Introduction
The intestinal endocrine cells have been reported to
be abnormal in patients with inflammatory bowel disease (IBD) and
in animal models of IBD (1–20).
Recent discussion on the interaction between the hormones secreted
by gut endocrine cells and the immune system has led to the
hypothesis that this interaction plays an important role in the
pathophysiology of IBD (21,22, unpublished data). One recent study
found that the densities of all colonic endocrine cell types were
affected in rats with dextran sodium sulfate (DSS)-induced colitis,
and that this change strongly correlated with changes in the
densities of mucosal immune cells caused by the inflammation
(unpublished data). These findings confirmed the likelihood of an
interaction between intestinal hormones and the immune cells.
3-[(Dodecylthiocarbonyl)-methyl]-glutarimide
(DTCM-G) is a synthetic derivative of 9-methylstreptimidone, which
is a potent anti-inflammatory agent that suppresses activator
protein 1 (AP-1), and dehydroxymethylepoxyquinomicin (DHME-Q) is a
low-molecular-weight nuclear factor κB (NF-κB) inhibitor that also
exhibits potent anti-inflammatory activity. Both of these
anti-inflammatory agents have been shown to be effective in animal
models of IBD (23–26). The aim of this study was to
determine whether the anti-inflammatory effects of these two agents
restore the densities of colonic endocrine cells to normal levels
in rats with DSS-induced colitis.
Materials and methods
Rats
Sixty male Wistar rats (Hannover GALAS; Taconic
Farms, Inc., Lille Skensved, Denmark) with a mean body weight of
290 g (range, 241–395 g) were housed in Makrolon III cages with
ad libitum access to water and food. They were fed a
standard diet (B&K Universal AS, Nittedal, Norway) and
maintained in an environment at 21±1°C, a relative humidity of
55±5% and a 12/12 h light/dark cycle. The animals were allowed to
acclimatize in the animal house for 8 days prior to the
experiments, and were then divided into 4 groups of 15 animals
each.
The animals in the control group were provided with
normal drinking water for 7 days, and colitis was induced in the
rats in the remaining 3 groups by providing the rats with distilled
water containing 5% DSS (molecular weight 40 kDa; TdB Consultancy,
Uppsala, Sweden), which was prepared daily, for 7 days, as
previously described (27,28).
The 3 DSS-treated groups were then randomized to receive the
vehicle [0.5 ml of 0.5% carboxymethyl cellulose (CMC; DSS group)],
DTCM-G at 20 mg/kg body weight in 0.5% CMC (DSS-G group), and
DHME-Q at 15 mg/kg body weight in 0.5% CMC (DSS-Q group),
intraperitoneally, twice daily for 5 days. The synthesis of DTCM-G
and DHME-Q is described elsewhere (23,27–31). The animals were monitored twice
daily, and any animals exhibiting signs of pain were administered a
subcutaneous injection of 1 ml of Temgesic solution (containing 0.3
g/ml Temgesic; Merck Pharmaceutical).
At the end of the 5-day treatment period, all the
animals were sacrificed by CO2 inhalation, and a
post-mortem laparotomies were carried out. The colon was dissected
out, and tissue samples were taken from the lower part of the colon
for histological examinations.
The local ethics committee for the Protection of
Vertebrate Animals used for Experimental and Other Scientific
Purposes approved the study protocols.
Histopathology and
immunohistochemistry
The tissue samples were fixed overnight in 4%
buffered paraformalde-hyde, embedded in paraffin and then sectioned
at a thickness of 5 µm. The sections were deparaffinized and then
stained with hematoxylin and eosin, or immunostained using the
ultraView Universal DAB Detection kit (version 1.02.0018) and the
BenchMark Ultra IHC/ISH staining module (both from Ventana Medical
Systems, Basel, Switzerland). The sections were immunostained by
incubating them with one of the primary antibodies for 32 min at
37°C. The primary antibodies used are summarized in Table I.
 | Table ISummary of the primary antibodies used
in this study. |
Table I
Summary of the primary antibodies used
in this study.
| Antibodies raised
against | Type of antibody | Source | Code no. | Detects |
|---|
| N-terminal of
purified CgA | Monoclonal, raised in
mouse | Dako, Glostrup,
Denmark | M869 | CgA |
| Serotonin | Monoclonal, raised in
mouse | Dako, Glostrup,
Denmark | 5HT-209 | Serotonin |
| PYY | Polyclonal, raised in
rabbit | Alpha-Diagnostica,
San Antonio, TX, USA | PYY 11A | PYY |
| Porcine
glicentin/glucagon | Polyclonal, raised in
rabbit | Acris Antibodies,
Herford, Germany | BP508 | Enteroglucagon
(oxyntomodulin) |
| Synthetic human
PP | Polyclonal, raised
in rabbit | Diagnostic
Biosystems,Pleasanton, CA, USA | #114 | PP |
| Synthetic human
somatostatin | Polyclonal, raised
in rabbit | Dako, Glostrup,
Denmark | A566 | Somatostatin |
| Human CD45 | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | M0701 | CD45 is considered
a common leukocyte antigen and is expressed exclusively on cells of
the hematopoietic system and their progenitors |
| Human CD5 | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | IS082 | B and T
lymphocytes |
| Human CD57 | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | IS647 | Subsets of natural
killer of cells and CD8+ lymphocytes, and by a small
proportion CD4+/CD45R0+ T lymphocytes |
| Human CD23 | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | IS781 | B lymphocytes |
| Human CD68 | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | M0814 | Monocytes,
macrophages, and myeloid cells |
| Human mast cell
tryptase | Monoclonal, raised
in mouse | Dako, Glostrup,
Denmark | M7052 | Mast cells |
Quantification of endocrine and immune
cells
The endocrine and immune cells were quantified using
Olympus cellSens imaging software (version 1.7) as described
elsewhere (32,33). A ×40 objective was used, which
meant that each frame (field) displayed on the monitor represented
a tissue area of 0.035 mm2. The data are presented as
the number of cells/mm2 of epithelium for endocrine
cells, and the number of cells per field for immune cells. The
sections were coded, and the measurements were made by the same
person (M.E.-S.) who was unaware of the identity of the
sections.
Statistical analysis
The Kruskal-Wallis non-parametric test, with Dunn's
test as a post-test was used to compare the findings obtained form
the animals in the control, DSS, DSS-G and DSS-Q groups. The data
are presented as the mean ± SEM values, and the cut-off for
statistical significance was set at P<0.05.
Results
The examination of the hematoxylin and eosin-stained
sections of the colonic tissues revealed normal histological
results in the control, DSS-G, and DSS-Q groups, while a disrupted
mucosal architecture, edema, bleeding, crypt abscesses and immune
cell infiltration into the mucosa and submucosa were observed in
the DSS group.
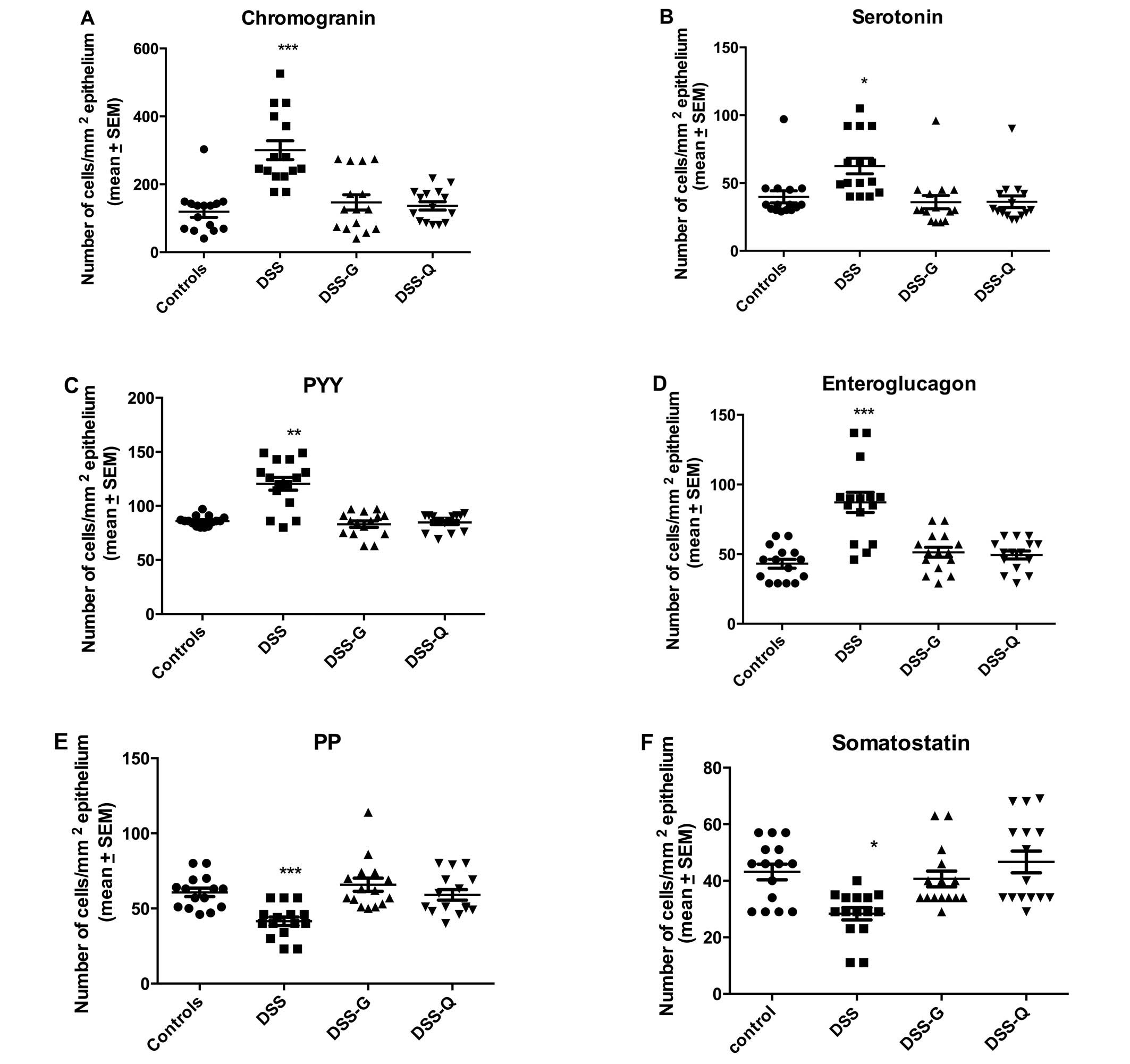
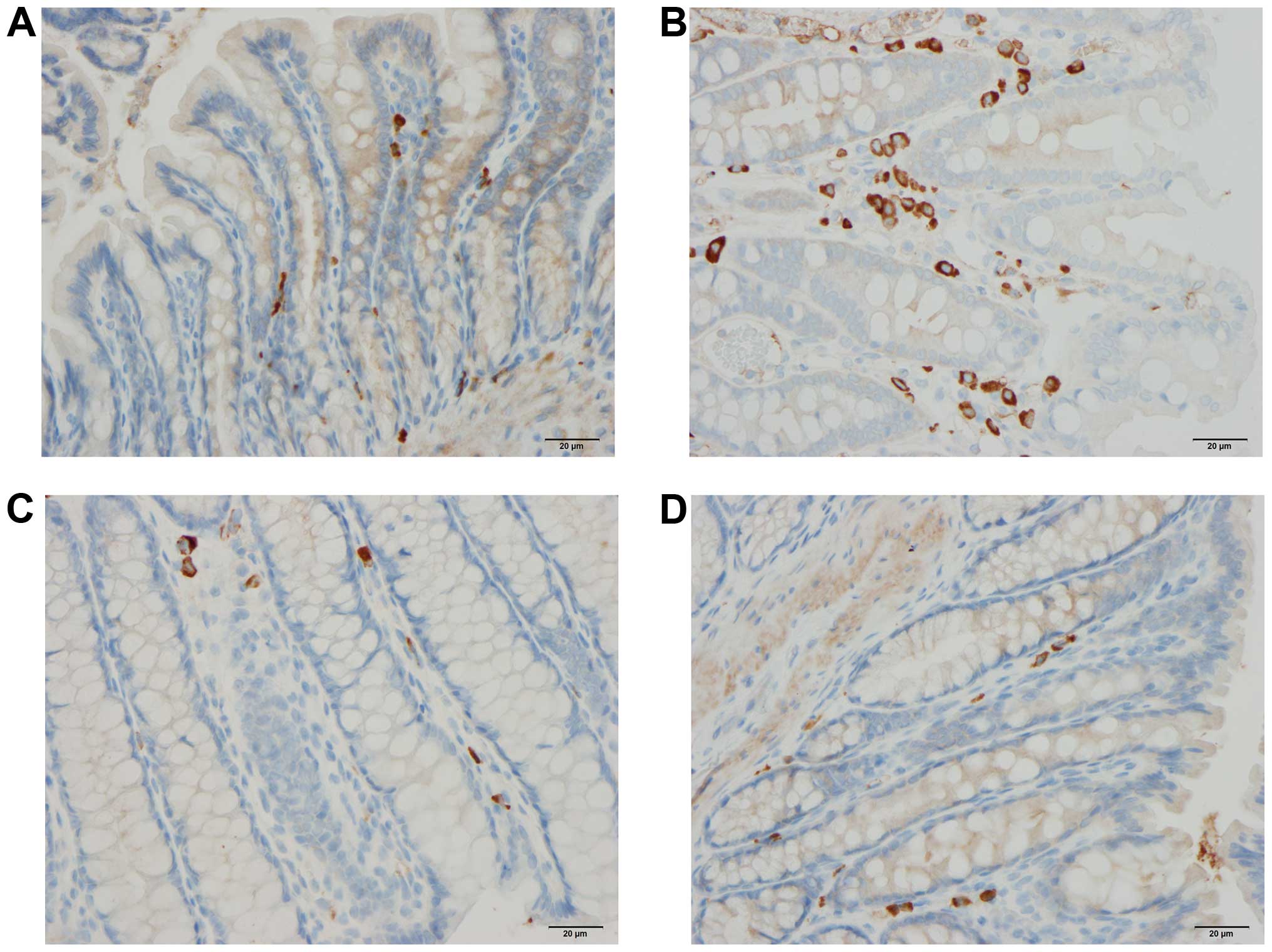
Endocrine cells
The cell densities of various endocrine cell types
in the control, DSS, DSS-G, and DSS-Q groups are summarized in
Table II and illustrated in
Figs. 1Figure 2–3.
 | Table IIDensities of the different endocrine
cell types (number/mm2 of epithelium) in the control
animals, and in animals with DSS-induced colitis treated with the
vehicle (CgA, DSS group), DTCM-G (DSS-G group) and DHME-Q (DSS-Q
group). |
Table II
Densities of the different endocrine
cell types (number/mm2 of epithelium) in the control
animals, and in animals with DSS-induced colitis treated with the
vehicle (CgA, DSS group), DTCM-G (DSS-G group) and DHME-Q (DSS-Q
group).
| Animal group | Endocrine cell type
|
|---|
| CgA | Serotonin | PYY | Enteroglucagon | PP | Somatostatin |
|---|
| Control | 119.0±16.5 | 39.9±4.4 | 96.0±1.2 | 43.1±3.2 | 60.7±2.8 | 43.1±2.8 |
| DSS | 300.6±27.7a | 62.6±5.8a | 120.5±5.9b | 87.2±7.2a | 41.5±2.8a | 28.3±2.2c |
| DSS-G | 146.7±22.4 | 35.9±4.9 | 98.2±7.0 | 51.3±3.6 | 65.8±4.4 | 40.7±2.7 |
| DSS-Q | 136.6±12.2 | 36.2±4.3 | 84.8±2.0 | 49.5±2.9 | 59.1±3.5 | 46.7±3.8 |
Chromogranin A (CgA)-producing cells
The density CgA-producing cells was significantly
higher in the DSS group than in the control, DSS-G, and DSS-Q
groups (P<0.0001, 0.0009 and <0.0001, respectively). There
was no significant difference observed between the control group
and the DSS-G and DSS-Q groups (P=0.6 and 0.2, respectively).
Serotonin-producing cells
The density serotonin-producing cells was
significantly higher in the DSS group than in the control, DSS-G
and DSS-Q groups (P=0.0006, 0.0006 and 0.0003, respectively), and
it did not differ significantly between the control group and the
DSS-G and DSS-Q groups (P=0.1 for both).
Peptide YY (PYY)- and
enteroglucagon-producing cells
The densities of the PYY- and enteroglucagon-
producing cells were significantly higher in the DSS group than in
the control, DSS-G and DSS-Q groups (PYY, P=0.005, 0.04 and 0.005,
respectively; and enteroglucagon, P<0.0001, 0.0004 and 0.0004,
respectively). The cell density did not differ significantly
between the control group and the DSS-G and DSS-Q groups for either
PYY (P=0.2 and 0.9) or enteroglucagon (P=0.1 for both).
Pancreatic polypeptide (PP)- and
somatostatin-producing cells
Both the densities of PP- and somatostatin-producing
cells were significantly lower in the DSS group than in the
control, DSS-G, and DSS-Q groups (PP, P=0.0001, <0.0001 and
0.0008, respectively; and somatostatin, 0.02, 0.003 and 0.001,
respectively). The cell density did not differ significantly
between the control group and the DSS-G and DSS-Q groups for either
PP (P=0.5 and 0.6, respectively) or somatostatin (P=0.6 and 0.5,
respectively).
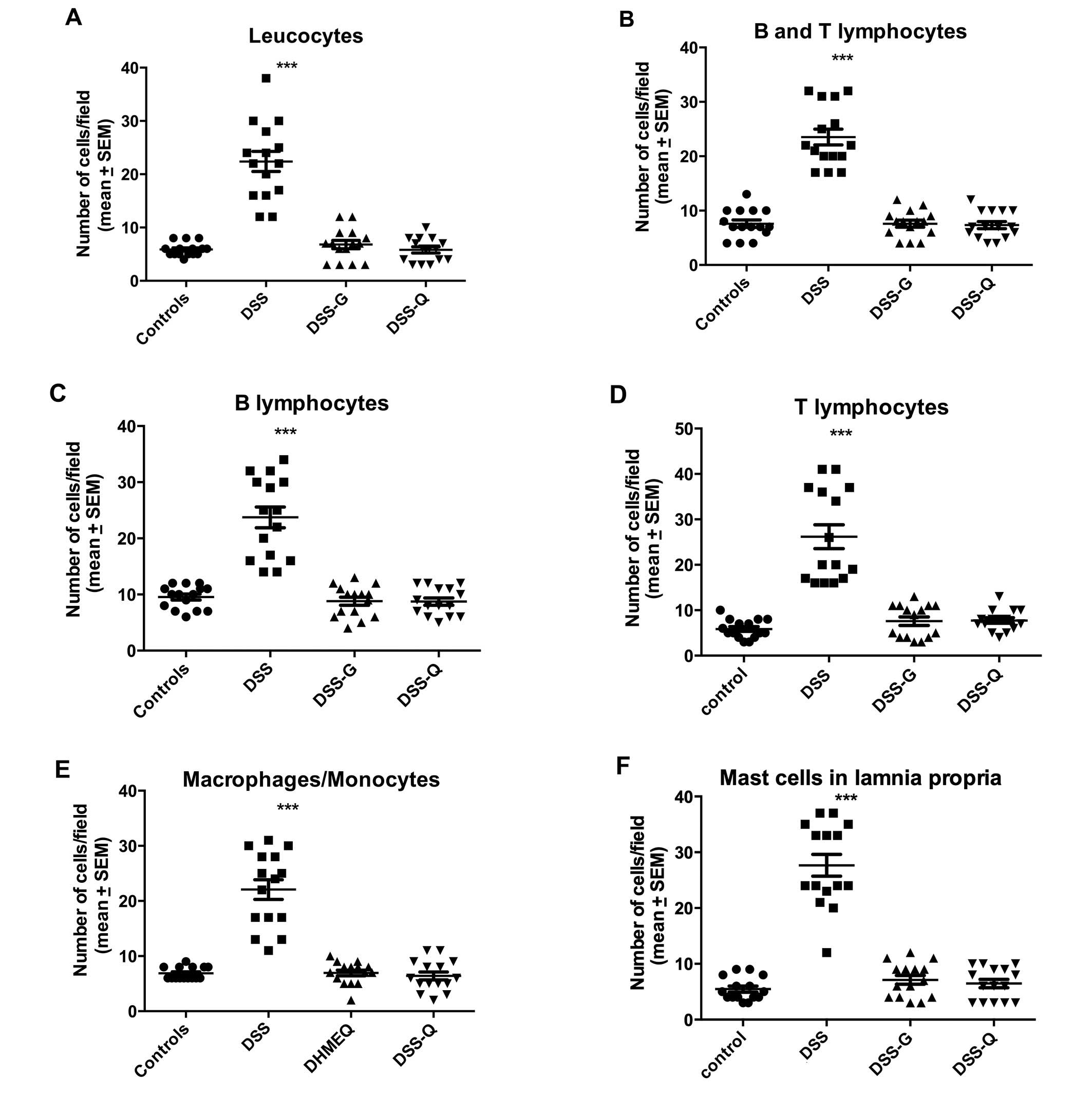
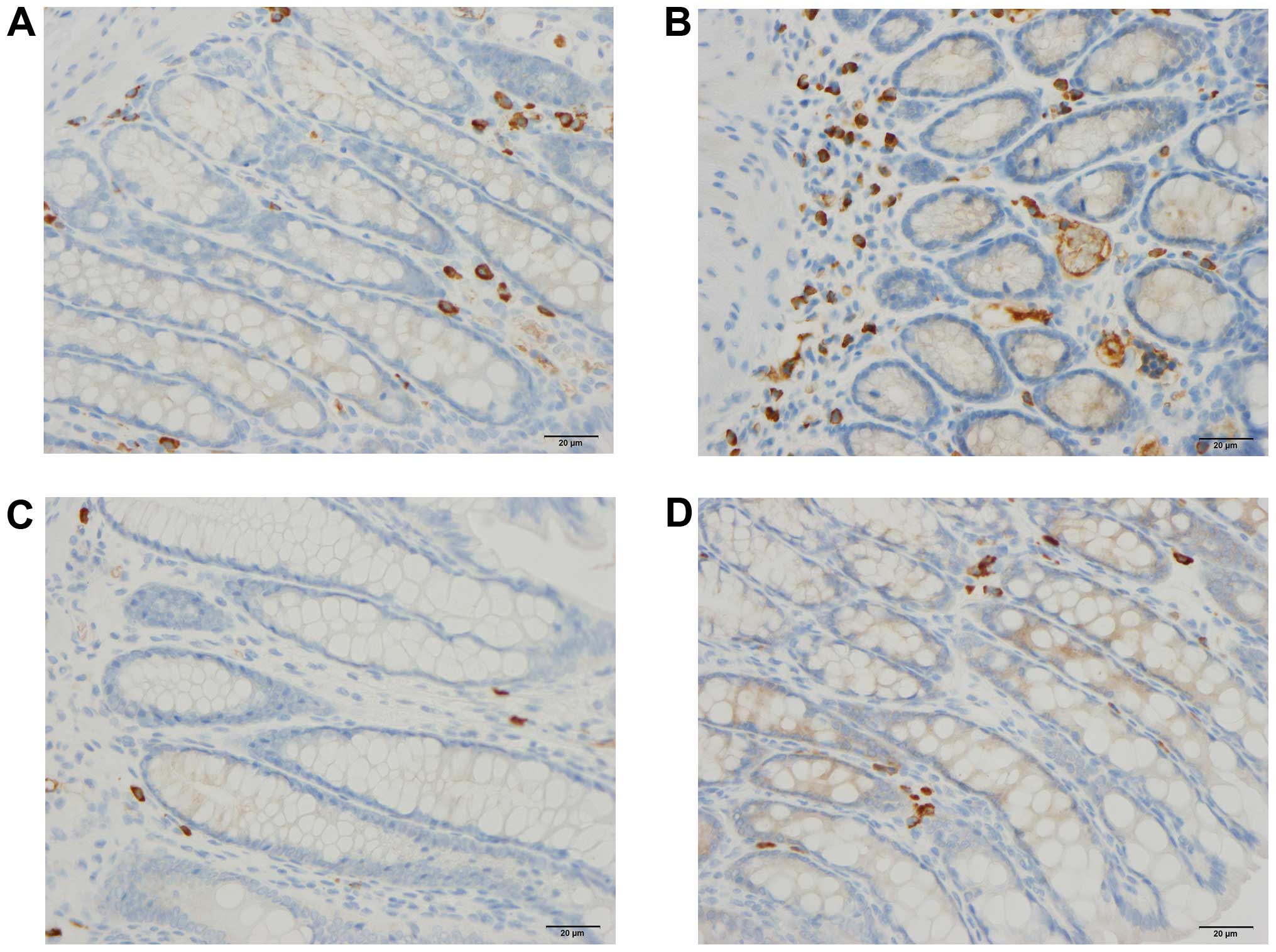
Immune cells
The densities of the immune cells in the control,
DSS, DSS-G, and DSS-Q groups are presented in Table III and Figs. 4Figure 5–6.
 | Table IIIDensities of the various immune cells
(number/field) in the control group and various experimental
treatment groups with DSS-induced colitis. |
Table III
Densities of the various immune cells
(number/field) in the control group and various experimental
treatment groups with DSS-induced colitis.
| Animal group | Immune cell type
|
|---|
| Leukocytes | B/T
lymphocytes | B lymphocytes | T lymphocytes |
Macrophages/monocytes | Mast cells |
|---|
| Control | 5.9±0.3 | 7.6±0.7 | 9.5±0.5 | 5.9±0.5 | 6.9±0.3 | 5.5±0.5 |
| DSS | 22.4±1.9a | 23.5±1.4a | 23.7±1.9a | 26.2±2.6a | 22.1±1.8a | 27.7±1.9a |
| DSS-G | 6.8±0.8 | 7.6±0.6 | 8.8±0.7 | 7.6±0.9 | 6.9±0.5 | 7.1±0.8 |
| DSS-Q | 5.8±0.6 | 7.3±0.6 | 8.7±0.6 | 7.7±0.6 | 6.4±0.7 | 6.5±0.7 |
Leukocytes
The density of leukocytes was significantly higher
in the DSS group than in the control, DSS-G, and DSS-Q groups
(P<0.0001 for all), and it did not differ significantly between
the control group and the DSS-G and DSS-Q groups (P=0.3 and 0.9,
respectively).
Lymphocytes
The density of B/T lymphocytes (Fig. 5) in the DSS group was increased
relative to the control, DSS-G, and DSS-Q groups (P<0.0001 for
all), as were the densities of the B lymphocytes (P<0.0001 for
all) and T lymphocytes (P<0.0001 for all). There were no
differences observed between the control group and the DSS-G and
DSS-Q groups with respect to the densities of B/T lymphocytes
(P=0.9 and 0.7, respectively), B lymphocytes (P=0.9 and 0.7,
respectively) and T lymphocytes (P=0.2, and 0.2, respectively).
Macrophages/monocytes
The density of macrophages/monocytes was higher in
the DSS group than in the control, DSS-G, and DSS-Q groups
(P<0.0001 for all), and did not differ significantly between the
control group and the DSS-G and DSS-Q groups (P=0.7 and 0.4,
respectively).
Mast cells
The density of mast cells (Fig. 6) was significantly higher in the
DSS group than in the control, DSS-G, and DSS-Q groups (P<0.0001
for all), and did not differ significantly between the control
group and the DSS-G and DSS-Q groups (P=0.2 and 0.4,
respectively).
Discussion
DSS-induced colitis is an animal model that closely
replicates both the clinical and morphological characteristics of
human ulcerative colitis (UC) (34). Thus, animals with DSS-induced
colitis suffer from bloody diarrhea, dehydration and the loss of
body weight (34). Furthermore,
the morphological features resemble human UC both macroscopically
and microscopically (35).
However, this model lacks the chronicity (i.e., disease relapse and
remission) observed in human UC (35). Therefore, care should be taken
when drawing conclusions about human UC based on this model.
In the present study, 5 days of treatment with
either DTCM-G or DHME-Q ameliorated the inflammation caused by DSS,
as indicated by the restored mucosal architecture and normal
histological appearance in the animals with DSS-induced colitis
treated with these agents compared to the untreated controls with
DSS-induced colitis. This finding is in line with the results of
previously published studies on colitis induced by trinitrobenzene
sulfonic acid (TNBS) and DSS in rats and mice (25,26, unpublished
data). This resolution of inflammation was associated with the
restoration of the normal densities of all colonic endocrine cell
types to normal levels. DTCM-G and DHME-Q are anti-inflammatory
agents with different modes of action: the former acts by
inhibiting AP-1, while the latter inhibits NF-κB. Thus, the effects
of these substances on colonic endocrine cell densities cannot be
attributed to a direct effect on the endocrine cells; rather, they
are more likely to be attributable to their effects on
inflammation.
There is a growing body of evidence indicating that
some of the hormones produced by the colonic endocrine cells
investigated herein interact with the immune cells during the
inflammation process. Thus, CgA peptides reduce the release of
interleukin (IL)-16 and IL-5, with the reduction of the number of
lymphocytes at the sites inflammation and hence the
pro-inflammatory action of lymphocytes and monocytes (36–38). Moreover, CgA inhibits the vascular
leakage caused by tumor necrosis factor α (TNF-α) (39). The serotonin cell number has been
reported to be reduced in mice lacking the T lymphocyte receptors
(36), IL-13 receptors have been
shown to be localized on serotonin-producing cells (40) and serotonin receptors have been
found in lymphocytes, monocytes, machrophages and dendritic cells
(41). Furthermore, serotonin the
inhibits apoptosis of immune cells and promotes the recruitment of
T cells, and affects the proliferation of lymphocytes, protects
natural killer cells (42–45).
The present observation that the immune cell densities were
normalized to the same extent as the colonic endocrine cells
following treatment with DTCM-G and DHME-Q, and the previous
finding of a strong correlation between the changes in immune cells
and endocrine cells in rats with DSS-induced colitis support the
suggested interaction between the two types of cell during the
inflammatory process (unpublished data).
It has been proposed that changes in the endocrine
cell density in response to or as a result of inflammation play a
major role in the manifestation of clinical symptoms such as
accelerated intestinal motility, decreased intestinal absorption of
water and electrolytes, and diarrhea (unpublished data). Treatment
with either DTCM-G or DHME-Q, administered under the same regimen
as that used in the present study, was also found to improve these
clinical symptoms in DSS-induced colitis in rats (unpublished
data). The present finding that treatment with these
anti-inflammatory agents restores the densities of the endocrine
cells supports the assumption regarding the importance of changes
in the endocrine cell populations in the development of the
clinical symptoms during colitis.
Acknowledgments
The present study was supported by grants from
Helse-Vest and Helse-Fonna.
References
|
1
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar
|
|
2
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin A cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Salhy M, Lomholt-Beck B and Gundersen
TD: High chromogranin A cell density in the colon of patients with
lymphocytic colitis. Mol Med Rep. 4:603–605. 2011.PubMed/NCBI
|
|
5
|
Moran GW, Pennock J and McLaughlin JT:
Enteroendocrine cells in terminal ileal Crohn's disease. J Crohn's
Colitis. 6:871–880. 2012. View Article : Google Scholar
|
|
6
|
Moran GW, Leslie FC and McLaughlin JT:
Crohn's disease affecting the small bowel is associated with
reduced appetite and elevated levels of circulating gut peptides.
Clin Nutr. 32:404–411. 2013. View Article : Google Scholar
|
|
7
|
Besterman HS, Mallinson CN, Modigliani R,
Christofides ND, Pera A, Ponti V, Sarson DL and Bloom SR: Gut
hormones in inflammatory bowel disease. Scand J Gastroenterol.
18:845–852. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (review). Int J Mol Med.
31:275–282. 2013.Review. PubMed/NCBI
|
|
9
|
Hirotani Y, Mikajiri K, Ikeda K, Myotoku M
and Kurokawa N: Changes of the peptide YY levels in the intestinal
tissue of rats with experimental colitis following oral
administration of mesalazine and prednisolone. Yakugaku Zasshi.
128:1347–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vona-Davis LC and McFadden DW: NPY family
of hormones: clinical relevance and potential use in
gastrointestinal disease. Curr Top Med Chem. 7:1710–1720. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tari A, Teshima H, Sumii K, Haruma K,
Ohgoshi H, Yoshihara M, Kajiyama G and Miyachi Y: Peptide YY
abnormalities in patients with ulcerative colitis. Jpn J Med.
27:49–55. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sciola V, Massironi S, Conte D, Caprioli
F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT and Piodi L:
Plasma chromogranin a in patients with inflammatory bowel disease.
Inflamm Bowel Dis. 15:867–871. 2009. View Article : Google Scholar
|
|
14
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manocha M and Khan WI: Serotonin and GI
Disorders: an update on clinical and experimental studies. Clin
Transl Gastroenterol. 3:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto H, Morise K, Kusugami K, Furusawa
A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T, et
al: Abnormal neuropeptide concentration in rectal mucosa of
patients with inflammatory bowel disease. J Gastroenterol.
31:525–532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Payer J, Huorka M, Duris I, Mikulecky M,
Kratochvílová H, Ondrejka P and Lukác L: Plasma somatostatin levels
in ulcerative colitis. Hepatogastroenterology. 41:552–553.
1994.PubMed/NCBI
|
|
19
|
Watanabe T, Kubota Y, Sawada T and Muto T:
Distribution and quantification of somatostatin in inflammatory
disease. Dis Colon Rectum. 35:488–494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koch TR, Carney JA, Morris VA and Go VL:
Somatostatin in the idiopathic inflammatory bowel diseases. Dis
Colon Rectum. 31:198–203. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan WI and Ghia JE: Gut hormones:
emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
22
|
Margolis KG and Gershon MD: Neuropeptides
and inflammatory bowel disease. Curr Opin Gastroenterol.
25:503–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ota E, Takeiri M, Tachibana M, Ishikawa Y,
Umezawa K and Nishiyama S: Synthesis and biological evaluation of
molecular probes based on the 9-methylstreptimidone derivative
DTCM-glutarimide. Bioorg Med Chem Lett. 22:164–167. 2012.
View Article : Google Scholar
|
|
24
|
Shibasaki S, Yamashita K, Goto R, Wakayama
K, Tsunetoshi Y, Zaitsu M, Igarashi R, Haga S, Ozaki M, Umezawa K
and Todo S: Immunosuppressive effects of DTCM-G, a novel inhibitor
of the mTOR downstream signaling pathway. Transplantation.
95:542–550. 2013. View Article : Google Scholar
|
|
25
|
Funakoshi T, Yamashita K, Ichikawa N,
Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T,
Ichihara S, et al: A novel NF-κB inhibitor,
dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic
injury in mice. J Crohn's Colitis. 6:215–225. 2012. View Article : Google Scholar
|
|
26
|
El-Salhy M, Umezawa K, Gilja OH, Hatlebakk
JG, Gundersen D and Hausken T: Amelioration of severe TNBS induced
colitis by novel AP-1 and NF-κB inhibitors in rats. Scientific
World Journal. 2014:1–8. 2014. View Article : Google Scholar
|
|
27
|
Takeiri M, Tachibana M, Kaneda A, Ito A,
Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata
G, et al: Inhibition of macrophage activation and suppression of
graft rejection by DTCM-Glutarimide, a novel piperidine derived
from the antibiotic 9-methylstreptimidone. Inflamm Res. 60:879–888.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishikawa Y, Tachibana M, Matsui C, Obata
R, Umezawa K and Nishiyama S: Synthesis and biological evaluation
on novel analogs of 9-methylstreptimidone, an inhibitor of
NF-kappaB. Bioorg Med Chem Lett. 19:1726–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ueki S, Yamashita K, Aoyagi T, Haga S,
Suzuki T, Itoh T, Taniguchi M, Shimamura T, Furukawa H, Ozaki M, et
al: Control of allograft rejection by applying a novel nuclear
factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin.
Transplantation. 82:1720–1727. 2006. View Article : Google Scholar
|
|
30
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Umezawa N, Matsumoto N, Iwama S, Kato N
and Higuchi T: Facile synthesis of peptide-porphyrin conjugates:
towards artificial catalase. Bioorg Med Chem. 18:6340–6350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Endocrine cells in the ileum of
patients with irritable bowel syndrome. World J Gastroenterol.
20:2383–2391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Low-Grade inflammation in the rectum of patients with
sporadic irritable bowel syndrome. Mol Med Rep. 7:1081–1085.
2013.PubMed/NCBI
|
|
34
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Low D, Nguyen DD and Mizoguchi E: Animal
models of ulcerative colitis and their application in drug
research. Drug Des Devel Ther. 7:1341–1357. 2013.PubMed/NCBI
|
|
36
|
Spiller R: Serotonin and GI clinical
disorders. Neuropharmacology. 55:1072–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egger M, Beer AG, Theurl M, Schgoer W,
Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J,
Patsch JR, et al: Monocyte migration: a novel effect and signaling
pathways of catestatin. Eur J Pharmacol. 598:104–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feistritzer C, Mosheimer BA, Colleselli D,
Wiedermann CJ and Kähler CM: Effects of the neuropeptide
secretoneurin on natural killer cell migration and cytokine
release. Regul Pept. 126:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrero E, Magni E, Curnis F, Villa A,
Ferrero ME and Corti A: Regulation of endothelial cell shape and
barrier function by chromogranin A. Ann N Y Acad Sci. 971:355–358.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Steeds J, Motomura Y, Deng Y,
Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK and Khan
WI: CD4+ T cell-mediated immunological control of
enterochro-maffin cell hyperplasia and 5-hydroxytryptamine
production in enteric infection. Gut. 56:949–957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cloëz-Tayarani I and Changeux JP: Nicotine
and serotonin in immune regulation and inflammatory processes: a
perspective. J Leukoc Biol. 81:599–606. 2007. View Article : Google Scholar
|
|
42
|
Stefulj J, Cicin-Sain L, Schauenstein K
and Jernej B: Serotonin and immune response: effect of the amine on
in vitro proliferation of rat lymphocytes. Neuroimmunomodulation.
9:103–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Betten A, Dahlgren C, Hermodsson S and
Hellstrand K: Serotonin protects NK cells against oxidatively
induced functional inhibition and apoptosis. J Leukoc Biol.
70:65–72. 2001.PubMed/NCBI
|
|
44
|
Laberge S, Cruikshank WW, Beer DJ and
Center DM: Secretion of IL-16 (lymphocyte chemoattractant factor)
from serotonin-stimulated CD8+ T cells in vitro. J
Immunol. 156:310–315. 1996.PubMed/NCBI
|
|
45
|
Soga F, Katoh N, Inoue T and Kishimoto S:
Serotonin activates human monocytes and prevents apoptosis. J
Invest Dermatol. 127:1947–1955. 2007. View Article : Google Scholar : PubMed/NCBI
|