum. To minimize discomfort and pain for the animals,
experimental procedures were carried out in accordance with the
European Community's Council Directive of 24 November 1986
(86/609/EEC). The mice were randomly divided into 3 groups (n=9):
normal saline-treated controls (NS), piperine-treated 10 mg/kg body
weight MPTP-induced group (P+M), and the group treated with MPTP
alone (MPTP). Piperine was dissolved in 5% carboxymethylcellulose
sodium solution. The mice were treated with piperine (10 mg/kg;
Selleck Chemicals, Houston, TX, USA) by daily intragastric
administration for 15 days. For the MPTP treatment, the mice
received intraperitoneal injection of MPTP hydrochloride (30 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) dissolved in normal saline once
daily for 7 successive days, starting the 8th day of piperine
treatment. After the behavioral testing was conducted, the animals
were sacrificed and their brains were dissected and prepared for
immunohistochemical staining (4 mice from each group were
sacrificed by cervical dislocation) or western blot analysis (the
remaining mice were sacrificed by perfused fixation) (for
experimental schedules, see Fig.
1).
Rotarod test
To measure motor coordination, the mice were
assessed on the rotarod apparatus (27). Prior to the test session, each
mouse received 30 min daily training for two successive days (speed
0.17 × g). The rotational speed was increased to 0.21 × g on the
third day. Each mouse was placed in a separate lane of the rotarod
(3 cm in diameter) and the time they remained on the rotating bar
was recorded. The maximum time was 6 min/trial. Each mouse was
given three trials on the rotating bar, and the average retention
time for each mouse was used for comparison. The examiner
conducting the rotarod test was blind to the treatment.
MWM test
For the MWM test, a stainless steel cylindrical tank
(120 cm in diameter) surrounded by a wall 40 cm high and filled
with homothermal water (22°C) was used. A plastic platform, 8 cm in
diameter, was submerged 1.5 cm below the water surface with its
base fixed to the floor of the tank. Four large unique navigation
markers were placed above the edge of each quadrant of the tank as
geographical cues prior to releasing the animals into the
water.
In the hidden platform acquisition test, on each of
four consecutive days mice underwent four swimming sessions. For
each session, the mice were placed facing the wall of the pool and
released from a starting point pseudo-randomly chosen from the four
predetermined positions into the water. The time mice spent to
reach the platform was recorded as the escape latency. If any mouse
failed to reach the platform within 60 sec, they were guided and
placed on the platform for 20 sec by the experimenter and the
escape latency was recorded as 60 sec. The platform location
remained constant throughout the test. For a particular day for an
individual mouse, the average time spent over the four sessions was
utilized as the latency score. The 4 day averages were then
measured for each group to evaluate spatial learning ability.
On the fifth day, an additional 1 min session
occurred with the platform removed (probe session). The mice were
placed in the diagonal quadrant of the hidden platform originally
located. Site crossings (the number of times animals crossed the
original platform location) were recorded and used to indicate the
degree of memory maintenance.
The results were analyzed with the analysis system
of Morris water Maze (Huaibei Zhenghua Biological Equipment, Anhui,
China). Two series of MWM tests were conducted by two professional
technicians who were blind to the treatments.
Immunohistochemical staining
procedures
Brain tissue was prepared for immunohistochemical
staining. Briefly, the mice were perfused under chloral hydrate
anesthesia through the ventriculus sinister with normal saline,
followed by 4% ice-cold paraformaldehyde for ~20 min. After antigen
retrieval and phosphate-buffered saline (PBS) rinse, the brain
sections were incubated at 37°C overnight with rabbit monoclonal
anti-tyrosine hydroxylase (TH) (1:600), rabbit anti-IL-1β (1:200)
or rabbit anti-Iba-1 (1:200) (all from Abcam, Qatar, Kingdom of
Saudi Arabia). The following day, the brain sections were again
rinsed with PBS and incubated with the appropriate biotinylated
secondary antibody, goat anti-mouse/rabbit HRP-labeled (K5007;
Dako, Glostrup, Denmark) for 50 min. The sections were then washed
and stained with a DAB staining kit (Dako). After Harris
hematoxylin staining and dehydration, the stained sections were
mounted and analyzed under an optical microscope (Nikon, Tokyo,
Japan).
Assay for MDA activity
Activity of the lipid peroxidation product MDA was
measured by using a thiobarbituric acid reactive substances assay
kit (Jiancheng Bioengineering, Nanjing, China) according to
manufacturer's instructions. The assay was performed using a
homogenate of midbrain tissues in physiological saline according to
the given protocol. The supernatant was prepared by centrifugation
at 9,184 × g for 10 min at 4°C (Smart R17; Hanil Science Inc.,
Incheon, Korea). Absorbance at 532 nm was measured using a
microplate reader (Synergy HT; BioTek, Winooski, VT, USA). The MDA
content was calculated according to the equation in the protocol
and expressed in nanomoles per milligram protein.
Assay for SOD activity
The aforementioned midbrain tissue homogenates were
also used to study SOD activity. Total SOD activity was determined
spectrophotometrically using a SOD assay kit (Jiancheng
Bioengineering) and calculated according to the manufacturer's
instructions. In this study, a SOD unit was defined as the amount
that reduced the absorbance at 550 nm by 50%. The result was
expressed as SOD units per milligram protein.
Western blot analysis of IL-1β, Bax and
Bcl-2
Brain tissues were prepared as previously described
(28–30). Briefly, the midbrain tissues were
homogenized overnight in an ice-cold lysis buffer containing a
protease inhibitor cocktail (Sigma-Aldrich). Homogenates were
centrifuged for 30 min at 13,225 × g at 4°C (Smart R17; Hanil
Science Inc.) and the resulting supernatant contained the cytosolic
fraction. Protein concentration was measured with a BCA Protein
assay kit (Biosharp, Shanghai, China). After boiling the samples at
95°C for 5 min, 35 µg of protein was loaded in each lane
with a gel loading buffer containing 10% glycerol, 62.5 mM
Tris-HCl, pH 6.8, 50 mM dithiothreitol, 2% sodium dodecyl sulfate
(SDS) and 0.1% (w/v) bromophenol blue. Western blot analysis was
conducted as described by Burnette (31), with little modification. Proteins
were resolved using SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) with 12% acrylamide resolving gel for IL-1β, Bcl-2 and
Bax, and then transferred to polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked in TNE
containing 5% skim dry milk in Tris-buffered saline and 0.05%
Tween-20 detergent for 4 h. The primary antibodies were diluted in
blocking buffer. Anti-IL-1β (1:500; rabbit monoclonal), anti-Bax
(1:500; mouse monoclonal), and anti-Bcl-2 (1:500; mouse polyclonal)
(all from Abcam) were used. The nitrocellulose membranes were then
incubated overnight with primary antibodies at 4°C. After washing
three times, the membranes were incubated with secondary
antibodies, goat-anti-mouse-HRP-labeled (1:1,000; Boster
Biongineering, Wuhan, China) for 2 h at room temperature. The color
was revealed by recovering DAB (50 mg/100 ml + 30%
H2O2) reagent on the membranes. Conserved
protein (β-actin) was measured to assay equal loading of protein.
The pixels were measured using the Chemidoc XRS imaging system
(Universal Hood II; Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Results are presented as mean ± standard deviation.
The comparisons among groups were evaluated by one-way ANOVA,
followed by LSD tests using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered statistically significant.
Results
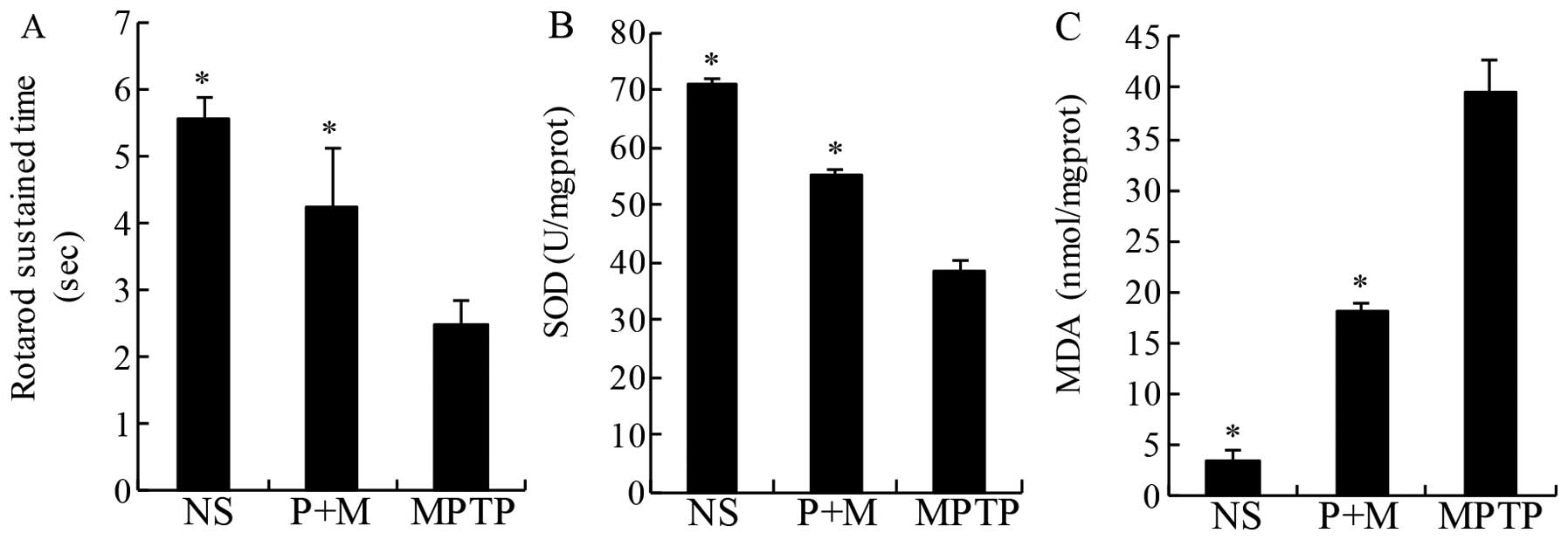
Effect of piperine on rotarod in
MPTP-induced parkinsonian mice
The aim of the antioxidant therapy in PD is to
decrease functional impairments (32). Thus, in the present study, the
rotarod was used to directly assess motor coordination. As
expected, MPTP treatment significantly decreased the latency to
fall off the rotating rod relative to NS mice, an effect that was
significantly prevented by pretreatment with piperine (10 mg/kg)
(Fig. 2A).
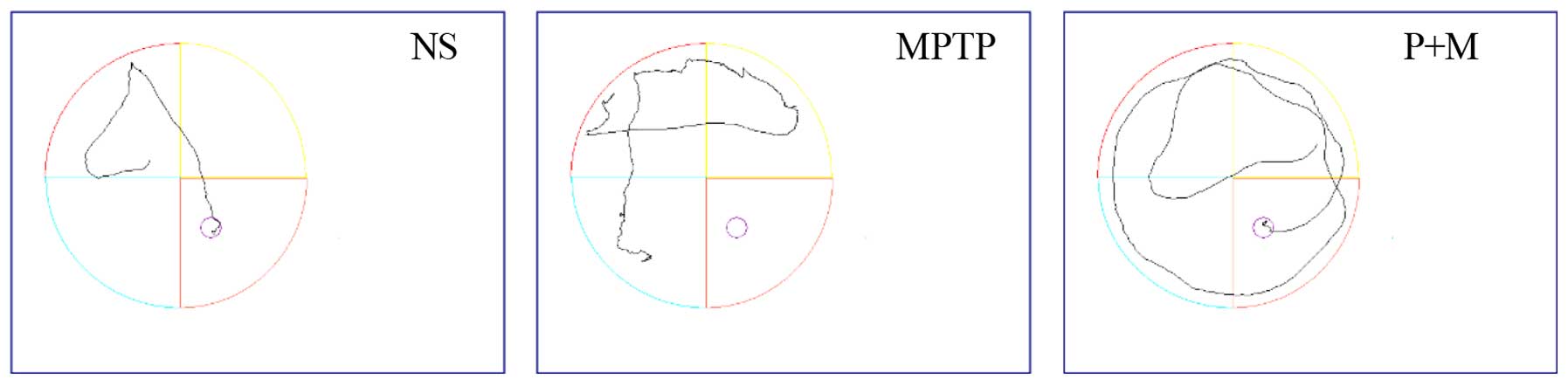
Effect of piperine on the MWM test in
MPTP-induced parkinsonian mice
Various cognitive impairments including deficits in
learning and memory are common clinical symptoms of PD, so the MWM
was used to assess learning and recall. The mean escape latency
decreased gradually over repeated days in all the groups (Table I and Fig. 3), and the mean escape latencies of
the NS and piperine groups were significantly shorter compared to
the MPTP group. These results indicated that MPTP impairs learning
ability in the MWM test and piperine pretreatment was able to
protect against this impairment. In the probe trial, the MPTP group
showed decreased site crossings (P<0.05), suggesting that
piperine is capable of improving the spatial memory ability of PD
mice in the MWM test (Table
I).
 | Table IThe performance of the mouse model in
the probe trial of the MWM test. |
Table I
The performance of the mouse model in
the probe trial of the MWM test.
| Group (n=9) | Escape latency
(second)
| Site crossings
|
|---|
| 1st day | 2nd day | 3rd day | 4th day | 5th day |
|---|
| NS | 41.43±6.24a | 31.07±4.20a | 30.75±1.76a | 23.23±1.31a | 2.75±1.00a |
| MPTP | 58.06±3.82 | 48.1±6.00 | 47.36±2.10 | 46.65±5.73 | 0.5±0.58 |
| Piperine | 50.77±8.84a | 40.21±4.25a | 38.37±2.13a | 37.42±1.44a | 1.83±0.31a |
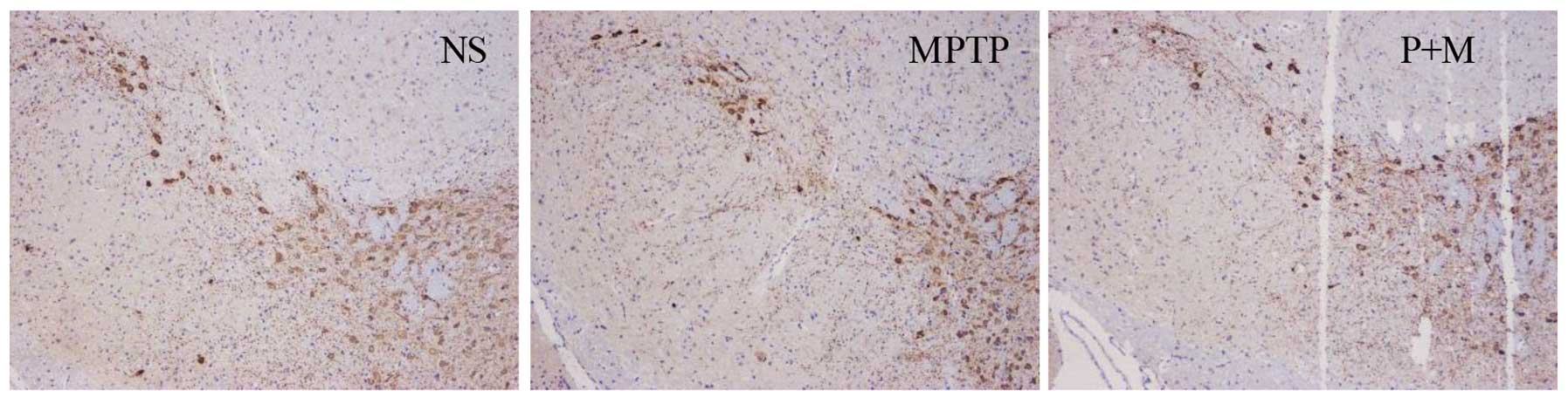
Piperine protects DA neurons against
MPTP-induced neurotoxicity
Brain sections were immunostained for TH, a marker
for DA neurons. The result was expressed as the number of
TH-positive neurons in the SNpc. TH-positive cells were
significantly decreased in the SNpc after MPTP administration
relative to the NS group (P<0.05), demonstrating that MPTP
induced dopaminergic neuronal toxicity. However, piperine treatment
(10 mg/kg) clearly protected against MPTP-induced dopaminergic
neuronal death in the SNpc (Fig.
4).
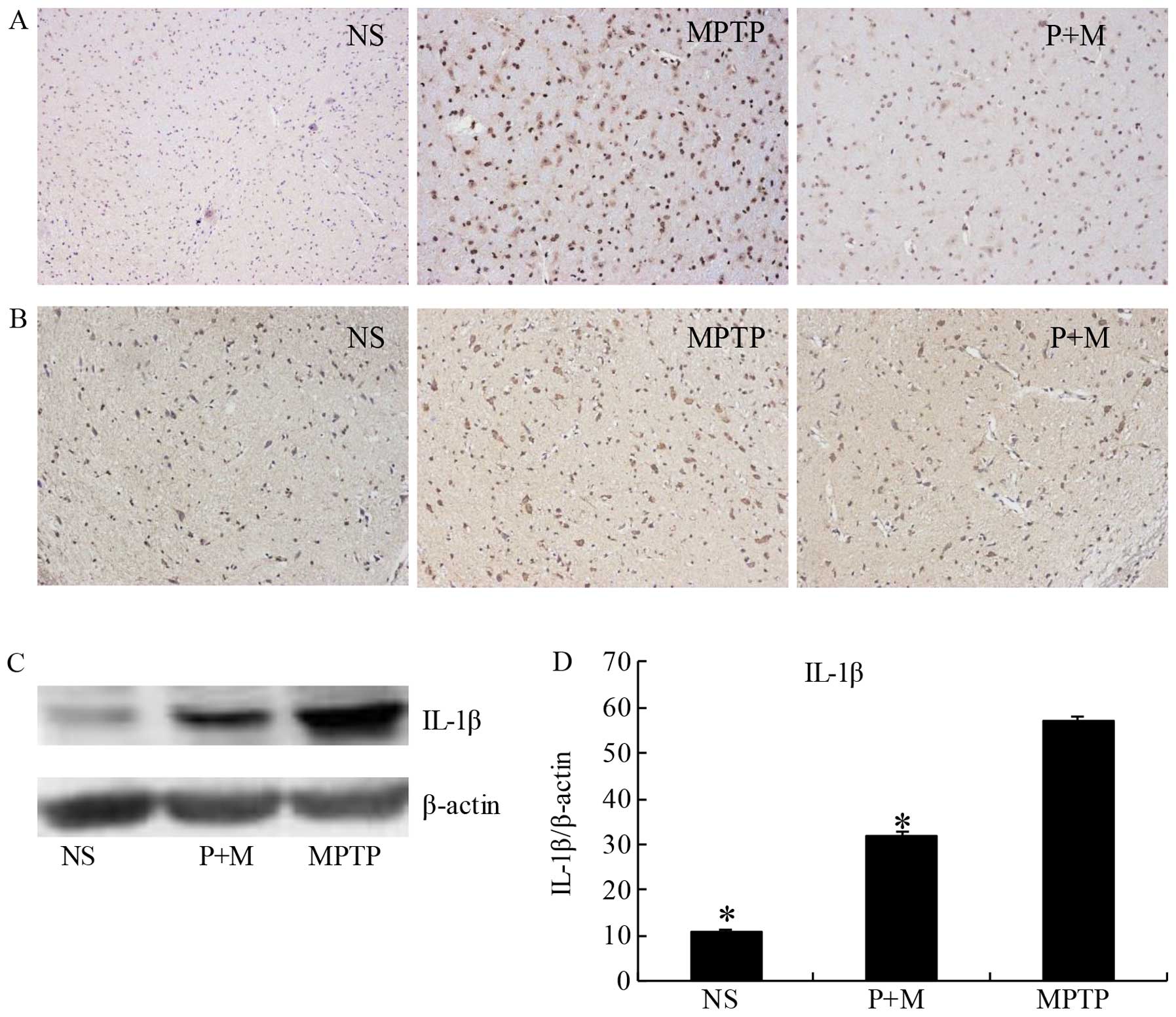
Piperine attenuates inflammation in
MPTP-treated brains
Previous findings have shown that MPTP treatment
induces degeneration of DA neurons due to the induction of
pro-inflammatory cytokine secretion by activated microglia
(33,34). Therefore, we examined the
expression of IL-1β, a representative pro-inflammatory cytokine,
and Iba-1 as a marker of activated microglia. Immunohistochemical
data (Fig. 5B) and western blot
analysis (Fig. 5C and D)
demonstrated that piperine significantly suppressed the expression
of IL-1β in the SNpc of MPTP-treated brains. The Iba-1-positive
cells showed that activated microglia were apparently present in
the SNpc of the MPTP group. The number and morphological phenotype
of activated microglia were obviously alleviated following piperine
pretreatment (Fig. 5A).
Piperine attenuates oxidative stress in
MPTP-treated brains
MDA is a marker of lipid peroxidation and oxidative
stress. The level of lipid peroxidation indicated by MDA was
significantly increased in the midbrain of MPTP-treated mice
compared to the NS group (Fig.
3). The increase was significantly prevented by piperine
pretreatment (Fig. 2B).
Similarly, treatment with piperine markedly increased SOD activity
in the mouse midbrain samples while MPTP only induced modest
increases compared to NS-treated mice (Fig. 2C). These results suggested that
piperine attenuated the oxidative stress induced by MPTP.
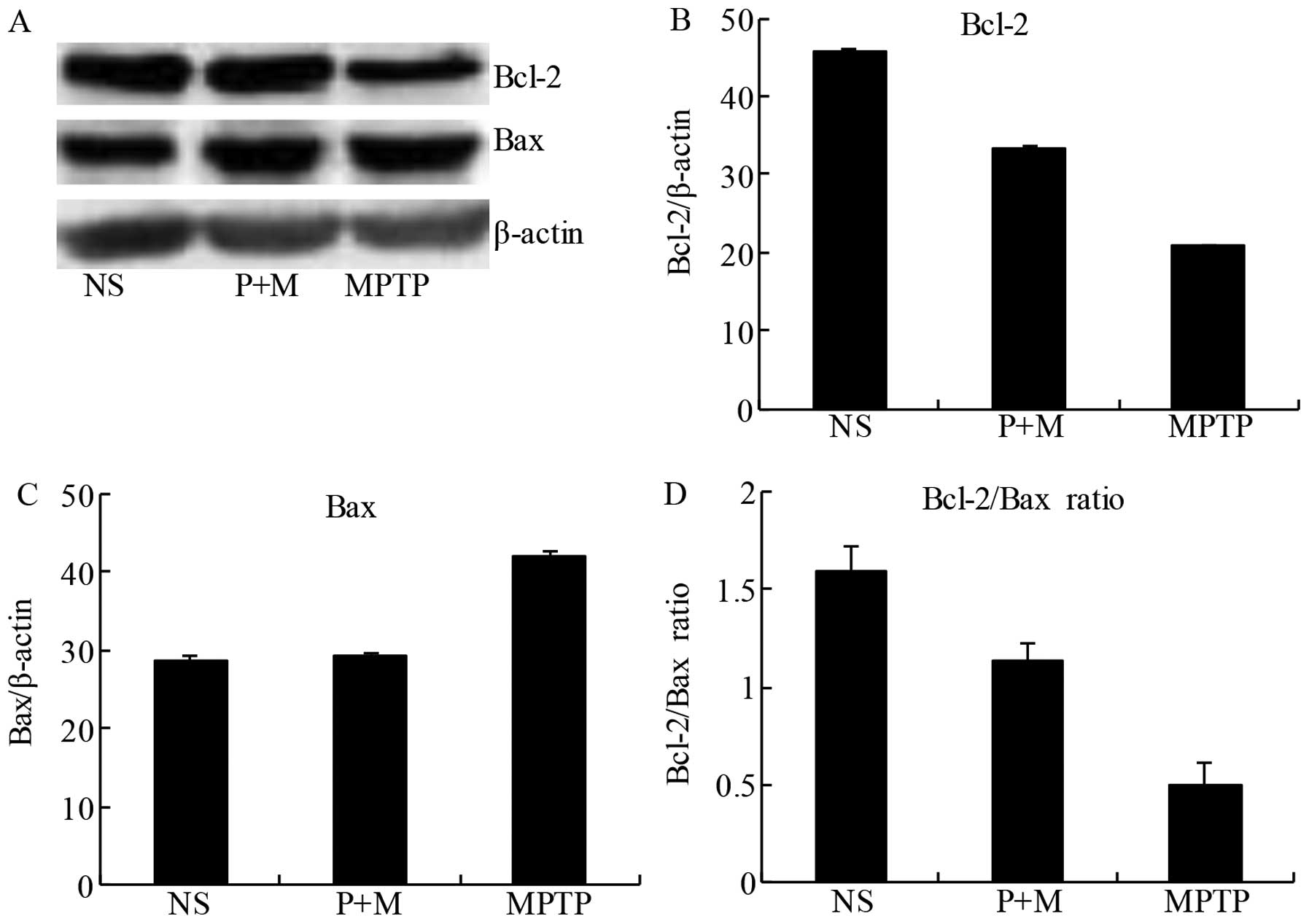
Piperine attenuates apoptosis in the
brains of MPTP-treated mice
To confirm the anti-apoptotic potential of piperine,
we evaluated the expression of the pro-apoptotic protein Bax and
the anti-apoptotic protein Bcl-2 in the midbrain tissue. The
balance of pro-apoptotic and anti-apoptotic signals from the Bcl-2
family can be influenced by members of the caspase family, which
can determine whether neurons undergo apoptosis. Following MPTP
treatment, Bcl-2 expression was reduced in the midbrain compared to
the NS group, while piperine pretreatment prevented this decrease
(Fig. 6A and B). Similarly, MPTP
treatment alone increased Bax expression relative to the NS group,
an effect significantly attenuated by pretreatment with piperine
(Fig. 6A and C). The ratio of the
anti-apoptotic/pro-apoptotic proteins demonstrated that piperine
significantly attenuated MPTP-induced apoptosis by a reduced
expression of pro-apoptotic proteins (Fig. 6D). These results suggested that
piperine had an anti-apoptotic effect on MPTP-induced neuronal cell
death.
Discussion
It has been reported that piperine has antioxidant
and anti-inflammatory activity (35,36). Lee et al studied the
inhibitory effect of piperine against the cytotoxicity of
MPP+ in PC12 cells (26). Previous findings have shown that
piperine plays a novel role in the neuroprotection for PD. In the
present study, the results demonstrated that piperine pretreatment
improved motor ability and cognitive performance, and the mechanism
of its neuroprotection may be by inhibiting MPTP-induced
neurotoxicity due to a reduction of oxidative stress, apoptosis,
and inflammation. Similar to our results, Shrivastava et al
(17) have reported that piperine
exerts a protective effect through antioxidant, anti-apoptotic, and
anti-inflammatory mechanisms in the 6-OHDA-induced Parkinson's rat
model. These results suggested that piperine is a promising therapy
for PD patients. However, in some studies on C57BL/6J mice, MPTP
has been reported to produce an almost complete, permanent and
selective nigrostriatal DA depletion similar to that observed in
humans with PD and primates (37,38), particularly in chronic
administration paradigms (39,40). Thus, in this study, we used a
MPTP-induced Parkinson's disease mouse model to explore the
neuroprotective effects of piperine.
Motor function impairments have been identified
following MPTP treatment in mice (41,42). In the present results, we have
shown that in the rotarod test, a widely used method to assess
motor coordination in laboratory rodents, piperine pretreatment
attenuated MPTP-induced reduction in the fall latency. However,
similar to our results, Shrivastava et al have shown that
piperine showed motor deficit improvement in 6-OHDA-treated rats
(17). This finding is of crucial
importance in providing information concerning the qualitative
aspects of walking movements (43). Cognitive impairment and dementia
have been particular challenges in addition to the functional
impairment caused by motor symptoms for patients with PD, placing
patients under increasing strain (44). The impairment may be mild (mild
cognitive impairment) or severe enough to be defined as dementia
(PD dementia) (45,46). The results of the MWM test showed
that piperine may improve learning and recall in the
MPTP-intoxicated mice. To the best of our knowledge, this is the
first study to show that piperine is capable of improving cognitive
impairment in a PD mouse model and, at least in part, can improve
the learning and memory of animals.
Accumulating evidence suggests that
neuroinflammation in the brain plays an important role in the
pathogenesis of PD (47–49). Autopsy studies have shown that
greater numbers of reactive microglia were identified in the
substantia nigra of PD patients, particularly in areas of maximal
neurode-generation (50). A large
number of activated microglia have also been detected in
MPTP-induced PD animal models (51). Overactivation of microglia is an
important element of neuroinflammation. Activated microglia can
release deleterious compounds such as pro-inflammatory cytokines
(IL-1β), which may exert a direct deleterious effect on DA neurons
(52), and are believed to
contribute to neurodegenerative processes (53,54). It has been shown that piperine has
anti-inflammatory activity and is capable of suppressing
lipopolysaccharide-induced inflammation (55). Thus, we examined the activation of
glial cells and the level of cytokines IL-1β in the midbrain of PD
mice. We observed that piperine pretreatment significantly
prevented MPTP-induced activation of microglia and ameliorated the
levels of IL-1β. These results demonstrate that piperine played a
neuroprotective role mediated by anti-inflammatory effects in the
MPTP-induced mouse model of PD.
Oxidative stress is widely accepted to play a role
in the development and progression of PD and MPTP-mediated
parkinsonism (35,36). Previous findings have demonstrated
that neuronal lipids (56),
nucleic acids (57) and proteins
(58), which are extensive in the
brains of PD patients, are particularly damaged by free radical
oxidation. Our results have shown that pretreatment with piperine
clearly elevated the SOD levels and decreased lipid peroxidation.
These results suggest that this antioxidant property may be
involved in the neuroprotective effects against MPTP
neurotoxicity.
Apoptosis is involved in the pathogenesis of cell
death in PD (59). Mitochondrial
defects following cytotoxic stimuli can be closely associated with
apoptosis (60). The
proapoptogenic Bax, which can form a channel by itself for
translocation to mitochondria, can trigger the release of the
apoptogenic factor cytochrome c from mitochondria into the
cytoplasm (61,62). Contrary to Bax, the anti-apoptotic
Bcl-2 is able to prevent the release of cytochrome c from
mitochondria by functioning as a docking protein or by direct
blockade of the MTP opening (61,63). The balance between pro- and
anti-apoptotic proteins is crucial in apoptosis and cell survival.
We observed that the expression of Bax and Bcl-2 in the midbrain
from the piperine and MPTP groups showed opposite trends.
Furthermore, the ratio between anti-apoptotic Bcl-2 and
pro-apoptotic Bax suggested that piperine played a positive role in
cell survival by inhibiting apoptosis. Thus, the results suggest
that piperine has anti-apoptotic activity against MPTP
neurotoxicity in this mouse model of PD.
In conclusion, results of the present study have
shown that piperine inhibits MPTP-induced neurotoxicity in mice.
Piperine reduced MPTP-induced oxidative stress by decreasing the
expression of lipid peroxidation (shown by a reduction of MDA
expression) while increasing SOD. It also prevented MPTP-induced
alterations in the balance of Bcl-2 and Bax. In addition, piperine
controlled the overactivation of microglia and inhibited
inflammation by reducing the levels of cytokine IL-1β. Taken
together, the results suggest that piperine has therapeutic
potential as a treatment for PD and other neurodegenerative
disorders.
Acknowledgments
This study was supported by the Fund of Education
Department of Anhui, China (KJ2104A163) to H.-D.Q.
References
|
1
|
Meissner WG, Frasier M, Gasser T, Goetz
CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, et
al: Priorities in Parkinson's disease research. Nat Rev Drug
Discov. 10:377–393. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noelker C, Bacher M, Gocke P, Wei X,
Klockgether T, Du Y and Dodel R: The flavanoide caffeic acid
phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity.
Neurosci Lett. 383:39–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jankovic J: Parkinson's disease: Clinical
features and diagnosis. J Neurol Neurosurg Psychiatry. 79:368–376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engler H, Doenlen R, Riether C, Engler A,
Niemi MB, Besedovsky HO, del Rey A, Pacheco-López G, Feldon J and
Schedlowski M: Time-dependent alterations of peripheral immune
parameters after nigrostriatal dopamine depletion in a rat model of
Parkinson's disease. Brain Behav Immun. 23:518–526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dardiotis E, Xiromerisiou G,
Hadjichristodoulou C, Tsatsakis AM, Wilks MF and Hadjigeorgiou GM:
The interplay between environmental and genetic factors in
Parkinson's disease susceptibility: The evidence for pesticides.
Toxicology. 307:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch EC, Hunot S, Faucheux B, Agid Y,
Mizuno Y, Mochizuki H, Tatton WG, Tatton N and Olanow WC:
Dopaminergic neurons degenerate by apoptosis in Parkinson's
disease. Mov Disord. 14:383–385. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller RL, James-Kracke M, Sun GY and Sun
AY: Oxidative and inflammatory pathways in Parkinson's disease.
Neurochem Res. 34:55–65. 2009. View Article : Google Scholar
|
|
8
|
Shimura H, Hattori N, Kubo S, Mizuno Y,
Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, et
al: Familial Parkinson disease gene product, parkin, is a
ubiquitin-protein ligase. Nat Genet. 25:302–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dugan LL, Tian L, Quick KL, Hardt JI,
Karimi M, Brown C, Loftin S, Flores H, Moerlein SM, Polich J, et
al: Carboxyfullerene neuroprotection postinjury in Parkinsonian
nonhuman primates. Ann Neurol. 76:393–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cassarino DS, Parks JK, Parker WD Jr and
Bennett JP Jr: The parkinsonian neurotoxin MPP+ opens
the mitochondrial permeability transition pore and releases
cytochrome c in isolated mitochondria via an oxidative mechanism.
Biochim Biophys Acta. 1453:49–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lotharius J, Dugan LL and O'Malley KL:
Distinct mechanisms underlie neurotoxin-mediated cell death in
cultured dopaminergic neurons. J Neurosci. 19:1284–1293.
1999.PubMed/NCBI
|
|
12
|
Lee CS, Han ES, Jang YY, Han JH, Ha HW and
Kim DE: Protective effect of harmalol and harmaline on MPTP
neurotoxicity in the mouse and dopamine-induced damage of brain
mitochondria and PC12 cells. J Neurochem. 75:521–531. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee CS, Park SY, Ko HH, Song JH, Shin YK
and Han ES: Inhibition of MPP+-induced mitochondrial
damage and cell death by trifluoperazine and W-7 in PC12 cells.
Neurochem Int. 46:169–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rojas P and Rios C: Increased striatal
lipid peroxidation after intracerebroventricular MPP+
administration to mice. Pharmacol Toxicol. 72:364–368. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdel-Salam OM: Drugs used to treat
Parkinson's disease, present status and future directions. CNS
Neurol Disord Drug Targets. 7:321–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wattanathorn J, Chonpathompikunlert P,
Muchimapura S, Priprem A and Tankamnerdthai O: Piperine, the
potential functional food for mood and cognitive disorders. Food
Chem Toxicol. 46:3106–3110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shrivastava P, Vaibhav K, Tabassum R, Khan
A, Ishrat T, Khan MM, Ahmad A and Islam F, Safhi MM and Islam F:
Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA
induced Parkinson's rat model. J Nutr Biochem. 24:680–687. 2013.
View Article : Google Scholar
|
|
18
|
Vijayakumar RS1, Surya D and Nalini N:
Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine
in rats with high fat diet induced oxidative stress. Redox Rep.
9:105–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bajad S, Bedi KL, Singla AK and Johri RK:
Antidiarrhoeal activity of piperine in mice. Planta Med.
67:284–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai YF and Xu H: Protective action of
piperine against experimental gastric ulcer. Acta Pharmacol Sin.
21:357–359. 2000.
|
|
21
|
Selvendiran K, Prince Vijeya Singh J and
Sakthisekaran D: In vivo effect of piperine on serum and tissue
glycoprotein levels in benzo(a)pyrene induced lung carcinogenesis
in Swiss albino mice. Pulm Pharmacol Ther. 19:107–111. 2006.
View Article : Google Scholar
|
|
22
|
Gupta SK, Bansal P, Bhardwaj RK and
Velpandian T: Comparative anti-nociceptive, anti-inflammatory and
toxicity profile of nimesulide vs. nimesulide and piperine
combination. Pharmacol Res. 41:657–662. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atal CK, Dubey RK and Singh J: Biochemical
basis of enhanced drug bioavailability by piperine: Evidence that
piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp
Ther. 232:258–262. 1985.PubMed/NCBI
|
|
24
|
Hu Y, Liao HB, Liu P, Guo DH and Wang YY:
Antidepressant effects of piperine and its neuroprotective
mechanism in rats. Zhong Xi Yi Jie He Xue Bao. 7:667–670. 2009.In
Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chonpathompikunlert P, Wattanathorn J and
Muchimapura S: Piperine, the main alkaloid of Thai black pepper,
protects against neurodegeneration and cognitive impairment in
animal model of cognitive deficit like condition of Alzheimer's
disease. Food Chem Toxicol. 48:798–802. 2010. View Article : Google Scholar
|
|
26
|
Lee CS, Han ES and Kim YK: Piperine
inhibition of 1-methyl-4-phenylpyridinium-induced mitochondrial
dysfunction and cell death in PC12 cells. Eur J Pharmacol.
537:37–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rozas G, López-Martín E, Guerra MJ and
Labandeira-García JL: The overall rod performance test in the
MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods.
83:165–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SR, Chen X, Oo TF, Kareva T, Yarygina
O, Wang C, During M, Kholodilov N and Burke RE: Dopaminergic
pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann
Neurol. 70:110–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SR, Kareva T, Yarygina O, Kholodilov N
and Burke RE: AAV transduction of dopamine neurons with
constitutively active Rheb protects from neurodegeneration and
mediates axon regrowth. Mol Ther. 20:275–286. 2012. View Article : Google Scholar :
|
|
30
|
Kim SR, Chung ES, Bok E, Baik HH, Chung
YC, Won SY, Joe E, Kim TH, Kim SS, Jin MY, et al: Prothrombin
kringle-2 induces death of mesencephalic dopaminergic neurons in
vivo and in vitro via microglial activation. J Neurosci Res.
88:1537–1548. 2010.
|
|
31
|
Burnette WN: 'Western blotting':
Electrophoretic transfer of proteins from sodium dodecyl sulfate -
polyacrylamide gels to unmodified nitrocellulose and radiographic
detection with antibody and radioiodinated protein A. Anal Biochem.
112:195–203. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Casani S, Gómez-Pastor R, Matallana E and
Paricio N: Antioxidant compound supplementation prevents oxidative
damage in a Drosophila model of Parkinson's disease. Free Radic
Biol Med. 61:151–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Block ML and Hong JS: Microglia and
inflammation-mediated neurodegeneration: Multiple triggers with a
common mechanism. Prog Neurobiol. 76:77–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang T, Zhang W, Pei Z, Block M, Wilson B,
Reece JM, Miller DS and Hong JS: Reactive microgliosis participates
in MPP+-induced dopaminergic neurodegeneration: role of
67 kDa laminin receptor. FASEB J. 20:906–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baillet A, Chanteperdrix V, Trocmé C,
Casez P, Garrel C and Besson G: The role of oxidative stress in
amyotrophic lateral sclerosis and Parkinson's disease. Neurochem
Res. 35:1530–1537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carvalho AN, Marques C, Rodrigues E,
Henderson CJ, Wolf CR, Pereira P and Gama MJ: Ubiquitin-proteasome
system impairment and MPTP-induced oxidative stress in the brain of
C57BL/6 wild-type and GSTP knockout mice. Mol Neurobiol.
47:662–672. 2013. View Article : Google Scholar
|
|
37
|
Jakowec MW and Petzinger GM:
1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine-lesioned model of
parkinson's disease, with emphasis on mice and nonhuman primates.
Comp Med. 54:497–513. 2004.PubMed/NCBI
|
|
38
|
Sundström E, Fredriksson A and Archer T:
Chronic neurochemical and behavioral changes in MPTP-lesioned
C57BL/6 mice: a model for Parkinson's disease. Brain Res.
528:181–188. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kurz MJ, Pothakos K, Jamaluddin S,
Scott-Pandorf M, Arellano C and Lau YS: A chronic mouse model of
Parkinson's disease has a reduced gait pattern certainty. Neurosci
Lett. 429:39–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petroske E, Meredith GE, Callen S,
Totterdell S and Lau YS: Mouse model of Parkinsonism: a comparison
between subacute MPTP and chronic MPTP/probenecid treatment.
Neuroscience. 106:589–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matheus FC, Aguiar AS Jr, Castro AA,
Villarinho JG, Ferreira J, Figueiredo CP, Walz R, Santos AR, Tasca
CI and Prediger RD: Neuroprotective effects of agmatine in mice
infused with a single intranasal administration of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Behav Brain
Res. 235:263–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hutter-Saunders JA, Gendelman HE and
Mosley RL: Murine motor and behavior functional evaluations for
acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
intoxication. J Neuroimmune Pharmacol. 7:279–288. 2012. View Article : Google Scholar :
|
|
43
|
Whishaw IQ, Li K, Whishaw PA, Gorny B and
Metz GA: Use of rotorod as a method for the qualitative analysis of
walking in rat. J Vis Exp. 22:10302008.
|
|
44
|
Emre M, Ford PJ, Bilgic B and Uc EY:
Cognitive impairment and dementia in Parkinson's disease: practical
issues and management. Mov Disord. 29:663–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Emre M, Aarsland D, Brown R, Burn DJ,
Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier
S, et al: Clinical diagnostic criteria for dementia associated with
Parkinson's disease. Mov Disord. 22:1689–1707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Litvan I, Goldman JG, Tröster AI, Schmand
BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K,
Williams-Gray CH, et al: Diagnostic criteria for mild cognitive
impairment in Parkinson's disease: Movement Disorder Society Task
Force guidelines. Mov Disord. 27:349–356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gyoneva S, Shapiro L, Lazo C,
Garnier-Amblard E, Smith Y, Miller GW and Traynelis SF: Adenosine
A2A receptor antagonism reverses inflammation-induced impairment of
microglial process extension in a model of Parkinson's disease.
Neurobiol Dis. 67:191–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deleidi M and Gasser T: The role of
inflammation in sporadic and familial Parkinson's disease. Cell Mol
Life Sci. 70:4259–4273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR,
Zeng YL, Li SN, Huang BX, Lv QK, Wang W, et al: Anti-inflammatory
effects of BHBA in both in vivo and in vitro Parkinson inverted
question marks disease models are mediated by GPR109A-dependent
mechanisms. J Neuroinflammation. 12:92015. View Article : Google Scholar
|
|
50
|
Hirsch EC, Hunot S, Damier P and Faucheux
B: Glial cells and inflammation in Parkinson's disease: A role in
neurodegeneration? Ann Neurol. 44(Suppl 1): S115–S120. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kitamura Y, Itano Y, Kubo T and Nomura Y:
Suppressive effect of FK-506, a novel immunosuppressant, against
MPTP-induced dopamine depletion in the striatum of young C57BL/6
mice. J Neuroimmunol. 50:221–224. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sriram K and O'Callaghan JP: Divergent
roles for tumor necrosis factor-alpha in the brain. J Neuroimmune
Pharmacol. 2:140–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang MJ, Huang HY, Chen WF, Chang HF and
Kuo JS: Glycogen synthase kinase-3β inactivation inhibits tumor
necrosis factor-α production in microglia by modulating nuclear
factor κB and MLK3/JNK signaling cascades. J Neuroinflammation.
7:992010. View Article : Google Scholar
|
|
54
|
Fu SP, Li SN, Wang JF, Li Y, Xie SS, Xue
WJ, Liu HM, Huang BX, Lv QK, Lei LC, et al: BHBA suppresses
LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB
activation. Mediators Inflamm. 2014:9834012014. View Article : Google Scholar
|
|
55
|
Bae GS, Kim MS, Jung WS, Seo SW, Yun SW,
Kim SG, Park RK, Kim EC, Song HJ and Park SJ: Inhibition of
lipopolysaccharide-induced inflammatory responses by piperine. Eur
J Pharmacol. 642:154–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lovell MA, Ehmann WD, Butler SM and
Markesbery WR: Elevated thiobarbituric acid-reactive substances and
antioxidant enzyme activity in the brain in Alzheimer's disease.
Neurology. 45:1594–1601. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hirai K, Aliev G, Nunomura A, Fujioka H,
Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M,
et al: Mitochondrial abnormalities in Alzheimer's disease. J
Neurosci. 21:3017–3023. 2001.PubMed/NCBI
|
|
58
|
Lyras L, Perry RH, Perry EK, Ince PG,
Jenner A, Jenner P and Halliwell B: Oxidative damage to proteins,
lipids, and DNA in cortical brain regions from patients with
dementia with Lewy bodies. J Neurochem. 71:302–312. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Olanow CW: The pathogenesis of cell death
in Parkinson's disease - 2007. Mov Disord. 22(Suppl 17): S335–S342.
2007. View Article : Google Scholar
|
|
60
|
Tatton WG and Olanow CW: Apoptosis in
neurodegenerative diseases: The role of mitochondria. Biochim
Biophys Acta. 1410:195–213. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Eskes R, Antonsson B, Osen-Sand A,
Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A and Martinou
JC: Bax-induced cytochrome C release from mitochondria is
independent of the permeability transition pore but highly
dependent on Mg2+ ions. J Cell Biol. 143:217–224. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|