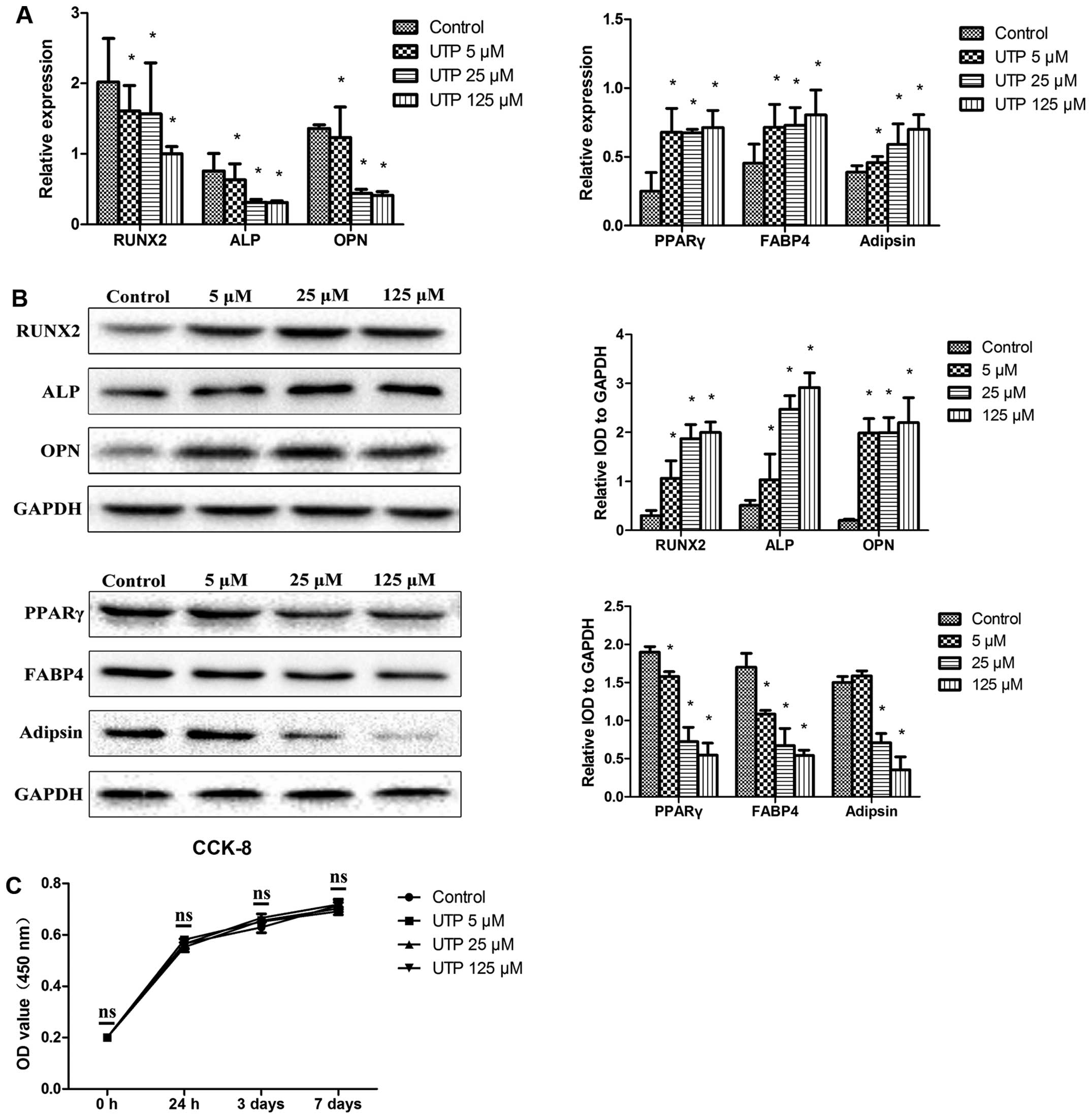
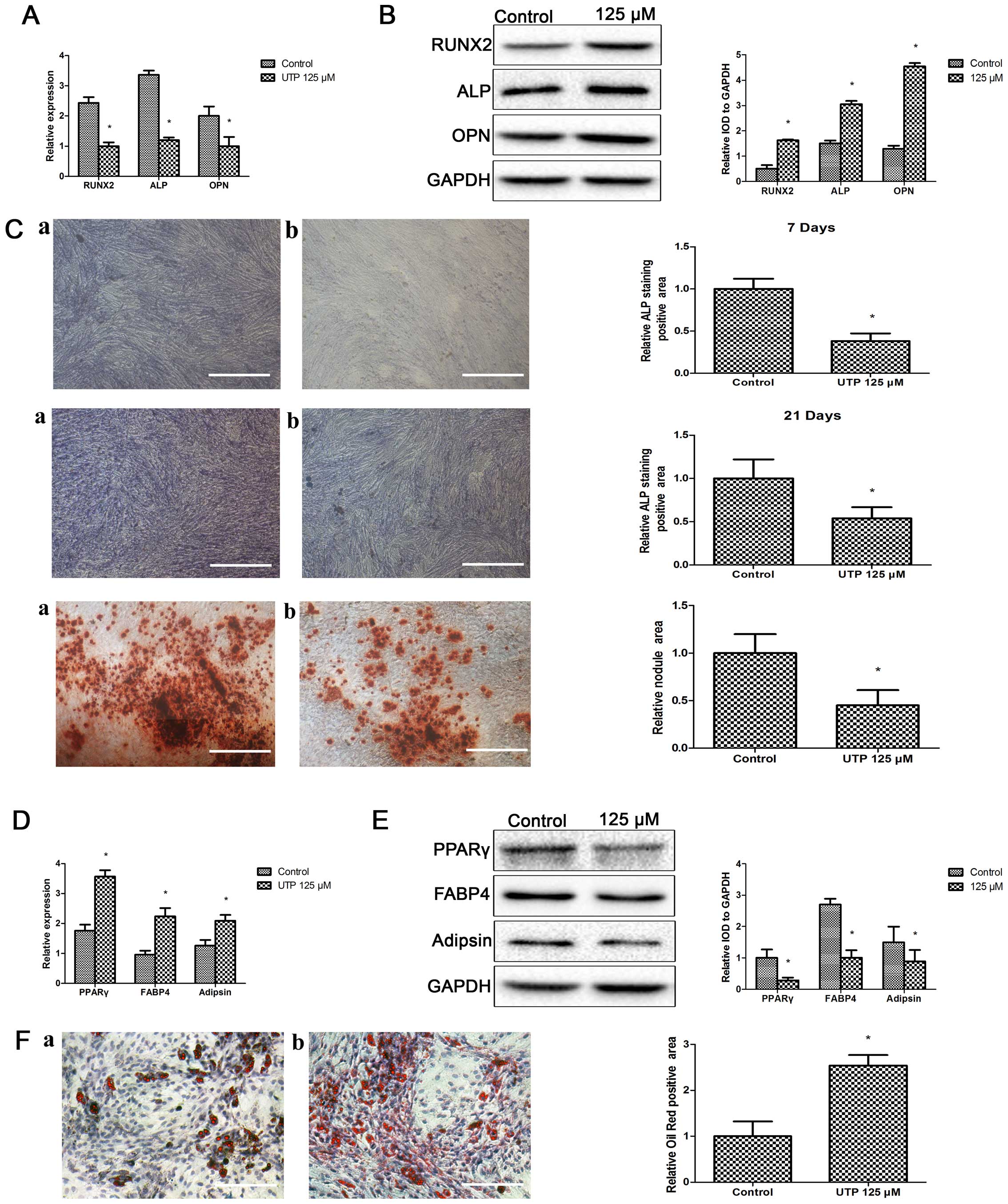
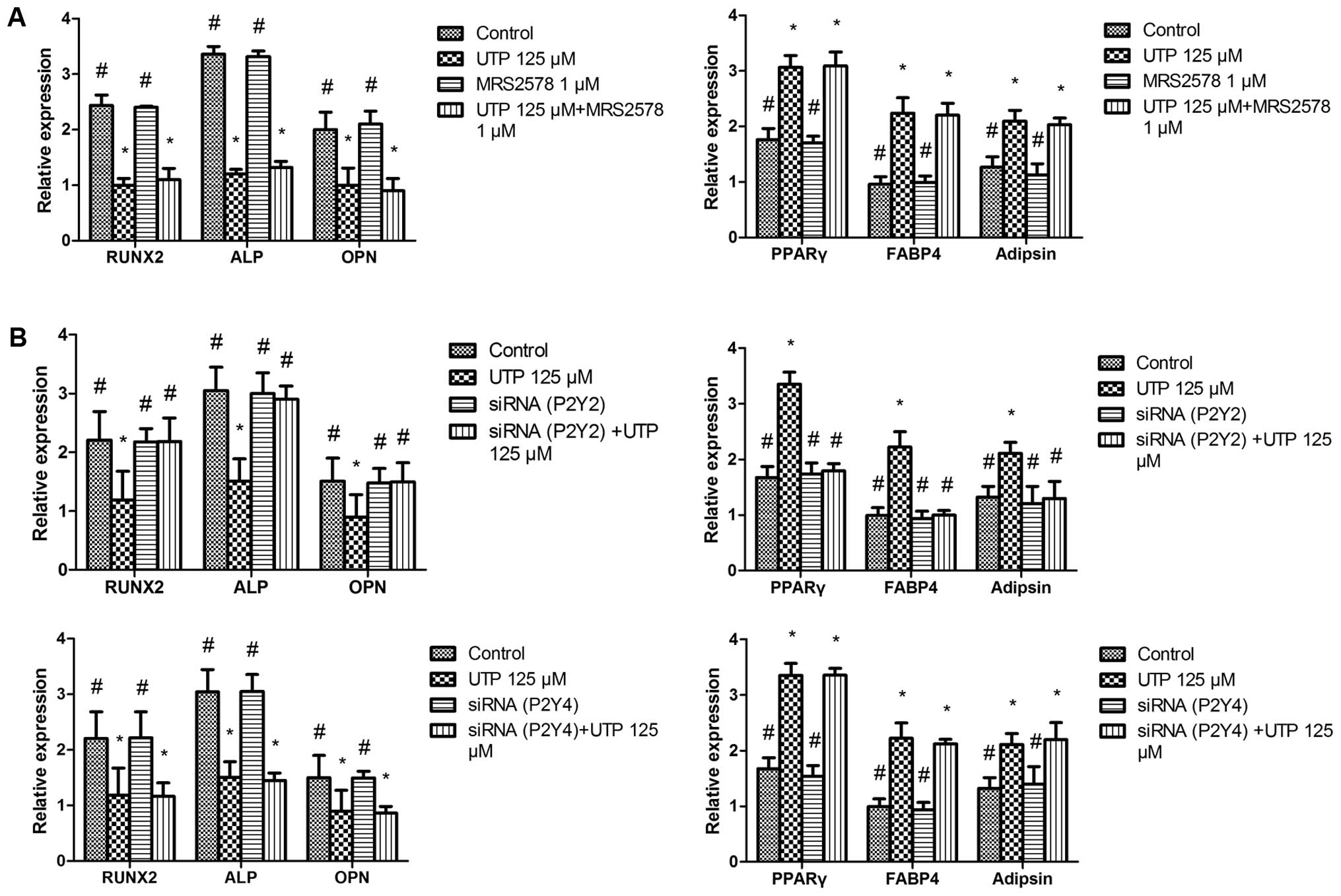
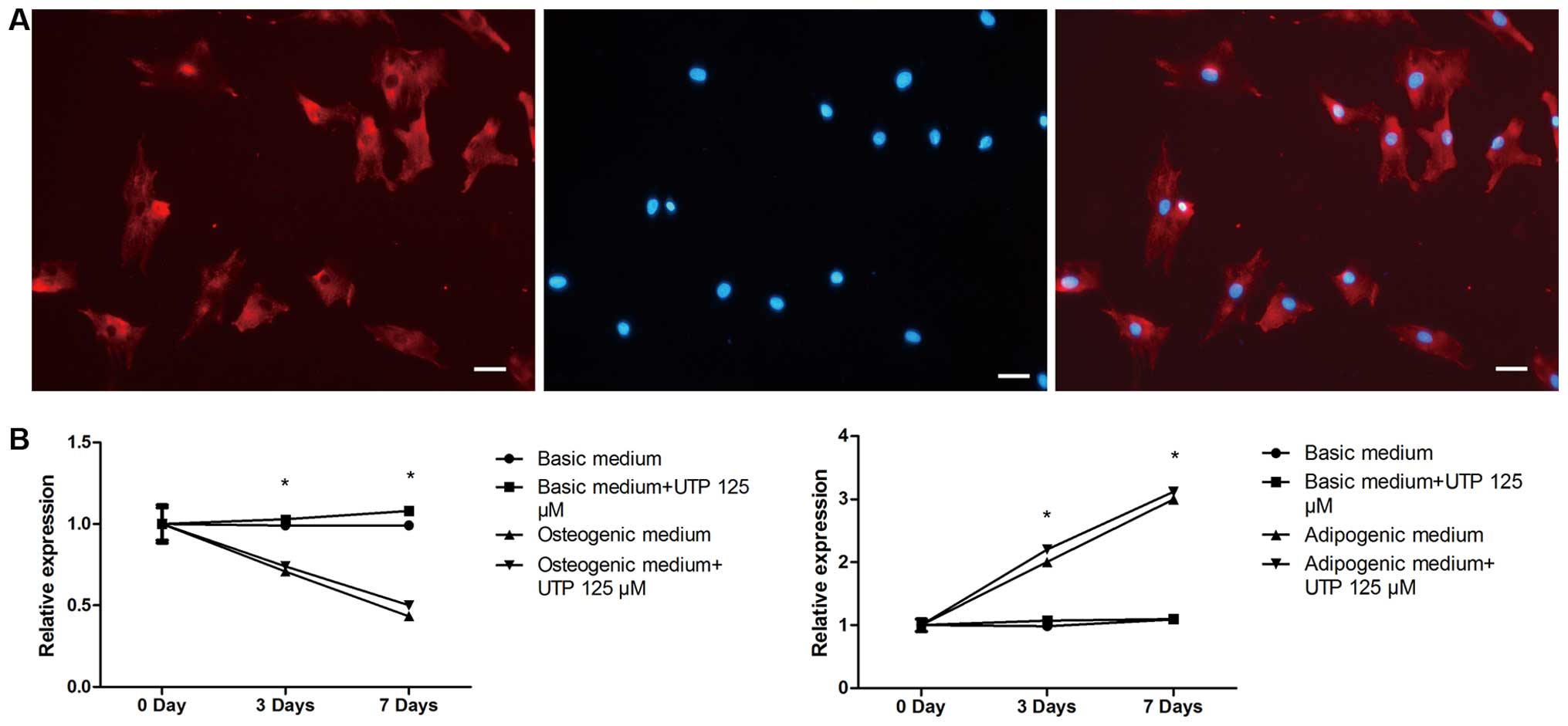
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hess R, Pino AM, Ríos S, Fernández M and
Rodríguez JP: High affinity leptin receptors are present in human
mesenchymal stem cells (MSCs) derived from control and osteoporotic
donors. J Cell Biochem. 94:50–57. 2005. View Article : Google Scholar
|
|
3
|
Dalle Carbonare L, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egermann M, Heil P, Tami A, Ito K, Janicki
P, Von Rechenberg B, Hofstetter W and Richards PJ: Influence of
defective bone marrow osteogenesis on fracture repair in an
experimental model of senile osteoporosis. J Orthop Res.
28:798–804. 2010.
|
|
5
|
Nuttall ME and Gimble JM: Controlling the
balance between osteoblastogenesis and adipogenesis and the
consequent therapeutic implications. Curr Opin Pharmacol.
4:290–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Post S, Abdallah BM, Bentzon JF and Kassem
M: Demonstration of the presence of independent pre-osteoblastic
and pre-adipocytic cell populations in bone marrow-derived
mesenchymal stem cells. Bone. 43:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maurin AC, Chavassieux PM, Frappart L,
Delmas PD, Serre CM and Meunier PJ: Influence of mature adipocytes
on osteoblast proliferation in human primary cocultures. Bone.
26:485–489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan Y, Chong LW and Evans RM: PPAR-gamma
regulates osteoclastogenesis in mice. Nat Med. 13:1496–1503. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robling AG, Hinant FM, Burr DB and Turner
CH: Improved bone structure and strength after long-term mechanical
loading is greatest if loading is separated into short bouts. J
Bone Miner Res. 17:1545–1554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duncan RL and Turner CH:
Mechanotransduction and the functional response of bone to
mechanical strain. Calcif Tissue Int. 57:344–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoebertz A, Arnett TR and Burnstock G:
Regulation of bone resorption and formation by purines and
pyrimidines. Trends Pharmacol Sci. 24:290–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riddle RC, Taylor AF, Rogers JR and
Donahue HJ: ATP release mediates fluid flow-induced proliferation
of human bone marrow stromal cells. J Bone Miner Res. 22:589–600.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rumney RM, Sunters A, Reilly GC and
Gartland A: Application of multiple forms of mechanical loading to
human osteoblasts reveals increased ATP release in response to
fluid flow in 3D cultures and differential regulation of immediate
early genes. J Biomech. 45:549–554. 2012. View Article : Google Scholar :
|
|
16
|
Ferrari D, Gulinelli S, Salvestrini V,
Lucchetti G, Zini R, Manfredini R, Caione L, Piacibello W,
Ciciarello M, et al: Purinergic stimulation of human mesenchymal
stem cells poten-tiates their chemotactic response to CXCL12 and
increases the homing capacity and production of proinflammatory
cytokines. Exp Hematol. 39:360–374. 2011. View Article : Google Scholar
|
|
17
|
Noronha-Matos JB, Costa MA,
Magalhães-Cardoso MT, Ferreirinha F, Pelletier J, Freitas R, Neves
JM, Sévigny J and Correia-de-Sá P: Role of ecto-NTPDases on
UDP-sensitive P2Y(6) receptor activation during osteogenic
differentiation of primary bone marrow stromal cells from
postmenopausal women. J Cell Physiol. 227:2694–2709. 2012.
View Article : Google Scholar
|
|
18
|
Zippel N, Limbach CA, Ratajski N, Urban C,
Luparello C, Pansky A, Kassack MU and Tobiasch E: Purinergic
receptors influence the differentiation of human mesenchymal stem
cells. Stem Cells Dev. 21:884–900. 2012. View Article : Google Scholar
|
|
19
|
Erlinge D and Burnstock G: P2 receptors in
cardiovascular regulation and disease. Purinergic Signal. 4:1–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burnstock G: Purinergic signalling: past,
present and future. Braz J Med Biol Res. 42:3–8. 2009. View Article : Google Scholar
|
|
21
|
Abbracchio MP, Burnstock G, Boeynaems JM,
Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C,
Jacobson KA and Weisman GA: International Union of Pharmacology
LVIII: update on the P2Y G protein-coupled nucleotide receptors:
from molecular mechanisms and patho-physiology to therapy.
Pharmacol Rev. 58:281–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ayala-Peña VB, Scolaro LA and Santillán
GE: ATP and UTP stimulate bone morphogenetic protein-2,-4 and -5
gene expression and mineralization by rat primary osteoblasts
involving PI3K/AKT pathway. Exp Cell Res. 319:2028–2036. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orriss IR, Utting JC, Brandao-Burch A,
Colston K, Grubb BR, Burnstock G and Arnett TR: Extracellular
nucleotides block bone mineralization in vitro: evidence for dual
inhibitory mechanisms involving both P2Y2 receptors and
pyrophosphate. Endocrinology. 148:4208–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orriss IR, Knight GE, Ranasinghe S,
Burnstock G and Arnett TR: Osteoblast responses to nucleotides
increase during differentiation. Bone. 39:300–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoebertz A, Mahendran S, Burnstock G and
Arnett TR: ATP and UTP at low concentrations strongly inhibit bone
formation by osteoblasts: a novel role for the P2Y2 receptor in
bone remodeling. J Cell Biochem. 86:413–419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SC, Vielhauer NS, Leaver EV and
Pappone PA: Differential regulation of ca(2+) signaling and
membrane trafficking by multiple p2 receptors in brown adipocytes.
J Membr Biol. 207:131–142. 2005. View Article : Google Scholar
|
|
27
|
Lee H, Jun DJ, Suh BC, Choi BH, Lee JH, Do
MS, Suh BS, Ha H and Kim KT: Dual roles of P2 purinergic receptors
in insulin-stimulated leptin production and lipolysis in
differentiated rat white adipocytes. J Biol Chem. 280:28556–28563.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sreejit P, Dilip KB and Verma RS:
Generation of mesenchymal stem cell lines from murine bone marrow.
Cell Tissue Res. 350:55–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barragán-Iglesias P, Mendoza-Garcés L,
Pineda-Farias JB, Solano-Olivares V, Rodríguez-Silverio J,
Flores-Murrieta FJ, Granados-Soto V and Rocha-González HI:
Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in
formalin-induced inflammatory pain in rats. Pharmacol Biochem
Behav. 128:23–32. 2015. View Article : Google Scholar
|
|
30
|
Rodrigues-Ribeiro R, Alvarenga EC, Calio
ML, Paredes-Gamero EJ and Ferreira AT: Dual role of P2 receptors
during osteoblast differentiation. Cell Biochem Biophys.
71:1225–1233. 2015. View Article : Google Scholar
|
|
31
|
Orriss IR, Knight GE, Utting JC, Taylor
SE, Burnstock G and Arnett TR: Hypoxia stimulates vesicular ATP
release from rat osteoblasts. J Cell Physiol. 220:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yong Y, Ming ZD, Feng L, Chun ZW and Hua
W: Electromagnetic fields promote osteogenesis of rat mesenchymal
stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen
Med. Mar 16–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Yan M, Wang Z, Zheng Y, Li J, Ma S,
Liu G and Yu J: 17beta-estradiol promotes the odonto/osteogenic
differentiation of stem cells from apical papilla via
mitogen-activated protein kinase pathway. Stem Cell Res Ther.
5:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu FF, Zhu H, Li XM, Yang F, Chen JD, Tang
B, Sun HG, Chu YN, Zheng RX, Liu YL, et al: Intercellular adhesion
molecule-1 inhibits osteogenic differentiation of mesenchymal stem
cells and impairs bio-scaffold-mediated bone regeneration in vivo.
Tissue Eng Part A. 20:2768–2782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu D, Yi C, Fong CC, Jin Q, Wang Z, Yu
WK, Sun D, Zhao J and Yang M: Activation of multiple signaling
pathways during the differentiation of mesenchymal stem cells
cultured in a silicon nanowire microenvironment. Nanomedicine
(Lond). 10:1153–1163. 2014.
|
|
36
|
Yu Y, Wang L, Yu J, Lei G, Yan M, Smith G,
Cooper PR, Tang C, Zhang G and Smith AJ: Dentin matrix proteins
(DMPs) enhance differentiation of BMMSCs via ERK and P38 MAPK
pathways. Cell Tissue Res. 356:171–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashimoto R, Katoh Y, Miyamoto Y, Itoh S,
Daida H, Nakazato Y and Okada T: Increased extracellular and
intracellular Ca2+ lead to adipocyte accumulation in
bone marrow stromal cells by different mechanisms. Biochem Biophys
Res Commun. 457:647–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kusuyama J, Bandow K, Shamoto M, Kakimoto
K, Ohnishi T and Matsuguchi T: Low intensity pulsed ultrasound
(LIPUS) influences the multilineage differentiation of mesenchymal
stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK
signaling pathway. J Biol Chem. 289:10330–10344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang YY, Li X, Qian SW, Guo L, Huang HY,
He Q, Liu Y, Ma CG and Tang QQ: Down-regulation of type I Runx2
mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol
Endocrinol. 26:798–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naito M, Omoteyama K, Mikami Y, Takahashi
T and Takagi M: Inhibition of Wnt/β-catenin signaling by
dexamethasone promotes adipocyte differentiation in mesenchymal
progenitor cells, ROB-C26. Histochem Cell Biol. 138:833–845. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Menuki K, Mori T, Sakai A, Sakuma M,
Okimoto N, Shimizu Y, Kunugita N and Nakamura T: Climbing exercise
enhances osteoblast differentiation and inhibits adipogenic
differentiation with high expression of PTH/PTHrP receptor in bone
marrow cells. Bone. 43:613–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ciciarello M, Zini R, Rossi L, Salvestrini
V, Ferrari D, Manfredini R and Lemoli RM: Extracellular purines
promote the differentiation of human bone marrow-derived
mesenchymal stem cells to the osteogenic and adipogenic lineages.
Stem Cells Dev. 22:1097–1111. 2013. View Article : Google Scholar :
|
|
43
|
Schödel J, Weise I, Klinger R and Schmidt
M: Stimulation of lipogenesis in rat adipocytes by ATP, a ligand
for P2-receptors. Biochem Biophys Res Commun. 321:767–773. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kubota N, Terauchi Y, Miki H, Tamemoto H,
Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et
al: PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy
and insulin resistance. Mol Cell. 4:597–609. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawai M and Rosen CJ: PPARγ: a circadian
transcription factor in adipogenesis and osteogenesis. Nat Rev
Endocrinol. 6:629–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Costessi A, Pines A, D'Andrea P, Romanello
M, Damante G, Cesaratto L, Quadrifoglio F, Moro L and Tell G:
Extracellular nucleotides activate Runx2 in the osteoblast-like
HOBIT cell line: a possible molecular link between mechanical
stress and osteoblasts' response. Bone. 36:418–432. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katz S, Boland R and Santillán G:
Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in
osteoblasts: involvement of mechanical stress-activated calcium
influx, PKC and Src activation. Int J Biochem Cell Biol.
38:2082–2091. 2006. View Article : Google Scholar : PubMed/NCBI
|