Introduction
Mesenchymal stem cells (MSCs) are multipotent
somatic stem cells that have the potential to differentiate into
mesodermal and even non-mesodermal lineages and are known to
produce trophic factor for tissue repair/regeneration (1–4).
Due to their ease of isolation, culture expansion, multipotential
differentiation and immunomodulatory properties, MSCs have the
potential for use in regenerative medicine and have therapeutic
application (5). Indeed, MSCs
have been investigated in a number of clinical trials for presently
untreatable diseases, such as bone and cartilage defects,
myocardial infarction, stroke, graft-versus-host disease (GvHD) and
autoimmune diseases (6,7).
Although MSCs were first reported to be derived from
bone marrow, a number of studies have reported similar cell types
in a wide range of tissues, e.g., umbilical cord blood, the
placenta, adipose tissue, amniotic fluid, dental tissue, skin, hair
follicles and tonsils (8–14). Given the observed clinical
efficacy of MSCs and a number of comparative analyses of MSCs
derived from different tissues, it is surprising that so little is
known about the identity and characteristics of MSCs derived from
different tissues (15,16). Although the International Society
for Cellular Therapy (ISCT) proposed the minimal criteria of MSCs
in 2006, this definition is non-specific and fails to address the
differences between MSCs (derived from different tissues) and
fibroblasts (17).
Currently, there is no consensus on the markers that
identify or distinguish MSCs derived from different tissues and
fibroblasts. Furthermore, a precise characterization of MSCs
derived from different tissues and their properties relating to
their therapeutic potential represent an essential requirement for
the exploitation and development of optimal MSC-based therapies,
since the biological capacity of MSCs (i.e., immunomodulatory
capacity, differentiation potential to a specific cell type and
endogenous stem cell mobilizing capacity) of one tissue may be
superior to others.
The aim of the present study was to compare the
biological characteristics of MSCs originating from different
tissues, i.e., bone marrow (BM-MSCs), umbilical cord blood
(CB-MSCs), placenta (P-MSCs) and adipose tissue (A-MSCs), with
respect to cell morphology, growth rate, immunophenotype, gene
expression profile, immunomodulatory capacity and differentiation
potential under the same conditions. The characterization of MSCs
derived from different tissues with identifying molecular
signatures may prove to be helpful for selecting a suitable source
for a specified clinical application.
Materials and methods
Cells
Bone marrow samples (from 3 male donors, aged 21, 26
and 27 years, respectively) were obtained from normal allogeneic
hematopoietic stem cell donors after obtaining written informed
consent. Umbilical cord blood was collected in a bag with CPDA
anticoagulant following delivery (from 3 donor babies, 1 male and 2
females). This study was approved by the Institutional Review
Boards of Severance Hospital (an affiliated hospital of Yonsei
University Health System, Seoul, Korea). The mononuclear cell (MNC)
fraction was separated by Ficoll-Hypaque density gradient
centrifugation (Pharmacia Biotech, Uppsala, Sweden), and the MSCs
were cultured as previously described (18). Human dermal fibroblasts (from 3
donors, a 22 year-old female, 26 year-old female and 31 year-old
male) were provided by Dr Dong-Wook Kim (Yonsei University College
of Medicine, Korea). In this study, fibroblasts were used as a
negative control. Placental- (from 3 donors, 28-, 32- and
33-year-old females) and adipose tissue-derived MSCs (from 3
donors, 34-, 41- and 46-year old females) were kindly provided by
Dr Ja Young Kwon (Yonsei University College of Medicine) and Dr
Kyoung Sik Kim (Yonsei University College of Medicine),
respectively. The isolated MSCs were frozen until the cells were
used. To permit an exact analysis, all cells were used at passage
3–5 and cultured under standardized conditions; DMEM-low glucose
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (P/S) (all from Invitrogen, Carlsbad, CA,
USA). The cells were cultured at 37°C with 5% CO2, and
the media were replaced every 3 or 4 days. Over the course of
expansion, we examined the differences in cell morphology under an
inverted phase microscope (Olympus IX-71; Olympus, Tokyo,
Japan).
Growth characteristics
To compare the growth characteristics of the cells,
the growth rate and population doubling time (PDT; period of time
required for cells to proliferate or grow) were measured. All cells
were plated at a density of 2×104 cells in 12-well
plates. On days 2 and 4, the cells were harvested and counted by
Trypan blue staining. The PDT was calculated based on a previously
reported formula (19). The
finite population doublings were defined as the cumulative number
of serial cell passages until the cells reached senescence.
Colony-forming unit-fibroblast (CFU-F)
assay
The capacity of the cells for self-renewal can be
evaluated by CFU-F assay. To assess the self-renewal capacity of
the cells, 1×103 cells at passage 3 were seeded in
100-mm plates (Corning Inc., Corning, NY, USA). Following
cultivation for 14 days, the cells were washed with
phosphate-buffered saline (PBS; Invitrogen) and stained with 0.5%
crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 5 min at
room temperature. Stained colonies with >50 cells were
counted.
Immunophenotyping
The cells were stained with the following
antibodies: CD14-FITC (555397), CD29-FITC (556048), CD31-PE
(555446), CD34-FITC (560942), CD44-PE (555479), CD45-PE (561866),
CD73-PE (550257), CD90-FITC (555595), CD105-PE (560839) and
CD106-FITC (551146) (all from BD Pharmingen, San Diego, CA, USA).
Additionally, phycoerythrin-conjugated and FITC-conjugated isotype
controls were applied. The cells were stained with the antibodies
for 20 min at 4°C. The stained cells were washed with PBS and fixed
with 1% paraformaldehyde (Biosesang, Seongnam, Korea).
Subsequently, the labeled cells were analyzed using a flow
cytometer (Cytomics Flow Cytometer; Beckman Coulter, Fullerton, CA,
USA).
RNA isolation and RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Standard reverse transcription (RT) was performed
using transcriptase II (Invitrogen). RT-PCR was performed using PCR
primers (Bioneer, Daejeon, Korea) under the conditions listed in
Table I. The glyceraldehyde
3-phosphate dehydrogenase (GAPDH) level was used as an
internal control. Human induced pluripotent stem (hiPS) cell cDNA
was used as a positive control (kindly provided by Dr Dong-Wook
Kim, Yonsei University College of Medicine). The signal intensity
of the product was normalized to its respective GAPDH signal
intensity.
 | Table IPrimer sets used for RT-PCR. |
Table I
Primer sets used for RT-PCR.
| Gene | Primer sequence
(5′→3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| GAPDH | Forward:
GTGGTCTCCTCTGACTTCAACA | | |
| Reverse:
CTCTTCCTCTTGTGCTCTTGCT | 62 | 210 |
| OCT4 | Forward:
GACAACAATGAGAACCTTCAGGAGA | | |
| Reverse:
TTCTGGCGCCGGTTACAGAACCA | 62 | 218 |
| SOX2 | Forward:
AACCAAGACGCTCATGAAGAAG | | |
| Reverse:
GCGAGTAGGACATGCTGTAGGT | 62 | 341 |
| c-Myc | Forward:
TCGGATTCTCTGCTCTCCTC | | |
| Reverse:
CGCCTCTTGACATTCTCCTC | 62 | 413 |
| KLF4 | Forward:
ATTCTCTCCAATTCGCTGACCC | | |
| Reverse:
TTCAGCACGAACTTGCCCAT | 62 | 376 |
| NANOG | Forward:
ATAGCAATGGTGTGACGCAG | | |
| Reverse:
GATTGTTCCAGGATTGGGTG | 62 | 219 |
| REX1 | Forward:
CTGAAGAAACGGGCAAAGAC | | |
| Reverse:
GAACATTCAAGGGAGCTTGC | 58 | 344 |
| LIN28 | Forward:
GCTCCGTGTCCAACCAGCAG | | |
| Reverse:
TTTCCTTTTGGCCGCCTCTC | 58 | 376 |
| GD2 synthase | Forward:
CCAACTCAACAGGCAACTAC | | |
| Reverse:
GATCATAACGGAGGAAGGTC | 59 | 230 |
| DLX5 | Forward:
ACCATCCGTCTCAGGAATCG | | |
| Reverse:
ACCTTCTCTGTAATGCGGCC | 60 | 384 |
| CBFA1 | Forward:
TTGCAGCCATAAGAGGGTAG | | |
| Reverse:
GTCACTTTCTTGGAGCAGGA | 58 | 470 |
| PPARG | Forward:
TCTCTCCGTAATGGAAGACC | | |
| Reverse:
GCATTATGAGACATCCCCAC | 55 | 474 |
| C/EBPA | Forward:
CCAAGAAGTCGGTGGACAAGAA | | |
| Reverse:
TCATTGTCACTGGTCAGCTCCA | 62 | 145 |
| BMP7 | Forward:
CCAACGTCATCCTGAAGAAATAC | | |
| Reverse:
GCTTGTAGGATCTTGTTCATTGG | 60 | 271 |
| SOX9 | Forward:
GGTTGTTGGAGCTTTCCTCA | | |
| Reverse:
TAGCCTCCCTCACTCCAAGA | 61 | 400 |
| HLA-ABC | Forward:
CAGATACCTGGAGAACGG | | |
| Reverse:
TGGCCTCATGGTCAGAGA | 56 | 96 |
| HLA-DR | Forward:
CCCCACAGCACGTTTCTTG | | |
| Reverse:
CCGCTGCACTGTGAAGCTCT | 60 | 274 |
| HLA-G | Forward:
GCGGCTACTACAACCAGAGC | | |
| Reverse:
GCACATGGCACGTGTATCTC | 58 | 900 |
| IL10 | Forward:
ACCTGGTAGAAGTGATGCCCCAGGCA | | |
| Reverse:
CTATGCAGTTGATGAAGATGTCAA | 58 | 237 |
| TNFAIP6 | Forward:
GGTGTGTACCACAGAGAAGCA | | |
| Reverse:
GGGTTGTAGCAATAGGCATCC | 60 | 284 |
| TSG-6 | Forward:
GGTGTGTACCACAGAGAAGCA | | |
| Reverse:
GGGTTGTAGCAATAGGCATCC | 60 | 284 |
| IL6 | Forward:
ATGAACTCCTTCTCCACAAGC | | |
| Reverse:
GTTTCTGCCAGTGCCTCTTTG | 60 | 264 |
| TGFB1 | Forward:
GAGGTGACCTGGCCACCATT | | |
| Reverse:
TCCGCAAGGACCTCGGCTGG | 55 | 194 |
| INHBA | Forward:
GATGTACCCAACTCTCAGCCA | | |
| Reverse:
GCCGATGTCCTTGAAACTGAC | 55 | 866 |
Differentiation assay
To induce osteogenic, adipogenic and chondrogenic
differentiation, the cells derived from each type of tissue were
seeded simultaneously in osteogenic induction medium, chondrogenic
induction medium, and adipogenic induction medium (Cambrex, Lonza,
MD, USA). The cells were then cultured for 3 weeks, and the medium
was changed every 3 or 4 days. Whenever the medium was changed
during chondrogenesis, 10 ng/ml transforming growth factor (TGF)-β3
(Cambrex) was added. After 3 weeks, the cells were analyzed for
osteogenesis, adipogenesis and chondrogenesis by von Kossa
staining, Oil Red O staining, and Safranin O staining. The stained
cells were photographed using a phase microscope (Olympus IX-71;
Olympus).
T cell proliferation assay
To assess the ability of MSCs to suppress T cell
proliferation, the MSCs were treated with 50 ng/ml of mitomycin C
(Sigma-Aldrich) for 60 min to inactivate their proliferation.
Subsequently, 2×105 cells of human peripheral blood MNCs
were co-cultured with 2×104 MSCs of each type in a
96-well plate. To activate T cells, 10 µg/ml
phytohaemagglutinin (PHA; Sigma-Aldrich) was applied for 72 h. To
examine the inhibition of T cells, a BrdU cell proliferation assay
(Millipore, Billerica, MA, USA) was performed according to the
manufacturer's instructions. Activated T cells alone without MSCs
were used as a positive control.
Statistical analysis
Quantitative data are expressed as the means ± SD.
All statistical comparisons between groups were performed by
one-way analysis of variance (ANOVA) with post hoc Bonferroni
corrections. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
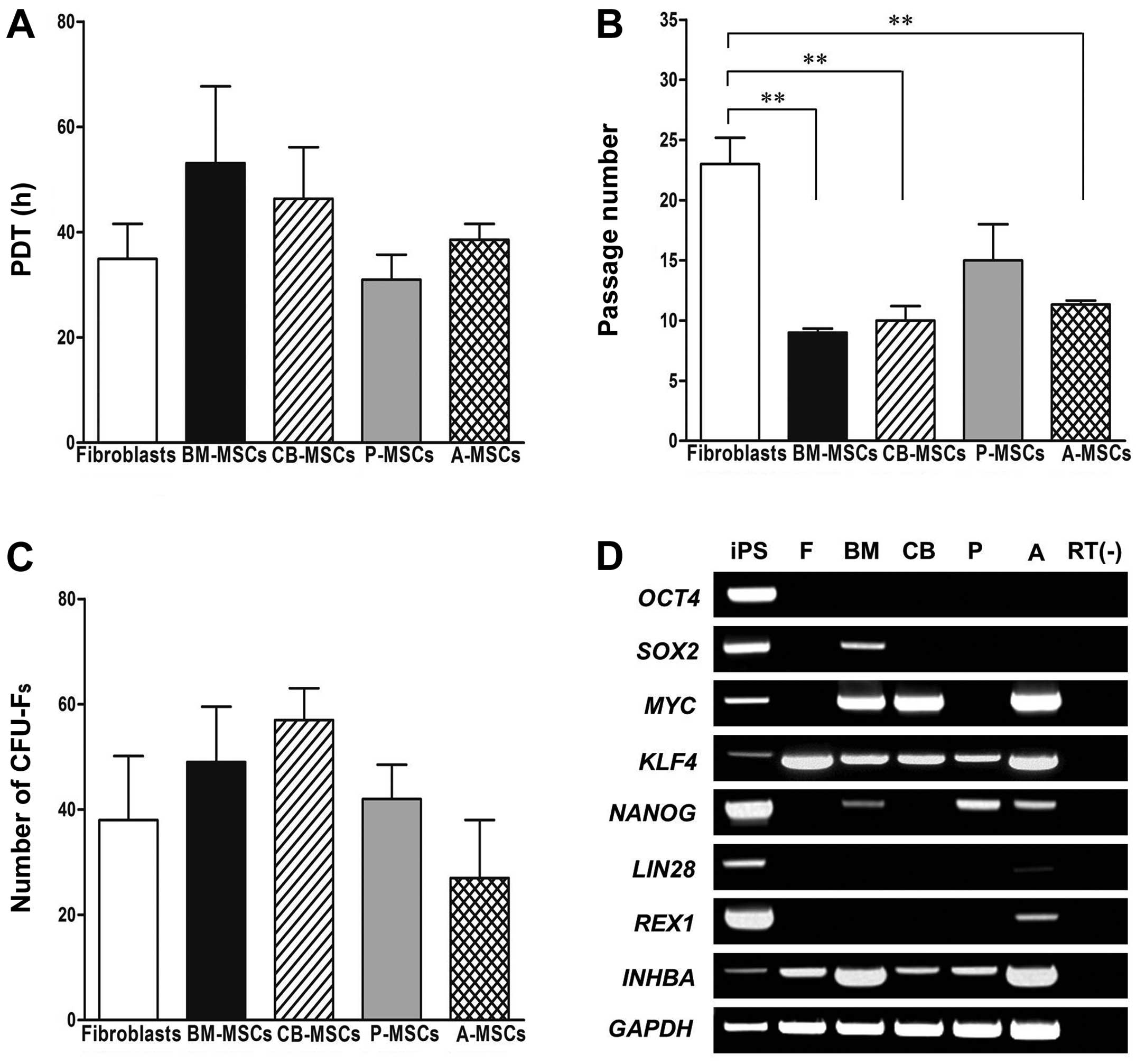
Growth characteristics of MSCs derived
from different tissues
All MSCs and fibroblasts exhibited similar growth
properties on day 2. However, of the MSCs derived from different
tissues, the P-MSCs displayed the highest proliferative capacity
between days 2 and 4 (Fig. 1A),
as they had the lowest PDT. Although the P-MSCs showed a slight
increase in growth compared to the controls (shown by the decrease
in the PDT), the differences in population doubling time between
the tested cells were not statistically significant. Although the
cells were isolated from different tissues, we did not find any
differences in our morphological examination (data not shown). To
determine the maximum proliferative capacity, all cell types were
serially passaged until they displayed replicative senescence with
a loss of proliferation. Of the MSCs derived from different
tissues, the P-MSCs could withstand longer periods of culture,
whereas the BM-, CB- and A-MSCs exhibited a similar maximum culture
period (Fig. 1B). The CFU-F assay
was used to examine the self-renewal capacity of the cells.
Although the fibroblasts and P-MSCs exhibited better growth
characteristics than the other cells, there were no significant
differences in the number of CFU-Fs following cell seeding at
1×103 cells in 100-mm plates after 14 days (Fig. 1C). The BM- and CB-MSCs displayed a
higher self-renewal capacity regardless of growth rate, although
the differences were not significant.
In order to identify the molecular signature, we
examined the expression of stemness markers in the MSCs derived
from different tissues (Fig. 1D).
The octamer-binding transcription factor 4 (OCT4) gene was
not detected in any of the MSCs, or the fibroblasts. Sex
determining region Y-box 2 (SOX2) was only expressed in the
BM-MSCs; NANOG was detected in the BM-, P- and A-MSCs.
Compared to the hiPS cells, the expression of SOX2 and
NANOG was much lower in the BM-MSCs. Krüppel-like factor 4
(KLF4) was expressed in all types of cells and fibroblasts,
whereas MYC was expressed in all cells apart from the
fibroblasts and P-MSCs. Activin A [inhibin, beta A (INHBA)]
was strongly detected in the BM-and A-MSCs, as compared to the
fibroblasts and MSCs derived from other tissues. Compared to the
hiPS cells, MYC, KLF4 and INHBA expression was
much stronger in the other MSCs tested. In the A-MSCs we noted a
basal expression of LIN28 and REX1, which was much
lower than that expressed in the hiPS cells. These results suggest
that BM- and A-MSCs possess the highest capacity for self-renewal
and differentiation potential in multiple lineages, whereas P-MSCs
have the least functionality as stem cells of those which were
tested.
Immunophenotype and differentiation
potential
Flow cytometric analysis was performed with the MSCs
derived from different tissues, and we revealed that all cell types
displayed similar immunophenotypic patterns. The cells were
negative for CD14, CD31, CD34, CD45 and CD106, which are known
markers of hematopoietic and endothelial cells, whereas the MSCs
were positive for CD29, CD44, CD73, CD90 and CD105, which are known
markers of MSCs. Positive MSC markers were expressed in all of the
cell types, even in fibroblasts (Table II). These results confirm that
cells from diverse sources express MSC surface markers, as defined
by the ISCT. However, the expression of CD90, a typical MSC marker,
was less obvious in the P-MSCs than in the other cells.
 | Table IIImmunophenotyping of cells derived
from various sources by flow cytometry. |
Table II
Immunophenotyping of cells derived
from various sources by flow cytometry.
| Surface marker | Fibroblasts | BM-MSCs | CB-MSCs | P-MSCs | A-MSCs |
|---|
| CD14 | 1.0±1.5a | 1.4±0.8 | 1.3±0.6 | 0.8±0.8 | 2.0±1.1 |
| CD29 | 82.3±15.6 | 97.5±1.9 | 94.4±7.9 | 98.6±1.9 | 68.5±17.9 |
| CD31 | 1.2±0.4 | 1.8±1.4 | 1.0±0.9 | 0.4±0.5 | 0.6±0.5 |
| CD34 | 0.8±1.2 | 2.3±1.6 | 6.1±8.4 | 0.7±0.7 | 1.8±1.1 |
| CD44 | 93.3±11.7 | 100±0.0 | 96.5±6.0 | 93.0±12.0 | 99.8±0.3 |
| CD45 | 0.7±0.6 | 1.7± 1.5 | 0.5±0.4 | 0.4±0.3 | 0.9±0.4 |
| CD73 | 99.3±0.3 | 99.1±0.9 | 93.7±6.3 | 99.5±0.8 | 90.9±3.0 |
| CD90 | 90.9±10.1 | 83.1±20.3 | 66.4±11.2 | 22.2±12.7 | 62.5±16.9 |
| CD105 | 91.7±0.6 | 90.3±12.1 | 68.2±27.2 | 73.5±36.3 | 75.0±14.1 |
| CD106 | 1.0±1.6 | 4.3±1.6 | 6.3±7.5 | 0.9±1.1 | 2.0±1.2 |
To investigate the differentiation potential of the
MSCs, the cells were subjected to osteogenic, adipogenic and
chondrogenic differentiation (Fig.
2A). Osteogenic differentiation, which was evaluated by calcium
deposition and von Kossa staining, was evident in the BM- and
A-MSCs, whereas the other MSCs did not differentiate into
osteoblasts under osteogenic induction. No osteogenic
differentiation was induced in the fibroblasts. Adipogenic
differentiation, verified by the accumulation of cytoplasmic lipid
vacuoles and Oil Red O staining, was distinctly observed in the BM-
and A-MSCs, whereas theCB- and P-MSCs were only weakly positive.
Only a few or very small Oil Red O-stained granules were detected
in the fibroblasts, and this could be explained by the findings of
a previous study which suggested that human dermal fibroblasts
exhibit delayed adipogenic differentiation compared with MSCs (as
also shown in Fig. 3A) (20). Chondrogenesis, verified by
cartilage-specific proteoglycans and Safranin O staining, was
demonstrated in all the tested cells (Fig. 3B). The BM- and A-MSCs exhibited
only tri-lineage potency, whereas the CB- and P-MSCs had the
capacity to differentiate into only 2 cell lineages. The
fibroblasts also differentiated into adipocytes and chondrocytes,
although the results were weakly positive. Therefore, we suggest
that only the BM- and A-MSCs can differentiate into 3 mesodermal
lineages, i.e., osteoblasts, adipocytes and chondrocytes, thus
demonstrating that of the cells from diverse sources, only the BM-
and A-MSCs have multipotency as true MSCs.
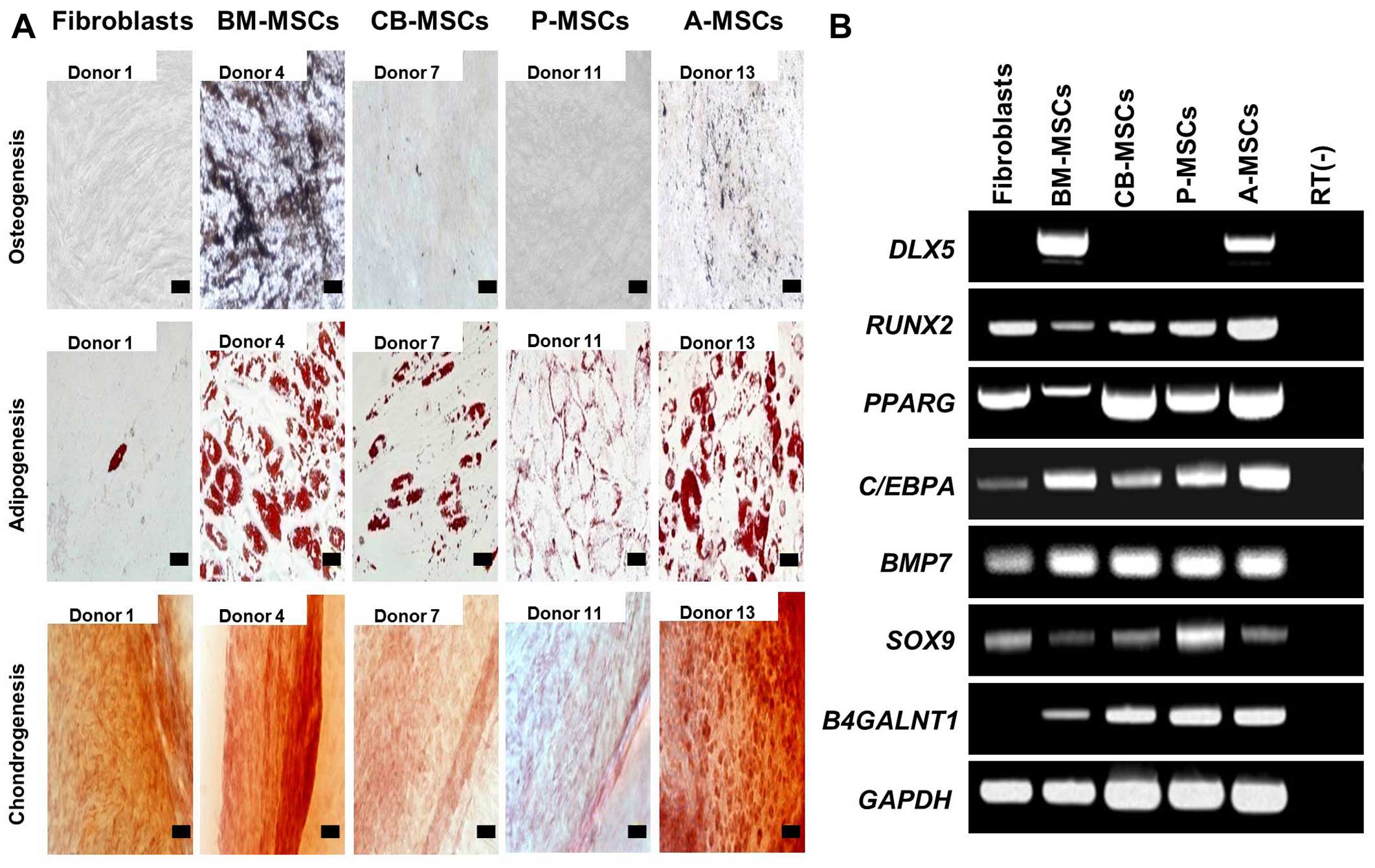 | Figure 2Tri-lineage differentiation of
mesenchymal stem cells (MSCs) derived from different tissues. (A)
In vitro differentiation assay. MSCs were induced to
differentiate toward osteogenic lineage and verified by von Kossa
staining after induction (magnification, ×200; scale bar, 100
µm), adipogenic lineage and verified by Oil Red O
(magnification, ×400; scale bar, 50 µm), and chondrogenic
lineage and verified by Safranin O staining (magnification, ×200;
scale bar, 100 µm). One representative of 3 independent
experiments is shown. (B) RT-PCR analysis for tri-lineage
differentiation-associated markers in MSCs derived from bone marrow
(BM-MSCs), umbilical cord blood (CB-MSCs), the placenta (P-MSCs)
and adipose tissue (A-MSCs) compared to fibroblasts. The expression
of osteogenic (DLX5 and RUNX2), adipogenic
(PPARG and C/EBPA) and chondrogenic-associated genes
(BMP7 and SOX9) was assayed. The expression of
B4GALNT1 was confined to MSCs, and was not noted in
fibroblasts. One representative of 3 independent experiments is
shown. |
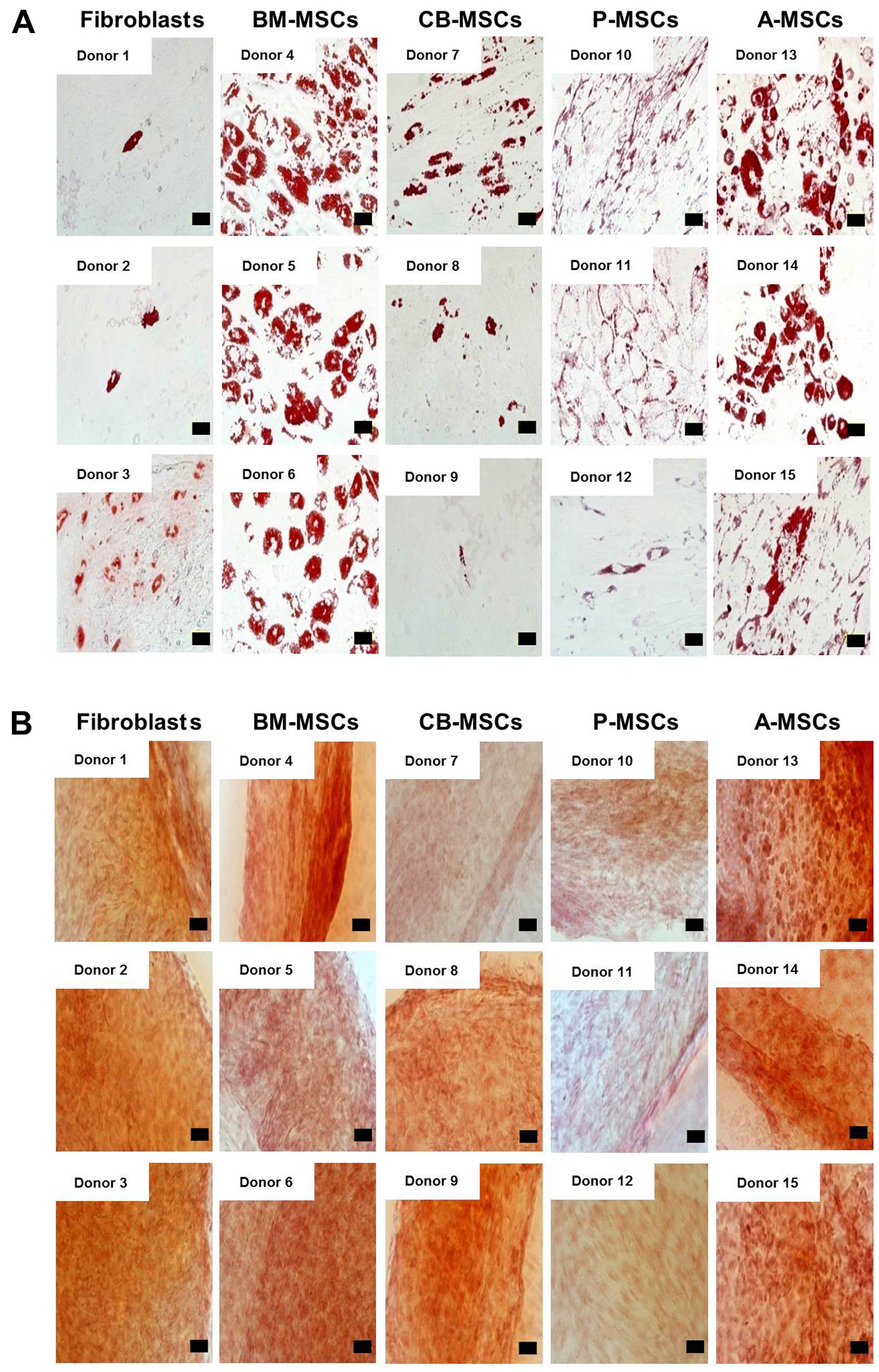 | Figure 3(A) Adipogenenic differentiation
potential of mesenchymal stem cells (MSCs) derived from different
tissue sources. Adipogenic differentiation was carried out for MSCs
and fibroblasts isolated from different donors and terminated after
21 days. Fibroblast, bone marrow (BM)-, cord blood (CB)-, placental
(P)-, adipose tissue (A)-derived MSCs from different donors were
stained by Oil Red O for intracellular lipid vesicles after
induction (×400). (Scale bar, 50 µm). (B) Chondrogenic
potential of MSCs derived from different tissue sources.
Chondrogenic differentiation was induced for 21 days. Fibroblasts,
and bone marrow, cord blood, placental, and adipose tissue-derived
MSCs from different donors were induced and analyzed by Safranin-O
staining (×200 magnification). (Scale bar, 100 µm). |
Subsequently, we evaluated the osteogenic,
adipogenic and chondrogenic gene expression in the cells by RT-PCR
(Fig. 2B). Osteogenesis-related
gene runt-related transcription factor 2 (RUNX2),
adipogenesis-related genes peroxisome proliferator-activated
receptor gamma (PPARG), CCAAT/enhancer-binding protein alpha
(C/EBPA), and chondrogenesis-related genes bone
morphogenetic protein 7 (BMP7) and sex determining region
Y-box 9 (SOX9) were similarly expressed in the majority of
cell types, whereas distal-less homeobox 5 (DLX5), which
plays a key role in the development of skeletal elements and the
commitment of MSCs to the osteoblast lineage was only expressed in
the BM-MSCs and A-MSCs. RUNX2 and PPARG expression in
the BM-MSCs were lower than in the other cell types. These results
again support our theory that BM- and A-MSCs possess tri-lineage
differentiation potential.
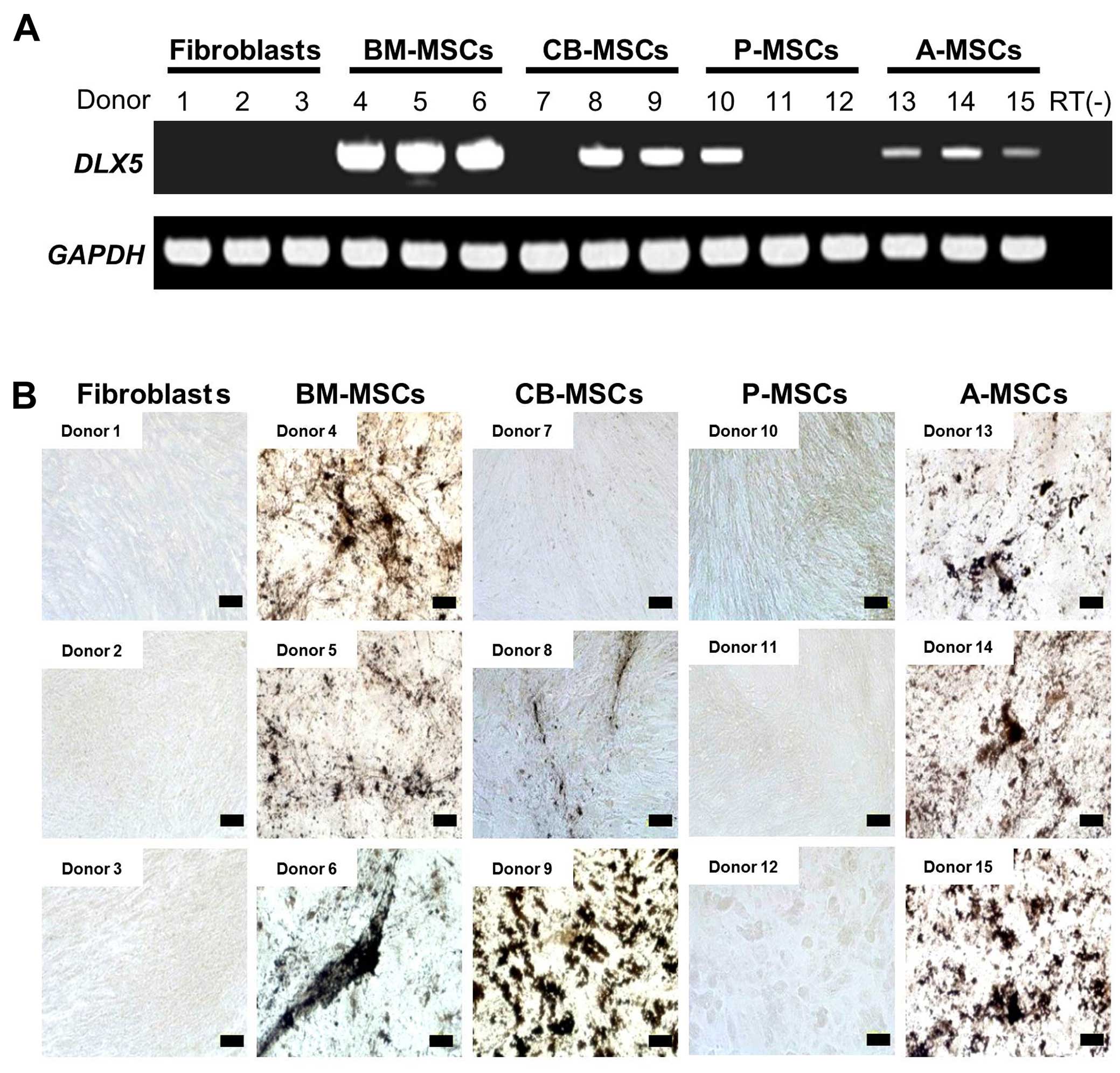
DLX5 expression and osteogenic
potential
To confirm the differential expression of
DLX5 and osteogenic potential, we performed RT-PCR analysis
of DLX5 in various MSCs derived from 3 different donors.
DLX5 was expressed in all assessed BM-MSCs and A-MSCs
(Fig. 4A). However, DLX5
was also detected in 2 out of 3 CB-MSCs (donors 8 and 9) and 1 of 3
P-MSCs (donor 10), indicating the heterogeneity of MSCs between
donors and/or preparations. We analyzed the in vitro
osteogenic potential of those MSCs tested for DLX5 gene
expression (Fig. 4B). Following
osteogenic induction, the BM- and A-MSCs from all 3 donors
possessed cells with an osteogenic phenotype. By contrast, the
DLX5-expressing CB-MSCs developed an osteogenic phenotype,
albeit at varying degrees and this coincided with DLX5
expression (donors 8 and 9). Only a weak osteogenic phenotype was
observed in one of the DLX5-expressing P-MSCs, and no
osteogenic phenotype was induced in the fibroblasts. It is clear
that the levels of DLX5 expression do not necessarily
correlate with osteogenic potential. The discrepancy in DLX5
expression and the osteogenic potential of A-MSCs may be explained
by the differences in the expression of growth factors, growth
factor receptors and transcription factors involved in
osteogenesis. Our data suggest that DLX5, one of the key
transcription factors for osteoblast differentiation, is a
predictive marker for the osteogenic potential of MSCs. In
addition, we noted great inter-individual variation in the degree
of osteogenic potential between the MSCs obtained from different
tissues.
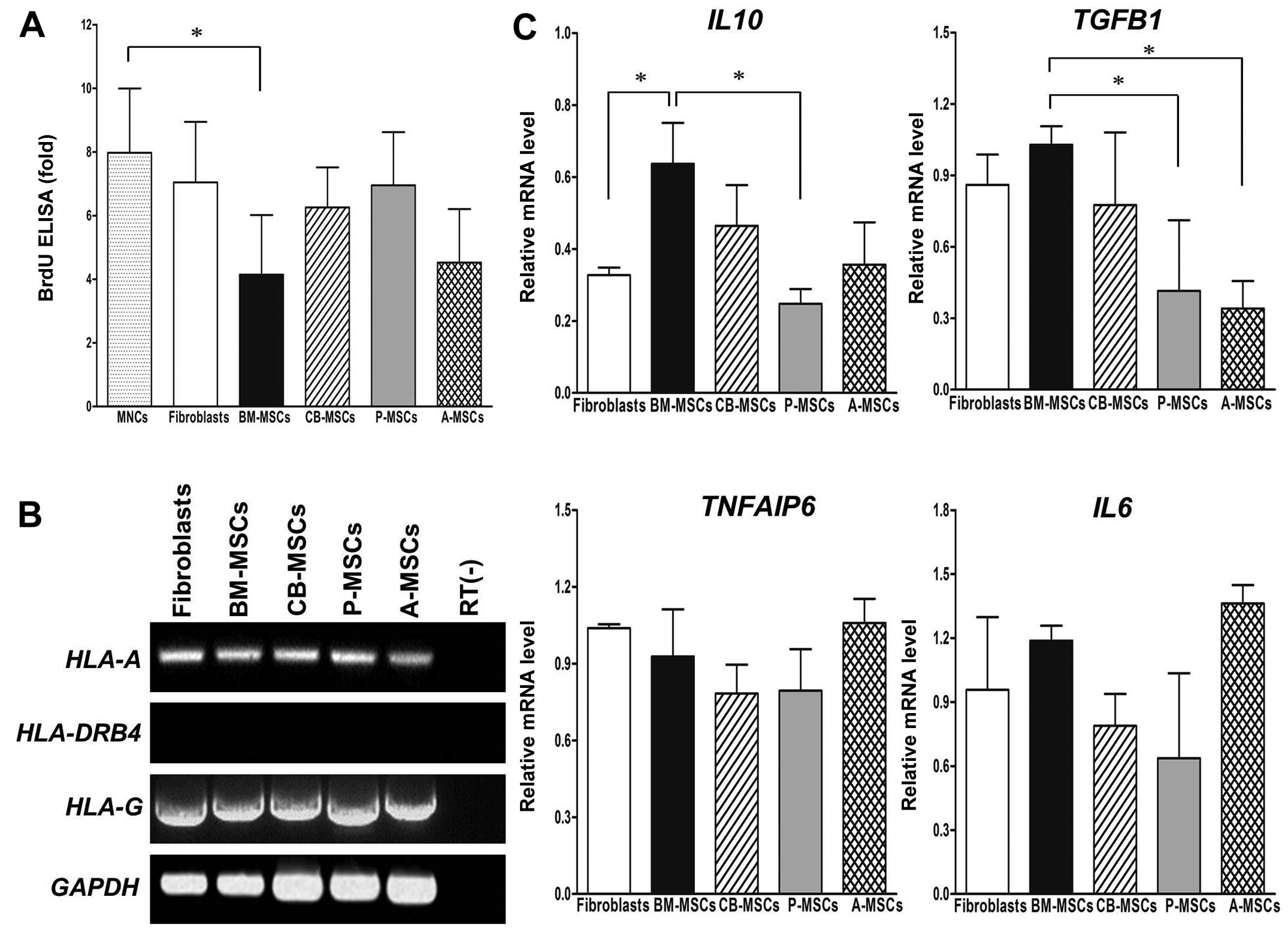
Suppression of T cell proliferation by
MSCs derived from different tissues
To assess the immunomodulatory effects of MSCs on
activated T cells, we performed a BrdU ELISA assay in T cells
co-cultured with various MSCs. The proliferation of T cells was
suppressed by MSCs derived from different tissues to varying
degrees (Fig. 5A). While the
fibroblasts and P-MSCs only weakly inhibited the cell proliferation
induced by PHA, a clear reduction in cell proliferation was
observed in the BM- and A-MSCs.
It is well known that the immunomodulatory
properties of MSCs are mediated by HLA and soluble cytokines. The
expression of HLA-A and HLA-G was readily detectable
in all tested cells, implying that the expression level of
HLA-G and MHC class I proteins (HLA-A) in MSCs and
fibroblasts could not account for the observed inhibition of T cell
proliferation (Fig. 5B).
Expression of HLA-DRB4 was negative in all cells. We then
analyzed the gene expression profiles of cytokines related to
immunomodulation by RT-PCR that included interleukin 10
(IL10), TGFB1, tumor necrosis factor, alpha-induced
protein 6 [(TNFAIP6), tumor TNF-stimulated gene 6
(TSG-6)] and interleukin 6 (IL6) (Fig. 5C). The relative quantification of
gene expression from the MSCs was normalized to the internal
control, GAPDH. The expression of TGFB1 was higher in
the BM-MSCs when compared with the P-MSCs and A-MSCs. Compared to
the fibroblasts, no significant differences were detected in the
expression of TNFAIP6 and IL6 in the MSCs derived
from different tissues. Notably, a strong IL10 expression
was observed in the BM-MSCs compared to that of fibroblasts and
P-MSCs, implying that BM-MSCs exert immunosuppressive activity
primarily via IL10.
Discussion
Due to their regenerative and immunosuppressive
properties, MSCs derived from adult tissues have become a preferred
cell type in the field of regenerative medicine and are being
extensively investigated for their clinical applications (21). Although bone marrow is considered
a universal source of multipotent MSCs, the invasive procedure
necessary to harvest these cells, the risks of complications and
the age-dependent decline of the self-renewal capacity of MSCs has
led to a search for alternate sources for MSCs (22,23). CB-MSCs, P-MSCs and A-MSCs have
been suggested as alternative sources of MSCs for experimental and
clinical purposes since they are free from ethical concerns, easy
to procure and are available in large quantities (24–26). Currently, BM-, CB-, P- and A-MSCs
are the representative candidates for stem cell therapy (27). As MSCs are being isolated from
different tissue sources with different protocols of isolation and
culture expansion, it is unclear whether these MSCs share common
properties or are dissimilar in terms of certain characteristics
that may affect their clinical utilization and outcome. Thus, the
comparative analysis of cellular behaviour in vitro,
phenotypes, differentiation potential, and immunosuppressive
capacity is useful for their potential utilization in clinical
settings. In order to characterize MSCs derived from various tissue
sources in a number of parameters, all cell preparations in the
present study were treated under identical conditions to minimize
variables that affect cellular characteristics.
The data obtained demonstrated that MSCs derived
from different tissues and the fibroblasts (used as controls)
exhibited a similar morphology, clonogenic capacity and
immunophenotype, but differed in terms of proliferative rates and
differentiation potential. The P-MSCs consistently grew faster and
more robustly than the cells derived from other tissues, with a
rapid population doubling time. MSCs have a limited life span and
enter replicative senescence during in vitro culture, as
indicated by enlarged and irregular cell shapes and cessation of
proliferation (28). The BM-, CB-
and A-MSCs exhibited replicative senescence when they reached
passage 10 on average, whereas the P-MSCs expanded until passage
15. Thus, MSCs are theoretically capable of long-term culture in
vitro without losing their fundamental stem cell properties;
however, we noted that the growth capacity of the MSCs was
generally inferior to that of fibroblasts. Our results demonsrated
that P-MSCs are superior to the other MSC types with regard to
growth, but more CFU-F colonies were observed among the BM- and
CB-MSCs. These results suggest that rapid and long-term growth is
not required for the 'stem' properties of MSCs.
Although a list of surface molecules was proposed by
ISCT as one of the minimal criteria for MSC identification, all
tested markers did not distinguish MSCs from fibroblasts. Thus, the
identification of a single definitive marker and precise
characterization of MSCs derived from various tissues with regard
to their multipotency will be a significant advance for their
clinical application. In our phenotypic analysis, we noted that
MSCs derived from various sources were positive for the expression
of the MSC markers, CD44, CD73, CD90, and CD105, and were negative
for CD14, CD34 and CD45. However, CD90 expression, which is known
to be associated with haematopoiesis and cell migration, was
slightly different among the P-MSCs, and its biological
significance needs to be determined. As the function of MSCs is
governed by differential molecular profiles, we analyzed the
expression of pluripotency genes in order to provide further
insight into the differences between MSCs from different tissues.
In this study, SOX2, which is involved in self-renewal in
pluripotent stem cells and multipotency in MSCs, was only expressed
in BM-MSCs, implying the more primitive status of BM-MSCs, as has
also been previously noted (29).
Since SOX2 functions as a molecular switch in neuronal
development, its expression in BM-MSCs may reflect the neuronal
differentiation potential (30).
BM-MSCs expressed detectable amounts of the majority of core
transcription factors, as evidenced by RT-PCR, such as SOX2,
MYC, KLF4 and NANOG, even in the absence of
exogenous stimuli, whereas A-MSCs expressed MYC,
KLF4, NANOG, LIN28 and REX1. The
amplified transcripts were of the same size as those in human iPS
cells. It was previously demonstrated that INHBA is required
for the chondrogenic and osteogenic differentiation of MSCs
(31), and our data indicated
that the BM- and A-MSCs exhibited a higher expression of
INHBA than the other MSCs. Thus, these data demonstrate that
BM- and A-MSCs have properties of primitive multipotent stem cells.
KLF4 was ubiquitously expressed in MSCs, as well as
fibroblasts.
It is well known that MSCs possess immunosuppressive
properties and can inhibit the proliferation and function of major
immune cell populations, including T cells (32). In the present study, in activated
T cell co-cultures with MSCs in vitro, only the BM- and
A-MSCs significantly inhibited T cell proliferation induced by PHA.
While HLA-G expression is known to be involved in the
immunomodulation induced by MSCs, we also found that all MSCs and
fibroblasts were positive for HLA-A and HLA-G, and
negative for HLA-DRB4 (as shown by RT-PCR), indicating that
the expression of HLA molecules is not associated with the
inhibitory capacity of PHA-induced T cell proliferation (33,34). However, the possible involvement
of HLA-G in the immunosuppression of MSCs via other immune
cells cannot be excluded. Other factors associated with the
immunomodulatory effects of MSCs include IL10, TGFB1,
IL6 and TNFAIP6, TSG-6 (35,36). In the present study, BM-MSCs
displayed the greatest suppressive effects on T cells, and elevated
levels of IL10 and TGFB1 were noted in the BM-MSCs
compared to the other MSCs and the fibroblasts, and this is in
agreement with the findings of previous studies (37–39).
Concerning the multipotency of MSCs derived from
different tissues, their multilineage differentiation capacity was
confirmed by in vitro differentiation into osteoblasts,
adipocytes and chondrocytes. All of the cells had differentiation
potential for at least 2 lineages. In our study, fibroblasts also
differentiated toward adipocyte and chondrocyte lineages, as has
also been reported previously (20). Only the BM- and A-MSCs
differentiated into 3 lineages, including osteoblasts. To identify
functional regulator(s) that govern the differentiation potential
of MSCs into a specific lineage, we selected 6 genes that are known
to play key roles in mesodermal lineage differentiation and
verified that only DLX5 is differentially expressed in MSCs
with osteogenic potential. Our findings suggest that only BM- and
A-MSCs have tri-lineage differentiation potential and thus meet the
minimal criteria for an MSC, as defined by the ISCT. We also
demonstrated that B4GALNT1 (GM2/GS2 synthase), the
neural ganglioside GD2 synthase, is expressed by MSCs derived from
different tissues. This finding is consistent with the findings of
Martinez et al, that GD2 is a valuable marker that uniquely
distinguishes MSCs from fibroblasts (40).
DLX5, one of the mammalian homologs of the
Drosophila Distal-less (DLL/DLX) genes, is a
homeodomain transcription factor that regulates the development of
multiple cell types, including osteoblasts and neural cells
(41,42). Since DLX5 expression has
the potential to identify cells with lineage-specific
differentiation capacity, in the present study this was further
evaluated in MSCs from multiple donors. In all donors tested,
DLX5 was expressed in MSCs with dominant osteogenic
potential, i.e., BM- and A-MSCs. By contrast, 2 of 3 donors of
CB-MSC and 1 of 3 donors of P-MSCs expressed DLX5, and the
same donors exhibited a concurrent osteogenic phenotype, albeit to
varying degrees. Thus, the osteogenic potential of MSCs, regardless
of their tissue origin, appears to be related to DLX5
expression. To the best of our knowledge, this is the first study
that suggests that DLX5 expression is a predictive maker for
MSCs with osteogenic potential. However, it remains to be
determined, using a larger number of donors, whether DLX5
expression firmly characterizes a subset of MSCs with osteogenic
potential, although studies of inter-donor variation with regard to
growth rate, marker expression and multipotency have already been
undertaken (43,44).
Our finding of the variation in DLX5
expression between MSCs adds further support to the accumulating
evidence that points to substantial diversity both within and
between MSCs from various tissue sources (45–47), although little is known regarding
the functional differences between MSCs from different tissue
and/or different donors. Differences in donor age, gender,
genetics, epigenetics and environmental factors have been
postulated as the basis for this heterogeneity (48). The issue of MSC heterogeneity has
profound implications for clinical application of MSCs, such as
establishing standardized protocols that can generate functionally
equivalent cellular therapeutics (49,50). Thus, the characterization of MSCs
derived from various tissues with standardized protocols will have
a great impact on clinical outcomes, such as homing, repairing
and/or regenerating damaged tissues.
In conclusion, in this study, we demonstrated that
there are significant differences in the characteristics of MSCs
derived from various tissue sources and fibroblasts (used as
controls), including their multipotency, stemness signature and
lineage associated markers. Specifically, the BM- and A-MSCs
exhibited full tri-lineage (osteogenic, adipogenic and
chondrogenic) differentiation potential, and this ability was
associated with the expression of DLX5. In addition, there
was a donor-related variation of osteogenic potential in the CB-
and P-MSCs, and this potential appeared to be associated with
DLX5 expression. In conclusion, the findings of this
comparative study contribute to the development of MSC-based cell
therapies and regenerative medicine by providing valuable
information which can be used when selecting the optimal MSCs for
specified clinical applications.
Acknowledgments
This study was supported by a grant from the Korean
Health Technology R&D Project, Ministry of Health and Welfare,
Republic of Korea (HI13C1270).
References
|
1
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petersen BE, Bowen WC, Patrene KD, Mars
WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS and Goff JP:
Bone marrow as a potential source of hepatic oval cells. Science.
284:1168–1170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prockop DJ, Gregory CA and Spees JL: One
strategy for cell and gene therapy: harnessing the power of adult
stem cells to repair tissues. Proc Natl Acad Sci USA. 100(Suppl 1):
11917–11923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwartz RE, Reyes M, Koodie L, Jiang Y,
Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS and Verfaillie CM:
Multipotent adult progenitor cells from bone marrow differentiate
into functional hepatocyte-like cells. J Clin Invest.
109:1291–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan JM, Barry FP, Murphy JM and Mahon BP:
Mesenchymal stem cells avoid allogeneic rejection. J Inflamm
(Lond). 2:82005. View Article : Google Scholar
|
|
6
|
Mastri M, Lin H and Lee T: Enhancing the
efficacy of mesenchymal stem cell therapy. World J Stem Cells.
6:82–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma RR, Pollock K, Hubel A and McKenna
D: Mesenchymal stem or stromal cells: a review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedenstein AJ, Piatetzky-Shapiro II and
Petrakova KV: Osteogenesis in transplants of bone marrow cells. J
Embryol Exp Morphol. 16:381–390. 1966.PubMed/NCBI
|
|
9
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bajpai VK, Mistriotis P and Andreadis ST:
Clonal multipotency and effect of long-term in vitro expansion on
differentiation potential of human hair follicle derived
mesenchymal stem cells. Stem Cell Res (Amst). 8:74–84. 2012.
View Article : Google Scholar
|
|
11
|
Campagnoli C, Roberts IA, Kumar S, Bennett
PR, Bellantuono I and Fisk NM: Identification of mesenchymal
stem/progenitor cells in human first-trimester fetal blood, liver,
and bone marrow. Blood. 98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
In 't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetral or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004. View Article : Google Scholar
|
|
13
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC, Chung SM, et al: Tonsil-derived
mesenchymal stromal cells: Evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner W, Wein F, Seckinger A, Frankhauser
M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W
and Ho AD: Comparative characteristics of mesenchymal stem cells
from human bone marrow, adipose tissue, and umbilical cord blood.
Exp Hematol. 33:1402–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sohn HS, Heo JS, Kim HS, Choi Y and Kim
HO: Duration of in vitro storage affects the key stem cell features
of human bone marrow-derived mesenchymal stromal cells for clinical
transplantation. Cytotherapy. 15:460–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choudhery MS, Khan M, Mahmood R, Mehmood
A, Khan SN and Riazuddin S: Bone marrow derived mesenchymal stem
cells from aged mice have reduced wound healing, angiogenesis,
proliferation and anti-apoptosis capabilities. Cell Biol Int.
36:747–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jääger K and Neuman T: Human dermal
fibroblasts exhibit delayed adipogenic differentiation compared
with mesenchymal stem cells. Stem Cells Dev. 20:1327–1336. 2011.
View Article : Google Scholar
|
|
21
|
Ménard C and Tarte K: Immunoregulatory
properties of clinical grade mesenchymal stromal cells: evidence,
uncertainties, and clinical application. Stem Cell Res Ther.
4:642013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kemp KC, Hows J and Donaldson C: Bone
marrow-derived mesenchymal stem cells. Leuk Lymphoma. 46:1531–1544.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bieback K, Kern S, Klüter H and Eichler H:
Critical parameters for the isolation of mesenchymal stem cells
from umbilical cord blood. Stem Cells. 22:625–634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Evangelista M, Soncini M and Parolini O:
Placenta-derived stem cells: new hope for cell therapy?
Cytotechnology. 58:33–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikegame Y, Yamashita K, Hayashi S, Mizuno
H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, et
al: Comparison of mesenchymal stem cells from adipose tissue and
bone marrow for ischemic stroke therapy. Cytotherapy. 13:675–685.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sousa BR1, Parreira RC, Fonseca EA, Amaya
MJ, Tonelli FM, Lacerda SM, Lalwani P, Santos AK, Gomes KN, Ulrich
H, et al: Human adult stem cells from diverse origins: an overview
from multiparametric immunophenotyping to clinical applications.
Cytometry A. 85:43–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wagner W, Horn P, Castoldi M, Diehlmann A,
Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V and Ho
AD: Replicative senescence of mesenchymal stem cells: a continuous
and organized process. PLoS One. 3:e22132008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon DS, Kim YH, Jung HS, Paik S and Lee
JW: Importance of Sox2 in maintenance of cell proliferation and
multipotency of mesenchymal stem cells in low-density culture. Cell
Prolif. 44:428–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kishi M, Mizuseki K, Sasai N, Yamazaki H,
Shiota K, Nakanishi S and Sasai Y: Requirement of Sox2-mediated
signaling for differentiation of early Xenopus neuroectoderm.
Development. 127:791–800. 2000.PubMed/NCBI
|
|
31
|
Djouad F, Jackson WM, Bobick BE, Janjanin
S, Song Y, Huang GT and Tuan RS: Activin A expression regulates
multipotency of mesenchymal progenitor cells. Stem Cell Res Ther.
1:112010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nasef A, Mathieu N, Chapel A, Frick J,
François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D
and Fouillard L: Immunosuppressive effects of mesenchymal stem
cells: involvement of HLA-G. Transplantation. 84:231–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selmani Z, Naji A, Zidi I, Favier B,
Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et
al: Human leukocyte antigen-G5 secretion by human mesenchymal stem
cells is required to suppress T lymphocyte and natural killer
function and to induce CD4+CD25highFOXP3+
regulatory T cells. Stem Cells. 26:212–222. 2008. View Article : Google Scholar
|
|
35
|
Bunnell BA, Betancourt AM and Sullivan DE:
New concepts on the immune modulation mediated by mesenchymal stem
cells. Stem Cell Res Ther. 1:342010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HO, Choi SM and Kim HS: Mesenchymal
stem cell-derived secretome and microvesicles as a cell-free
therapeutics for neurodegenerative disorders. Tissue Eng Reg Med.
10:93–101. 2013. View Article : Google Scholar
|
|
37
|
Grütz G: New insights into the molecular
mechanism of interleukin-10-mediated immunosuppression. J Leukoc
Biol. 77:3–15. 2005.
|
|
38
|
Marie JC, Letterio JJ, Gavin M and
Rudensky AY: TGF-beta1 maintains suppressor function and Foxp3
expression in CD4+CD25+ regulatory T cells. J
Exp Med. 201:1061–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nasef A, Chapel A, Mazurier C, Bouchet S,
Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D and
Fouillard L: Identification of IL-10 and TGF-beta transcripts
involved in the inhibition of T-lymphocyte proliferation during
cell contact with human mesenchymal stem cells. Gene Expr.
13:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martinez C, Hofmann TJ, Marino R, Dominici
M and Horwitz EM: Human bone marrow mesenchymal stromal cells
express the neural ganglioside GD2: a novel surface marker for the
identification of MSCs. Blood. 109:4245–4248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long JE, Garel S, Depew MJ, Tobet S and
Rubenstein JL: DLX5 regulates development of peripheral and central
components of the olfactory system. J Neurosci. 23:568–578.
2003.PubMed/NCBI
|
|
43
|
Phinney DG, Kopen G, Righter W, Webster S,
Tremain N and Prockop DJ: Donor variation in the growth properties
and osteogenic potential of human marrow stromal cells. J Cell
Biochem. 75:424–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Portalska KJ, Groen N, Krenning G, Georgi
N, Mentink A, Harmsen MC, van Blitterswijk C and de Boer J: The
effect of donor variation and senescence on endothelial
differentiation of human mesenchymal stromal cells. Tissue Eng Part
A. 19:2318–2329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ho AD, Wagner W and Franke W:
Heterogeneity of mesenchymal stromal cell preparations.
Cytotherapy. 10:320–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Z, Zhang C, Weiner LP, Zhang Y and
Zhong JF: Molecular characterization of heterogeneous mesenchymal
stem cells with single-cell transcriptomes. Biotechnol Adv.
31:312–317. 2013. View Article : Google Scholar :
|
|
47
|
Sivasubramaniyan K, Lehnen D, Ghazanfari
R, Sobiesiak M, Harichandan A, Mortha E, Petkova N, Grimm S,
Cerabona F, de Zwart P, et al: Phenotypic and functional
heterogeneity of human bone marrow- and amnion-derived MSC subsets.
Ann NY Acad Sci. 1266:94–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Noer A, Sørensen AL, Boquest AC and Collas
P: Stable CpG hypomethylation of adipogenic promoters in freshly
isolated, cultured, and differentiated mesenchymal stem cells from
adipose tissue. Mol Biol Cell. 17:3543–3556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dominici M, Paolucci P, Conte P and
Horwitz EM: Heterogeneity of multipotent mesenchymal stromal cells:
from stromal cells to stem cells and vice versa. Transplantation.
87(Suppl): S36–S42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Phinney DG: Functional heterogeneity of
mesenchymal stem cells: Implications for cell therapy. J Cell
Biochem. 113:2806–2812. 2012. View Article : Google Scholar : PubMed/NCBI
|