Introduction
Hepatitis C virus (HCV) infection has become a
global public health problem worldwide: it infects approximately 3%
of the population worldwide, which may eventually lead to
hepatitis, cirrhosis, and liver cancer (1). HCV, which is a member of the
Flaviviridae family, is a positive-stranded RNA with a high
infection rate (2). In recent
years, with advances in knowledge and technology, treatments for
HCV infection have improved to a certain degree (3,4).
However, effective and secure therapeutic approaches are still
lacking.
The HCV genome encodes a polyprotein of ~3,010 amino
acids, which is processed into core protein C, envelope
glycoprotein 1 (E1) and 2 (E2), and non-structural proteins such as
non-structural proteins 3 and -5B (NS3 and NS5B) (5,6).
Of these proteins, it has been suggested that HCV E2 glycoproteins
play an important role in regulating HCV entry (7). E2 is a transmembrane glycoprotein
containing the amino acid residues 384–746 that mediates HCV entry
through interacting with various surface receptors on hepatic
cells, including CD81, low-density lipoprotein receptor, scavenger
receptor class B type I (SR-BI), claudin 1, and occludin (8,9).
CD81 is the first identified essential HCV receptor that has been
extensively studied (10,11). Various studies have suggested that
targeting and inhibiting CD81 inhibits HCV infection (8,12,13). Thus, CD81 may be a potential
molecular target for developing novel and promising anti-HCV
therapies.
It has been suggested that the conserved E2
(502–520) segment plays a critical role in cell entry by
influencing interaction with HCV receptors (14). Antibodies to the E2 (412–423)
regions have been reported to play broadly neutralizing roles
(15). Mutations of the two
conserved histidines (490 and 621) located in E2 have been noted to
block the binding of CD81 with the E2 protein (16). Albecka et al have
demonstrated that a segment from 705 to 715 located in the stem
region of E2 is involved in regulating HCV entry and infection
(17).
The use of synthesized peptide to inhibit HCV
infection has been widely investigated. It has been suggested that
synthetic anti-lipopolysaccharide peptides bind to heparan sulfate
moieties on the cell surface and inhibit infection with a variety
of enveloped viruses, including HCV (18). Human apolipoprotein E peptides
were found to inhibit HCV entry by blocking virus binding (19). Bukong et al reported that a
novel human radixin peptide suppressed HCV infection at the level
of cell entry (20). It has
previously been noted that synthesized peptide C18 (WPWHNHR) with
the highest affinity for binding HCV E2 protein effectively
inhibited HCV infection (21).
In the present study, we synthesized a short peptide
of E2 (710–725) from the E2 glycoprotein and investigated its
effects on HCV infection. The lack of a cell culture system to
maintain the effective various replications has been a major
obstacle for studying HCV. Usage of the developed cell
culture-derived HCV (HCVcc) has become a reliable system for
investigating HCV replication and infection (6,22,23). We evaluated the possible role of
the synthesized peptide E2 (710–725) in HCV infection by using
HCVcc. Our results showed that treatment with E2 (710–725)
dose-dependently decreased HCVcc entry, RNA replication, and
protein expression in hepatocytes. Furthermore, the inhibitory
effect of HCVcc on hepatocyte viability was reversed by the
synthesized peptide E2 (710–725). Using a co-immunoprecipitation
assay, we found that the interaction between CD81 and HCV E2 was
interrupted by the peptide E2 (710–725). In addition, our results
indicated that the synthesized peptide E2 (710–725) was not
cytotoxic to hepatocytes. Taken together, our results provided
novel insights into the design of anti-HCV drugs, and we suggest
that the synthesized E2 (710–725) peptide is a potential candidate
for use in anti-HCV therapy.
Materials and methods
Cell culture
Human hepatoma Huh7.5 cells, which were provided by
Dr Charlie Rice (Rockefeller University, New York, NY, USA), were
maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS)
(Gibco Life Technologies, Rockville, MD, USA), 2 mM L-glutamine,
and 1% penicillin and streptomycin. Primary human hepatocytes (pHH)
(purchased from Lonza, Basel, Switzerland) were grown in Williams'
medium E (AppliChem GmbH, Darmstadt, Germany) containing 5% fetal
calf serum, 1 µM dexamethason/fortecortin, 1 µM
insulin, 1 mM sodium pyruvate, 4 mM L-glutamine, and 1% penicillin
and streptomycin. All cells were cultured in a humidified
atmosphere containing 5% CO2 at 37°C.
Peptide synthesis
The E2 (710–725) peptide representing amino acids
710–725 of the E2 region from the HCV subtype 1a (ASWAIK WEYVV
LLFLL) was synthesized by solid phase peptide synthesis, as
previously described (24). The
protein sequences were verified by protein sequencing (Protech,
Suzhou, China).
HCVcc production
In the present study, HCVcc was produced according
to a method previously described (6). Briefly, plasmid pFLJ6/JFH1
containing the full-length genomic cDNA of HCV J6 and JFH-1 was
linearized and used as the template for transcription using an
in vitro Megascript kit (Promega Corp., Madison, WI, USA)
according to the manufacturer's instructions. Thereafter, the in
vitro transcribed RNA (25 µg) was delivered to Huh7.5
cells (1.25×106) by electroporation. After incubation
for 8–12 days, the supernatants containing HCVcc were collected,
filtered (0.45 mm), and stored at −80°C for further use.
HCV infection and evaluation
The viral RNA was extracted using an RNeasy Qiagen
kit (Qiagen, Dusseldorf, Germany). The viral RNA in collected
supernatants was measured and normalized for equal genome
equivalents which were then used to infect Huh7.5 cells and pHH in
the presence or absence of synthesized peptide. After an infection
of 48 h, the cells were harvested and lysed for luciferase activity
assay (Promega Corp.) according to the manufacturer's instructions.
The viral RNA was extracted from the cells and quantified using
reverse transcription-quantitative PCR (RT-qPCR) according to a
method previously described (25). The HCV specific primers were as
follows: 5′-AGCGTCTAGCCATGGCGT-3′ (forward) and
5′-GGTGTACTCACCGGTTCCG-3′ (reverse). The relative gene expression
was obtained by normalization with GAPDH (forward,
5′-CGGATTTGGTCGTATTGG-3′ and reverse,
5′-AGATGGTGATGGGATTTC-3′).
Cell viability assay
Cell viability was detected by MTT assay. Briefly,
cells were seeded in 96-well plates infected with HCVcc in the
presence or absence of synthesized peptide. After incubation for 72
h, the old medium was replaced by fresh medium containing 20
µl MTT (Sigma-Aldrich, St. Louis, MO, USA) solution (0.5
mg/ml diluted in PBS). After 4 h incubation, the medium was
discarded and 150 µl dimethyl sulfoxide was added to
dissolve the formazan crystals for 15 min. Finally, absorbance at
490 nm was measured using a microplate reader (Thermo Electron
Corp., Vantaa, Finland).
Co-immunoprecipitation assay
Huh7.5 cells were infected with HCVcc in the
presence or absence of synthesized peptide for 72 h. Subsequently,
cells were harvested and lysed in RIPA buffer (Beyotime Institute
of Biotechnology, Haimen, China) containing protein inhibitor in an
ice bath for 30 min. After centrifugation at 12,000 × g for 30 min,
the supernatant was collected. Protein A-Sepharose beads (GE
Healthcare, Piscataway, NJ, USA) mixed with a mouse monoclonal
anti-CD81 antibody (sc-23962; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) were incubated with the lysis buffer for 1 h at 4°C.
Mouse monoclonal IgG antibody (sc-69786; Santa Cruz Biotechnology,
Inc.) was used as control. Subsequently, the antibody-beads mixture
was added to the collected supernatant and incubated for 2 h at 4°C
followed by centrifugation at 3,000 × g for 5 min. The bead
complexes were then harvested and washed three times with lysis
buffer. The protein complexes on the beads were eluted by boiling
with sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) sample buffer and then separated by SDS-PAGE. The target
proteins were examined by western blot analysis.
Western blot analysis
Proteins were extracted from cells using a total
protein extraction kit (Applygen Technologies, Inc., Beijing,
China). Protein concentrations in different samples were detected
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of protein (25 µg) were
separated by electrophoresis on 12.5% SDS-PAGE and then transferred
to nitrocellulose membranes (Miltenyi Biotec, Auburn, CA, USA).
After being blocked with 3% non-fat milk, the membranes were
blotted with primary antibodies at 4°C overnight. Subsequently, the
membranes were washed three times with Tris-buffered saline with
Tween-20. The secondary antibodies conjugated with horseradish
peroxidase (1:2,000; BIOSS, Beijing, China) were added to the
membranes and incubated for 1 h at room temperature. The band color
was developed with an enhanced chemiluminescence reagent (GE
Healthcare) according to the manufacturer's instructions. The band
intensity was quantified with Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). In the present study, the
primary antibodies anti-HCV E2 (IT-004-005), anti-HCV
non-structural protein 3 (NS3; IT-004-014), and anti-HCV NS5B
(IT-004-021) were purchased from Immune Tech (New York, NY, USA);
anti-CD81 (sc-9158) and anti-GAPDH (sc-25778) were purchased from
Santa Cruz Biotechnology, Inc.
Statistical analysis
In the present study, all data are expressed as the
means ± standard deviation (SD). The differences were studied by
one-way analysis of variance with SPSS software package version
11.5 (SPSS, Inc., Chicago, IL, USA). Finally, a P-value <0.05
was considered to indicate a statistically significant
difference.
Results
Synthesized peptide E2 (710–725) is not
cytotoxic to cells
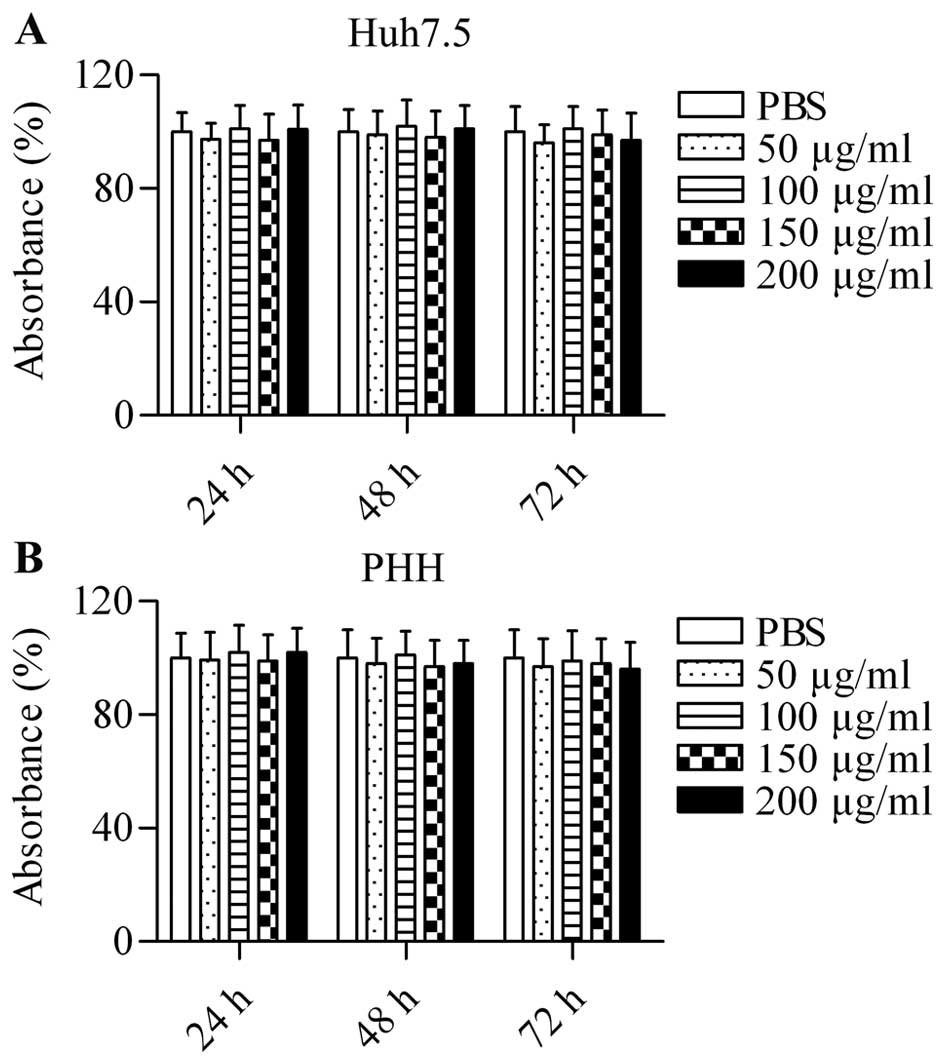
To explore the potential of the small molecular
weight peptide E2 (710–725) from HCV E2 to act as an inhibitor of
HCV infection, we first assessed the toxicity of this heterologous
protein using an MTT assay. Huh7.5 cells were treated with
different concentrations of E2 (710–725) (50, 100, 150 and 200
µg/ml) for 24, 48 and 72 h. The results demonstrated that
the synthesized peptide E2 (710–725) had no obvious cytotoxicity
for Huh7.5 cells at any concentration (Fig. 1A). Furthermore, even with
increases in time, E2 (710–725) was not cytotoxic to Huh7.5 cells
(Fig. 1A). Similarly, no
cytotoxic effect was observed in pHH treated with different
concentrations of E2 (710–725) for 24, 48 and 72 h (Fig. 1B). The data excluded the
possibility that the suppression of HCVcc infection was due to
toxicity of the heterologous protein.
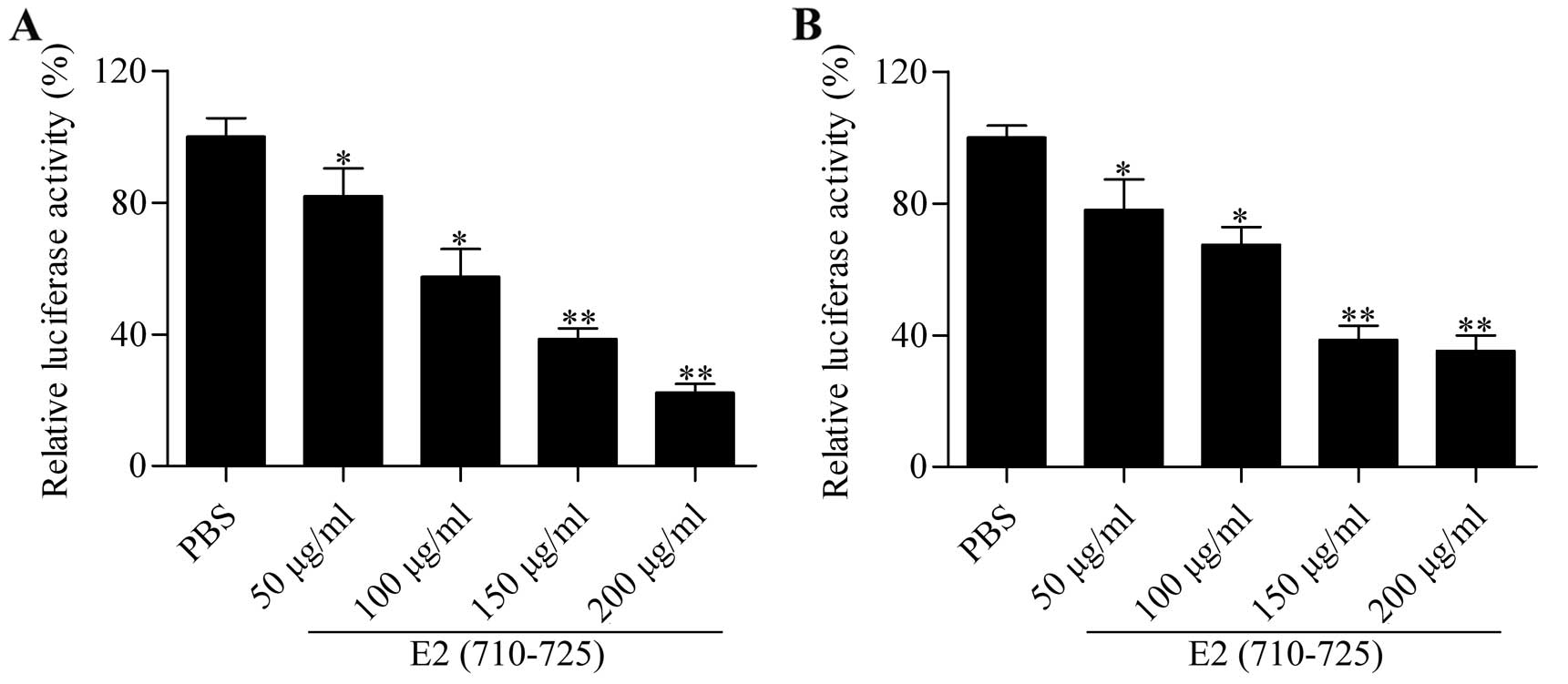
We hypothesized that the synthesized peptide E2
(710–725) blocks the infection of HCVcc. Thus, we evaluated the
effect of E2 (710–725) on the entry of HCVcc into hepatocytes.
Huh7.5 cells were administrated with 50% tissue culture infective
dose (TCID50) of HCVcc plus different concentrations of the E2
(710–725) peptide. Since HCVcc carries the luciferase gene, we
measured luciferase activity to assess the entry of HCVcc. The
results showed that the E2 (710–725) peptide significantly
inhibited luciferase activity in a dose-dependent manner in Huh7.5
cells infected with HCVcc for 72 h (Fig. 2A). The results were further
validated using pHH: the luciferase activity was also
dose-dependently decreased by treatment with different
concentrations of E2 (710–725) peptide (Fig. 2B). These results showed that the
synthesized peptide E2 (710–725) blocked the entry of HCVcc into
hepatocytes.
Synthesized peptide E2 (710–725) inhibits
HCV RNA and HCV-specific protein expression
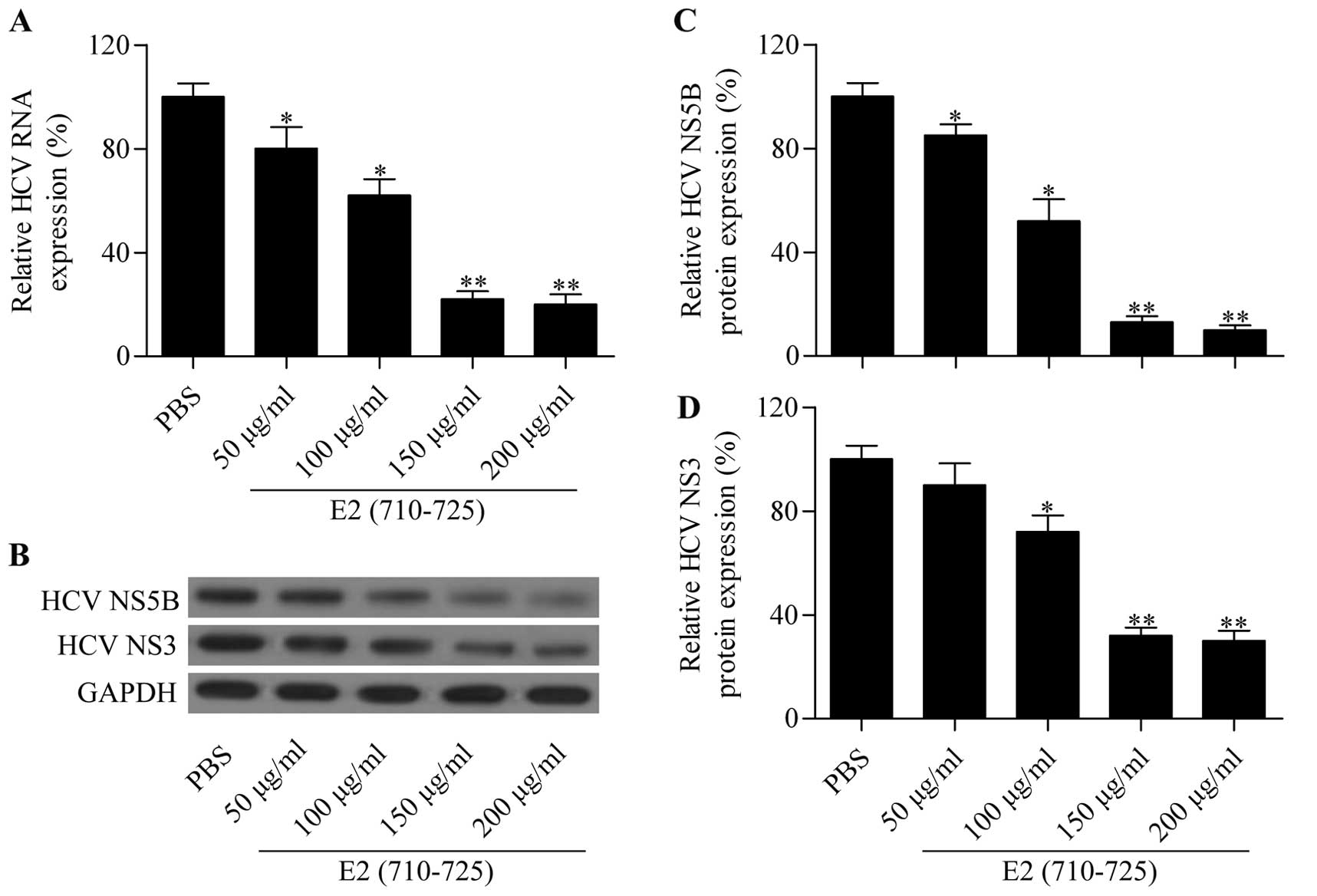
To further validate the inhibitory effect of the E2
(710–725) peptide on HCVcc infection, we detected HCV RNA
expression in the infected cells treated with different
concentrations of E2 (710–725) peptide. The RT-qPCR results showed
that the E2 (710–725) peptide significantly reduced intracellular
HCV RNA expression in Huh7.5 (Fig.
3A) cells in a dose-dependent manner. Furthermore, the
expression level of HCV-specific proteins NS3 and NS5B was examined
by western blot analysis (Fig.
3B). The results demonstrated that the protein expression of
NS3 and NS5B (Fig. 3C and D) in
Huh7.5 was also markedly reduced by the synthesized peptide E2
(710–725) in a dose-dependent manner. These results further
confirmed that the synthesized peptide E2 (710–725) inhibited HCV
infection.
Synthesized peptide E2 (710–725)
attenuates the inhibitory effect of HCVcc on hepatocyte
viability
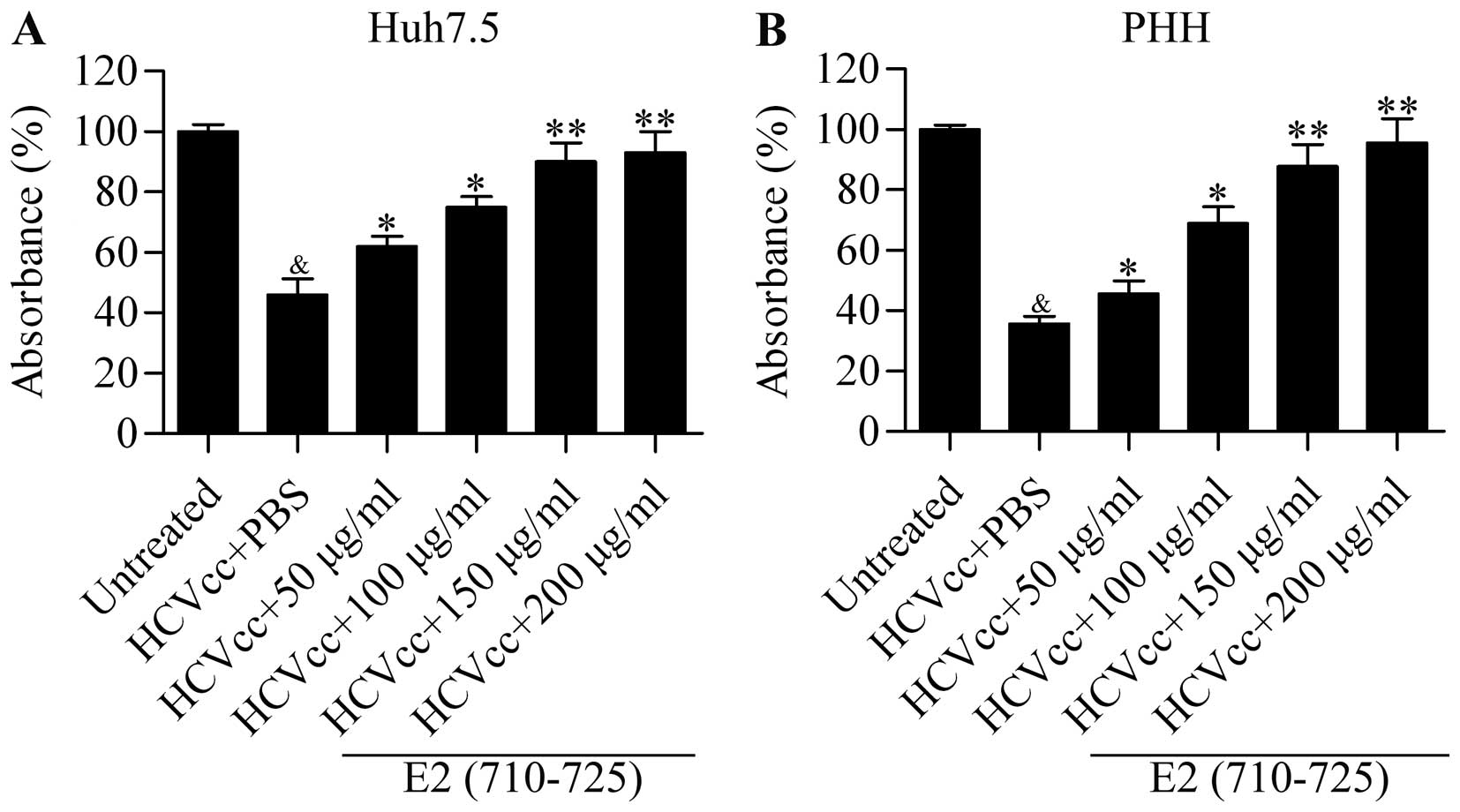
To further assess the role of the synthesized
peptide E2 (710–725) as an effective inhibitor of HCVcc infection,
we examined its effect on cell growth arrest induced by HCVcc. The
results showed that HCVcc infection significantly decreased the
cell viability of Huh7.5 cells, which was then reversed by E2
(710–725) treatment in a dose-dependent manner (Fig. 4A). Moreover, the E2 (710–725)
peptide also significantly attenuated the inhibition of hepatocyte
viability induced by HCVcc infection in pHH in a dose-dependent
manner (Fig. 4B). In summary, our
data suggested that the synthesized peptide E2 (710–725)
effectively blocked HCVcc infection. In order to gain a better
understanding of the underlying mechanism of the E2 (710–725)
peptide in preventing HCVcc infection, we performed subsequent
experiments.
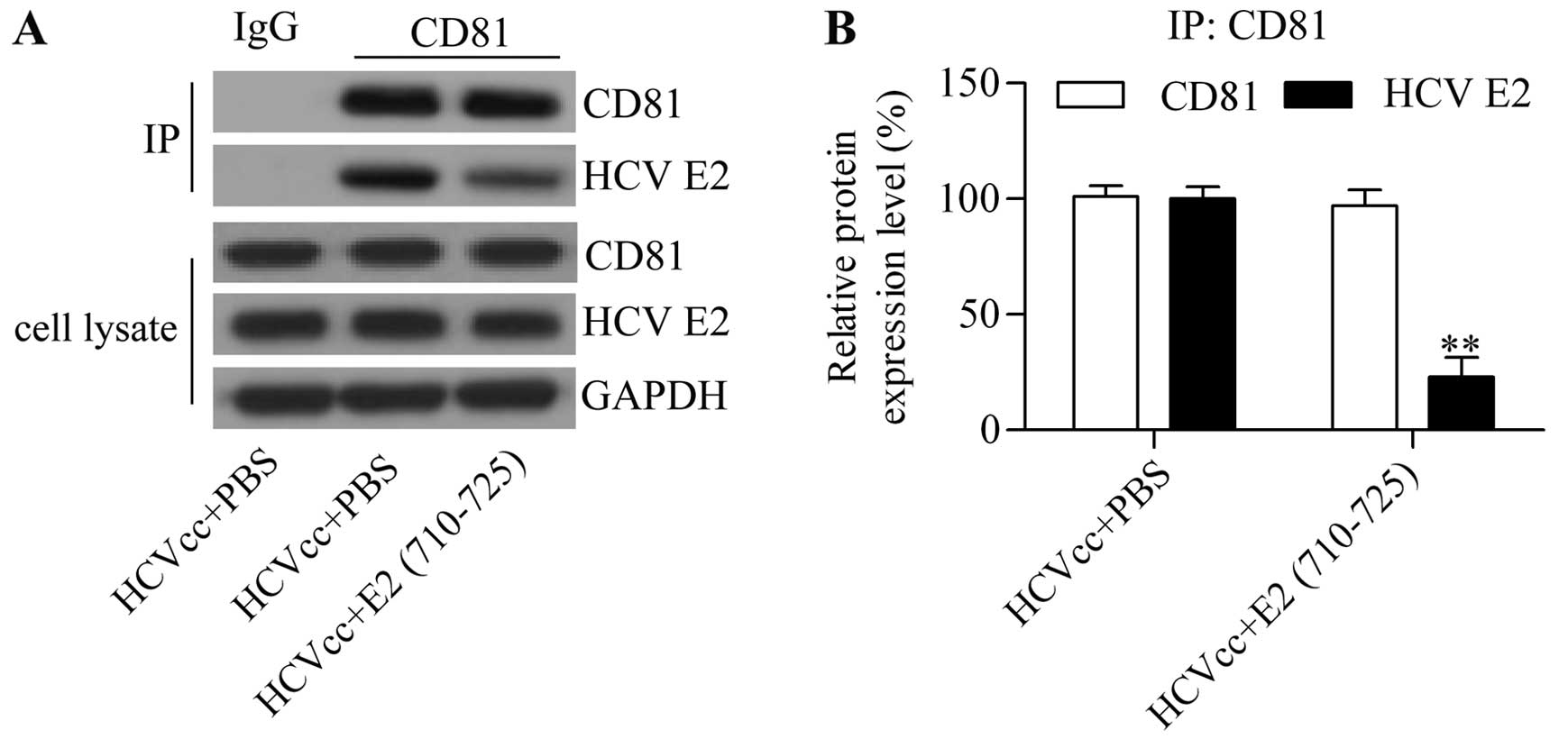
We hypothesized that the synthesized peptide E2
(710–725) abrogates the interaction between CD81 and HCV E2
envelope protein. CD81 has been suggested to act as an essential
HCV receptor that interacts with HCV E2 envelope protein for
subsequent HCV entry (10,26).
In order to investigate whether the E2 (710–725) peptide derived
from the HCV E2 protein blocked interaction between CD81 and the
HCV E2 protein, we performed a co-immunoprecipitation assay. Thus,
using the CD81 antibody, we analyzed HCV E2 protein expression in
the immune complex by western blot analysis. The results showed
that HCV E2 protein expression was significantly reduced in the
immune complex from cells treated with E2 (710–725) whereas CD81
protein expression level was barely altered (Fig. 5). The results suggested that E2
(710–725) competitively binds to CD81, and thus interrupted the
interaction between CD81 and HCV E2 envelope protein by which E2
(710–725) blocked HCV cell entry.
Discussion
In the present study, we demonstrated that the
synthesized peptide E2 (710–725) markedly inhibited HCV infection,
possibly due to its ability to disrupt the interaction between the
HCV E2 envelope protein and CD81 receptor. The results suggest that
the segment from 710–725 amino acid residues of E2 plays an
important role in regulating HCV entry.
Previous research has proposed that the HCV E2
envelope protein plays a major role in regulating HCV entry into
cells (7). It has been reported
that W420, Y527, W529, G530 and D535 in the E2 protein are the
critical binding sites for the CD81 receptor (27). The E2 (436–443) motif plays an
important role in viral entry in a CD81-dependent manner (28). The conserved E2 (502–520) segment
has been suggested to play a critical role in cell entry by
influencing the interaction with HCV receptors (14). Mutations of the two conserved
histidines (490 and 621) located in E2 have been shown to block the
binding of CD81 with the E2 protein (16). Albecka et al have
demonstrated that a segment from 705 to 715 located in the stem
region of E2 is involved in regulating HCV entry and infection
(17). However, the majority of
studies have focused on the N-terminal and core region of E2
(27,28). It remains largely unknown whether
the amino acid sites located in the carboxyl terminus of E2 play a
role in regulating HCV entry. In the present study, we synthesized
a peptide from 710–725 amino acids of E2 and investigated its
effect on HCV infection. We observed that the E2 (710–725) peptide
blocked HCV infection in both human hepatoma Huh7.5 cells and also
pHH. Our data suggest that the amino acid residues in the
carboxyl-terminal of the HCV E2 protein also play an important role
in terms of regulating HCV infection.
To date, various cell surface receptors have been
identified as HCV entry receptors, including CD81, low-density
lipoprotein receptor, SR-BI, claudin 1 and occludin (8,9).
Of these receptors, CD81 is the first identified HCV receptor which
interacts with the HCV E2 protein to facilitate the endocytosis of
HCV (10,11). Numerous studies have aimed to
target CD81 to block HCV infection (12,29–31). It has previously been reported
that blocking CD81 using anti-CD81 monoclonal antibodies
dose-dependently inhibited HCV entry (12,29). Furthermore, treatment with
anti-CD81 monoclonal antibodies has been noted to effectively
prevent HCV infection and spread in chimeric mice with human liver
(30). Notably, the silencing of
CD81 by small interfering RNA significantly suppressed HCV
pseudotype infection in Huh7.5 hepatoma cells, whereas
overexpression of CD81 in infection-resistant human liver cells
promoted HCV infection (29).
More recently, it has been reported that microRNA-194 functions as
a hepatocyte gatekeeper that blocks HCV entry by targeting and
inhibiting CD81 expression (31).
Additionally, it has also been suggested that CD81 plays an
important role in HCV replication (32). Cells exhibiting low expression of
CD81 had limited replication capacity, which was restored by
overexpression of CD81 (32). In
the present study, we demonstrated that the E2 (710–725) peptide is
capable of blocking the interaction between CD81 and the HCV E2
protein, and this leads to inhibition of HCV entry and
infection.
The potential use of synthesized peptides to treat
HCV infection has previously been studied. For example, Krepstakies
et al have reported that synthetic anti-lipopolysac-charide
peptides bind to heparan sulfate moieties on the cell surface and
inhibit HCV infection (18). It
has also been noted that the human apolipoprotein E peptide
directly blocked the binding of HCV to hepatocytes (19). A human radixin hinge region
peptide has been demonstrated to inhibit HCV infection through
disrupting HCV engagement of CD81 (20). Interestingly, a synthesized
peptide C18 (WPWHNHR) with the highest affinity for binding HCV E2
protein has been demonstrated to be capable of inhibiting HCV
infection (21). In the present
study, we detected the effect of the synthesized peptide from HCV
E2 glycoprotein and observed that this peptide blocked HCV cell
entry and also inhibited HCV infection. Our results indicated that
the synthesized E2 (710–725) peptide serves as a potential
candidate for anti-HCV therapy.
Previous research has focused on HCV E2 in order to
develop new and potentially therapeutic drugs for the treatment of
HCV infection. For instance, it was previously noted that blocking
the signaling lymphocytic activation molecule family 3 expression
on the hepatocytes by specific antibody abrogated its interaction
with HCV E2, thus leading to the inhibition of HCV entry (33). A new compound, 281816, has been
screened using surface plasmon resonance detection, which binds the
HCV E2 protein and blocks E2 binding to CD81 and inhibits HCV
infection (34). In the present
study, we demonstrated that the peptide E2 (710–725) derived from
the HCV E2 protein acted as an inhibitor of HCV E2 entry, and we
also provided useful insights which should be utilized to fiurther
the development of new drugs for preventing HCV infection.
Moreover, we confirmed that the synthesized peptide E2 (710–725)
exerted no cytotoxicity and has the potential to be used for the
treatment of HCV infection. However, further studies are needed to
investigate its safety and effectiveness using in vivo
animal models.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81170394).
References
|
1
|
Wasley A and Alter MJ: Epidemiology of
hepatitis C: Geographic differences and temporal trends. Semin
Liver Dis. 20:1–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keck ZY, Li TK, Xia J, Bartosch B, Cosset
FL, Dubuisson J and Foung SK: Analysis of a highly flexible
conformational immunogenic domain a in hepatitis C virus E2. J
Virol. 79:13199–13208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobson IM, Gordon SC, Kowdley KV,
Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz
E, Everson G, Bennett M, et al POSITRON Study: FUSION Study:
Sofosbuvir for hepatitis C genotype 2 or 3 in patients without
treatment options. N Engl J Med. 368:1867–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeuzem S, Dusheiko GM, Salupere R, Mangia
A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM,
Symonds WT, et al VALENCE Investigators: Sofosbuvir and ribavirin
in HCV genotypes 2 and 3. N Engl J Med. 370:1993–2001. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carrère-Kremer S, Montpellier C, Lorenzo
L, Brulin B, Cocquerel L, Belouzard S, Penin F and Dubuisson J:
Regulation of hepatitis C virus polyprotein processing by signal
peptidase involves structural determinants at the p7 sequence
junctions. J Biol Chem. 279:41384–41392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindenbach BD, Evans MJ, Syder AJ, Wölk B,
Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR,
McKeating JA and Rice CM: Complete replication of hepatitis C virus
in cell culture. Science. 309:623–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan AG, Miller MT and Marcotrigiano J:
HCV glycoprotein structures: what to expect from the unexpected.
Curr Opin Virol. 12:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dorner M, Horwitz JA, Robbins JB, Barry
WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR,
et al: A genetically humanized mouse model for hepatitis C virus
infection. Nature. 474:208–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meredith LW, Wilson GK, Fletcher NF and
McKeating JA: Hepatitis C virus entry: beyond receptors. Rev Med
Virol. 22:182–193. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pileri P, Uematsu Y, Campagnoli S, Galli
G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G
and Abrignani S: Binding of hepatitis C virus to CD81. Science.
282:938–941. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartosch B, Dubuisson J and Cosset FL:
Infectious hepatitis C virus pseudo-particles containing functional
E1–E2 envelope protein complexes. J Exp Med. 197:633–642. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Molina S, Castet V, Pichard-Garcia L,
Wychowski C, Meurs E, Pascussi JM, Sureau C, Fabre JM, Sacunha A,
Larrey D, et al: Serum-derived hepatitis C virus infection of
primary human hepatocytes is tetraspanin CD81 dependent. J Virol.
82:569–574. 2008. View Article : Google Scholar :
|
|
13
|
Meuleman P, Hesselgesser J, Paulson M,
Vanwolleghem T, Desombere I, Reiser H and Leroux-Roels G: Anti-CD81
antibodies can prevent a hepatitis C virus infection in vivo.
Hepatology. 48:1761–1768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavie M, Sarrazin S, Montserret R,
Descamps V, Baumert TF, Duverlie G, Séron K, Penin F and Dubuisson
J: Identification of conserved residues in hepatitis C virus
envelope glycoprotein E2 that modulate virus dependence on CD81 and
SRB1 entry factors. J Virol. 88:10584–10597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keck ZY, Angus AG, Wang W, Lau P, Wang Y,
Gatherer D, Patel AH and Foung SK: Non-random escape pathways from
a broadly neutralizing human monoclonal antibody map to a highly
conserved region on the hepatitis C virus E2 glycoprotein
encompassing amino acids 412–423. PLoS Pathog. 10:e10042972014.
View Article : Google Scholar
|
|
16
|
Qin ZL, Ju HP, Gao TT, Wang WB, Ren H,
Zhao P and Qi ZT: Two conserved histidines (His490 and His621) on
the E2 glycoprotein of hepatitis C virus are critical for
CD81-mediated cell entry. J Gen Virol. 96:1389–1399. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Albecka A, Montserret R, Krey T, Tarr AW,
Diesis E, Ball JK, Descamps V, Duverlie G, Rey F, Penin F and
Dubuisson J: Identification of new functional regions in hepatitis
C virus envelope glycoprotein E2. J Virol. 85:1777–1792. 2011.
View Article : Google Scholar :
|
|
18
|
Krepstakies M, Lucifora J, Nagel CH,
Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T,
Baumert TF, Brand-enburg K, et al: A new class of synthetic peptide
inhibitors blocks attachment and entry of human pathogenic viruses.
J Infect Dis. 205:1654–1664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu S, McCormick KD, Zhao W, Zhao T, Fan D
and Wang T: Human apolipoprotein E peptides inhibit hepatitis C
virus entry by blocking virus binding. Hepatology. 56:484–491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bukong TN, Kodys K and Szabo G: A novel
human radixin peptide inhibits hepatitis C virus infection at the
level of cell entry. Int J Pept Res Ther. 20:269–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lü X, Yao M, Zhang JM, Yang J, Lei YF,
Huang XJ, Jia ZS, Ma L, Lan HY, Xu ZK and Yin W: Identification of
peptides that bind hepatitis C virus envelope protein E2 and
inhibit viral cellular entry from a phage-display peptide library.
Int J Mol Med. 33:1312–1318. 2014.PubMed/NCBI
|
|
22
|
Wakita T, Pietschmann T, Kato T, Date T,
Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami
M, et al: Production of infectious hepatitis C virus in tissue
culture from a cloned viral genome. Nat Med. 11:791–796. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong J, Gastaminza P, Cheng G, Kapadia S,
Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T and Chisari
FV: Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci
USA. 102:9294–9299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blanco-Canosa JB and Dawson PE: An
efficient Fmoc-SPPS approach for the generation of thioester
peptide precursors for use in native chemical ligation. Angew Chem
Int Ed Engl. 47:6851–6855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alsaleh K, Delavalle PY, Pillez A,
Duverlie G, Descamps V, Rouillé Y, Dubuisson J and Wychowski C:
Identification of basic amino acids at the N-terminal end of the
core protein that are crucial for hepatitis C virus infectivity. J
Virol. 84:12515–12528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krieger SE, Zeisel MB, Davis C, Thumann C,
Harris HJ, Schnober EK, Mee C, Soulier E, Royer C, Lambotin M, et
al: Inhibition of hepatitis C virus infection by anti-claudin-1
antibodies is mediated by neutralization of E2-CD81-claudin-1
associations. Hepatology. 51:1144–1157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Owsianka AM, Timms JM, Tarr AW, Brown RJ,
Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH
and Ball JK: Identification of conserved residues in the E2
envelope glycoprotein of the hepatitis C virus that are critical
for CD81 binding. J Virol. 80:8695–8704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drummer HE, Boo I, Maerz AL and
Poumbourios P: A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif
in hepatitis C virus glycoprotein E2 is a determinant of CD81
binding and viral entry. J Virol. 80:7844–7853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Randall G, Higginbottom A, Monk
P, Rice CM and McKeating JA: CD81 is required for hepatitis C virus
glycoprotein-mediated viral infection. J Virol. 78:1448–1455. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji C, Liu Y, Pamulapati C, Bohini S,
Fertig G, Schraeml M, Rubas W, Brandt M, Ries S, Ma H and Klumpp K:
Prevention of hepatitis C virus infection and spread in human liver
chimeric mice by an anti-CD81 monoclonal antibody. Hepatology.
61:1136–1144. 2015. View Article : Google Scholar
|
|
31
|
Mekky RY, El-Ekiaby NM, Hamza MT, Elemam
NM, El-Sayed M, Esmat G and Abdelaziz AI: Mir-194 is a hepatocyte
gate keeper hindering HCV entry through targeting CD81 receptor. J
Infect. 70:78–87. 2015. View Article : Google Scholar
|
|
32
|
Zhang YY, Zhang BH, Ishii K and Liang TJ:
Novel function of CD81 in controlling hepatitis C virus
replication. J Virol. 84:3396–3407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cartier F, Marcq I, Douam F, Ossart C,
Regnier A, Debuysscher V, Lavillette D and Bouhlal H: The
expression of the hepatocyte SLAMF3 (CD229) receptor enhances the
hepatitis C virus infection. PLoS One. 9:e996012014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al Olaby RR, Cocquerel L, Zemla A, Saas L,
Dubuisson J, Vielmetter J, Marcotrigiano J, Khan AG, Vences Catalan
F, Perryman AL, et al: Identification of a novel drug lead that
inhibits HCV infection and cell-to-cell transmission by targeting
the HCV E2 glycoprotein. PLoS One. 9:e1113332014. View Article : Google Scholar : PubMed/NCBI
|