Introduction
Osteoblasts are involved in normal skeletal growth
and homeostasis (1). Bone can be
produced by the direct differentiation of osteoblasts from
mesenchymal progenitors (2).
Osteoblast differentiation is regulated by various transcription
factors and signaling proteins (3). It has been demonstrated that the
transcription factors, β-catenin (β-cat) and osterix (OSX, also
known as SP7), are essential for osteoblast differentiation and
bone formation during embryonic development (1). During development, OSX is
specifically expressed in osteoblasts, but not in osteoclast
lineage cells (1). As OSX is
associated with bone mineral density (BMD) in both children and
adults, it is likely that OSX also plays an important role in the
development of the postnatal skeleton (4,5).
OSX has been found to play an essential multifunctional role in
postnatal bone growth and homeostasis (1).
β-cat is a key component of the Wnt signaling
pathway (6), which is essential
to osteoblast differentiation during embryonic development
(3) and has been implicated in
the regulation of BMD (7). In the
majority of cells, β-cat is predominantly located at the plasma
membrane, which is resistant to mild detergents and is referred to
as the insoluble pool of β-cat (8). Small amounts of soluble β-cat are
normally present in the cytoplasm (8,9).
The activation of the 'canonical' Wnt pathway involves the
stabilization of cytoplasmic/soluble β-cat, which interacts with
the T cell factor (Tcf) family of transcription factors to activate
downstream target genes, such as c-Myc and c-Jun (8–10).
A previous study demonstrated that β-cat signaling in osteoblasts
coordinates postnatal bone acquisition by controlling the
differentiation and activity of osteoblasts (7).
Thus, it seems that both OSX and β-cat are essential
for embryonic and postnatal osteoblast differentiation and bone
growth. In the present study, we explored the crosstalk between
β-cat signaling and OSX, and assessed its effect on osteoblast
differentiation in human pre-osteoblastic and bone marrow stromal
cells.
Materials and methods
Cell culture
Human MG-63 pre-osteoblastic/osteosarcoma cell
(CRL-1427) and human HS-27A bone marrow stromal cells (CRL-2496)
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The HS-27A and MG-63 cells were respectively
cultured in DMEM and RPMI-1640 medium (Life Technologies, Carlsbad,
CA, USA) containing 10% heat-inactivated FBS (Life Technologies)
and 100 U/ml penicillin-streptomycin (Sigma-Aldrich, Beijing,
China) in an incubator with a humidified atmosphere of 95% air and
5% CO2 at 37°C. In order to induce osteoblast
differentiation, the HS-27A and MG-63 cells (5,000 cells/well) were
cultured in osteoblastogenic medium containing 50 mg/l ascorbic
acid, 10 mmol/l β-glycerol phosphate disodium and 100 nmol/l
dexamethasone. To block β-cat signaling, the cells were treated
with the selective β-cat signaling inhibitor, CCT031374 (50
µM), during the entire osteoblastogenic culture period.
Plasmids and reagents
The human OSX cDNA clone (SC328709) was purchased
from OriGene Technologies (Beijing, China), and the full-length OSX
cDNA sequence was subcloned into the pcDNA 3.1 plasmid (Life
Technologies). The human β-cat cDNA clone (SC107921) was purchased
from OriGene, and the β-cat cDNA sequence lacking those encoding
151 amino-terminal residues was subcloned into the pcDNA 3.1
plasmid to generate a constitutively active (∆N151) β-cat
expression vector. The human OSX promoter/luciferase reporter
(S713117) and LightSwitch luciferase assay kit (LS010) were both
purchased from SwitchGear Genomics (Shanghai, China). Mutant OSX
promoter/luciferase reporter constructs were generated by
polymerase chain reaction (PCR) and confirmed by sequencing. OSX
(sc-43984-V), c-Jun (sc-29223-V) and control (sc-108080) shRNA
lentiviral particles, and goat poly-clonal anti-β-cat (C-18)
(sc-1496) antibody (epitope matched to the carboxyl terminal of
human β-cat), rabbit polyclonal anti-OSX (Y-21) (sc-133871)
antibody, mouse monoclonal anti-cJun (G-4) (sc-74543) antibody,
mouse monoclonal anti-cFos (6-2H-2F) (sc-447) antibody and mouse
monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
(6C5; sc-32233) antibody were all purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Lipofectamine 2000
transfection reagent, TRIzol reagent and SuperScript II reverse
transcriptase were purchased from Life Technologies. The
colorimetric alkaline phosphatase (ALP) assay kit (ab83369) was
purchased from Abcam (Cambridge, MA, USA). The calcium (CPC)
liquicolor kit (#0150-250) was purchased from Stanbio Laboratory
(Boerne, TX, USA). The selective β-cat signaling inhibitor,
CCT031374, was purchased from Tocris Bioscience (Bristol, UK).
Puromycin and G418 were purchased from Sigma-Aldrich. Putative
transcription factor binding sites in the human OSX gene promoter
sequence were identified using online PROMO software (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3),
as previously described (11,12).
Stable transfection and lentiviral
transduction
The constitutively active (∆N151) β-cat and the OSX
expression vectors were respectively transfected into the cells
using Lipofectamine 2000 transfection reagent (Life Technologies)
according to the manufacturer's instructions. Pools of stable
transductants were generated via selection with G418 (800
µg/ml) according to the manufacturer's instructions. The OSX
and c-Jun shRNA lentiviral particles produce target-specific shRNA
designed to specifically knockdown OSX and c-Jun expression,
respectively, whereas control shRNA lentiviral particles contain a
scrambled shRNA sequence that will not lead to the specific
degradation of any cellular mRNA. Lentiviral transduction was
performed, and pools of stable transductants were generated via
selection with puromycin (5 µg/ml) according the
manufacturer's instructions (Santa Cruz Biotechnology). Cells
stably transfected with the constitutively active (ΔN151) β-cat
expression vector were stably transfected with lentiviral OSX shRNA
to knock down OSX. Cells stably transfected with the OSX expression
vector were treated with the selective β-cat signaling inhibitor,
CCT031374 (50 µM), during the entire osteoblastogenic
culture period to block β-cat signaling.
Western blot analysis
For whole cell lysates, the cells were lysed with a
hypotonic buffer containing 2% Nonidet-P-40 and a protease
inhibitor cocktail (Sigma) by sonication 3 times for 3 sec on ice.
The supernatant obtained following centrifugation at 2,000 x g for
15 min at 4°C was used to determine the protein concentration by
the Coomassie blue method and also for subsequent steps. For the
detection of soluble β-cat, the cells were lysed in 0.1% Nonidet
P-40 lysis buffer (0.1% Nonidet P-40, 10 mM HEPES, pH 7.5, 142.5 mM
KCl, 5 mM MgCl2 and 1 mM EGTA). The lysates were
centrifuged at 14,000 × g for 10 min, and the supernatants were
saved as soluble cell lysate, as previously described (8,10).
Equal amounts of proteins for each sample were separated by 8–15%
SDS-polyacrylamide gel and blotted onto polyvinylidene difluoride
microporous membranes (Millipore, Billerica, MA, USA). The
membranes were incubated for 1 h with a 1:1,000 dilution of primary
antibody, and then washed and revealed using secondary antibodies
with horseradish peroxidase conjugate (1:5,000, 1 h). Peroxidase
was revealed using an ECL kit (GE Healthcare, Shanghai, China).
Three independent experiments were performed.
Transient transfection and luciferase
reporter assay
The cells were transfected with the human OSX
promoter/luciferase reporter or TOPflash or FOPflash plasmids
(Upstate Cell Signaling Solutions, Billerica, MA, USA) using
Lipofectamine 2000 transfection reagent (Life Technologies). The
luciferase assays were performed 30 h after transfection using the
LightSwitch Luciferase assay kit (SwitchGear Genomics) following
the manufacturer's instructions. The pRL-CMV plasmid (Promega,
Madison, WI, USA) encoding Renilla reniformis luciferase (at
one fifth molar ratio to test plasmids) was co-transfected with the
test plasmids in each transfection as an internal control for data
normalization. Each experiment was repeated 3 times in
duplicate.
Reverse-transcription-quantitative PCR
(RT-qPCR)
RNA was prepared from the cells using TRIzol
reagent, and cDNA was synthesized using SuperScript II reverse
transcriptase (Life Technologies). Quantitative (real-time) PCR
(qPCR) was performed on an ABI PRISM 7700 Sequence detection
system, with the fluorescent dye SYBR-Green Master Mix (Applied
Biosystems, Beijing, China), according to the instructions provided
by the manufacturer. The primers used were as follows: for OSX,
5′-TGCTTGAGGAGGAAGTTCAC-3′ (forward) and 5′-AGGTCACTGCCCACAGAGTA-3′
(reverse); for c-Myc, 5′-GCAAACCTCCTCACAGCCCACT-3′ (forward) and
5′-AACTTGACCCTCTTGGCAGCA-3′ (reverse); for c-Jun,
5′-CAAAGTTTGGATTGCATCAAGTG-3′ (forward) and
5′-TAACATTATAAATGGTCACAGCACATG-3′ (reverse); for GAPDH,
5′-GACTCATGACCACAGTCCATGC-3′ (forward) and
5′-AGAGGCAGGGATGATGTTCTG-3′ (reverse). Relative quantification of
the mRNA levels was determined using the 2−ΔΔCt method,
which normalizes the expression levels of genes of interest against
that of GAPDH in the same samples, as previously described
(13). Each experiment was
repeated 3 times in duplicate.
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts were prepared from the HS-27A and
MG-63 cells, as previously described in the study by Johnson et
al (14). EMSA was performed
with 32P-labeled double-stranded oligonucleotides
incubated with nuclear extract in EMSA buffer [10 mM Tris, pH 7.5,
5% glycerol, 1 mM EDTA, pH 7.1, 50 mM NaCl, 1 mM DTT, 1 mM EDTA and
0.1 mg/ml poly(dI-dC)]. For oligonucleotide competition analysis, a
100-fold molar excess of unlabeled competitor oligonucleotides was
also added to the mixture and incubated at room temperature for 30
min. For antibody supershift assays, 1 µl monoclonal
antibodies to c-Jun or c-Fos (Santa Cruz Biotechnology) was added
to the mixture. The reaction was then incubated on ice for 1 h.
Protein-DNA complexes and free DNA were fractionated on 5%
polyacrylamide gels in 1X Tris-glycine EDTA buffer at 4°C and were
visualized by autoradiography.
Quantitative assessment of osteoblast
differentiation and mineralization
In order to induce osteoblast differentiation, the
HS-27A and MG-63 cells (5,000 cells/well) were cultured in
osteoblastogenic medium. The cells were not passaged during the
experiment (maximum 28 days), but the culture medium and
supplements were changed twice each week. Osteoblast
differentiation was determined quantitatively by measuring ALP
activity on day 14 of culture with a colorimetric ALP assay kit
(Abcam), as previously described (15,16). Data are presented relative to the
total protein concentration. Osteoblast differentiation was also
assessed by in vitro mineralization. On day 14 (HS-27A
cells) and on day 28 (MG-63 cells), calcium was extracted from the
monolayers by incubating the cells overnight in 0.6 N HCl and
measured quantitatively as µg/well using a calcium (CPC)
liquicolor kit (Stanbio Laboratory), as previously described
(17). Each experiment was
repeated 3 times in duplicate.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 19.0 (IBM, Inc., Chicago, IL, USA). All continuous variable
values are expressed as the means ± SD. A comparison of the means
between 2 groups was performed using the Student's t-test.
Comparisons of the means between multiple groups were performed
with one-way ANOVA followed by post hoc pairwise comparisons using
Tukey's tests. A two-tailed value of P<0.05 was considered to
indicate a statistically significant difference.
Results
β-cat signaling upregulates the
expression of OSX in human pre-osteoblastic and bone marrow stromal
cells
To explore the potential crosstalk between β-cat
signaling and OSX in human pre-osteoblastic and bone marrow stromal
cells, we stably overexpressed a constitutively active β-cat
mutant, which lacks 151 amino-terminal residues (∆N151), in the
HS-27A human bone marrow stromal cells and MG-63 human
pre-osteoblastic cells. We also used CCT031374, a selective β-cat
signaling inhibitor, which decreases the cytoplasmic/soluble β-cat
level (8,18) in order to inhibit β-cat signaling
in the cells. In addition, we stably transduced lentiviral shRNA to
knock down OSX in the cells overexpressing the ∆N151/active β-cat
mutant, and stably overexpressed OSX in the cells treated with
CCT031374. As shown in Fig. 1,
∆N151/active β-cat was overexpressed in the HS-27A and MG-63 cells
compared with the levels of wild-type soluble β-cat in the
controls. Compared with the controls, CCT031374 decreased the
soluble β-cat level by approximately 60 and 75% in the HS-27A and
MG-63 cells, respectively (Fig.
1). In the cells overexpressing active β-cat, the protein level
of OSX increased by approximately 2.9- and 3.4-fold in HS-27A and
MG-63 cells, respectively; in cells treated with CCT031374, the
protein level of OSX decreased approximately 55 and 64% in the
HS-27A and MG-63 cells, respectively (Fig. 1). In addition, the overexpression
and knockdown of OSX had no significant effect on the soluble β-cat
levels. These results suggest that β-cat signaling upregulates the
expression of OSX in human pre-osteoblastic and bone marrow stromal
cells.
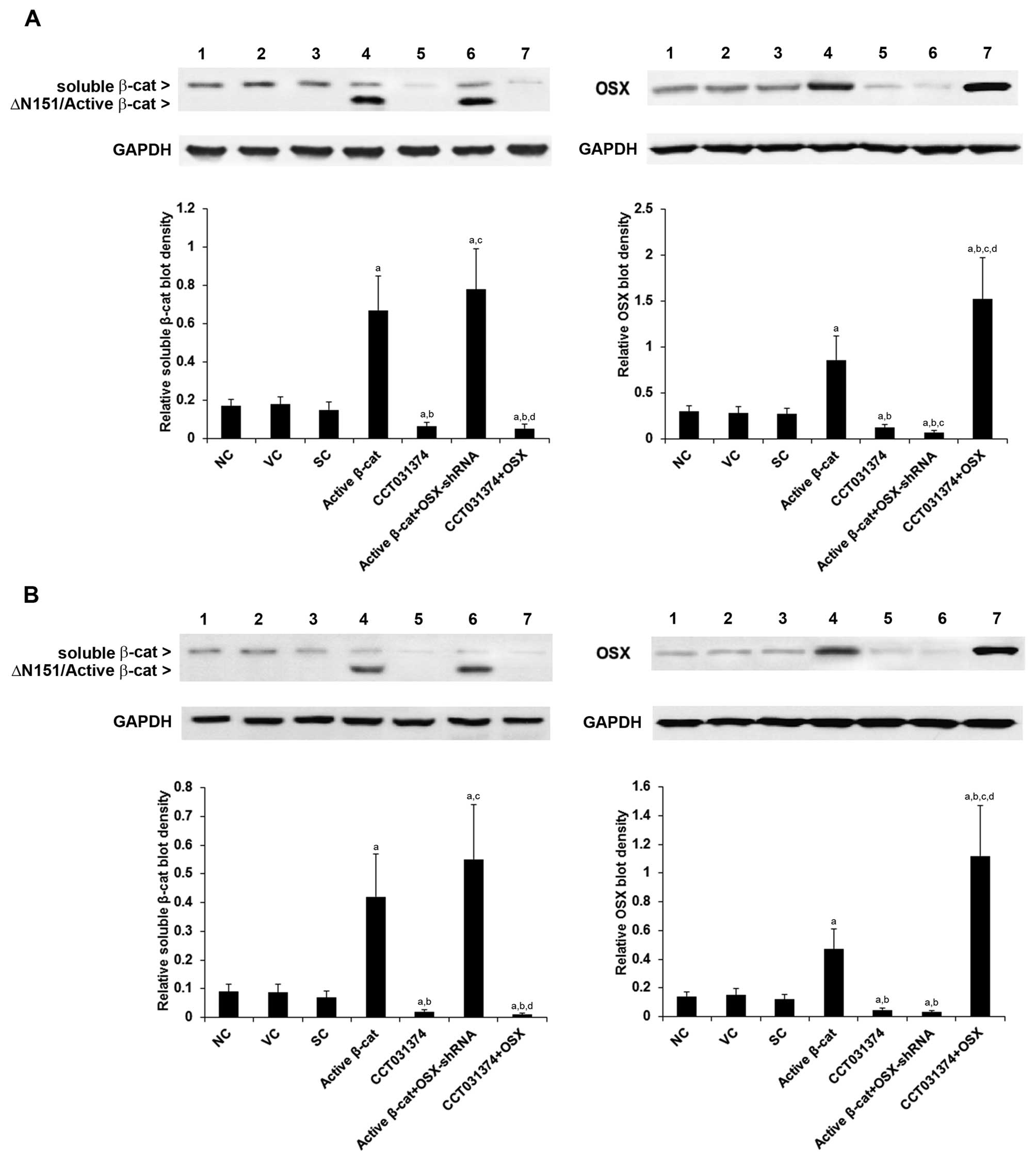 | Figure 1Protein levels of β-catenin (β-cat)
and osterix (OSX) in human pre-osteoblastic and bone marrow stromal
cells. In (A) HS-27A human bone marrow stromal cells and (B) MG-63
human pre-osteoblastic cells, the protein levels of
cytoplasmic/soluble β-cat were measured by western blot analyses in
normal control cells (NC, lane 1), cells stably transfected with
the empty pcDNA3.1 vector (VC, lane 2), cells stably transduced
with scramble control shRNA (SC, lane 3), cells stably transfected
with constitutively active (∆N151) β-cat (active β-cat, lane 4),
cells treated with selective β-cat signaling inhibitor CCT031374
(50 µM) for 30 h (lane 5), cells stably transfected with
constitutively active (∆N151) β-cat and stably transduced with
OSX-shRNA (active β-cat + OSX-shRNA, lane 6), and cells stably
transfected with OSX and treated with CCT031374 (50 µM) for
30 h (lane 7). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
blotting was used as a loading control. Density of the OSX and the
cytoplasmic/soluble β-cat blots was normalized against that of the
GAPDH blot to obtain a relative blot density. In cells
overexpressing ∆N151/active β-cat, the relative density of
∆N151/active β-cat instead of that of wild-type soluble β-cat was
calculated and is shown in the bar graph. Three independent
experiments were performed for each western blot analysis. Data are
expressed as the means + SD. aP<0.05 vs. controls
(NC, VC and SC); bP<0.05 vs. active β-cat;
cP<0.05 vs. CCT031374; dP<0.05 vs.
active β-cat + OSX-shRNA. |
We then examined whether β-cat signaling induces the
expression of OSX through the transcriptional activation of the OSX
gene. The transcriptional activity of β-cat signaling in the HS-27A
and MG-63 cells was measured with TOPflash, a synthetic luciferase
reporter for β-cat/Tcf signaling activity (8,10).
As shown in Fig. 2A, compared
with the controls, the active β-cat and the inhibitor, CCT031374,
markedly increased and decreased the luciferase activity of
TOPflash, respectively; little change was observed with FOPflash, a
negative control reporter (8,10).
In agreement with the results of the TOPflash/FOPflash experiments,
RT-qPCR revealed that active β-cat and the inhibitor, CCT031374,
markedly increased and decreased the mRNA levels of the established
β-cat signaling target genes, c-Myc and c-Jun (8–10),
respectively (Fig. 2B). The mRNA
levels of OSX followed a similar trend as those of c-Myc and c-Jun,
under the effects of the active β-cat and the inhibitor, CCT031374
(apart from the cells in which OSX was overexpressed or knocked
down, which served as positive controls in the experiment)
(Fig. 2B), suggesting that the
OSX gene is a target of β-cat signaling.
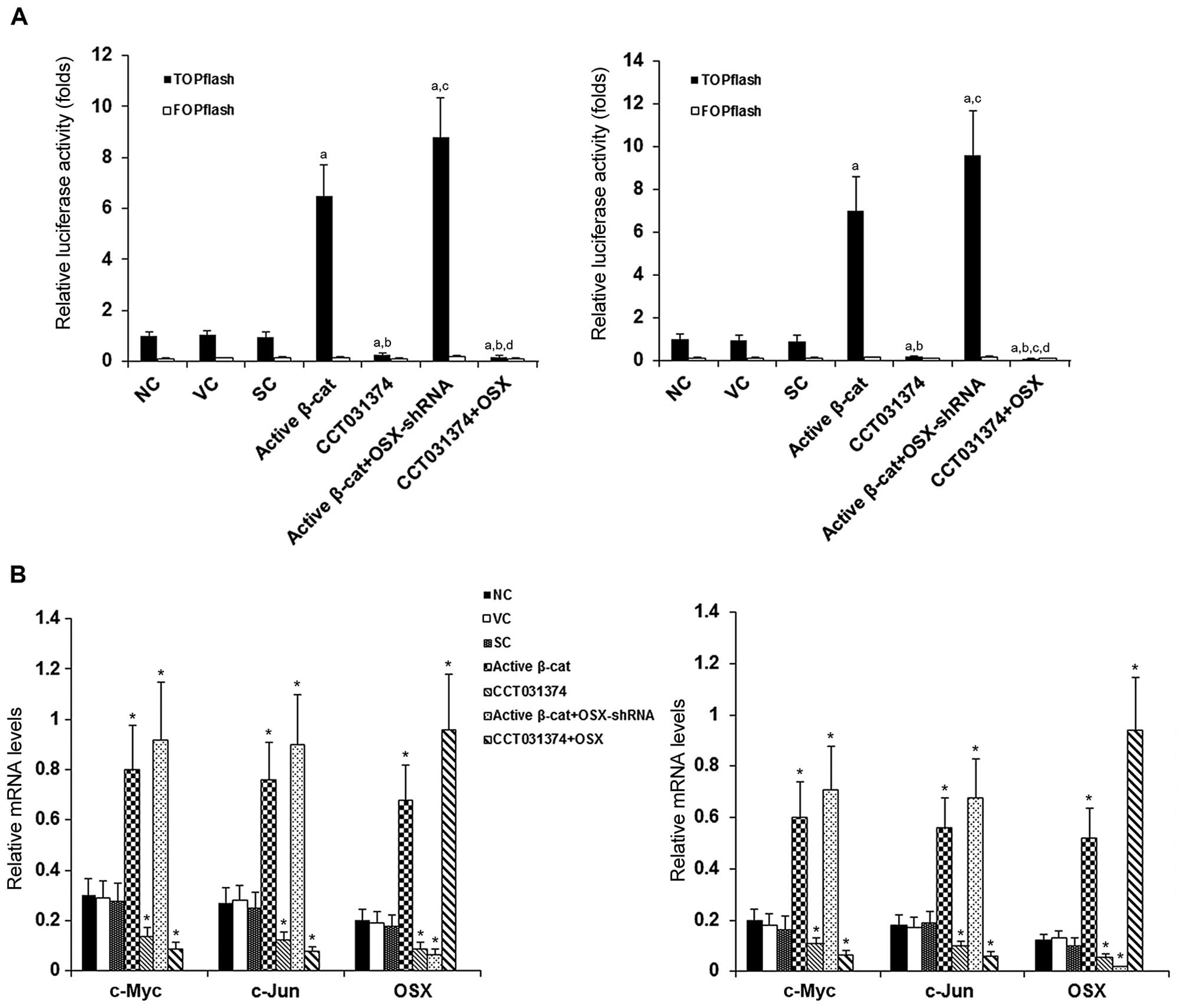 | Figure 2β-catenin (β-cat) signaling luciferase
reporter activities and target gene mRNA levels in human
pre-osteoblastic and bone marrow stromal cells. (A) HS-27A (left
panel) and MG-63 (right panel) cells were transfected with
TOPflash, a synthetic β-cat signaling luciferase reporter, or
FOPflash, a negative control reporter. Thirty hours later,
luciferase activity was determined in normal control cells (NC),
cells stably transfected with the empty pcDNA3.1 vector (VC), cells
stably transduced with scramble control shRNA (SC), cells stably
transfected with constitutively active (∆N151) β-cat (active
β-cat), cells treated with selective β-cat signaling inhibitor
CCT031374 (50 µM) for 30 h, cells stably transfected with
constitutively active (∆N151) β-cat and stably transduced with
osterix (OSX)-shRNA (active β-cat + OSX-shRNA), and cells stably
transfected with OSX and treated with CCT031374 (50 µM) for
30 h. Luciferase activity was measured 30 h after transfection and
expressed as a fold change to that of NC (designated as 1).
aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. active β-cat; cP<0.05 vs.
CCT031374; dP<0.05 vs. active β-cat + OSX-shRNA. (B)
The mRNA levels of OSX and established β-cat signaling target genes
c-Myc and c-Jun were measured by RT-qPCR in the HS-27A (left panel)
and MG-63 (right panel) cells. The mRNA levels of c-Myc, c-Jun and
OSX were normalized against those of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). *P<0.05 vs. controls (NC, VC
and SC). |
β-cat signaling transactivates the human
OSX gene promoter
We then investigated whether β-cat signaling
trans-activates the human OSX gene promoter, as well as the
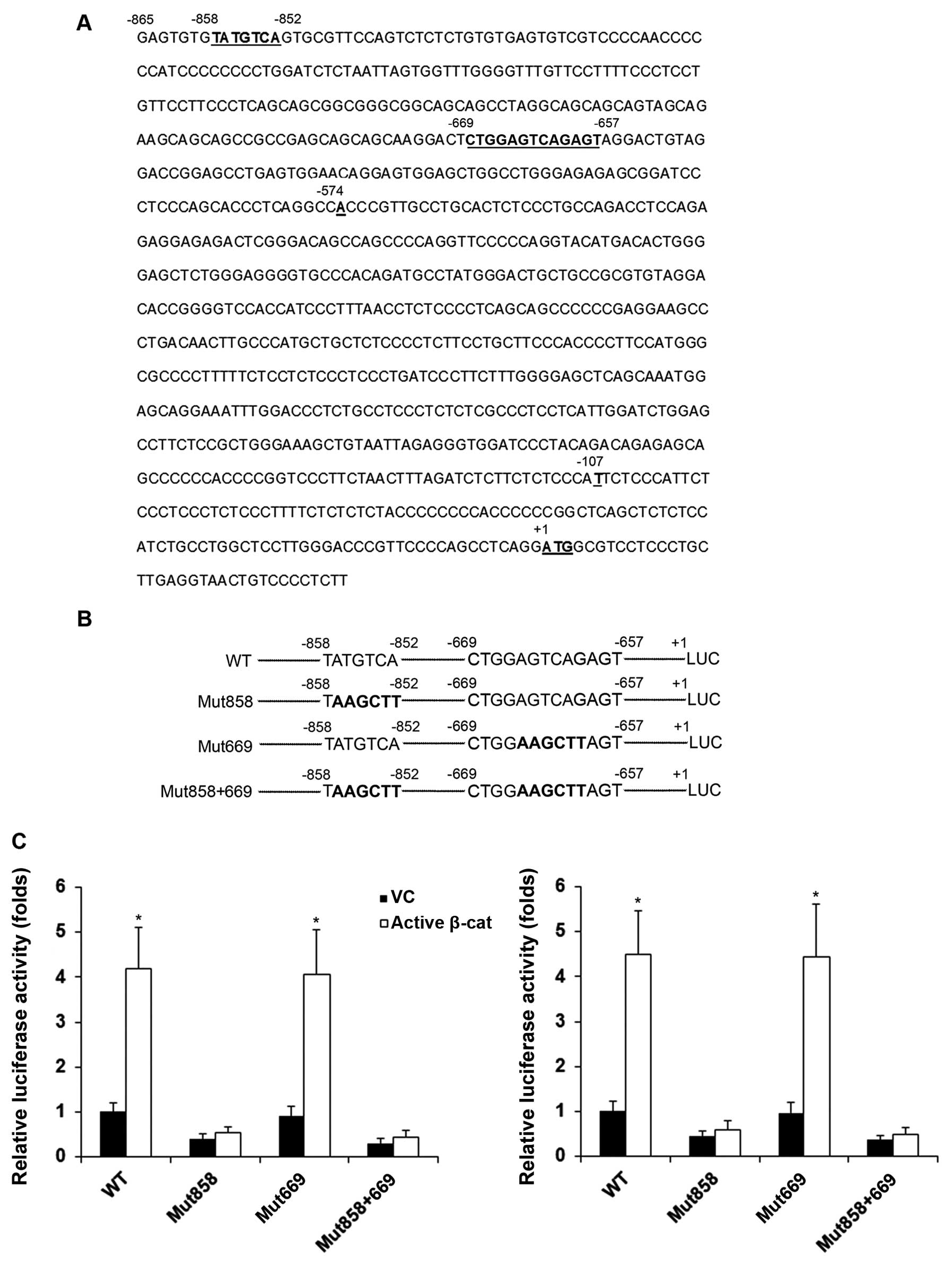
possible mechanisms involved. We employed a commercial human OSX
promoter/luciferase reporter (SwitchGear Genomics), which had 865
bp of 5′-untranslated region (UTR) immediately upstream of the OSX
gene translation start codon inserted in frame with the luciferase
cDNA. We screened for putative transcription factor binding sites
in the 865-bp human OSX promoter sequence with high stringency
(factors predicted within a 5% dissimilarity margin from the
consensus binding sequence) using online PROMO software (11,12). As shown in Fig. 3A, with the OSX gene ATG
translation start codon designated as +1, a putative c-Jun binding
site at −858/−852 and a putative AP-1/c-Jun/c-Fos binding site at
−669/−657 (AP-1, −669/−661; c-Jun, −667/−661; c-Fos, −666/−657)
were identified in the −865 bp human OSX promoter region. We then
introduced mutations to disrupt the putative transcription factor
binding sites in the OSX promoter/luciferase reporter, respectively
(Fig. 3B). As shown in Fig. 3C, compared with the controls, the
overexpression of active β-cat increased OSX promoter activity
4-fold, which was completely abolished by mutation at the
−858/−852, but not the −669/−657 putative transcription factor
binding site in both the HS-27A and MG-63 cells. These results
suggested that the −858/−852 putative c-Jun binding site was
functional.
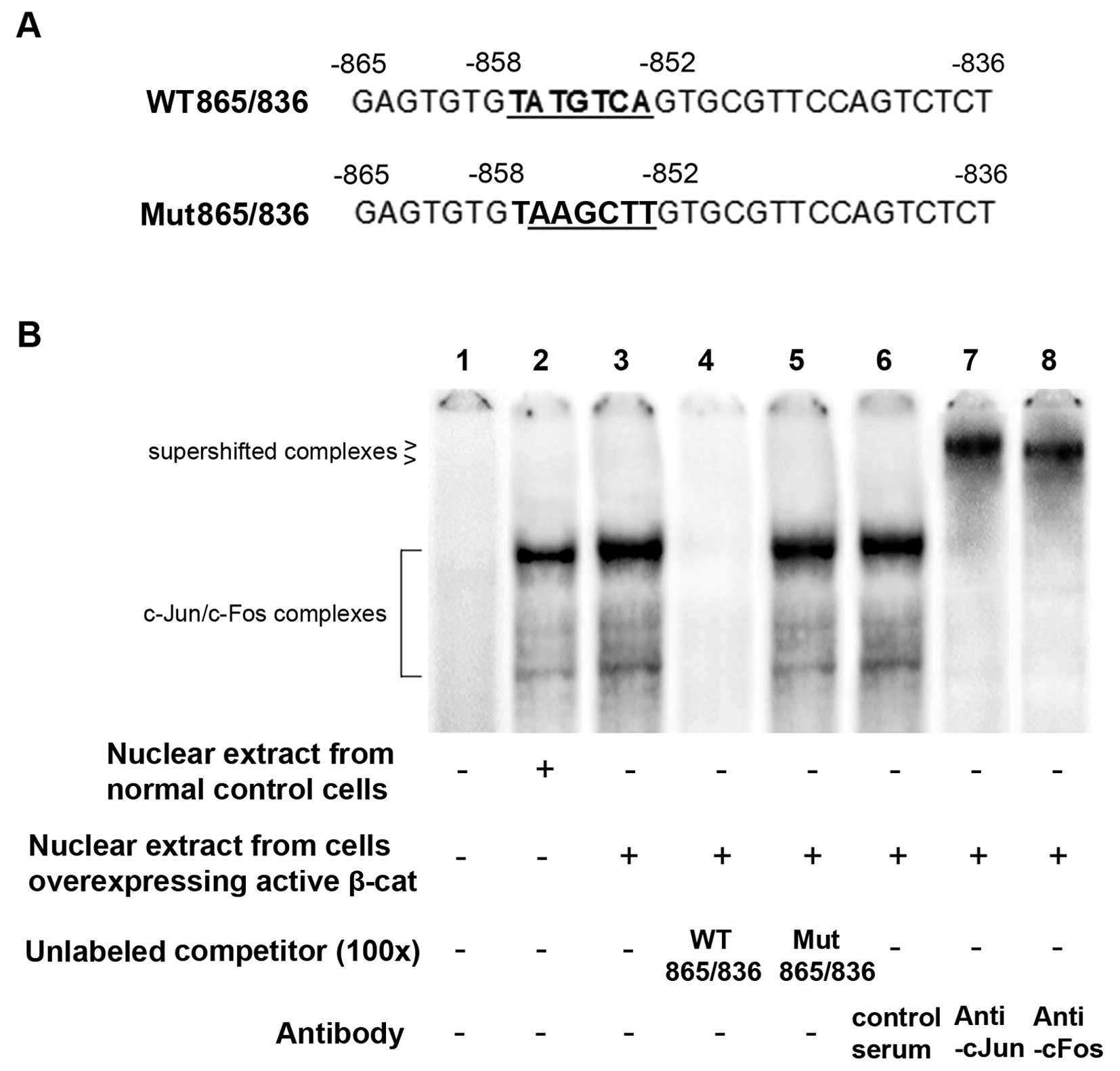
We then performed EMSAs to determine whether c-Jun
specifically binds to the −858/−852 putative binding site.
Oligonucleotide WT865/836 (Fig.
4A), corresponding to the human OSX promoter sequence
−865/−836, was radiolabeled and used as the probe to incubate with
HS-27A cell nuclear extracts in EMSAs. Unlabeled WT865/836 and
Mut865/836, another oligonucleotide with the same sequence as
WT865/836 apart from a mutated −858/−852 putative c-Jun binding
site (Fig. 4A), were used as
competitors to the probe. As shown in Fig. 4B, the nuclear extract from the
cells overexpressing active β-cat exhibited significantly stronger
binding activity with the probe than that from the control cells. A
100-fold molar excess of unlabeled WT865/836, but not Mut865/836,
completely abolished the binding activity (Fig. 4B), suggesting specific protein
binding at the −858/−852 putative c-Jun binding site. In addition,
although the control serum seemingly had no effect, anti-cJun and
anti-cFos antibodies supershifted the major protein-DNA complexes
to higher positions, respectively (Fig. 4B). These results suggest that
c-Jun specifically binds to the −858/−852 putative c-Jun binding
site in the form of c-Jun/c-Fos heterodimers.
To determine the functional role of c-Jun in the
β-cat signaling-induced expression of OSX, we stably transduced
lentiviral cJun shRNA into the HS-27A and MG-63 cells
over-expressing active β-cat. As shown in Fig. 5, compared with the controls,
active β-cat significantly increased the expression of OSX at both
the mRNA and the protein level, which was abolished by knocking
down c-Jun with shRNA.
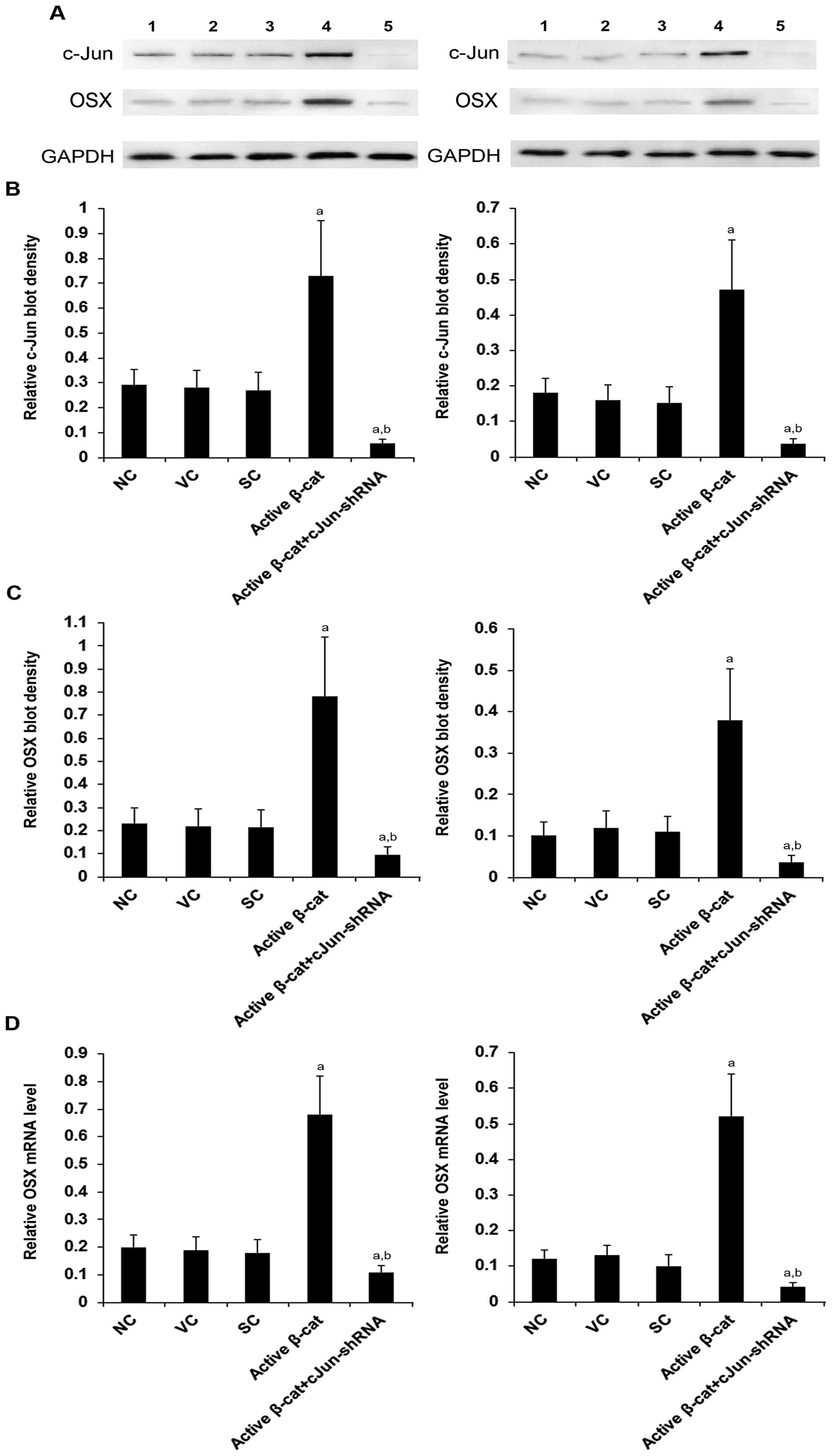 | Figure 5Knockdown of c-Jun abolished the
effect of β-catenin (β-cat) on the expression of osterix (OSX) in
human pre-osteoblastic and bone marrow stromal cells. (A) The
protein levels of c-Jun and OSX in HS-27A (left panel) and MG-63
(right panel) cells were measurd by western blot analyses in normal
control cells (NC, lane 1), cells stably transfected with the empty
pcDNA3.1 vector (VC, lane 2), cells stably transduced with scramble
control shRNA (SC, lane 3), cells stably transfected with
constitutively active (∆N151) β-cat (active β-cat, lane 4), and
cells stably transfected with constitutively active (∆N151) β-cat
and stably transduced with lentiviral shRNA against c-Jun (active
β-cat + cJun-shRNA, lane 5). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) blotting was used as a loading control.
Density of (B) c-Jun and (C) OSX blots was normalized against that
of the GAPDH blot to obtain a relative blot density, respectively.
Three independent experiments were performed for each western blot
analysis. Data are expressed as the means + SD. (D) The mRNA levels
of OSX were measured by RT-qPCR assays in the HS-27A (left panel)
and MG-63 (right panel) cells and normalized against those of
GAPDH. aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. active β-cat. |
Taken together, the above results suggest that β-cat
signaling upregulates the expression of OSX in human
pre-osteoblastic and bone marrow stromal cells by transactivating
the OSX gene promoter mainly through increased c-Jun binding
activity at the −858/−852 putative c-Jun binding site; c-Jun is an
essential mediator of the β-cat signaling-induced expression of OSX
in human pre-osteoblastic and bone marrow stromal cells.
Effect of β-cat/OSX signaling on
osteoblast differentiation and mineralization
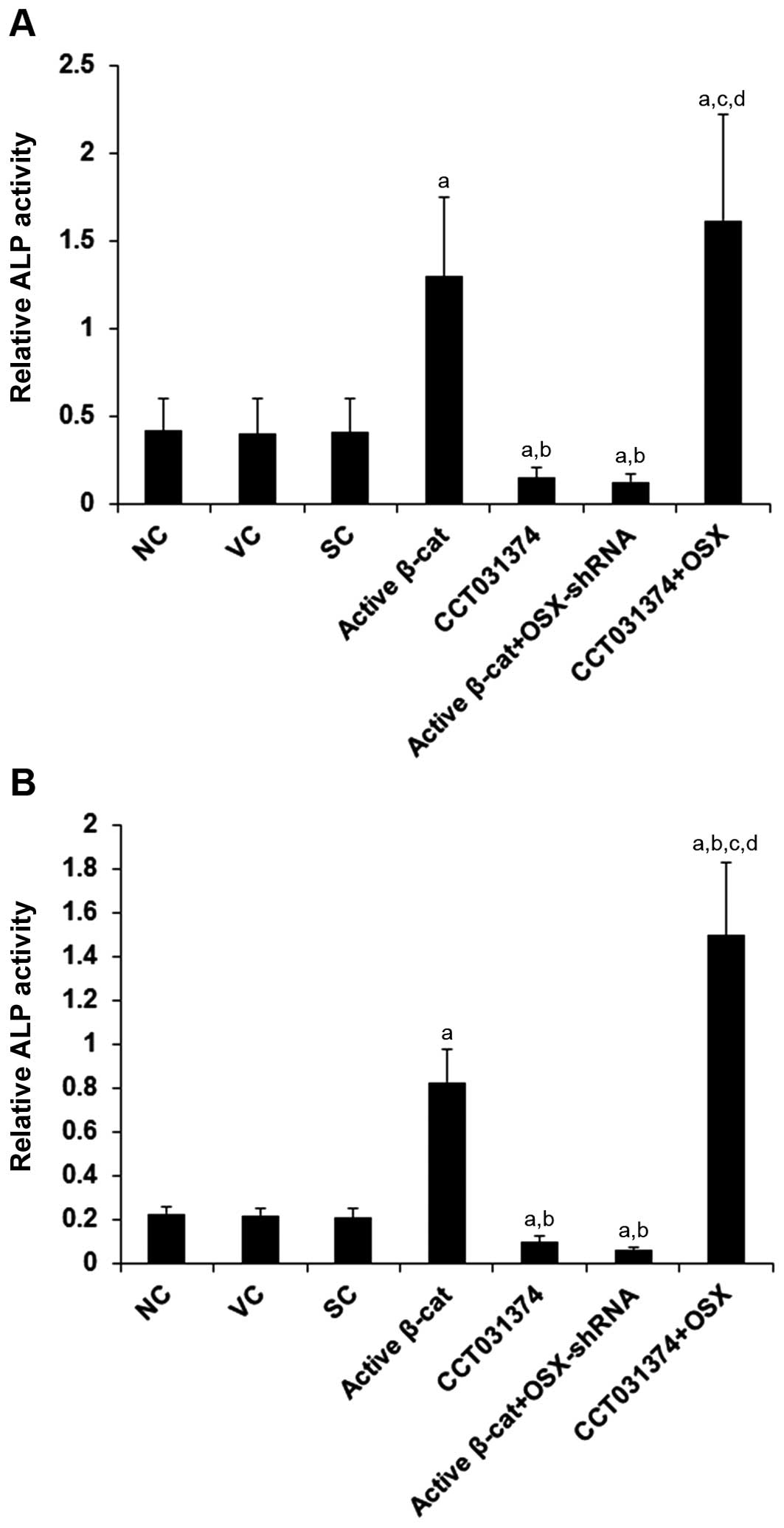
To examine the effects of β-cat/OSX signaling on
osteoblast differentiation and mineralization, we cultured the
HS-27A and MG-63 cells in osteoblastogenic medium. ALP activity, a
marker of early osteoblast differentiation and the commitment of
bone marrow stromal cells toward the osteoblastic phenotype
(19), was measured using a
colorimetric ALP assay kit (Abcam) on day 14 of osteoblastogenic
culture. As shown in Fig. 6,
compared with the controls, stimulating β-cat signaling activity by
the overexpression of active β-cat increased ALP activity by
approximately 3.0-fold in the HS-27A cells and by 3.6-fold in the
MG-63 cells, which was abolished by the knockdown of OSX with
shRNA. On the other hand, inhibiting β-cat signaling activity with
CCT031374 decreased ALP activity by approximately 63% in the HS-27A
cells and by approximately 54% in the MG-63 cells, which was
abolished by the overexpression of OSX.
We also assessed the effects of β-cat/OSX signaling
on osteoblast differentiation by measuring in vitro
mineralization. During the osteoblastogenic culture period, calcium
deposition was measured using a calcium (CPC) liquicolor kit
(Stanbio Laboratory) on day 14 in the HS-27A cells and on day 28 in
the MG-63 cells. As shown in Fig.
7, compared with the controls, the overexpression of active
β-cat increased calcium deposition by approximately 2.0-fold in the
HS-27A cells and by approximately 3.0-fold in the MG-63 cells,
which was abolished by the knockdown of OSX. On the other hand,
CCT031374 decreased calcium deposition by approximately 46% in the
HS-27A cells and 48% in the MG-63 cells, which was abolished by the
over-expression of OSX.
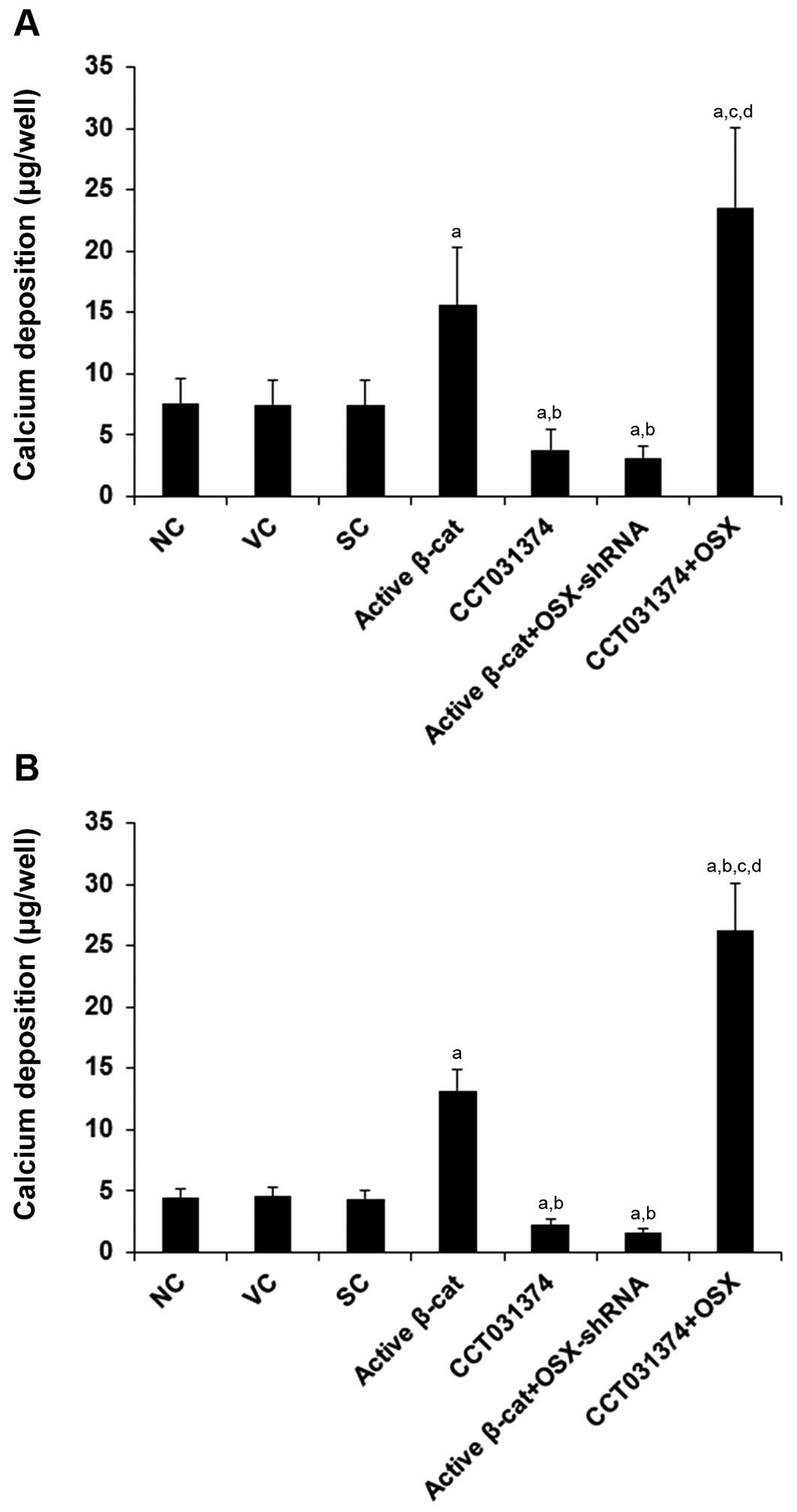 | Figure 7Effect of overexpression and
inhibition of β-catenin (β-cat) and/or osterix (OSX) on calcium
deposition in human pre-osteoblastic and bone marrow stromal cells.
For osteoblast differentiation, (A) HS-27A and (B) MG-63 cells
(5,000 cells/well) were cultured in osteoblastogenic medium. On day
14 in HS-27A cells and on day 28 in MG-63 cells, calcium deposition
was measured using a calcium (CPC) liquicolor kit (Stanbio
Laboratory) in normal control cells (NC), cells stably transfected
with the empty pcDNA3.1 vector (VC), cells stably transduced with
scramble control shRNA (SC), cells stably transfected with
constitutively active (∆N151) β-cat (active β-cat), cells treated
with selective β-cat signaling inhibitor CCT031374 (50 µM)
during the entire osteoblastogenic culture period, cells stably
transfected with constitutively active (∆N151) β-cat and stably
transduced with Osterix (OSX)-shRNA (active β-cat + OSX-shRNA), and
cells stably transfected with OSX and treated with CCT031374 (50
µM) during the entire osteoblastogenic culture period.
aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. active β-cat; cP<0.05 vs.
CCT031374; dP<0.05 vs. active β-cat + OSX-shRNA. |
Taken together, these results suggest that OSX may
be a major downstream mediator of the osteoblastogenic effect of
β-cat signaling on human pre-osteoblastic and bone marrow stromal
cells.
Discussion
Accumulating evidence has indicated that both OSX
and β-cat are essential for embryonic and postnatal osteoblast
differentiation and bone growth (1,3–5,7).
In the present study, for the first time and to the best of our
knowledge, we provide evidence that β-cat signaling induces
osteoblastogenic differentiation largely by upregulating the
expression of OSX in human pre-osteoblastic and bone marrow stromal
cells. The human MG-63 pre-osteoblastic/osteosarcoma cell line
(20) and human HS-27A bone
marrow stromal cell line have previously been used as cell models
in osteoblastogenic differentiation studies (21,22). In addition, as shown in the
present study, the two cell lines exhibited modest differences in
constitutive OSX and cytoplasmic/soluble β-cat levels, with HS-27A
cells demonstrating readily detectable, and MG-63 showing
relatively low, OSX and soluble β-cat levels. We employed both cell
lines to demonstrate generalizable findings.
The stabilization/accumulation of
cytoplasmic/soluble β-cat is essential for the canonical Wnt/β-cat
signaling pathway, which then leads to transcriptional activation
of β-cat/Tcf-regulated genes (8–10).
In the present study, we increased and decreased the
cytoplasmic/soluble β-cat levels with an active β-cat mutant and a
selective β-cat signaling inhibitor, respectively. The effects were
reflected in the luciferase activities of TOP/FOPflash β-cat
signaling reporters and the mRNA levels of the established β-cat
signaling target genes, c-Myc and c-Jun (8–10).
Similarly, the activation and inhibition of β-cat signaling
increased and decreased the expression of OSX at both the mRNA and
protein level, respectively, suggesting that OSX was a target gene
of β-cat signaling. This was confirmed by subsequent findings that
β-cat signaling transactivated the OSX gene promoter mainly through
increased c-Jun binding activity at a putative c-Jun binding site.
As c-Jun itself is a transcriptional target of β-cat/Tcf signaling,
the effect of β-cat on Osx expression is likely exerted through an
indirect effect of β-cat/Tcf signaling. We noted that both
anti-cJun and anti-cFos antibodies supershifted the major
protein-DNA complexes in EMSAs, suggesting that c-Jun specifically
bound to the putative c-Jun binding site in the form of c-Jun/c-Fos
heterodimers. This is in agreement with previous research that
demonstrated c-Fos can only form heterodimers with c-Jun and that
Jun-Fos heterodimers are more stable and have stronger DNA-binding
activity than Jun-Jun homodimers (23). This was also the reason that we
knocked down rather than overexpressed c-Jun to determine the
functional role of c-Jun in the β-cat signaling-induced expression
of OSX, as the overexpression of c-Jun increases the formation and
binding of Jun-Jun homodimers to the putative c-Jun binding site
and thus tends to generate artifacts.
Using a Cre-based conditional β-cat knockout mouse
model, in a previous study, Rodda et al (2) suggested that β-cat regulates Osx
downstream signaling. In agreement with their study, in our study,
we demonstrated that endogenous β-cat signaling contributed to
50–55% at the mRNA level and 55–64% at the protein level of the
expression of OSX in the HS-27A and MG-63 cells. Our results
revealed that a 55–64% decrease in Osx expression led to
significant alteration in osteoblast differentiation in
vitro. However, whether such a decrease in Osx expression would
affect osteoblast differentiation in vivo requires further
investigation.
Osteoblast differentiation can be divided into 3
stages, namely cell proliferation, matrix maturation and matrix
mineralization (24). The matrix
maturation phase is characterized by maximal expression/activity of
ALP. Once mineralization is complete, calcium deposition can be
quantified. In this study, we induced the osteoblastogenic
differentiation of HS-27A and MG-63 cells using osteoblastogenic
medium, and evaluated the functional role of β-cat/OSX signaling on
osteoblastogenic differentiation by measuring ALP activity and
calcium deposition. Clearly, while the inducing effects of β-cat
signaling on ALP activity and calcium deposition were completely
abolished by the knockdown of OSX in the HS-27A and MG-63 cells,
the inhibitory effect of the selective β-cat signaling inhibitor,
CCT031374, was completely reversed by the overexpression of OSX,
suggesting that OSX is an essential downstream mediator of the
osteoblastogenic effect of β-cat signaling in human
pre-osteoblastic and bone marrow stromal cells. In addition, our
results revealed that the HS-27A cells had higher levels of Osx and
soluble β-cat, as well as higher ALP activity and calcium
deposition than the MG-63 cells, corroborating that β-cat/Osx
signaling correlates with osteoblast differentiation. Nakashima
et al (25) demonstrated
that OSX functions downstream of runt-related transcription factor
2 (Runx2), another transcription factor which is required for
osteoblast differentiation and bone formation during embryonic
development (1). It will be
interesting to explore whether and how β-cat signaling interacts
with Runx2 upstream of OSX in the osteogenic program.
Zhang et al (3) reported that OSX inhibited β-cat/Tcf
signaling activity in Xenopus embryos and human embryonic
kidney 293 (HEK293) cells. Indeed, in this study, we noted that the
knockdown of OSX enhanced the effect of active β-cat (statistically
insignificant in both HS-27A and the MG-63 cells) and that the
overexpression of OSX enhanced the effect of CCT031374
(statistically significant in the MG-63 cells) (Fig. 2), suggesting that OSX inhibits
β-cat/Tcf signaling in human pre-osteoblastic and bone marrow
stromal cells. Based on these findings, it is likely that the
inhibitory effect of OSX on β-cat signaling is a feedback mechanism
for β-cat signaling-induced expression of OSX and that the dynamic
equilibrium between the two reverse processes may help determine
the direction of the osteogenic program. Verification of the
feedback mechanism and further exploration of its underlying
molecular mechanisms needs to be elaborated on in future
studies.
In conclusion, the findings of the present study
suggest that β-cat signaling upregulates the expression of OSX in
human pre-osteoblastic and bone marrow stromal cells by
transacti-vating the OSX gene promoter mainly through increased
c-Jun binding at a putative c-Jun binding site. In addition, our
data indicate that OSX largely mediates β-cat signaling-induced
osteoblastogenic differentiation. This study provides new insight
into the molecular mechanisms underlying osteoblast
differentiation.
References
|
1
|
Zhou X, Zhang Z, Feng JQ, Dusevich VM,
Sinha K, Zhang H, Darnay BG and de Crombrugghe B: Multiple
functions of Osterix are required for bone growth and homeostasis
in postnatal mice. Proc Natl Acad Sci USA. 107:12919–12924. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodda SJ and McMahon AP: Distinct roles
for Hedgehog and canonical Wnt signaling in specification,
differentiation and maintenance of osteoblast progenitors.
Development. 133:3231–3244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Cho K, Huang Y, Lyons JP, Zhou X,
Sinha K, McCrea PD and de Crombrugghe B: Inhibition of Wnt
signaling by the osteoblast-specific transcription factor Osterix.
Proc Natl Acad Sci USA. 105:6936–6941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timpson NJ, Tobias JH, Richards JB,
Soranzo N, Duncan EL, Sims AM, Whittaker P, Kumanduri V, Zhai G,
Glaser B, et al: Common variants in the region around Osterix are
associated with bone mineral density and growth in childhood. Hum
Mol Genet. 18:1510–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Styrkarsdottir U, Halldorsson BV,
Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T,
Jonsdottir T, Saemundsdottir J, Snorradóttir S, Center JR, et al:
New sequence variants associated with bone mineral density. Nat
Genet. 41:15–17. 2009. View
Article : Google Scholar
|
|
6
|
Chesire DR and Isaacs WB: Beta-catenin
signaling in prostate cancer: an early perspective. Endocr Relat
Cancer. 10:537–560. 2003. View Article : Google Scholar
|
|
7
|
Holmen SL, Zylstra CR, Mukherjee A, Sigler
RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL and Williams BO:
Essential role of beta-catenin in postnatal bone acquisition. J
Biol Chem. 280:21162–21168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Jiang YG: Podocalyxin promotes
glioblastoma multiforme cell invasion and proliferation via
β-catenin signaling. PLoS One. 9:e1113432014. View Article : Google Scholar
|
|
9
|
Cawthorn WP, Heyd F, Hegyi K and Sethi JK:
Tumour necrosis factor-alpha inhibits adipogenesis via a
beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ.
14:1361–1373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Xiong H, Kim TH, Ren B and Zhang Z:
Positive inter-regulation between beta-catenin/T cell factor-4
signaling and endothelin-1 signaling potentiates proliferation and
survival of prostate cancer cells. Mol Pharmacol. 69:520–531. 2006.
View Article : Google Scholar
|
|
11
|
Messeguer X, Escudero R, Farré D, Núñez O,
Martínez J and Albà MM: PROMO: detection of known transcription
regulatory elements using species-tailored searches.
Bioinformatics. 18:333–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farré D, Roset R, Huerta M, Adsuara JE,
Roselló L, Albà MM and Messeguer X: Identification of patterns in
biological sequences at the ALGGEN server: PROMO and MALGEN.
Nucleic Acids Res. 31:3651–3653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Zhou M, Brand J and Huang L:
Inflammation activates the interferon signaling pathways in taste
bud cells. J Neurosci. 27:10703–10713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson DR, Levanat S and Bale AE: Direct
molecular analysis of archival tumor tissue for loss of
heterozygosity. Biotechniques. 19:190–192. 1995.PubMed/NCBI
|
|
15
|
Baylan N, Bhat S, Ditto M, Lawrence JG,
Lecka-Czernik B and Yildirim-Ayan E: Polycaprolactone nanofiber
interspersed collagen type-I scaffold for bone regeneration: a
unique injectable osteogenic scaffold. Biomed Mater. 8(045011)2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stratford EW, Daffinrud J, Munthe E,
Castro R, Waaler J, Krauss S and Myklebost O: The
tankyrase-specific inhibitor JW74 affects cell cycle progression
and induces apoptosis and differentiation in osteosarcoma cell
lines. Cancer Med. 3:36–46. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferreira E, Porter RM, Wehling N,
O'Sullivan RP, Liu F, Boskey A, Estok DM, Harris MB, Vrahas MS,
Evans CH and Wells JW: Inflammatory cytokines induce a unique
mineralizing phenotype in mesenchymal stem cells derived from human
bone marrow. J Biol Chem. 288:29494–29505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ewan K, Pajak B, Stubbs M, Todd H, Barbeau
O, Quevedo C, Botfield H, Young R, Ruddle R, Samuel L, et al: A
useful approach to identify novel small-molecule inhibitors of
Wnt-dependent transcription. Cancer Res. 70:5963–5973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sikavitsas VI, Bancroft GN, Holtorf HL,
Jansen JA and Mikos AG: Mineralized matrix deposition by marrow
stromal osteoblasts in 3D perfusion culture increases with
increasing fluid shear forces. Proc Natl Acad Sci USA.
100:14683–14688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dias NJ and Selcer KW: Steroid sulfatase
mediated growth Sof human MG-63 pre-osteoblastic cells. Steroids.
88:77–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HK, Kim MG and Leem KH: Collagen
hydrolysates increased osteogenic gene expressions via a MAPK
signaling pathway in MG-63 human osteoblasts. Food Funct.
5:573–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vallet S, Pozzi S, Patel K, Vaghela N,
Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT,
et al: A novel role for CCL3 (MIP-1α) in myeloma-induced bone
disease via osteocalcin downregulation and inhibition of osteoblast
function. Leukemia. 25:1174–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halazonetis TD, Georgopoulos K, Greenberg
ME and Leder P: c-Jun dimerizes with itself and with c-Fos, forming
complexes of different DNA binding affinities. Cell. 55:917–924.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein GS and Lian JB: Molecular mechanisms
mediating developmental and hormone-regulated expression of genes
in osteoblasts: an integrated relationship of cell growth and
differentiation. Cellular and molecular biology of bone. Noda M:
Academic Press; Tokyo: pp. 47–95. 1993
|
|
25
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|