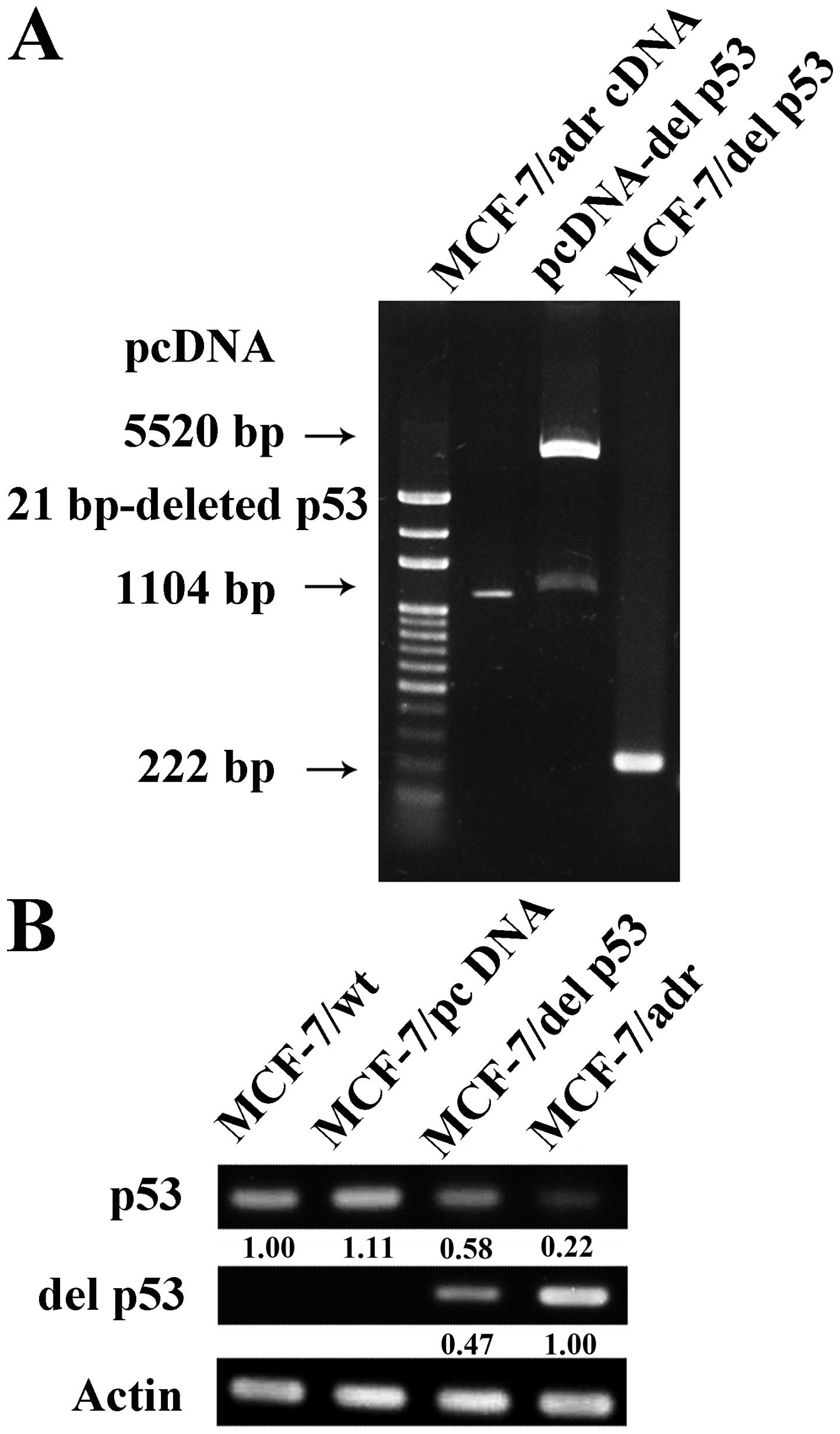
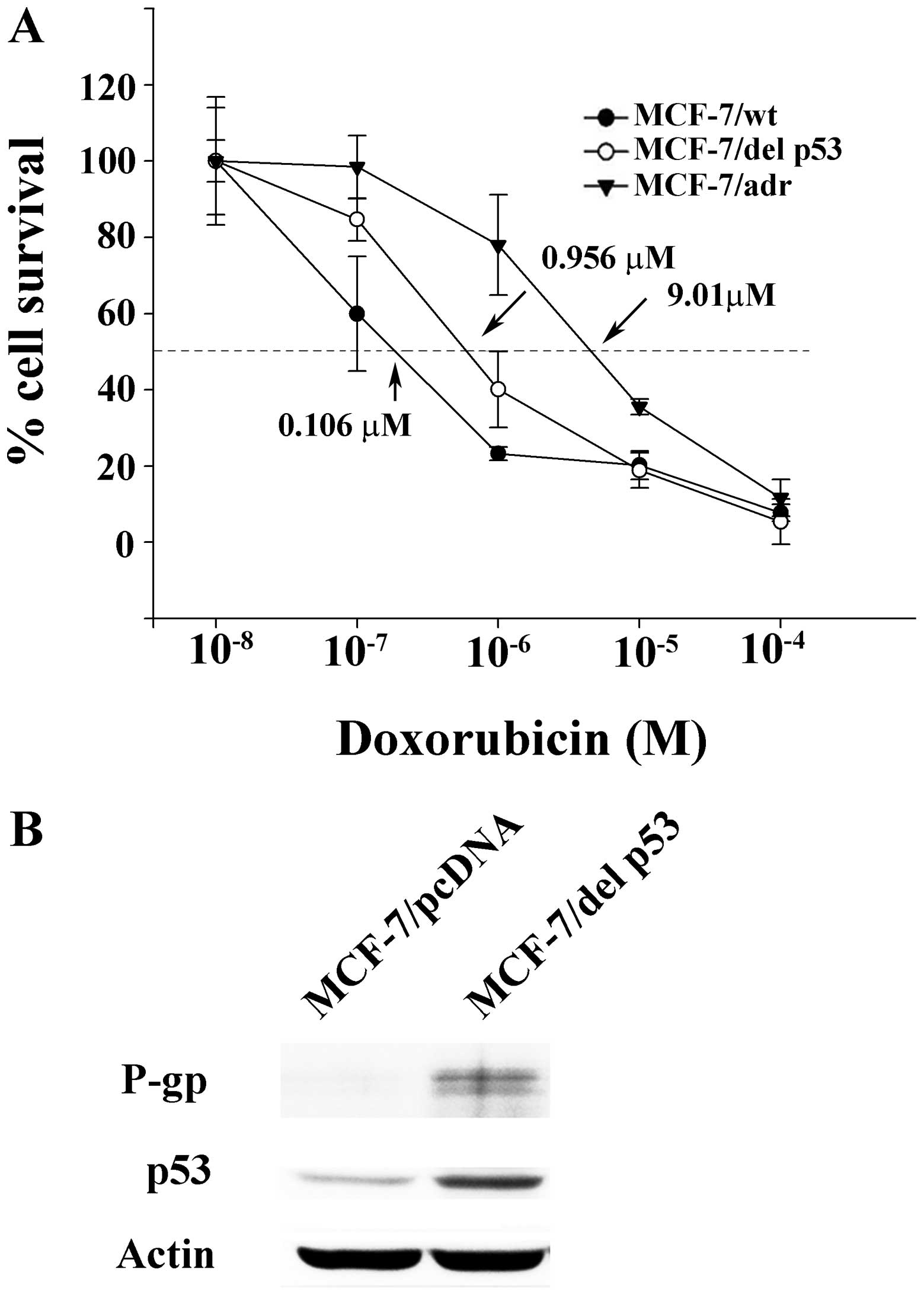
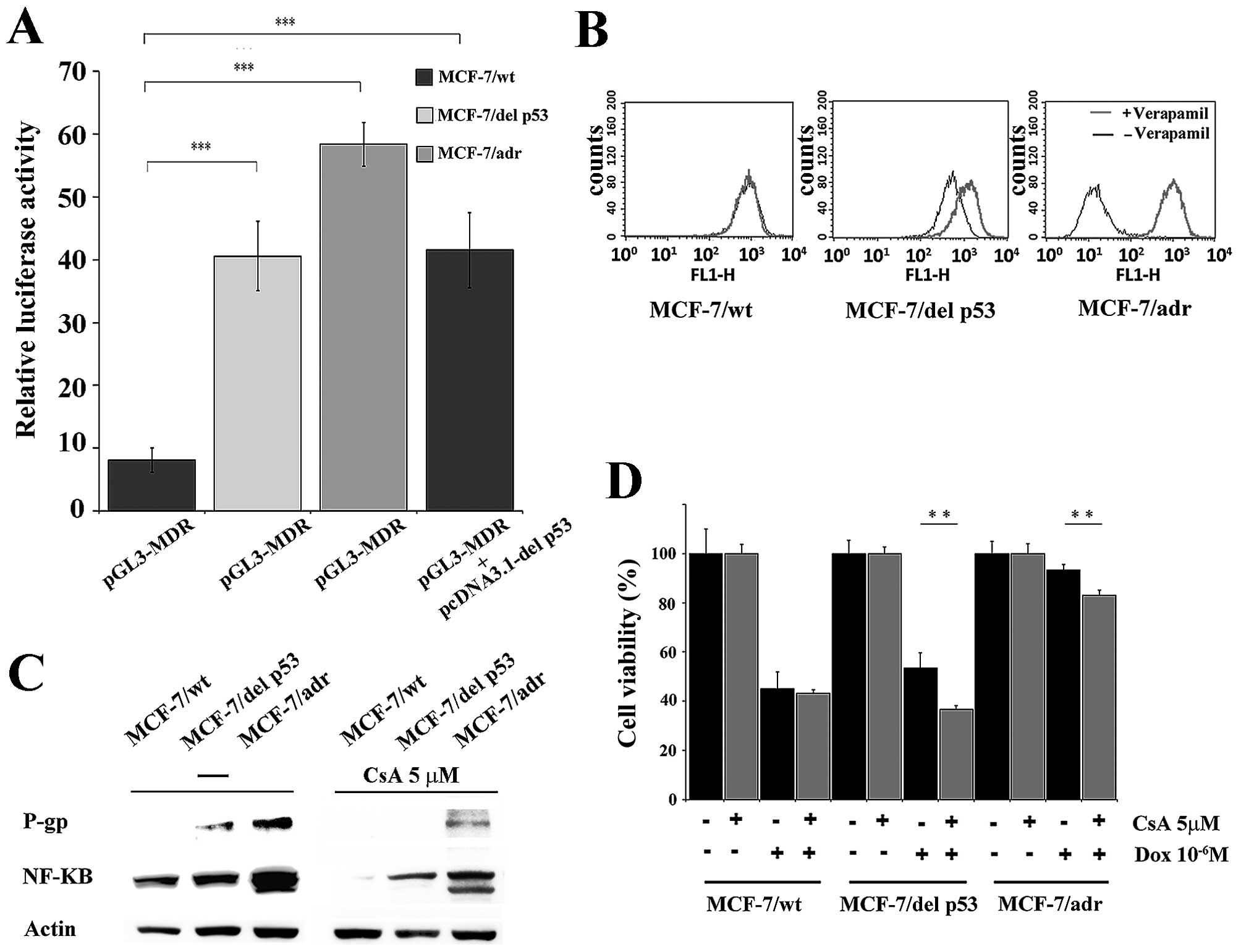
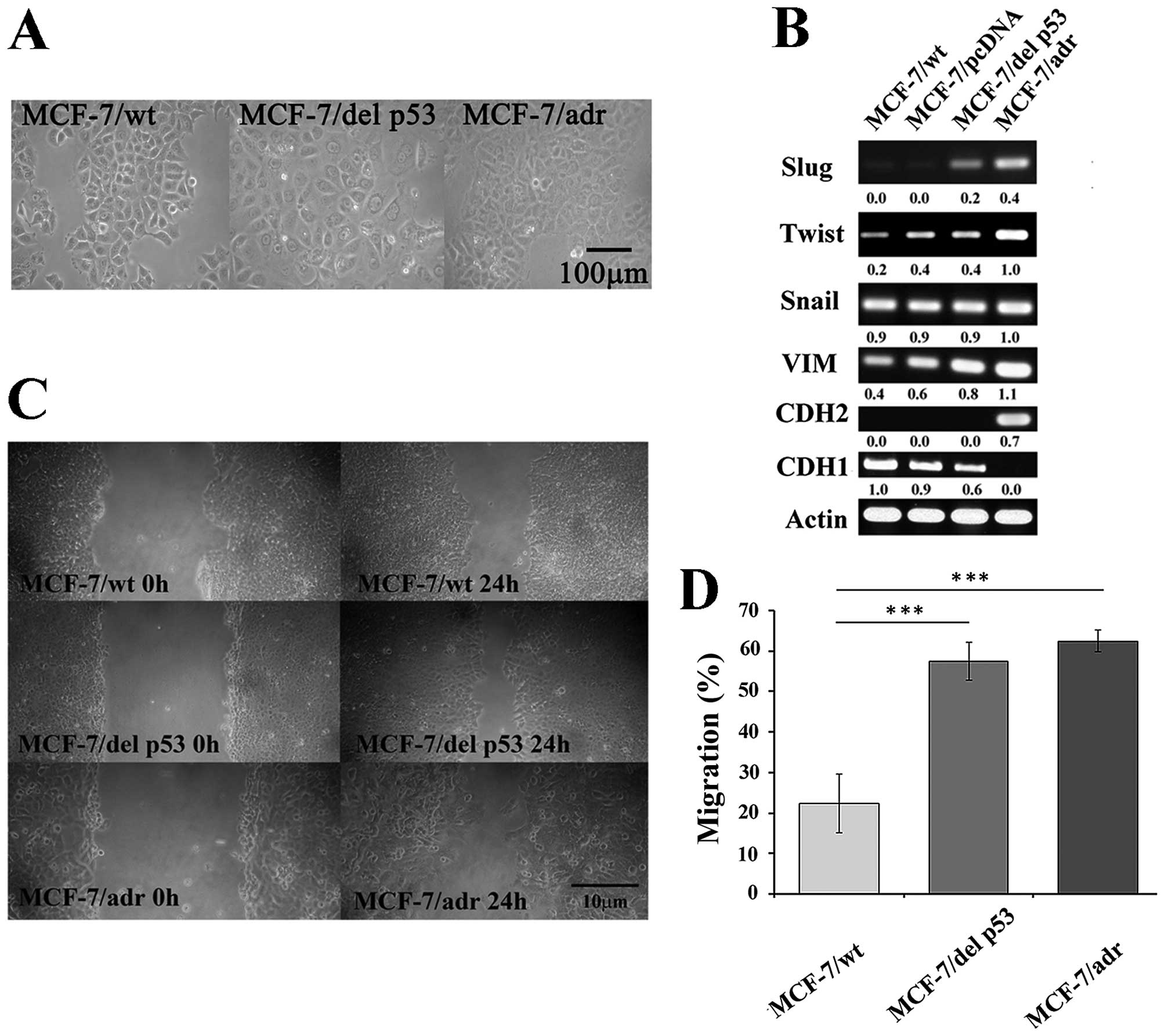
|
1
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar
|
|
2
|
Petitjean A, Achatz MI, Borresen-Dale AL,
Hainaut P and Olivier M: TP53 mutations in human cancers:
functional selection and impact on cancer prognosis and outcomes.
Oncogene. 26:2157–2165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keshelava N, Zuo JJ, Waidyaratne NS,
Triche TJ and Reynolds CP: p53 mutations and loss of p53 function
confer multidrug resistance in neuroblastoma. Med Pediatr Oncol.
35:563–568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sturm I, Bosanquet AG, Hermann S, Güner D,
Dörken B and Daniel PT: Mutation of p53 and consecutive selective
drug resistance in B-CLL occurs as a consequence of prior
DNA-damaging chemotherapy. Cell Death Differ. 10:477–484. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pritchard JR, Lauffenburger DA and Hemann
MT: Understanding resistance to combination chemotherapy. Drug
Resist Updat. 15:249–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glavinas H, Krajcsi P, Cserepes J and
Sarkadi B: The role of ABC transporters in drug resistance,
metabolism and toxicity. Curr Drug Deliv. 1:27–42. 2004. View Article : Google Scholar
|
|
7
|
Warmann S, Hunger M, Teichmann B, Flemming
P, Gratz KF and Fuchs J: The role of the MDR1 gene in the
development of multidrug resistance in human hepatoblastoma:
clinical course and in vivo model. Cancer. 95:1795–1801. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bargou RC, Jürchott K, Wagener C, Bergmann
S, Metzner S, Bommert K, Mapara MY, Winzer KJ, Dietel M, Dörken B
and Royer HD: Nuclear localization and increased levels of
transcription factor YB-1 in primary human breast cancers are
associated with intrinsic MDR1 gene expression. Nat Med. 3:447–450.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chin KV, Ueda K, Pastan I and Gottesman
MM: Modulation of activity of the promoter of the human MDR1 gene
by Ras and p53. Science. 255:459–462. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamura H, Yoshida K, Sasaki E, Morimoto H
and Haneji T: Transcription factor NF-Y regulates mdr1 expression
through binding to inverted CCAAT sequence in drug-resistant human
squamous carcinoma cells. Int J Oncol. 25:1031–1037.
2004.PubMed/NCBI
|
|
11
|
Rohlff C and Glazer RI: Regulation of the
MDR1 promoter by cyclic AMP-dependent protein kinase and
transcription factor Sp1. Int J Oncol. 12:383–386. 1998.PubMed/NCBI
|
|
12
|
Sampath J, Sun D, Kidd VJ, Grenet J,
Gandhi A, Shapiro LH, Wang Q, Zambetti GP and Schuetz JD: Mutant
p53 cooperates with ETS and selectively up-regulates human MDR1 not
MRP1. J Biol Chem. 276:39359–39367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arlt A and Schäfer H: NFkappaB-dependent
chemoresistance in solid tumors. Int J Clin Pharmacol Ther.
40:336–347. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deb D, Scian M, Roth KE, Li W, Keiger J,
Chakraborti AS, Deb SP and Deb S: Hetero-oligomerization does not
compromise 'gain of function' of tumor-derived p53 mutants.
Oncogene. 21:176–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanagasabai R, Krishnamurthy K, Druhan LJ
and Ilangovan G: Forced expression of heat shock protein 27 (Hsp27)
reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene
expression in Adriamycin-resistant human breast cancer cells. J
Biol Chem. 286:33289–33300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooks T, Pateras IS, Tarcic O, Solomon H,
Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld
N, et al: Mutant p53 prolongs NF-κB activation and promotes chronic
inflammation and inflammation-associated colorectal cancer. Cancer
Cell. 23:634–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferris RL and Grandis JR: NF-kappaB gene
signatures and p53 mutations in head and neck squamous cell
carcinoma. Clin Cancer Res. 13:5663–5664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Ahmed F, Ali S, Philip PA, Kucuk O
and Sarkar FH: Inactivation of nuclear factor kappaB by soy
isoflavone genistein contributes to increased apoptosis induced by
chemotherapeutic agents in human cancer cells. Cancer Res.
65:6934–6942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braeuer SJ, Büneker C, Mohr A and Zwacka
RM: Constitutively activated nuclear factor-kappaB, but not induced
NF-kappaB, leads to TRAIL resistance by up-regulation of X-linked
inhibitor of apoptosis protein in human cancer cells. Mol Cancer
Res. 4:715–728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Godwin P, Baird AM, Heavey S, Barr MP,
O'Byrne KJ and Gately K: Targeting nuclear factor-kappa B to
overcome resistance to chemotherapy. Front Oncol. 3:1202013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bentires-Alj M, Barbu V, Fillet M, Chariot
A, Relic B, Jacobs N, Gielen J, Merville MP and Bours V: NF-kappaB
transcription factor induces drug resistance through MDR1
expression in cancer cells. Oncogene. 22:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iseri OD, Kars MD, Arpaci F, Atalay C, Pak
I and Gunduz U: Drug resistant MCF-7 cells exhibit
epithelial-mesenchymal transition gene expression pattern. Biomed
Pharmacother. 65:40–45. 2011. View Article : Google Scholar
|
|
23
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporters. Cell Death Dis. 2:e1792011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
25
|
Yang HW, Menon LG, Black PM, Carroll RS
and Johnson MD: SNAI2/Slug promotes growth and invasion in human
gliomas. BMC Cancer. 10:3012010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Storci G, Sansone P, Trere D, Tavolari S,
Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M,
Montanaro L, et al: The basal-like breast carcinoma phenotype is
regulated by SLUG gene expression. J Pathol. 214:25–37. 2008.
View Article : Google Scholar
|
|
28
|
Lowe SW, Bodis S, McClatchey A, Remington
L, Ruley HE, Fisher DE, Housman DE and Jacks T: p53 status and the
efficacy of cancer therapy in vivo. Science. 266:807–810. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong P, Karaayvaz M, Jia N, Kaneuchi M,
Hamada J, Watari H, Sudo S, Ju J and Sakuragi N: Mutant p53
gain-of-function induces epithelial-mesenchymal transition through
modulation of the miR-130b-ZEB1 axis. Oncogene. 32:3286–3295. 2013.
View Article : Google Scholar :
|
|
30
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24− breast cancer cells
exhibit enhanced invasive properties: an early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
31
|
van Oijen MG and Slootweg PJ:
Gain-of-function mutations in the tumor suppressor gene p53. Clin
Cancer Res. 6:2138–2145. 2000.PubMed/NCBI
|
|
32
|
Tsou SH, Chen TM, Hsiao HT and Chen YH: A
critical dose of doxorubicin is required to alter the gene
expression profiles in MCF-7 cells acquiring multidrug resistance.
PLoS One. 10:e01167472015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogretmen B and Safa AR: Expression of the
mutated p53 tumor suppressor protein and its molecular and
biochemical characterization in multidrug resistant MCF-7/Adr human
breast cancer cells. Oncogene. 14:499–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu ST, Chen TM, Tseng SY and Chen YH:
Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in
breast cancer cells. Biochem Biophys Res Commun. 358:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berglind H, Pawitan Y, Kato S, Ishioka C
and Soussi T: Analysis of p53 mutation status in human cancer cell
lines: a paradigm for cell line cross-contamination. Cancer Biol
Ther. 7:699–708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nygren P, Larsson R, Gruber A, Peterson C
and Bergh J: Doxorubicin selected multidrug-resistant small cell
lung cancer cell lines characterised by elevated cytoplasmic
Ca2+ and resistance modulation by verapamil in absence
of P-glycoprotein overexpression. Br J Cancer. 64:1011–1018. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Norberg T, Klaar S, Lindqvist L, Lindahl
T, Ahlgren J and Bergh J: Enzymatic mutation detection method
evaluated for detection of p53 mutations in cDNA from breast
cancers. Clin Chem. 47:821–828. 2001.PubMed/NCBI
|
|
38
|
Takahashi RU, Takeshita F, Honma K, Ono M,
Kato K and Ochiya T: Ribophorin II regulates breast tumor
initiation and metastasis through the functional suppression of
GSK3β. Sci Rep. 3:24742013. View Article : Google Scholar
|
|
39
|
Li W, Liu C, Tang Y, Li H, Zhou F and Lv
S: Overexpression of Snail accelerates adriamycin induction of
multidrug resistance in breast cancer cells. Asian Pac J Cancer
Prev. 12:2575–2580. 2011.
|
|
40
|
Ogretmen B and Safa AR: Negative
regulation of MDR1 promoter activity in MCF-7, but not in multidrug
resistant MCF-7/Adr, cells by cross-coupled NF-kappa B/p65 and
c-Fos transcription factors and their interaction with the CAAT
region. Biochemistry. 38:2189–2199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nurwidya F, Takahashi F, Murakami A and
Takahashi K: Epithelial mesenchymal transition in drug resistance
and metastasis of lung cancer. Cancer Res Treat. 44:151–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Menon V and Povirk L: Involvement of p53
in the repair of DNA double strand breaks: multifaceted Roles of
p53 in homologous recombination repair (HRR) and non-homologous end
joining (NHEJ). Subcell Biochem. 85:321–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Z and Sun Y: Targeting p53 for novel
anticancer therapy. Transl Oncol. 3:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oka M, Kounoura K, Narasaki F, Sakamoto A,
Fukuda M, Matsuo I, Ikeda K, Tsurutani J, Ikuno N, Omagari K, et
al: P-glycoprotein is positively correlated with p53 protein
accumulation in human colorectal cancers. Jpn J Cancer Res.
88:738–742. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Labialle S, Gayet L, Marthinet E, Rigal D
and Baggetto LG: Transcriptional regulators of the human multidrug
resistance 1 gene: recent views. Biochem Pharmacol. 64:943–948.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang LH, Okaichi K, Ihara M and Okumura Y:
Sensitivity of anticancer drugs in Saos-2 cells transfected with
mutant p53 varied with mutation point. Anticancer Res. 18A:321–325.
1998.
|
|
48
|
Chan KT and Lung ML: Mutant p53 expression
enhances drug resistance in a hepatocellular carcinoma cell line.
Cancer Chemother Pharmacol. 53:519–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sosa AJ, Chavez P and Dyke KV: Inibitory
effect of tetrandrine and paxlitaxel or doxorubicin on
multi-drug-resistant (MDR) cancer cells associated with MDR-ATPase.
Int J Pharmacother. 4:102014.
|
|
50
|
Kim HG, Hien TT, Han EH, Hwang YP, Choi
JH, Kang KW, Kwon KI, Kim BH, Kim SK, Song GY, et al: Metformin
inhibits P-glycoprotein expression via the NF-κB pathway and CRE
transcriptional activity through AMPK activation. Br J Pharmacol.
162:1096–1108. 2011. View Article : Google Scholar :
|
|
51
|
Kajita M, McClinic KN and Wade PA:
Aberrant expression of the transcription factors snail and slug
alters the response to genotoxic stress. Mol Cell Biol.
24:7559–7566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fenouille N, Tichet M, Dufies M, Pottier
A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari
A, et al: The epithelial-mesenchymal transition (EMT) regulatory
factor SLUG (SNAI2) is a downstream target of SPARC and AKT in
promoting melanoma cell invasion. PLoS One. 7:e403782012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roger L, Jullien L, Gire V and Roux P:
Gain of oncogenic function of p53 mutants regulates E-cadherin
expression uncoupled from cell invasion in colon cancer cells. J
Cell Sci. 123:1295–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dhasarathy A, Phadke D, Mav D, Shah RR and
Wade PA: The transcription factors Snail and Slug activate the
transforming growth factor-beta signaling pathway in breast cancer.
PLoS One. 6:e265142011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Catalano A, Rodilossi S, Rippo MR, Caprari
P and Procopio A: Induction of stem cell factor/c-Kit/slug signal
transduction in multidrug-resistant malignant mesothelioma cells. J
Biol Chem. 279:46706–46714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bhat-Nakshatri P, Appaiah H, Ballas C,
Pick-Franke P, Goulet R Jr, Badve S, Srour EF and Nakshatri H:
SLUG/SNAI2 and tumor necrosis factor generate breast cells with
CD44+/CD24− phenotype. BMC Cancer.
10:4112010. View Article : Google Scholar
|
|
58
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gordon LA, Mulligan KT, Maxwell-Jones H,
Adams M, Walker RA and Jones JL: Breast cell invasive potential
relates to the myoepithelial phenotype. Int J Cancer. 106:8–16.
2003. View Article : Google Scholar : PubMed/NCBI
|