Introduction
Type 2 diabetes mellitus (T2DM), which accounts for
>90% of diabetes cases, is a common metabolic disease that is
characterized by the resistance of target tissues to insulin
stimulation (1). T2DM is usually
associated with hyperglycemia, dyslipidemia, obesity, hypertension,
fatty liver disease, atherosclerosis, certain types of cancer and
cardiovascular diseases (2). The
peptide hormone, insulin, lowers blood glucose levels by
facilitating glucose uptake, mainly into skeletal muscle and fat
tissue, and by inhibiting endogenous glucose production in the
liver; insulin resistance occurs when a normal dose of the hormone
is incapable of eliciting these metabolic responses (3). Multiple defects in intracellular
events, including an impairment of the insulin signaling axis,
disrupt glucose metabolism and reduce glycogen synthesis, thus
contributing to insulin resistance (4–6).
Relieving insulin resistance has been considered a
promising approach to the treatment of T2DM (7). Thiazolidinediones (TZDs) and
fibrates are some of the most commonly used medications in the
treatment of T2DM, hyperlipidemia and insulin resistance. These
drugs bind to and activate peroxisome proliferator-activated
receptors (PPARs), which results in the upregulation of several
genes involved in glucose and lipid metabolism (8). The PPAR family, belonging to the
nuclear hormone receptor family, consists of 3 isoforms, PPARα,
PPARβ/δ and PPARγ (9). PPARγ is
mainly expressed in adipose tissue and vascular tissue/macrophages
(10), and is present in muscle
and β cells at low levels (11);
it affects genes involved in lipid synthesis and storage, and
glucose homeostasis. It is the functioning receptor for TZDs,
including rosiglitazone and pioglitazone (8). PPARα is predominantly expressed in
the liver (12) and stimulates
lipid consumption by enhancing the expression of fatty acid
oxidation genes, resulting in the amelioration of hyperlipidemia
(13). PPARα agonists, including
fenofibrate have been shown to exert potent effects by reducing
plasma triglyceride levels (14).
The distinct metabolic effects of PPARα and PPARγ agonists on
insulin sensitivity and lipid metabolism has encouraged the
development of novel drugs which target both PPARα and PPARγ
(15–17). It has been proposed that the dual
activation of PPARα and PPARγ may guarantee more desirable effects
with limited adverse effects (15–17). A number of PPARα/γ dual agonists
have been identified and tested in obese and insulin-resistant
individuals (18,19); however, the majority of these
drugs have been shown to have unexpected side-effects, including
heart failure, renal failure, urinary cancer and anemia (18,19). Therefore, the development of novel
PPARα/γ dual agonists with few adverse effects is urgently
required.
Amorpha fruticosa (A. fruticosa)
(Leguminosae) has been applied traditionally in the treatment of
hypertension, hematomas and contusions in China, Japan and Korea.
Previous studies on this plant have reported a number of phenolic
compounds, including stilbenes, flavonoids and rotenoids. In
addition, their pharmacological activities, such as antimicrobial,
antitumor and tumor necrosis factor (TNF)-α inhibitory activities
have been investigated (20,21). In our previous studies, we
demonstrated the therapeutic potential of amorphastilbol isolated
from A. fruticosa in metabolic disorders through the dual
activation of PPARα/γ in 3T3-L1 cell systems and animal models
(22,23). In the present study, we identified
another active ingredient, 5,7-dihydroxy-6-geranylflavanone (DGF;
chemical structure shown in Fig.
1), isolated from A. fruticosa which promotes the dual
activation of PPARα/γ and explored its pharmacological
properties.
Materials and methods
Cell culture and chemicals
The CV-1 kindey cells, 3T3-L1 preadipocyte mouse
embryonic fibroblasts and C2C12 mouse myoblasts were obtained from
ATCC (Manassas, VA, USA) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT,
USA) and 1% penicillin/streptomycin (Invitrogen). The HepG2
hepatocellular carcinoma cells were purchased from ATCC and
cultured in minimum essential medium with Earle's balanced salts
(MEM/EBSS), supplemented with 10% FBS, 1% penicillin/streptomycin,
1X non-essential amino acid and 1 mM sodium pyruvate (both from
Welgene, Daegu, Korea) at 37°C with 5% CO2 in air.
Troglitazone (TRO; PPARγ agonist) and WY14643 (PPARα agonist) were
purchased from Sigma-Aldrich (St. Louis, MO, USA), and GW501516
(PPARδ agonist) was purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Extraction, isolation and identification
of DGF from A. fruticosa
Fruits of A. fruticosa were collected in
Gangwon Province, Korea in December 2012. The plant was
authenticated and deposited at the KIST Gangneung Institute
Herbarium, Gangneung, Korea. The fruit of A. fruticosa (300
g) was extracted 3 times with ethanol and evaporated under a vacuum
at 40°C. The extract was reconstituted with 0.5 liter of water and
re-extracted with 0.5 liter of ethyl acetate. A total of 5 pure
compounds, amorphastilbol, DGF, 4,4′-dimethoxy-2′-hydroxychalcone,
4′,7-dimethoxy-5-hydroxyisoflavone and 12α-hydroxy-α-toxicarol,
were isolated by preparative high-performance liquid chromatography
(DeltaPrep 150 ml System, SunFire Silica 150×10 mm Column, Waters,
MA, USA) and identified from this extract, the chemical structures
of which are shown in Fig. 1.
Cell-based transactivation assay
The CV-1 cells were seeded in 24-well plates and
cultured for 24 h prior to transfection. After 24 h, the medium was
changed to 10% charcoal dextran-treated FBS-DMEM; 4 h following the
change in medium, a DNA mixture containing a 3X multimerized
PPRE-luciferase reporter plasmid (0.3 µg), pcDNA3-hPPAR (30
ng) and an internal control plasmid, pRL-SV-40 (5 ng), was
transfected into the cells using the TransFast™ transfection
reagent (Promega, Madison, WI, USA) (23). For RXRα reporter gene analysis,
RXRα plasmid (30 ng) and RXRE-luciferase reporter plasmid (0.3
µg) were transfected into the CV-1 cells. Following 24 h of
transfection, the cells were treated with 10 µM TRO or the
indicated concentrations of plant extracts (1, 3, 10 and 30
µg/ml) or DGF (1, 3, 10 and 30 µM) and incubated for
an additional 24 h. The luciferase activities of the cell lysates
were measured using the Dual-Luciferase® reporter assay
system according to the manufacturer's instructions (Promega). The
relative luciferase activity was normalized to the corresponding
Renilla luciferase activity to determine the transfection
efficiency.
Adipocyte differentiation
Adipocyte differentiation was induced by treating
the cells with differentiation medium (DM) containing hormonal
coctail (10 µg/ml insulin, 1 µM dexamethasone and 0.5
mM 3-isobutyl-1-methylxanthine) with 10% FBS in DMEM for 48 h
before the cells were switched to a maintenance medium with 10% FBS
and 10 µg/ml insulin. After an additional 48 h, the medium
was replaced with 10% FBS-DMEM. Thereafter, the medium was changed
every 2 days. The test drugs were administered at the initiation of
differentiation and at every medium change for 8 days. The lipid
accumulation in the cells was detected by Oil Red O staining.
Ligand binding assay
The LanthaScreen™ TR-FRET PPAR competitive binding
assay (Invitrogen) was performed according to the manufacturer's
instructions (24). In brief, a
mixture of 5 ng glutathione S-transferase fused to the PPAR
ligand-binding domain (GST-PPAR-LBD), 5 nM Tb-GST antibody, 5 nM
Fluormone Pan-PPAR Green, and serial dilutions of DGF (≤100
µM) was added to the wells of black 384-well plates to a
total volume of 18 ml. Following 2 h of incubation in the dark, the
FRET signal was measured by excitation at 340 nm and emission at
520 nm using the Infinite® M1000 microplate reader
(Tecan Group Ltd., Männedorf, Switzerland).
Measurement of triglyceride content
Triglyceride contents were measured using the
triglyceride determination kit (Sigma-Aldrich). Following
treatment, the differentiated 3T3-L1 adipocytes were rinsed twice
with PBS, scraped in 200 µl of saline solution (150 mM NaCl,
2 mM EDTA, 50 mM sodium phosphate, pH 7.4), sonicated to homogenize
the cell suspension and the total triglyceride content was then
measured. The total triglyceride content was determined by
measuring the increase in absorbance at 540 nm using a microplate
reader (PowerWave XS; BioTek Instruments Inc., Winooski, VT, USA)
according to the manufacturer's instructions. The results were
expressed as milligrams of triglyceride per milligram of cellular
protein. The residual cell lysate was centrifuged at 12,000 rpm to
remove the fatty layer, and was then assayed for the measurement of
the protein content using a BCA protein assay kit
(Sigma-Aldrich).
Measurement of glycerol-3-phosphate
dehydrogenase (GPDH) activity
GPDH activity was measured using a GPDH) activity
assay kit (MK426; Takara Bio Inc., Shiga, Japan). Following
treatment, the differentiated 3T3-L1 adipocytes were rinsed twice
with PBS, scraped into 200 µl enzyme extraction buffer
(provided with the kit) and sonicated. GPDH activity was determined
according to the decrease in NADH activity by measuring the
decrease in the absorbance at 340 nm using a microplate reader,
according to the manufacturer's instructions.
Gene expression analysis
Total RNA was isolated from the 3T3-L1 adipocytes or
HepG2 cells transfected with pcDNA3-hPPARα plasmid using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions.
The RNA concentration of each sample was determined using a
spectrophotometer (Nanodrop 2000; Thermo Fischer Scientific, Inc.,
Wilmington, DE, USA) at 260 nm; the integrity of each RNA sample
was evaluated using the Agilent 2100 BioAnalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). cDNA synthesis was
performed using 1 µg of total RNA in 20 µl with
random primers and SuperScript II reverse transcriptase.
Quantitative PCR (q-PCR) was performed with SYBR-Green fluorescent
dye using the 7500 Real-Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). Data analyses were performed
using the 7500 system SDS software version 1.3.1 (Applied
Biosystems Life Technologies). In some cases, conventional PCR was
performed using the GeneAmp PCR system 9700 (Applied Biosystems
Life Technologies), and the integrity of the PCR product was
verified by agarose gel electrophoresis and ethidium bromide
staining. The sequences of the primers used in this study are
listed in Table I.
 | Table IPrimer sequences used for RT-PCR and
qPCR. |
Table I
Primer sequences used for RT-PCR and
qPCR.
| Gene name | Oligonucleotide
sequence (5′→3′) | Accession no. | Gene expression
analysis |
|---|
| mC/EBPα | F:
AGGTGCTGGAGTTGACCAGT | NM_007678 | qPCR/RT-PCR |
| R:
CAGCCTAGAGATCCAGCGAC | | |
| mPPARγ | F:
CAAGAATACCAAAGTGCGATCAA | NM_011146 | qPCR |
| R:
GAGCTGGGTCTTTTCAGAATAATAAG | | |
| mPPARγ | F:
TTCGCTGATGCACTGCCTAT | NM_011146 | RT-PCR |
| R:
GCCAACAGCTTCTCCTTCTC | | |
| maP2 | F:
AGTGAAAACTTCGATGATTACATGAA | NM_024406 | qPCR |
| R:
GCCTGCCACTTTCCTTGTG | | |
| maP2 | F:
ATGTGTGATGCCTTTGTGGGA | NM_024406 | RT-PCR |
| R:
TGCCCTTTCATAAACTCTTGT | | |
| mGLUT4 | F:
AGAGTCTAAAGCGCCT | NM_009204 | qPCR/RT-PCR |
| R:
CCGAGACCAACGTGAA | | |
| mAdiponectin | F:
AGCCTGGAGAAGCCGCTTAT | NM_009605 | qPCR/RT-PCR |
| R:
TTGCAGTAGAACTTGCCAGTGC | | |
| mResistin | F:
TCAACTCCCTGTTTCCAAATGC | NM_022984 | qPCR/RT-PCR |
| R:
TCTTCACGAATGTCCCACGA | | |
| mGAPDH | F:
TTGTTGCCATCAACGACCCC | NM_008084 | qPCR |
| R:
GCCGTTGAATTTGCCGTGAG | | |
| mGAPDH | F:
ACCACAGTCCATGCCATCAC | NM_008084 | RT-PCR |
| R:
TCCACCACCCTGTTGCTGTA | | |
| hACO2 | F:
GCGGACATGGCTACTCAAAGC | NM_003500.1 | RT-PCR |
| R:
GCAGTGCACCTTAGCAGCCTG | | |
| hCPT1α | F:
AGACGGTGGAACAGAGGCTGAAG | NM_001876.1 | RT-PCR |
| R:
TGAGACCAAACAAAGTGATGATGTCAG | | |
| hGAPDH | F:
ACCACAGTCCATGCCATCAC | NM_002046 | RT-PCR |
| R:
TCCACCACCCTGTTGCTGTA | | |
Myotube formation and western blot
analysis
The C2C12 myoblasts were cultured in DMEM until
reaching 90% confluence. The cells were differentiated into
myotubes with DMEM containing 2% horse serum for 4 days, and then
incubated for 16 h in DMEM containing 2% BSA and 10% FBS in the
absence or presence of 0.75 mM palmitate to induce insulin
resistance. Subsequently, the DGF-treated cells were stimulated
with 100 nM insulin for 10 min. Following stimulation, the cells
were washed twice with PBS and harvested. For western blot
analysis, antibodies to Akt (cs9272; 1:1,000), phospho-Ser473 Akt
(cs4060; 1:1,000), insulin receptor (IR) β (cs3020; 1:1,000),
phospho-Tyr1135/1136 insulin growth factor (IGF)-1Rβ/Tyr1150/1151
IRβ (cs3024; 1:1,000) were used (Cell Signaling Technology,
Beverly, MA, USA).
2-NBDG glucose uptake assay
The myotubes, which were obtained from the
above-mentioned procedures, were stimulated with 100 nM insulin for
1 h. Following insulin stimulation, the myotubes were incubated
with 50 µM 2-NBDG (Invitrogen) for 15 min and then washed
with PBS 3 times to remove free 2-NBDG. The fluorescence intensity
of the cells containing 2-NBDG was measured using the Infinite
M1000 microplate reader (Tecan Group Ltd.) with excitation at 485
nm and emission at 535 nm.
Statistical analysis
Data are expressed as the means ± SD. Differences
between the mean values in the 2 groups were analyzed using one-way
analysis of variance (ANOVA). A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Dual activation of PPARα/γ
transcriptional activity by A. fruticosa
To explore the pharmacological properties of A.
fruticosa, we examined the effects of A. fruticosa on
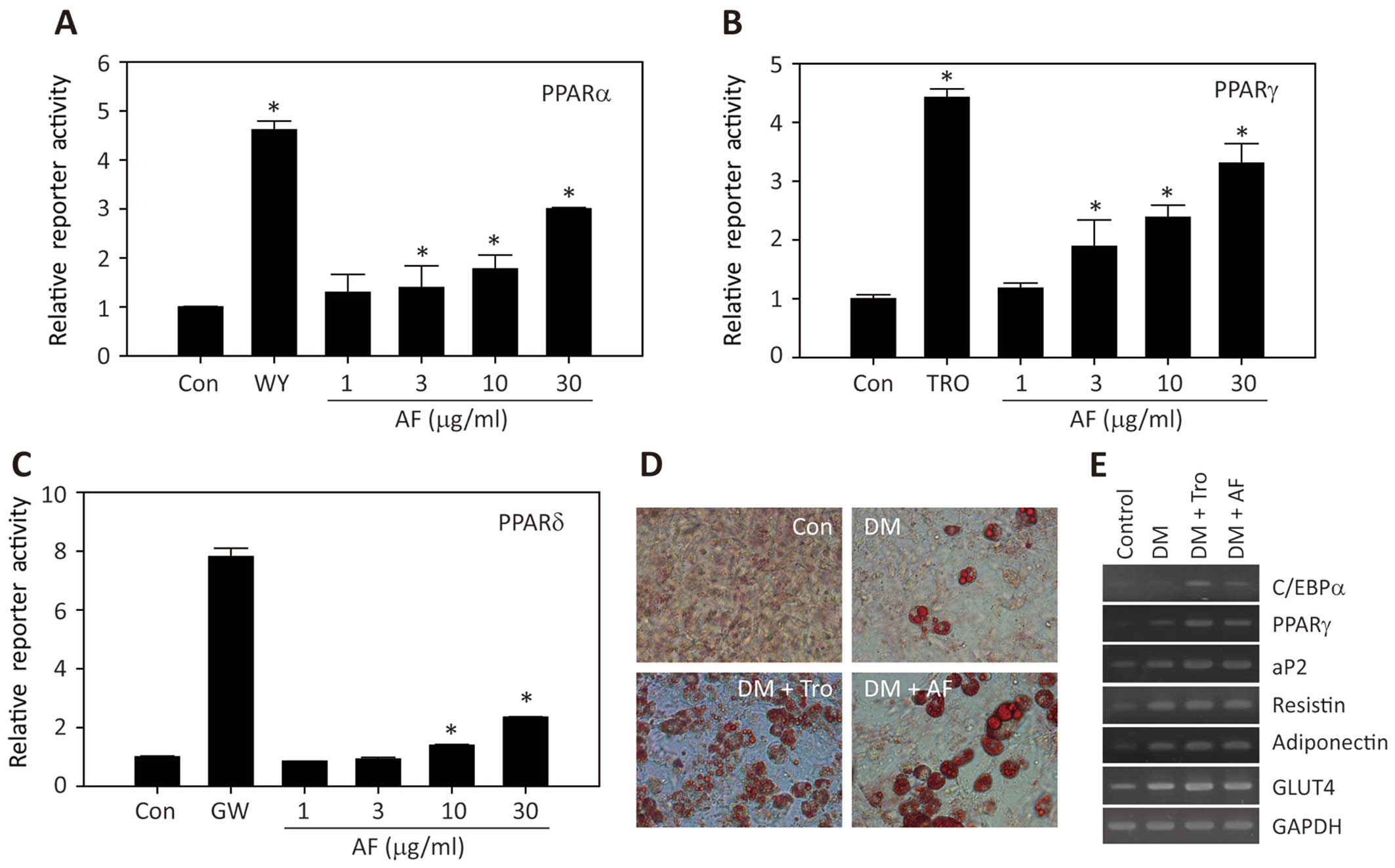
the transcriptional activation of PPARs. As shown in Fig. 2A and B, treatment with the extract
of A. fruticosa led to an increase in PPARα- and
PPARγ-reporter gene activities in a dose-dependent manner; however,
it had a minimal effect on the transcriptional activation of PPARδ
(Fig. 2C). In addition, treatment
with A. fruticosa extract markedly enhanced the adipocyte
differentiation of the 3T3-L1 cells, as shown by Oil Red O staining
(Fig. 2D), which was accompanied
by an increase in the expression of adipocyte marker genes,
including CCAAT-enhancer-binding protein (C/EBP)α, PPARγ,
adipocyte protein 2 (aP2), resistin, adiponectin, and glucose
transporter type 4 (GLUT4) (Fig. 2E). These results strongly indicate
the presence of a novel PPARα/γ agonist in the fruit extract of
A. fruticosa.
DGF is a novel PPARα/γ dual
activator
As the PPARα/γ-reporter gene activities and
adipocyte differentiation were markedly enhanced by the extract of
A. fruticosa, we wished to identify the active ingredients
of A. fruticosa. Thus, we isolated and identified 5
compounds from A. fruticosa and evaluated their activities
on PPARα/γ transcriptional activation. As shown in Table II, although amorphastilbol
exhibited the most potent promoting effect on PPARα/γ
transactivation, DGF also had a similar effect. The other
compounds, 4,4′-dimethoxy-2′-hydroxychalcone,
4′,7-dimethoxy-5-hydroxyisoflavone and 12α-hydroxy-α-toxicarol,
exerted weaker effects than those of amorphastilbol and DGF. Since
the pharmacological properties of amorphastilbol have already been
reported (22), in this study, we
focused on the properties of DGF. As shown in Fig. 3A and B, treatment with DGF led to
an increase in both PPARα- and PPARγ-reporter gene activities in a
dose-dependent manner. However, DGF exerted a minimal effect on
PPARδ transcription (Fig. 3C) and
no detectable effect on retinoid X receptor (RXRα) was obsrved
(Fig. 3D). The DGF binding
affinities to PPARα and PPARγ were weaker than those of the
positive controls, GW501516 and TRO (Fig. 3E), which is in agreement with the
weak ability of DGF to activate the transcription of PPARα/γ in
comparison to that of the positive control (Fig. 3A and B).
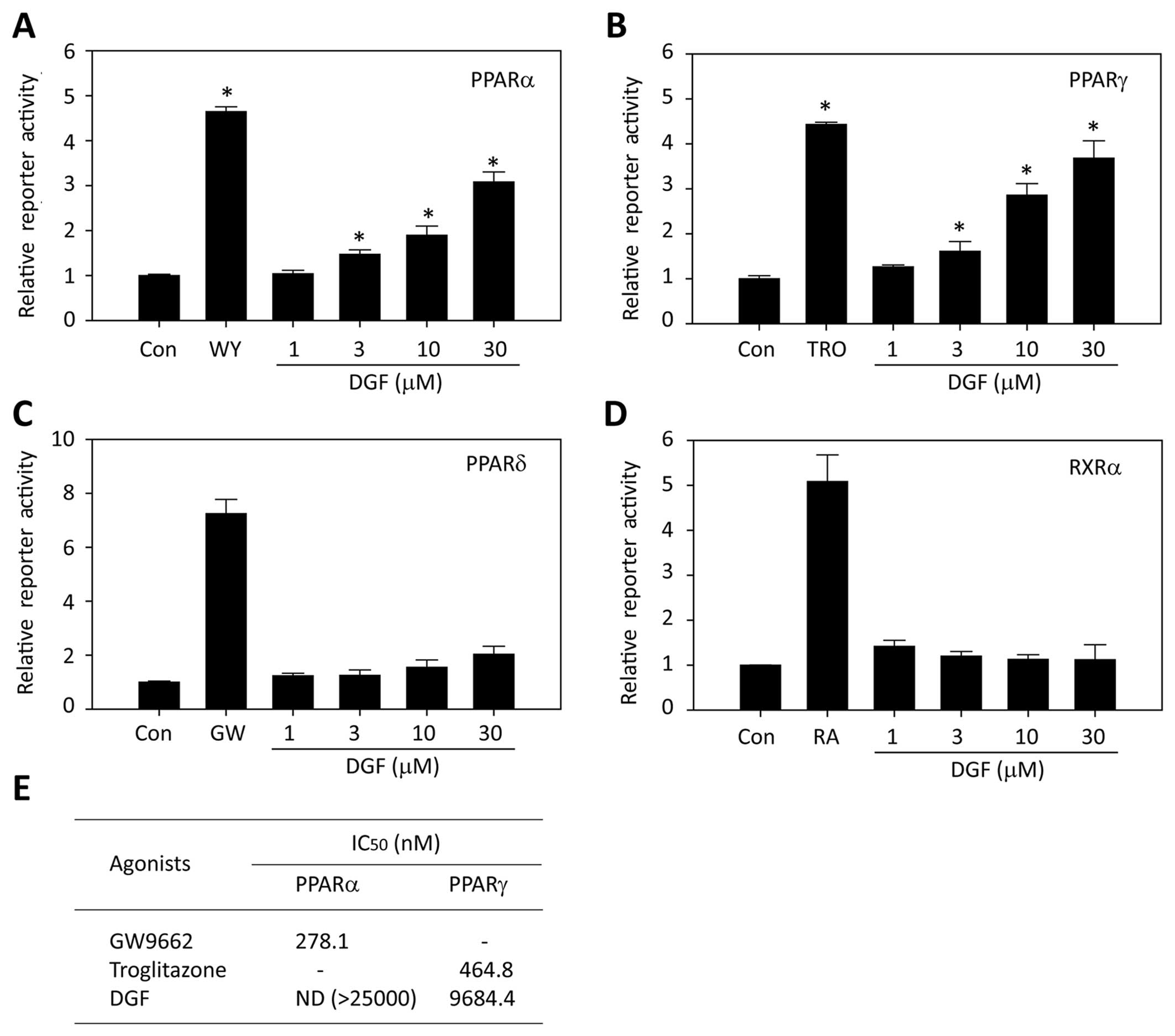 | Figure 35,7-Dihydroxy-6-geranyl flavanone
(DGF) from Amorpha fruticosa (A. fruticosa) selectively
activates peroxisome proliferator-activated receptor (PPAR)α and
PPARγ in vitro. Human (A) PPARα, (B) PPARγ, or (C) PPARδ or
(D) RXRα expression vector, PPRE-luciferase reporter construct, and
pRL-SV40 vector were transiently co-transfected into the CV-1
cells. The cells were then treated with 10 µM WY14643 (WY),
10 µM troglitazone (TRO), 1 µM GW501516 (GW), or
various concentrations of DGF (1,3,10 or 20 µM) for 24 h. A
reporter assay was performed as described in the Materials and
methods. Each bar represents the mean ± SD of duplicates.
*P<0.05 vs. control. (E) Binding affinities of DGF to
PPARα and PPARγ were analyzed using the LanthaScreen™ TR-FRET PPAR
competitive binding assay, as described in the Materials and
methods. |
 | Table IIEffects of the compounds from A.
fruticosa on PPARα/γ transcriptional activity. |
Table II
Effects of the compounds from A.
fruticosa on PPARα/γ transcriptional activity.
| Compounds | Transactivation
(fold)
|
|---|
| PPARα | PPARγ |
|---|
| DGF | 2.97 | 3.68 |
| Amorphastilbol | 3.94 | 3.61 |
|
4,4′-Dimethoxy-2′-hydroxychalcone | 1.38 | 1.27 |
|
4′,7-Dimethoxy-5-hydroxyisoflavone | 1.65 | 2.38 |
|
12α-Hydroxy-α-toxicarol | 2.01 | 2.85 |
| WY14643 | 4.71 | – |
| TRO | – | 4.4 |
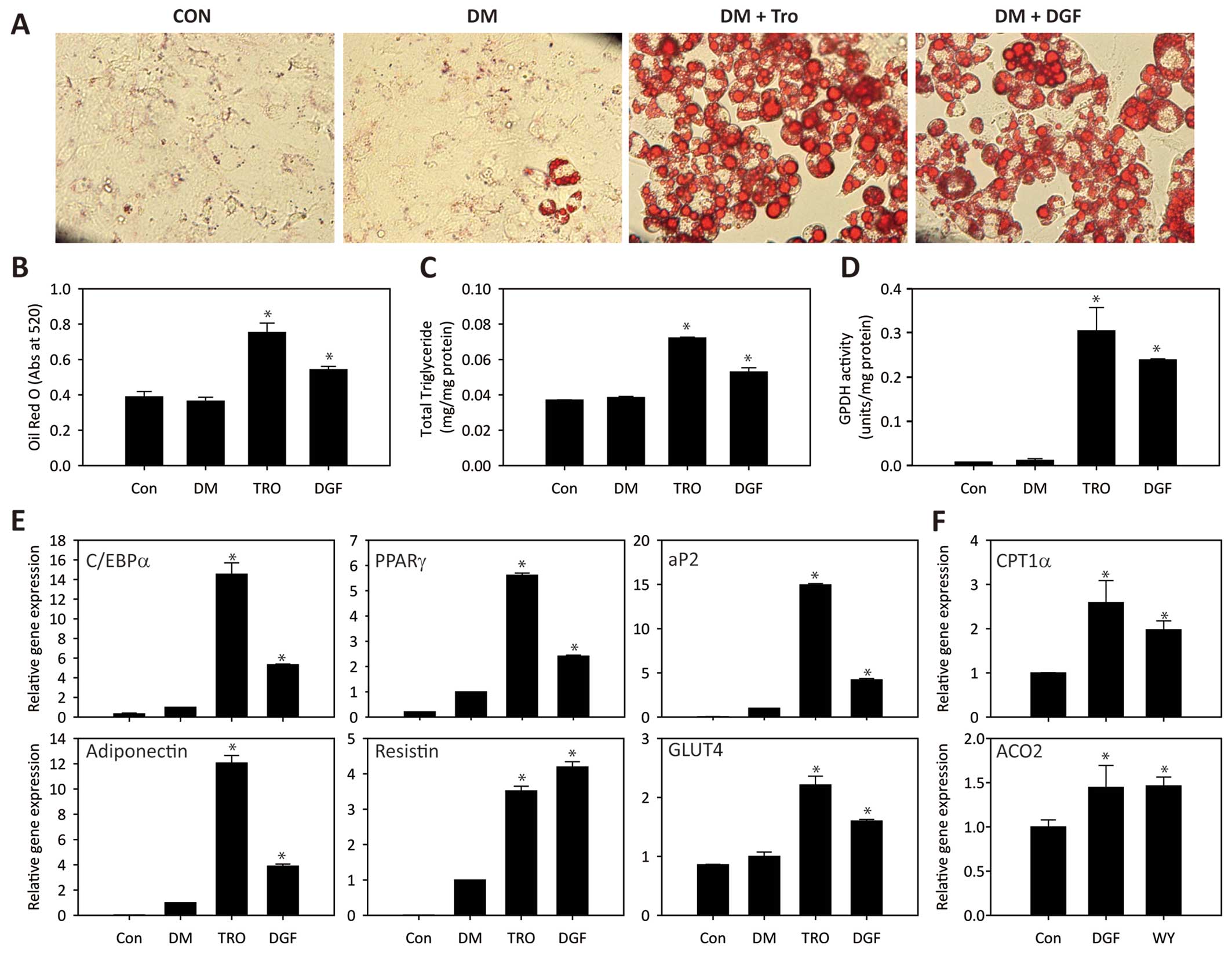
DGF upregulates specific genes involved
in both adipocyte differentiation and fatty acid oxidation
To evaluate the potential effects of DGF on
adipogenesis, 3T3-L1 preadipocytes were differentiated for 8 days
in the presence of DGF. As shown in Fig. 4A and B, treatment with DGF induced
a marked increase in lipid accumulation in the differentiated
adipocytes, which was comparable to the effects of TRO. To further
characterize the adipogenic potential of DGF, the cellular
triglyceride content and GPDH enzyme activity were measured. The
triglyceride content in the differentiated adipocytes was also
increased by DGF treatment up to 1.5-fold (Fig. 4C); this observation was in
accordance with the result that DGF induced lipid droplet
accumulation in adipocytes, as shown in Fig. 4A. In addition, GPDH activity was
significantly enhanced by DGF treatment (Fig. 4D). Taken together, these results
strongly support the notion that DGF promotes the adipocyte
differentiation of 3T3-L1 cells. As adipogenesis is governed by the
increased expression of various transcription factors and
adipocyte-specific genes (25),
we examined the effects DGF on the expression of adipogenic
transcription factors and marker genes in the differentiated
adipocytes. Following the induction of adipocyte differentiation in
the presence of DGF, the mRNA levels of C/EBPα, PPARγ, aP2,
adiponectin and resistin were measured by qPCR. In the
DGF-treated adipocytes, the mRNA levels of these genes were
markedly increased compared to their levels in the absence of DGF
(Fig. 4E). In addition, treatment
with DGF induced an increase in the mRNA level of GLUT4,
which is responsible for insulin-mediated glucose uptake (26), indicating that the insulin
sensitivity of adipose tissue may be enhanced by DGF treatment.
Subsequently, we examined the effects of DGF on fatty acid
oxidation to confirm its PPARα agonistic effect. In the HepG2 cells
transfected with the PPARα overexpression vector, the expression
levels of two different PPARα target genes involved in β-oxidation,
carnitine palmitoyltransferase 1α (CPT1α) and acyl-CoA
oxidase 2 (ACO2), were increased by DGF treatment (Fig. 4F), supporting the hypothesis that
DGF promotes fatty acid oxidation through the activation of
PPARα.
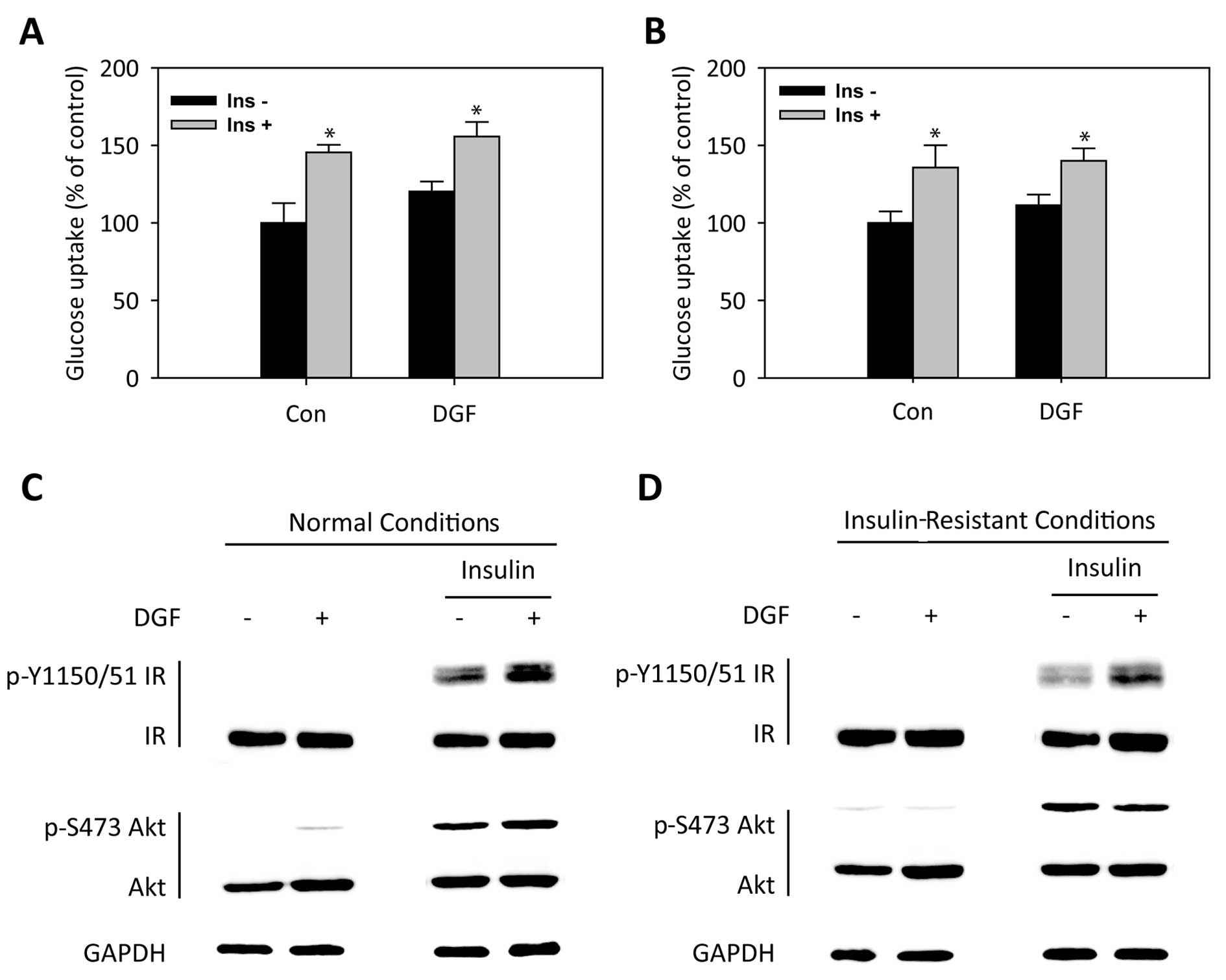
DGF improves insulin sensitivity
To further ascertain the positive effects of DGF on
insulin sensitivity, we first examined glucose uptake in C2C12
myotubes under normal conditions. As shown in Fig. 5A, glucose uptake in C2C12 myotubes
in the absence or presence of insulin was significantly enhanced by
DGF treatment under normal conditions. Correspondingly, treatment
with DGF also enhanced the insulin-induced phosphorylation of IR
and Akt under normal conditions (Fig.
5C). We then examined the effects of DGF on glucose uptake
under palmitate-induced insulin-resistant conditions. DGF enhanced
glucose uptake under insulin-resistant conditions as well (Fig. 5B). The effects of DGF on insulin
sensitivity under insulin-resistant conditions were further
confirmed by the elevated phosphorylation levels of these
downstream proteins in insulin signaling (Fig. 5D). All these results suggest that
the enhancement of insulin sensitivity by DGF is mediated by the
insulin-induced activation of the IR-Akt signaling axis and
enhanced glucose uptake.
Discussion
T2DM and related metabolic diseases pose a serious
health concern in modern societies. We have recently reported that
amorphastilbol isolated from A. fruticosa exerts beneficial
effects on glucose and lipid metabolism by selectively activating
both PPARα and PPARγ, thus ameliorating metabolic disorders without
being associated with any severe adverse reactions that have been
observed for other PPAR agonists, such as weight gain and
hepatomegaly (22). In this
study, we demonstrated that DGF, as another novel PPARα/γ dual
agonist isolated from A. fruticosa, promoted adipogenesis
through the upregulation of adipocyte specific marker gene
expression, and improved insulin sensitivity. However, the binding
affinities of DGF to both PPARα (>25 µM) and PPARγ (9.7
µM) were much weaker than those of amorphastilbol; the
binding affinities of amorphastilbol to both PPARα and PPARγ were
reported as 1.5 µM and 0.84 µM, respectively
(22). Although its binding
affinities to PPARα/γ were much weaker than those of
amorphastilbol, DGF significantly increased their transcriptional
activities, which resulted in the upregulation of adipocyte
specific marker gene expression and the improvement of insulin
sensitivity, indicating that DGF is one of the active ingredients
of A. fruticosa with anti-diabetic properties.
It has been demonstrated that rosiglitazone, a full
agonist of PPARγ, is associated with an increased risk of heart
attacks, which potentially limits its appeal and further clinical
use, in spite of its potent improvement of glucose metabolism
(27). However, recently, FDA
demonstrated that there is no direct evidence for the
cardiovascular risk of rosiglitazone after reviewing the results of
clinical trials (28). In
addition, pioglitazone, another PPARγ agonist, is less frequently
associated with increased cardiovascular risk (29). All these facts imply that PPARγ
activity itself may not be directly associated with this
cardiovascular risk. Therefore, the development of novel partial
PPAR agonists that are structurally unrelated to TZDs, particularly
rosiglitazone, remains appealing. From this point of view, it is
noteworthy that amorphastilbol and DGF isolated from A.
fruticosa are partial dual agonists of PPARα/γ and that their
chemical structures are unique from those of TZDs. In addition,
amorphastilbol and DGF do not affect hERG K+ channel
activity, which is associated with cardiovascular toxicity (data
not shown). Taken together, these data support, in part, the
relative safe use of amorphastilbol and DGF with regard to
cardiovascular risk.
Insulin resistance occurs when a normal level of
insulin is incapable of eliciting its metabolic responses (3) and results from multiple
intracellular defects, including an impairment of the insulin
signaling axis (4–6). Our data demonstrated that DGF
ameliorated insulin resistance by activating the insulin signaling
axis, resulting in the enhancement of glucose uptake (Fig. 5A and B).
In conclusion, in this study, we demonstrate that
DGF exerts beneficial effects on glucose and lipid metabolism, thus
ameliorating metabolic disorders by selectively activating both
PPARα and PPARγ. These results strongly suggest that DGF is one of
active components of A. fruticosa with anti-diabetic and
anti-metabolic properties. Therefore, our data provide strongly
support the further development of A. fruticosa and its
component DGF as anti-metabolic agents for the treatment of glucose
and lipid abnormalities, as well as insulin resistance.
Acknowledgments
The present study was supported by grants from the
Korea Institute of Science and Technology, Republic of Korea (no.
2Z04371) and the Sookmyung Women's University Research Grants
(1-1403-0042).
References
|
1
|
Rathmann W and Giani G: Global prevalence
of diabetes: estimates for the year 2000 and projections for 2030.
Diabetes Care. 27:2568–2569; author reply 2569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olokoba AB, Obateru OA and Olokoba LB:
Type 2 diabetes mellitus: a review of current trends. Oman Med J.
27:269–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltiel AR: New perspectives into the
molecular pathogenesis and treatment of type 2 diabetes. Cell.
104:517–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bajaj M and Defronzo RA: Metabolic and
molecular basis of insulin resistance. J Nucl Cardiol. 10:311–323.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pendergrass M, Bertoldo A, Bonadonna R,
Nucci G, Mandarino L, Cobelli C and Defronzo RA: Muscle glucose
transport and phosphorylation in type 2 diabetic, obese
nondiabetic, and genetically predisposed individuals. Am J Physiol
Endocrinol Metab. 292:E92–E100. 2007. View Article : Google Scholar
|
|
6
|
Cusi K, Maezono K, Osman A, Pendergrass M,
Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR and Mandarino LJ:
Insulin resistance differentially affects the PI 3-kinase- and MAP
kinase-mediated signaling in human muscle. J Clin Invest.
105:311–320. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilcox G: Insulin and insulin resistance.
Clin Biochem Rev. 26:19–39. 2005.PubMed/NCBI
|
|
8
|
Michalik L, Auwerx J, Berger JP,
Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T,
Lazar MA, O'Rahilly S, et al: International Union of Pharmacology.
LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev.
58:726–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Olson P and Evans RM: Minireview:
lipid metabolism, metabolic diseases, and peroxisome
proliferator-activated receptors. Endocrinology. 144:2201–2207.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vidal-Puig AJ, Considine RV, Jimenez-Liñan
M, Werman A, Pories WJ, Caro JF and Flier JS: Peroxisome
proliferator-activated receptor gene expression in human tissues.
Effects of obesity, weight loss, and regulation by insulin and
glucocorticoids. J Clin Invest. 99:2416–2422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dubois M, Pattou F, Kerr-Conte J, Gmyr V,
Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K and Lefebvre J:
Expression of peroxisome proliferator-activated receptor gamma
(PPARgamma) in normal human pancreatic islet cells. Diabetologia.
43:1165–1169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bünger M, Hooiveld GJ, Kersten S and
Müller M: Exploration of PPAR functions by microarray technology -
a paradigm for nutrigenomics. Biochim Biophys Acta. 1771:1046–1064.
2007. View Article : Google Scholar
|
|
13
|
Superko HR: A review of combined
hyperlipidaemia and its treatment with fenofibrate. J Int Med Res.
17:99–112. 1989.PubMed/NCBI
|
|
14
|
Bajaj M, Suraamornkul S, Hardies LJ, Glass
L, Musi N and DeFronzo RA: Effects of peroxisome
proliferator-activated receptor (PPAR)-alpha and PPAR-gamma
agonists on glucose and lipid metabolism in patients with type 2
diabetes mellitus. Diabetologia. 50:1723–1731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pickavance LC, Brand CL, Wassermann K and
Wilding JP: The dual PPARalpha/gamma agonist, ragaglitazar,
improves insulin sensitivity and metabolic profile equally with
pioglitazone in diabetic and dietary obese ZDF rats. Br J
Pharmacol. 144:308–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reifel-Miller A, Otto K, Hawkins E, Barr
R, Bensch WR, Bull C, Dana S, Klausing K, Martin JA,
Rafaeloff-Phail R, et al: A peroxisome proliferator-activated
receptor alpha/gamma dual agonist with a unique in vitro profile
and potent glucose and lipid effects in rodent models of type 2
diabetes and dyslipidemia. Mol Endocrinol. 19:1593–1605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrity T, Farrelly D, Tieman A, Chu C,
Kunselman L, Gu L, Ponticiello R, Cap M, Qu F, Shao C, et al:
Muraglitazar, a novel dual (alpha/gamma) peroxisome
proliferator-activated receptor activator, improves diabetes and
other metabolic abnormalities and preserves beta-cell function in
db/db mice. Diabetes. 55:240–248. 2006. View Article : Google Scholar
|
|
18
|
Mittra S, Sangle G, Tandon R, Sharma S,
Roy S, Khanna V, Gupta A, Sattigeri J, Sharma L, Priyadarsiny P, et
al: Increase in weight induced by muraglitazar, a dual
PPARalpha-gamma agonist, in db/db mice: adipogenesis/or oedema? Br
J Pharmacol. 150:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adeghate E, Adem A, Hasan MY, Tekes K and
Kalasz H: Medicinal Chemistry and Actions of Dual and Pan PPAR
Modulators. Open Med Chem J. 5(Suppl 2): 93–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dat NT, Lee JH, Lee K, Hong YS, Kim YH and
Lee JJ: Phenolic constituents of Amorpha fruticosa that inhibit
NF-kappaB activation and related gene expression. J Nat Prod.
71:1696–1700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho JY, Kim PS, Park J, Yoo ES, Baik KU,
Kim YK and Park MH: Inhibitor of tumor necrosis factor-alpha
production in lipopolysaccharide-stimulated RAW264.7 cells from
Amorpha fruticosa. J Ethnopharmacol. 70:127–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee W, Ham J, Kwon HC, Kim YK and Kim SN:
Anti-diabetic effect of amorphastilbol through PPARα/γ dual
activation in db/db mice. Biochem Biophys Res Commun. 432:73–79.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee W, Ham J, Kwon HC, Yoon G, Bae GU, Kim
YK and Kim SN: Amorphastilbol exerts beneficial effects on glucose
and lipid metabolism in mice consuming a high-fat-diet. Int J Mol
Med. 36:527–533. 2015.PubMed/NCBI
|
|
24
|
Kim T, Lee W, Jeong KH, Song JH, Park SH,
Choi P, Kim SN, Lee S and Ham J: Total synthesis and dual PPARα/γ
agonist effects of amorphastilbol and its synthetic derivatives.
Bioorg Med Chem Lett. 22:4122–4126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soukas A, Socci ND, Saatkamp BD, Novelli S
and Friedman JM: Distinct transcriptional profiles of adipogenesis
in vivo and in vitro. J Biol Chem. 276:34167–34174. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zierath JR, He L, Gumà A, Odegoard
Wahlström E, Klip A and Wallberg-Henriksson H: Insulin action on
glucose transport and plasma membrane GLUT4 content in skeletal
muscle from patients with NIDDM. Diabetologia. 39:1180–1189. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nissen SE and Wolski K: Effect of
rosiglitazone on the risk of myocardial infarction and death from
cardiovascular causes. N Engl J Med. 356:2457–2471. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
U.S. Food and Drug Administration:
Rosiglitazone-containing diabetes medicines: Drug Safety
Communication - Removal of some prescribing and dispensing
restrictions. FDA Drug Safety Communication. 2013.
|
|
29
|
Lincoff AM, Wolski K, Nicholls SJ and
Nissen SE: Pioglitazone and risk of cardiovascular events in
patients with type 2 diabetes mellitus: a meta-analysis of
randomized trials. JAMA. 298:1180–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|