Introduction
Green tea and coffee are the most commonly consumed
beverages worldwide. Green tea has been consumed mainly in Eastern
countries, while coffee is consumed throughout the world.
(-)-Epigallocatechin gallate (EGCG), the most abundant catechin and
a major bioactive component in green tea, and chlorogenic acid
(CGA), a main phenolic compound in coffee, have beneficial
properties for human health, such as antioxidation,
anti-inflammation and cancer prevention (1–3).
Bone metabolism is regulated strictly by two types of functional
cells, osteoblasts and osteoclasts, which are responsible for bone
formation and resorption, respectively (4). Bone tissue in the skeleton is
continuously renewed through a process, known as bone remodeling
(5). A decrease in bone mineral
density results from an unbalanced bone remodeling process
(5). This remodeling process
begins with osteoclast bone resorption, followed by osteoblast bone
formation (6). Bone remodeling
disorder causes metabolic bone disease, including osteoporosis, and
the increased risk of fracture. It has been shown that ingestion of
green tea prevents age-related bone loss and fractures in elderly
individuals (7). Accumulating
evidence indicates that catechin suppresses bone resorption and
supports osteoblastic bone formation (7,8).
Additionally, catechin stimulates alkaline phosphatase activity, a
mature osteoblast phenotype, and reduces apoptosis in osteoblasts
(7). By contrast, it has been
shown that CGA increases mineralization in the tibia, and improves
mechanical properties of the femoral diaphysis in rat model
(9). In addition, CGA reportedly
inhibits osteoclast differentiation and bone resorption by
downregulation of receptor activator of nuclear factor κB (RANK)
ligand-induced nuclear factor of activated T cell c1 expression
(10). However, the exact
mechanisms underlying the effects of EGCG or CGA on bone metabolism
remain to be elucidated.
Tumor necrosis factor (TNF)-α, a multifunctional
cytokine, is firmly established to have an important role in
inflammation, infection and cancer (11). With regard to bone metabolism,
TNF-α stimulates the activation of RANK in osteoclasts through RANK
ligand expression in osteoblasts, which acts as a potent stimulator
of osteoclastogenesis (12). It
has been reported that TNF-α stimulates the synthesis of
interleukin-6 (13).
Interleukin-6 is a member of the gp130 cytokine family and has
important physiological effects on a wide range of functions, such
as promotion of B-cell differentiation, T-cell activation and
induction of acute-phase proteins (11,14,15). As for the association between
interleukin-6 and bone metabolism, it is well recognized that
interleukin-6 stimulates bone resorption and induces osteoclast
formation (11,14). By contrast, interleukin-6 has a
pivotal role in the process of bone fracture repair (16). Thus, interleukin-6 is currently
considered to also be an osteotropic factor and influence bone
formation under the condition of increased bone turnover (17). Our previous studies have shown
that interleukin-6 synthesis induced by TNF-α is negatively
regulated through the p70 S6 kinase pathway in osteoblast-like
MC3T3-E1 cells (18,19).
In the present study, the effects of EGCG or CGA on
TNF-α-induced interleukin-6 synthesis in osteoblast-like MC3T3-E1
cells were investigated.
Materials and methods
Materials
EGCG and CGA were obtained from Sigma-Aldrich (St.
Louis, MO, USA). TNF-α and mouse interleukin-6 enzyme-linked
immunosorbent assay (ELISA) kit were obtained from R&D Systems,
Inc. (Minneapolis, MN, USA). Phospho-specific p70 S6 kinase
(Thr-389) antibodies (#9205; polyclonal; rabbit anti-mouse;
1:1,000) and p70 S6 kinase antibodies (#9202; polyclonal; rabbit
anti-mouse; 1:1,000) were obtained from Cell Signaling Technology,
Inc. (Beverly, MA, USA). An ECL western blotting detection system
was obtained from GE Healthcare UK Ltd. (Buckinghamshire, UK).
Other materials and chemicals were obtained from commercial
sources. EGCG was dissolved in dimethyl sulfoxide and CGA was
dissolved in ethanol. The maximum concentration of dimethyl
sulfoxide or ethanol was 0.1%, which did not affect the assay for
interleukin-6 or the western blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (20) were
maintained as previously described (21). Briefly, the cells were cultured in
α-minimum essential medium (α-MEM) containing 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2/95% air. The cells were seeded in 35-mm diameter
dishes (5×104 cells/dish) or 90-mm diameter dishes
(2×105 cells/dish) in α-MEM containing 10% FBS. After 5
days, the medium was exchanged for α-MEM containing 0.3% FBS. The
cells were used for experiments after 48 h.
Assay for interleukin-6
The cultured cells were pretreated with various
doses of EGCG or CGA for 60 min, and subsequently stimulated by 30
ng/ml of TNF-α or vehicle in 1 ml of α-MEM containing 0.3% FBS for
the indicated periods. The conditioned medium was collected at the
end of the incubation, and the interleukin-6 concentration was
subsequently measured using mouse interleukin-6 ELISA kit according
to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cultured cells were pretreated with 100
µM of EGCG, 50 µM of CGA or vehicle for 60 min, and
subsequently stimulated by 30 ng/ml of TNF-α or vehicle in α-MEM
containing 0.3% FBS for 3 h. Total RNA was isolated and transcribed
into complementary DNA using TRIzol reagent (Invitrogen Corp.,
Carlsbad, CA, USA) and Omniscript reverse transcriptase kit (Qiagen
Inc., Valencia, CA, USA), respectively. RT-qPCR was performed using
a LightCycler system in capillaries and Fast Start DNA Master
SYBR-Green I provided with the kit (Roche Diagnostics, Basel,
Switzerland). Sense and antisense primers for mouse interleukin-6
mRNA were purchased from Takara Bio Inc. (Tokyo, Japan) (primer set
ID: MA039013), while mouse glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) mRNA primers were synthesized based on the study by Simpson
et al (22). The amplified
products were determined using melting curve analysis and agarose
electrophoresis. The interleukin-6 mRNA levels were normalized to
those of GAPDH mRNA.
Western blot analysis
The cultured cells were pretreated with various
doses of EGCG or CGA for 60 min, and subsequently stimulated by 30
ng/ml of TNF-α or vehicle in α-MEM containing 0.3% FBS for 10 min.
The cells were washed twice with phosphate-buffered saline and
lysed, homogenized and sonicated in a lysis buffer containing 62.5
mM Tris/HCl (pH 6.8) 2% sodium dodecyl sulfate (SDS), 50 mM
dithiothreitol and 10% glycerol. SDS-polyacrylamide gel
electrophoresis was performed by the method of Laemmli (23) in 10% polyacrylamide gels. The
proteins were fractionated and transferred onto Immun-Blot
polyvinyl difluoride (PVDF) membranes (Bio-Rad Laboratories,
Hercules, CA, USA). The membranes were blocked with 5% fat-free dry
milk in Tris-buffered saline-Tween [TBS-T; 20 mM Tris-HCl (pH 7.6),
137 mM NaCl and 0.1% Tween-20] for 2 h before incubation with
primary antibodies. A western blot analysis was performed as
previously described (24) using
phospho-specific p70 S6 kinase antibodies or p70 S6 kinase
antibodies as primary antibodies, with peroxidase-labeled
antibodies raised in goat against rabbit immunoglobulin G (no.
074-1506; KPL, Inc., Gaithersburg, MD, USA) used as the secondary
antibodies. The primary and secondary antibodies were diluted at
1:1,000 with 5% fat-free dry milk in TBS-T. The peroxidase activity
on the PVDF sheet was visualized on X-ray film by means of the ECL
Western blotting detection system.
Determination
The absorbance of the enzyme immunosorbent assay
samples was measured at 450 nm with the EL 340 Bio Kinetic Reader
(Bio-Tek Instruments, Inc., Winooski, VT, USA). A densitometric
analysis was performed using a scanner and image analysis software
package (ImageJ version 1.47; National Institutes of Health,
Bethesda, MD, USA). The phosphorylated protein levels were
calculated as follows: The background-subtracted signal intensity
of each phosphorylation signal was respectively normalized to the
total protein signal and plotted as the fold increase in comparison
with that of the control cells treated without stimulation.
Statistical analysis
The data were analyzed by an analysis of variance
followed by Bonferroni method for multiple comparisons between
pairs, and P<0.05 was considered to indicate a statistically
significant difference. All the data are presented as the means ±
standard error of the mean of triplicate determinations from three
independent cell preparations.
Results
Effect of EGCG on the TNF-α-stimulated
interleukin-6 release in MC3T3-E1 cells
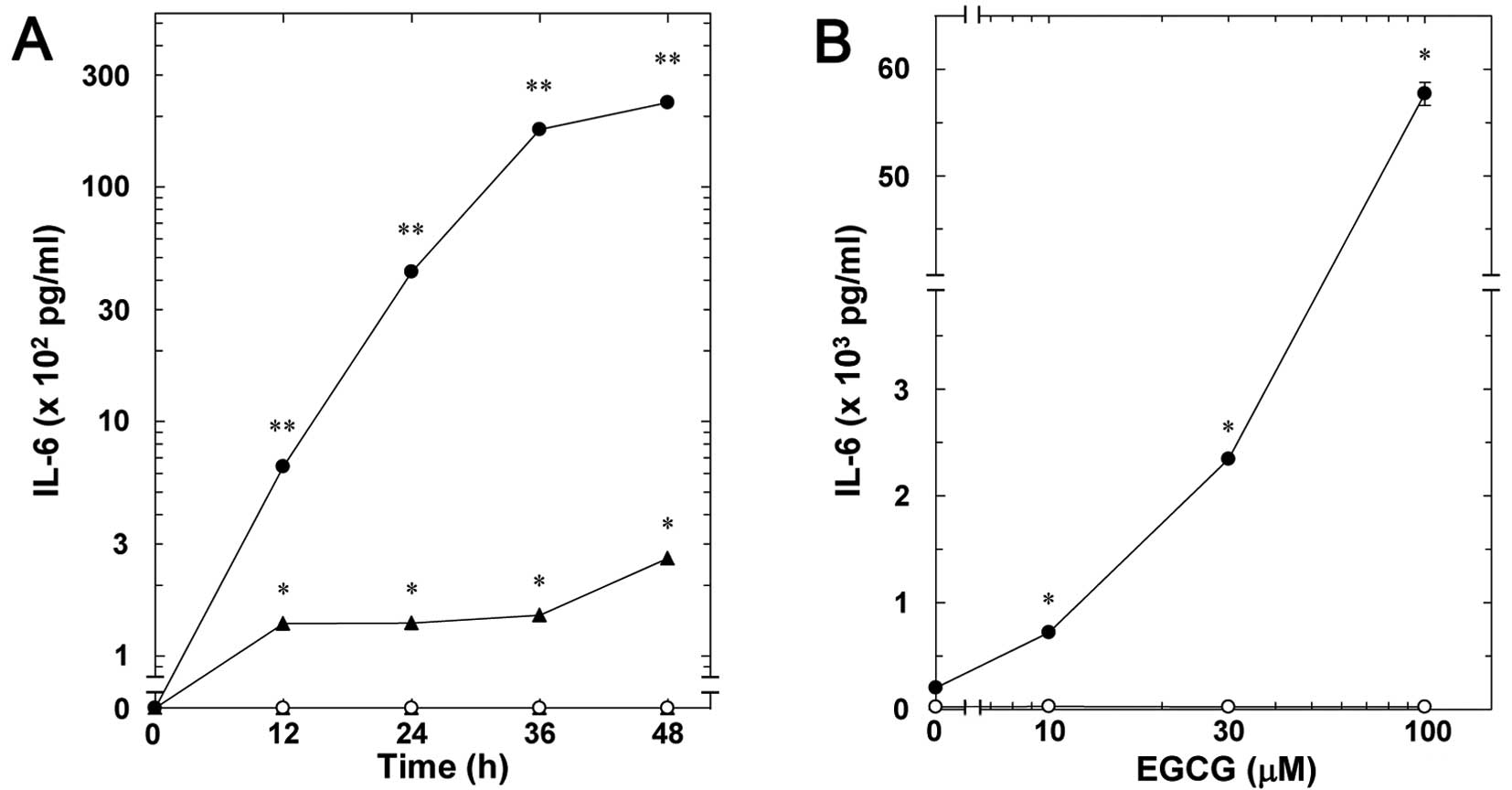
Our previous study showed that TNF-α stimulates
interleukin-6 synthesis in osteoblast-like MC3T3-E1 cells (18). Firstly, the effect of EGCG on the
TNF-α-stimulated interleukin-6 release in MC3T3-E1 cells was
examined. EGCG significantly amplified the TNF-α-stimulated
interleukin-6 release in a time-dependent manner ≤48 h (Fig. 1A). The enhancing effect of EGCG
was dose-dependent between 10 and 100 µM (Fig. 1B). The maximum effect of EGCG on
the interleukin-6 release was observed at 100 µM.
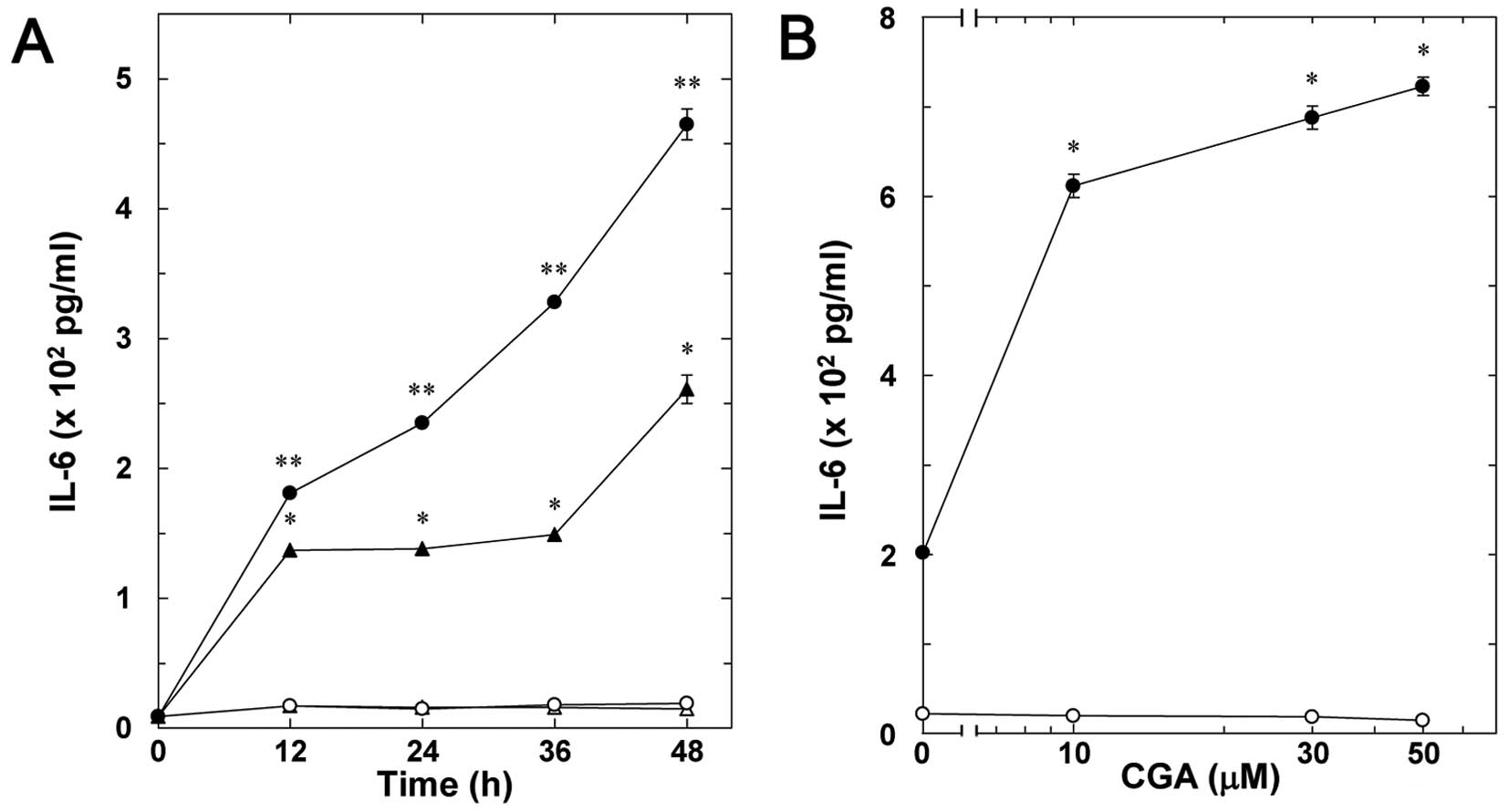
Effect of CGA on the TNF-α-stimulated
interleukin-6 release in MC3T3-E1 cells
The effect of CGA on the TNF-α-stimulated
interleukin-6 release in osteoblast-like MC3T3-E1 cells was
examined. CGA significantly enhanced the TNF-α-stimulated
interleukin-6 release in a time-dependent manner ≤48 h (Fig. 2A). The enhancement by CGA on the
release was dose-dependent between 10 and 50 µM (Fig. 2B).
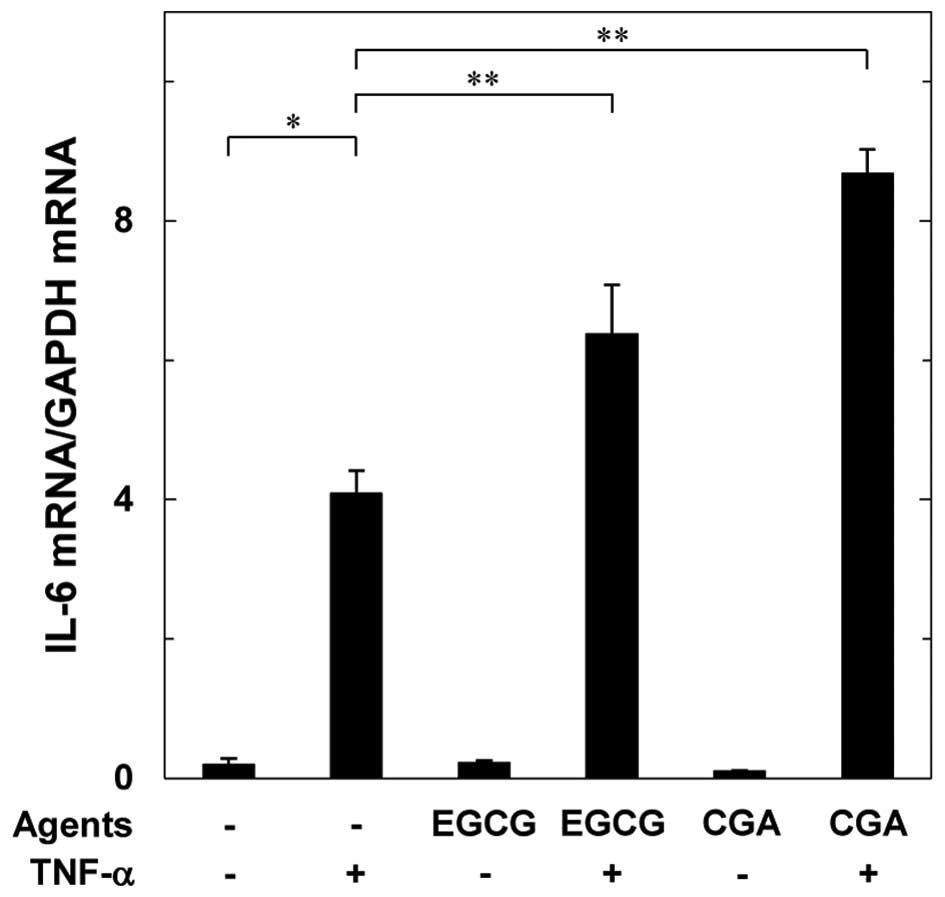
Effects of EGCG or CGA on the
TNF-α-induced expression of interleukin-6 mRNA in MC3T3-E1
cells
In order to investigate whether the amplifying
effect of EGCG or CGA on the TNF-α-stimulated interleukin-6 release
is mediated via transcriptional events in MC3T3-E1 cells, the
effects of EGCG or CGA on the TNF-α-induced interleukin-6 mRNA
expression were examined. EGCG or CGA, which by itself had little
effect on the interleukin-6 mRNA levels, markedly upregulated the
TNF-α-induced interleukin-6 mRNA expression levels (Fig. 3).
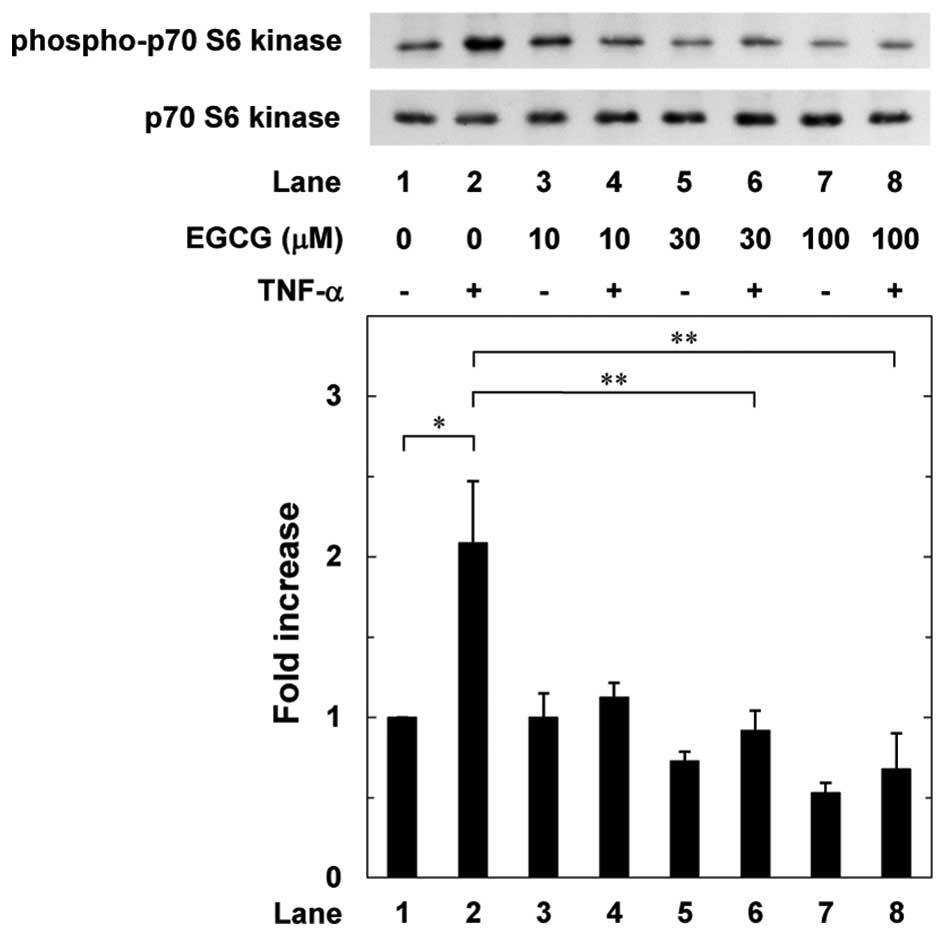
Effects of EGCG or CGA on the
TNF-α-induced phosphorylation of p70 S6 kinase in MC3T3-E1
cells
Our previous study reported that p70 S6 kinase has a
suppressive role in the TNF-α-stimulated interleukin-6 synthesis in
osteoblast-like MC3T3-E1 cells (19). Therefore, to clarify whether the
effects of EGCG or CGA on the TNF-α-stimulated interleukin-6
synthesis are associated with the activation of p70 S6 kinase in
MC3T3-E1 cells, the effects of EGCG or CGA on the TNF-α-induced
phosphorylation of p70 S6 kinase were further examined. EGCG
significantly suppressed the TNF-α-induced phosphorylation of p70
S6 kinase in a dose-dependent manner between 10 and 100 µM
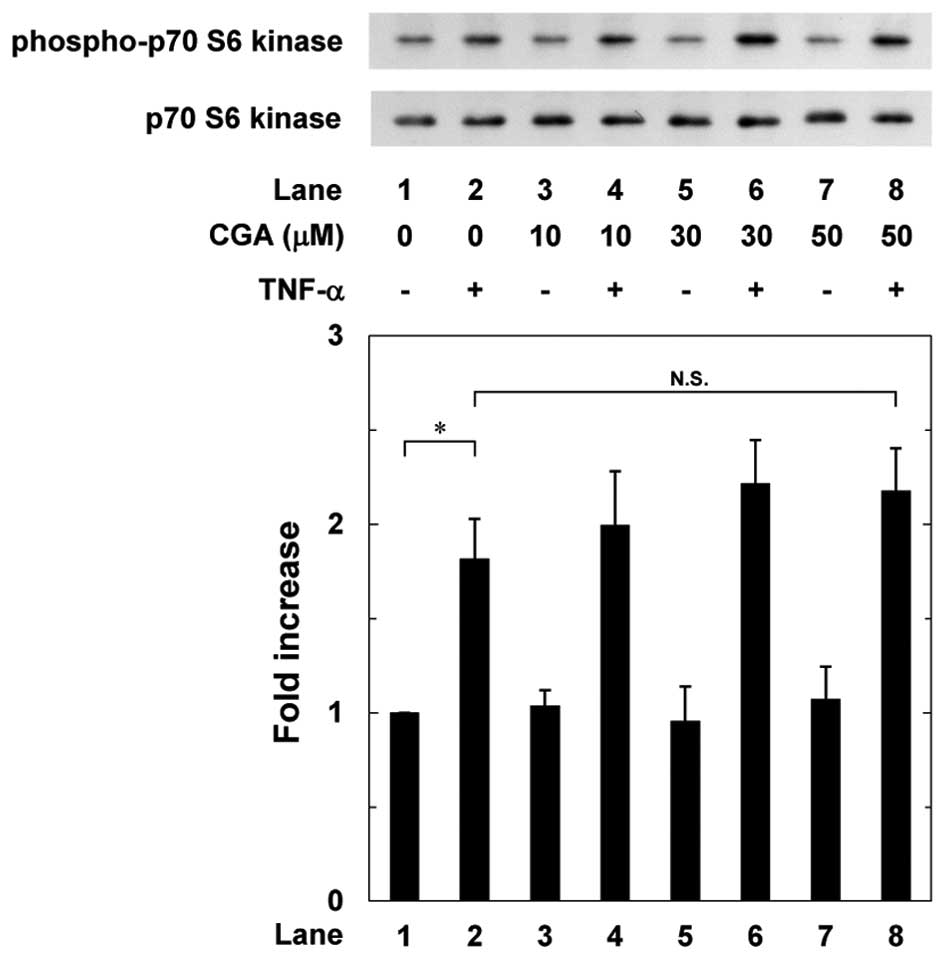
(Fig. 4). By contrast, CGA did
not affect the TNF-α-induced phosphorylation of p70 S6 kinase
between 10 and 50 µM (Fig.
5).
Discussion
In the present study, EGCG, a main flavonoid in
green tea, and CGA, a bioactive compound in coffee, were
demonstrated to enhance the TNF-α-stimulated interleukin-6 release
in osteoblast-like MC3T3-E1 cells. Additionally, the expression
levels of interleukin-6 mRNA induced by TNF-α were amplified by
EGCG, as well as CGA. Thus, it appears that the enhancing effects
of EGCG and CGA on the TNF-α-stimulated interleukin-6 release are
mediated through transcriptional levels in MC3T3-E1 cells. Taking
the present findings into account, it is most likely that EGCG and
CGA in beverages upregulate TNF-α-stimulated synthesis of
interleukin-6 in osteoblast-like MC3T3-E1 cells.
Regarding the intracellular signaling of
TNF-α-stimulated interleukin-6 synthesis in osteoblasts, our
previous study reported that TNF-α stimulates the activation of p70
S6 kinase in osteoblast-like MC3T3-E1 cells, resulting in the
limitation of the TNF-α-stimulated interleukin-6 synthesis
(19). Based on these results,
the precise mechanism behind the enhancement by EGCG and CGA of the
TNF-α-stimulated interleukin-6 synthesis in MC3T3-E1 cells was
investigated. EGCG markedly suppressed the TNF-α-stimulated
phosphorylation of the p70 S6 kinase. However, CGA failed to affect
the phosphorylation of p70 S6 kinase. Therefore, it is most likely
that EGCG strengthens the TNF-α-stimulated interleukin-6 synthesis
through the inhibition of p70 S6 kinase in osteoblast-like MC3T3-E1
cells. By contrast, it appears unlikely that the enhancing effect
of CGA on the TNF-α-induced interleukin-6 synthesis is mediated
through p70 S6 kinase. In our previous studies, we have reported
that p70 S6 kinase negatively regulates the interleukin-6 synthesis
stimulated by platelet-derived growth factor (PDGF), a bone
remodeling modulator in osteoblast-like MC3T3-E1 cells (25). Additionally, our previous study
showed that EGCG reduces the interleukin-6 synthesis by
platelet-derived growth factor without affecting the p70 S6 kinase
activation (26). It is generally
recognized that platelet-derived growth factor receptor has an
intrinsic protein tyrosine kinase activity, whereas TNF-α receptor
belongs to the cytokine receptor superfamily, and that the signal
transduction mechanisms are quite different from each other
(12,27). Thus, it appears likely that
different and rather opposite effects of EGCG on p70 S6 kinase
activation and interleukin-6 synthesis induced by TNF-α or PDGF-BB
in osteoblast-like MC3T3-E1 cells are due to the difference between
each receptor-mediated intracellular signaling. Further
investigations are required to clarify the details underlying the
effects of EGCG and CGA on the interleukin-6 synthesis in
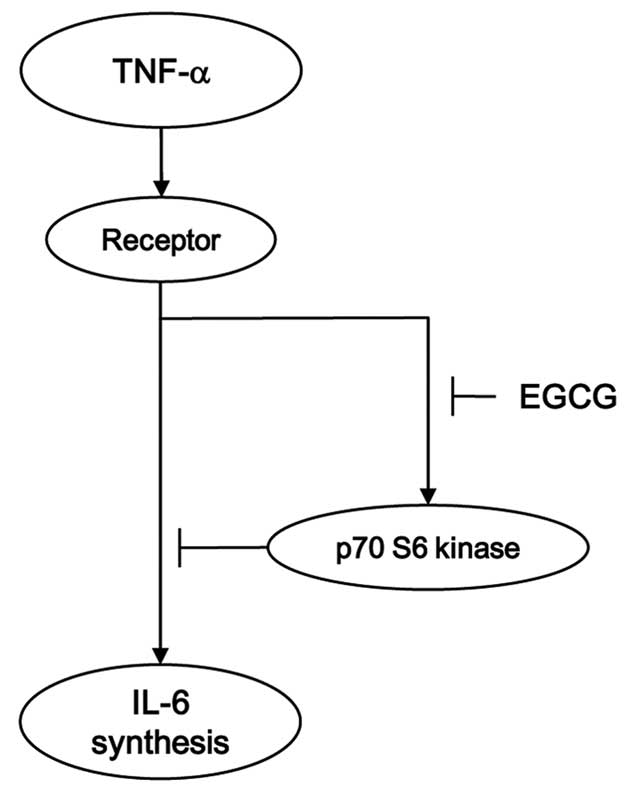
osteoblasts. The potential mechanism of EGCG in the
TNF-α-stimulated interleukin-6 synthesis in osteoblasts is
summarized in Fig. 6.
Polyphenolic compounds in beverages, including green
tea and coffee, have beneficial properties for human health. With
regard to bone metabolism, accumulating evidence indicates that
green tea consumption contributes bone formation, improves bone
mineral density and decreases the risk of fracture (7). Additionally, it is currently known
that coffee consumption reduces the risk of osteoporosis and
osteoporotic fracture in elderly people (28). In the present study, EGCG and CGA
amplified the TNF-α-stimulated interleukin-6 synthesis in
osteoblast-like MC3T3-E1 cells. In bone metabolism, interleukin-6
has long been recognized to act as a powerful bone resorptive agent
and promotes osteoclast formation (29). However, interleukin-6 is currently
considered as an osteotropic factor under the condition of
increased bone turnover and induces bone formation (17). Bone resorption is the first step
of bone remodeling, and subsequently bone formation is initiated
(4). To obtain the quantity and
quality of bone, adequate bone remodeling processed by osteoclasts
and osteoblasts is essential. With regard to bone metabolism as a
whole, it is possible that interleukin-6 functions as a bone
remodeling mediator. In addition, it has been reported that
interleukin-6 has a crucial role in the initiation of bone
formation in an early stage of bone fracture repair (16). Therefore, the present findings
that EGCG and CGA enhance the TNF-α-stimulated interleukin-6
synthesis in osteoblasts provide a new insight in fracture
prevention in elderly people. Further investigations are required
to elucidate the exact roles of EGCG and CGA in bone
metabolism.
In conclusion, the present results strongly suggest
that EGCG and CGA enhance the TNF-α-stimulated interleukin-6
synthesis in osteoblasts and that the enhancement by EGCG, but not
CGA, is exerted via inhibiting p70 S6 kinase.
Acknowledgments
The authors are extremely grateful to Mrs. Yumiko
Kurokawa for her skillful technical assistance. The present study
was supported in part by a Grant-in-Aid for Scientific Research
(grant no. 19591042) from the Ministry of Education, a Grant-in-Aid
for Scientific Research (grant no. H25-Aging-General-004) from the
Ministry of Health, Labour and Welfare, and the Research Funding
for Longevity Sciences (grant no. 25–4, 26–12) from the National
Center for Geriatrics and Gerontology, Japan.
References
|
1
|
George SE, Ramalakshmi K and Mohan Rao LJ:
A perception on health benefits of coffee. Crit Rev Food Sci Nutr.
48:464–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thielecke F and Boschmann M: The potential
role of green tea catechins in the prevention of the metabolic
syndrome - a review. Phytochemistry. 70:11–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu M, Adachi S, Masuda M, Kozawa O
and Moriwaki H: Cancer chemoprevention with green tea catechins by
targeting receptor tyrosine kinases. Mol Nutr Food Res. 55:832–843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastogenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann NY Acad Sci. 1092:385–396. 2006. View Article : Google Scholar
|
|
7
|
Shen CL, Yeh JK, Cao JJ and Wang JS: Green
tea and bone metabolism. Nutr Res. 29:437–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh R, Akhtar N and Haqqi TM: Green tea
polyphenol epigallocatechin-3-gallate: Inflammation and arthritis.
[corrected]. Life Sci. 86:907–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folwarczna J, Pytlik M, Zych M, Cegieła U,
Nowinska B, Kaczmarczyk-Sedlak I, Sliwinski L, Trzeciak H and
Trzeciak HI: Effects of caffeic and chlorogenic acids on the rat
skeletal system. Eur Rev Med Pharmacol Sci. 19:682–693.
2015.PubMed/NCBI
|
|
10
|
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee
MS, Rho MC and Oh J: Chlorogenic acid inhibits osteoclast
differentiation and bone resorption by down-regulation of receptor
activator of nuclear factor kappa-B ligand-induced nuclear factor
of activated T cells c1 expression. Biol Pharm Bull. 36:1779–1786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou W, Hakim I, Tschoep K, Endres S and
Bar-Shavit Z: Tumor necrosis factor-alpha mediates RANK ligand
stimulation of osteoclast differentiation by an autocrine
mechanism. J Cell Biochem. 83:70–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe
E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, et al:
IL-6 is produced by osteoblasts and induces bone resorption. J
Immunol. 145:3297–3303. 1990.PubMed/NCBI
|
|
14
|
Akira S, Taga T and Kishimoto T:
Interleukin-6 in biology and medicine. Adv Immunol. 54:1–78. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heymann D and Rousselle AV: gp130 Cytokine
family and bone cells. Cytokine. 12:1455–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fazzalari NL: Bone fracture and bone
fracture repair. Osteoporos Int. 22:2003–2006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franchimont N, Wertz S and Malaise M:
Interleukin-6: An osteotropic factor influencing bone formation?
Bone. 37:601–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozawa O, Suzuki A, Kaida T, Tokuda H and
Uematsu T: Tumor necrosis factor-α autoregulates interleukin-6
synthesis via activation of protein kinase C. Function of
sphingosine 1-phosphate and phosphatidylcholine-specific
phospholipase C. J Biol Chem. 272:25099–25104. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minamitani C, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Yamauchi J, Kato K, Natsume H, Mizutani J,
Kozawa O and Otsuka T: p70 S6 kinase limits tumor necrosis
factor-alpha-induced interleukin-6 synthesis in osteoblast-like
cells. Mol Cell Endocrinol. 315:195–200. 2010. View Article : Google Scholar
|
|
20
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
23
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takai S, Tokuda H, Hanai Y and Kozawa O:
Limitation by p70 S6 kinase of PDGF-BB-induced IL-6 synthesis in
osteoblast-like MC3T3-E1 cells. Metabolism. 56:476–483. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takai S, Matsushima-Nishiwaki R, Adachi S,
Natsume H, Minamitani C, Mizutani J, Otsuka T, Tokuda H and Kozawa
O: (-)-Epigallocatechin gallate reduces platelet-derived growth
factor-BB-stimulated interleukin-6 synthesis in osteoblasts:
Suppression of SAPK/JNK. Mediators Inflamm. 2008:2918082008.
View Article : Google Scholar
|
|
27
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI
|
|
28
|
Higdon JV and Frei B: Coffee and health: A
review of recent human research. Crit Rev Food Sci Nutr.
46:101–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blair HC and Athanasou NA: Recent advances
in osteoclast biology and pathological bone resorption. Histol
Histopathol. 19:189–199. 2004.PubMed/NCBI
|