Introduction
T cell maturation is an important phase in the
development of an effective cell-mediated immune response. The
thymus is the organ where T cells differentiate and mature
(1). Two functional thymic
compartments, known as the cortex and the medulla, are important
for the positive selection of immature thymocytes and the deletion
of self-reactive T cell clones, respectively (2). It has been shown that thymic atrophy
occurs under several conditions. Since the first descriptions
(3,4), thymic involution has been
classically considered as an irreversible, inevitable age-related
deterioration process of this organ (5,6).
Although thymic atrophy has been observed in various diseases such
as graft-versus-host disease (GVHD) (7) and microbial infections (8), and it is affected by changes in
hormone production (9), the
mechanisms involved in this phenomenon remain to be elucidated.
We have studied for several years a model system of
a mammary tumor named D1-DMBA-3, developed in BALB/c mice by
D1-7,12-dimethylbenzanthracene by Medina and DeOme (10). An in vitro cell line
(DA-3), was derived in our laboratory from this in vivo
tumor (11). Our studies have
shown a profound tumor-associated immunosuppression (11) and a progressive thymic atrophy in
tumor-bearing mice (12). The
tumor-induced immunosuppression observed in our model is associated
in part with an accumulation of myeloid-derived suppressor cells in
several peripheral organs (13).
In addition, the mammary tumor cells used in these studies secreted
several molecules which are known to have effects on the immune
system (14–16). In a previous study, we observed a
decrease in the level of interferon (IFN)-γ in the circulation and
in its production by peripheral T cells, mainly due to the
downregulation of interleukin (IL)-12 (17). Tumor-induced thymic involution is
accompanied by a severe depletion of CD4+CD8+
double-positive (DP) immature thymocytes and an increase in the
percentages of CD4+CD8− and
CD4−CD8+ single-positive populations and
CD4−CD8− double-negative (DN) populations
(18). Previous studies from our
laboratory have shown that an early block in the maturation of the
thymus is associated with severe thymic atrophy in mammary tumor
bearers (12,18). In addition, there are changes in
the levels of crucial cytokines expressed in the thymic
microenvironment (19) and in
anti-apoptotic proteins (20), as
well as alterations in IFNs and Jak/Stat signaling pathways
(21).
Leptin is an adipocyte-derived peptide hormone that
plays a central role in regulating body weight by inhibiting food
intake and stimulating energy expenditure (22). In addition, leptin appears to
modulate innate and adaptive immunity by regulating CD4 Th cell
cytokine production, promoting the proliferation of naïve T cells
and regulating thymic development (23). Thymopoiesis is an essential
process for the development and maintenance of the immune system.
This process involves multiple thymocyte differentiation steps
(24). Maturing thymocytes
differentiate from the CD4/CD8 DN population into DP cells
(25). DP thymocytes undergo
further selection processes that involve MHC class II or MHC class
I restriction between immature thymocytes and thymic stromal cells
or other cells, such as macrophages and dendritic cells (26). Some soluble factors, such as IL-7,
human growth hormone (hGH), keratinocyte growth factor (KGF), and
thymic stromal-derived lymphopoietin (TSLP), can also positively
impact both thymopoiesis and thymocyte development (27).
It has been postulated that leptin can provide a
survival signal to developing immature thymocytes (23). In leptin-deficient obese mice, the
distribution of thymocyte subpopulations differs markedly from that
in wild-type mice. Upon the administration of leptin, however, the
imbalances in thymic subpopulations are rapidly recovered, with a
decrease in the percentage of DN thymocytes and an increase in DP
thymocytes (23). Thus, leptin
may significantly affect the differentiation of immature
thymocytes. Taking into consideration the leptin-mediated signaling
effects on cell survival and proliferation, it is plausible that
adipocyte infiltration and the expression of leptin in the thymuses
of tumor-bearing mice may be closely related to thymic involution.
In the present study, we explored this issue by using in
vivo and in vitro assay systems. Collectively, our data
suggest that the heavy infiltration of adipocytes and the presence
of leptin in the thymuses of tumor bearers may affect normal
thymopoiesis, leading to the impairment of T cell development
observed during tumor-induced thymic atrophy.
Materials and methods
Mice and tumor implantation
BALB/c mice (n=60) were bred and housed at the
Division of Veterinary Resources at the University of Miami Miller
School of Medicine, with full compliance to the USA Federal
Regulations and Institutional Policies. They were placed in cages
under pathogen-free conditions and allowed free access to food and
water. Two groups of 6 mice in each were tested in each experiment.
The groups were the normal control mice and mice that were
implanted with mammary tumors for 4 weeks. Mice (aged 10–14 weeks)
were used for tumor implantation. BALB/c mice were subcutaneously
injected with 1×106 tumor cells in 0.9% saline. The
D1-DMBA-3 tumor is a transplantable mammary adenocarcinoma, derived
from a non-viral, non-carcinogen-induced preneoplastic nodule in a
BALB/c mouse treated with 7,12-dimethylbenzanthracene (10). The D1-DMBA-3 tumor (10) was a generous gift from Dr Daniel
Medina and we have maintained and used it in vivo and in vitro in
our laboratory for over 25 years. The immunogenic D1-DMBA-3 tumor
was routinely transplanted into BALB/c mice by the subcutaneous
injection of tumor cells as previously described (12). Palpable tumors were apparent
within approximately 8 days following implantation and the mice
were sacrificed by cervical dislocation 4 weeks following tumor
implantation. All experiments were carried out according to the
guidelines of the Animal Care and Use Committee of the University
of Miami Miller School of Medicine, Miami, FL. USA.
Thymus collection and histological
analysis
The mice were sacrificed and both lobes of the
thymus were carefully dissected from the chest cavity. Histological
examinations were performed after fixing the thymic lobes in 10%
neutral-buffered formalin and embedding the tissues in paraffin. To
assess adipocyte infiltration, the fixed thymic tissues were
stained with Oil Red O (Sigma-Aldrich, St. Louis, MO, USA)
according to the manufacturer's instructions. In other experiments,
the thymuses were placed in a petri dish containing 1X Hanks'
balanced salt solution, 1% calf serum, 10 mM HEPES, pH 7.2. The
thymic lobes were weighed and placed in a cell strainer in a petri
dish with a drop of medium on the top, and gently compressed with
the base of a 3 ml syringe followed by a wash with cold medium and
were then transferred to polypropylene tubes. Cells were counted
before being used in the various assays described below.
Analysis of mRNA expression
The Mouse Common Cytokines PCR Array was purchased
from Qiagen (Valencia, CA, USA). Total RNA was extracted from the
entire thymus using TRIzol (ThermoFisher Scientific, Grand Island,
NY, USA) using a tissue homogenizer from Omni International
(Marietta, GA, USA). cDNA was prepared from this total RNA and
hybridized to the arrayed filters according to the manufacturer's
instructions. The resulting hybridization signal was visualized by
chemiluminescence. Data were subjected to densitometric analysis
using Scion Image Software (Scion, Frederick, MD, USA). RNA levels
were expressed as relative optical density (OD) measurements after
normalizing to the hybridization signals to GAPDH that served as a
control.
Western blot analysis
Whole-cell extracts from thymuses of the normal and
tumor-bearing mice were used. The thymuses were lysed using cold
RIPA assay buffer supplemented with protease inhibitor cocktail
tablets and sodium vanadate (1 mmol/l final concentration) (both
from Roche, Indianapolis, IN, USA). The protein concentration was
determined using the BCA protein assay kit (Pierce Biotechnology
Inc., Rockford, IL, USA) before analyzing the samples by western
blot analysis as previously described (19). The primary antibodies used were
two rabbit polyclonal antibodies, anti-leptin (Cat. no. 500-P68;
PeProtech, Rocky Hill, NJ, USA) and anti-adiponectin (Cat. no.
2789; Cell Signaling Technology, Inc., Danvers. MA, USA). The
levels of β-actin (Cat. no. A1978; Sigma-Aldrich, St. Louis, MO,
USA) were detected by a rabbit anti-mouse polyclonal antibody
(Sigma-Aldrich). The secondary antibody was a peroxidase-conjugated
donkey anti-goat IgG from Jackson ImmunoResearch (West Grove, PA,
USA). Visualization of the complexes was performed with the
chemiluminescence method (West Pico Chemiluminescent Substrate;
Pierce Biotechnology Inc.) and the membranes were exposed to X-ray
film. The films were scanned and the data was subjected to
densitometric analysis using Scion Image Software (NIH). The
protein levels were normalized to the hybridization signals of
β-actin, and reported as the relative intensity.
Ex vivo isolation of adipocytes
Adipocytes were separated from other cell types
present in the thymuses of the tumor-bearing mice by enzymatic
digestion of the tissue with collagenase. Briefly, approximately
10–15 thymuses from the tumor-bearing were minced into small pieces
(~1 mm) and incubated in 4 volumes of 1 mg/ml collagenase IV
(Worthington Biochemical Corp., Lakewood, NJ, USA) in
phosphate-buffered saline (PBS) for 30 min at 37°C. The samples
were centrifuged at 600 × g for 2 min to obtain a floating fraction
of adipocytes. The ex vivo isolated adipocytes were counted
and mixed with thymocytes from the normal mice as described
below.
Cell culture
The isolated total thymocytes from the normal mice
(5×105 cells/well) were cultured in 0.2 ml of RPMI-1640
containing 5% fetal calf serum, glutamine (30 µg/ml),
penicillin (100 U/ml), streptomycin (100 µg/ml), and
2-mercap-toethanol (5×10−5 M) in 96-well flat-bottom
culture plates (Costar, Cambridge, MA, USA), with the addition of
anti-CD3 (1 µg/ml) and anti-CD28 (3 µg/ml). In some
experiments, the thymic T cells were left untreated (controls) or
treated with 50 or 100 ng of leptin prior to incubation for 48 h
before collecting the culture supernatants. In co-culture
experiments, thymocytes from normal mice were cultured alone
(controls) or with thymic adipocytes isolated from tumor bearers as
described above. When the ex vivo isolated adipocytes were
used, the floating fraction of adipocytes was isolated from
thymocytes as described above, and 5×105 adipocytes from
this fraction were mixed with 5×105 thymocytes.
Co-cultures were carried out for 48 h, and the conditioned medium
was harvested, centrifuged and the supernatants were frozen at
−80°C for further analyses.
Cytokine enzyme-linked immunosorbent
assay (ELISA)
The amounts of IFN-γ, IL-2 and
granulocyte-macrophage colony-stimulating factor (GM-CSF) were
analyzed using standard ELISA kits (BD Biosciences, San Diego, CA,
USA) according to the manufacturer's instructions. The amounts of
the cytokine present in each well were quantified by measuring the
absorbance at 450–550 nm using a Tecan SLT Rainbow Reader (SLT Lab
Instruments, Research Triangle Park, NC, USA). The OD values were
converted to pg/ml by including dilutions of known amounts of
recombinant murine cytokines in the ELISA. A standard curve was
generated by plotting the OD value of the standards versus their
known cytokine concentration.
Data analysis
Statistical evaluations were conducted using the
Student's t-test. Error bars represent the standard error of the
mean (SEM), and all p-values were two-sided. A probability value of
p≤0.05 considered to indicate a statistically significant
difference.
Results
In previous studies of ours, we described profound
alterations in the thymuses of tumor-bearing mice (12,18). Thus, while the thymuses from the
normal mice exhibited discrete cortical and medullar zones, the
mice implanted with D1-DMBA-3 tumors displayed progressively
reduced thymuses with the loss of demarcation between the cortex
and the medulla (19). In order
to determine the possible presence of adipocytes, histological
analyses of paraffin-embedded thymic lobes from the normal and
tumor-bearing mice were stained with Oil Red O as described in the
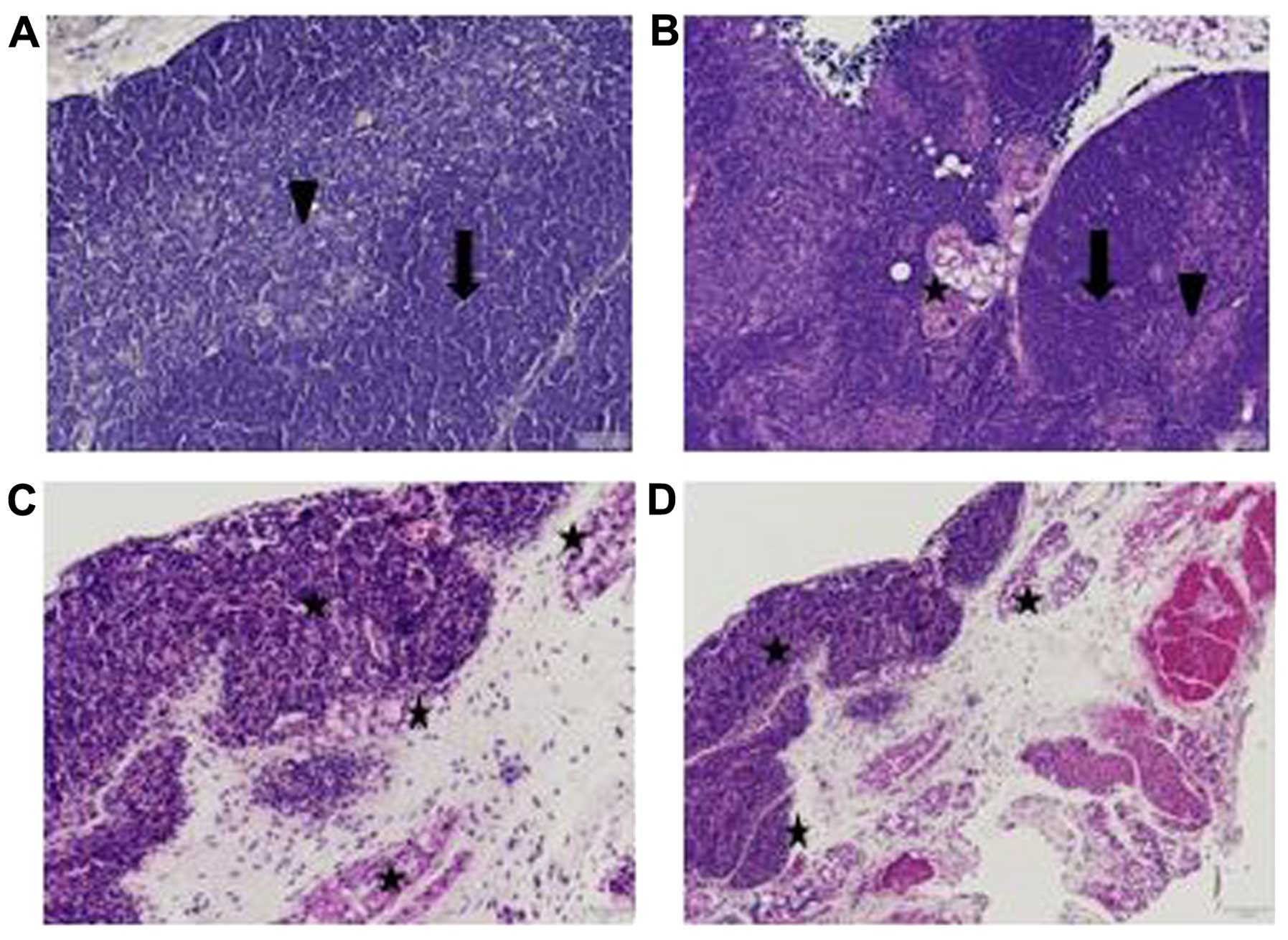
Materials and methods. As shown in Fig. 1A (×100 magnification),
histopathological analysis of the thymuses from a normal mouse
thymus revealed a well-defined cortex with a thick layer of
thymocytes and a distinct medullar zone. By contrast, as shown in
Fig. 1B (×100 magnification),
profound thymic disruption was observed in a tumor-bearing mouse at
4 weeks and the accumulation of adipocytes appeared in the tissue
surrounding the thymus. In addition, as can be clearly observed at
a higher magnification (×400), the accumulation of adipocytes did
not occur solely outside the organ, but was also found inside the
thymuses of the tumor-bearing mice (Fig. 1C and D) as detected by the red
staining of these cells.
Two adipokines that are prominently produced by
adipocytes are adiponectin (28),
which tends to have anti-inflammatory properties, and leptin, which
tends to have pro-inflammatory properties (29). Experiments were conducted to
determine the expression of these two molecules, which may be
involved in thymic involution in tumor-bearing mice, due to the
abnormal functioning of the stromal cell microenvironment in this
organ. No changes in the levels of adiponectin were observed in the
thymuses of the normal and tumor-bearing mice (data not shown). The
expression of leptin in the thymuses was detected by western blot
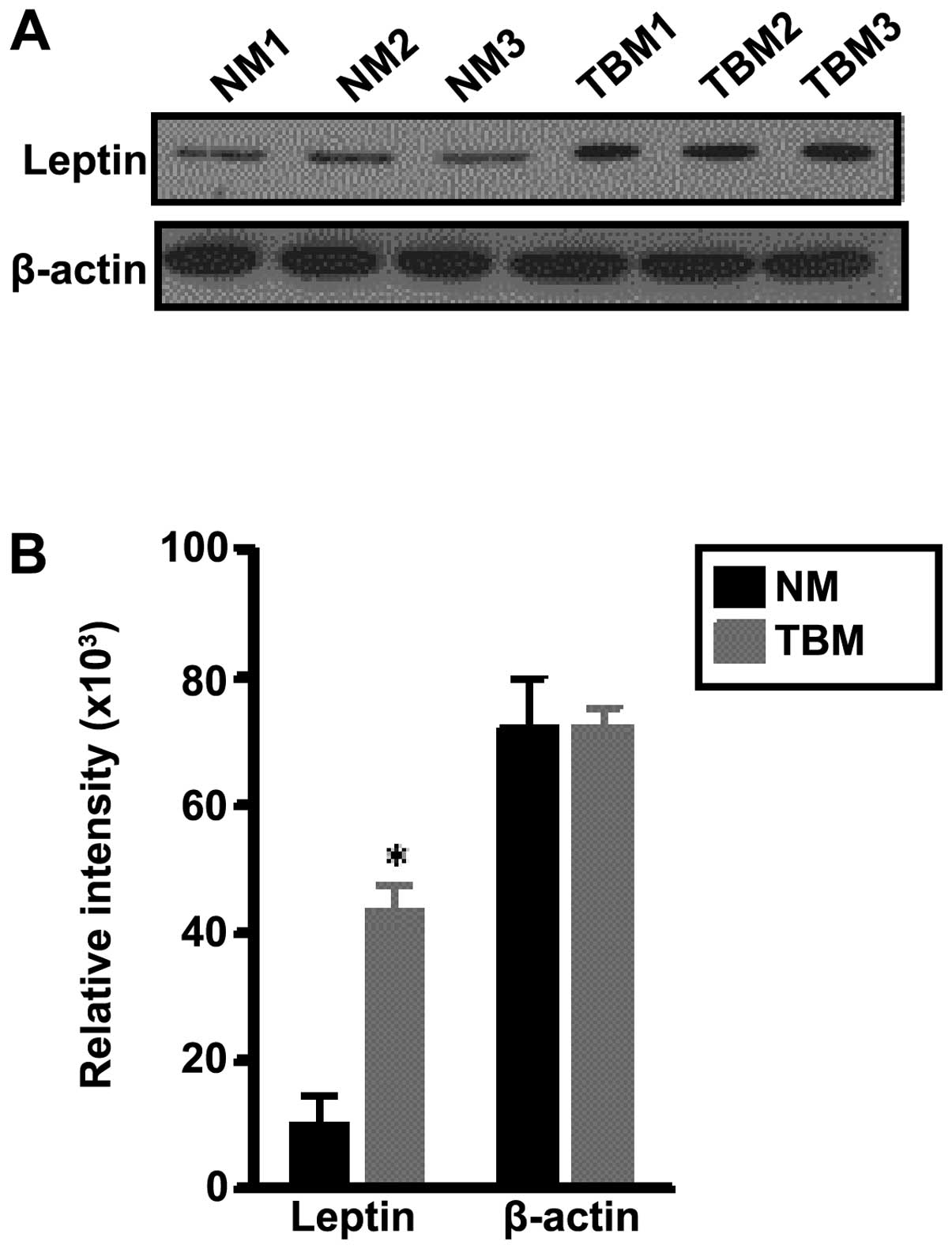
analysis and β-actin was used as a loading control. Fig. 2A shows one of two experiments in
which the leptin protein levels of normal mice and tumor bearers
were investigated. It can be observed that the thymuses of the
tumor-bearing mice (TBM1, TBM2 and TBM3) expressed higher levels of
leptin than the thymuses from normal mice (NM1, NM2 and NM3).
Densitometric analyses were performed to determine the relative
intensity of the adipokine in the thymuses of the two types of
mice. Fig. 2B is based on two
western blot anlaysis experiments with a total of 6 individual
animals per group. The levels of leptin were significantly higher
in the thymuses from the tumor-bearing than in the thymuses from
normal mice, while the levels of β-actin remained unaltered. These
findings suggest that the heavy presence of adipocytes may be
linked to the overexpression of leptin in the thymuses of tumor
bearers, and may thus alter the functioning of the thymuses.
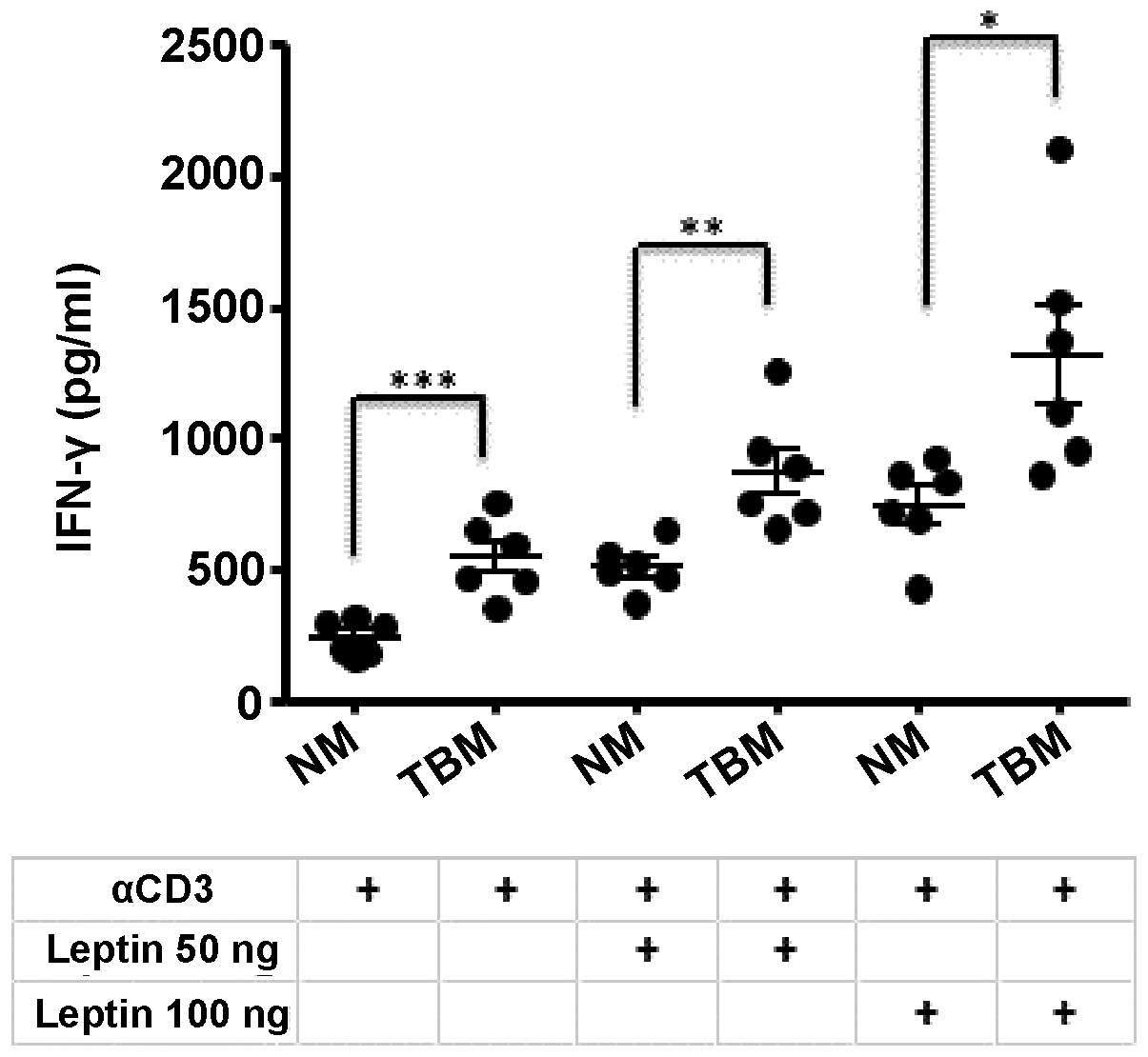
We have previously reported an upregulation of IFN-γ
levels in the thymocytes of tumor-bearing mice (21). In the present study, experiments
were performed to evaluate whether the increased expression of
leptin in the thymuses from tumor-bearing mice may affect this
elevation of IFN-γ levels. Thymocytes from normal and tumor-bearing
mice were cultured for 48 h in the presence of IL-2 and
anti-CD3/anti-CD28 as co-stimulators, and with or without the
addition of leptin. The leptin concentrations used were selected
based on the levels of leptin in the serum of BALB/c mice bearing
4T1 mammary tumors, as previously described by Kim et al
(30). The protein expression of
IFN-γ in the untreated and in the leptin-treated cultures is shown
in Fig. 3. Untreated thymocytes
from the thymuses of tumor-bearing mice (TBM) had higher levels of
IFN-γ than those from normal mice (NM), as previously described
(19,21). The addition of 50 ng leptin
increased the expression level of IFN-γ in both normal and
tumor-bearing mice, and the addition of 100 ng leptin further
increased the production of this cytokine. These results indicate
that leptin may be involved in the thymic changes occurring during
mammary tumorigenesis.
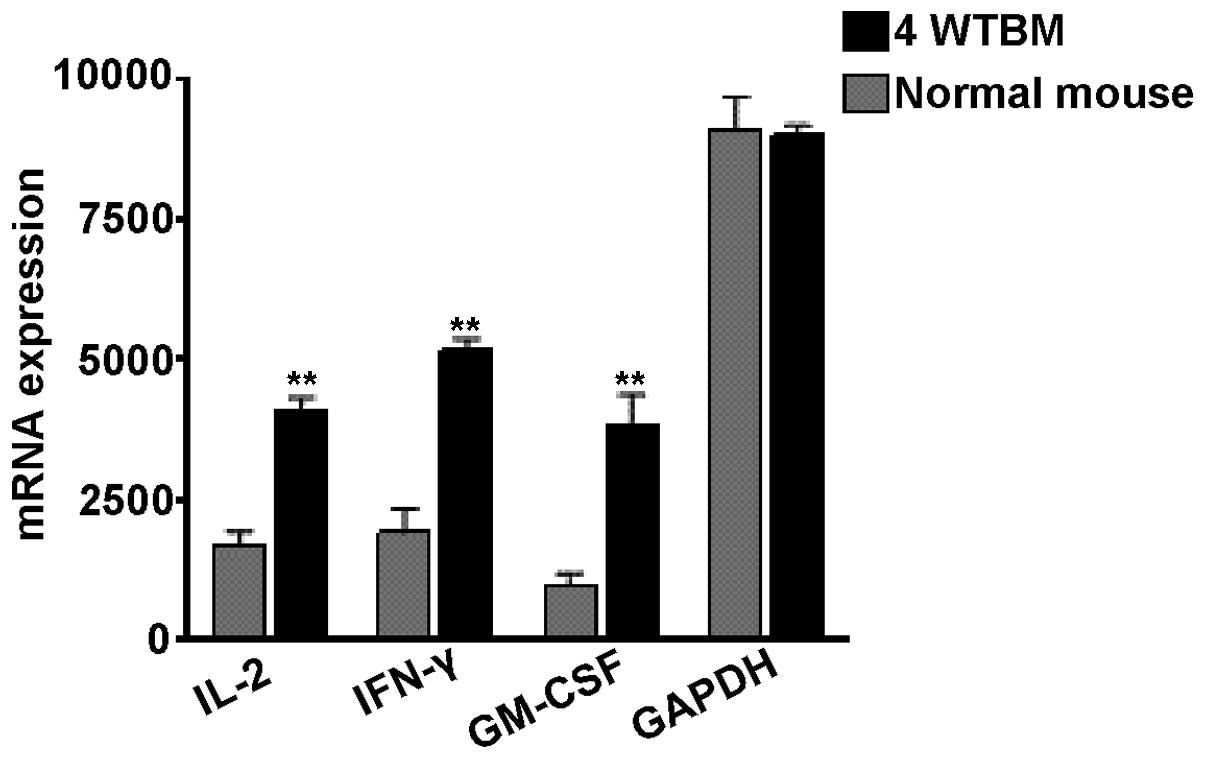
Leptin has been shown to modulate the production of
pro-inflammatory molecules (23).
Since this molecule is overproduced in the thymuses of tumor
bearers, we analyzed the mRNA levels of GM-CSF and those of the Th1
cell cytokines, IL-2 and IFN-γ. We decided to examine the effects
of these molecules based on the following reasons: Th cells are
very important for the activation of immune responses, and IL-2 and
IFN-γ are two important cytokines produced by these lymphocytes
(31). GM-CSF is expressed by
several types of immune cells, including dendritic cells,
macrophages and helper and cytotoxic T lymphocytes. The mammary
tumor cells used in our experiments have been shown to produce high
levels of GM-CSF (11,16) and to induce the expansion of
immunoregulatory macrophages (13). In addition, a novel subset of Th
cells named ThGM cells has been described, which promotes immune
responses by secreting GM-CSF (32). As shown in Fig. 4, the mRNA expression of the three
factors tested was found to be elevated in the thymuses of
tumor-bearing mice as compared with those from normal mice.
The experiments described above suggest that the
high numbers of adipocytes that infiltrated the thymuses of
tumor-bearing mice may be influencing the changes in the
microenvironment that appear to be involved in thymic involution.
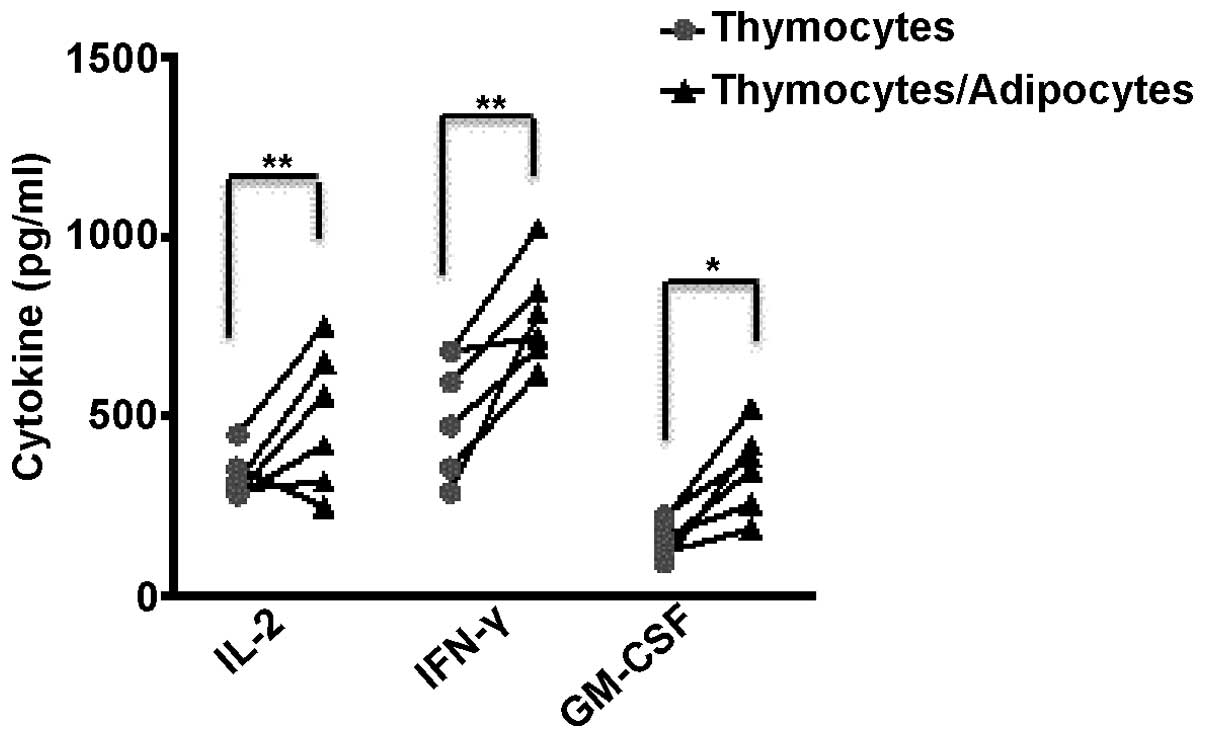
Thymocytes from normal mice were cultured by themselves or
co-cultured with the ex vivo isolated adipocytes. GM-CSF,
IL-2 and IFN-γ cytokine expression levels in the thymic cells from
6 individual normal mice were measured by ELISA following 48 h of
incubation. An increase in the levels of IFN-γ and GM-CSF was
observed in the 6 individual cell cultures that contained
adipocytes (Fig. 5). A total of 4
of the 6 samples co-cultured with the ex vivo isolated
adipocytes also had increased levels of IL-2. These results suggest
that the accumulation of adipocytes in the thymuses of tumor
bearers modifies the cytokine profiles observed in these
organs.
Discussion
The increased infiltration of adipocytes has been
shown to be associated with thymic atrophy in aging (33). Indeed, it has been reported that
with increasing age, adipocytes constitute the majority of cells in
the thymic space, while the number of thymocytes substantially
decreases (34,35). Furthermore, there is evidence that
progenitor lymphoid cells from young mice develop into
non-functional T lymphocytes when they are exposed to and come into
contact with aging thymic cells (36). Although thymics involution has
been shown to occur in obesity (37), infections (8) and cancer (12,38) the accumulation of thymic
adipocytes under these conditions has not been carefully analyzed
to date, to the the best of our knowledge.
Adipose tissues and adipocytes are known to be
important sources of pro-inflammatory cytokines as reviewed by
Gilbert and Slingerland (39). It
has also been shown that adipocytes have a direct impact on immune
cells (40,41). In a previous study, the number of
bone marrow adipocytes increased following treatment with
chemotherapy or following irradiation and the adipocytes acted as
negative regulators of the hematopoietic microenvironment (42). Vielma et al (40) demonstrated that medium conditioned
with adipocytes stimulated the release of certain cytokines, such
as IL-2, IFN-γ and GM-CSF from the cultures of normal spleen cells.
In our study, we also found elevated levels of these three
cytokines in the thymocytes of tumor bearing-mice in comparison
with the levels observed in the thymic cells of normal mice
(Fig. 4), where there was no
heavy infiltration of adipocytes. Furthermore, the co-culture of
normal thymocytes with adipocytes isolated from the thymuses of
tumor-bearing mice for 48 h also resulted in increased levels of
IL-2, IFN-γ and GM-CSF (Fig. 5).
We have previously described that the levels of IFN-γ are elevated
in the thymic cells of tumor bearing-mice (21). This finding appeared to be
paradoxical, as we have shown in several previous studies that
tumor-bearing mice have an overall decreased level of IFN-γ in the
circulation (17,38). However, it is important to
emphasize that thymic involution is associated with a severe
depletion of DP CD4+CD8+ thymocytes due to an
arrest at an early stage of differentiation (18). By contrast, the percentages of DN
CD4−CD8− thymocytes remains largely
unaltered, while the number of single positive CD4+ and
CD8+ cells increases. Since after 4 weeks of tumor
implantation the remaining thymic cells in the involuted organs are
approximately 5% of the normal numbers, the composition of
thymocytes is quite different in tumor-bearing mice with an
increase in the number of single positive cells (18). Indeed, it has been shown that in
aging, an expanded peripheral CD8+ T cell population
correlates with an increase in IFN-γ production (41).
The present study found that the expression of
leptin was higher in the thymuses of tumor-bearing mice than in
normal mice as measured by western blot analysis (Fig. 2). Leptin is an adipokine that has
multiple effects in obesity (43), as well as the neuroendocrine and
reproductive systems (44), among
others. Recently, it has been shown to be an important modulator of
the immune system (23). Thymic
development has been proposed to be positively influenced by leptin
levels (45). Of note, Dixit
et al (46) demonstrated
that leptin infusion into aged, but not young mice, significantly
enhanced thymopoiesis. In our model system, elevated levels of this
adipokine correlated with advanced thymic involution. However, we
have found previously that there is a profound decrease in
hepatocyte growth factor (HGF) in the thymuses of tumor bearers
(19). In connection with this,
Yamaji et al (47)
demonstrated that leptin inhibited the HGF-induced ductal
morphogenesis of bovine mammary epithelial cells. Thus, this
adipokine may be causing the decrease in HGF that is associated
with mammary tumor thymic involution, that we have previously shown
is reversible, by restoring the normal levels of this growth factor
(19). In future studies, we plan
to extensively analyze the possible effects of leptin on the
expression of thymic HGF in tumor bearers. In this study, we
demonstrated that high leptin levels are associated with a heavy
infiltration of adipocytes into and around the involuted thymuses;
thus, it appears that these two factors are important contributors
to the thymic atrophy observed in tumor-bearing mice.
Acknowledgments
This study was supported by grants from the Florida
Breast Cancer Foundation and from the Cancer Link.
References
|
1
|
Gill J, Malin M, Sutherland J, Gray D,
Hollander G and Boyd R: Thymic generation and regeneration. Immunol
Rev. 195:28–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahama Y: Journey through the thymus:
stromal guides for T-cell development and selection. Nat Rev
Immunol. 6:127–135. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyd E: The weight of the thymus gland in
health and disease. Am J Dis Child. 43:1162–1214. 1932.
|
|
4
|
Metcalf D, Moulds R and Pike B: Influence
of the spleen and thymus on immune responses in ageing mice. Clin
Exp Immunol. 2:109–120. 1967.PubMed/NCBI
|
|
5
|
Aspinall R and Andrew D: Thymic involution
in aging. J Clin Immunol. 20:250–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linton PJ and Dorshkind K: Age-related
changes in lymphocyte development and function. Nat Immunol.
5:133–139. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lapp WS, Ghayur T, Mendes M, Seddik M and
Seemayer TA: The functional and histological basis for
graft-versus-host-induced immunosuppression. Immunol Rev.
88:107–133. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haynes BF, Markert ML, Sempowski GD, Patel
DD and Hale LP: The role of the thymus in immune reconstitution in
aging, bone marrow transplantation, and HIV-1 infection. Annu Rev
Immunol. 18:529–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montecino-Rodriquez E, Min H and Dorshkind
K: Reevaluating current models of thymic involution. Semin Immunol.
17:356–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medina D and DeOme KB: Response of
hyperplastic alveolar nodule outgrowth-line D1 to mammary tumor
virus, nodule-inducing virus, and prolonged hormonal stimulation
acting singly and in combination. J Natl Cancer Inst. 42:303–310.
1969.PubMed/NCBI
|
|
11
|
Sotomayor EM, Fu YX, Lopez-Cepero M,
Herbert L, Jimenez JJ, Albarracin C and Lopez DM: Role of
tumor-derived cytokines on the immune system of mice bearing a
mammary adenocarcinoma. II. Down-regulation of macrophage-mediated
cytotoxicity by tumor-derived granulocyte-macrophage
colony-stimulating factor. J Immunol. 147:2816–2823.
1991.PubMed/NCBI
|
|
12
|
Fu YX, Altman N and Lopez DM: Thymic
atrophy induced by murine mammary adenocarcinoma in vivo. In Vivo.
3:1–5. 1989.PubMed/NCBI
|
|
13
|
Ilkovitch D and Lopez DM: The liver is a
site for tumor-induced myeloid-derived suppressor cell accumulation
and immunosuppression. Cancer Res. 69:5514–5521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watson GA, Fu YX and Lopez DM: Splenic
macrophages from tumor-bearing mice co-expressing MAC-1 and MAC-2
antigens exert immunoregulatory functions via two distinct
mechanisms. J Leukoc Biol. 49:126–138. 1991.PubMed/NCBI
|
|
15
|
Ilkovitch D, Carrio R and Lopez DM:
Mechanisms of antitumor and immune-enhancing activities of
MUC1/sec, a secreted form of mucin-1. Immunol Res. 57:70–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu YX, Watson G, Jimenez JJ, Wang Y and
Lopez DM: Expansion of immunoregulatory macrophages by
granulocyte-macrophage colony-stimulating factor derived from a
murine mammary tumor. Cancer Res. 50:227–234. 1990.PubMed/NCBI
|
|
17
|
Handel-Fernandez ME, Cheng X, Herbert LM
and Lopez DM: Down-regulation of IL-12, not a shift from a T
helper-1 to a T helper-2 phenotype, is responsible for impaired
IFN-gamma production in mammary tumor-bearing mice. J Immunol.
158:280–286. 1997.PubMed/NCBI
|
|
18
|
Adkins B, Charyulu V, Sun QL, Lobo D and
Lopez DM: Early block in maturation is associated with thymic
involution in mammary tumor-bearing mice. J Immunol. 164:5635–5640.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrio R, Altman NH and Lopez DM:
Downregulation of interleukin-7 and hepatocyte growth factor in the
thymic microenvironment is associated with thymus involution in
tumor-bearing mice. Cancer Immunol Immunother. 58:2059–2072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carrio R and Lopez DM: Impaired
thymopoiesis occurring during the thymic involution of
tumor-bearing mice is associated with a down-regulation of the
antiapoptotic proteins Bcl-XL and A1. Int J Mol Med. 23:89–98.
2009.
|
|
21
|
Carrio R, Torroella-Kouri M,
Iragavarapu-Charyulu V and Lopez DM: Tumor-induced thymic atrophy:
alteration in interferons and Jak/Stats signaling pathways. Int J
Oncol. 38:547–553. 2011.
|
|
22
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Procaccini C, Jirillo E and Matarese G:
Leptin as an immunomodulator. Mol Aspects Med. 33:35–45. 2012.
View Article : Google Scholar
|
|
24
|
Miller JF: The golden anniversary of the
thymus. Nat Rev Immunol. 11:489–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciofani M and Zúñiga-Pflücker JC: The
thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev
Biol. 23:463–493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carpenter AC and Bosselut R: Decision
checkpoints in the thymus. Nat Immunol. 11:666–673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Germain RN: T-cell development and the
CD4-CD8 lineage decision. Nat Rev Immunol. 2:309–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Bao HG, Han L, Liu L and Wang X:
Effects of adiponectin on mortality and its mechanism in a sepsis
mouse model. J Invest Surg. 25:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Canavan B, Salem RO, Schurgin S, Koutkia
P, Lipinska I, Laposata M and Grinspoon S: Effects of physiological
leptin administration on markers of inflammation, platelet
activation, and platelet aggregation during caloric deprivation. J
Clin Endocrinol Metab. 90:5779–5785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EJ, Choi MR, Park H, Kim M, Hong JE,
Lee JY, Chun HS, Lee KW and Yoon Park JH: Dietary fat increases
solid tumor growth and metastasis of 4T1 murine mammary carcinoma
cells and mortality in obesity-resistant BALB/c mice. Breast Cancer
Res. 13:R782011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Roberts AI, Liu C, Ren G, Xu G,
Zhang L, Devadas S and Shi Y: A novel subset of helper T cells
promotes immune responses by secreting GM-CSF. Cell Death Differ.
20:1731–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Youm YH, Yang H, Sun Y, Smith RG, Manley
NR, Vandanmagsar B and Dixit VD: Deficient ghrelin
receptor-mediated signaling compromises thymic stromal cell
microenvironment by accelerating thymic adiposity. J Biol Chem.
284:7068–7077. 2009. View Article : Google Scholar :
|
|
34
|
Yang H, Youm YH and Dixit VD: Inhibition
of thymic adipogenesis by caloric restriction is coupled with
reduction in age-related thymic involution. J Immunol.
183:3040–3052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixit VD: Adipose-immune interactions
during obesity and caloric restriction: reciprocal mechanisms
regulating immunity and health span. J Leukoc Biol. 84:882–892.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clise-Dwyer K, Huston GE, Buck AL, Duso DK
and Swain SL: Environmental and intrinsic factors lead to antigen
unresponsiveness in CD4(+) recent thymic emigrants from aged mice.
J Immunol. 178:1321–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang H, Youm YH, Vandanmagsar B, Ravussin
A, Gimble JM, Greenway F, Stephens JM, Mynatt RL and Dixit VD:
Obesity increases the production of proinflammatory mediators from
adipose tissue T cells and compromises TCR repertoire diversity:
implications for systemic inflammation and insulin resistance. J
Immunol. 185:1836–1845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carrio R and Lopez DM: Insights into
thymic involution in tumor-bearing mice. Immunol Res. 57:106–114.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gilbert CA and Slingerland JM: Cytokines,
obesity, and cancer: new insights on mechanisms linking obesity to
cancer risk and progression. Annu Rev Med. 64:45–57. 2013.
View Article : Google Scholar
|
|
40
|
Vielma SA, Klein RL, Levingston CA and
Young MR: Adipocytes as immune regulatory cells. Int
Immunopharmacol. 16:224–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koethe JR, Hulgan T and Niswender K:
Adipose tissue and immune function: a review of evidence relevant
to HIV infection. J Infect Dis. 208:1194–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bandrés E, Merino J, Vázquez B, Inogés S,
Moreno C, Subirá ML and Sánchez-Ibarrola A: The increase of
IFN-gamma production through aging correlates with the expanded
CD8(+high)CD28(−) CD57(+) subpopulation. Clin Immunol. 96:230–235.
2000. View Article : Google Scholar
|
|
43
|
Sáinz N, Barrenetxe J, Moreno-Aliaga MJ
and Martínez JA: Leptin resistance and diet-induced obesity:
central and peripheral actions of leptin. Metabolism. 64:35–46.
2015. View Article : Google Scholar
|
|
44
|
Zhang F, Chen Y, Heiman M and Dimarchi R:
Leptin: structure, function and biology. Vitam Horm. 71:345–372.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gruver AL and Sempowski GD: Cytokines,
leptin, and stress-induced thymic atrophy. J Leukoc Biol.
84:915–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dixit VD, Yang H, Sun Y, Weeraratna AT,
Youm YH, Smith RG and Taub DD: Ghrelin promotes thymopoiesis during
aging. J Clin Invest. 117:2778–2790. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamaji D, Kamikawa A, Soliman MM, Ito T,
Ahmed MM, Makondo K, Watanabe A, Saito M and Kimura K: Leptin
inhibits hepatocyte growth factor-induced ductal morphogenesis of
bovine mammary epithelial cells. Jpn J Vet Res. 54:183–189.
2007.PubMed/NCBI
|