Introduction
Worldwide, breast cancer is the leading type of
cancer in women, and is much more common in developed countries,
due to greater wealth and related dietary habits. Long-term use of
oral contraceptives and low body mass index (BMI) are associated
with an increased risk of premenopausal breast cancer (1,2).
Breast cancer in young women is thought to be associated with
high-grade tumors, negative hormone receptors and overexpression of
human epidermal growth factor receptor 2 (HER2) (3). The overall worldwide burden of
breast cancer has increased significantly, with the mortality rates
steadily decreasing, owing to early detection and improved
therapies (3). Survival rates are
higher in the developed world, with nearly 80% of affected patients
in England and the United States surviving for at least 5 years;
however, in developing countries, survival rates are poorer
(4–6).
Mammalian target of rapamycin (mTOR) plays a central
role in the regulation of cell fate and cancer progression
(7,8). In particular, mTOR activation is one
of the most frequent events in human malignancies, and inhibition
of mTOR by rapamycin is an effective and promising strategy in
anticancer treatments. mTOR activity is also critical for
sustaining the self-renewal ability of cancer stem cells (CSCs)
(9–11). mTOR inhibition is known to protect
normal oral epithelial cells from radiation-induced epithelial stem
cell depletion via the increased expression of manganese superoxide
dismutase (MnSOD/SOD2), suggesting that interaction occurs between
mTOR and MnSOD. MnSOD is a nuclear-encoded mitochondrial
antioxidant enzyme, which is essential for the removal of
superoxide radicals and governs the types of reactive oxygen
species (ROS) egressing from the organelle (12), the accumulation of which damage
DNA and the mitochondrial membrane, leading to tumorigenesis. The
aberrant expression of MnSOD has been implicated in carcinogenesis
and tumor resistance to therapy (13,14); however, its roles in CSCs are
still poorly understood.
Tumor groups are composed of heterogeneous cancer
cells, of which the CSCs account only for a small population
although they are crucial for tumorigenesis and treatment
resistance. The CSCs are thought of as the roots of cancer, have
low proliferative status and slow cell cycles, and remain steady
throughout chemo-radiotherapy. Due to the negative response to
major treatments, the elimination of CSCs has proven to be a key
obstacle in curing cancer, and the existence of CSCs contributes to
tumor relapse and resistance to clinical therapies (11,15). The general perception is that CSCs
are inherently resistant to radiation therapy, and this resistance
is considered to be a general property of the stem cell group
(11). However, diverse results
have been detected in certain studies: on the one hand, CSCs have
been found to be resistant to common chemo-radiotherapies,
contributing to tumor occurrence and relapse (16–18); on the other hand, previous
research has suggested that the tumor-derived stem cells have
different characteristics, and respond to radiotherapy in different
ways (19). ROS activity is
thought to be linked to the response to therapies: high levels of
ROS are related to stronger productive properties of cancer cells,
and are closely related to tumor recurrence and therapy resistance,
whereas lower ROS levels are closely related to the signatures of
CSCs (19–21).
Radiation is known to act as a powerful tool in the
fight against breast cancer, and high doses of radiation are often
used to eradicate tumor resistance to chemotherapies, acting as the
last part of clinical treatments. However, studies have found that
radiation increases therapy resistance by increasing the number of
stem cells in cancer groups (22). On the one hand, radiation
treatment can kill the majority of tumor cells, but, on the other
hand, it can also transform cancer cells into treatment-resistant
CSCs. The elimination of the majority of cancer cells paves the way
for self-renewal of stem cells, making it more difficult to cure
the tumor in the future (23).
Controlling the radiation-resistant breast cancer stem cells
(BrCSCs) during radiation treatment may ultimately improve
curability and reduce the high radiation doses currently
administered to breast cancer patients, and thus decrease acute and
long-term side effects by decreasing the administration of
high-dose radiation. The elimination of a smaller pool of breast
CSCs in massive pools of cancer cells will eventually help to
irradiate the remaining cancer cells, killing the cancer. We
hypothesize that mTOR inhibition with rapamycin could then
synergize with the antitumoral effects of radiation, which is one
of the most frequent approaches in the treatment of triple-negative
breast cancer (TNBC). Increased sensitization of tumors to
radiotherapy will help to improve the prognosis of patients with
breast cancer, particularly those patients with TNBC, which is more
malignant and resistant to clinical therapies than other cancers.
If we uncover the mechanisms through which the stem cells generate
and transform, we may be able to block these happening and make the
radiation therapy more powerful and less harmful.
Materials and methods
Cell culture, transfection and
treatment
The human breast cancer cell lines SK-BR-3, T47-D,
ZR-75-1, ZR-75-30, BT20, BT-549, MDA-MB-231, MDA-MB-453,
MDA-MB-468, HCC1143 and HS-578T were all purchased from the Cell
Bank of Shanghai Institute (Shanghai, China), and cultured in
RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA,
USA), containing 10% fetal bovine serum (FBS) (Thermo Fisher
Scientific) and 1% penicillin and streptomycin (Gibco, Thermo
Fisher Scientific). The mammospheres (CSCs) were cultured in 1X
DMEM/Ham's F12 medium, with 10 ng/ml epidermal growth factor (EGF),
10 ng/ml human basic fibroblast growth factor (hbFGF), 1
µg/ml hydrocortisone, 4 µg/ml insulin and 1%
penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA), as
previously reported (24). Cancer
cells were plated in ultra-low attachment dishes (Corning, Inc.,
Corning, NY, USA) to test their ability to form primary
mammospheres in stem cell medium. On the 7th day, the number of
mammospheres was counted as previously described (7,25–27). Briefly, a sphere is identified if
it contains >50 cells, as was observed and counted under a
microscope. The obtained mammospheres of different groups were
disaggregated and then seeded into ultra-low attachment dishes to
test their self-renewal ability for subsequent generation in
continous culturation. Three individual pairs of siRNAs against
mTOR and MnSOD and RFP-based shRNAs against Akt1 were all designed
and synthesized by Gene Pharma (Shanghai, China). Transfection with
siRNAs was performed using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. The cells were
irradiated using a Cs-137 irradiator (GSM:GSR D1). Ionizing
radiation was carried out in strict accordance with the clinical
criteria. The cells were exposed to ionizing radiation prior to use
in the experiments. Rapamycin (ab120224; Abcam, Shanghai, China)
was used to inhibit mTOR activity, and was prepared at a
concentration of 20 µM. An Akt inhibitor (Akti-1/2,
ab142088; Abcam, Cambridge, MA, USA) was used to inhibit Akt
phosphorylation.
Western blot analysis and
immunofluorescence assay
For western blot analysis, proteins of different
groups were harvested in RIPA lysis buffer (Beijing Biotech,
Beijing, China), with protease/phosphatase inhibitor cocktail
(100X, no. 5872; Cell Signaling Technology, Inc., Danvers, MA,
USA), and subsequently subjected to 10% SDS-PAGE separation.
Monoclonal or polyclonal anti-p-mTOR (Ser2481, no. 2974; Cell
Signaling Technology, Inc.), anti-MnSOD (DD-17, no. S5069,
Sigma-Aldrich, St. Louis, MO, USA) and anti-β-actin (no. 4967; Cell
Signaling Technology, Inc.) were diluted to 1:1,000–1:5,000 for
western blot analysis. HRP goat anti-mouse (no. 554002) and HRP
goat anti-rat (no. 554017) were purchased from BD Pharmingen (San
Diego, CA, USA).
For the immunofluorescence assay, the cells were
planted in chambers and then fixed with 10% formalin for 15 min.
The cells were subsequently blocked in 2% normal goat serum
(ab7481; purchased from Abcam, Cambridge, MA, USA), and incubated
with the primary antibody (the same one used for western blot
analysis) for 1 h in PBST, and sequentially with goat anti-rabbit
or goat anti-mouse secondary antibody for a least 30 min with Alexa
Fluor® 488, 568 or 633 dye; finally, the cells were
incubated for 15 min with DAPI nuclear dye (no. 62248) (both from
Life Technologies, Thermo Fisher Scientific). In order to study the
division modes of CSCs, the mammospheres were disaggregated and
seeded in chambers 24 h prior to staining. Akt-pan (C67E7, no.
4691; Cell Signaling Technology, Inc.) was used to identify the
asymmetrically divided stem cells (21), as previously described, and the
uneven or asymmetric distribution of pan-Akt was taken to indicate
the occurrence of asymmetric division (AD) in CSCs, as previously
described (21,28,29).
Dihydroethidium (DHE) staining
DHE (Molecular Probes, Vigorous Inc., Beijing,
China) is an oxidative fluorescent dye, and was used to evaluate
theROS levels in the cells. ROS production was assessed using a
FACSAria flow cytometer. The cells were cultured with 50 µM
of DHE for 60 min at 37°C, and were kept in the dark. The cells
were then trypsinized and subjected to flow cytometry at an
excitation wavelength of 515 nm, and a waudio videoelength of 600
nm. The ROS-positive cells presented strong red fluorescence,
compared to the ROS-negative group.
Statistical analysis
All data in this study were obtained from three
independent experiments, and are expressed as the means ± SD.
Statistical analysis was performed using a Student's t-test and
χ2 test with SPSS 16.0 for Windows (IBM, Chicago, IL,
USA) and Excel 2007 (Microsoft Corporation, Redmond, WA, USA). A
P-value <0.01 was deemed to indicate a statistically significant
difference.
Results
Activation of mTOR phosphorylation is
crucial to the self-renewal ability of HS587-T and MDA-MB-231 stem
cells
In order to identify the roles of mTOR in BrCSCs, we
first detected endogenous mTOR activity in multiple breast cancer
cell lines, as previously described (30). In breast cancer cell lines
SK-BR-3, T47-D, ZR-75-1, ZR-75-30, BT20, BT-549, MDA-MB-231,
MDA-MB-453, MDA-MB-468, HCC1143 and HS-578T, the mTOR levels were
positively correlated with the self-renewal ability of BrCSCs, and
of the cell lines, endogenous mTOR activity was much higher in
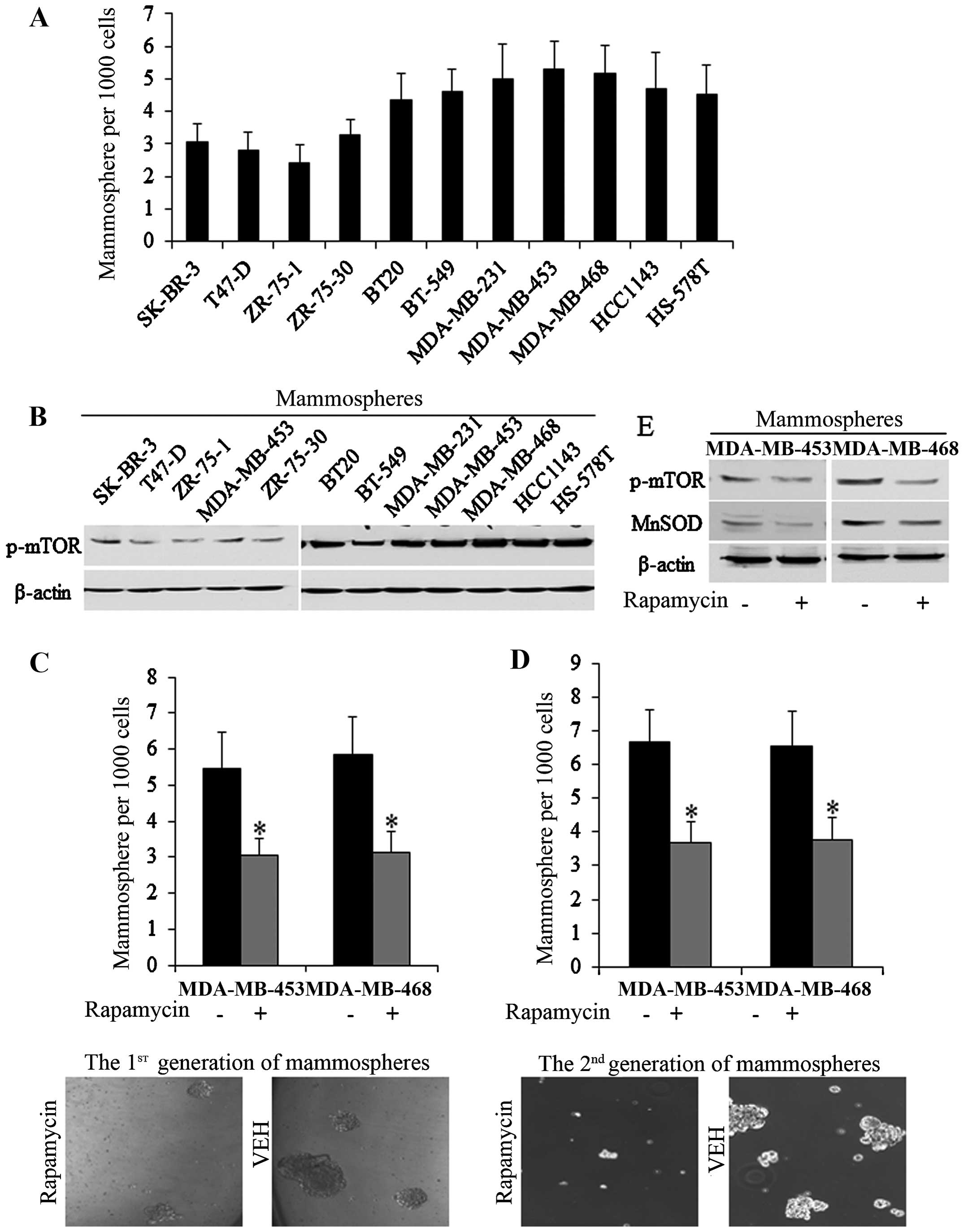
MDA-MB-453 and MDA-MB-468 stem cells (Fig. 1A and B). Rapamycin (ab120224;
Abcam, Shanghai, China) at 20 µM significantly decreased the
number of mammospheres of 1st and 2nd generations (Fig. 1C and D), while mTOR
phosphorylation decreased (Fig.
1E), proving that mTOR plays crucial roles in sustaining the
stem cell pool of MDA-MB-453 and MDA-MB-468. On the basis of these
results, MDA-MB-468. MDA-MB-453 and MDA-MB-468 stem cells were used
subsequently.
mTOR inhibition sensitizes BrCSCs to
radiation-induced inhibition of self-renewal
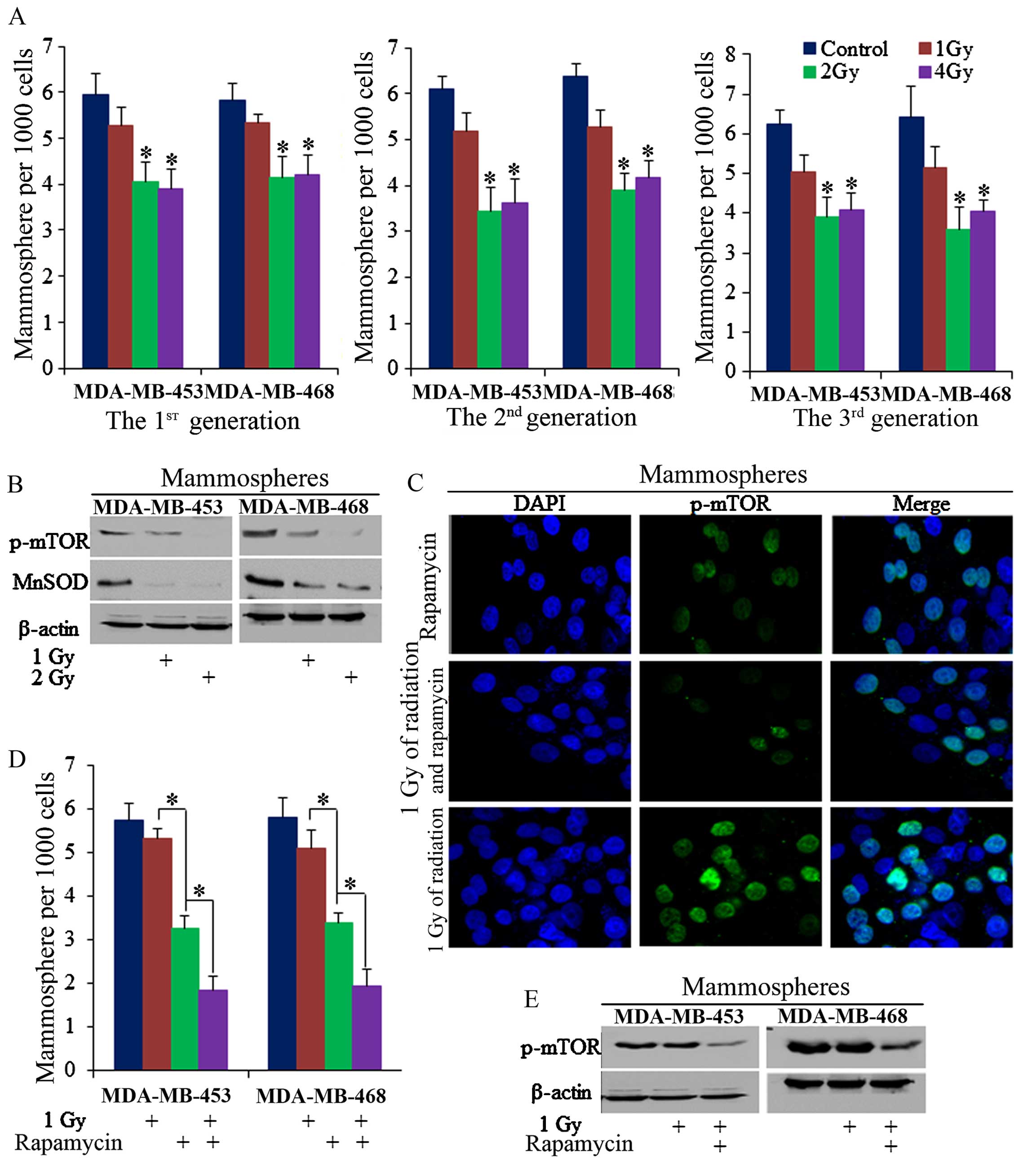
Radiation of 2 Gy decreased the number of
mammospheres of MDA-MB-453 and MDA-MB-468 cells in continuous
mammosphere culture (Fig. 2A),
with mTOR phosphorylation also being inhibited (Fig. 2B and C). However, 1 Gy of ionizing
radiation failed to markedly inhibit the number of mammospheres in
these two cell lines, P>0.05 (Fig.
2A), despite the fact that it is known to play a role in the
induction of cell apoptosis, as has been previously described
(31–33). However, when 1 Gy of radiation and
20 µM rapamycin were combined, 1 Gy of radiation effectively
reduced mammosphere formation efficiency (Fig. 2D), and effectively inhibited mTOR
phosphorylation in the mammospheres (Fig. 2C and E).
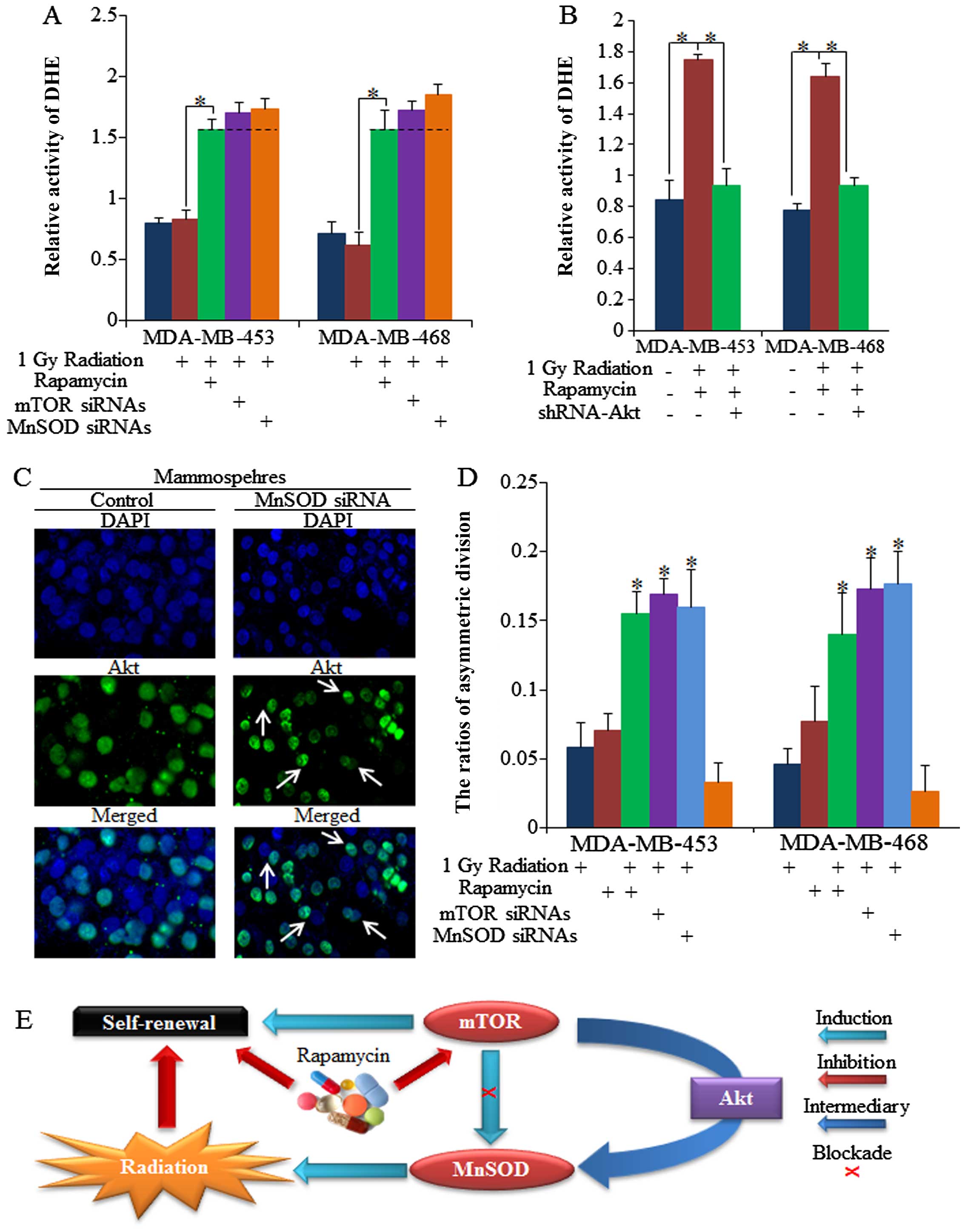
Decreased MnSOD is critical for
rapamycin-induced inhibition of self-renewal of BrCSCs treated with
ionizing radiation
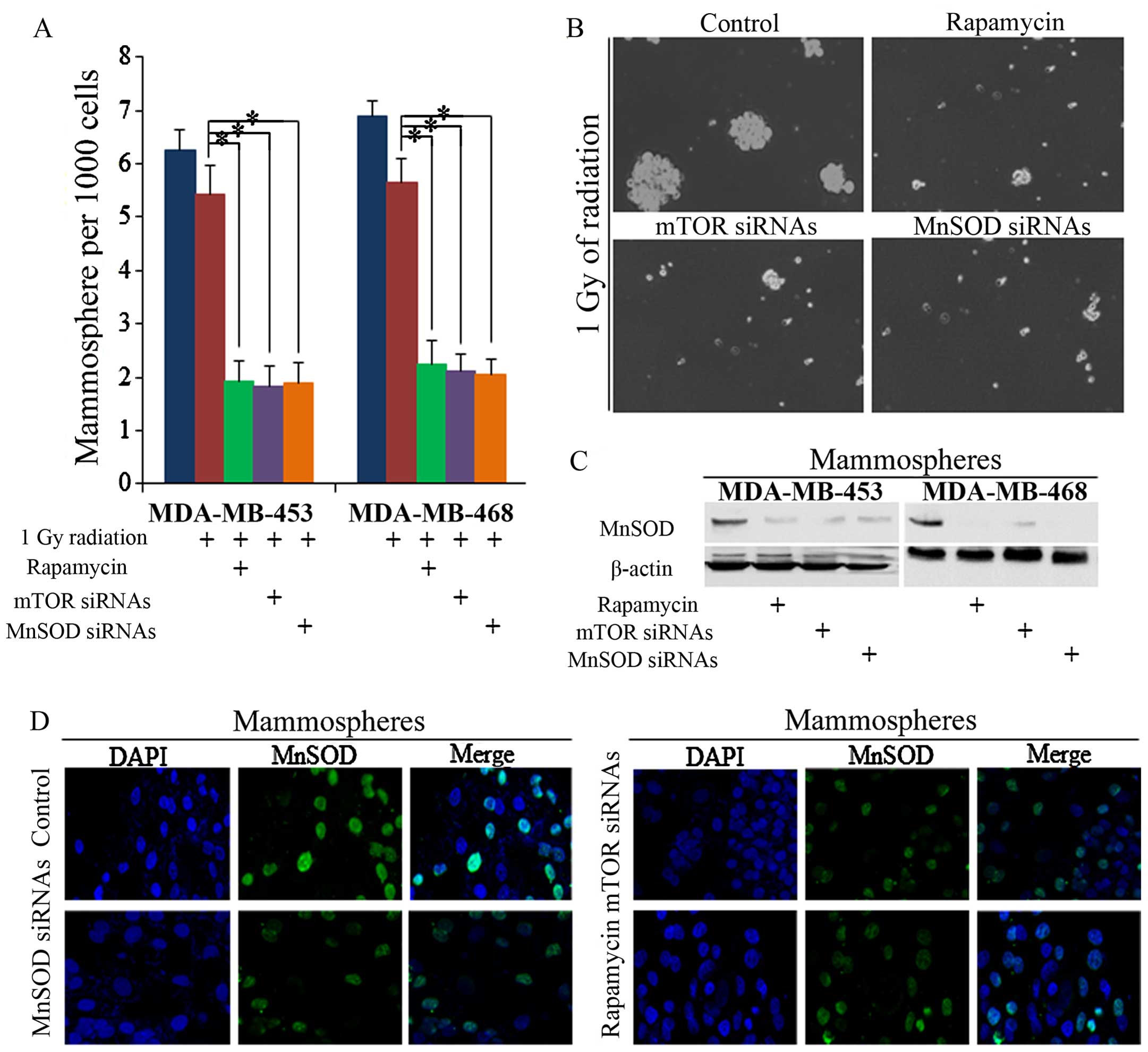
MnSOD is associated with mTOR activity in stem
cells, and we noted that it was suppressed by both rapamycin and
radiation (Figs. 1E and 2B). The inhibition of MnSOD sensitized
BrCSCs to 1 Gy of radiation, and we noted that no significant
difference between rapamycin- or mTOR siRNA-treated cells was
observable (Fig. 3A and B). Both
rapamycin and mTOR siRNAs decreased the self-renewal of cells, as
did MnSOD siRNAs (Fig. 3C and
D).
Akt is required for rapamycin function
and sensitization of cells to effects of radiation
Although it is known that mTOR functions through Akt
in many ways, it was not known whether mTOR inhibition induces
repression of self-renewal through Akt, and the roles which Akt
plays in the regulation of mTOR and MnSOD had not previously been
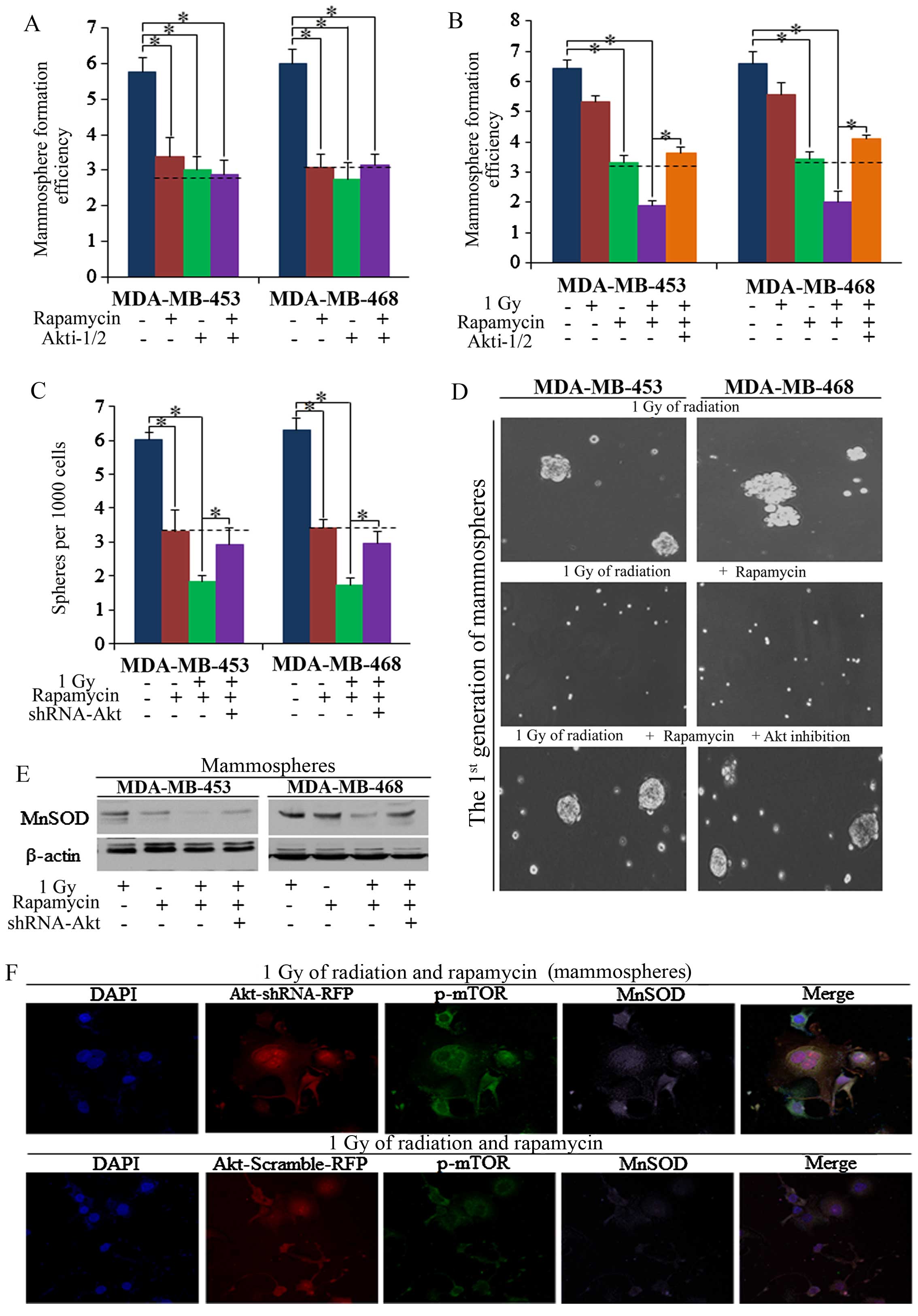
explored. In the present study, we found that Akt inhibition
decreased the mammo-sphere formation efficiency (MFE) of BrCSCs
(Fig. 4A), and functioned the
same way as mTOR inhibition, as had also been previously reported
(34–36). The knockdown of Akt by Akt
inhibitors (Akti-1/2, ab142088; Abcam, Cambridge, MA, USA)
abolished the effects of rapamycin on radiation sensitization
(Fig. 4B). To confirm the role of
Akt in rapamycin-induced radiation sensitization, we subsequently
used shRNA-Akt1 lentivirals, and similar results were detected
compared to the usage of Akt inhibitors (Fig. 4C and D), and downstream MnSOD was
not markedly influenced in Akt knockdown cells (Fig. 4E). Thus, we posit that the
existence of Akt is required for the rapamycin regulation of MnSOD
in the sensitization of BrCSCs to radiation-induced suppression
(Fig. 4F).
The existence of Akt is critical for
rapamycin-induced asymmetric cell division
mTOR functions via the regulation of MnSOD in normal
epithelial stem cell senescence and cancer cell fate (7); however, its roles in CSCs had not
previously been discussed. Low ROS activity is one of the most
effective biomarkers of a high ability to self-renew, something
which has previously been achieved by induction of symmetric cell
division (21). Our results
showed that ROS activity was upregulated by rapamycin (Fig. 5A), and this was achieved by
inhibition of MnSOD, as was also previously suggested (12,37). Moreover, we noted that Akt is also
required for rapamycin sensitization of the radiotherapy response
and ROS regulation (Fig. 5B).
Increased ROS has been shown to promote the AD of CSCs, and results
in repression of self-renewal, helping to sensitize CSCs to the
effects of radiation (21,38,39).
Asymmetric cell division was recognized through Akt distribution in
dividing cells, as was also previously reported (21,29,40,41), and we identified the
asymmetrically dividing stem cells by Akt staining at the division
stage (Fig. 5C). As shown in
Fig. 5D, we found that rapamycin
increased the AD of stem cells, and enhanced the functions of
low-dose radiation in relation to the induction of AD, which then
resulted in the number of mammospheres decreasing. However, the
combined functions of single factors failed to influence the ratio
of symmetric division (SD), which may have been caused by the large
number of symmetrically divided stem cells (data not shown). The
undefined dividing cells in each group did not vary significantly
(data not shown). The association between mTOR, MnSOD and Akt in
stem cell regulation and treatment sensitizations, and the
importance of Akt, are depicted in Fig. 5E.
Discussion
Chemotherapies, endocrine therapies, surgical
removal and targeted therapies are all commonly used in clinical
settings, but it is inevitable that some patients will relapse with
resistant cancer (42). The
undisturbed, growing tumors maintain a small number of CSCs, which
are responsible for the ability to self-renew, the resistance to
common chemo-radiotherapies, and contribute to maintaining the
tumor group, therapy resistance and long-term tumor survival and
relapse. These stem cells are silent and steady, but, when
challenged by various stressors that threaten their numbers,
including ionizing radiation, the breast cancer cells begin to
generate more stem cells (43),
and together with the surviving CSCs repopulate the tumor (44).
Radiotherapy is commonly used in clinical
treatments, and new strategies for helping to sensitize breast
cancer cells to radiation will thus improve the prospects of
patients with worse receptor status (45–48). Sensitization of breast CSCs to the
inhibitive effects of chemo-radiotherapies by adjuvant therapy
targeting oncogenic pathways represents a step toward improving the
outcome of radiation in patients with TNBC. The activation of the
mTOR pathway has been recognized as occurring frequently in
neoplastic transformation, and our research aims to determine new
methods that will improve patient prognosis by inhibiting the
expansion of CSCs with mTOR inhibitors (49–51).
Studies of the role of MnSOD in tumorigenesis and
cancer progression have yielded conflicting results in relation to
cancer risk, prognosis and susceptibility to therapy; however,
recent results indicated that MnSOD contributes to the progression
of tumors toward aggressive phenotypes by creating a cellular
environment conducive to the decrease of ROS in mitochondria in
CSCs (12,52,53). Oxidative stress caused by the
accumulation of ROS functions in many ways and influences the
self-renewal ability of stem cells in cancer (13).
In this study, we examined whether mTOR blockade by
rapamycin increased radiation-induced self-renewal inhibition of
CSCs in multiple breast cancer cell lines, and our results
demonstrated that mTOR activity is closely related to the
self-renewal ability of BrCSCs. In mammospheres from MDA-MB-453 and
MDA-MB-468 cells, rapamycin repression of mTOR phosphorylation
decreased the number of mammospheres, and helped to sensitize the
resistant CSCs to low-dose radiation therapy. By using siRNAs
targeting mTOR and MnSOD, we confirmed that rapamycin functioned
via the mTOR/MnSOD/ROS signaling pathway, and the existence of Akt
is necessary for rapamycin induction of AD of the stem cells in
radiation-treated breast cancer. The synergic effects of rapamycin
and low-dose radiation induced the AD of stem cells, which then
resulted in the decrease of number of mammospheres, and are
critical for improved radiotherapy responses in clinical treatment.
To the best of our knowledge, for the first time, it has been
demonstrated that different doses of radiotherapy cause different
outcomes in relation to self-renewal of cells, and that 1 Gy of
radiation did not significantly influence the accumulation of
mammospheres of breast cancer, but this effect was improved and
strengthened by mTOR inhibition, and we also noted that Akt is
required for the sensitization of stem cells to low-dose radiation.
Thus, we posit that an in-depth understanding of the interaction of
radiation with CSCs has the potential to make radiation even better
and more effective.
Acknowledgments
The authors are grateful for the help and support of
the colleagues and staff of the Department of Thyroid and Breast
Surgery, and the Department of Obstetrics and Gynecology, the First
Affiliated Hospital of Sun Yat-sen University. The authors are also
grateful for the guidance from the administrators of the central
laboratories of the First Affiliated Hospital of Sun Yat-sen
University.
References
|
1
|
Kanavos P: The rising burden of cancer in
the developing world. Ann Oncol Suppl. 8:viii15–viii23
|
|
2
|
Sun X, Qin S, Fan C, Xu C, Du N and Ren H:
Let-7: A regulator of the ERα signaling pathway in human breast
tumors and breast cancer stem cells. Oncol Rep. 29:2079–2087.
2013.PubMed/NCBI
|
|
3
|
Assi HA, Khoury KE, Dbouk H, Khalil LE,
Mouhieddine TH and El Saghir NS: Epidemiology and prognosis of
breast cancer in young women. J Thorac Dis. 5(Suppl 1): S2–S8.
2013.PubMed/NCBI
|
|
4
|
Ohno S, Takanashi M, Sudo K, Ueda S,
Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et
al: Systemically injected exosomes targeted to EGFR deliver
antitumor microRNA to breast cancer cells. Mol Ther. 21:185–191.
2013. View Article : Google Scholar :
|
|
5
|
Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y,
Cong H, Wang X, Qiu W and Yue L: MiR-200b expression in breast
cancer: a prognostic marker and act on cell proliferation and
apoptosis by targeting Sp1. J Cell Mol Med. 19:760–769. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu FJ, Wang XB and Cao AG: Screening and
functional analysis of a differential protein profile of human
breast cancer. Oncol Lett. 7:1851–1856. 2014.PubMed/NCBI
|
|
7
|
Iglesias-Bartolome R, Patel V, Cotrim A,
Leelahavanichkul K, Molinolo AA, Mitchell JB and Gutkind JS: mTOR
inhibition prevents epithelial stem cell senescence and protects
from radiation-induced mucositis. Cell Stem Cell. 11:401–414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castilho RM, Squarize CH, Chodosh LA,
Williams BO and Gutkind JS: mTOR mediates Wnt-induced epidermal
stem cell exhaustion and aging. Cell Stem Cell. 5:279–289. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martelli AM, Evangelisti C, Follo MY,
Ramazzotti G, Fini M, Giardino R, Manzoli L, McCubrey JA and Cocco
L: Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target
of rapamycin signaling network in cancer stem cells. Curr Med Chem.
18:2715–2726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsubara S, Ding Q, Miyazaki Y, Kuwahata
T, Tsukasa K and Takao S: mTOR plays critical roles in pancreatic
cancer stem cells through specific and stemness-related functions.
Sci Rep. 3:32302013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun X, Jiao X, Pestell TG, Fan C, Qin S,
Mirabelli E, Ren H and Pestell RG: MicroRNAs and cancer stem cells:
the sword and the shield. Oncogene. 33:4967–4977. 2014. View Article : Google Scholar
|
|
12
|
Hart PC, Mao M, de Abreu ALP,
Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A,
Diamond AM, Minshall RD, Consolaro ME, et al: MnSOD upregulation
sustains the Warburg effect via mitochondrial ROS and
AMPK-dependent signalling in cancer. Nat Commun. 6:60532015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhar SK, Tangpong J, Chaiswing L, Oberley
TD and St Clair DK: Manganese superoxide dismutase is a
p53-regulated gene that switches cancers between early and advanced
stages. Cancer Res. 71:6684–6695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch HA, Iliopoulos D and Struhl K:
Metformin inhibits the inflammatory response associated with
cellular transformation and cancer stem cell growth. Proc Natl Acad
Sci USA. 110:972–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rupaimoole R, Han HD, Lopez-Berestein G
and Sood AK: MicroRNA therapeutics: principles, expectations, and
challenges. Chin J Cancer. 30:368–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24−/low/CD44+ breast
cancer-initiating cells to radiation. J Natl Cancer Inst.
98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zielske SP, Spalding AC, Wicha MS and
Lawrence TS: Ablation of breast cancer stem cells with radiation.
Transl Oncol. 4:227–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dey-Guha I, Wolfer A, Yeh ACG, G Albeck J,
Darp R, Leon E, Wulfkuhle J, Petricoin EF III, Wittner BS and
Ramaswamy S: Asymmetric cancer cell division regulated by AKT. Proc
Natl Acad Sci USA. 108:12845–12850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: the 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delort L, Perrier S, Dubois V, Billard H,
Mracek T, Bing C, Vasson MP and Caldefie-Chézet F:
Zinc-α2-glycoprotein: a proliferative factor for breast cancer? In
vitro study and molecular mechanisms. Oncol Rep. 29:2025–2029.
2013.PubMed/NCBI
|
|
25
|
Sun X, Tang SC, Xu C, Wang C, Qin S, Du N,
Liu J, Zhang Y, Li X, Luo G, et al: DICER1 regulated let-7
expression levels in p53-induced cancer repression requires cyclin
D1. J Cell Mol Med. 19:1357–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveras-Ferraros C, Cufí S,
Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B
and Menendez JA: Micro(mi) RNA expression profile of breast cancer
epithelial cells treated with the anti-diabetic drug metformin:
induction of the tumor suppressor miRNA let-7a and suppression of
the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 10:1144–1151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G
and Wei J: Estrogen promotes stemness and invasiveness of
ER-positive breast cancer cells through Gli1 activation. Mol
Cancer. 13:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henle SJ, Wang G, Liang E, Wu M, Poo MM
and Henley JR: Asymmetric PI(3,4,5)P3 and Akt signaling mediates
chemotaxis of axonal growth cones. J Neurosci. 31:7016–7027. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung CY, Potikyan G and Firtel RA:
Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase
through the regulation of PAKa. Mol Cell. 7:937–947. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: one tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010.
|
|
31
|
Mirzayans R, Andrais B, Scott A, Wang YW
and Murray D: Ionizing radiation-induced responses in human cells
with differing TP53 status. Int J Mol Sci. 14:22409–22435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cataldi A, Di Giacomo V, Rapino M, Zara S
and Rana RA: Ionizing radiation induces apoptotic signal through
protein kinase Cδ (delta) and survival signal through Akt and
cyclic-nucleotide response element-binding protein (CREB) in Jurkat
T cells. Biol Bull. 217:202–212. 2009.PubMed/NCBI
|
|
33
|
Landsverk KS, Lyng H and Stokke T: The
response of malignant B lymphocytes to ionizing radiation: cell
cycle arrest, apoptosis and protection against the cytotoxic
effects of the mitotic inhibitor nocodazole. Radiat Res.
162:405–415. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
McDermott SP and Wicha MS: Targeting
breast cancer stem cells. Mol Oncol. 4:404–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suman S, Das TP and Damodaran C: Silencing
NOTCH signaling causes growth arrest in both breast cancer stem
cells and breast cancer cells. Br J Cancer. 109:2587–2596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lombardo Y, Filipović A, Molyneux G,
Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yagüe E, Michel L
and Coombes RC: Nicastrin regulates breast cancer stem cell
properties and tumor growth in vitro and in vivo. Proc Natl Acad
Sci USA. 109:16558–16563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Shi K, Guan L, Cao T, Jiang Q, Yang
Y and Xu C: ROS leads to MnSOD upregulation through ERK2
translocation and p53 activation in selenite-induced apoptosis of
NB4 cells. FEBS Lett. 584:2291–2297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi X, Zhang Y, Zheng J and Pan J:
Reactive oxygen species in cancer stem cells. Antioxid Redox
Signal. 16:1215–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ito K and Suda T: Metabolic requirements
for the maintenance of self-renewing stem cells. Nat Rev Mol Cell
Biol. 15:243–256. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neumüller RA and Knoblich JA: Dividing
cellular asymmetry: asymmetric cell division and its implications
for stem cells and cancer. Genes Dev. 23:2675–2699. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dey-Guha I, Alves CP, Yeh AC, Salony, Sole
X, Darp R and Ramaswamy S: A mechanism for asymmetric cell division
resulting in proliferative asynchronicity. Mol Cancer Res.
13:223–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lagadec C, Vlashi E, Della Donna L,
Dekmezian C and Pajonk F: Radiation-induced reprogramming of breast
cancer cells. Stem Cells. 30:833–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Printz C: Radiation treatment generates
therapy-resistant cancer stem cells from less aggressive breast
cancer cells. Cancer. 118:32252012.PubMed/NCBI
|
|
45
|
Wu L, Shao L, Li M, Zheng J, Wang J, Feng
W, Chang J, Wang Y, Hauer-Jensen M and Zhou D: BMS-345541
sensitizes MCF-7 breast cancer cells to ionizing radiation by
selective inhibition of homologous recombinational repair of DNA
double-strand breaks. Radiat Res. 179:160–170. 2013. View Article : Google Scholar :
|
|
46
|
Lai CH, Chang CS, Liu HH, Tsai YS, Hsu FM,
Yu YL, Lai CK, Gandee L, Pong RC, Hsu HW, et al: Sensitization of
radio-resistant prostate cancer cells with a unique cytolethal
distending toxin. Oncotarget. 5:5523–5534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J,
Zhu W, Cao H, Geng Y, Xu J, et al: Hypoxia-inducible factor 1α
(HIF-1α) and reactive oxygen species (ROS) mediates
radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in
non-small cell lung carcinoma cells. Oncotarget. 6:10893–10907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun X, Jiang S, Liu J, Wang H, Zhang Y,
Tang SC, Wang J, Du N, Xu C, Wang C, et al: MiR-208a stimulates the
cocktail of SOX2 and β-catenin to inhibit the let-7 induction of
self-renewal repression of breast cancer stem cells and formed
miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget.
6:32944–32954. 2015.PubMed/NCBI
|
|
49
|
Cruceru ML, Neagu M, Demoulin J-B and
Constantinescu SN: Therapy targets in glioblastoma and cancer stem
cells: lessons from haematopoietic neoplasms. J Cell Mol Med.
17:1218–1235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bartalucci N, Tozzi L, Bogani C,
Martinelli S, Rotunno G, Villeval JL and Vannucchi AM: Co-targeting
the PI3K/mTOR and JAK2 signalling pathways produces synergistic
activity against myeloproliferative neoplasms. J Cell Mol Med.
17:1385–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rosner M, Hanneder M, Siegel N, Valli A,
Fuchs C and Hengstschlager M: The mTOR pathway and its role in
human genetic diseases. Mutat Res. 659:284–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Britschgi A, Bill A, Brinkhaus H, Rothwell
C, Clay I, Duss S, Rebhan M, Raman P, Guy CT, Wetzel K, et al:
Calcium-activated chloride channel ANO1 promotes breast cancer
progression by activating EGFR and CAMK signaling. Proc Natl Acad
Sci USA. 110:E1026–E1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou D, Shao L and Spitz DR: Reactive
oxygen species in normal and tumor stem cells. Adv Cancer Res.
122:1–67. 2014. View Article : Google Scholar : PubMed/NCBI
|