Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1,2), and non-small cell lung cancer
(NSCLC) accounts for the majority of all lung cancer-related deaths
(3). The prognosis of lung cancer
remains unfavorable, with a 5-year overall survival rate of
approximately 11%, despite recent advances in clinical and
experimental oncology (4). Thus,
detailed research into the development and progression of NSCLC is
essential for improving the diagnosis, prevention and treatment of
this disease. Cisplatin (DDP) remains the most widely used
first-line chemotherapeutic agent for the treatment of NSCLC.
However, the continuous and multiple-dose administration of DDP
often causes severe side-effects and cancer cells often become
resistant; thus, this has limited the use of this drug (5). Therefore, enhancing the sensitivity
of cancer cells to DDP (perhaps to lower doses of the drug) remains
a challenge for the efficacty of chemotherapy. Recently, an
increasing number of studies has demonstrated that small,
non-coding RNAs may be involved in the pathogenesis of NSCLC
(6,7,14),
thereby providing new insight into the biology of the disease.
Previous studies have indicated that the dysregulation of microRNAs
(miRNAs or miRs) contributes to the resistance of human cancer
cells to DDP (8,9). Novel therapeutic modalities
combining miRNAs have the potential to be effective in the
treatment of NSCLC in the future.
miRNA-196a (miR-196a) was one of the first miRNAs to
be discovered in human cells; it is highly conserved in mammals.
Previous studies have indicated that the expression of miR-196a is
significantly upregulated in different solid tumors (10–12) and have revealed that miR-196a is
involved in the proliferation, detachment, migration and invasion
of a number of cancer cells (colorectal cancer, breast cancer,
pancreatic cancer, gastric cancer and NSCLC cells) (12–16). Huang et al reported that
miR-196a promotes the progression of pancreatic cancer (13); Liu et al found that the
downregulation of miR-196a inhibited NSCLC cell proliferation and
invasion (14). However, to the
best of our knowledge, there are no studies available to date on
the association between miR-196a expression and the sensitivity of
NSCLC cells to DDP.
In the present study, we found that miR-196a was
upregulated in human NSCLC tissues and cell lines; the
downregulation of miR-196a enhanced the sensitivity of NSCLC cell
lines (SPC-A-1, A549) to DDP through the induction of apoptosis by
targeting homeobox A5 (HOXA5). Taken together, these findings
suggest that miR-196a is a valid therapeutic target with the
potential to be employed as a novel multimodality therapy as part
of a strategy for the treatment of patients with NSCLC.
Materials and methods
Patients and tissue samples
A total of 23 pairs of matched NSCLC and
non-cancerous tissue samples were obtained from patients undergoing
surgical procedures at the Sixth People's Hospital of Chongqing
(Chongqing, China), and tumor diagnosis was performed by an
independent pathologist. None of the patients had received
chemotherapy or radiotherapy prior to surgery. The samples were
snap-frozen in liquid nitrogen and stored at ‒80°C until RNA
extraction. Written informed consent was obtained from all patients
prior to surgery. This study was approved by the Research Ethics
Committee of the Sixth People's Hospital of Chongqing, China.
Animals
Female BALB/c nude mice (n=48, 4–6 weeks of age)
were purchased from the Shanghai Laboratory Animal Research Center
(Shanghai, China) and maintained under pathogen-free conditions.
All experimental procedures involving animals were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and were performed according to the Institutional Ethical
Guidelines for Animal Experiments.
Cell culture
Four NSCLC adenocarcinoma cell lines (A549, SPC-A-1,
NCI-H1650 and NCI-H1299), a NSCLC squamous carcinoma cell line
(SK-MES-1) and a normal human bronchial epithelial cell line
(16HBE) were purchased from the Institute of Biochemistry and Cell
Biology of the Chinese Academy of Sciences (Shanghai, China). The
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) (both from Gibco, Grand Island, NY, USA), 100
U/ml penicillin and 100 mg/ml streptomycin (both from Invitrogen,
Carlsbad, CA, USA) in humidified air with 5% CO2 at
37°C.
Locked nucleic acid (LNA)-anti-miR-196a
oligonucle-otidetransfection assay
The SPC-A-1 and A549 cells were maintained in
RPMI-1640 medium. For transfection, LNA-anti-miR-196a or
LNA-control oligonucleotides were delivered at a final
concentration of 50 nM [as previously described (17)] using Lipofectamine 2000 reagent
(Invitrogen). LNA-anti-miR-196a and LNA-control oligonucleotides
were purchased from Exiqon A/S (Vedbaek, Denmark). A group of mock
cells (untransfected cells) was also used.
Treatment of cells with DDP
The mock SPC-A-1/A549 cells and the SPC-A-1/A549
cells transfected with LNA-anti-miR-196a/LNA-control
oligonucleotide were treated with various concentrations (0, 5, 10
and 20 μg/ml) of DDP for 12 h or 5 μg/ml of DDP for
0, 24, 48, 72 and 96 h.
Cell viability assay
Cell viability was assessed using the CCK8 assay
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions. Briefly, the treated cells were
cultured in triplicate in a 96-well plate. CCK8 reagent was added
to each well 2 h prior to the termination of the experiment, and
the absorbance (OD450) was expressed as the viability percentages
of the cells compared with the controls. All tests were performed
in triplicate and the data are presented as the means ± SD.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the mock-transfected
SPC-A-1/A549 or stably-transfected SPC-A-1/A549 cells
(5×106 cells) and the tissue samples, using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions.
To quantify the miRNA levels, RT-qPCR was performed using TaqMan
microRNA assays (Applied Biosystems Life Technologies, Foster City,
CA, USA). Briefly, 10 ng of total RNA was reverse transcribed using
an miRNA-specific looped RT primer for each miRNA and a
corresponding TaqMan® microRNA Reverse Transcription kit
(Applied Biosystems Life Technologies; prime sequences included in
kit). qPCR was performed using the generated cDNA in gene-specific
TaqMan miRNA Real-Time PCR assay solution on a StepOnePlus
Real-Time PCR system (Applied Biosystems Life Technologies). The
reaction was performed at 95°C for 10 min, followed by 45 cycles at
95°C for 15 sec, and 60°C for 60 sec. RNA U6 (RNU6B; Applied
Biosystems Life Technologies) was used as an internal control. The
relative expression was calculated using the comparative cycle
threshold (Ct) method. All qPCR reactions were performed in
triplicate and the data are presented as the means ± SD.
Colony formation assay
Approximately 500 mock-transfected SPC-A-1/A549 or
stably-transfected SPC-A-1/A549 cells were placed in a fresh 6-well
plate with or without DDP for 12 h and maintained in RPMI-1640
containing 10% FBS for another 2 weeks. The colonies were fixed
with methanol and stained with 0.1% crystal violet in 20% methanol
for 15 min. All samples were analyzed in triplicate and the data
are presented as the means ± SD.
Flow cytometric analysis of
apoptosis
The mock-transfected SPC-A-1/A549 or
stably-transfected SPC-A-1/A549 cells were treated with DDP and
harvested. The cells were double-stained with FITC-Annexin V and
propidium iodide (PI), and then analyzed using a flow cytometer
equipped with CellQuest software (both from BD Biosciences,
Franklin Lakes, NJ, USA), as previously described (18). The relative ratio of apoptotic
cells was compared with the control from each experiment. All
samples were analyzed in triplicate and the data are presented as
the means ± SD.
Caspase-3 activity assay
Briefly, the cells were seeded in a 6-well plate,
cultured for 24 h and then treated with or without DDP for a
further 12 h, and harvested. The activity of caspase-3 was measured
using a Caspase-3 Colorimetric assay kit (Sigma-Aldrich, St. Louis,
MO, USA) according to the manufacturer's instructions. Caspase-3
activity was quantified spectrophotometrically at a wavelength of
405 nm. All samples were analyzed in triplicate and the data are
presented as the means ± SD.
Western blot analysis
Protein extracts (40 μg) from the treated
cells were separated by 15% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE; Invitrogen) and electrophoretically
transferred onto PDVF membranes (GE Healthcare Life Sciences,
Pittsburgh, PA, USA). The membranes were blocked with 5% non-fat
dried milk for 2 h, and then incubated for 2 h with specific
primary antibodies HOXA5 (sc-365784), p53 (sc-126) and β-actin
(sc-376421) (all from Santa Cruz Biotechnology, Santa Cruz, CA,
USA). After washing with TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and
0.1% Tween-20), the membranes were incubated with horseradish
peroxidase-linked antibody (#7076; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h. The membranes were washed and the
proteins were visualized using ECL chemiluminescence and exposed to
X-ray film. All of the samples were analyzed in triplicate.
In vivo experiments using mice
BALB/c nude mice were randomly divided into 6 groups
[mock + vehicle (normal saline), mock + DDP, LNA-NC + vehicle,
LNA-NC + DDP, LNA-anti-miR196a + vehicle and LNA-anti-miR196a +
DDP; 8 mice/group]. The mice were injected with either
mock-transfected or stably-transfected SPC-A-1 cells by
subcutaneous injection (3×106 cells/0.2 ml). One day
after tumor cell implantation, the mice were treated with DDP [3.0
mg/kg; intraperitoneally (i.p.) every other day (qod)] or the
vehicle. Tumor volume was examined for 5 weeks and measured once a
week. The volume formed was calculated using the following formula:
volume = 0.4 × D × d2 (D, longitudinal diameter; d,
latitudinal diameter). All mice were sacrificed by exposure to
carbon dioxide and the tumors were harvested, and TUNEL staining
assay was then performed.
TUNEL staining assay
The tissues were harvested and plated on
polylysine-coated slides. The slides were then fixed with 4%
paraformaldehyde for 1 h at room temperature, and were then rinsed
with 0.1 M PBS, and finally permeabilized with 1% Triton X-100. DNA
fragmentation was detected using the TUNEL Apoptosis Detection kit
(KeyGen, Nanjing, China), which specifically labeled 3′-hydroxyl
termini of DNA strand breaks using fluorescein isothiocyanate
(FITC)-conjugated dUTP. The DNA was labeled with FITC DNA-binding
dye for 5 min. The FITC fluorescence was measured using a
fluorescence microscope. The percentage of apoptotic cells was
calculated as the number of apoptotic cells per the number of total
cells ×100.
Statistical analysis
In the present study, the data are expressed as the
means ± SD. Statistical analysis was performed using a t-test with
SPSS 13.0 software to evaluate the significance of differences
between groups. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
miR-196a expression is upregulated in
human NSCLC tissues and cell lines
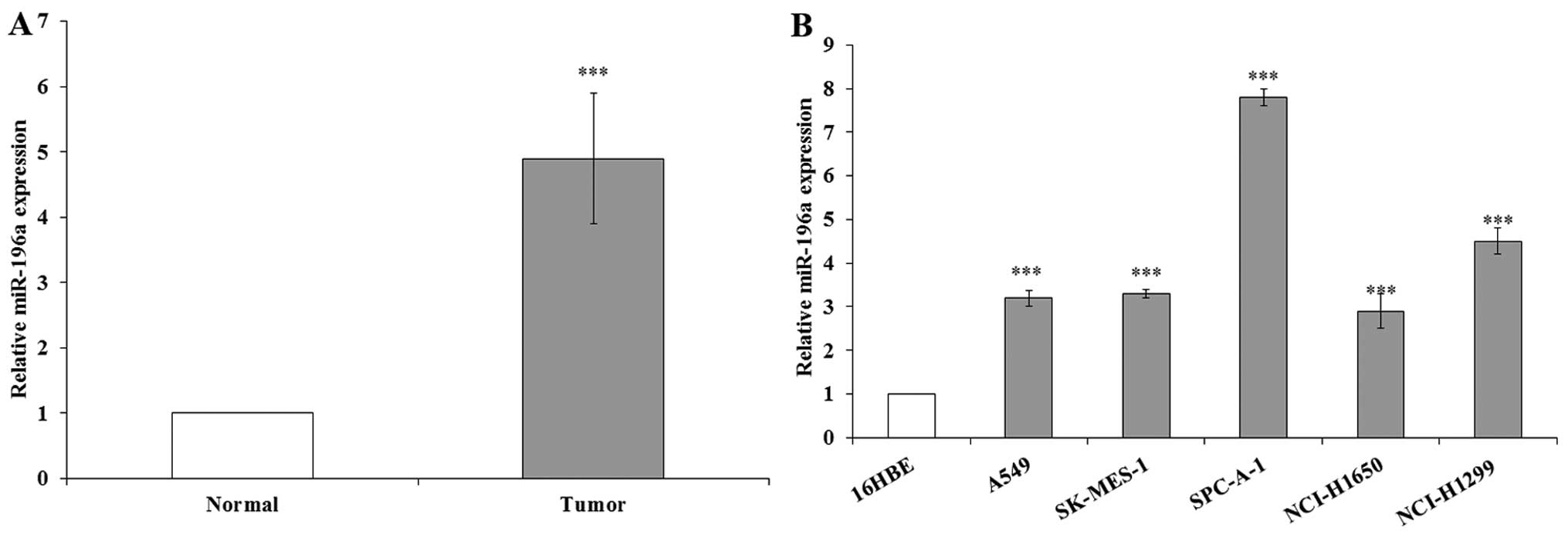
In this study, the miR-196a levels were measured in
23 NSCLC samples and adjacent normal tissues by RT-qPCR, and
normalized to U6. The results indicated that miR-196a expression
was significantly upregulated in the NSCLC samples compared with
the expression levels in the corresponding normal tissue samples
(Fig. 1A). We also performed
RT-qPCR to examine the expression of miR-196a in the human NSCLC
cell lines, including both adenocarcinoma and squamous carcinoma
subtypes. The results revealed that miR-196a expression was also
significantly upregulated in the human NSCLC cell lines compared
with the expression levels in the normal cell line, 16HBE (Fig. 1B). These results indicate that the
overexpression of miR-196a may play an important role in the
progression and development of NSCLC.
Manipulation of miR-196a levels in NSCLC
cells by performing LNA-anti-miR-196a oligonucleotide transfection
assay
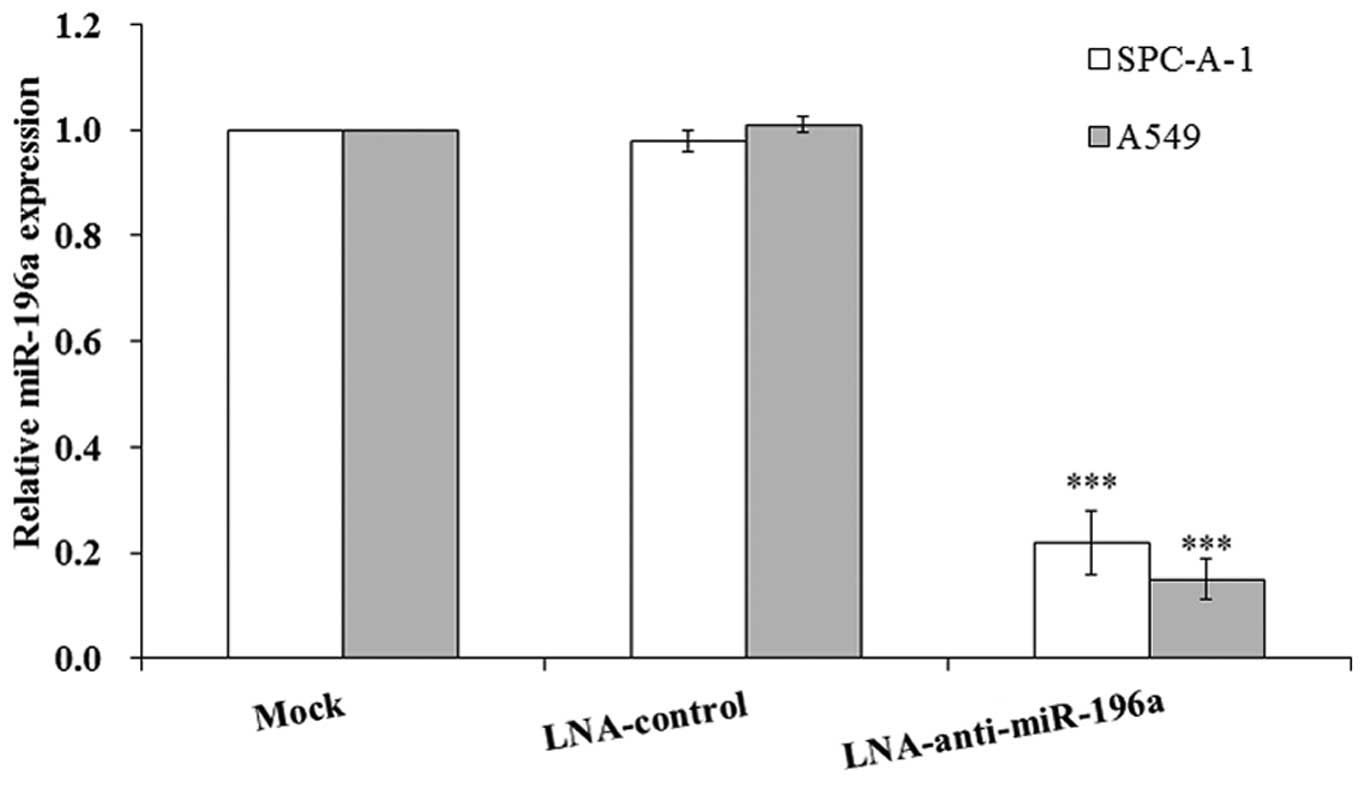
To selectively downregulate miR-196a in the NSCLC
cell lines, SPC-A-1/A549, the LNA-anti-miR-196a oligonucleotide
transfection assay was used in this study. The SPC-A-1/A549 cells
were transfected with LNA-anti-miR-196a or LNA-control
oligonucleotide; 48 h after transfection, the cells were harvested
and RT-qPCR was performed. The results revealed that the expression
of miR-196a was significantly downregulated by approximately
4-5-fold following transfection with LNA-anti-miR-196a
oligonucleotide compared with that in the mock-transfected cells
(Fig. 2); therefore, transfection
with LNA-anti-miR-196a oligonucleotide was used to manipulate the
miR-196a level to investigate the biological effects of miR-196a in
the subsequent experiments.
Effect of miR-196a on NSCLC cell
proliferation and apoptosis
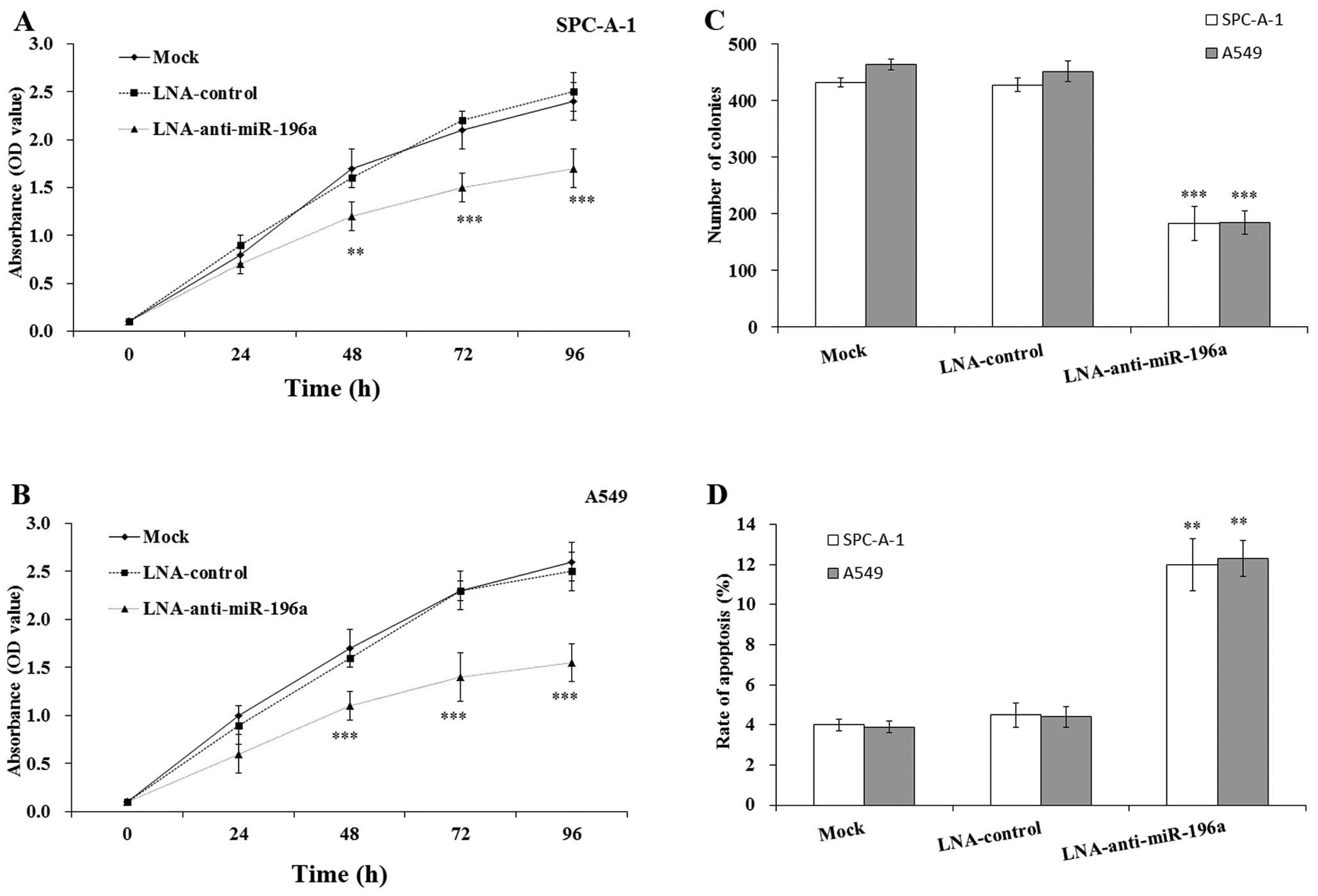
To examine the biological role of miR-196a in NSCLC
cells, we examined the effects of downregulating miR-196a on cell
proliferation and apoptosis by CCK8 assay, colony formation assay
and FACS analysis. As shown in Fig.
3A and B, the SPC-A-1 and A549 cells transfected with
LNA-anti-miR-196a oligonucleotide exhibited a significant decrease
in cell viability compared with the mock- or
LNA-control-transfected cells, particularly after 96 h
(p<0.001). Similarly, the results of colony formation assay
revealed that clonogenic survival was decreased following the
downregulation of miR-196a in the SPC-A-1 and A549 cells compared
with the the mock- or LNA-control-transfected cells (p<0.001;
Fig. 3C). To determine whether
apoptosis was a contributing factor to cell growth inhibition, we
performed flow cytometric analysis of the SPC-A-1 and A549 cells
following transfection with LNA-anti-miR-196a oligonucleotide. The
results revealed that the apoptotic rate was significantly
increased in the SPC-A-1/A549 cells transfected with
LNA-anti-miR-196a oligonucleotide compared with that in the mock-
or LNA-control-transfected cells (p<0.01) (Fig. 3D). Taken together, these results
indicate that the inhibition of miR-196a may inhibit the growth and
induce the apoptosis of SPC-A-1 and A549 cells.
Downregulation of miR-196a enhances the
sensitivity of NSCLC cells to DDP in vitro
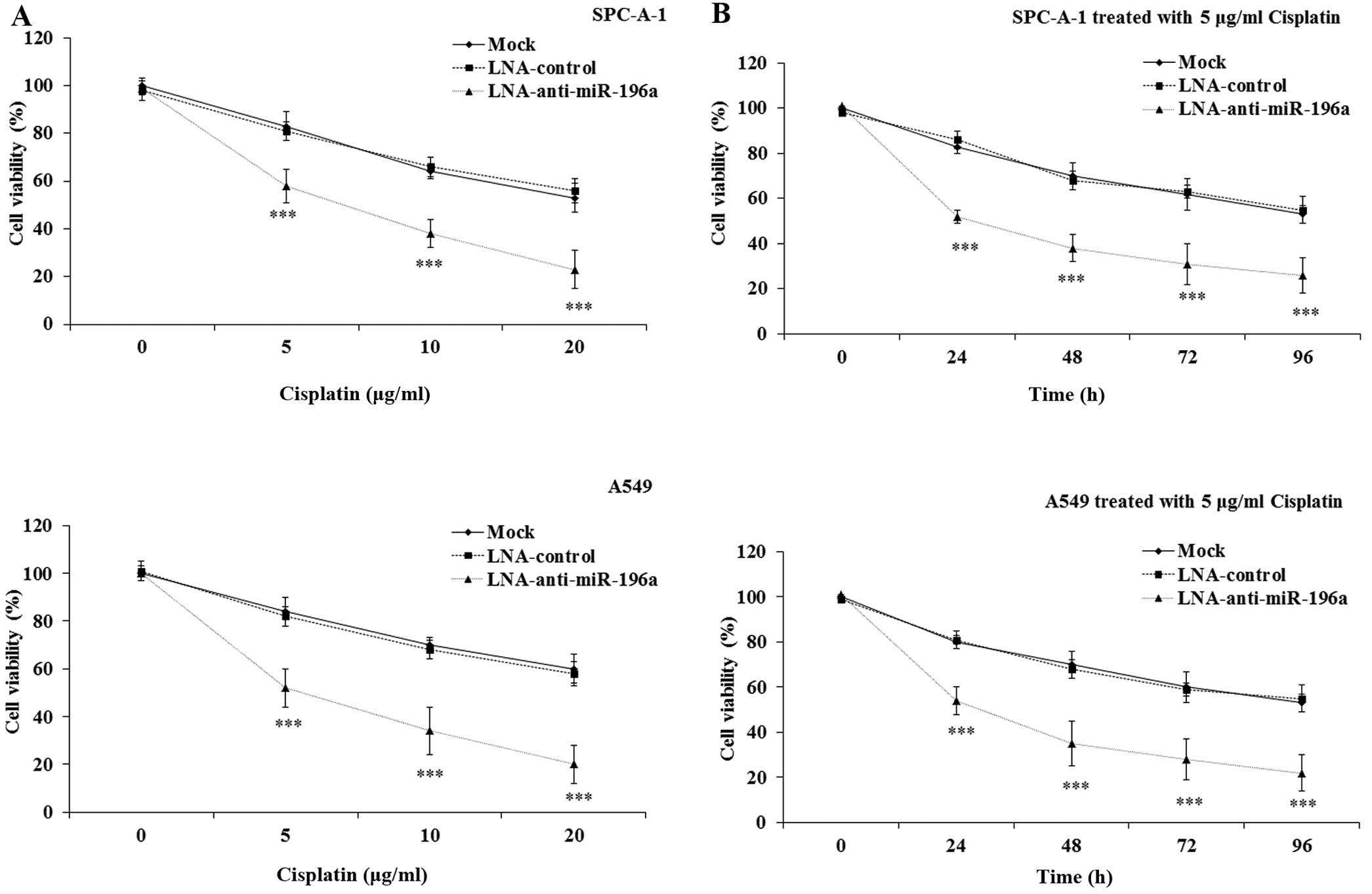
The dysregulation of miRNA expression has been
reported to be associated with chemoresistance in human cancers
(19). However, whether miR-196a
expression affects the sensitivity of NSCLC cells to DDP is not yet
fully understood. In the present study, we hypothesized that there
was an association between the dysregulation of miR-196a and the
sensitivity of NSCLC cells to DDP. To examine this hypothesis, the
mock-transfected SPC-A-1/A549 cells and the SPC-A-1/A549 cells
transfected with LNA-anti-miR-196a/LNA-control oligonucleotide were
treated with various concentrations (0, 5, 10 and 20 μg/ml)
of DDP for 12 h or 5 μg/ml of DDP for 0, 24, 48, 72 and 96
h. The CCK8 assay was performed to determine cell viability. The
results indicated that the downregulation of miR-196a led to a
significant decrease in the viability of the SPC-A-1/A549 cells
treated with DDP, in a dose- and time-dependent manner compared
with that in the LNA-control and the mock-transfected cells
(p<0.001; Fig. 4). These data
clearly demonstrate that the downregulation of miR-196a may
effectively enhance the sensitivity of NSCLC cells to DDP.
Downregulation of miR-196a enhances the
DDP-induced apoptosis of NSCLC cells
To determine whether apoptosis was a contributing
factor to the enhanced sensitivity of SPC-A-1/A549 cells to DDP, we
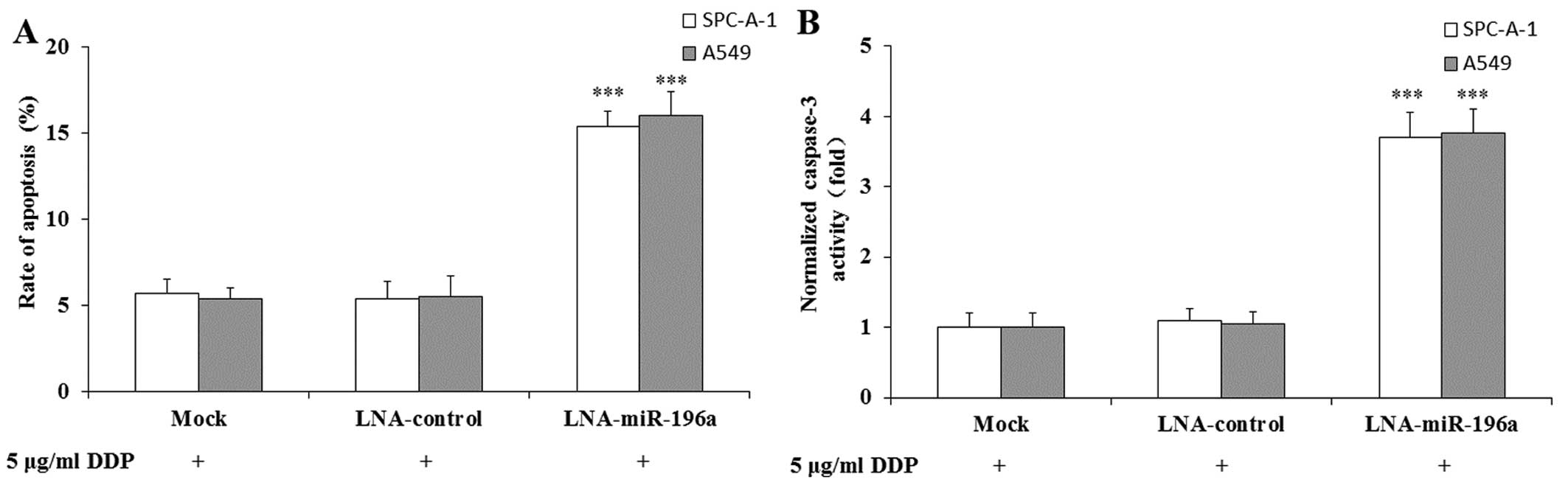
performed flow cytometric analysis. As shown in Fig. 5A, the apoptotic rate was
significantly increased in the SPC-A-1/A549 cells transfected with
LNA-anti-miR-196a oligonucleotide and treated with 5 μg/ml
DDP compared with that in the mock-transfected cells treated with 5
μg/ml DDP. The apoptotic rate of the SPC-A-1/A549 cells
transfected with LNA-control oligonucleotide and treated with DDP
did not differ significantly compared with that of the
mock-transfected cells treated with DDP. We then examined caspase-3
activity using a colorimetric assay. The results revealed that
caspase-3 activity in the SPC-A-1/A549 cells transfected with
LNA-anti-miR-196a oligonucleotide and treated with DDP
significantly increased compared with that in the mock- or
LNA-control-transfected cells treated with DDP (Fig. 5B). Therefore, the downregulation
of miR-196a may increase the sensitivity of SPC-A-1/A549 cells to
DDP by enhancing DDP-induced apoptosis.
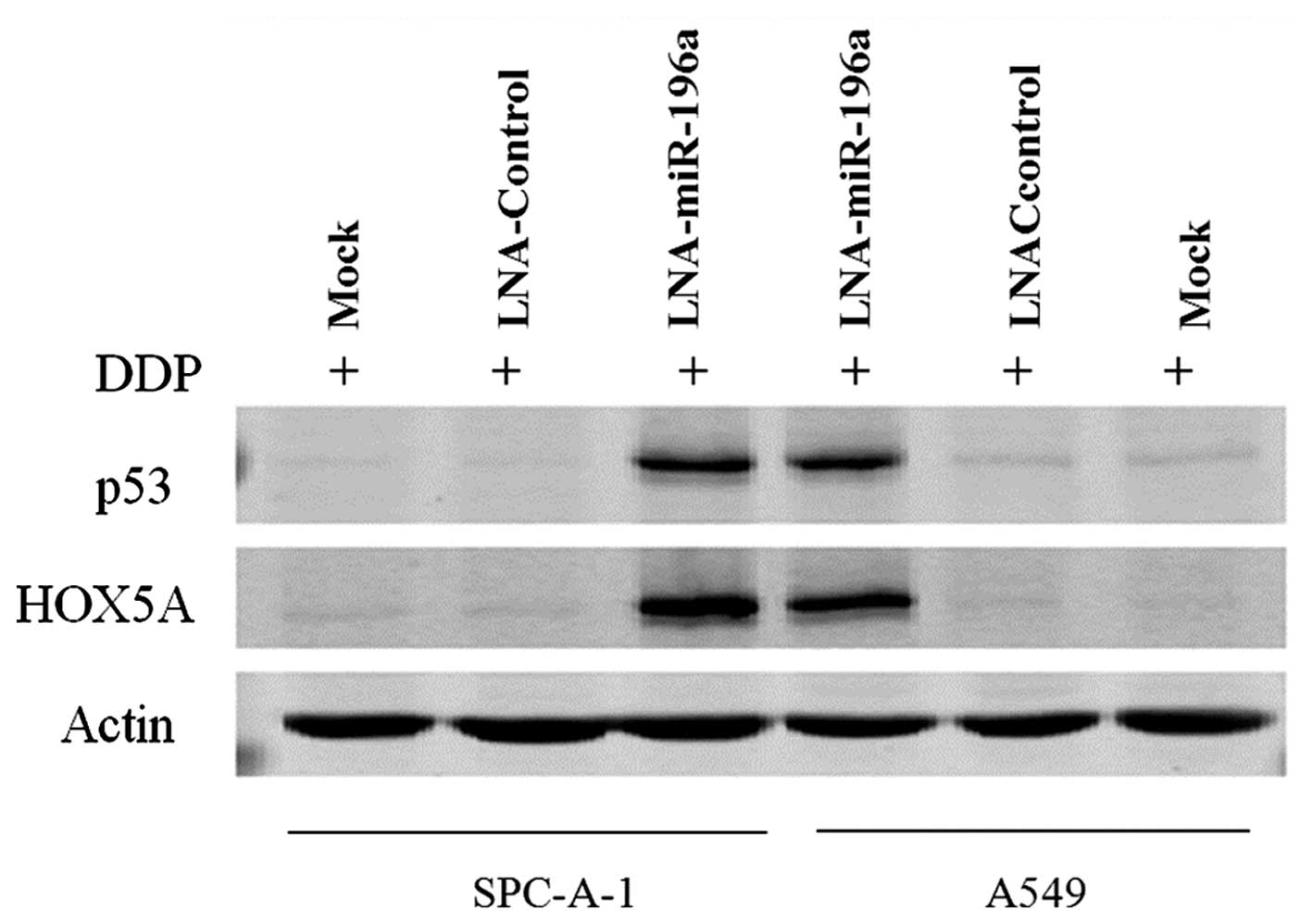
Downregulation of miR-196a enhances HOXA5
expression in NSCLC cells
A previous study indicated that miR-196a promotes
NSCLC cell proliferation and invasion by targeting HOXA5 (14). Raman et al reported that
the overexpression of HOXA5 induced cell apoptosis and the
overexpression of p53 concomitantly (20). Thus, to determine whether the
apoptosis induced by the downregulation of miR-196a was affected by
HOXA5, we performed western blot analysis to determine the HOXA5
and p53 levels in the cisplatin-treated NSCLC cells. The results
revealed that the downregulation of miR-196a enhanced the
expression of HOXA5 and p53 in the SPC-A-1/A549 cells treated with
5 μg/ml DDP (Fig. 6).
RT-qPCR was then performed to determine the mRNA levels of HOXA5
and p53, and the results of RT-qPCR were similar to those of
western blot analysis (data not shown). Therefore, the
downregulation of miR-196a induced the expression of HOXA5 and p53
at the protein and mRNA level. Taken together, these findings
suggest that the downregulation of miR-196a enhances the
sensitivity of NSCLC cells to DDP through apoptotic signaling by
targeting HOXA5. We aim to elucidate the precise mechanism of this
interaction with apoptotic signaling in future studies.
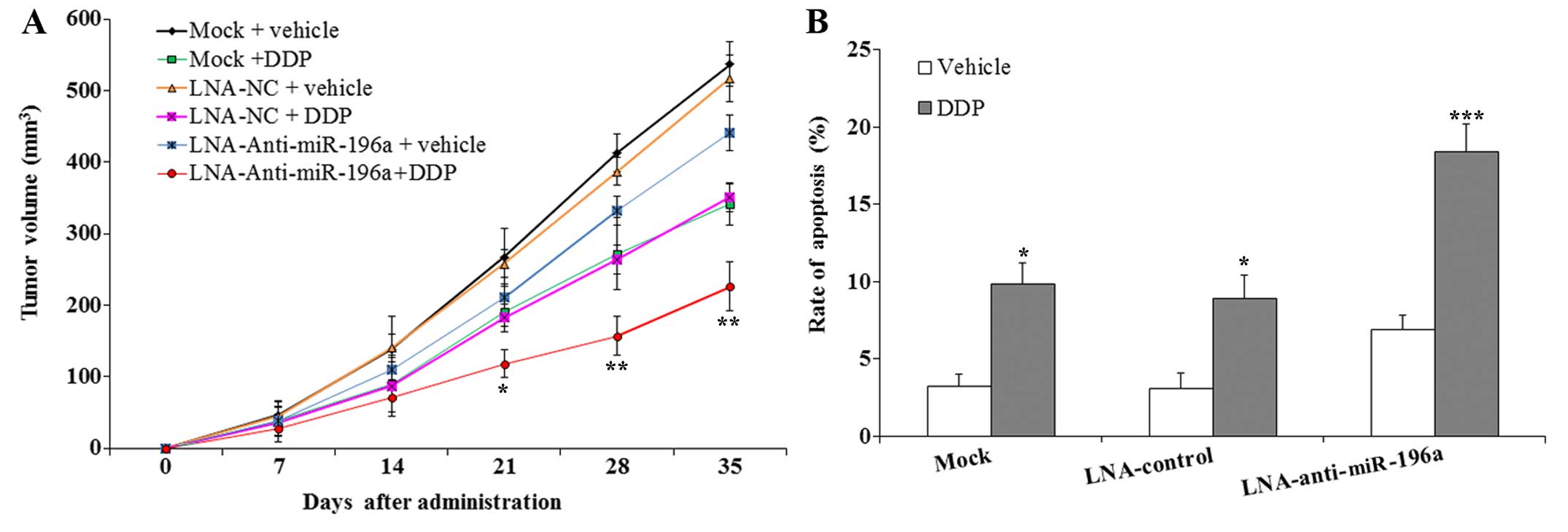
Downregulation of miR-196a enhyances the
sensitivity of SPC-A-1 cells to DDP in vivo
To examine the effects of downregulating miR-196a on
the sensitivity of SPC-A-1 cells to DDP in vivo, nude mice
were subcutaneously injected with SPC-A-1 cells to form tumors.
This was followed by the administration of either DDP or the
vehicle. The results revealed that the tumors formed following
transfection with LNA-anti-miR-196a oligonucleotide grew
significantly slower than those from the mice injected with the
mock- or LNA-control-transfected SPC-A-1 cells (Fig. 7A). Following treatment with DDP,
the inhibition of tumor growth in the mice injected with the
SPC-A-1 cells transfected with LNA-anti-miR-196a oligonucleotide
was much greater than that in the mice injected with mock- or
LNA-control-transfected SPC-A-1 cells (p<0.05). The results of
TUNEL assay revealed that the apoptotic rate in the tumors of mice
injected with the SPC-A-1 cells transfected with LNA-anti-miR-196a
oligonucleotide was significantly higher than that of the tumors
from the mice injected with the mock- or LNA-control-transfected
SPC-A-1 cells following treatment with DDP (p<0.001; Fig. 7B). These data clearly indicate
that the downregulation of miR-196a may effectively enhance the
sensitivity of SPC-A-1 cells to DDP by inducing apoptosis in
vivo.
Discussion
It is well known that miRNAs are a series of small
(19–24 nt in length), non-coding RNAs which are involved in
post-transcriptional gene regulation or degradation (21,22). In a wide range of plant and animal
cells, miRNAs have been shown to play an important role in various
processes, including cell proliferation, differentiation and
metabolism (23,24). They bind to target mRNAs at the
3′-untranslated region (UTR) and/or 5′-UTR of target mRNA, to block
translation or contribute to target mRNA degradation (25). There is increasing evidence to
suggest that the deregulation of miRNAs frequently occurs in tumor
tissues, and that they target genes involved in cancer cell
proliferation, differentiation, apoptosis, metastasis and
resistance (26–29). It has also been demonstrated that
miRNAs play an important role in modulating sensitivity and
resistance to anticancer drugs in substantial ways (19).
Recently, the function of miR-196a in the
pathogenesis of tumors has been widely investigated; a number of
studies have suggested that miR-196a exhibits an oncogenic function
in cancer (30–33,40). Higher levels of miR-196a have been
found in pancreatic cancer, leukemia and esophageal adenocarcinoma,
and have been shown to be negatively associated with survival
(34–38). In esophageal cancer, miR-196a
overexpression has been shown to promote cell proliferation and to
suppress apoptosis by directly regulating annexin A1 (39). In colorectal cancer, high levels
of miR-196a have been shown to promote cancer cell detachment,
migration, invasion and chemosensitivity, and to promote the
development of lung metastases in mice by activating the Akt
signaling pathway (11,40). However, the mechanisms responsible
for the chemosensitivity mediated by miR196a have not yet been
clearly defined and evidence of an association between miR-196a and
chemoresistance remains limited. In addition, the potential
involvement of miR-196a in the induction of drug resistance,
particularly in resistance to DDP, remains to be determined.
Inspired by the above-mentioned observations, in this study, we
investigated the biological role of miR-196a in mediating
resistance to DDP in NSCLC cells.
In this study, we demonstrated that miR-196a
expression was upregulated in NSCLC tissue samples and NSCLC cell
lines. In addition, we attempted to explore the role of miR-196a in
NSCLC; the results revealed that the targeted knockdown of miR-196a
expression in NSCLC cells led to the significant inhibition of cell
proliferation and colony formation by inducing caspase-3-dependent
apoptosis. Furthermore, we found that the downregulation of
miR-196a significantly enhanced the sensitivity of NSCLC cells to
DDP in vitro and in vivo, by targeting HOXA5.
However, further studies exploring the role of miR-196a in the
regulation of NSCLC cell growth and metastasis are warranted in the
future.
miR-196a plays critical roles in the pathogenesis of
cancer by targeting several genes, including high mobility group
AT-hook 2 (HMGA2), annexin A1 and HOX; however, miR-196a is not
only expressed in cancer cells, but also in normal cells (40–43), and the functions and targets of
miR-196a have not yet been fully analyzed. The downregulation of
miR-196a may induce abnormal gene expression in normal cells. As a
potential concern, the miRNA antagonist used may also
non-specifically bind to other RNAs, and one miRNA may have
multiple target genes due to the sequence homology of the binding
sites, which may result in unwanted side-effects. It has been
reported that single-nucleotide polymorphism (SNP) in miRNA-196a is
associated with severe toxicity in lung cancer patients,
particularly in individuals treated with cisplatin or gemcitabine
(44). In spite of the multiple
potential side-effects, miR-196a gene therapy may prove to be a
novel multimodality treatment for NSCLC with continued
research.
Taken together, the findings of the present suggest
that the downregulation of miR-196a enhances the sensitivity of
NSCLC cells to DDP both in vitro and in vivo. Thus,
the appropriate application of DDP in combination with the
regulation of miR-196a expression may prove to be a potential
therapeutic strategy for the treatment of NSCLC in the future.
References
|
1
|
World Cancer Report 2014. World Health
Organization. Chapter 1.1. 2014
|
|
2
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I; EUROCARE-4 Working
Group: Recent cancer survival in Europe: a 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kostova I: Platinum complexes as
anticancer agents. Recent Pat Anticancer Drug Discov. 1:1–22. 2006.
View Article : Google Scholar
|
|
6
|
Liu Y, Li M, Zhang G and Pang Z:
MicroRNA-10b overexpression promotes non-small cell lung cancer
cell proliferation and invasion. Eur J Med Res. 18:412013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma Q, Jiang Q, Pu Q, Zhang X, Yang W, Wang
Y, Ye S, Wu S, Zhong G, Ren J, et al: MicroRNA-143 inhibits
migration and invasion of human non-small-cell lung cancer and its
relative mechanism. Int J Biol Sci. 9:680–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT
and Zhang CP: MicroRNAs contribute to the chemoresistance of
cisplatin in tongue squamous cell carcinoma lines. Oral Oncol.
46:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorrentino A, Liu C-G, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hui AB, Shi W, Boutros PC, Miller N,
Pintilie M, Fyles T, McCready D, Wong D, Gerster K, Waldron L, et
al: Robust global micro-RNA profiling with formalin-fixed
paraffin-embedded breast cancer tissues. Lab Invest. 89:597–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun M, Liu XH, Li JH, Yang JS, Zhang EB,
Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, et al: MiR-196a is
upregulated in gastric cancer and promotes cell proliferation by
downregulating p27(kip1). Mol Cancer Ther. 11:842–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang F, Tang J, Zhuang X, Zhuang Y, Cheng
W, Chen W, Yao H and Zhang S: MiR-196a promotes pancreatic cancer
progression by targeting nuclear factor kappa-B-inhibitor alpha.
PLoS One. 9:e878972014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XH, Lu KH, Wang KM, Sun M, Zhang EB,
Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, et al: MicroRNA-196a
promotes non-small cell lung cancer cell proliferation and invasion
through targeting HOXA5. BMC Cancer. 12:3482012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du W, Ma XL, Zhao C, Liu T, Du YL, Kong
WQ, Wei BL, Yu JY, Li YY, Huang JW, et al: Associations of single
nucleotide polymorphisms in miR-146a, miR-196a, miR-149 and miR-499
with colorectal cancer susceptibility. Asian Pac J Cancer Prev.
15:1047–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffman AE, Zheng T, Yi C, Leaderer D,
Weidhaas J, Slack F, Zhang Y, Paranjape T and Zhu Y: microRNA
miR-196a-2 and breast cancer: a genetic and epigenetic association
study and functional analysis. Cancer Res. 69:5970–5977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu H, Zhu X, Cheng G, Zhou M and Lou W:
Downregulation of microRNA-21 enhances radiosensitivity in
nasopharyngeal carcinoma. Exp Ther Med. 9:2185–2189.
2015.PubMed/NCBI
|
|
18
|
Zhang SZ, Pan FY, Xu JF, Yuan J, Guo SY,
Dai G, Xue B, Shen WG, Wen CJ, Zhao DH and Li CJ: Knockdown of
c-Met by adenovirus-delivered small interfering RNA inhibits
hepatocellular carcinoma growth in vitro and in vivo. Mol Cancer
Ther. 4:1577–1584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raman V, Martensen SA, Reisman D, Evron E,
Odenwald WF, Jaffee E, Marks J and Sukumar S: Compromised HOXA5
function can limit p53 expression in human breast tumours. Nature.
405:974–978. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
et al: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Cell. 124:1169–1181.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu L, Fan J and Belasco JG: MicroRNAs
direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA.
103:4034–4039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amaral JD, Xavier JM, Steer CJ and
Rodrigues CM: Targeting the p53 pathway of apoptosis. Curr Pharm
Des. 16:2493–2503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dykxhoorn DM: MicroRNAs and metastasis:
little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar
|
|
29
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar
|
|
30
|
Chen C, Zhang Y, Zhang L, Weakley SM and
Yao Q: MicroRNA-196: critical roles and clinical applications in
development and cancer. J Cell Mol Med. 15:14–23. 2011. View Article : Google Scholar
|
|
31
|
Suh YE, Raulf N, Gäken J, Lawler K, Urbano
TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M:
MicroRNA-196a promotes an oncogenic effect in head and neck cancer
cells by suppressing annexin A1 and enhancing radioresistance. Int
J Cancer. 137:1021–1034. 2015. View Article : Google Scholar
|
|
32
|
Yang G, Han D, Chen X, Zhang D, Wang L,
Shi C, Zhang W, Li C, Chen X, Liu H, et al: MiR-196a exerts its
oncogenic effect in glioblastoma multiforme by inhibition of IκBα
both in vitro and in vivo. Neuro Oncol. 16:652–661. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Zheng F, Yu G, Yin Y and Lu Q:
miR-196a targets netrin 4 and regulates cell proliferation and
migration of cervical cancer cells. Biochem Biophys Res Commun.
440:582–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce
CM: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szafranska AE, Doleshal M, Edmunds HS,
Gordon S, Luttges J, Munding JB, Barth RJ Jr, Gutmann EJ,
Suriawinata AA, Marc Pipas J, et al: Analysis of microRNAs in
pancreatic fine-needle aspirates can classify benign and malignant
tissues. Clin Chem. 54:1716–1724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Chen J, Chang P, LeBlanc A, Li D,
Abbruzzesse JL, Frazier ML, Killary AM and Sen S: MicroRNAs in
plasma of pancreatic ductal adenocarcinoma patients as novel
blood-based biomarkers of disease. Cancer Prev Res (Phila).
2:807–813. 2009. View Article : Google Scholar
|
|
37
|
Wang Y, Li Z, He C, Wang D, Yuan X, Chen J
and Jin J: MicroRNAs expression signatures are associated with
lineage and survival in acute leukemias. Blood Cells Mol Dis.
44:191–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luthra R, Singh RR, Luthra MG, Li YX,
Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, et al:
MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of
annexin A1 downregulation in cancers. Oncogene. 27:6667–6678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schimanski CC, Frerichs K, Rahman F,
Berger M, Lang H, Galle PR, Moehler M and Gockel I: High miR-196a
levels promote the oncogenic phenotype of colorectal cancer cells.
World J Gastroenterol. 15:2089–2096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L,
Zeng Y, Miao R, Jin G, Ma H, et al: Genetic variants of miRNA
sequences and non-small cell lung cancer survival. J Clin Invest.
118:2600–2608. 2008.PubMed/NCBI
|
|
42
|
Hu Z, Liang J, Wang Z, Tian T, Zhou X,
Chen J, Miao R, Wang Y, Wang X and Shen H: Common genetic variants
in pre-microRNAs were associated with increased risk of breast
cancer in Chinese women. Hum Mutat. 30:79–84. 2009. View Article : Google Scholar
|
|
43
|
Yekta S, Shih IH and Bartel DP:
MicroRNA-directed cleavage of HOXB8 mRNA. Science. 304:594–596.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhan X, Wu W, Han B, Gao G, Qiao R, Lv J,
Zhang S, Zhang W, Fan W, Chen H, et al: Hsa-miR-196a2 functional
SNP is associated with severe toxicity after platinum-based
chemotherapy of advanced nonsmall cell lung cancer patients in a
Chinese population. J Clin Lab Anal. 26:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|