Introduction
Acute lung injury (ALI) is the basis of acute
respiratory distress syndrome (ARDS) and is involved in the
development of multiple organ failure, which leads to death in
patients with sepsis, shock and pneumonia (1–3).
ALI is characterized by neutrophil accumulation in the lung, lung
tissue destruction, lung edema and diffuse lung inflammation, which
is accompanied by the overexpression of inflammatory mediators,
including cytokines and chemokines (4,5).
The search for an effective ALI therapy has been ongoing for
decades (6,7). However, there are few effective
therapies for ALI (8). Therefore,
it is essential to develop novel effective therapies for ALI.
Lipopolysaccharide (LPS), a constituent of the outer
membrane of gram-negative bacteria, is a key pathogen in animal
models of ALI (9,10). Previous studies have reported that
LPS induces direct/indirect neutrophil and macrophage activation,
resulting in the release of pro-inflammatory mediators (11,12). This cascade eventually aggravates
inflammatory responses on various lesions that have been damaged by
stimuli.
Previous findings have demonstrated that LPS exerts
its inflammatory responses by activating specific inflammatory
signaling pathways, such as the nuclear factor-κB (NF-κB) signaling
pathway. NF-κB, a central regulator of inflammation, and the NF-κB
signaling pathway are considered to be involved in the pathogenesis
of ALI (12). Once NF-κB is
activated, NF-κB signaling proceeds through phosphorylation and the
subsequent phosphorylation and degradation of the inhibitor of κB
(IκB), resulting in the cytoplasmic release and nuclear
translocation of NF-κB (13,14). Thus, NF-κB activation induces the
transcription of pro-inflammatory mediators. These mediators
include tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6,
cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2)
(15–18), which play critical roles in ALI
(12). Thus, NF-κB is a potential
therapeutic target in ALI.
Clausena anisata (Willd.) Hook.f. ex Benth.
(CA), locally known as 'isifudu' in the South African isiZulu
language, is widely used in traditional medicine to treat many
diseases. Various parts of CA, such as leaves, roots and bark, are
reportedly useful as effective remedies against parasitic
infections, eye complaints, heart disorders, hypertension,
constipation, gastroenteritis, malaria, other febrile conditions,
other inflammatory conditions, toothaches, swollen gums, mental
disorders, impotence and sterility (19–25).
However, the anti-inflammatory effect of CA has yet
to be studied in ALI. Thus, the present study aimed to evaluated
the effects of CA, and to elucidate the underlying mechanisms of
action of CA, in a mouse model of LPS-induced ALI and in
LPS-stimulated RAW 264.7 cells.
Materials and methods
Preparation of plant extract
Clausena anisata (Willd.) Hook.f. ex Benth.
of the family Rutaceae was collected from Ninh Thuan province,
Thuan Bac district, Cong Hai, Vietnam. Plant samples were
identified by Tran-The Bach, Institute of Ecology and Biological
Resources. Voucher specimens (KRIB 0034836,7,8) have been deposited
in the herbarium (KRIB) of the Korea Research Institute of
Bioscience and Biotechnology (KRIBB). After grinding the twigs and
leaves of CA, the powder (220 g) was treated with MeOH and
sonicated several times at room temperature for 3 days to produce
an extract (8.61 g). CA extract was dissolved in DMSO and stored at
−20°C.
In vitro experiment
Cell culture
RAW 264.7 macrophages were maintained at
1×105 cells/ml in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% heat-inactivated fetal bovine serum
(FBS) (both from Sigma-Aldrich, St. Louis, MO, USA) and 1% (w/v)
antibiotic-antimycotic solution (Invitrogen, Grand Island, NY, USA)
in a 95% air and 5% CO2 humidified atmosphere at 37°C.
The cells were seeded in 96-well plates at 5×104
cells/well and incubated in serum-free medium in the presence of
different CA concentrations (5, 10, 20 and 40 µg/ml). CA was
obtained from the Plant Extract Bank at the Korea Research
Institute of Bioscience and Biotechnology (KRIBB) (BVN12106;
Daejeon, Korea).
Cell viability assay
Cell viability was evaluated by determining the
mitochondrial reductase function using with an assay based on the
reduction of tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(CAS#298-93-1; Amresco, LLC, Solon, OH, USA) into formazan
crystals. Formazan formation is proportional to the number of
functional mitochondria in living cells. To determine cell
viability, 50 mg/ml of MTT was added to 5 µl of cell
suspension (1×105 cells/ml in 96-well plates) for 4 h.
The formazan formed was dissolved in acidic 2-propanol, and the
optical density was measured at 570 nm using a microplate reader
(Benchmark; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
optical density of formazan formed in the control (untreated) cells
was set to 100% viability.
Measurement of nitric oxide (NO)
production
NO production in RAW 264.7 cell culture supernatants
was determined using the Griess reaction. The cells were incubated
in the presence of LPS (0.5 µg/ml) at 37°C for 24 h. The
cells were then dispensed into 96-well plates, and 100 µl of
each supernatant was mixed with the same volume of Griess reagent
(1% sulfanil-amide, 0.1% N-(1-naphathyl)-ethylenediamine
dihydrochloride and 5% phosphoric acid) and agitated gently for 10
min at room temperature. The nitrite concentration was measured at
540 nm using sodium nitrite to generate a standard curve.
Measurement of PGE2 and
pro-inflammatory cytokine production
Cultures were serum-starved for 24 h and treated
with experimental agents. Following culture incubation under
experimental conditions, the media from each sample were collected
and assessed for IL-6 synthesis using a commercial enzyme-linked
immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis,
MN, USA) according to the manufacturer's protocol, and the assay
was calibrated spectrophotometrically with a standard curve.
Supernatant PGE2 levels were determined using a
PGE2 enzyme immuno assay (EIA) kit (Cayman Chemical Co.,
Ann Arbor, MI, USA) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was reverse transcribed using AccuPower RT
Premix (Bioneer Corporation, Daejeon, Korea) according to the
manufacturer's instructions. The resulting cDNA (equivalent to 40
ng total RNA) was used for qPCR using FastStart DNA
MasterPLUS SYBR-Green I and LightCycler (both from Roche
Diagnostics, Indianapolis, IN, USA) according to the manufacturer's
instructions. qPCR was performed using the primers: COX-2, sense,
5′-GAAGTCTTTGGTCTGGTCTCCTG-3′ and antisense,
5′-GTCTGCTGGTTTGGAATAGTTGC-3′; and β-actin sense,
5′-CGCTCATTGCCGATAGTGAT-3′ and antisense
5′-TGTTTGAGACCTTCAACACC-3′. All of the samples were normalized to
β-actin. qPCR products were subjected to electrophoresis on 1.5%
agarose gels and stained with ethidium bromide. Images were
captured with an Olympus C4000 zoom camera system (Olympus America
Inc., Melville, NY, USA).
Western blot analysis
Cells (1×106) were harvested and washed
twice in cold Tris-buffered saline (TBS). The cells were
solubilized in ice-cold 1% Triton X-100 lysis buffer. After 30 min
on ice, the lysates were clarified by centrifugation at 600 × g for
20 min. Proteins (20 µg) were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%
acrylamide) and transferred to nitrocellulose membranes. The
membranes were incubated with blocking solution (5% skim milk) and
then incubated overnight at 4°C with the appropriate primary
antibodies. The primary antibodies and dilutions used were:
anti-β-actin (1:2,000 dilution; Cat. no. P60709; Cell Signaling
Technology, Inc., Beverly, MA, USA) and anti-COX-2 (1:1,000
dilution; Cat. no. SC-1745; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). The membranes were washed three times with TBS
containing Tween-20 (TBST) and then incubated with a 1:3,000
dilution of horseradish peroxidase (HRP)-conjugated secondary
antibodies (Cat. no. SC-2027; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. The membranes were again washed three times
with TBST and then developed using an enhanced chemiluminescence
(ECL) kit (Thermo Fisher Scientific, Waltham, MA, USA). For
quantitative analysis, densitometric band values were determined
using ChemiDoc (Bio-Rad Laboratories, Inc.).
In vivo experiment
Animals
Male C57BL/6 mice (6–8 weeks of age) were obtained
from Orient Co. (Seoul, Korea). The mice were provided with food
and water ad libitum in an animal facility at a temperature
of 22–24 °C and on a 12 h light/dark cycle under specific
pathogen-free conditions. The mice were housed for a minimum of one
week for environmental adaptation prior to experimentation.
Experimental procedures were approved by the Institutional Animal
Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology.
LPS-induced ALI in mice
Specific pathogen-free male C57BL/6 mice were
randomly divided into four groups (n=7/group): i) NC (normal
control); ii) LPS (LPS treatment); iii) Dex (LPS treatment + oral
gavage of 5 mg/kg dexamethasone for 3 days); and iv) CA (LPS
treatment + oral gavage of 30 mg/kg CA for 3 days). The mice from
the control and LPS groups received an equal volume of
phosphate-buffered saline (PBS) instead of dexamethasone or CA.
Three hours after drug administration on the third day, the mice
were slightly anesthetized with diethyl ether inhalation, and 10
µg of LPS in 50 µl of PBS was instilled intranasally
(i.n.) to induce lung injury. The mice in the control group were
given 50 µl of PBS without LPS. Twenty-four hours after the
LPS treatment, the mice were sacrificed by an i.p. injection of
pentobarbital (50 mg/kg; Hanlim Pharm. Co. Ltd., Seoul, Korea) and
bronchoalveolar lavage fluid (BALF) and lung tissue samples were
harvested for further studies.
BALF collection and cell counting
BALF collection was performed three times through a
tracheal cannula with auto-claved PBS and instilled up to a total
volume of 1.4 ml. The BALF was centrifuged at 200 × g for 15 min at
4°C. Following centrifugation, the supernatant was stored at −70°C
for subsequent cytokine assays. The total inflammatory cell numbers
were assessed by counting cells in at least five squares of a
hemocytometer after excluding dead cells by trypan blue staining.
To determine differential cell counts, 100 µl of BALF was
centrifuged onto slides using a Cytospin (Hanil Science Industrial,
Seoul, Korea). After the slides were dried, the cells were fixed
and stained using Diff-Quik® staining reagent (B4132-1A;
IMEB Inc., Deerfield, IL, USA).
Measurement of reactive oxygen species
(ROS)
Detection of cellular ROS in inflammatory cells was
performed using 2′,7′-dichlorofluorescein diacetate (DCF-DA)
reagent as part of a kit purchased from Abcam (ab112851; Cambridge,
MA, USA). After cell counting, 100 µl of inflammatory cells
were seeded in 96-well plates. The cells were stained with 20
µM DCFDA for 30 min at 37°C. Fluorescence intensity was then
measured at 485 nm excitation and 530 nm emission using a
microplate reader (Bio-Rad Laboratories, Inc.).
Measurement of cytokine levels in
BALF
TNF-α, IL-1β and IL-6 levels in BALF were measured
using ELISA kits (BioSource International, Camarillo, CA, USA). The
procedures were performed according to the manufacturer's
instructions.
Histopathological lung
examination
After obtaining the BALF samples, lung tissue was
fixed in 10% (v/v) neutral-buffered formalin. Lung tissues were
embedded in paraffin, cut into 4-µm sections, and stained
with hematoxylin and eosin (H&E) solution (both from
Sigma-Aldrich; hematoxylin, MHS-16; eosin, HT110-1-32) to estimate
inflammation.
Western blot analysis
Lung tissues were harvested and homogenized (1/10
w/v) using a homogenizer and tissue lysis/extraction reagent
containing a protease inhibitor cocktail (both from Sigma-Aldrich).
Protein concentrations were determined using a Bradford reagent
(Bio-Rad Laboratories, Inc.,). Western blotting was performed as
described above, and the following primary antibodies and dilutions
used were: anti-IκB (1:1,000 dilution; Cell Signaling Technology,
Inc.,), anti-NF-κB/p65 (1:1,000 dilution; Santa Cruz Biotechnology,
Inc.) and anti-β-actin (1:2,000 dilution; Cell Signaling
Technology, Inc.).
Images captured and
photomicrography
The photomicrographs were obtained using a
Photometric Quantix digital camera running Windows, and images were
assembled in Adobe Photoshop 7.0. Images were cropped and corrected
for brightness and contrast but were not otherwise manipulated.
Statistical analysis
Data were presented as the means ± standard error of
the mean (SEM). Statistical significance was determined using
analysis of variance (ANOVA) followed by a multiple comparison test
with Dunnett's adjustment. P<0.05 was considered to indicate a
statistically significant difference.
Results
In vitro evaluation
Effects of CA on NO production in
LPS-stimulated RAW 264.7 cells
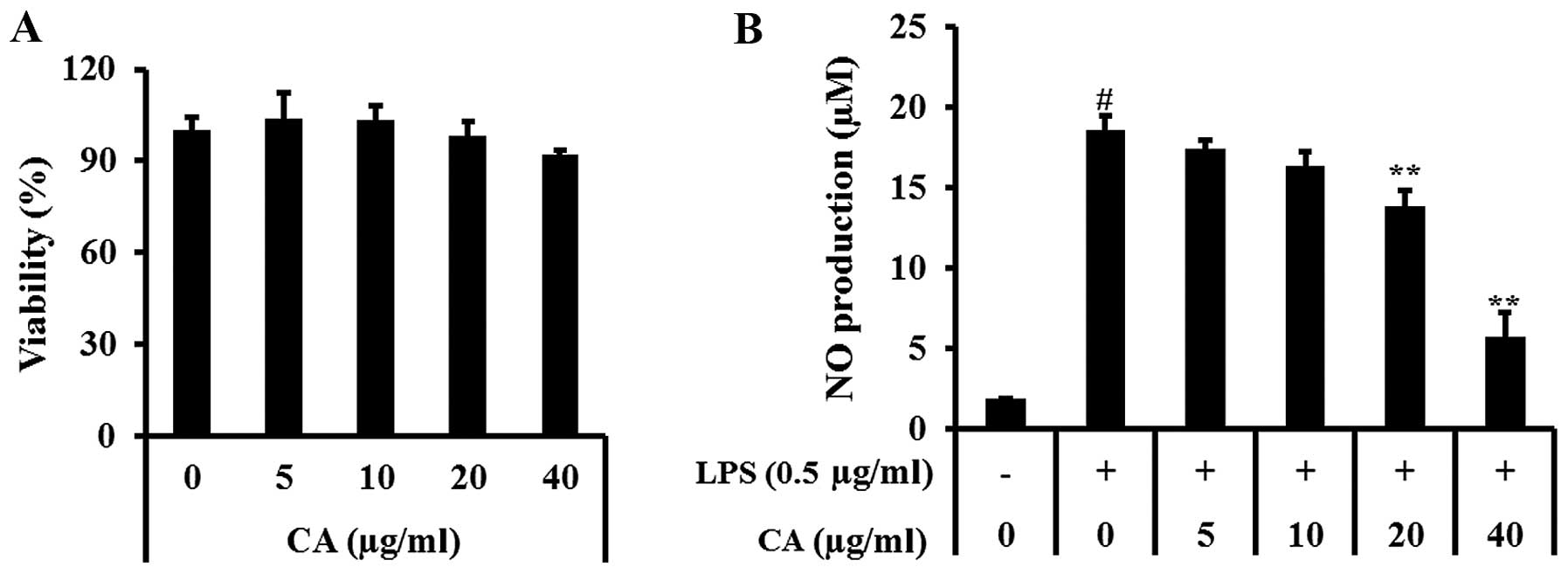
Non-toxic CA concentrations (5, 10, 20 and 40
µg/ml) (Fig. 1A) were
assessed for their ability to inhibit LPS-stimulated NO production.
NO production was significantly increased in LPS-stimulated RAW
264.7 cells. By contrast, CA at 20 and 40 µg/ml induced a
significant decrease in LPS-induced NO production; NO production
decreased in a concentration-dependent manner (Fig. 1B).
Effect of CA on PGE2 and
pro-inflammatory cytokine production in LPS-stimulated RAW 264.7
cells
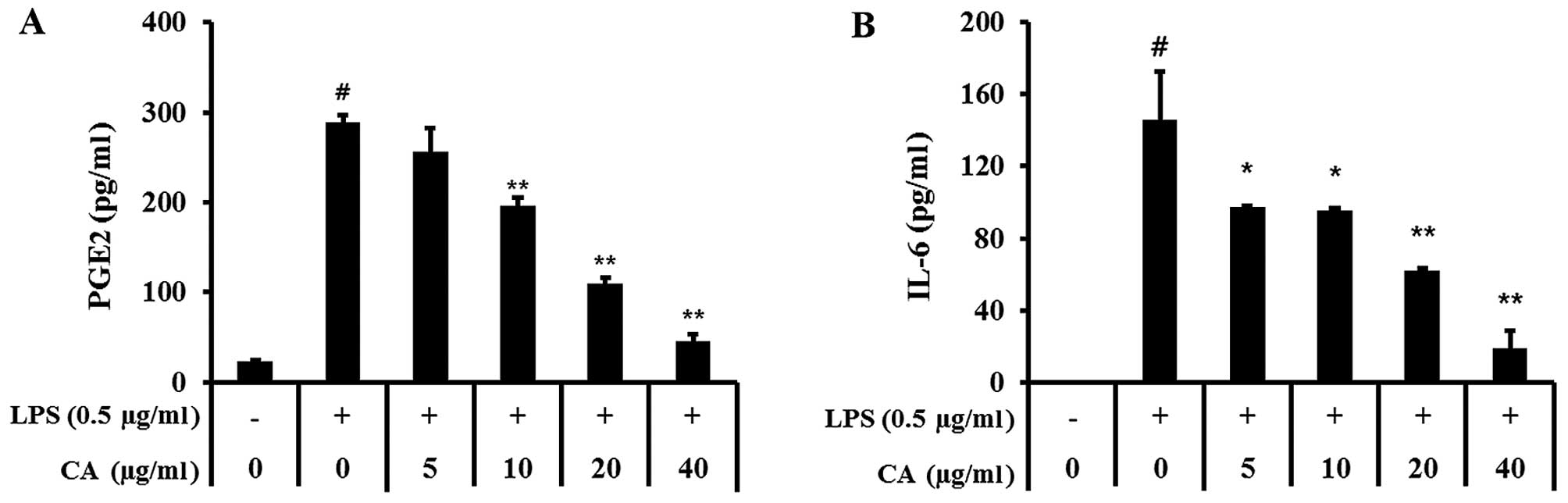
PGE2 levels in the culture supernatant
increased with LPS stimulation, and this effect was markedly
reduced by CA treatment (Fig.
2A). Treatment of RAW 264.7 cells with LPS alone resulted in a
significant increase in IL-6 production compared with the control
group (Fig. 2B). However, CA
treatment significantly inhibited LPS induction of IL-6, in a
dose-dependent manner.
Effect of CA on mRNA and protein
expression levels of inflammatory mediators
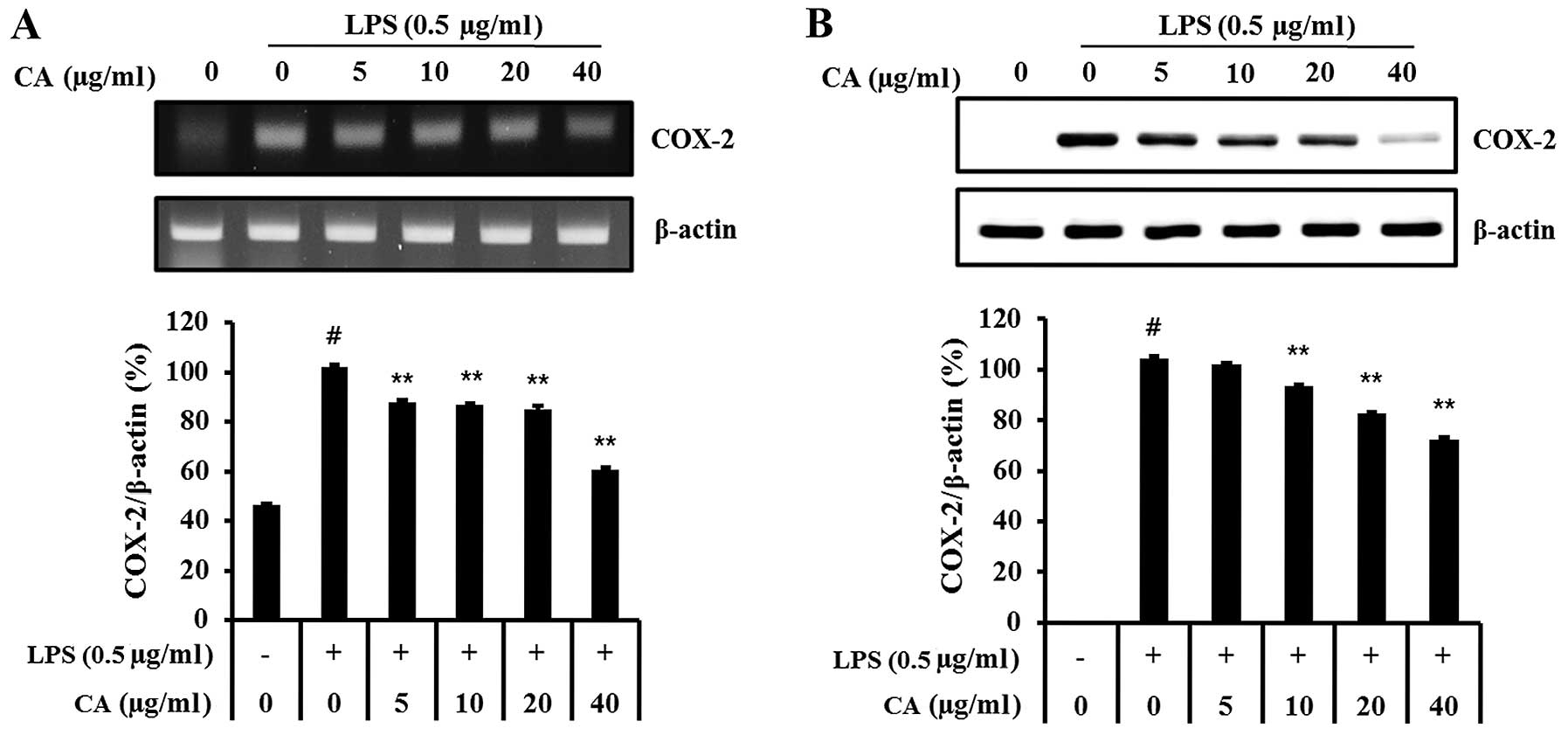
COX-2 mRNA and protein production was significantly
increased in LPS-stimulated RAW 264.7 cells (Fig. 3). However, CA-treated cells
exhibited significantly decreased COX-2 mRNA and protein levels
compared with LPS-stimulated cells in a concentration-dependent
manner.
In vivo experiment
Effect of CA on inflammatory cell
counts in the BALF isolated from mice with LPS-induced ALI
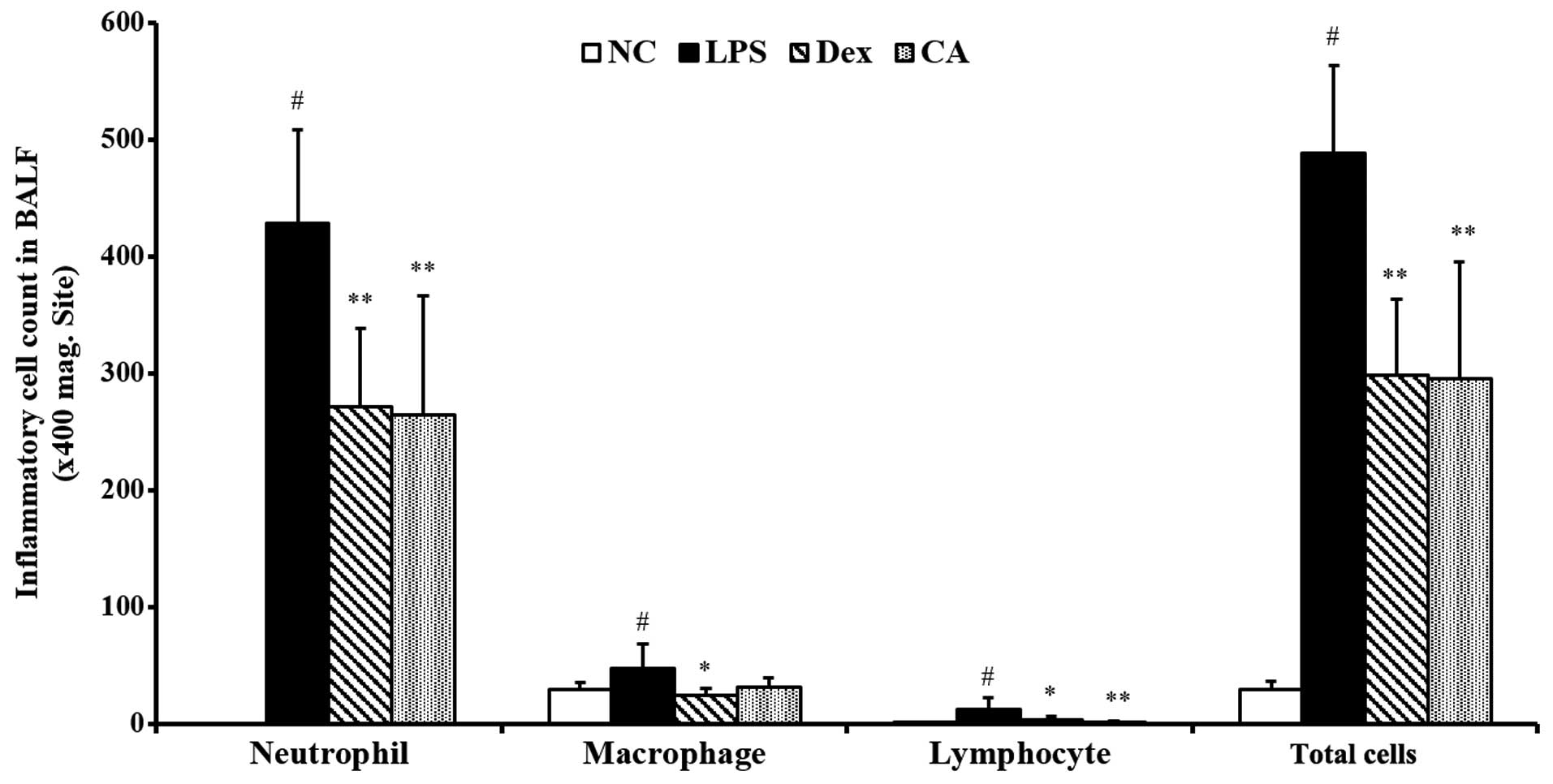
The total number of inflammatory cells in the BALF
comprised neutrophils, macrophages and lymphocytes. LPS challenge
significantly increased the number of total cells, neutrophils,
macrophages and lymphocytes compared with the control group.
However, BALF samples from the CA-treated mice exhibited a
significantly decreased influx of total cells, neutrophils,
macrophages and lymphocytes compared with the LPS-treated mice
(Fig. 4). The reductions were
similar to the results obtained in the dexa methasone-treated
mice.
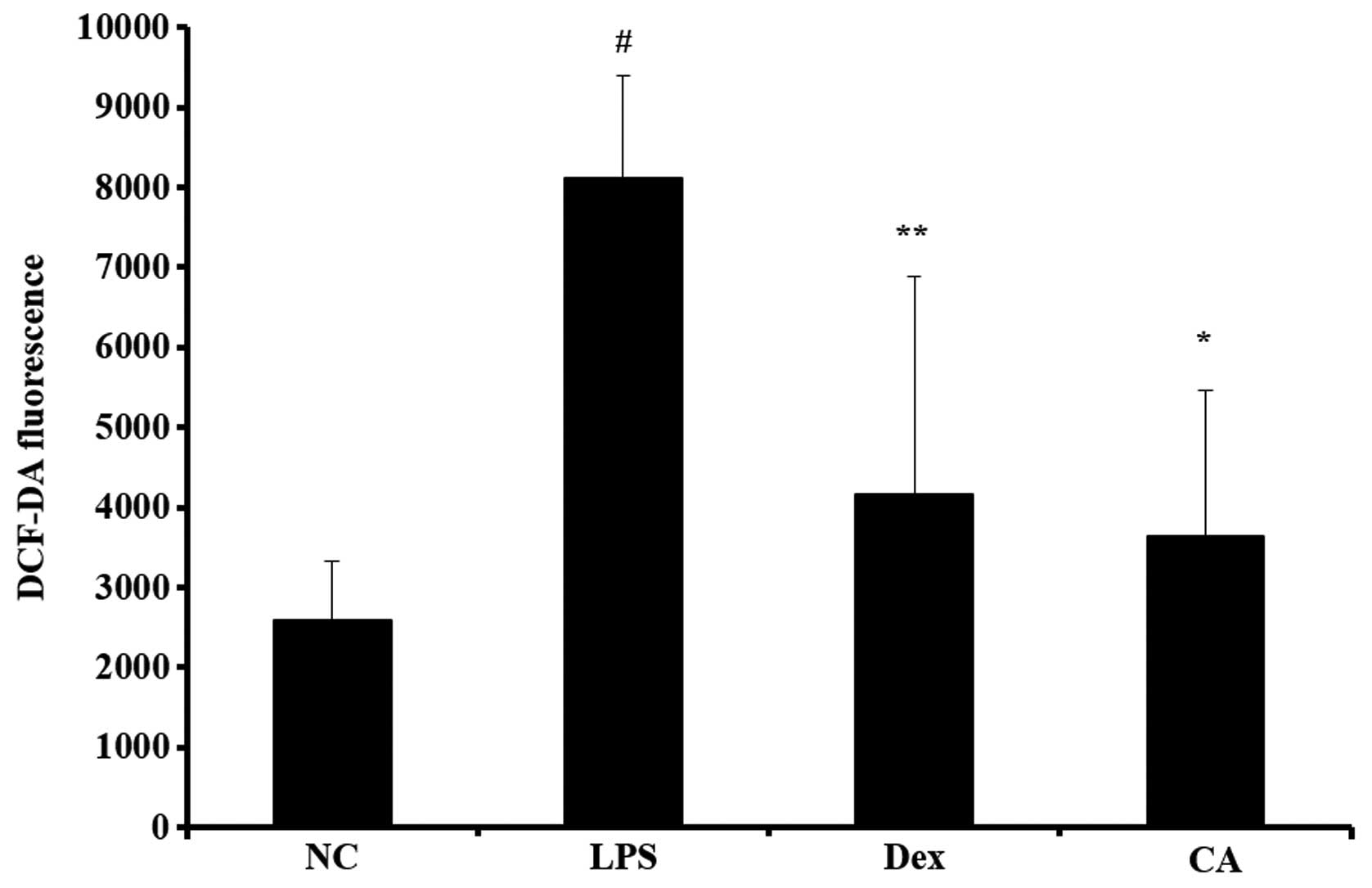
Effect of CA on ROS production in the
BALF isolated from mice with LPS-induced ALI
ROS levels in the BALF were significantly increased
in LPS-treated mice compared with the negative controls group
(Fig. 5). By contrast, the
CA-treated mice exhibited a significant decrease in ROS production
compared with the LPS-treated mice. The reductions were similar to
the results obtained for the dexamethasone-treated mice.
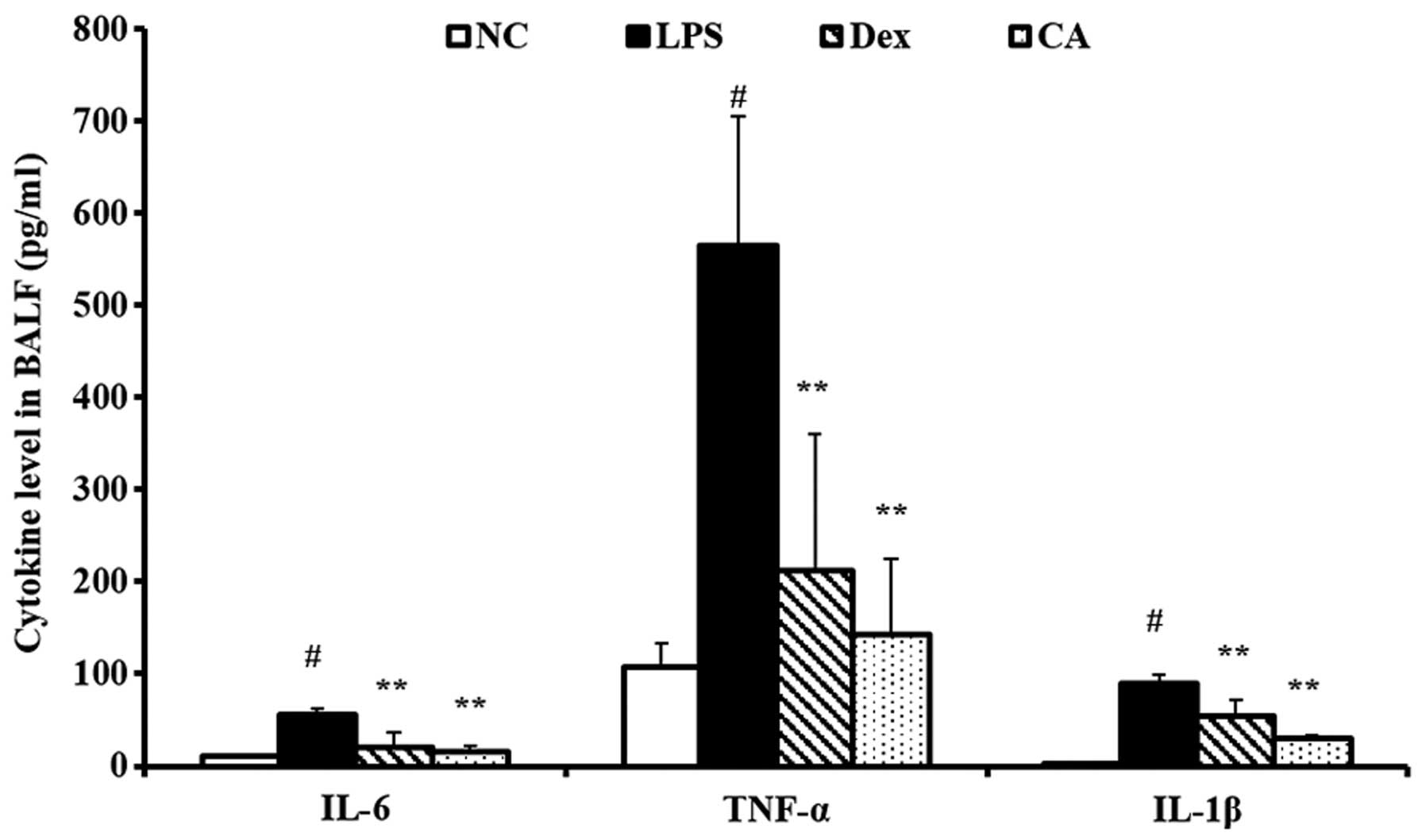
Effect of CA on pro-inflammatory
cytokine production in the BALF isolated from mice with LPS-induced
ALI
TNF-α, IL-1β and IL-6 levels in the BALF were
significantly increased in LPS-treated mice compared with the
negative control group (Fig. 6).
By contrast, CA-treated mice exhibited a significant decrease in
TNF-α, IL-1β and IL-6 levels compared with the LPS-treated mice.
The reductions were similar to the results obtained in the
dexamethasone-treated mice.
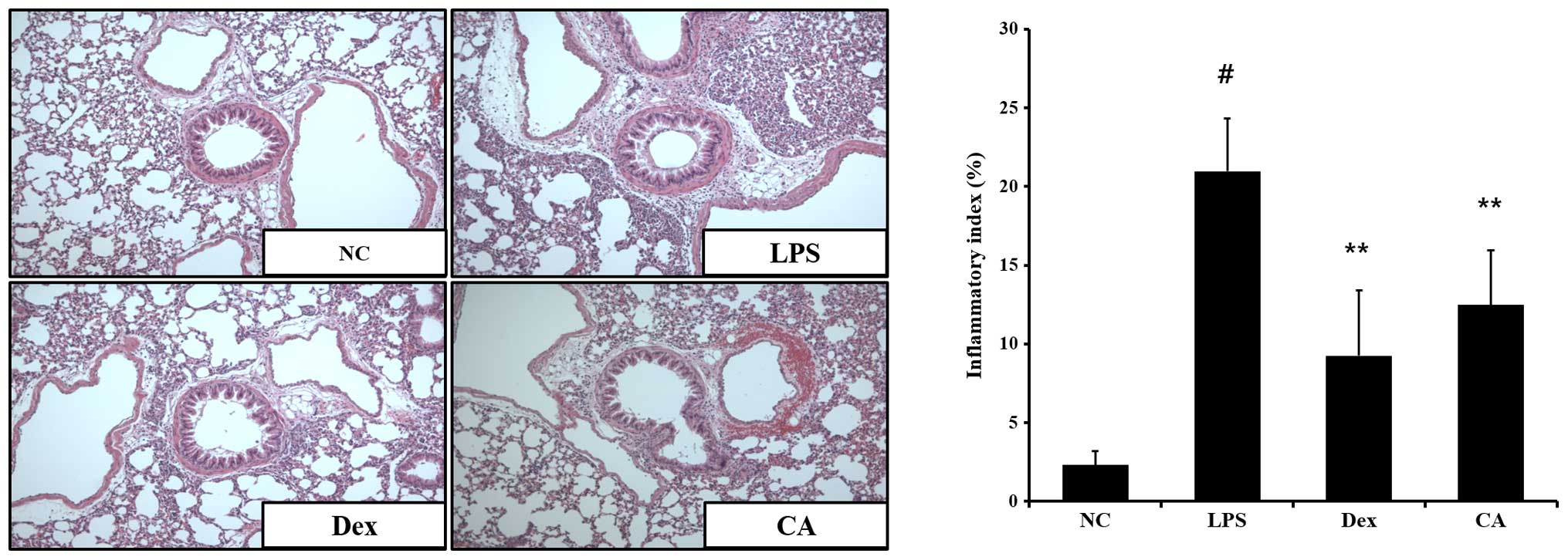
Effects of CA on the inflammatory
response in the lung tissue of mice with LPS-induced ALI
To evaluate histological changes following LPS
treatment of LPS-induced lung inflammation, the lung sections were
subjected to H&E staining. Significant pathological changes
were observed in the lungs of LPS-treated mice including
inflammatory cell infiltration, lung tissue damage, and alveolar
wall thickening. However, fewer histopathological changes were
observed in the lung tissues of CA-treated mice compared with the
LPS-treated mice (Fig. 7). The
reductions were similar to the results obtained in the
dexamethasone-treated mice.
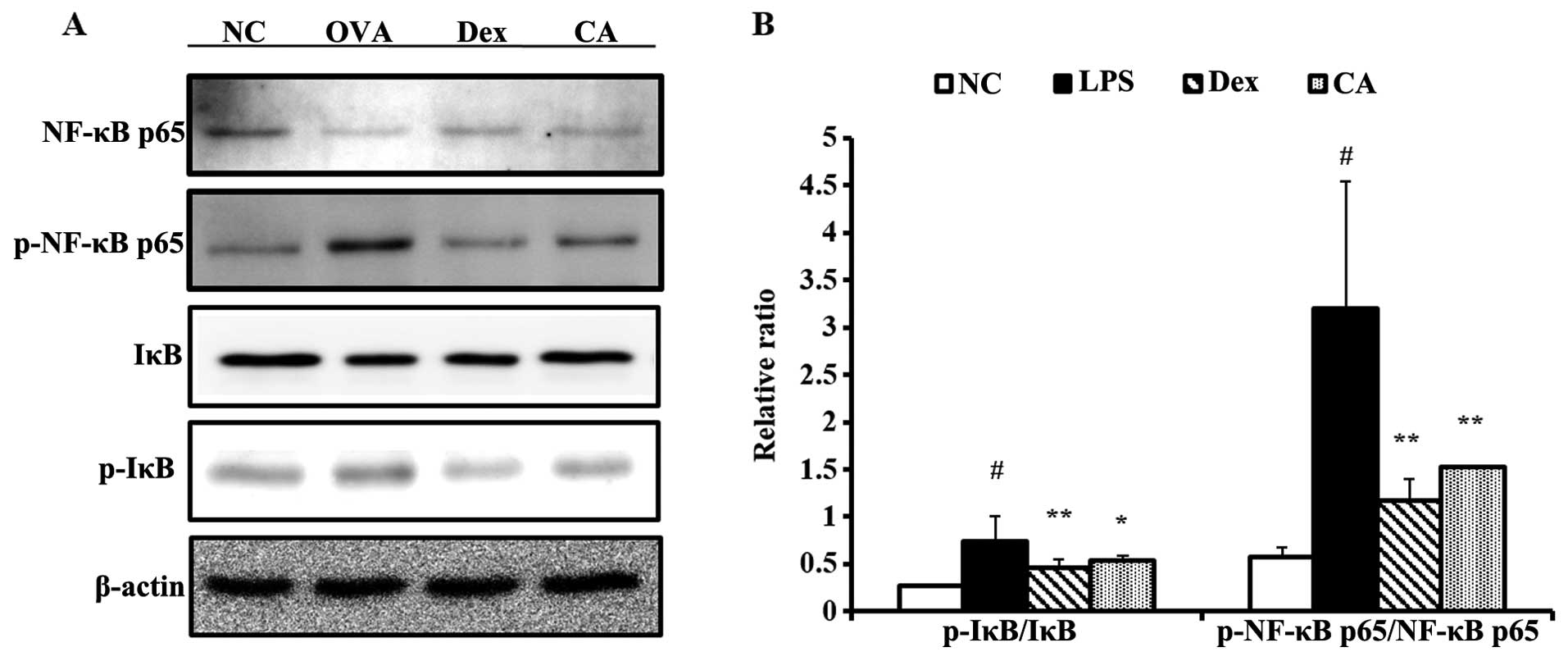
Effect of CA on protein expression of
pro-inflammatory mediators in the lung tissue of mice with
LPS-induced ALI
Mice with LPS-induced ALI exhibited increased IκB
and NF-κB phosphorylation in the lung tissue samples compared with
the negative control group. However, there was significantly
decreased IκB and NF-κB phosphorylation in the lung tissue of
CA-treated mice compared with the LPS-treated mice (Fig. 8). The reductions were similar to
the results obtained in the dexamethasone-treated mice.
Discussion
CA is a traditional medicine that is widely used for
its reported antitumor and anti-inflammatory activities (19–25). The aim of the present study was to
investigate the potential therapeutic effects of CA in a mouse
model of LPS-induced ALI and in LPS-stimulated RAW 264.7 cells.
In vitro, CA decreased NO production and pro-inflammatory
cytokines, such as IL-6, as well as PGE2 in
LPS-stimulated RAW 264.7 cells. CA also reduced the expression of
pro-inflammatory mediators such as COX-2. In vivo,
CA-treated mice had significantly reduced inflammatory cell counts
in the BALF and suppressed pro-inflammatory cytokine levels,
including TNF-α, IL-6 and IL-1β, as well as decreased production of
ROS in the BALF. CA effectively reduced airway inflammation in the
lung tissue, in addition to decreasing IκB and NF-κB p65
phosphorylation, compared with the LPS-treated mice.
Previous studies have demonstrated that LPS
stimulates alveolar macrophages and it is important in the
development of ALI. Stimulated alveolar macrophages produce NO,
PGE2 and pro-inflammatory cytokines, such as TNF-α, IL-6
and IL-1β (11,12). These cytokines play critical roles
in the inflammatory responses that are featured in ALI and
contribute to the severity of the lung injury (26–28). TNF-α, IL-6 and IL-1β amplify the
entire, or focal, inflammatory responses as well as the recruitment
of neutrophils into the lung in ALI (29). TNF-α, the earliest primary
endogenous mediator of an inflammatory reaction, has been
demonstrated to damage vascular endothelial cells and induce
alveolar epithelial cells to produce other cell factors, such as
IL-6 (30,31). TNF-α also elevates levels of
intracellular ROS, which is an important factors in the
pathogenesis of ALI. IL-6 is a product of TNF-α-induced epithelial
cells and in ALI it is one of the most common inflammatory
cytokines and it is also a significant predictor of morbidity and
mortality in patients with ARDS (29). Moreover, IL-1β inhibits fluid
transportations across the distal lung epithelium to cause
surfactant disorders and to increase protein permeability across
the alveolar-capillary barrier (32). In the present study, we determined
that CA decreased TNF-α, IL6, and IL-1β production compared with
LPS-treatment alone. The inhibition of pro-inflammatory cytokine
production was in accordance with the effects of CA on LPS-induced
histological changes and ROS production. CA significantly
suppressed the LPS-induced increase in ROS production in the BALF.
CA administration also attenuated the extensive LPS-induced
inflammatory responses in the lung tissues. These finding indicate
that CA has protective effects on LPS instillation-induced ALI.
Previous studies (33,34) have indicated that pro-inflammatory
cytokine production is modulated by the NF-κB signaling pathway.
NF-κB is an important nuclear transcription factor that is
responsible for inflammation, and it plays a critical role in
regulating immune and inflammatory responses. In normal conditions,
heterodimers of NF-κB complexes, which are mostly p50/p65, are
present as an inactive cytoplasmic form with IκB in the cytoplasm
(35,37). However, LPS stimulation is known
to cause IκB phosphorylation by the IκB kinase complex, which
results in degradation via the ubiquitin-proteasome pathway. IκB
degradation leads to NF-κB dimer phosphorylation and translocation
to the nucleus where it activates the transcription of specific
target genes, including TNF-α, IL-1β, IL-6, COX-2 and
PGE2 (15–18). In patients with ALI, NF-κB
activation increases pro-inflammatory cytokines, which promotes
inflammatory responses and neutrophil recruitment into the lung
(37). COX-2 expression, has also
been demonstrated to increase inflammatory cells and induce the
expression of prostaglandins, which aggravate inflammatory
responses (38). In the present
study, we investigated whether the anti-inflammatory activity of CA
was exerted via the NF-κB signaling pathway, in the lung tissue of
mice with ALI. In vivo, CA demonstrated a significant
reduction in NF-κB phosphorylation and IκB degradation. In
vitro, CA markedly decreased COX-2 expression and reduced
PGE2 production in LPS-stimulated RAW 264.7 cells. Thus,
CA possesses anti-inflammatory activity in mice with ALI and
LPS-stimulated RAW 264.7 cells. These effects are associated with
downregulation of the NF-κB signaling pathway.
In conclusion, CA demonstrated effective suppression
of the pro-inflammatory cytokines, ROS, NO and PGE2 in
LPS-stimulated RAW 264.7 cells and in mice with LPS-induced ALI.
These effects were closely associated with the suppression of NF-κB
phosphorylation. Thus, CA is a potential candidate for development
as an adjunctive treatment forof inflammatory disorders, such as
ALI.
Acknowledgments
This study was supported by the grants from the
Ministry of Science, ICT and Future Planning (FGC1011534) and the
KRBB Research Initiative Program (KGM1221622) of the Republic of
Korea.
References
|
1
|
Basu RK, Chawla LS, Wheeler DS and
Goldstein SL: Renal angina: an emerging paradigm to identify
children at risk for acute kidney injury. Pediatr Nephrol.
27:1067–1078. 2012. View Article : Google Scholar :
|
|
2
|
Atabai K and Matthay MA: The pulmonary
physician in critical care. 5: Acute lung injury and the acute
respiratory distress syndrome: definitions and epidemiology.
Thorax. 57:452–458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghavendran K and Napolitano LM:
Definition of ALI/ARDS. Crit Care Clin. 27:429–437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chignard M and Balloy V: Neutrophil
recruitment and increased permeability during acute lung injury
induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol.
279:L1083–L1090. 2000.PubMed/NCBI
|
|
5
|
Shang Y, Li X, Prasad PV, Xu S, Yao S, Liu
D, Yuan S and Feng D: Erythropoietin attenuates lung injury in
lipopolysaccharide treated rats. J Surg Res. 155:104–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diaz JV, Brower R, Calfee CS and Matthay
MA: Therapeutic strategies for severe acute lung injury. Crit Care
Med. 38:1644–1650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imai Y, Kuba K, Neely GG,
Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen
R, Leung YH, Wang H, et al: Identification of oxidative stress and
Toll-like receptor 4 signaling as a key pathway of acute lung
injury. Cell. 133:235–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zambon M and Vincent JL: Mortality rates
for patients with acute lung injury/ARDS have decreased over time.
Chest. 133:1120–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitamura Y, Hashimoto S, Mizuta N,
Kobayashi A, Kooguchi K, Fujiwara I and Nakajima H:
Fas/FasL-dependent apoptosis of alveolar cells after
lipopolysaccharide-induced lung injury in mice. Am J Respir Crit
Care Med. 163:762–769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abraham E, Nick JA, Azam T, Kim SH, Mira
JP, Svetkauskaite D, He Q, Zamora M, Murphy J, Park JS, et al:
Peripheral blood neutrophil activation patterns are associated with
pulmonary inflammatory responses to lipopolysaccharide in humans. J
Immunol. 176:7753–7760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouwmeester T, Bauch A, Ruffner H, Angrand
PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J,
Ghidelli S, et al: A physical and functional map of the human
TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol.
6:97–105. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auron PE: The interleukin 1 receptor:
Ligand interactions and signal transduction. Cytokine Growth Factor
Rev. 9:221–237. 1998. View Article : Google Scholar
|
|
14
|
Creager MA, Luscher TF, Cosentino F and
Beckman JA: Diabetes and vascular disease: pathophysiology,
clinical consequences, and medical therapy: Part I. Circulation.
108:1527–1532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kagan JC and Medzhitov R:
Phosphoinositide-mediated adaptor recruitment controls Toll-like
receptor signaling. Cell. 125:943–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson SJ, Leone BA, Anderson D, Manning A
and Holgate ST: Immunohistochemical analysis of the activation of
NF-kappaB and expression of associated cytokines and adhesion
molecules in human models of allergic inflammation. J Pathol.
189:265–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richmond A: NF-kappa B, chemokine gene
transcription and tumour growth. Nat Rev Immunol. 2:664–674. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
19
|
Senthikumar A and Venkatesalu V:
Phytochemical analysis and antibacterial activity of essential oil
of Clausena anisata (Wild). Hook. F. ex Benth. Int J Intergrat
Biol. 5:116–120. 2009.
|
|
20
|
Ajibesin KK, Ekpo BA, Bala DN, Essien EE
and Adesanya SA: Ethnobotanical survey of Akwa Ibom State of
Nigeria. J Ethnopharmacol. 115:387–408. 2008. View Article : Google Scholar
|
|
21
|
Hutchings A, Scott AH, Lewis G and
Cunningham AB: Zulu Medicinal Plants – An Inventory. 1st edition.
University of Natal Press; Pietermaritzburg: 1996
|
|
22
|
Lakshmi V, Prakash D, Raj K, Kapil RS and
Popli SP: Monoterpenoid furanocoumarin lactones from Clausena
anisata. Phytochemistry. 23:2629–2631. 1984. View Article : Google Scholar
|
|
23
|
Adesina SK and Adewunmi CO: Molluscicidal
agents from the root of Clausena anisata. Fitoterapia. 56:289–292.
1985.
|
|
24
|
Makanju OO: Behavioral and anticonvulsant
effects of an aqueous extract from the roots of Clausena anisata.
Int J Crude Drug Res. 21:29–32. 1983. View Article : Google Scholar
|
|
25
|
Watt JM and Breyer Brandwijk MG: The
Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd
edition. E. and S. Livingstone; London: 1962
|
|
26
|
Ganter MT, Roux J, Miyazawa B, Howard M,
Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, et
al: Interleukin-1beta causes acute lung injury via alphavbeta5 and
alphavbeta6 integrin-dependent mechanisms. Circ Res. 102:804–812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolb M, Margetts PJ, Anthony DC, Pitossi F
and Gauldie J: Transient expression of IL-1β induces acute lung
injury and chronic repair leading to pulmonary fibrosis. J Clin
Invest. 107:1529–1536. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giebelen IA, van Westerloo DJ, LaRosa GJ,
de Vos AF and van der Poll T: Local stimulation of alpha7
cholinergic receptors inhibits LPS-induced TNF-alpha release in the
mouse lung. Shock. 28:700–703. 2007.PubMed/NCBI
|
|
29
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: Four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan
HM, Yan J, Li Z, Liu H, Hua F, et al: TLR4 activity is required in
the resolution of pulmonary inflammation and fibrosis after acute
and chronic lung injury. Am J Pathol. 180:275–292. 2012. View Article : Google Scholar
|
|
33
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu Y, Liu B, Zhang N, Liu Z, Liang D, Li
F, Cao Y, Feng X, Zhang X and Yang Z: Magnolol inhibits
lipopolysaccharide-induced inflammatory response by interfering
with TLR4 mediated NF-κB and MAPKs signaling pathways. J
Ethnopharmacol. 145:193–199. 2013. View Article : Google Scholar
|
|
35
|
Moine P, McIntyre R, Schwartz MD, Kaneko
D, Shenkar R, Le Tulzo Y, Moore EE and Abraham E: NF-kappaB
regulatory mechanisms in alveolar macrophages from patients with
acute respiratory distress syndrome. Shock. 13:85–91. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuo MY, Liao MF, Chen FL, Li YC, Yang ML,
Lin RH and Kuan YH: Luteolin attenuates the pulmonary inflammatory
response involves abilities of antioxidation and inhibition of MAPK
and NFκB pathways in mice with endotoxin-induced acute lung injury.
Food Chem Toxicol. 49:2660–2666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Redington AE, Meng QH, Springall DR, Evans
TJ, Créminon C, Maclouf J, Holgate ST, Howarth PH and Polak JM:
Increased expression of inducible nitric oxide synthase and
cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects
and regulation by corticosteroid treatment. Thorax. 56:351–357.
2001. View Article : Google Scholar : PubMed/NCBI
|