Introduction
Osteoporosis is a serious health problem that is
related to aging; it is characterized by decreased bone density and
increased risk of fracture (1).
Bone remodeling is a continuous process between bone resorption
(activity of osteoclasts) and formation (activity of osteoblasts).
Disintegration due to these opposing processes may cause bone
diseases (2–4). Osteoclasts are activated for many
reasons, one of which is the imbalance of hormones caused by the
menopause (5). The absence of
estrogen, induced by the menopause, increases the formation and the
activity of osteoclasts, which play key roles in bone loss, and
osteoclasts ultimately increase the risk of menopausal osteoporosis
(6). Therefore, inhibiting
osteoclast formation and function is an important therapeutic
strategy.
Osteoclasts are multinucleated cells generated from
mono-cyte/macrophage precursor cells, and osteoclast formation
requires receptor activator of nuclear factor-κB (NF-κB) ligand
(RANKL). RAW 264.7 cells have been demonstrated to play an
important role, using in vitro studies, on osteoclast
formation and function (7).
Adding RANKL to RAW 264.7 cells induces osteoclast differentiation
(4). The receptor activator of
NF-κB (RANK) is expressed on RAW 264.7 cell surfaces and conjugates
with RANKL, which is essential for osteoclastogenesis (8). RANKL to RANK interaction activates
tumor necrosis factor receptor-associated factor 6 (TRAF6), and
TRAF6 then activates c-Fos, as an important transcription factor
for osteoclastogenesis, and downregulates osteoclastogenesis via
nuclear factor of activated T-cells cytoplasmic 1 (NFATc1)
activation (9). As a master
transcription factor of osteoclastogenesis, NFATc1 regulates
diverse osteoclastogenesis-related genes such as tartrate-resistant
acid phosphatase (TRAP), cathepsin K (CTK), calcitonin receptor
(CTR) and matrix metallopeptidase-9 (MMP-9) (4,10–12).
Various therapies are available for post-menopausal
osteoporosis, such as estrogen replacement therapy, bisphosphonate
and calcitonin, and it has previously been noted that estrogen
replacement therapy is commonly used in cases of post-menopausal
osteoporosis (3,13). However, long-term estrogen
replacement therapy has been demonstrated to cause various
side-effects such as breast cancer and endometrial cancer (3,14).
An alternative therapy which is thus worthy of consideration is
natural herbs, which exert positive effects on osteoporosis and
have fewer harmful side-effects.
Lycii Radicis Cortex (LRC) is the dried root bark of
Lycium chinense Mill. and is termed 'Jigolpi' in Korea.
Traditionally, LRC has been used to treat lung fever and reduce
fever of the blood, lower blood pressure, decrease blood sugar,
and, particularly, decrease steaming bone disorder (15,16). LRC has been reported to perform
various biological roles, and acts as an anti-inflammatory
(17), anti-oxidant (18), anti-depressant (19), tumor growth inhibitor (20) and blood glucose regulator
(21). However, the inhibitory
effect of LRC on osteoclastogenesis has not been previously
investigated, to the best of our knowledge.
In the present study, we aimed to investigate the
effect of LRC on osteoclastogenesis, bone resorption activity and
expression levels of osteoclastic markers in RAW 264.7 cells and on
post-menopausal osteoporosis induced by ovariectomy in
ovariectomized (OVX) rats.
Materials and methods
Reagents
RANKL was purchased from PeproTech (London, UK).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
Welgene (Daejeon, Korea). Cell medium minimum essential medium-α
(α-MEM) and fetal bovine serum (FBS) were both purchased from Gibco
(Gaithersburg, NY, USA). Penicillin/streptomycin was purchased from
Invitrogen (Carlsbad, CA, USA). Dulbecco's phosphate-buffered
saline (DPBS) was obtained from Gibco. An aqueous non-radioactive
cell proliferation kit (for MTS assay) was purchased from Promega
(Madison, WI, USA). A TRAP staining kit was purchased from
Sigma-Aldrich (St. Louis, MI, USA). An Osteo Assay Stripwell plate
was purchased from Corning Inc. (New York, NY, USA). We obtained
the reverse transcription kit from Invitrogen. Taq polymerase was
obtained from Kapa Biosystems (Woburn, MA, USA). PCR primers were
from Genotech (Daejeon, Korea). Primary antibodies against c-Fos
(Cat. no. sc-447) and β-actin (Cat. no. sc-8432) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and
NFATc1 antibody (Cat. no. 556602) was obtained from BD Pharmingen
(San Diego, CA, USA). Peroxidase IgG secondary antibody (Cat. no.
115-035-062) was purchased from Jackson ImmunoResearch (West Grove,
PA, USA). Protease inhibitor cocktail and phosphatase inhibitor
cocktail were both purchased from Sigma-Aldrich.
Sample preparation
LRC was purchased from Kyung Hee University Medical
Center. The extract was prepared by decocting 300 g of the dried
herb using 3 liters of boiling distilled water for 2 h and filtered
using filter paper (no. 3; Whatman, Maidstone, UK). The extract was
concentrated using a rotary evaporator (Eyela, Tokyo, Japan),
lyophilized, and it yielded 11.1 g dried powder (yield ratio 3.7%),
and the extract was then stored at −20°C until use.
Cell culture and cytotoxicity assay
Murine macrophage RAW 264.7 cells were cultured in
DMEM with 10% FBS and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere of 5% CO2 in air. For the
cytoxicity assay, RAW 264.7 cells were cultured in DMEM with 10%
FBS and 1% penicillin/streptomycin and plated in a 96-well plate at
5×103 cells/well. After 24 h, various concentrations of
LRC were added to the medium for 24 h. MTS solution was added at 20
μl/well. The plate was incubated for 2 h at 37°C. Cell
viability was measured using an enzyme-linked immunosorbent assay
(ELISA reader; Molecular Devices, Sunnyvale, CA, USA) at 562 nm
optical density.
TRAP staining and the TRAP activity
assay
In order to study osteoclast differentiation, RAW
264.7 cells were cultured in α-MEM with 10% FBS and 1%
penicillin/streptomycin in a 96-well plate 5×103
cells/well. After 24 h, the α-MEM was changed for 10% FBS and 1%
penicillin/streptomycin. RANKL (100 ng/ml) and various
concentrations of LRC were added to the media for 5 days. The
medium was changed every 2 days. After 5 days, multinucleated
osteoclasts were observed. Mature osteoclasts were washed with DPBS
and fixed in 10% formaldehyde for 10 min. After being fixed, cells
were stained using a TRAP staining kit (Sigma-Aldrich) according to
the manufacturer's instructions. Cells were washed with deionized
water. We then counted TRAP-positive multinucleated (>3 nuclei)
under an inverted microscope (Olympus, Tokyo, Japan). In order to
measure TRAP activity, we added 50 μl supernatant to a
96-well plate, dissolved 4.93 mg Pnpp in 850 μl 0.5 M
acetate, mixed it with 150 μl tartrate acid solution and
separated it (50 μl each) into 96-well plates. After 30 min,
we added 50 μl 0.5 M NaOH and measured optical density at
405 nm using an ELISA reader.
Pit formation assay
RAW 264.7 cells were cultured in α-MEM with 10% FBS
and 1% penicillin/streptomycin and plated in an Osteo Assay
Stripwell plate at 5×103 cells/well. After 24 h, α-MEM
was changed for 10% FBS and 1% penicillin/streptomycin. RANKL (100
ng/ml) and various concentrations of LRC were added to the medium
for 5 days. The medium was changed every 2 days. After 5 days,
multinucleated osteoclasts were observed. The mature osteoclasts
were then lysed by 4% NaClO and lysates were washed with deionized
water. The pit area in each plate was studied and measured under an
inverted microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In order to generate osteoclasts, RAW 264.7 cells
were cultured with α-MEM, 10% FBS and 1% penicillin/streptomycin.
RANKL (100 ng/ml) was added for 4 days in the presence of various
concentrations of LRC. Total RNA was prepared using TRIzol (Takara
Bio, Otsu, Japan) according to the manufacturer's instructions. The
concentration of total RNA was determined (absorbance at 260 and
280 nm) with a NanoDrop 2.0 spectrophotometer (Thermo Fisher
Scientific, Pittsburgh, PA, USA), and 2 μg total RNA was
generated using a reverse transcription kit (Invitrogen) according
to the manufacturer's instructions. The PCR cycles were as follows:
22–40 cycles of 1 min at 94°C (denaturation), 1 min at 55–58°C
(annealing), and a 1-min 72°C (extension), using Taq polymerase.
Primer sequences are shown in Table
I. The cDNA samples after reaction were separated on a 1–1.2%
agarose gel, stained with SYBR-Green (Invitrogen) and studied using
ImageJ software.
 | Table IPrimer sequences for RT-qPCR. |
Table I
Primer sequences for RT-qPCR.
| Target | Primer sequences
(5′→3′) | Annealing
temperature (°C) | Cycle |
|---|
| NFATc1 | F: 5′-TGC TCC TCC
TCC TGC TGC TC-3′ | 58 | 32 |
| R: 5′-CGT CTT CCA
CCT CCA CGT CG-3′ | | |
| c-Fos | F: 5′-ATG GGC TCT
CCT GTC AAC AC-3′ | 58 | 33 |
| R: 5′-GGC TGC CAA
AAT AAA CTC CA-3′ | | |
| RANK | F: 5′-AAA CCT TGG
ACC AAC TGC AC-3′ | 53 | 32 |
| R: 5′-ACC ATC TTC
TCC TCC CHA GT-3′ | | |
| TRAP | F: 5′-ACT TCC CCA
GCC CTT ACT ACC G-3′ | 58 | 30 |
| R: 5′-TCA GCA CAT
AGC CCA CAC CG-3′ | | |
| CTK | F: 5′-AGG CGG CTA
TAT GAC CAC TG-3′ | 58 | 27 |
| R: 5′-CCG AGC CAA
GAG AGC ATA TC-3′ | | |
| CTR | F: 5′-TGC ATT CCC
GGG ATA CAC AG-3′ | 59 | 40 |
| R: 5′-AGG AAC GCA
GAC TTC ACT GG-3′ | | |
| MMP-9 | F: 5′-CGA CTT TTG
TGG TCT TCC CC-3′ | 58 | 33 |
| R: 5′-TGA AGG TTT
GGA ATC GAC CC-3′ | | |
| CAII | F: 5′-CTC TCA GGA
CAA TGC AGT GCT GA-3′ | 58 | 32 |
| R: 5′-ATC CAG GTC
ACA CAT TCC AGC A-3′ | | |
| GAPDH | F: 5′-ACT TTG TCA
AGC TCA TTT CC-3′ | 58 | 30 |
| R: 5′-TGC AGC GAA
CTT TAT TGA TG-3′ | | |
Western blot analysis
The cells incubated with various concentrations of
LRC (1, 10 and 100 μg/ml) were washed with cold DPBS, and
cells were lysed in lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 1%
NP-40, 0.5% Na-deoxycholate, 0.1% SDS, protease inhibitor cocktail,
phosphatase inhibitor cocktail) and incubated on ice for 30 min.
After centrifugation at 13,200 rpm for 20 min at 4°C, the
supernatants were stored at −70°C until use. Protein concentration
was calculated using a BCA protein assay kit (Thermo Fisher
Scientific). The protein samples (30 μg) were separated by
10–15% SDS-PAGE and transferred to a nitrocellulose membrane
(Whatman, Dassel, Germany). We then blocked them with 5% skimmed
milk for 1 h, and the membrane was then incubated with primary
antibodies, namely NFATc1, c-Fos and actin, in 1% BSA solution at
4°C overnight. Subsequently, the membrane was probed with the
secondary antibody. The protein was detected using ECL solution
(Santa Cruz Biotechnology, Inc.).
Animal model of OVX rats and
histopathological examination
The animal experiments were conducted in compliance
with the principles of the Institutional Animal Care and Use
Committee of Kyung Hee University Laboratory Animal Center: the
permission no. is KHUASP (SE)-13-051. Sprague-Dawley (SD) rats were
purchased from Nara Biotech (Seoul, Korea). The 17β-estradiol
(E2) was from Sigma-Aldrich. Twelve-week-old female
Sprague-Dawley rats weighing 240–250 g were used. Rats were housed
at 22±1°C in an atmosphere with 55±10% humidity on a 12 h
light/dark cycle with free access to food and water. After
acclimatization to the laboratory environment for 1 week, rats were
divided into 5 groups (8 rats/group), i) sham-operated rats, ii)
OVX rats, iii) OVX rats which received 17β-estradiol (100
μg/kg p.o.), iv) OVX rats treated with low doses of LRC (5
mg/kg p.o.), and v) OVX rats treated with high doses of LRC (50
mg/kg p.o.).
After the operation, sham-operated and OVX rats were
administrated distilled water orally. After the ovariectomy, the
rats were treated 5 times/week for 8 weeks and weighed each week.
At the end of the treatment, the rats were euthanized while under
deep anesthesia with high doses of pentobarbital sodium (80 mg/kg)
and blood samples were acquired by cardiac puncture for biochemical
analyses. The uteri and both sides of the femurs were dissected out
and immediately weighed on an electronic scale. The femurs were
then fixed in 10% neutral buffered formalin (NBF) for 2 days and
decalcified using 10% ethylene-diaminetetraacetate (EDTA) for 3
weeks. The samples, which were cut into 5-μm thick sections
using a microtome (Zeiss, Oberkochen, Germany), were embedded in
paraffin. Tissues were deparaffinized, rehydrated, and stained with
hematoxylin and eosin (H&E). Histopathological changes were
observed using light microscopy (at ×40 and ×100
magnification).
Measurement of bone density after
inducing OVX
At the end of treatment, the rats were sacrificed
and the bone density of the right femurs was measured using
Archimedes' principle, as previously described (22). Right femurs were briefly placed in
vials filled with deionized water. The vials were placed in a
vacuum for 90 min to ensure that all trapped air diffused from the
bones. Right femurs were removed from the vials, dried with gauze,
weighed, and placed in new vials containing deionized water. The
bones were reweighed in the water. As previously described, using
Archimedes' principle, bone density was calculated
(g/cm3 bone volume) (23).
Statistical analysis
In this study, the data are presented as the means ±
SEM. Statistical analysis was performed using the GraphPad Prism
software (GraphPad Software, Inc., San Diego, CA, USA). One-way
ANOVA was used to evaluate the treatment effect, followed by
Dunnett's multiple comparison test. A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
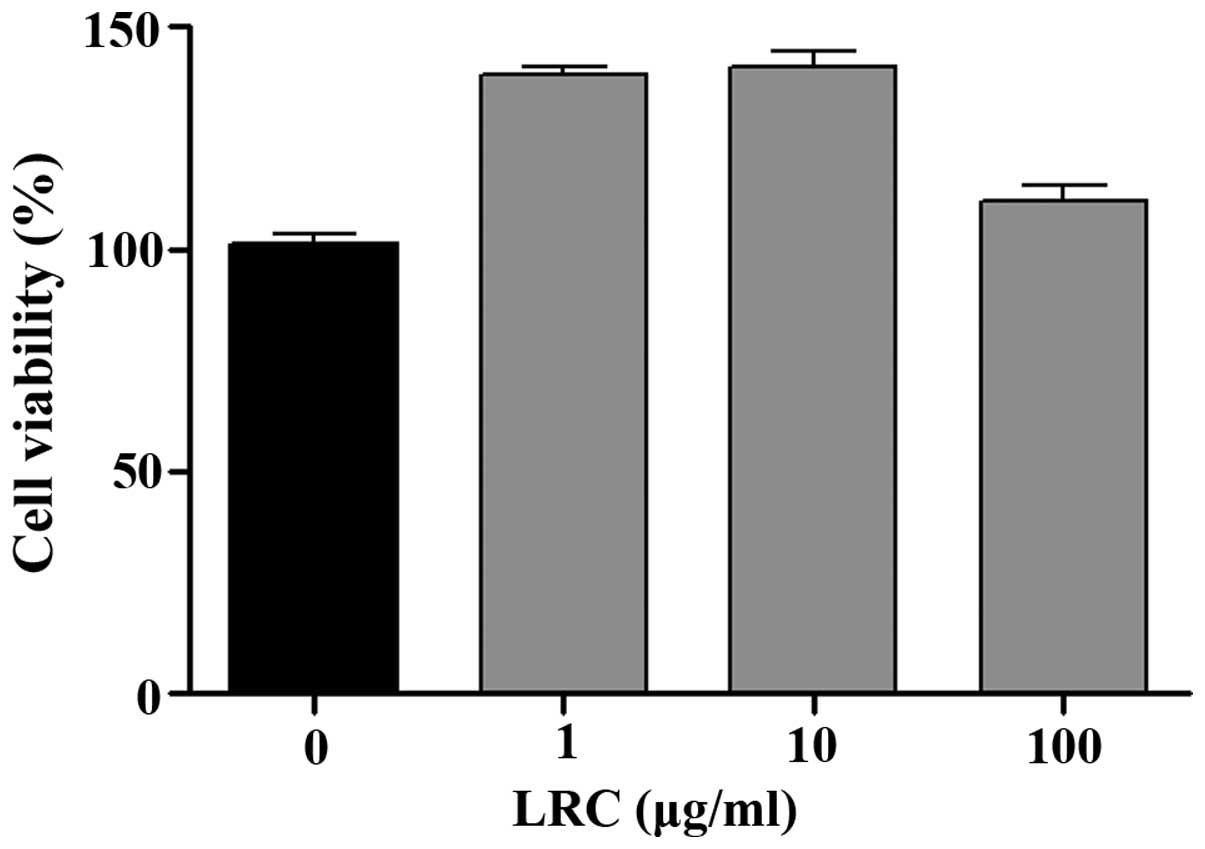
LRC is not cytotoxic to RAW 264.7
cells
Prior to the in vitro tests, we measured the
effects of LRC on cell viability. As shown in Fig. 1, all concentrations of LRC exerted
equivalent effects on cell viability. LRC was not cytotoxic to RAW
264.7 cells. Thus, the following experiments were undertaken using
a range of LRC concentrations (1, 10 and 100 μg/ml).
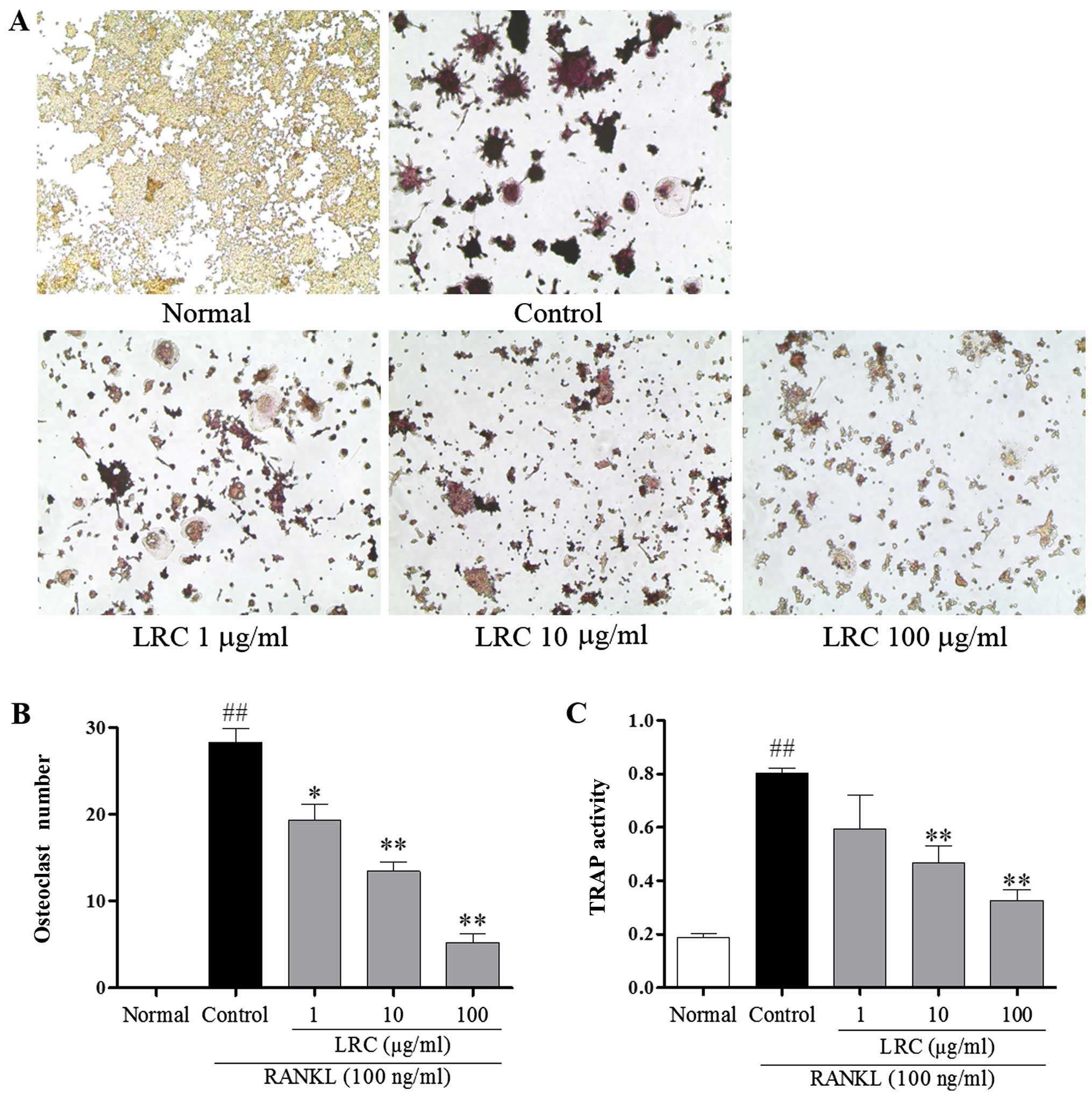
LRC inhibits osteoclastogenesis in RAW
264.7 cells induced by RANKL
To examine the effect of LRC on osteoclastogenesis,
RAW 264.7 cells exposed to RANKL were stained using a TRAP staining
kit. RAW 264.7 cells were stimulated with RANKL to differentiate
into TRAP-positive cells, and we noted that 100 μg/ml LRC
significantly decreased the number of TRAP-positive cells (Fig. 2A and B). Furthermore, as shown in
Fig. 2C, it was also clear that
LRC exerted an inhibitory effect on TRAP activity.
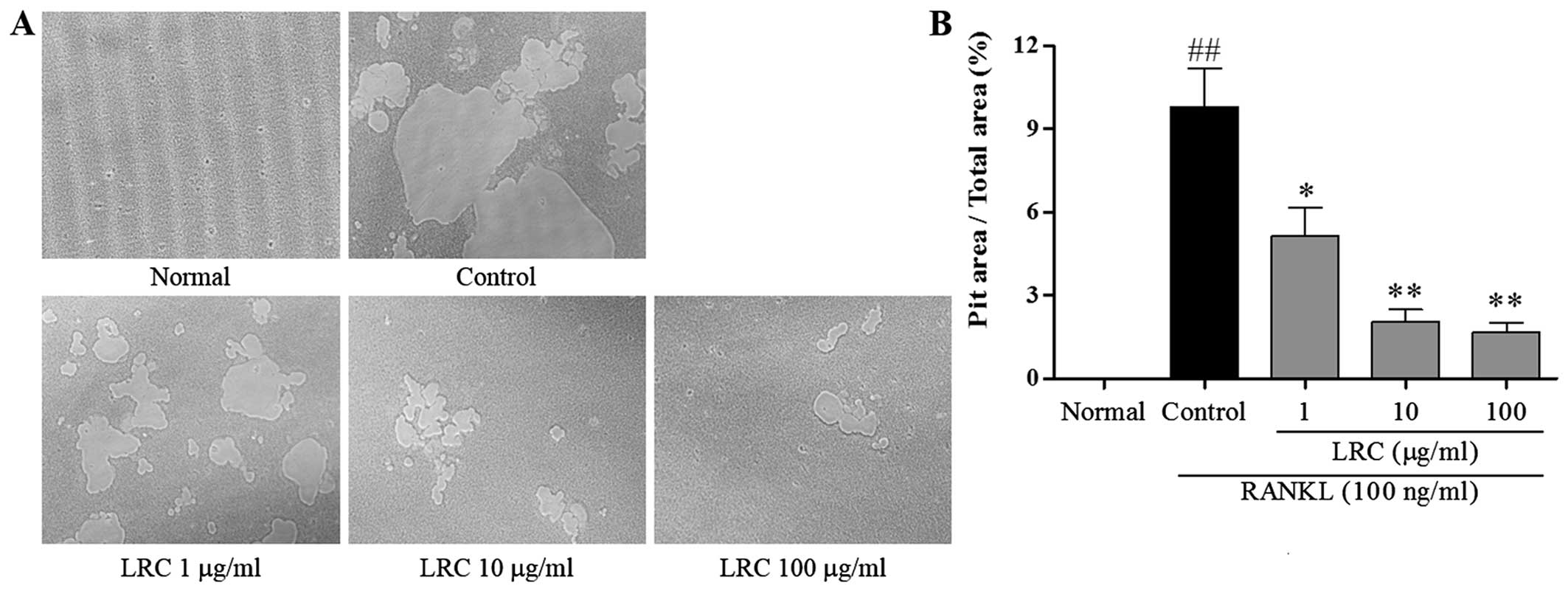
LRC inhibits pit formation
The effect of LRC on pit formation of mature
osteoclasts was studied. As shown in Fig. 3A, the area of resorption pit
lacunae was significantly reduced by treatment with LRC. It was
clear that the measured areas markedly decreased after treatment
with LRC, in a dose-dependant manner (Fig. 3B).
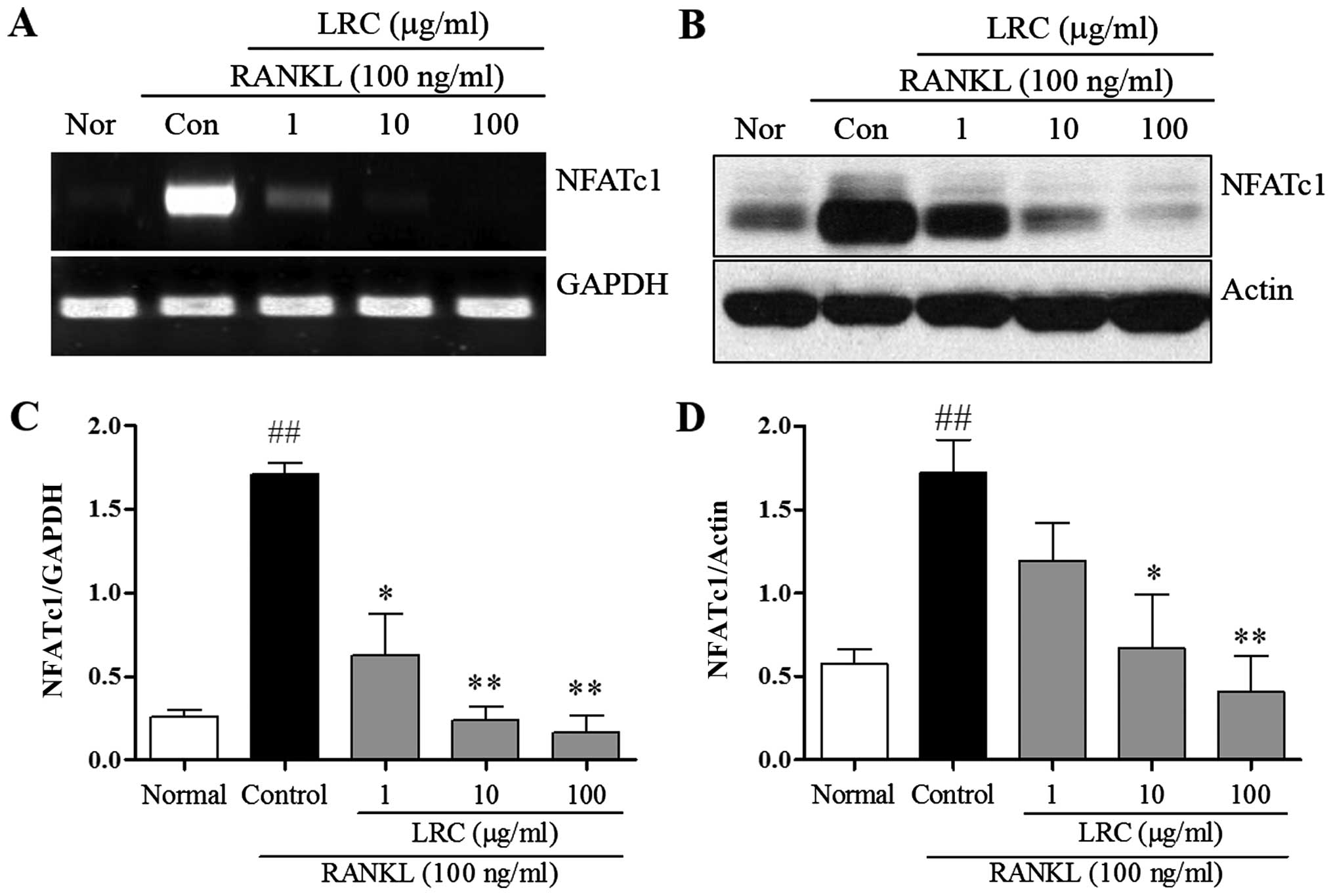
LRC inhibits NFATc1 expression
In order to confirm the inhibitory effect of LRC on
osteoclastogenesis and bone resorption, we measured the expression
of important osteoclast differentiation indicators, NFATc1. NFATc1
is known to be a master transcription factor in osteoclastogenesis
(4). Thus, in the present study,
mRNA and protein levels of NFATc1 were measured. As shown in
Fig. 4, we noted that the levels
were significantly upregulated upon exposure to RANKL, and LRC
exerted a marked inhibitory effect on mRNA and protein expression
levels. In addition, we noted that LRC did not markedly affect the
expression of housekeeping genes such as GAPDH and actin.
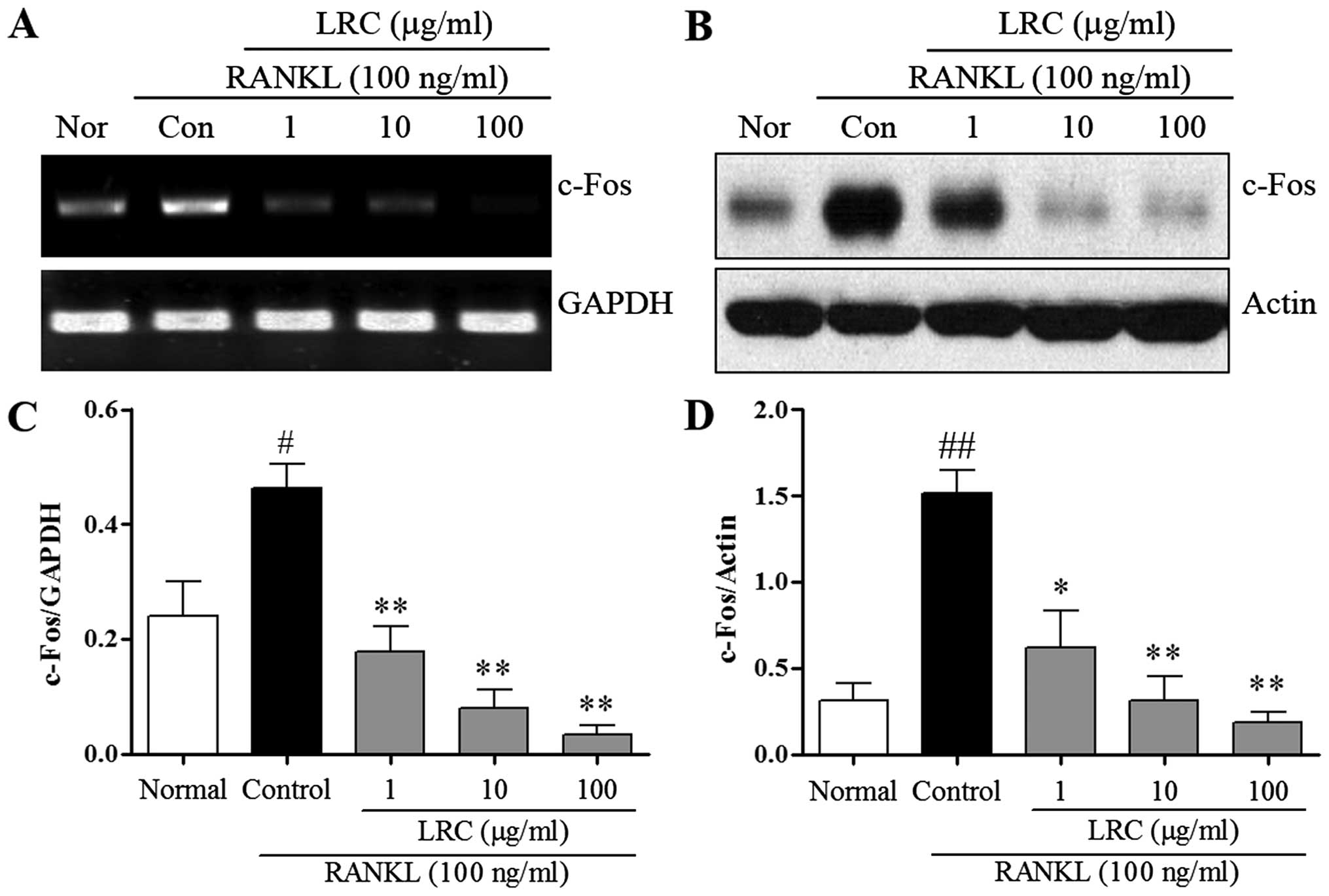
LRC inhibits c-Fos expression
We measured the expression of c-Fos, which
contributes to osteoclastogenesis via the downregulation of NFATc1.
We measured the effect of LRC on c-Fos mRNA and protein levels. As
shown in Fig. 5A, the mRNA
expression of c-Fos was induced slightly in normal cells, and in
RAW 264.7 cells exposed to RANKL we noted significantly increased
mRNA expression of c-Fos compared with normal cells. LRC exerted a
marked inhibitory effect on mRNA expression. As shown in Fig. 5B, the protein levels of c-Fos were
also significantly upregulated upon exposure to RANKL, whereas LRC
clearly exerted a significant inhibitory effect on the protein
levels. Moreover, we noted that LRC did not markedly affect the
expression of the housekeeping genes GAPDH and actin.
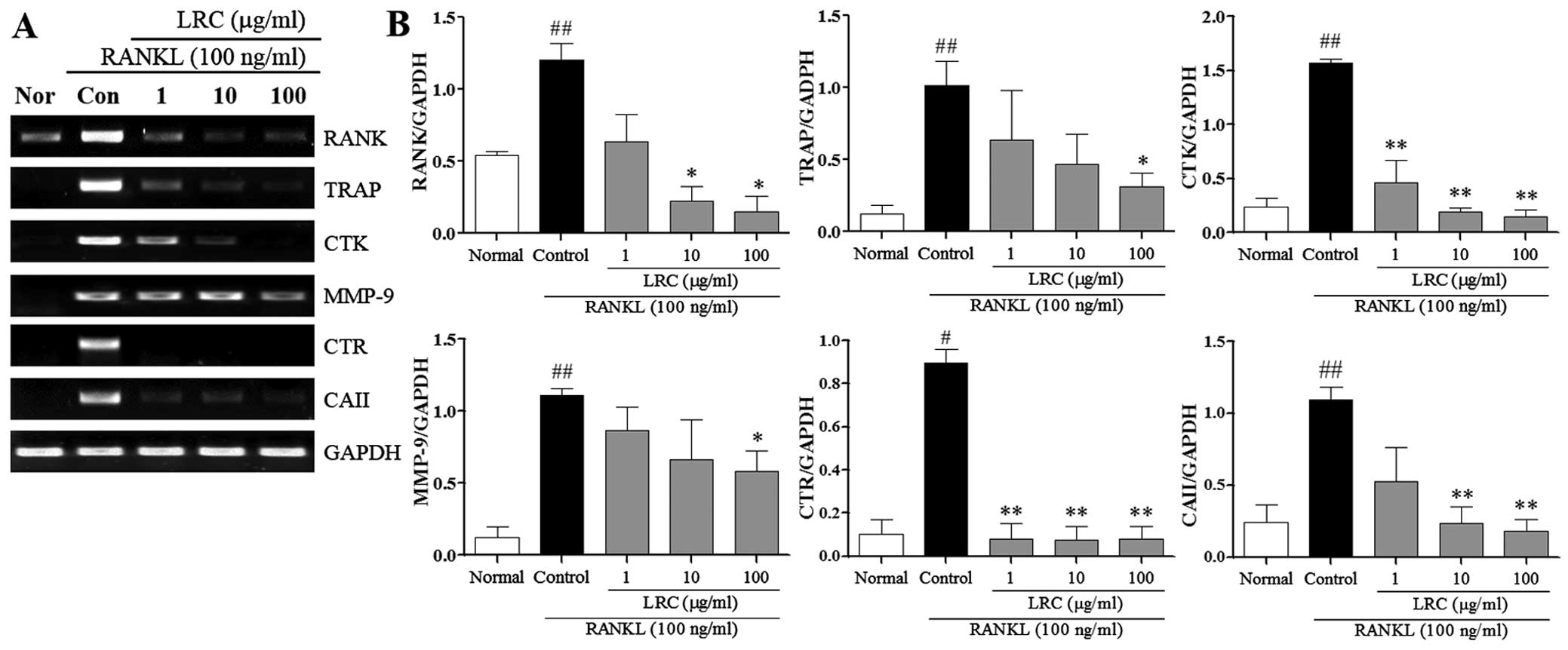
LRC inhibits the expression of
osteoclastogenesis-related genes
In the present study, we also examined the effect of
LRC on osteoclastogenesis-related genes stimulated by RANKL, namely
RANK, TRAP, CTK, MMP-9, CTR and carbonic anhydrase II (CAII). As
shown in Fig. 6, the mRNA
expression of RANK was induced, at a low level, in normal cells,
and in RAW 264.7 cells exposed to RANKL we noted significantly
increased mRNA expression of RANK compared with normal cells. LRC
exerted significant inhibitory effects on RANK expression at
concentrations of 10 and 100 μg/ml. Moreover, the mRNA
expression of TRAP, CTK, MMP-9, CTR and CAII were significantly
upregulated upon exposure to RANKL. LRC exerted a marked inhibitory
effect on TRAP and MMP-9 expression at concentrations of 100
μg/ml. In addition, we noted that LRC markedly reduced the
expression of CTK and CTR at all concentrations and significantly
inhibited the expression of CAII at concentrations of 10 and 100
μg/ml.
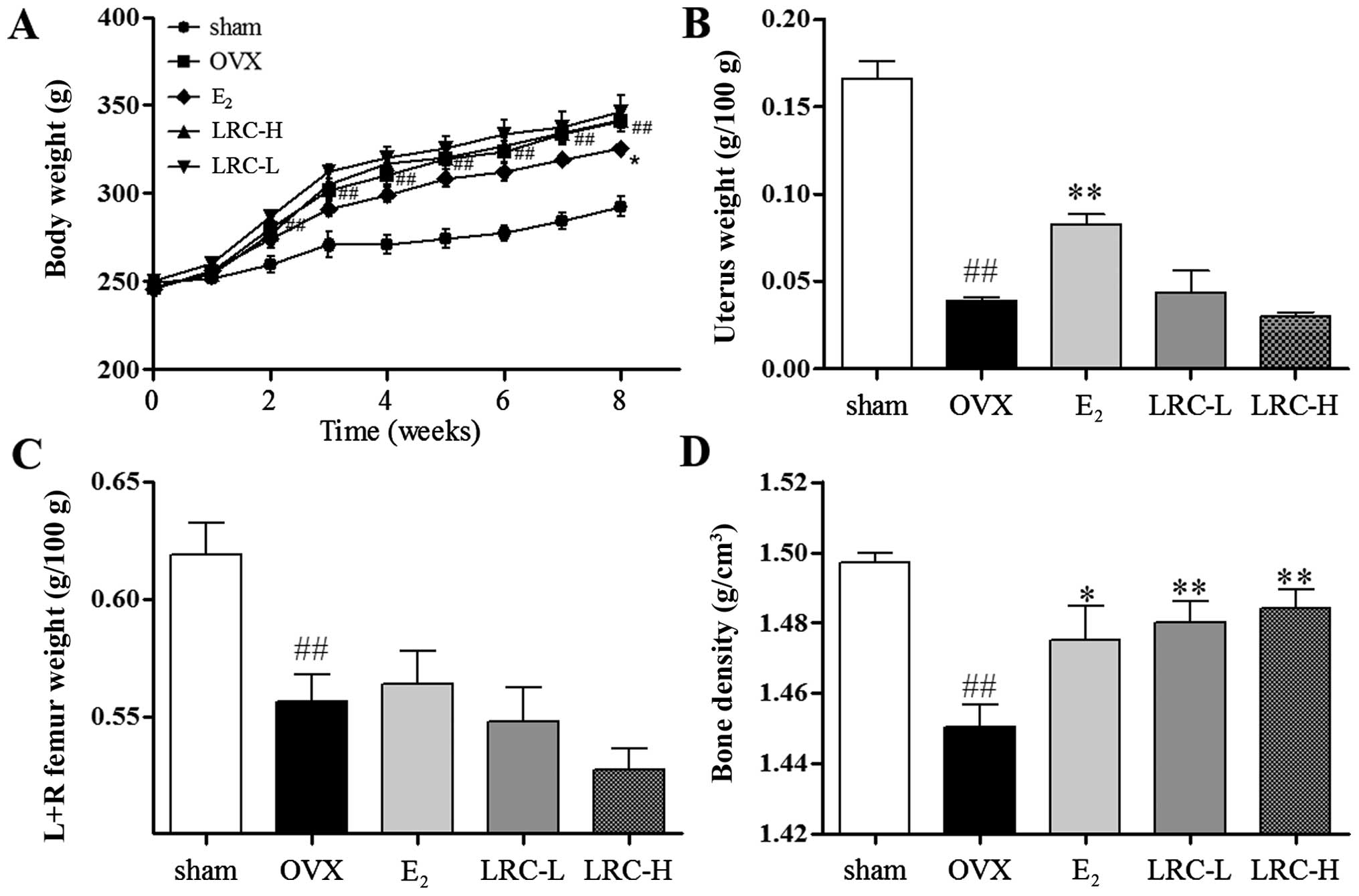
LRC increases bone density in the OVX rat
model
We aimed to investigate whether LRC prevents
ovariectomy-induced bone loss. As shown in Fig. 7A, after the rats underwent the
ovariectomy, the body weight of the OVX group significantly
increased (as measured weekly) compared with the sham-operated
group. In the LRC-treated groups, no significant changes in body
weight were noted, and in the E2-treated group body
weight was significantly inhibited compared with the OVX group. In
the OVX group, a significant decrease in the weight of the uterus
compared with the sham-operated group was noted (Fig. 7B). In the groups treated with low
and high concentrations of LRC, no significant changes in uterus
weight compared with the OVX group were noted. In the
E2-treated group, a significant decrease in uterus
weight loss compared with the OVX group was clear. We noted in the
OVX group significantly decreased femur weight compared with the
sham-operated group (Fig. 7C). In
the groups treated with low and high concentrations of LRC and the
E2-treated group, no significant changes in femur weight
were noted compared with the OVX group. We also measured the bone
density of the femurs using Archimedes' principle (Fig. 7D). The OVX group showed
significantly decreased bone density compared with the
sham-operated group. Moreover, in the LRC-treated groups, we noted
a significant decrease in bone density loss in femurs compared with
the OVX group. In the E2-treated group, we also noted
decreased bone density loss compared with the OVX group. Thus, our
results indicate that LRC decreased bone density loss without
markedly affecting body weight, femur weight or uterine weight.
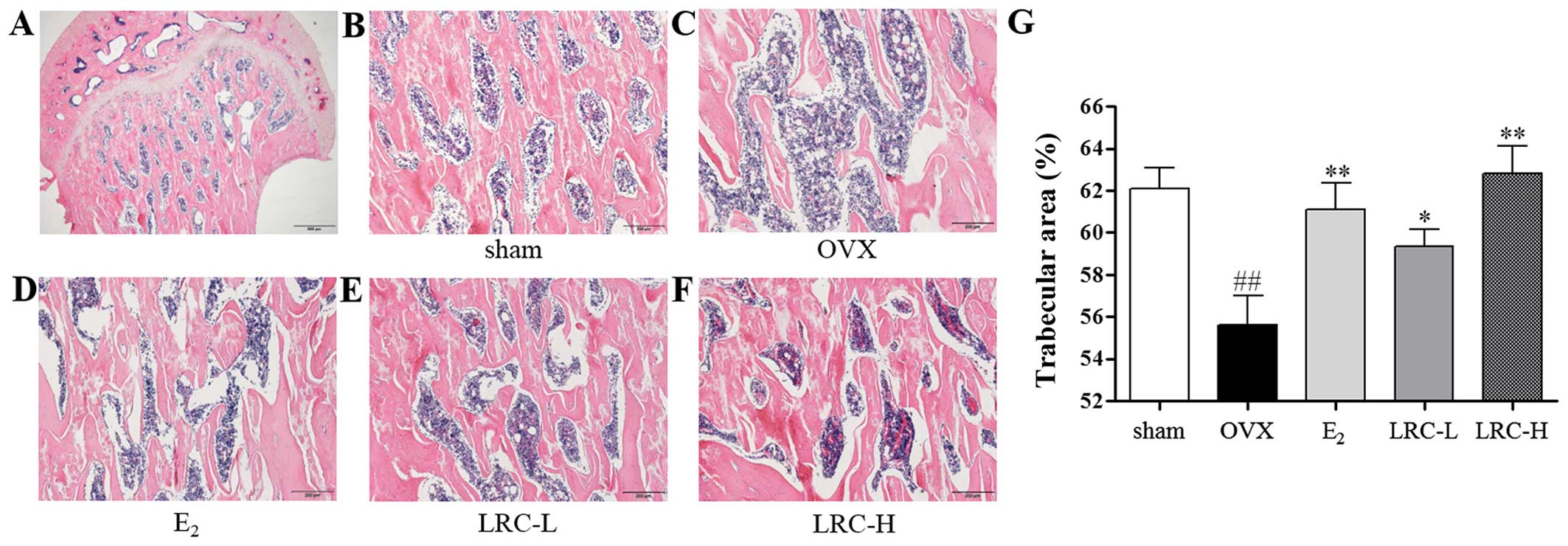
LRC exerts an inhibitory effect on
trabecular area loss in OVX rats
To determine the in vivo effect of LRC on
OVX-induced bone loss, we used histological staining on the femur
samples. In the OVX group, we noted a significantly decreased
trabecular area compared with the sham-operated group (Fig. 8B–F). In the E2-treated
group the trabecular area loss was decreased compared with the OVX
group. In the group treated with low concentrations of LRC, only a
small decrease in trabecular area loss was noted compared with the
OVX group. However, in the group treated with a high concentration
of LRC a significant decrease in trabecular area loss compared with
the OVX group was noted.
Discussion
In the present study, we demonstrated that LRC
exerted an inhibitory effect on osteoclastogenesis through the
reduction of key transcription factors such as NFATc1 and c-Fos. We
noted that LRC also suppressed expression of
osteoclastogenesis-related markers. Moreover, LRC inhibited bone
loss in the OVX rat model. Abnormal bone resorption of osteoclasts
is an important causal factor in osteoporosis, and as such,
suppressing osteoclastogenesis is a significant step in
osteoporosis treatment (6).
TRAPs are expressed particularly in osteoclasts and
are commonly used as phenotype markers of osteoclasts; treatment of
RAW 264.7 cells with RANKL has been shown to easily induce cell
differentiation into osteoclasts, which are TRAP-positive cells
(24). The study of TRAP-positive
cell formation and activity is a well-known method of determining
osteoclast formation and function (25,26). In the present study, we noted that
LRC inhibited osteoclast formation and TRAP activity. These results
indicate that LRC exerts an inhibitory effect on
osteoclastogenesis.
Mature osteoclasts result in resorption lacunae and
pit formation upon stimulation with RANKL (27,28). Functionally, it is important that
pit formation be used when identifying the osteoclast phenotype
(24). In the present study, in
order to investigate the effects of LRC on osteoclastic bone
resorption, RAW 264.7 cells were cultured with RANKL. The density
of pits was significantly reduced by LRC treatment. This suggests
that LRC also exerted an inhibitory effect on mature osteoclast
function.
In order to analyze molecular mechanisms, we
measured osteoclastogenesis-related markers. In previous research
it has been demonstrated that NFATc1 is an important transcription
factor for RANKL-mediated osteoclast differentiation, fusion, and
activation (9,10). It has also been noted that
overexpression of NFATc1 induces differentiation into osteoclasts
even in cases of RANKL deficiency (29). Moreover, in NFATc1 knock-out mice,
defective osteoclast differentiation and osteopetrosis have been
noted (30). In the present
study, we noted that LRC exerted an inhibitory effect on NFATc1
mRNA and protein levels. NFATc1 plays an important role in
osteoclast activation through the release of
osteoclastogenesis-related genes such as TRAP, CTK, MMP-9 and CTR;
the expression of TRAP, CTK and MMP-9 genes, which are the main
markers responsible for the degradation of bone mineral and
collagen matrices, are regulated by NFATc1 (4,10–12). Calcitonin suppresses both
osteoclast formation and bone resorption and is a primary treatment
for patients with hypercalcemia and increased bone turnover. The
binding of calcitonin to its receptor has long been known to reduce
osteoclast activation (4,31). In the present study, we
demonstrated that LRC inhibited expression of the TRAP, CTK, MMP-9
and CTR genes. These data indicate that LRC inhibits
osteoclastogenesis-related genes through suppression of NFATc1,
which is an important transcription factor in
osteoclastogenesis.
The interaction between RANK and RANKL is essential
for osteoclast differentiation and activation. Binding of RANKL to
RANK results in the recruitment of c-Fos, and subsequent
stimulation of c-Fos results in the activation of NFATc1 (9,10).
The deletion of the gene-encoding c-Fos in mice leads to defective
osteoclast differentiation and osteopetrosis (32). In the present study, LRC exerted
inhibitory effects on c-Fos mRNA and protein levels. The data
indicated that LRC inhibited osteoclastogenesis through suppression
of NFATc1 following inhibition of c-Fos expression in LRC-treated
cells. c-Fos has also previously been shown to regulate
osteoclastogenesis-related genes such as CAII. CAII influences bone
resorption and osteoclast formation (12,33). CAII affects the surface of the
bone in an acid environment (2,34).
The acidic environment stimulates bone mineralization, and the
demineralized organic ingredient of bone is resorbed by TRAP, CTK
and MMP-9 (2,4,35).
In the present study, LRC inhibited the expression of CAII mRNA
expression. These data indicate that LRC inhibits CAII through
suppression of c-Fos. We also studied the expression of RANK, which
regulates the expression of c-Fos. RANKL-RANK conjugation is
important in the early stages of RAW 264.7 cell differentiation
(4,12). We showed that LRC inhibited RANK
mRNA expression. The data demonstrated that LRC suppresses the
expression of c-Fos by decreasing the signaling cascades connected
with RANKL.
OVX is a widely used experimental method for
inducing post-menopausal osteoporosis in females (22). Estrogen deficiency is accompanied
by atrophy of organs such as the uterus (36), and atrophy of the uterus is
evidence of the success of the ovariectomy. In the present study,
OVX rats exhibited significantly reduced uterus weight, which was
also observed in other studies, and in the 17β-estradiol treated
group the uterus weight loss was reduced (37–39). However, in the LRC-treated groups
no significant changes were noted in uterus weight. In addition,
OVX dramatically increased body weight. The mechanisms by which OVX
induces an increase in body weight remain unclear; it seems that
body fat accumulation is due to estrogen deficiency (40). However, in the present study we
noted that LRC did not have a marked effect on body weight. The
results suggest that the effect of LRC on OVX-induced rats does not
conform to hormonal-related factors, but other factors.
Post-menopausal osteoporosis has been noted as being
correlated with ovarian hormone deficiency following menopause, and
can be induced by decreasing bone density, which increases bone
resorption and deficient bone formation (41,42). Reduced bone density is the main
cause of fractures (43). Bones
in OVX rats are also characterized by reduced bone density and
reduced trabecular area (41). In
the present study, LRC treatment exerted greater effects than
17β-estradiol. The data from our experiments demonstrate that LRC
is a beneficial therapeutic agent which prevents bone loss due to
post-menopausal osteoporosis.
In conclusion, the results of the present study
suggest that LRC exerts an inhibitory effect on menopausal
osteoporosis. LRC reduced the expression of
osteoclastogenesis-related markers NFATc1, c-Fos, RANK, TRAP, CTK,
CTR, MMP-9 and CAII and reduced bone density loss and trabecular
area loss in the OVX rat model. In terms of bone density loss and
trabecular area loss, levels in rats treated with high doses of LRC
in particular were notably higher than in the E2-treated
groups. However, it is possible that LRC has side-effects and this
aspect requires additional study.
References
|
1
|
Burge R, Dawson-Hughes B, Solomon DH, Wong
JB, King A and Tosteson A: Incidence and economic burden of
osteoporosis-related fractures in the United States, 2005–2025. J
Bone Miner Res. 22:465–475. 2007. View Article : Google Scholar
|
|
2
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wensel TM, Iranikhah MM and Wilborn TW:
Effects of denosumab on bone mineral density and bone turnover in
postmenopausal women. Pharmacotherapy. 31:510–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Villiers TJ: Bone health and
osteoporosis in postmenopausal women. Best Pract Res Clin Obstet
Gynaecol. 23:73–85. 2009. View Article : Google Scholar
|
|
7
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012. View Article : Google Scholar
|
|
8
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: a key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar
|
|
11
|
Choi HJ, Park YR, Nepal M, Choi BY, Cho
NP, Choi SH, Heo SR, Kim HS, Yang MS and Soh Y: Inhibition of
osteoclastogenic differentiation by Ikarisoside A in RAW 264.7
cells via JNK and NF-kappaB signaling pathways. Eur J Pharmacol.
636:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujisaki K, Tanabe N, Suzuki N, Kawato T,
Takeichi O, Tsuzukibashi O, Makimura M, Ito K and Maeno M: Receptor
activator of NF-kappaB ligand induces the expression of carbonic
anhydrase II, cathepsin K, and matrix metalloproteinase-9 in
osteoclast precursor RAW264.7 cells. Life Sci. 80:1311–1318. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wimalawansa SJ: Prevention and treatment
of osteoporosis: efficacy of combination of hormone replacement
therapy with other antiresorptive agents. J Clin Densitom.
3:187–201. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alexandersen P, Toussaint A, Christiansen
C, Devogelaer JP, Roux C, Fechtenbaum J, Gennari C and Reginster
JY; Ipriflavone Multicenter European Fracture S; Ipriflavone
Multicenter European Fracture Study: Ipriflavone in the treatment
of postmenopausal osteoporosis: a randomized controlled trial.
JAMA. 285:1482–1488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbal Pharmacology Compilation Committee:
Herbal Pharmacology. Shinil books, Seoul. 715–717. 2010.
|
|
16
|
Herbology Editorial Committee of Korean
Medicine schools: Boncho-hak [Herbology]. Younglimsa; Seoul: pp.
534–535. 2004
|
|
17
|
Song MY, Jung HW, Kang SY, Kim KH and Park
YK: Anti-inflammatory effect of Lycii radicis in LPS-stimulated RAW
264.7 macrophages. Am J Chin Med. 42:891–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho SH, Park EJ, Kim EO and Choi SW: Study
on the hypochlolesterolemic and antioxidative effects of tyramine
derivatives from the root bark of Lycium chenese Miller. Nutr Res
Pract. 5:412–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SJ, Lee L, Kim JH, Lee TH and Shim I:
Antidepressant-like effects of lycii radicis cortex and betaine in
the forced swimming test in rats. Biomol Ther (Seoul). 21:79–83.
2013. View Article : Google Scholar
|
|
20
|
Jeong JC, Kim SJ, Kim YK, Kwon CH and Kim
KH: Lycii cortex radicis extract inhibits glioma tumor growth in
vitro and in vivo through downregulation of the Akt/ERK pathway.
Oncol Rep. 27:1467–1474. 2012.PubMed/NCBI
|
|
21
|
Gao D, Li Q, Liu Z, Li Y, Liu Z, Fan Y, Li
K, Han Z and Li J: Hypoglycemic effects and mechanisms of action of
Cortex Lycii Radicis on alloxan-induced diabetic mice. Yakugaku
Zasshi. 127:1715–1721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arjmandi BH, Alekel L, Hollis BW, Amin D,
Stacewicz-Sapuntzakis M, Guo P and Kukreja SC: Dietary soybean
protein prevents bone loss in an ovariectomized rat model of
osteoporosis. J Nutr. 126:161–167. 1996.PubMed/NCBI
|
|
24
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka H, Tanabe N, Shoji M, Suzuki N,
Katono T, Sato S, Motohashi M and Maeno M: Nicotine and
lipopolysaccharide stimulate the formation of osteoclast-like cells
by increasing macrophage colony-stimulating factor and
prostaglandin E2 production by osteoblasts. Life Sci. 78:1733–1740.
2006. View Article : Google Scholar
|
|
26
|
Tanabe N, Maeno M, Suzuki N, Fujisaki K,
Tanaka H, Ogiso B and Ito K: IL-1 alpha stimulates the formation of
osteoclast-like cells by increasing M-CSF and PGE2
production and decreasing OPG production by osteoblasts. Life Sci.
77:615–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SN, Kim MH, Min YK and Kim SH:
Licochalcone A inhibits the formation and bone resorptive activity
of osteoclasts. Cell Biol Int. 32:1064–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jun AY, Kim HJ, Park KK, Son KH, Lee DH,
Woo MH, Kim YS, Lee SK and Chung WY: Extract of Magnoliae Flos
inhibits ovariectomy-induced osteoporosis by blocking
osteoclastogenesis and reducing osteoclast-mediated bone
resorption. Fitoterapia. 83:1523–1531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell-specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:176–185. 2008. View Article : Google Scholar
|
|
30
|
Winslow MM, Pan M, Starbuck M, Gallo EM,
Deng L, Karsenty G and Crabtree GR: Calcineurin/NFAT signaling in
osteoblasts regulates bone mass. Dev Cell. 10:771–782. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi S, Goldring S, Katz M,
Hilsenbeck S, Williams R and Roodman GD: Downregulation of
calcitonin receptor mRNA expression by calcitonin during human
osteoclast-like cell differentiation. J Clin Invest. 95:167–171.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang ZQ, Ovitt C, Grigoriadis AE,
Möhle-Steinlein U, Rüther U and Wagner EF: Bone and haematopoietic
defects in mice lacking c-fos. Nature. 360:741–745. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
David JP, Rincon M, Neff L, Horne WC and
Baron R: Carbonic anhydrase II is an AP-1 target gene in
osteoclasts. J Cell Physiol. 188:89–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sundaram K, Nishimura R, Senn J, Youssef
RF, London SD and Reddy SV: RANK ligand signaling modulates the
matrix metalloproteinase-9 gene expression during osteoclast
differentiation. Exp Cell Res. 313:168–178. 2007. View Article : Google Scholar
|
|
35
|
Andersen TL, del Carmen Ovejero M,
Kirkegaard T, Lenhard T, Foged NT and Delaissé JM: A scrutiny of
matrix metalloproteinases in osteoclasts: evidence for
heterogeneity and for the presence of MMPs synthesized by other
cells. Bone. 35:1107–1119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Versi E, Harvey MA, Cardozo L, Brincat M
and Studd JW: Urogenital prolapse and atrophy at menopause: a
prevalence study. Int Urogynecol J Pelvic Floor Dysfunct.
12:107–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lim DW, Kim JG, Lee Y, Cha SH and Kim YT:
Preventive effects of Eleutherococcus senticosus bark extract in
OVX-induced osteoporosis in rats. Molecules. 18:7998–8008. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim DW and Kim YT: Dried root of Rehmannia
glutinosa prevents bone loss in ovariectomized rats. Molecules.
18:5804–5813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hidaka S, Okamoto Y, Nakajima K, Suekawa M
and Liu SY: Preventive effects of traditional Chinese (Kampo)
medicines on experimental osteoporosis induced by ovariectomy in
rats. Calcif Tissue Int. 61:239–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dang ZC, van Bezooijen RL, Karperien M,
Papapoulos SE and Löwik CW: Exposure of KS483 cells to estrogen
enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res.
17:394–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wronski TJ, Lowry PL, Walsh CC and
Ignaszewski LA: Skeletal alterations in ovariectomized rats. Calcif
Tissue Int. 37:324–328. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalu DN, Liu CC, Salerno E, Hollis B,
Echon R and Ray M: Skeletal response of ovariectomized rats to low
and high doses of 17 beta-estradiol. Bone Miner. 14:175–187. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hui SL, Slemenda CW and Johnston CC Jr:
Baseline measurement of bone mass predicts fracture in white women.
Ann Intern Med. 111:355–361. 1989. View Article : Google Scholar : PubMed/NCBI
|