Introduction
Joint hypermobility syndrome (JHS) is a heritable
connective tissue disorder characterized by generalized joint
hypermobility (excessive range of movement in the joints),
complications due to joint instability, minor skin changes, and
chronic mild to severe pain (1–3).
Severe complaints such as back pain, myalgias/myofascial pain,
dysmenorrhea and fatigue have also been reported in patients with
JHS (3). Patients with JHS often
fall into social isolation, emotional distress and depression
(3). Collective opinion is that
JHS and Ehlers-Danlos syndrome, hypermobility type (EDS-HT) are not
mutually exclusive and are in fact clinically overlapping disorders
(4).
Zweers et al (5) reported that haploinsufficiency of a
non-collagenous extracellular matrix (ECM) molecule, tenascin-X
(TNX), is associated with JHS/EDS-HT. Of the 20 heterozygous
individuals who exhibited haploinsufficiency of TNX regardless of
clinical symptoms, 9 (45%) of the individuals presented with
generalized joint hypermobility, recurring joint dislocations and
chronic joint pain, as seen in patients with JHS/EDS-HT, whereas 6
(7.5%) of 80 patients with EDS-HT exhibited haploinsufficiency of
TNX with significantly reduced serum TNX levels (5). These findings indicated that
haploinsufficiency of TNX is present in but a small subset of
patients with JHS/EDS-HT. Therefore, the causative gene in the
majority of individuals with JHS remains unknown.
Joint hypermobility is measured by the Beighton
nine-point scale (6). JHS can be
defined by the Brighton criteria; the major criteria for this
classification include a Beighton score of 4/9 or greater and
arthralgia for more than 3 months in more than 4 joints, and the
diagnosis is also based on minor criteria including joint
dislocation, soft tissue lesions, and abnormal skin (7). However, at present there is no
precise biomarker or reliable diagnostic test for JHS.
The development of proteomic analysis has assisted
in comparisons of comprehensive differentially expressed proteins
under pathological conditions and controls (8,9).
Researchers have successfully applied quantitative proteome
analyses using isobaric tags for relative and absolute quantitation
(iTRAQ) technology (10),
followed by nano liquid chromatography (nano LC)-matrix-assisted
laser desorption ionization (MALDI)-time of flight (TOF/TOF)-tandem
mass spectrometry (MS/MS) to reveal differentially expressed
proteins in lesion tissues and sera of patients with cardiovascular
diseases such as aortic aneurysms and aortic valve stenosis. These
analyses are valuable, as they elucidated the molecular mechanisms
underlying these diseases and/or identified disease biomarkers in
both tissue samples (11,12) and serum samples (13). However, to the best of our
knowledge, proteomic analysis in the field of JHS had not been
carried out prior to this research.
In the present study, in order to elucidate the
distinct molecular alteration that will lead to the discovery of
novel biomarkers in sera of patients with JHS, serum proteins with
differential levels in patients with JHS compared with those in
healthy individuals were investigated with iTRAQ labeling followed
by nano LC-MALDI-TOF/TOF-MS/MS.
Patients and methods
Collection of blood samples from patients
with JHS and controls
Blood samples were collected after approval from the
Ethics Committee of Nippon Medical School (Tokyo, Japan). Blood
samples from patients with JHS and healthy control individuals who
came to Nippon Medical School Hospital were collected. The patients
and healthy individuals provided written informed consent.
Inclusion criteria for this study were based on JHS diagnostic
criteria (14,15), modified by our experience to
include the following: i) generalized joint hypermobility (Beighton
score >4), ii) chronic joint pain, and iii) recurring joint
dislocations. Clinical profiles of 18 patients with JHS (patient
no. 1, 3, 4, 7, 10, 12, 14, 22, 29, 31, 34, 37, 40, 45, 51, 53, 68
and 73) [all were female, aged between 13 and 64 years (average age
was 30.7 years)] in this study are shown in Table I. Blood samples were centrifuged
at 1,500 × g for 15 min at 4°C and the plasma layer was
removed.
 | Table IClinical profiles of patients with JHS
in this study. |
Table I
Clinical profiles of patients with JHS
in this study.
| Clinical profile | No. (%)(n=18) |
|---|
| Family history of
JHS | 9 (50) |
| Joint dislocations
(any) | 18 (100) |
| Shoulders | 16 (89) |
| Fingers | 15 (83) |
| Knees | 10 (56) |
| Wrists | 7 (39) |
| Elbows | 6 (33) |
| Ankles | 6 (33) |
| Pain (any) | 18 (100) |
| Joint pain | 17 (94) |
| Back pain | 16 (89) |
| Muscle pain | 14 (78) |
| Skin manifestation
(any) | 7 (39) |
| Skin
hyperextensibility | 7 (39) |
| Fragile skin | 3 (17) |
| Chronic fatigue | 18 (100) |
| Bruising easily | 12 (67) |
| Postural
hypotension | 18 (100) |
| Headache | 14 (78) |
| Temporomandibular
disorder | 13 (72) |
| Recurrent
caries | 9 (50) |
| Gum fragility | 11 (61) |
| Temporomandibular
joint hypermobility | 8 (44) |
| Gastritis | 7 (39) |
| Abdominal pain | 5 (28) |
| Chronic diarrhea | 7 (39) |
The blood samples for proteomic analyses and
examination of correlations of iTRAQ ratios with western blot
ratios were collected from 6 patients with JHS (patient no. 1, 4,
10, 12, 14 and 22) (average age, 25.2 years) and 6 control healthy
individuals (no. 11, 13, 25, 36, 52, and 60) (all females; average
age was 42 years) (Table II). In
addition, other blood samples for western blot analyses were
collected from 12 patients with JHS (patient no. 3, 7, 29, 31, 34,
37, 40, 45, 51, 53, 68 and 73) (average age, 33.9 years) and 7
control healthy individuals (no. 9, 21, 27, 35, 44, 55 and 57) (all
females; average age, 51.3 years) (Table II). For control serum mixtures
used in proteomic analyses and western blot analyses, sera mixed
with equal amounts of 6 and 13 control healthy individuals were
used, respectively.
 | Table IISera used for iTRAQ labeling followed
by nano LC-MALDI-TOF/TOF-MS/MS proteomic analyses and western blot
analyses. |
Table II
Sera used for iTRAQ labeling followed
by nano LC-MALDI-TOF/TOF-MS/MS proteomic analyses and western blot
analyses.
Sera used for
proteomic analyses
|
|---|
| JHS patient
no. | Gender | Age (years) |
|---|
| 1 | F | 22 |
| 4 | F | 34 |
| 10 | F | 16 |
| 12 | F | 19 |
| 14 | F | 32 |
| 22 | F | 28 |
| Control individual
no. | | |
| 11 | F | 45 |
| 13 | F | 53 |
| 25 | F | 44 |
| 36 | F | 16 |
| 52 | F | 44 |
| 60 | F | 50 |
| Sera used for
western blot analyses |
| JHS patient
no. | Gender | Age (years) |
| 3 | F | 42 |
| 7 | F | 27 |
| 29 | F | 25 |
| 31 | F | 64 |
| 34 | F | 57 |
| 37 | F | 13 |
| 40 | F | 31 |
| 45 | F | 26 |
| 51 | F | 25 |
| 53 | F | 22 |
| 68 | F | 42 |
| 73 | F | 33 |
| Control individual
no. | | |
| 9 | F | 61 |
| 21 | F | 58 |
| 27 | F | 50 |
| 35 | F | 49 |
| 44 | F | 45 |
| 55 | F | 52 |
| 57 | F | 44 |
Immunodepletion of abundant serum
proteins
Fifty microliters of serum was first processed with
an albumin and immunoglobulin (IgG) depletion SpinTrap column
according to the manufacturer's instructions (GE Healthcare,
Buckinghamshire, UK) and also according to our previous study
(13) to remove the two major
serum proteins, albumin and IgG. Thereafter, the serum samples were
equilibrated with 50 mM triethylammonium bicarbonate (TEAB; Sigma,
Tokyo, Japan) using Spin-X UF concentrators (Corning, Tokyo,
Japan). Protein concentration was then determined using a
bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher
Scientific, Rockford, IL, USA).
Trypsin digestion, iTRAQ labeling, strong
cation exchange (SCX) chromatography, and nano LC
Sample preparation was performed according to the
manufacturer's instructions (AB Sciex, Foster City, CA, USA) and
according to our previous study (13). In order to investigate the
differentially expressed serum proteins between a mixed sample of
control healthy individuals and each sample taken from patients
with JHS, 100 µg of the mixed sample consisting of sera of 6
control healthy individuals and an equivalent amount of each sample
from the 6 patients with JHS were denatured by sodium dodecyl
sulfate (SDS) and reduced by tris-(2-carboxyethyl) phosphine
(TCEP), followed by cysteine alkylation with
methylmethanethiosulfonate (MMTS). Subsequently, each sample was
digested by trypsin (AB Sciex). Each digest was labeled with a
different iTRAQ tag by an iTRAQ reagent multiplex kit (AB Sciex).
iTRAQ label 114 or 115 was used for labeling the control mixed
sample, whereas iTRAQ label 116 or 117 was used for each of the
samples from patients with JHS. The labeled control mixed sample
and sample from patients with JHS were combined. Subsequently, the
combined sample was fractionated into 6 fractions by SCX
chromatography according to the manufacturer's instructions (AB
Sciex). Subsequently, each fraction was desalted by a Sep-Pak C18
cartridge according to the manufacturer's instructions (Waters,
Milford, MA, USA). Further fractionation of each fraction from SCX
chromatography to 171 spots was undertaken while mixing directly
with a matrix [4 mg/ml alpha-cyano-4-hydroxycinnamic acid (CHCA);
Wako, Osaka, Japan] with a DiNa nanoLC system (KYA Technologies,
Tokyo, Japan) according to the manufacturer's instructions and our
previous study (13).
Subsequently, the spots were placed on an Opti-TOF LC/MALDI 384
target plate (AB Sciex) using a DiNa MaP fraction collector (KYA
Technologies). Similarly, as a control in order to examine
differentially expressed serum proteins between the mixed sample of
control healthy individuals and each sample of the control
individuals, the same experiments were also undertaken using these
samples.
MALDI-TOF/TOF MS/MS analysis
The spots were analyzed on a 5800 MALDI-TOF/TOF
MS/MS analyzer with TOF/TOF Series software (version 4.0; AB Sciex)
to obtain MS and MS/MS data according to the manufacturer's
instructions. The peptide data obtained from 5800 MALDI TOF/TOF
MS/MS were analyzed with ProteinPilot™ 3.0 software using the
Paragon protein database search algorithm (AB Sciex) (16). Search results were filtered for a
global false discovery rate (FDR) of 5%, employing a decoy search
strategy which utilized a reverse database constructed by AB Sciex
(version 20081216, 40,978 entries). In the present study, the
statistical analyses for iTRAQ were undertaken using ProteinPilot
software (AB Sciex).
Panther classification analysis
In the present study, the Panther system (version
9.0) (http://www.pantherdb.org/) was used for
protein classification analyses, as previously described (17). The statistical analyses of protein
classification were performed with a statistical overrepresentation
test using Panther software. The annotations of proteins were
acquired from the UniProt database (http://www.uniprot.org/) and mentioned based on our
knowledge.
Western blot analysis
Western blot analyses were completed as described in
our previous study (18).
Briefly, albumin/IgG-immunodepleted serum samples and crude sera
for western blot analyses of vitronectin (VTN) and complement C1r
subcomponent (C1R) were electrophoresed through SDS-polyacrylamide
gel (SDS-PAGE), and the proteins were then transferred onto Hybond
ECL nitrocellulose membranes (GE Healthcare Japan, Hino, Japan).
The amounts of albumin/IgG-immunodepleted sera (µg) and
crude sera (µl) used for each analysis were as follows: VTN
(10 µg and 0.75 µl) and C1R (10 µg and 0.5
µl), respectively. The membranes were reacted with a rabbit
polyclonal anti-VTN antibody (dilution 5,000) and rabbit polyclonal
anti-C1R antibody (dilution 2,500) (both from GeneTex, Irvine, CA,
USA). The membranes were then reacted with donkey IRDye
680-conjugated anti-rabbit IgG (H+L) (dilution 5,000; LI-COR,
Lincoln, NE, USA), followed by visualization using the infrared
imaging system Odyssey (LI-COR). The intensity of each band that
reacted with a corresponding antibody was measured for
densitometric analysis of each protein level. To confirm equal
levels of proteins per lane, nonspecific proteins were stained with
Coomassie Brilliant Blue (CBB).
Statistical analysis
Data from triplicate experiments were subjected to
unpaired Student's t-test for statistical significance. A p-value
<0.05 was considered to indicate a statistically significant
difference. Results are expressed as the means ± standard error
(SE). The correlation coefficient between the iTRAQ value and
western blot value was calculated by CORREL function in Excel 2010
(Microsoft, Redmond, WA, USA).
Results
Proteomic analyses of serum proteins with
differential levels in patients with JHS and in healthy control
individuals
Protein levels in serum from each of the patients
with JHS were compared with mixed sera of the 6 healthy control
individuals using iTRAQ labeling coupled with nano
LC-MALDI-TOF/TOF-MS/MS followed by ProteinPilot analysis. Relative
quantitation by ProteinPilot analysis was based on statistical
analysis, as previously described (16). We set global FDR to 5%. The
average iTRAQ ratios of peptides in sera of patients with JHS and
those in sera of mixed control individuals were calculated. Aside
from albumin and Ig family members, a total of 106 differential
level proteins found in the sera of 6 patients with JHS were
identified in at least one patient's serum compared with those in
the mixture of the sera from 6 control individuals (data not
shown). Of these proteins, 64 differential level proteins were
identified as being in common with the sera of at least 4 patients,
with an unused ProtScore of ≥3.4 (99.96% confidence) (data not
shown). Subsequently, we examined the differential levels of the 64
identified proteins in the 6 healthy control individuals. Finally,
6 of the 64 proteins were identified as proteins with significantly
different expression level in patient sera than in the sera of
control individuals (p<0.05, JHS patient group vs. control
group). In Table III, the six
proteins [complement C1r subcomponent (C1R), apolipoprotein B-100
(APOB), VTN, complement component C9 (C9), C4b-binding protein
alpha chain (C4BPA), and transthyretin (TTR)] are listed in order
of iTRAQ ratios.
 | Table IIIProteins with differential levels in
patients with JHS and in healthy control individuals. |
Table III
Proteins with differential levels in
patients with JHS and in healthy control individuals.
| Unused
ProtScorea | Coverageb (%) | Peptidesc (95%) | UniProt no. | Gene symbol | Protein name | iTRAQ ratiod average ± SE | P-valuee | Molecular
function |
|---|
| 3.4 | 12.8 | 3 | P00736 | C1R | Complement C1r
subcomponent | 1.40±0.11 | 0.0100 | Complement |
| 211.3 | 42.8 | 133 | P04114 | APOB | Apolipoprotein
B-100 | 1.26±0.05 | 0.0195 | Ligand for LDL
receptor |
| 19.8 | 28.7 | 12 | P04004 | VTN | Vitronectin | 1.23±0.06 | 0.0344 | Cell adhesion
molecule |
| 6.0 | 16.3 | 4 | P02748 | C9 | Complement
component C9 | 1.15±0.12 | 0.0402 | Complement |
| 10.6 | 22.8 | 6 | P04003 | C4BPA | C4b-binding protein
α chain | 1.10±0.09 | 0.0372 | Complement |
| 6.2 | 52.4 | 4 | P02766 | TTR | Transthyretin | 1.06±0.10 | 0.0171 | Thyroxine and
retinol-binding protein |
Classfication analyses of the six
identified proteins
We examined the functional classification of each of
the six identified proteins in sera of patients with JHS with
Panther protein class analyses and Panther software, which sorts
the proteins into respective classes based on their biological
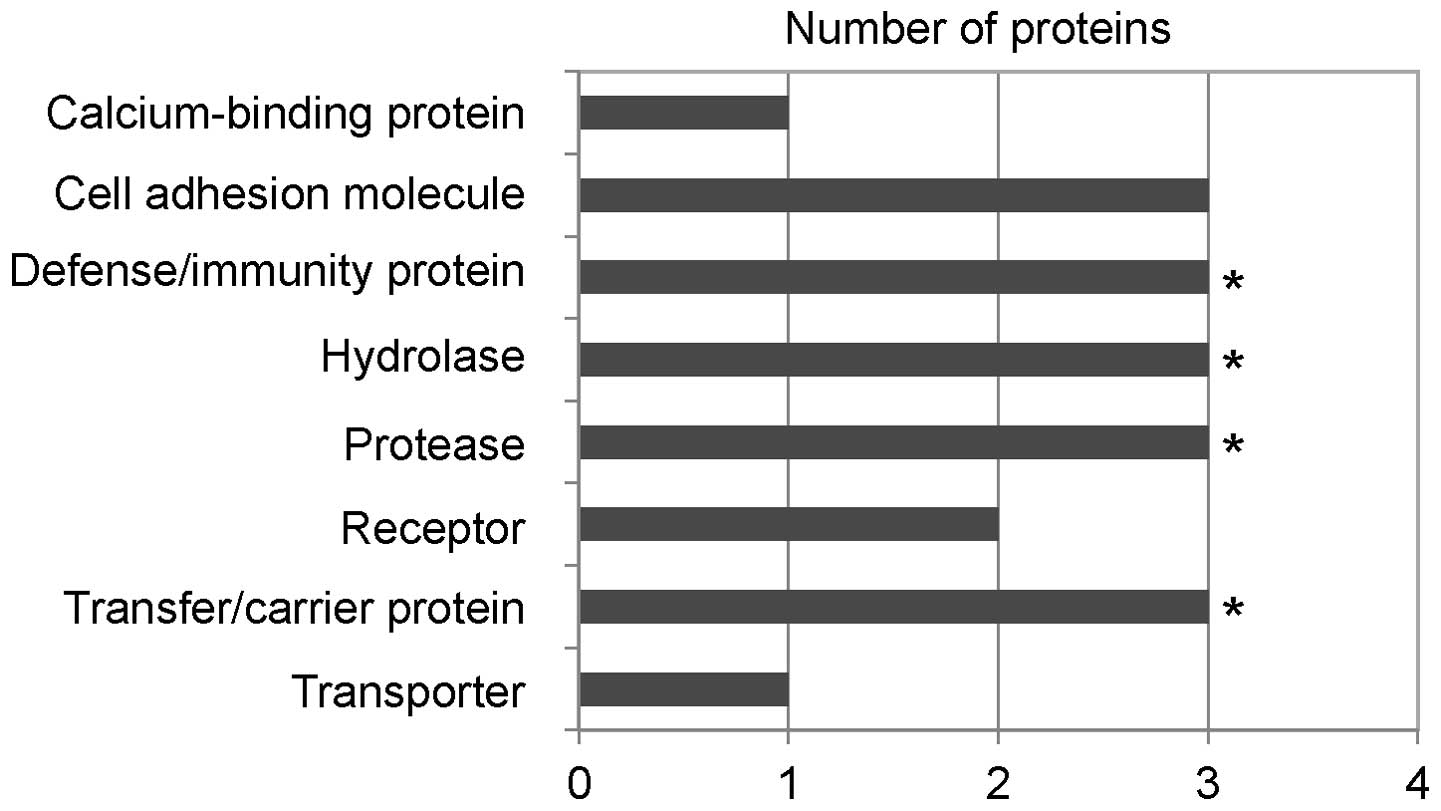
functions (Fig. 1).
Defense/immunity proteins (p=0.00018) including complement
components (C1R, C9 and C4BPA), hydrolase/protease including serine
protease (p=0.0073) (C1R, C9 and C4BPA), and transfer/carrier
proteins (p=0.028) (C9, C4BPA and TTR) were found to be
statistically significant. VTN had previously been shown to be
involved in the complement system: it acts as a potent inhibitor of
complement activation by forming an inactive terminal complement
complex, C5b-9 (19). Therefore,
we suggest that at least four proteins (C1R, VTN, C9 and C4BPA) of
the six identified proteins participate in the complement
system.
Confirmation of iTRAQ ratios by western
blot analysis
To validate the accuracy of quantitative results for
the differentially expressed proteins that were identified, we
focused on VTN and C1R due to their high iTRAQ ratios among the six
proteins, as shown in Table
III, and the participation of both proteins in the complement
system. We quantified again VTN and C1R with an iTRAQ quantitative
ratio by western blot analyses and investigated the correlations
between iTRAQ ratios and relative quantitative ratios by western
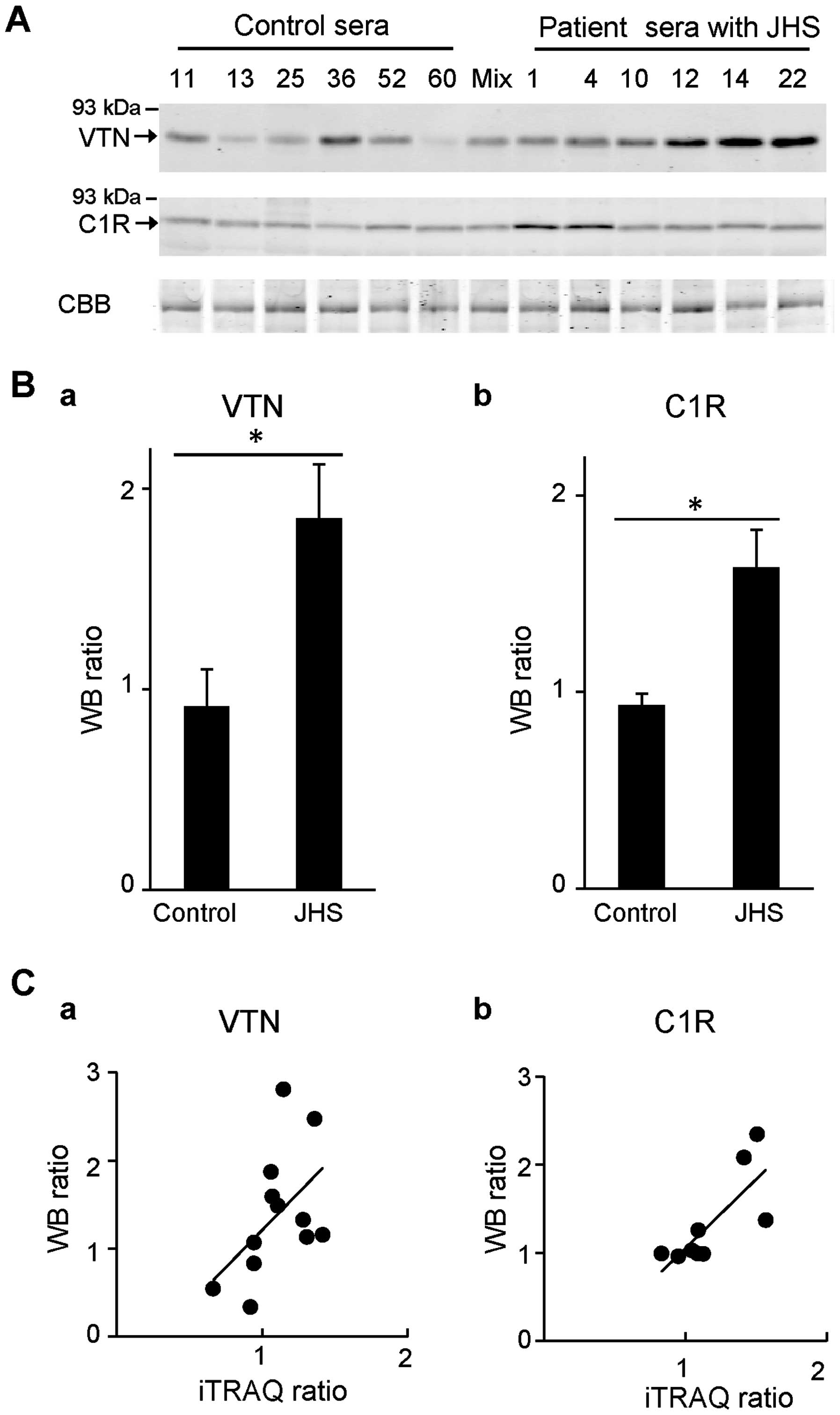
blot analyses (Fig. 2). The
levels of VTN (iTRAQ ratio=1.23) and C1R (iTRAQ ratio=1.40) in
albumin/IgG-immunodepleted serum samples of each of the 6 patients
with JHS and the 6 control individuals used for iTRAQ analyses were
examined by western blot analyses (Fig. 2A). Subsequently, the ratios of
levels of VTN and C1R in these samples compared with those in the
mixed control samples (1.0) were determined by examining band
intensity, and the mean value of each group, as shown in Fig. 2B. We noted that relative
quantitative ratios of the samples of patients with JHS compared to
the mixed control samples were 1.85-fold for VTN and 1.63-fold for
C1R. In addition, the correlations between iTRAQ ratios and western
blot ratios of each JHS and control sample compared to the mixed 6
control samples were investigated (Fig. 2C). Both proteins demonstrated a
tendency for positive correlation (VTN, n=12, r=0.51, p=0.094; C1R,
n=9, r=0.77, p=0.015). These results indicated that iTRAQ ratios
are almost consistent with quantitative results obtained by western
blot analyses.
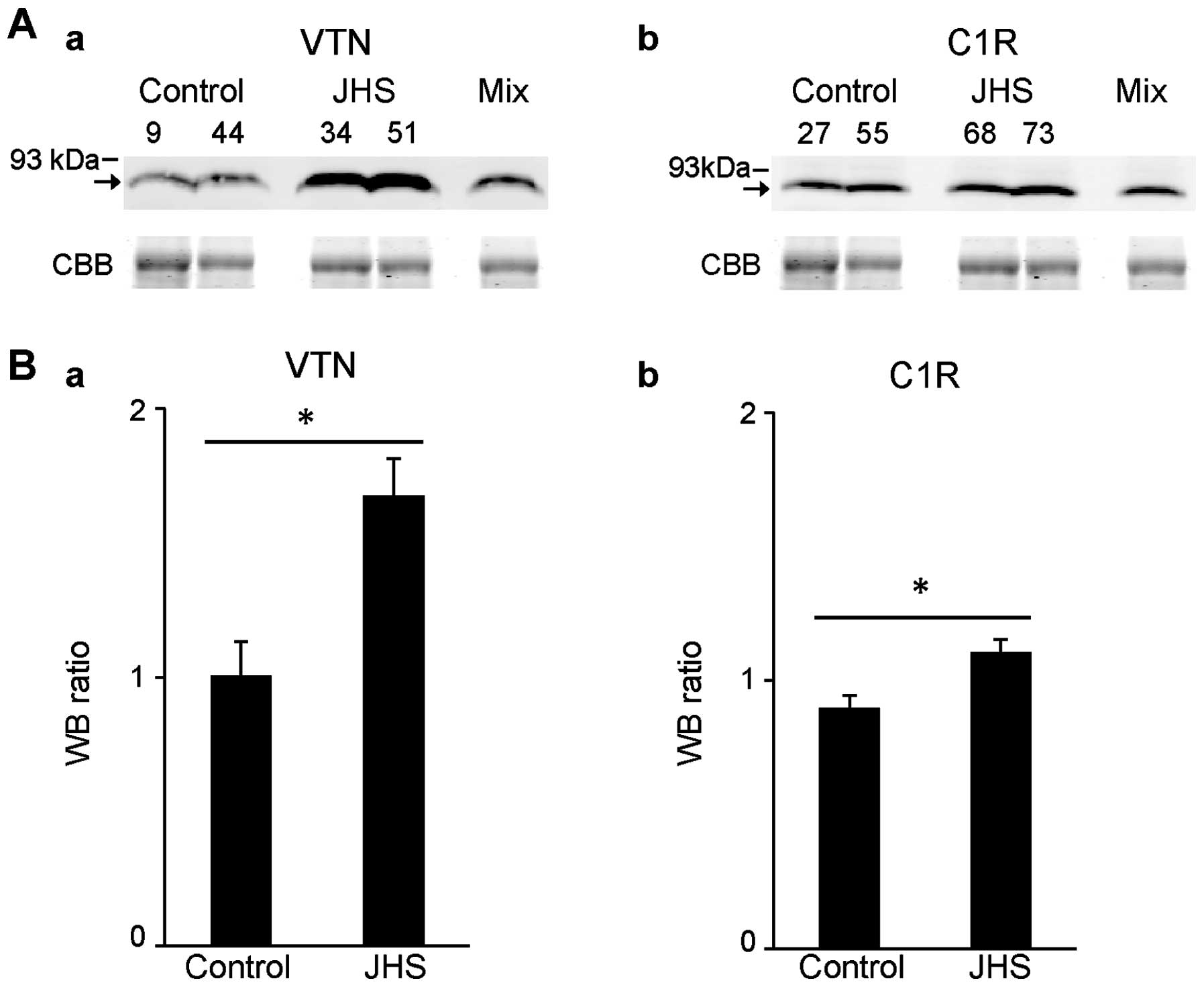
Verification of increased levels of VTN
and C1R in patients with JHS compared with other sera
To further confirm the increased levels of VTN and
C1R in sera of patients with JHS compared with those of control
individuals, we performed western blot analyses using sera of 12
other patients with JHS and compared them with sera of 7 other
control individuals, in addition to the sera of each of the 6
patients with JHS and control individuals which were used for the
proteomic analyses. As a result, we confirmed increased levels of
VTN and C1R in the sera of patients with JHS compared with those
from the control individuals by western blot analyses (Fig. 3A). The ratios of levels of VTN and
C1R in the sera of 18 patients with JHS compared with those in the
mixed sera of the 13 control individuals (1.0) were measured by the
intensity of each band, and mean ratios of the two groups were
1.68- and 1.11-fold, respectively (Fig. 3B). These results confirmed the
increased levels of VTN and C1R in sera of other patients with JHS
compared with those of control individuals.
Discussion
The molecular defect underlying JHS/EDS-HT remains
largely unknown, and TNX has been identified as a gene in
only 5–10% of patients with JHS/EDS-HT (5). As for serum disease markers for JHS,
to the best of our knowledge, no attempt has yet been made to
identify markers. In the present study, using iTRAQ labeling
followed by nano LC-MALDI-TOF/TOF-MS/MS analysis, we determined the
proteomic profiles of proteins with differential levels in sera of
patients with JHS and in control individuals. Consequently, we
identified six proteins, including four proteins involved in the
complement system as proteins with differential levels in sera from
patients with JHS and control individuals.
The complement system is not only involved in host
defense recognition and the elimination of potentially harmful
exogenous and endogenous microbial pathogens but also in different
forms of acute and choronic inflammatory diseases such as sepsis
and rheumatic disease. Previous research has revealed a fascinating
interplay between the complement system and inflammatory network
(20). C3a and C5a are known to
be crucially involved in inflammatory responses. Activation of the
complement system has been shown to be related to the pathogenesis
of inflammatory arthritis and articular injury (21). However, the possible involvement
of the complement system in the development of JHS remained
unclear. In the present study, four proteins, C1R, VTN, C9 and
C4BPA, were identified as proteins with differential levels in sera
of patients with JHS and control individuals. Inflammation
associated with joint instability, degenerative joint disease, and
chronic pain is common in patients with JHS. C1R, which is a
component of C1, is the serine protease responsible for intrinsic
activation of the C1 complex of complement. It has been previously
reported that C1R is synthesized and secreted in tissue and primary
cell cultures of synovia from patients with rheumatoid arthritis,
indicating the involvement of C1R in the inflammatory process in
rheumatoid arthritis (22). VTN
is a plasma multifunctional glycoprotein which is implicated in
cell migration, blood coagulation, fibrinogenesis, the inflammatory
process, and also membrane attack complex (MAC) formation. VTN
binds directly to the C5b-7 complex and C9, with distinct binding
sites on VTN (23). VTN also acts
as an inhibitor of the cytolytic reactions of MAC. Notably, VTN and
C9 have been identified as the differential level proteins in the
sera of patients with JHS in the present study. It has previously
been reported that C9 deposition was noted in the synovial
vasculature of patients with acute arthritis and rheumatoid
arthritis (24). In addition, it
should be noted that C4BP is a potent circulating soluble inhibitor
of the classical and lectin pathways of the complement system
(25). C4BPA binds to C4b, and
this interaction inhibits the complement activation pathway by
reducing the formation and stability of C4bC2b (C3 convertase).
Intriguingly, it has been shown that C4BP lacking the β-chain
(α7β0 isoform) induced a semimature and
anti-inflammatory state in dendric cells activated by a
pro-inflammatory stimulus (26).
Certain components of the local inflammatory
response such as cytokines, neuropeptides and complement-related
proteins, particularly C3a and C5a, are known to play roles in pain
(27,28). Local injection of C5a and C3a
elicited nociception, and C5a and C3a activate and sensitize
cutaneous nociceptors (29).
These results indicated that C5a and C3a are involved in pain.
Patients with JHS exhibit chronic pain, distinct from that
associated with acute dislocations (3). The changes in expression levels of
some complement factors in patients with JHS compared with those in
control individuals may contribute to such symptoms of chronic
pain.
The six identified proteins include APOB and TTR as
well as the four complement-related proteins. Plasma concentration
of APOB is known to be a good marker of cardiovascular risk
(30). APOB is a ligand for the
low-density lipoprotein (LDL) receptor that participates in
cholesterol transport to peripheral tissues and its accumulation in
the arterial wall. Increased levels of apolipoprotein A-I (APOA1)
and APOB have been observed in patients with osteoarthritis
(31). TTR functions as a carrier
for thyroxin and retinol-binding protein (RBP). Wilson (32) has reported that exome analysis of
a patient with EDS classical type (type I) revealed three
heterozygous mutations in TTR, fibrillin 1 (FBN1), and
voltage-gated calcium channel subunit alpha Cav2.1 (CACNA1A)
genes.
Our quantitative proteomic strategy employed in the
present study allowed for the identification of six potential
biomarkers of JHS, four of which are proteins involved in
regulation of the complement system associated with inflammation
and chronic pain. Our results provide valuable information on the
underlying mechanisms of JHS and will contribute to the
establishment of a method for early diagnosis of JHS and to the
development of pharmacological therapies.
In conclusion, to the best of our knowledge, this is
the first comprehensive study on differentially expressed proteins
in the sera of patients with JHS and control individuals using an
approach involving iTRAQ labelling. Proteomic characterization of
the sera of patients with JHS revealed increased levels of four
proteins involved in the regulation of the complement system.
Future work should focus on deciphering the role of each of the 6
potential biomarker proteins identified in the pathogenesis of
JHS/EDS-HT.
Acknowledgments
This study was supported by the Ministry of Health
Labour and Welfare for Health Labour Sciences Research Grant
(Research on Measures for Intractable Diseases) grant no.
2011-Nanchi-Ippan-110 to A.W. and the Japan Society for the
Promotion of Science (JSPS) KAKENHI grant nos. 26462296 to K.M.
References
|
1
|
Grahame R: Joint hypermobility and genetic
collagen disorders: are they related? Arch Dis Child. 80:188–191.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malfait F, Hakim AJ, De Paepe A and
Grahame R: The genetic basis of the joint hypermobility syndromes.
Rheumatology (Oxford). 45:502–507. 2006. View Article : Google Scholar
|
|
3
|
Castori M, Morlino S, Celletti C,
Ghibellini G, Bruschini M, Grammatico P, Blundo C and Camerota F:
Re-writing the natural history of pain and related symptoms in the
joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility
type. Am J Med Genet A. 161A:2989–3004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tinkle BT, Bird HA, Grahame R, Lavallee M,
Levy HP and Sillence D: The lack of clinical distinction between
the hypermobility type of Ehlers-Danlos syndrome and the joint
hypermobility syndrome (a.k.a. hypermobility syndrome). Am J Med
Genet A. 149A:2368–2370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zweers MC, Bristow J, Steijlen PM, Dean
WB, Hamel BC, Otero M, Kucharekova M, Boezeman JB and Schalkwijk J:
Haploinsufficiency of TNXB is associated with hypermobility type of
Ehlers-Danlos syndrome. Am J Hum Genet. 73:214–217. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beighton P: Hypermobility scoring. Br J
Rheumatol. 27:1631988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grahame R, Bird HA and Child A: The
revised (Brighton 1998) criteria for the diagnosis of benign joint
hypermobility syndrome (BJHS). J Rheumatol. 27:1777–1779.
2000.PubMed/NCBI
|
|
8
|
Sutton CW, Rustogi N, Gurkan C, Scally A,
Loizidou MA, Hadjisavvas A and Kyriacou K: Quantitative proteomic
profiling of matched normal and tumor breast tissues. J Proteome
Res. 9:3891–3902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bijian K, Mlynarek AM, Balys RL, Jie S, Xu
Y, Hier MP, Black MJ, Di Falco MR, LaBoissiere S and Alaoui-Jamali
MA: Serum proteomic approach for the identification of serum
biomarkers contributed by oral squamous cell carcinoma and host
tissue microenvironment. J Proteome Res. 8:2173–2185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross PL, Huang YN, Marchese JN, Williamson
B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, et
al: Multiplexed protein quantitation in Saccharomyces cerevisiae
using amine-reactive isobaric tagging reagents. Mol Cell
Proteomics. 3:1154–1169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumoto K, Satoh K, Maniwa T, Araki A,
Maruyama R and Oda T: Noticeable decreased expression of tenascin-X
in calcific aortic valves. Connect Tissue Res. 53:460–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto K, Satoh K, Maniwa T, Tanaka T,
Okunishi H and Oda T: Proteomic comparison between abdominal and
thoracic aortic aneurysms. Int J Mol Med. 33:1035–1047.
2014.PubMed/NCBI
|
|
13
|
Satoh K, Maniwa T, Oda T and Matsumoto K:
Proteomic profiling for the identification of serum diagnostic
biomarkers for abdominal and thoracic aortic aneurysms. Proteome
Sci. 11:272013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beighton P, De Paepe A, Steinmann B,
Tsipouras P and Wenstrup RJ: Ehlers-Danlos syndromes: revised
nosology, Villefranche, 1997. Ehlers-Danlos National Foundation
(USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet.
77:31–37. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levy HP: Ehlers-Danlos Syndrome,
Hypermobility Type. Gene-Reviews (Internet). Pagon RA, Adam MP,
Ardinger HH, et al: Last Update: September 13, 2012. University of
Washington; Seattle: 1993–2015
|
|
16
|
Shilov IV, Seymour SL, Patel AA, Loboda A,
Tang WH, Keating SP, Hunter CL, Nuwaysir LM and Schaeffer DA: The
Paragon Algorithm, a next generation search engine that uses
sequence temperature values and feature probabilities to identify
peptides from tandem mass spectra. Mol Cell Proteomics.
6:1638–1655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mi H, Muruganujan A and Thomas PD: PANTHER
in 2013: modeling the evolution of gene function, and other gene
attributes, in the context of phylogenetic trees. Nucleic Acids
Res. 41:D377–D386. 2013. View Article : Google Scholar :
|
|
18
|
Nakamura Y, Takayama N, Minamitani T,
Ikuta T, Ariga H and Matsumoto K: Primary structure, genomic
organization and expression of the major secretory protein of
murine epididymis, ME1. Gene. 251:55–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Podack ER and Müller-Eberhard HJ:
Isolation of human S-protein, an inhibitor of the membrane attack
complex of complement. J Biol Chem. 254:9808–9814. 1979.PubMed/NCBI
|
|
20
|
Markiewski MM and Lambris JD: The role of
complement in inflammatory diseases from behind the scenes into the
spotlight. Am J Pathol. 171:715–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mizuno M: A review of current knowledge of
the complement system and the therapeutic opportunities in
inflammatory arthritis. Curr Med Chem. 13:1707–1717. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Breitner S, Störkel S, Reichel W and Loos
M: Complement components C1q, C1r/C1s, and C1INH in rheumatoid
arthritis. Correlation of in situ hybridization and northern blot
results with function and protein concentration in synovium and
primary cell cultures. Arthritis Rheum. 38:492–498. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milis L, Morris CA, Sheehan MC,
Charlesworth JA and Pussell BA: Vitronectin-mediated inhibition of
complement: evidence for different binding sites for C5b-7 and C9.
Clin Exp Immunol. 92:114–119. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konttinen YT, Ceponis A, Meri S,
Vuorikoski A, Kortekangas P, Sorsa T, Sukura A and Santavirta S:
Complement in acute and chronic arthritides: assessment of C3c, C9,
and protectin (CD59) in synovial membrane. Ann Rheum Dis.
55:888–894. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blom AM, Villoutreix BO and Dahlbäck B:
Complement inhibitor C4b-binding protein-friend or foe in the
innate immune system? Mol Immunol. 40:1333–1346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivar R, Luque A, Naranjo-Gómez M, Quer
J, García de Frutos P, Borràs FE, Rodríguez de Córdoba S, Blom AM
and Aran JM: The α7β0 isoform of the complement regulator
C4b-binding protein induces a semimature, anti-inflammatory state
in dendritic cells. J Immunol. 190:2857–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coutaux A, Adam F, Willer JC and Le Bars
D: Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone
Spine. 72:359–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang JH, Liang D, Kido K, Sun Y, Clark DJ
and Brennan TJ: Increased local concentration of complement C5a
contributes to incisional pain in mice. J Neuroinflammation.
8:802011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jang JH, Clark JD, Li X, Yorek MS, Usachev
YM and Brennan TJ: Nociceptive sensitization by complement C5a and
C3a in mouse. Pain. 148:343–352. 2010. View Article : Google Scholar :
|
|
30
|
Thompson A and Danesh J: Associations
between apolipoprotein B, apolipoprotein AI, the apolipoprotein
B/AI ratio and coronary heart disease: a literature-based
meta-analysis of prospective studies. J Intern Med. 259:481–492.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sánchez-Enríquez S, Torres-Carrillo NM,
Vázquez-Del Mercado M, Salgado-Goytia L, Rangel-Villalobos H and
Muñoz-Valle JF: Increase levels of apo-A1 and apo B are associated
in knee osteoarthritis: lack of association with VEGF-460 T/C and
+405 C/G polymorphisms. Rheumatol Int. 29:63–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilson GN: Exome analysis of connective
tissue dysplasia: death and rebirth of clinical genetics? Am J Med
Genet A. 164A:1209–1212. 2014. View Article : Google Scholar : PubMed/NCBI
|