Introduction
Renal cell carcinoma (RCC) is the most common renal
tumor. Approximately one-third of patients have metastases at
diagnosis, and up to 30% of patients develop metastases during
therapy. Once metastasized, the prognosis for patients is bleak. A
better understanding of the molecular modes of action underlying
RCC development and progression has contributed to the development
of targeted therapies, thus improving the outlook for patients in
the advanced stages of this disease. However, despite these
therapeutic advances, the prognosis for patients with RCC remains
poor, with 5-year survival remaining between 5 and 12%.
Dissatisfaction with conventional therapy and the desire to reduce
side-effects have led many patients to complementary and
alternative medicine (CAM). Up to 80% of cancer patients in the
United States, and more than 50% of cancer patients in Europe use
CAM alongside, or in place of, conventional therapy.
Information about the efficacy of natural compounds
is sparse, and certain of these compounds, such as the cyanogenic
diglycoside amygdalin (D-mandelonitrile-β-gentiobioside), remain
controversial. Amygdalin is derived from the fruit kernels of the
Rosaceae family, which includes Prunus persica (peach),
Prunus armeniaca (apricot) and Prunus amygdalus var.
amara (bitter almond). Amygdalin, mainly in the United States, has
been administered to cancer patients since the 1920s. In the 1950s,
an intravenous, chemically different form of amygdalin was
synthesized and patented as laetrile. Although laetrile differs
from amygdalin, the terms are often used interchangeably, making
data interpretation difficult. By 1978 approximately 70,000 cancer
patients in the US had been treated with amygdalin. Evidence-based
research on amygdalin, however, remains limited. A clinical study
sponsored by the National Cancer Institute over 30 years ago
revealed no signs of tumor regression (1), whereas a retrospective analysis of
67 tumor patients receiving amygdalin reported two complete and
four partial responses (2).
Ambivalence is also reflected in case reports: amygdalin was
ineffective in five cases, and effective in four. Randomized
clinical trials and follow-up studies have not been carried out, to
the best of our knowledge. Proponents consider amygdalin an
effective natural cancer treatment option, whereas opponents warn
of toxicity due to hydrogen cyanide metabolization.
Metastasis is the main cause of RCC-associated
mortality. Transendothelial migration and motile spread are
critical steps in tumor dissemination and progression (3), and the dissemination of cancer cells
to distant organs constitutes the major clinical challenge in
treating cancer. In the present study, the anti-neoplastic effect
of amygdalin on RCC cell adhesion and migration properties was
investigated. Since integrins activate a number of intracellular
signaling pathways involved in cell proliferation, differentiation
and motility, the expression pattern of α and β integrin adhesion
receptors between amygdalin-treated cells and untreated controls
was determined. Integrins are important in both health and disease
(4) and play a pivotal role in
carcinogenesis and cancer progression (4).
The present study is based on a previous
investigation dealing with the influence of amygdalin on the
metastatic properties of three bladder cancer cell lines (5). Since some dissimilarities were
observed regarding the action of amygdalin on the metastatic
properties of the different bladder cancer cell lines, the question
arose as to whether the different effects of amygdalin were
restricted to particular tumor entities or occur in others. Thus,
three RCC cell lines were chosen, since RCC tumors are the most
aggressive urologic tumor.
Materials and methods
Cell culture
Kidney carcinoma cells, Caki-1, KTC-26, and A498,
were purchased from LGC Promochem GmbH (Wesel, Germany). The cells
were grown and subcultured in RPMI-1640 medium (Seromed, Berlin,
Germany) supplemented with 10% fetal calf serum (FCS), 20 mM HEPES
buffer, 100 IU/ml penicillin and 100 µg/ml streptomycin at
37°C in a humidified incubator with 5% CO2. Subcultures
from passages 5–24 were selected for experimental use. Human
umbilical vein endothelial cells (HUVECs) were isolated from human
umbilical veins and harvested by enzymatic treatment with dispase
(1 U/ml; Gibco-Invitrogen, Carlsbad, CA, USA). HUVECs were grown in
Medium 199 (M199; Biozol, Munich, Germany), supplemented with 10%
FCS, 10% pooled human serum, 20 µg/ml endothelial cell
growth factor (Boehringer, Mannheim, Germany), 0.1% heparin, 100
ng/ml gentamycin and 20 mM HEPES buffer (pH 7.4). Subcultures from
passages 1–5 were selected for experimental use. The Institutional
Ethics Committee of Goethe-University Hospital, Frankfurt, Germany,
waived the need for consent since HUVECs were used anonymously for
in vitro assays and had no links with patient data.
Amygdalin treatment
Amygdalin from apricot kernels (Sigma-Aldrich,
Taufkirchen, Germany) was freshly dissolved in cell culture medium
and then added to tumor cells at a concentration of 10 mg/ml
[previously evaluated as optimal concentration (6)] for either 24 h or for 2 weeks
(treatment applied three times a week) to evaluate acute versus
chronic treatment. Controls remained untreated. In all experiments,
treated and non-treated tumor cell cultures were compared. To
examine the toxic effects of amygdalin, cell viability was
determined by trypan blue (Gibco-Invitrogen).
Tumor cell adhesion
To analyze tumor cell adhesion, HUVECs were
transferred to 6-well multiplates (Sarstedt, Nümbrecht, Germany) in
complete HUVEC medium. When they reached confluence, Caki-1, KTC-26
and A498 cells were detached from the culture flasks by treatment
with accutase (PAA Laboratories, Cölbe, Germany), and
0.5×106 cells were then added to and left on the HUVEC
monolayer for 1, 2 or 4 h. Subsequently, non-adherent tumor cells
were washed off using warmed (37°C) PBS (Ca2+ and
Mg2+). The remaining cells were fixed with 1%
glutaraldehyde. Adherent tumor cells were counted in five different
fields of a defined size (5×0.25 mm2) using a phase
contrast microscope (ID03, 471202-9903; Carl Zeiss Microscopy GmbH,
Goettingen, Germany), and the mean cellular adhesion rate was
calculated.
Attachment to immobilized extracellular
matrix proteins
The 24-well plates were coated with collagen G
(extracted from calfskin, consisting of 90% collagen type I and 10%
collagen type III, and diluted to 400 µg/ml in PBS;
Biochrom, Berlin, Germany) or fibronectin (extracted from mice and
diluted to 100 µg/ml in PBS; Becton-Dickinson, Heidelberg,
Germany) overnight. Plastic dishes served as the background
control. Plates were washed with 1% bovine serum albumin (BSA) in
PBS to block non-specific cell adhesion. Tumor cells
(0.1×106) were then added to each well and left for 30
min incubation. Subsequently, non-adherent tumor cells were washed
off, the remaining adherent cells were fixed with 2% glutaraldehyde
and counted microscopically. The mean cellular adhesion rate,
defined by adherent cellscoated well - adherent cellsbackground,
was calculated from five different observation fields.
Chemotactic activity
Serum-induced chemotactic movement was examined
using 6-well Transwell chambers (Greiner, Frickenhausen, Germany)
with 8-µm pores. The cells (0.5×106 Caki-1,
KTC-26 or A498 cells/ml) were placed in the upper chamber in
serum-free medium, either free of amygdalin (control) or containing
amygdalin. The lower chamber contained 10% serum. Following
overnight incubation, the upper surface of the transwell membrane
was gently wiped with a cotton swab to remove cells that had not
migrated. Cells moving to the lower surface of the membrane were
stained using hematoxylin and counted microscopically. The mean
migration rate was calculated from five different observation
fields.
Invasion
Invasion was examined by serum-induced chemotactic
movement through a membrane (Greiner) with 8-µm pores,
pre-coated with collagen G (extracted from calfskin, consisting of
90% collagen type I and 10% collagen type III; diluted to 400
µg/ml in PBS; Biochrom) and HUVECs, grown to confluence.
Caki-1, KTC-26 or A498 cells (0.5×106/ml) were placed in
the upper chamber in serum-free medium, either free of amygdalin
(control) or containing amygdalin. The lower chamber contained 10%
serum. After overnight incubation, the upper surface of the
transwell membrane was gently wiped with a cotton swab to remove
cells which had not migrated. Cells which had moved to the lower
surface of the membrane were stained using hematoxylin and counted
microscopically. The mean migration rate was calculated in five
different observation fields.
Integrin surface expression
Tumor cells were washed in blocking solution (PBS,
0.5% BSA) and then incubated for 60 min at 4°C with phycoerythrin
(PE)-conjugated monoclonal antibodies directed against the
following integrin subtypes: anti-α1 (mouse IgG1; clone SR84;
#559596), anti-α2 (mouse IgG2a; clone 12F1-H6; #555669), anti-α3
(mouse IgG1; clone C3II.1; #556025), anti-α4 (mouse IgG1; clone
9F10; #555503), anti-α5 (mouse IgG1; clone IIA1; #555617), anti-α6
(mouse IgG2a; clone GoH3; #555736), anti-β1 (mouse IgG1; clone
MAR4; #555443), anti-β3 (mouse IgG1; clone VI-PL2; #555754) or
anti-β4 (rat IgG2b; clone 439-9B; #555720) (all from BD Pharmingen,
Heidelberg, Germany). Integrin expression of tumor cells was then
measured using a FACScan (BD Biosciences, Heidelberg; FL-2H (log)
channel histogram analysis; 1×104 cells/scan) and
expressed as mean relative fluorescence intensity (RFI). Mouse
IgG1-PE (MOPC-21; #555749), IgG2a-PE (G155-178; #555574) and rat
IgG2b-PE (R35-38; #555848; all from BD Biosciences) were used as
isotype controls.
Western blotting
To investigate integrin content, tumor cell lysates
were applied to a 7–12% polyacrylamide gel (depending on protein
size) and electrophoresed for 90 min at 100 V. The protein was then
transferred to nitrocellulose membranes. After blocking with
non-fat dry milk for 1 h, the membranes were incubated overnight
with the following antibodies: integrin α1 (rabbit, polyclonal,
1:1,000; #AB1934; Chemicon/Millipore GmbH, Schwalbach, Germany),
integrin α2 (mouse IgG1, 1:250, clone 2; #611017; BD Biosciences),
integrin α3 (rabbit, polyclonal, 1:1,000; #AB1920;
Chemicon/Millipore GmbH), integrin α4 (mouse, 1:200, clone: C-20;
#sc-6589; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)],
integrin α5 (mouse IgG2a, 1:5,000, clone 1; #610634; BD
Biosciences), integrin α6 (rabbit, 1:200, clone H-87; #sc-10730;
Santa Cruz Biotechnology, Inc.,), and integrin β1 (mouse IgG1,
1:2,500, clone 18; #610468), integrin β3 (mouse IgG1, 1:2,500,
clone 1; #611141) and integrin β4 (mouse IgG1, 1:250, clone 7;
#611233) (all from BD Biosciences). HRP-conjugated goat anti-mouse
IgG and HRP-conjugated goat anti-rabbit IgG (both 1:5,000; Upstate
Biotechnology, Lake Placid, NY, USA) served as the secondary
antibodies. Additionally, integrin-related signaling was explored
by anti-integrin-linked kinase (ILK) (clone 3, dilution 1:1,000;
#611803), anti-focal adhesion kinase (FAK) (clone 77, dilution
1:1,000; #610088) and anti-p-specific FAK (pY397; clone 18,
dilution 1:1,000; #611807) antibodies (all from BD Biosciences).
HRP-conjugated goat-anti-mouse IgG (dilution 1:5,000; Upstate
Biotechnology) served as secondary antibodies. The membranes were
briefly incubated with ECL detection reagent (ECL™; Amersham, GE
Healthcare, München, Germany) to visualize the proteins and then
analyzed with the Fusion FX7 system (Peqlab, Erlangen, Germany).
β-actin (1:1,000; Sigma-Aldrich) served as the internal
control.
Gimp 2.8 software was used to perform pixel density
analysis of the protein bands. The ratio of protein
intensity/β-actin intensity was calculated, and expressed as a
percentage, related to controls set to 100%.
Blocking experiments
To determine whether integrin α5 and α6 impacted the
metastatic spread independently of amygdalin in Caki-1, KTC-26, and
A498 cell lines, cells were incubated for 60 min with 10
µg/ml function-blocking anti-integrin α5 (clone P1D6) mouse
mAb or anti-integrin α6 (clone NKI-GoH3) rat mAb (both from
Millipore). Controls were incubated with cell culture medium alone.
Subsequently, tumor cell adhesion to immobilized collagen as well
as chemotaxis were analyzed as described above.
Statistical analysis
In the present study, all experiments were performed
3–6 times. Stat istical significance was determined using the
Wilcoxon, Mann-Whitney U-test. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Amygdalin blocks interaction between the
tumor cell endothelium and tumor cell matrix
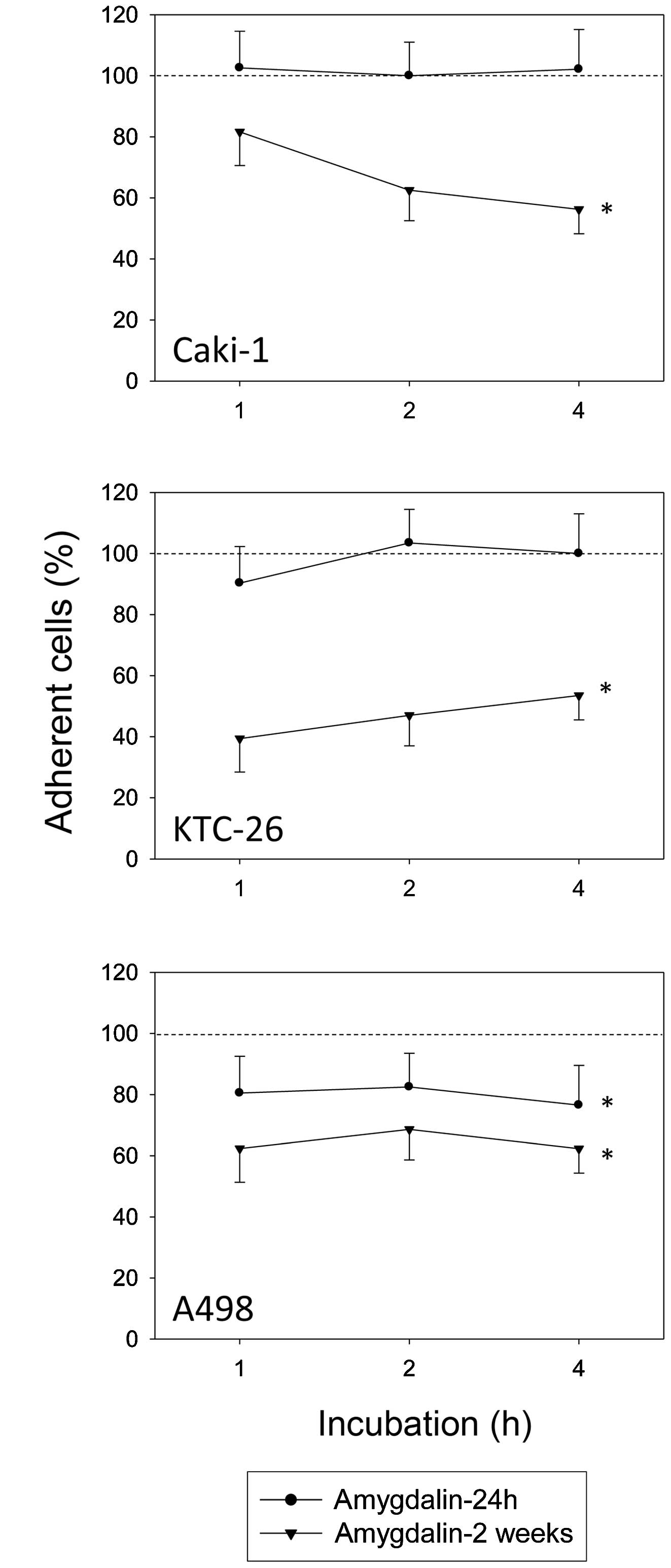
After 24 h of treatment with amygdalin, A498 tumor
cell adhesion to HUVECs was significantly diminished, but cell
adhesion of Caki-1 and KTC-26 cells was not (compared to untreated
controls set to 100%) (Fig. 1).
Extending the exposure time of amygdalin to 2 weeks significantly
decreased adhesion to HUVECs in all three tumor cell lines
(Fig. 1).
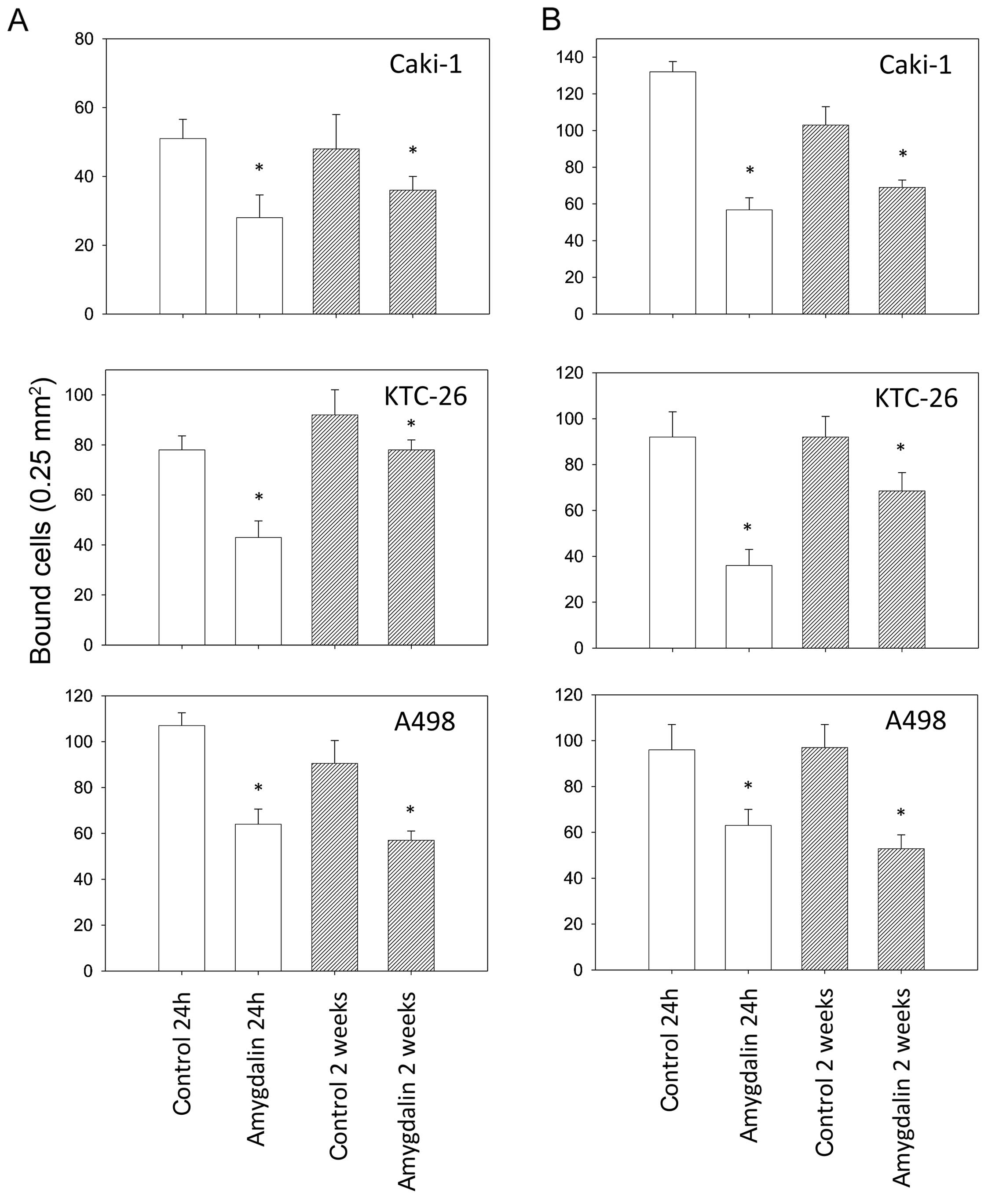
Amygdalin caused a significant decrease in the
binding capacity of all three RCC cell lines to immobilized
collagen and fibronectin, compared to controls (Fig. 2). Attachment of all three cell
lines to the matrix proteins was diminished after 24 h, as well as
after 2 weeks. In the KTC-26 cell line, short-term amygdalin
application (24 h) induced a greater decrease in adhesion than
long-term amygdalin application (2 weeks).
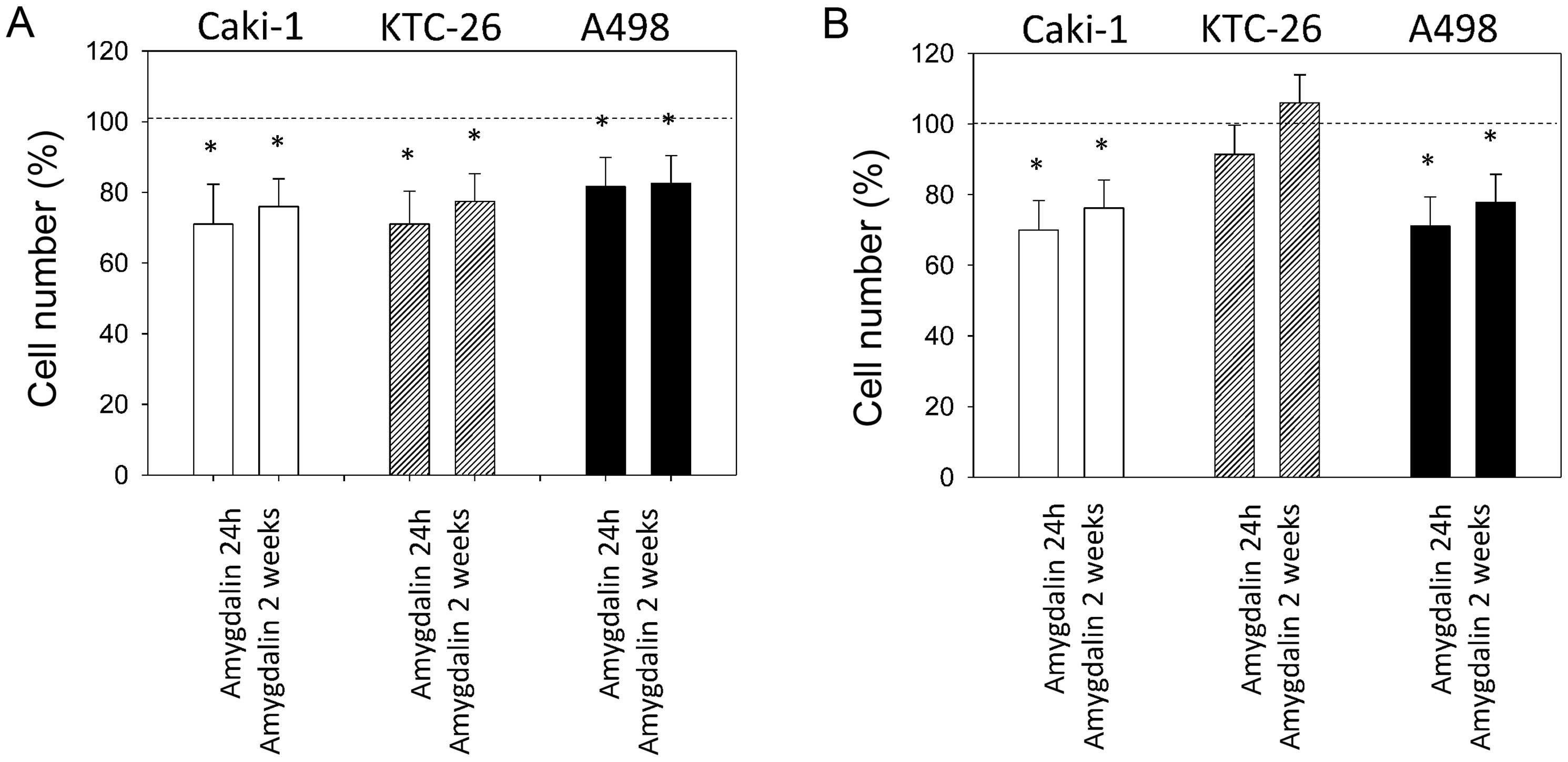
Amygdalin alters the tumor cell motility
of RCC cells
The chemotactic activity of Caki-1, KTC-26 and A498
cells significantly decreased after 24 h and 2 weeks of amygdalin
application, compared to the untreated control cells (Fig. 3A). Tumor cell invasion through the
collagen-coated transwell membranes was also significantly
diminished in Caki-1 and A498 cells after 24 h and 2 weeks of
amygdalin application (Fig. 3B).
However, 2 weeks of amygdalin did not reduce the invasive capacity
of KTC-26 cells.
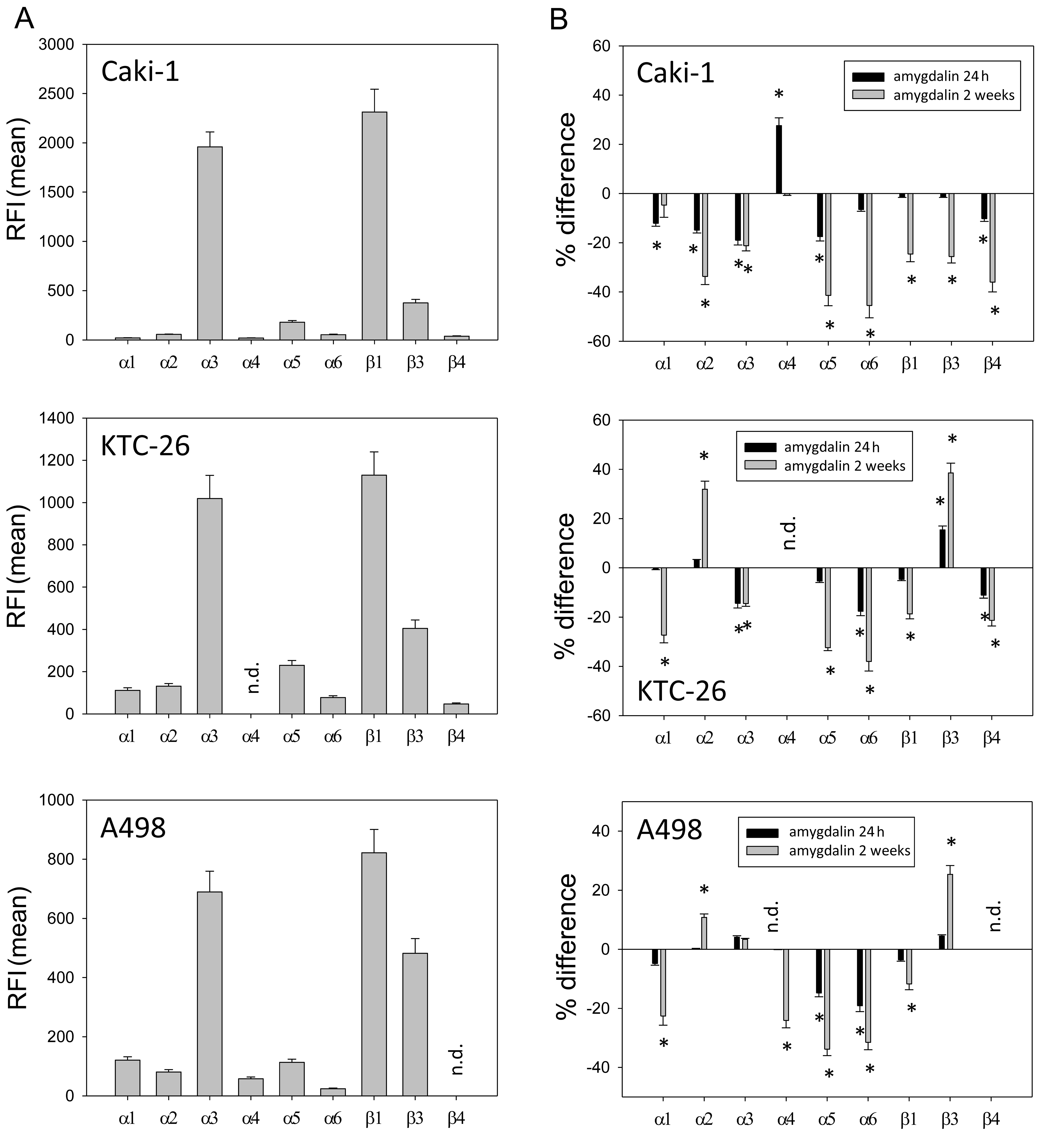
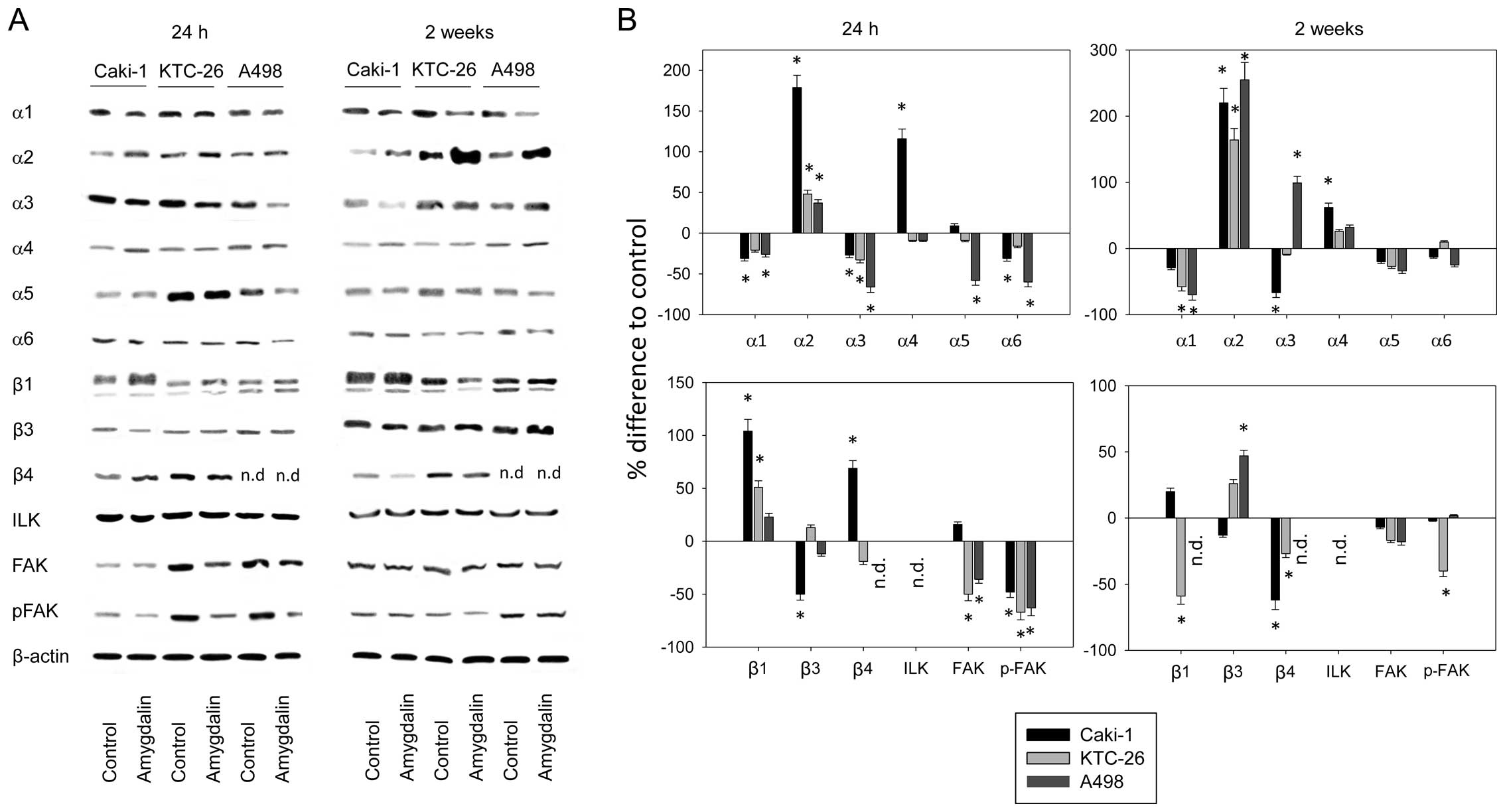
Amygdalin modulates integrin α and β
surface expression
Caki-1, KTC-26 and A498 cells were characterized by
different basal integrin α and β surface expression patterns
(Fig. 4A). Caki-1 markedly
expressed α3 and β1, moderately expressed α5 and β3, whereas α1,
α2, α4, α6 and β4 were only marginally detectable. KTC-26 strongly
expressed α3 and β1. The subtype members α1, α2, α5, α6, β3 and β4
were moderately expressed, and α4 was not detectable. The integrin
expression profile of A498 was similar to that of KTC-26, aside
from α4, which was detectable for A498. We also noted that β4 was
present in KTC-26 cells but not in A498 cells.
Amygdalin application for twenty-four hours and for
2 weeks altered the integrin surface profile, which is specific to
the cell type (Fig. 4B). We noted
that α5 and α6 were significantly down-regulated in all cell lines
after 2 weeks of amygdalin exposure. β1, which was strongly
expressed in all three cell lines, was also significantly reduced
following 2 weeks of amygdalin application. The high basal
expression of the α3 receptor was reduced in Caki-1 and KTC-26 but
not in A498 by amygdalin. Differences were also noted in relation
to α2 and β3, both of which were reduced in Caki-1 cells but
elevated in KTC-26 and A498 cells after 2 weeks. Diminished
expression levels of β4 were found in Caki-1 and KTC-26 cells after
amygdalin exposure.
Amygdalin influences the total cellular
integrin content
Evaluation of the integrin protein content after 24
h of amygdalin exposure revealed significant upregulation of α2 and
downregulation of α3 and p-FAK (Fig.
5). β1 was significantly elevated in Caki-1 and KTC-26, whereas
α6 was significantly decreased in Caki-1 and A498, and the total
content of α5 was reduced in A498 cells after 24 h. In Caki-1
cells, α4 and β4 increased and β3 decreased.
After 2 weeks of exposure to amygdalin, integrin α2
significantly increased, even more so than after exposure for 24 h.
In A498 cells, α3 and β3 increased after 2 weeks of amygdalin
exposure. Reduced β1 and p-FAK occurred in KTC-26 cells, and β4 was
downregulated in both Caki-1 and KTC-26 after 2 weeks of amygdalin
exposure (Fig. 5).
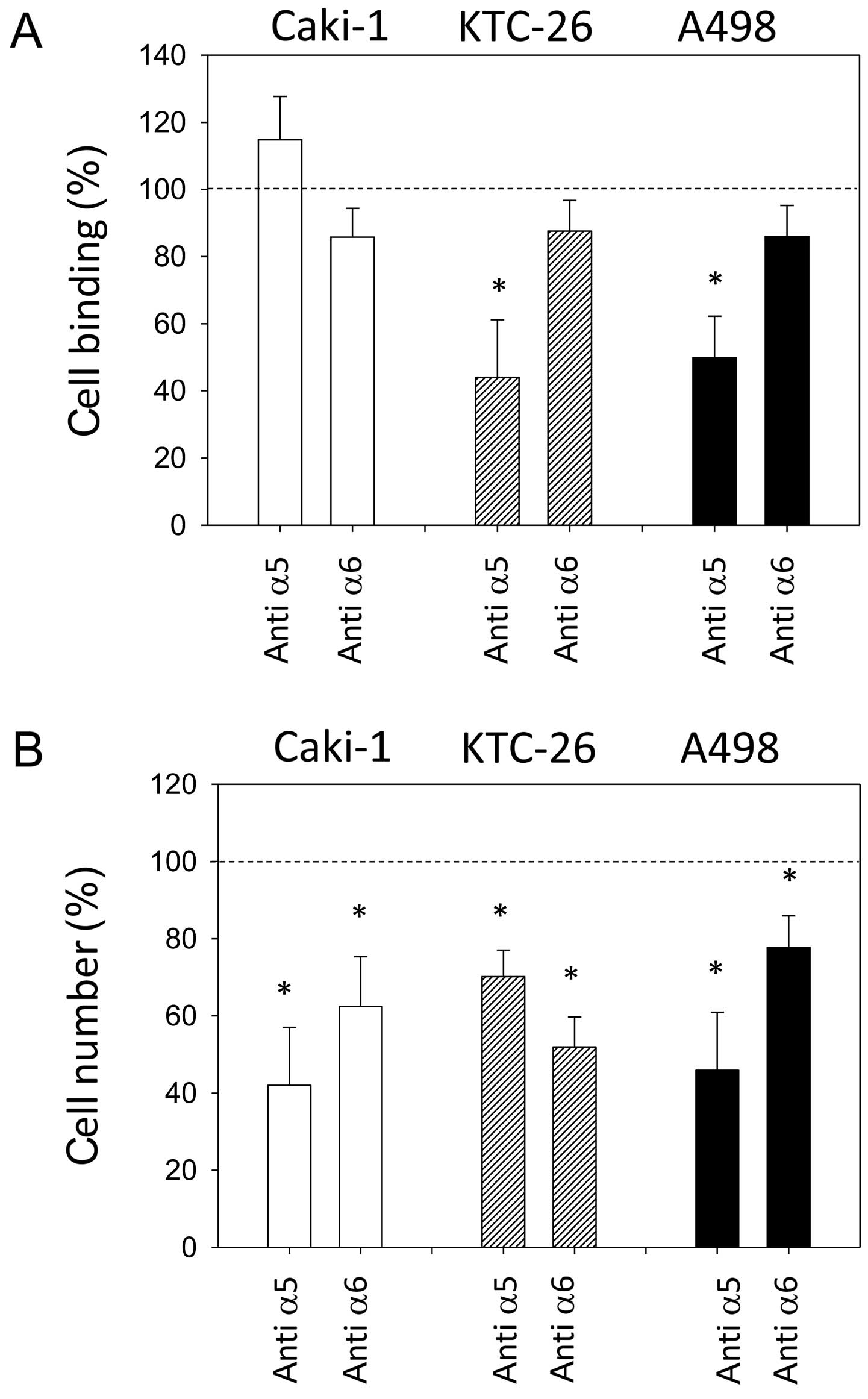
Blocking experiments
The integrin expression profiles of all three cell
lines were modified by amygdalin. Since surface integrin α5 and
integrin α6 were strongly reduced in all three cell lines following
amygdalin application, these integrins were chosen for functional
blocking studies, to investigate whether the reductions correlate
with changes in tumor cell adhesion and migration. Blocking α5 led
to the significant inhibition of KTC-26 and A498 cell adhesion to
collagen (Fig. 6A). However,
adhesion of Caki-1 cells to collagen was not significantly
influenced. Blocking integrin α5 resulted in reduced chemotactic
activity in all three cell lines (Fig. 6B). Blocking the α6 receptor did
not significantly affect adhesion to collagen in any of the cell
lines (Fig. 6A) but significantly
decreased chemotaxis in all three cell lines (Fig. 6B).
Discussion
It has been noted that interaction between tumor
cells and endothelium plays a crucial role in metastatic
progression; adhesion of non-small cell lung cancer (NSCLC) cells
to the vessel-wall endothelium has been associated with tumor cell
transmigration, which leads to brain and lymph node metastases
(7). A more aggressive,
metastasizing cancer phenotype has also been associated with
enhanced adhesion (7). In the
present study, we demonstrated that amygdalin exposure led to
significant inhibition of the binding interaction between RCC cells
and a HUVEC monolayer, collagen and fibronectin. The chemotactic
and invasive activity of RCC cells was thereby inhibited. Such
inhibition is clinically relevant since transendothelial migration
and motile spreading are critical steps in tumor dissemination and
progression (3) and correlate
with poor survival. Reducing migratory potential has been
associated with successful tumor therapy and a less malignant tumor
phenotype (8). Thus, we suggest
that inhibiting RCC cell adhesion and motility by amygdalin reduces
metastatic spread.
The adhesion- and migration-blocking effect of
amygdalin is not restricted to RCC cells. Amygdalin has recently
been demonstrated to also suppress the adhesive behavior of bladder
cancer cells (5). Although
amygdalin exerted a similar suppressive effect on the adhesion
properties in bladder cancer and RCC cells, bladder cancer cell
migration was affected differently by amygdalin. Chemotaxis was
downregulated in two, but upregulated in one bladder cancer cell
line following amygdalin exposure, indicating that the influence of
amygdalin probably depends on the tumor entity. Thus, it is
important to investigate the impact of amygdalin on different tumor
entities.
The integrin family has been implicated in all steps
of metastatic tumor progression (4,7).
Integrin α5 is upregulated in tumor cells of epithelial origin, and
a positive correlation between integrin α5 expression and RCC cell
adhesion has been established (9). In the present investigation,
amygdalin administration significantly decreased surface integrin
α5 in all three cell lines. Also, the total cellular content of
integrin α5 time-dependently decreased in the presence of
amygdalin. Blocking integrin α5 function caused significant
inhibition of KTC-26 and A498 cell adhesion to collagen and a
decrease in the chemotactic activity of all cell lines employed.
Consistent with the present data, downregulation of integrin α5 has
previously been associated with reduced adhesive and invasive
behavior of several cancer cell types (10–12).
In the present study, we noted that the collagen
adhesion of Caki-1 cells did not decrease after blocking integrin
α5, in contrast to the amygdalin-induced decrease in A498 and
KTC-26 cells. Such a difference in integrin function between tumor
cell types has previously been observed. Blocking α5 integrin has
been shown to inhibit cell-matrix interaction of the bladder cancer
cells HCV29 and BC3726 but enhance binding of the bladder cancer
cell lines T24 and Hu456 (equipped with a different integrin set)
(13). Similarly, blocking β1
integrin has been shown to inhibit UMUC-3 bladder cancer cell
attachment to collagen, but has the opposite adhesive effect on
TCCSUP cells, which are characterized by a different integrin
expression profile (5). In the
present investigation, amygdalin exposure caused surface β3
integrin to increase in KTC-26 and A498 cells, but decrease in
Caki-1 cells. Counter-regulation involving another integrin
subtype, in this case β3, may explain why blocking α5 failed to
stop adhesion in Caki-1 cells. Loss of integrin α5 caused
chemotaxic reduction in all three cell lines. Therefore, we suggest
that loss of surface integrin α5 is a mechanism by which amygdalin
acts on RCC cell migration, and fine-tuning integrin performance
depends on the specific integrin profile in the particular cell
line.
Integrin α6 has been shown to facilitate epithelial
cell migration and is correlated with progression risk, metastasis
and death in clinical trials (14). Other studies have shown that
integrin α6 promotes migration and invasion in colorectal cancer
(15), and pancreatic (16) and breast (17) carcinomas. Integrin α6 has been
noted to activate FAK (18) and
FAK-related downstream signaling, which is relevant to controlling
cell motility, survival and proliferation (4). In the present investigation, surface
expression of integrin α6 was significantly reduced and FAK was
deactivated by amygdalin in all three cell lines. Blocking surface
integrin α6 demonstrated that α6 does not interfere with tumor cell
adhesion but does regulate cell motility. Therefore, it is likely
that reduction of α6 represents a mechanism by which amygdalin
slows cell spreading.
Integrin β1 has been shown to promote cell invasion
in breast, lung, pancreatic and colorectal cancer, as well as
glioma, melanoma (4) and
neuroblastoma (19). In prostate
cancer cells, upregulation of integrin β1 has been shown to be
accompanied by elevated motile behavior, whereas integrin β1
blockade contributes to the downregulation of chemotaxis, migration
and cell adhesion (10).
Inhibition of integrin β1 has been associated with reduced invasion
and metastasis of ovarian cancer (12), and an integrin α5β1 peptide
inhibitor has been shown to block breast cancer metastasis in
vivo (20). In the present
investigation, surface integrin β1 was downregulated in all three
tumor cell lines following 2 weeks of amygdalin exposure.
Therefore, amygdalin may act on integrin β1 to slow the motile
spreading of RCC cells.
In vitro and in vivo investigations
indicate that α3 is another integrin involved in the invasion of
glioma, melanoma, hepatocellular and mammary carcinoma, and
promotes lung metastasis of breast carcinoma cells (4). Blocking integrin α3 has resulted in
adhesion inhibition of prostate cancer cells (21). In the present investigation,
surface integrin α3 was reduced in Caki-1 and KTC-26, but not in
A498 cells. This inhomogeneous reduction points to a cell
line-specific effect induced by amygdalin.
Amygdalin application, besides modulating surface
integrin expression, also changed total cellular integrin content.
In the present study, total cellular integrin α2 expression was
elevated in all three tumor cell lines following amydalin exposure.
Previous studies have shown that decreased levels of integrin α2 in
tumor cells potentially increase tumor cell dissemination, and
re-expression of integrin α2 has been shown to reverse malignant
properties of breast cancer cells (22). Hence, we suggest that the
amygdalin-induced inhibition of tumor cell adhesion and migration
observed here is associated with upregulation of integrin α2.
Total cellular α3 integrin was decreased 24 h after
amygdalin application in all three cell lines. This is in line with
the amygdalin-induced reduction in surface α3 in Caki-1 and KTC-26
cells. Since surface α3 was not modified in the A498 cells, it is
possible that amygdalin in this cell line acts through the
intracellular α3 signaling pathways. p-FAK, which was strongly
diminished in A498 cells 24 h after amygdalin application, supports
this hypothesis since the α3-FAK axis is involved in cancer
initiation and progression (23).
Lee et al have demonstrated that FAK is a critical mediator
of tumorigenesis and metastasis, which in part depend on integrin
α3 (24). Therefore, we suggest
that knocking down both integrin α3 and FAK deactivates the motile
machinery of A498 cells.
Total cellular integrin β1 was elevated in Caki-1
cells after amygdalin application, while surface expression was
diminished. This type of shift is not uncommon and points to
receptor translocation from the surface to the intracellular
compartment. Although the relevance of this process is not fully
understood, integrin β1 trafficking to the plasma membrane has been
shown to increase the metastatic potential of RCC cells, whereas
stopping β1 recycling by maintaining a high cyctoplasmic and a low
plasma membrane content decreases metastasis (25). Elevation of intracellular β1 has
been shown to be linked with FAK deactivation (25), which correlates with the present
findings.
The effects of amygdalin on integrin subtype
expression depended on the cell line, upon whether the application
time was acute (24 h) or chronic (2 weeks) and whether the integrin
location was the cell surface or in the cytoplasm. The molecular
mode of action of amygdalin in regard to integrin subtype
expression, therefore, is not homogeneous and may influence both
integrin-triggered mechanical cell-to-cell coupling and
integrin-controlled biochemical pathway activation.
In the three investigated RCC cell lines, amygdalin
application significantly reduced invasive and motile behavior.
However, 2 weeks of amygdalin did not reduce the invasive capacity
of KTC-26 cells. The reduction was predominantly associated with a
decrease in surface α5 and α6 integrins. This mechanism, however,
should not be generalized. Although amygdalin has been shown to
inhibit adhesion and migration in bladder cancer cells as well, the
integrins are altered differently. The α5 and α6 integrins seem to
be an important target of amygdalin in RCC cells, whereas
modulation of β1 or β4 integrins was most apparent in bladder
cancer cells (5). Further in
vitro investigations have been initiated to evaluate whether
amygdalin also influences RCC cell growth, as has been observed for
bladder cancer cells (6).
In conclusion, exposing RCC cells to amygdalin
inhibits metastatic spread and is associated with downregulation of
α5 and α6 integrins. Therefore, amygdalin exerts antitumor activity
in vitro in RCC. This in vitro activity must be
evaluated in an animal model.
Acknowledgments
The present study was supported by the 'Brigitta und
Norbert Muth Stiftung' and the 'Freunde und Förderer der
Goethe-Universität Frankfurt'.
References
|
1
|
Moertel CG, Fleming TR, Rubin J, Kvols LK,
Sarna G, Koch R, Currie VE, Young CW, Jones SE and Davignon JP: A
clinical trial of amygdalin (laetrile) in the treatment of human
cancer. N Engl J Med. 306:201–206. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newell GR and Ellison NM: Ethics and
designs: laetrile trials as an example. Cancer Treat Rep.
64:363–365. 1980.PubMed/NCBI
|
|
3
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun CC, Qu XJ and Gao ZH: Integrins:
players in cancer progression and targets in cancer therapy.
Anticancer Drugs. 25:1107–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makarević J, Rutz J, Juengel E, Kaulfuss
S, Tsaur I, Nelson K, Pfitzenmaier J, Haferkamp A and Blaheta RA:
Amygdalin influences bladder cancer cell adhesion and invasion in
vitro. PLoS One. 9:e1102442014. View Article : Google Scholar
|
|
6
|
Makarević J, Rutz J, Juengel E, Kaulfuss
S, Reiter M, Tsaur I, Bartsch G, Haferkamp A and Blaheta RA:
Amygdalin blocks bladder cancer cell growth in vitro by diminishing
cyclin A and cdk2. PLoS One. 9:e1055902014. View Article : Google Scholar
|
|
7
|
Salvo E, Garasa S, Dotor J, Morales X,
Peláez R, Altevogt P and Rouzaut A: Combined targeting of TGF-β1
and integrin β3 impairs lymph node metastasis in a mouse model of
non-small-cell lung cancer. Mol Cancer. 13:1122014. View Article : Google Scholar
|
|
8
|
White NM, Masui O, Newsted D, Scorilas A,
Romaschin AD, Bjarnason GA, Siu KW and Yousef GM: Galectin-1 has
potential prognostic significance and is implicated in clear cell
renal cell carcinoma progression through the HIF/mTOR signaling
axis. Br J Cancer. 110:1250–1259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juengel E, Makarević J, Reiter M, Mani J,
Tsaur I, Bartsch G, Haferkamp A and Blaheta RA: Resistance to the
mTOR inhibitor temsirolimus alters adhesion and migration behavior
of renal cell carcinoma cells through an integrin α5- and integrin
β3-dependent mechanism. Neoplasia. 16:291–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsaur I, Makarević J, Juengel E, Gasser M,
Waaga-Gasser AM, Kurosch M, Reiter M, Wedel S, Bartsch G, Haferkamp
A, et al: Resistance to the mTOR-inhibitor RAD001 elevates integrin
α2- and β1-triggered motility, migration and invasion of prostate
cancer cells. Br J Cancer. 107:847–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imanishi Y, Hu B, Jarzynka MJ, Guo P,
Elishaev E, Bar-Joseph I and Cheng SY: Angiopoietin-2 stimulates
breast cancer metastasis through the alpha(5)beta(1)
integrin-mediated pathway. Cancer Res. 67:4254–4263. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin
K and Lengyel E: Ligand-independent activation of c-Met by
fibronectin and α(5) β(1)-integrin regulates ovarian cancer
invasion and metastasis. Oncogene. 30:1566–1576. 2011. View Article : Google Scholar :
|
|
13
|
Lityńska A, Przybyło M, Pocheć E and
Laidler P: Adhesion properties of human bladder cell lines with
extracellular matrix components: the role of integrins and
glycosylation. Acta Biochim Pol. 49:643–650. 2002.
|
|
14
|
Ricci E, Mattei E, Dumontet C, Eaton CL,
Hamdy F, van der Pluije G, Cecchini M, Thalmann G, Clezardin P and
Colombel M: Increased expression of putative cancer stem cell
markers in the bone marrow of prostate cancer patients is
associated with bone metastasis progression. Prostate.
73:1738–1746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao C, Lotz MM, Clarke AC and Mercurio
AM: A function for the integrin alpha6beta4 in the invasive
properties of colorectal carcinoma cells. Cancer Res. 56:4811–4819.
1996.PubMed/NCBI
|
|
16
|
Cruz-Monserrate Z and O'Connor KL:
Integrin alpha 6 beta 4 promotes migration, invasion through Tiam1
upregulation, and subsequent Rac activation. Neoplasia. 10:408–417.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim TH, Kim HI, Soung YH, Shaw LA and
Chung J: Integrin (alpha6beta4) signals through Src to increase
expression of S100A4, a metastasis-promoting factor: implications
for cancer cell invasion. Mol Cancer Res. 7:1605–1612. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XH, Flores LM, Li Q, Zhou P, Xu F,
Krop IE and Hemler ME: Disruption of laminin-integrin-CD151-focal
adhesion kinase axis sensitizes breast cancer cells to ErbB2
antagonists. Cancer Res. 70:2256–2263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee S, Qiao J, Paul P and Chung DH:
Integrin β1 is critical for gastrin-releasing peptide
receptor-mediated neuroblastoma cell migration and invasion.
Surgery. 154:369–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khalili P, Arakelian A, Chen G, Plunkett
ML, Beck I, Parry GC, Doñate F, Shaw DE, Mazar AP and Rabbani SA: A
non-RGD-based integrin binding peptide (ATN-161) blocks breast
cancer growth and metastasis in vivo. Mol Cancer Ther. 5:2271–2280.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsaur I, Rutz J, Makarević J, Juengel E,
Gust KM, Borgmann H, Schilling D, Nelson K, Haferkamp A, Bartsch G
and Blaheta RA: CCL2 promotes integrin-mediated adhesion of
prostate cancer cells in vitro. World J Urol. 33:1051–1056. 2015.
View Article : Google Scholar
|
|
22
|
Zutter MM, Santoro SA, Staatz WD and Tsung
YL: Re-expression of the alpha 2 beta 1 integrin abrogates the
malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci
USA. 92:7411–7415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cagnet S, Faraldo MM, Kreft M, Sonnenberg
A, Raymond K and Glukhova MA: Signaling events mediated by α3β1
integrin are essential for mammary tumorigenesis. Oncogene.
33:4286–4295. 2014. View Article : Google Scholar
|
|
24
|
Lee S, Qiao J, Paul P, O'Connor KL, Evers
MB and Chung DH: FAK is a critical regulator of neuroblastoma liver
metastasis. Oncotarget. 3:1576–1587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tringali C, Lupo B, Silvestri I, Papini N,
Anastasia L, Tettamanti G and Venerando B: The plasma membrane
sialidase NEU3 regulates the malignancy of renal carcinoma cells by
controlling β1 integrin internalization and recycling. J Biol Chem.
287:42835–42845. 2012. View Article : Google Scholar : PubMed/NCBI
|