Introduction
Diabetic nephropathy (DN), as a common complication
of diabetes mellitus, is the leading cause of end-stage renal
disease and chronic renal failure (1). Although glomerular injury is thought
to be the initiating factor of kidney damage in DN, previous
research has indicated that tubular injury is also a key cause of
chronic kidney injury (2), which
correlates with the progression of DN (3). Tubular epithelial cell (TEC)
apoptosis is considered a critical detrimental event which leads to
kidney injury in cases of DN, and is closely associated with
tubular atrophy and interstitial fibrosis (4). However, the mechanisms of tubular
apoptosis during the progression of DN remain poorly
understood.
Electron transfer flavoprotein β (ETFβ) is a subunit
of ETF that is comprised of α and β subunits containing a flavin
adenine dinucleotide (FAD) cofactor in the mitochondrial matrix. In
the mitochondria, the oxidation of fatty acids is coupled with the
mitochondrial transport chain through an electron transfer pathway,
comprised of ETF-ubiquinone oxidoreductase (ETF-QO) and ubiquinone.
Therefore, ETF acts as an electron acceptor of energy production
from amino acid and fatty acids (5), which accepts electrons from
flavoprotein dehydrogenases (6)
and transfers them to ETF-QO in the inner mitochondrial membrane
(7–9). Deficiency in human ETF results in a
metabolic disease known as multiple acyl-CoA dehydrogenation
deficiency (MADD) or glutaric acidemia type 2, and can even cause
death in the neonatal period (10).
In the present study, in order to examine the role
of ETFβ in DN, a spontaneous type 2 diabetic model using Otsuka
Long-Evans Tokushima fatty (OLETF) rats was used in the present
study. This rat model is characterized by obesity, insulin
resistance, hyperglycemia, dyslipidemia and renal complications
(11). More importantly, the
renal abnormalities noted in OLETF rats resemble those of type 2
diabetic patients (11).
Long-Evans Tokushima Otsuka (LETO) rats are the control counterpart
animal model; these rats were developed from the same colony but do
not develop diabetes. We previously observed a mutation of the ETFβ
gene in the renal cortex of diabetic OLETF rats, which occured in
conservative regions. The ETFβ gene mutation of H88L in OLETF rats
suggested that ETFβ possibly plays a role in DN (unpublished data).
Thus, in the present study, we examined the expression of ETFβ in
DN patients and rats, and explored the mechanism of action in cell
lines.
Materials and methods
Human kidney biopsy samples
Human kidney tissues were collected from patients in
the Chinese Medicine Hospital of Shaanxi (Xi'an, China). The
medical information of patients was obtained by review of medical
records and the tissues for clinical diagnosis were obtained from
archived kidney biopsy specimens or discarded nephrectomy samples.
The kidney tissues were from patients diagnosed with DN or kidney
carcinoma without diabetes as the control. All patients with DN
were diagnosed based on the presence of diabetes, massive
proteinuria, and other histological changes typical of DN. Patients
were aged between 48 and 65 years, the female:male ratio was 1:1,
and proteinuria ranged from 2.3 to 4.3 g/24 h. Normal tissues
around the tumor from kidney carcinoma patients were employed as
the normal control; these patients were aged 51–65 years, the
female:male ratio was 1:2, and proteinuria <0.2 g/24 h. The
patients were matched in terms of age and gender information.
Kidney tissues from the patients were fixed in 10%
phosphate-buffered formalin solution, embedded in paraffin, and
then sectioned to 3-µm thickness and stained with periodic
acid-Schiff (PAS), TUNEL or used for immunohistochemical analyses.
The study was approved by the Research Ethics Board of the Chinese
Medicine Hospital of Shaanxi, and all patients provided informed
consent.
Animals
Male OLETF rats and corresponding controls,
4-week-old LETO rats, were kindly provided by the Tokushima
Research Institute (Otsuka Pharmaceutical Co., Ltd., Tokushima,
Japan). During the experiments, rats were housed at 22±3°C in an
atmosphere with 50±10% humidity and a 12-h light/dark cycle.
Animals had free access to standard rat chow and tap water. Seven
rats from the LETO control group and seven rats from the OLETF
group were sacrificed using chloral hydrate at 36 weeks of age. The
remaining seven rats of each group were sacrificed at 56 weeks of
age. After sacrifice, blood samples were collected from the
abdominal aorta, and kidneys were removed and separated for
histopathological examination and biological analysis. Paraffin
sections from rats were made as described above. The severity of DN
was assessed by measuring urinary protein levels and PAS staining
of kidney tissues with subsequent histopathological analysis.
Animal experiments were performed in accordance with the Guide for
the Care and Use of Laboratory Animal of the National Institutes of
Health. Procedures were approved by the Ethics Committee of Animal
Experiments of the China-Japan Friendship Hospital (Beijing, China)
(no. 08013).
Measuring body weight, blood glucose,
blood lipids and urinary protein levels
The body weight of rats was measured at 4-week
intervals. Blood was sampled from the tail vein also at 4-week
intervals, and blood glucose was measured using a OneTouch Ultra II
Blood Glucose monitoring system (LifeScan, Inc., Milpitas, CA,
USA). Total cholesterol (CHO) and triglyceride (TG) concentrations
were measured using an AutoAnalyzer (Abbott Diagnostics, Abbott
Park, IL, USA). Rats were housed in individual metabolic cages
(Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, Jiangsu,
China) for 24 h urine collection at 4-week intervals. Urinary
protein was assessed using the Bradford method.
Kidney histological and
immunohistochemical analyses
In the present study, PAS staining was performed on
paraffin-embedded tissues from patients and rats using standard
protocols. The degree of glomerulosclerosis, which was defined as a
thickening of the basement membrane and mesangial expansion, was
evaluated in 40 counted glomeruli using a microscope at ×400
magnification (BX51; Olympus, Tokyo, Japan), as previously
described (12). The observer was
blinded to all tissue samples. Tubulointerstitial injury was
assessed in PAS-stained paraffin sections from rats at ×200
magnification using a similar scoring system (grades 1–6) as has
been previously described (12).
Briefly, 10 random cortical fields under ×200 magnification were
outlined and positive staining patterns were subsequently
identified. The percentage of positive area in the examined field
was then measured, as previously described (13). Data are expressed as the means ±
SE. All counting was performed on blinded slides.
Immunohistochemical analysis was performed as
described in our previous study (12). Briefly, rat kidneys from each
group were fixed in 10% formalin, embedded in paraffin, and cut
into 3-µm thick sections followed by rehydration. Antigen
retrieval was undertaken in 10 mM citrate buffer (pH 6.0) at
90–95°C for 25 min. Kidney sections were incubated with polyclonal
goat ETFβ antibody (1:200; sc-242638) (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), overnight at 4°C. Following washing, slides
were incubated with anti-rabbit secondary antibody in the same
buffer. Immunohistochemical staining was performed using the GT
Vision III Detection System/Mo Rb (Gene Tech Biotechnology Co.,
Ltd., Shanghai, China) and captured using a light microscope
(Olympus) and quantified using Image Pro-Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Cell culture and RNA interference
The normal rat kidney epithelial cell line (NRK-52E)
was purchased from the Institute of Biochemistry and Cell Biology
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (DMEM; HyClone, Logan, UT, USA) supplemented with 25 mM
D-glucose, 5% fetal bovine serum (FBS; Gibco Life Technologies,
Carlsbad, CA, USA) in an incubator with 5% CO2 at 37°C.
Cells were cultured until they reached 70% confluence, and this was
followed by transfection with siRNA for 48 h.
NRK-52E cells were transfected with siRNA (20
µm) targeting ETFβ or scrambled siRNA as negative control
using Lipofectamine 3000 reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The siRNA sequences were as follows: sense,
5′-GGUGACUGCUGACUUAAGATT-3′ and antisense,
5′-UCUUAAGUCAGCAGUCACCTT-3′ (purchased from GenePharma, Shanghai,
China). The scrambled siRNA sequences were as follows: sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. After transfection for 48 h, cells
were harvested for total protein extraction or TUNEL detection.
Untreated cells with no transfection were used as the nomal
group.
TUNEL assay
For in vivo detection of apoptosis, apoptotic
cells in the human and rat kidneys were examined by TUNEL assay
according to the manufacturer's instructions (Roche Diagnostics
GmbH, Mannheim, Germany). After formalin-fixed sections were
dewaxed, the slides were heated with a microwave for 1 min in 0.1 M
citrate buffer (pH 6.0), and rapidly cooled by adding
doubled-distilled water and then transferred to PBS. The slides
were blocked with buffer comprising 0.1 M Tris-Hcl, 3% BSA and 20%
bovine serum (pH 7.5) for 30 min at room temperature. DNA fragments
were then labeled by terminal transferase dUTP for 30 min at 37°C.
In order to quantify apoptosis, the positively stained cells were
calculated in 10 randomly selected fields in each slide as
previously described (14).
For in vitro detection of apoptosis, NRK-52E
cells were seeded in 96-well plates and transfected with ETFβ siRNA
for 48 h as mentioned above. The cells were then rinsed twice,
fixed in 4% paraformaldehyde for 20 min, and subsequently
permeabilized with 0.1% Triton X-100 in PBS/BSA solution. TUNEL
assay was performed according to the manufacturer's instructions
and quantified as above.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from rat kidney cortices
with TRIzol reagent (Invitrogen Life Technologies). cDNA was
synthesized using a RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Waltham, MA, USA), and RT-qPCR was
performed using UltraSYBR Mixture (CWBio, Inc., Beijing, China).
Amplification was performed as follows: initial denaturation at
95°C for 2 min, followed by 40 cycles of denaturation at 95°C for
30 sec, annealing at 60°C for 30 sec and final extension at 72°C
for 2 min, using an ABI 7500 thermocycler (Applied Biosystems,
Foster, CA, USA). The primers used to detect ETFβ were: forward,
5′-GTACATTCGCCTCTCAGGTG-3′ and reverse, 5′-TACTCCTGCTAAGCGCTGAG-3′.
The β-actin primers were forward, 5′-GACATCCGTAAAGACCTCTATGCC-3′
and reverse, 5′-ATAGAGCCACCAATCCACACAGAG-3′. Results were
normalized to β-actin expression using the ΔΔC(t) method.
Western blot analysis
In the present study, proteins for western blot
analysis were extracted from the rat kidney tissues or NRK-52E
cells by lysing with radioimmunoprecipitation assay (RIPA) buffer,
as previously described (12).
Mitochondrial proteins from rat kidneys were extracted and
separated from cytosolic proteins as previously described (15). Briefly, the tissues were
homogenized in 0.25 M sucrose and 10 mM Hepes, pH 7.0, in a glass
homogenizer equipped with a motor-driven Teflon Pestle (1,000 rpm,
five up-and-down). Mitochondria were prepared by centrifugation for
70 min at 70,000 × g twice. Mitochondria were first collected at
the interface 0.25 M/1.6 M sucrose and then recovered under 1.45 M
sucrose. As final purification steps, mitochondria pellets were
submitted to successive washes at 23,000 × g, for 10 min and stored
in liquid nitrogen. Protein concentration was tested by Bradford
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
extracts (30–40 µg) were loaded on 12% SDS-PAGE and analyzed
by western blot analysis. After blocking with 5% skim milk,
membranes were then incubated with the following primary
antibodies: ETFβ goat antibody at a 1:1,000 dilution (sc-242638),
BAX mouse antibody at a dilution of 1:1,000 (sc-7480) and Bcl-2
mouse antibody at a dilution of 1:1,000 (sc-7382) (all purchased
from Santa Cruz Biotechnology, Inc.) and cleaved caspase-3 rabbit
antibody at a dilution of 1:500 (SAB 4503294) (Sigma-Aldrich, St.
Louis, MO, USA). β-actin mouse antibody was used as the loading
control for total protein at a dilution of 1:3,000 (KM 9001)
(Tianjin Sungene Biotech Co., Ltd., Tianjin, China), and VDAC
rabbit antibody (#4866) (Cell Signaling Technology, Danvers, MA,
USA) was used as the loading control for mitochondrial protein at a
dilution of 1:1,000, overnight. Bands were detected using an ECL
Western Blot Detection kit (Amersham Pharmacia Biotech, San
Francisco, CA, USA) and subsequently quantified by densitometry
using Quantity One software.
Statistical analyses
All values are expressed as the means ± SEM. Results
were analyzed using ANOVA or two-tailed Student's t-test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Decreased expression of ETFβ in kidneys
of patients with DN is accompanied by excessive numbers of
apoptotic cells
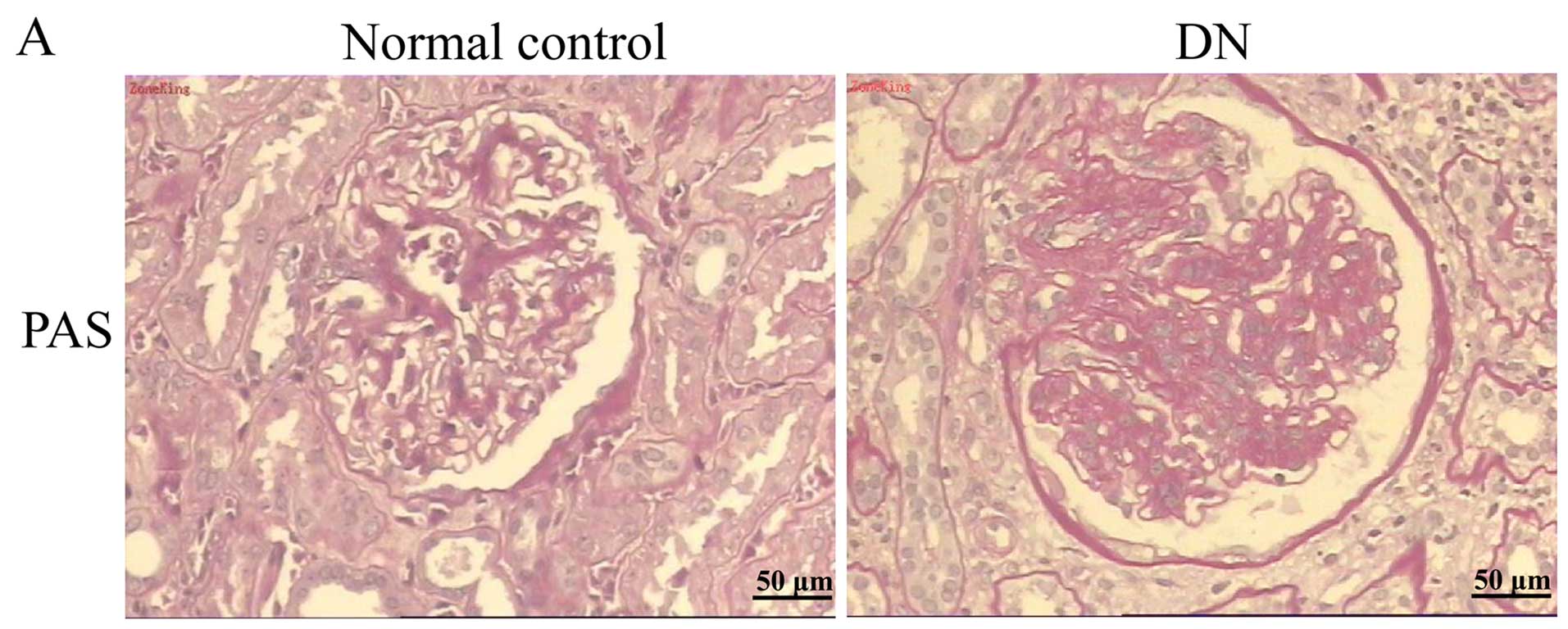
Peri-carcinoma tissues from patients were used as a
control, and kidney tissues from patients with DN were enrolled in
the study; ten patients with DN and three patients with kidney
carcinoma were enrolled in the study. Biopsies confirmed the
presence of the disease, and PAS staining provided evidence for
these pathological features (Fig.
1A). We examined expression patterns of ETFβ protein in the
kidney cortices of non-diabetic and diabetic humans. Compared with
the normal tissue around the tumors, the expression of ETFβ was
greatly reduced in kidney biopsy samples from patients with DN
(Fig. 1B). In addition, we noted
that the distribution of ETFβ was primarily in the TECs, but rarely
in the glomeruli. Excessive apoptotic nuclei were observed in the
kidney tissues of DN patients using a TUNEL assay (Fig. 1C). These findings are suggestive
of a possible association between decreased tubular ETFβ expression
and TEC apoptosis in cases of human DN.
Progression of DN in OLETF rats
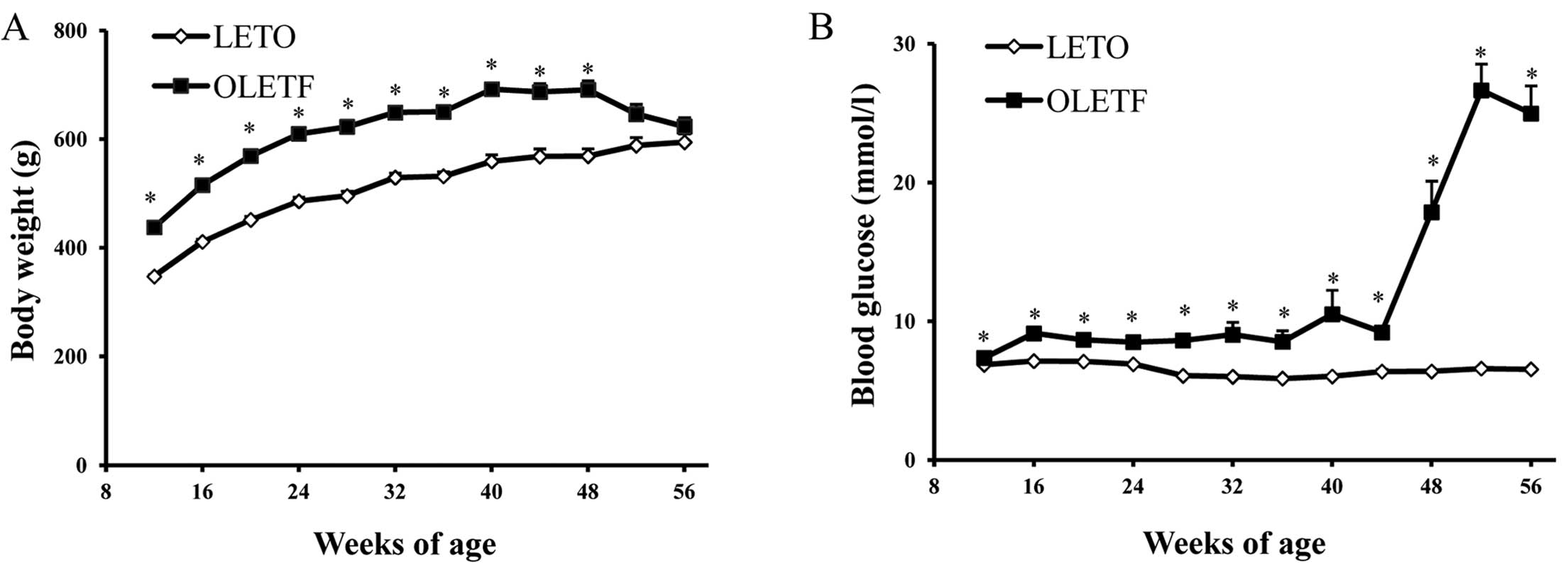
In the present study, the body weight of OLETF rats
was significantly greater than that of LETO rats from 12 to 48
weeks (Fig. 2A). However, as
diabetes developed, no significant difference was observed between
53 and 56 weeks. The blood glucose levels of OLETF rats were
significantly elevated at 48 weeks and peaked at 53 weeks (Fig. 2B), which was associated with the
development of proteinuria (Fig.
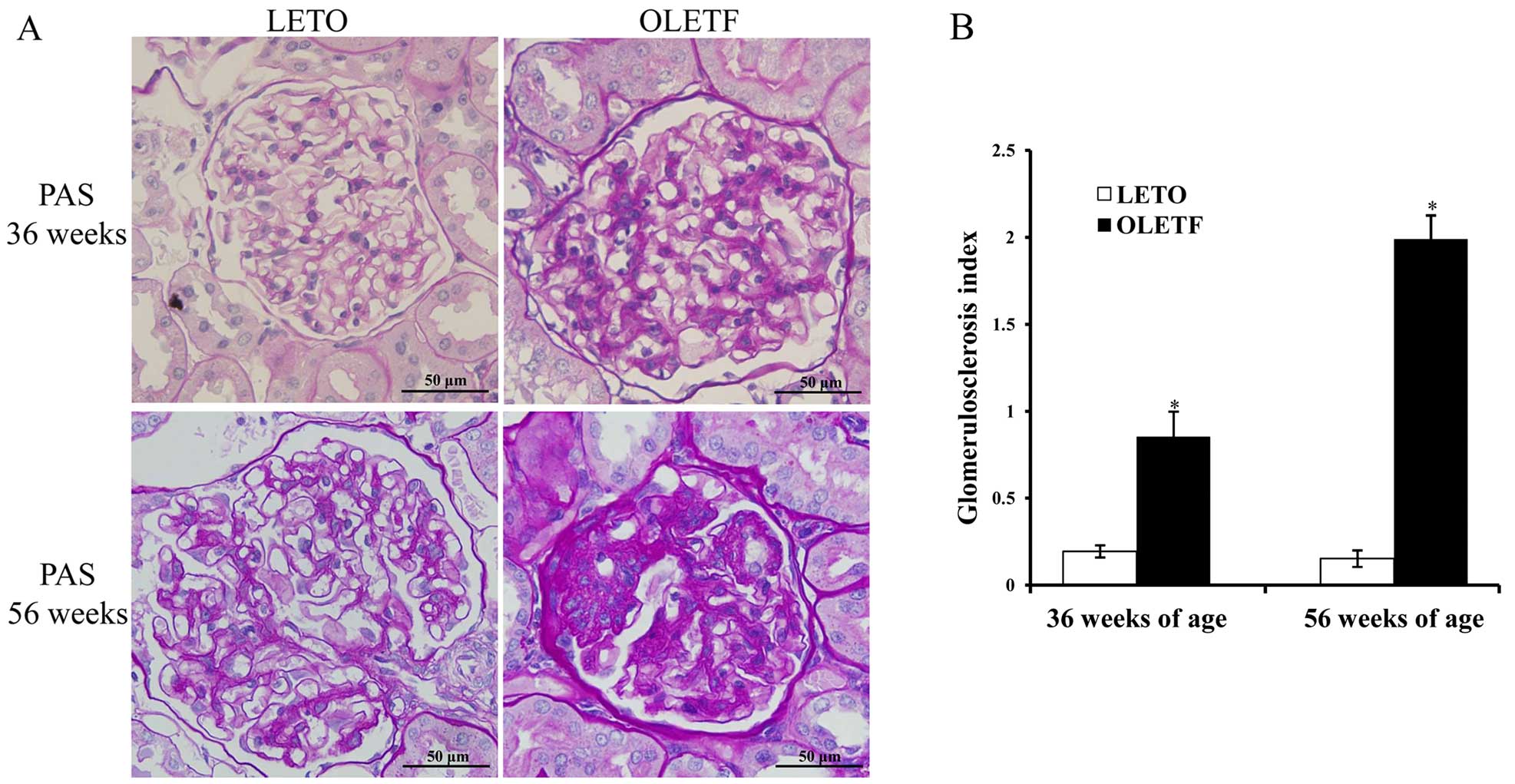
2D), glomerulosclerosis index and tubulointerstitial injury
index (Fig. 3). Moreover, high
levels of blood lipids, including serum CHO and TG concentration
were marked at 56 weeks of age (Fig.
2C). Serum TG level increased at an early stage, whereas serum
CHO levels were elevated later. There was mild glomerular and
tubular injury as early as 36 weeks of age, and this then
progressed to severe glomerulosclerosis and tubular atrophy and
tubulointerstitial fibrosis at 56 weeks (Fig. 3).
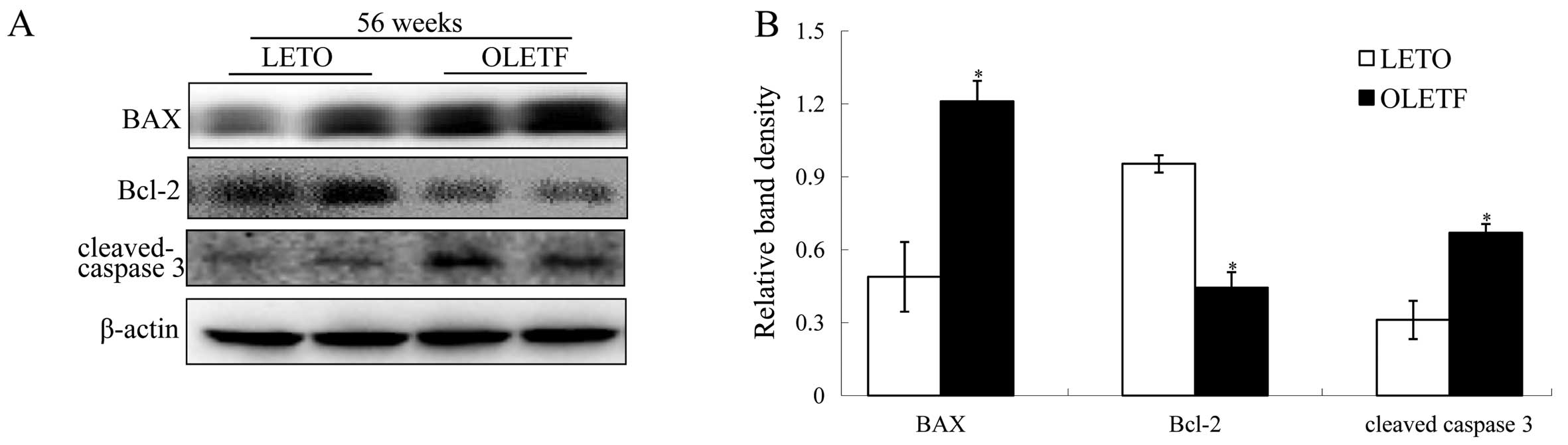
In the kidney cortices of OLETF rats, elevated
expression of BAX/Bcl-2 and cleaved caspase-3 occurred only at 56
weeks of age (Fig. 4A and B),
whereas there was no significant change in expression at 36 weeks
of age (P>0.05, data not shown). In agreement with the molecular
changes involved in apoptosis, the occurrence of apoptosis in the
diabetic kidneys also increased in 56-week-old OLETF rats, as
evidenced by the results of the TUNEL assay (Fig. 4C and D).
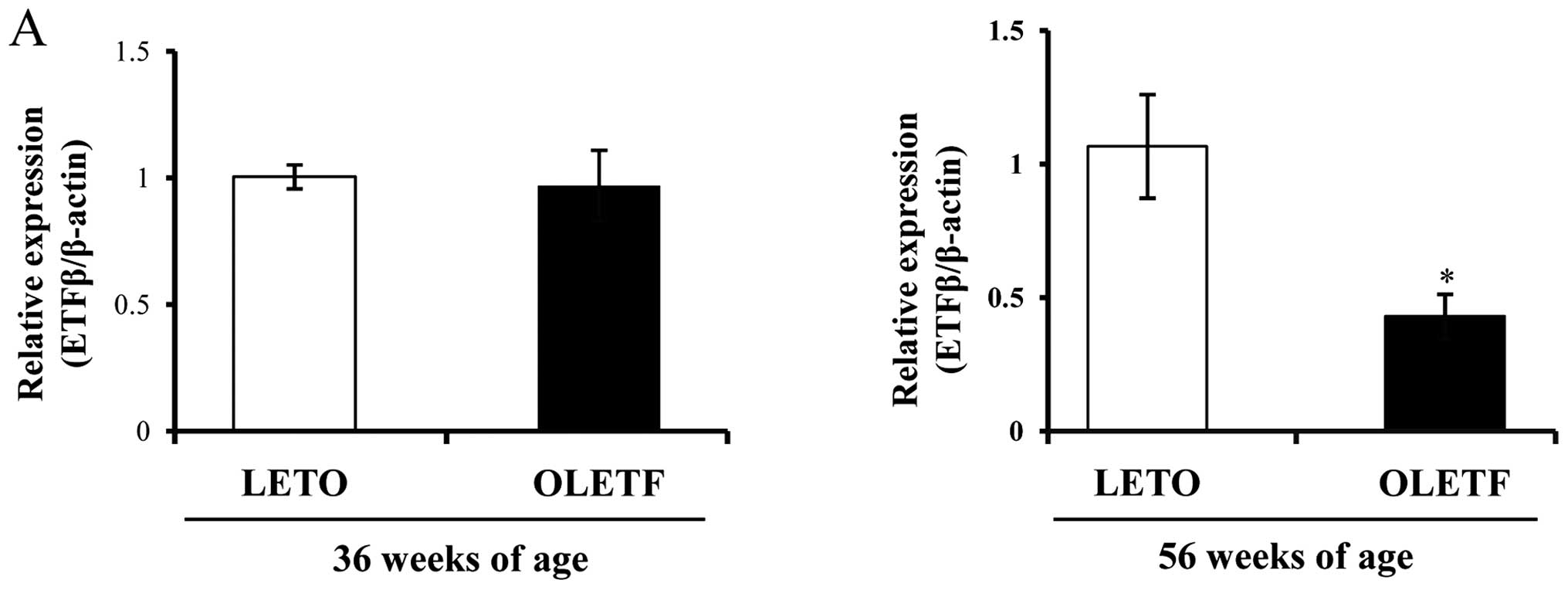
Decreased expression of ETFβ in diabetic
kidney and the progression of DN in OLETF rats
By contrast, we noted that the expression of ETFβ
was high in the kidneys of control LETO rats, but it was markedly
decreased in the kidneys of diabetic OLETF rats (Fig. 5). ETFβ mRNA and protein levels
were both markedly downregulated only at 56 weeks of age (Fig. 5A and B). ETFβ expression was also
reduced in mitochondria, which confirmed the ETFβ decrease prior to
loss of mitochondria, since we separated the mitochondrial fraction
and tested ETFβ expression with VDAC as a mitochondria control
(Fig. 5C). Immunohistochemistry
results indicated that ETFβ was widely expressed and located in
normal tubular region, compared with glomerular region (Fig. 5D and E).
In 56-week-old OLETF rats we noted increased levels
of BAX/Bcl-2 ratio and cleaved caspase 3, and decreased ETFβ in
kidneys, which was linked with TEC apoptosis, as demonstrated by
the significant increase in TUNEL-positive cells (Fig. 4).
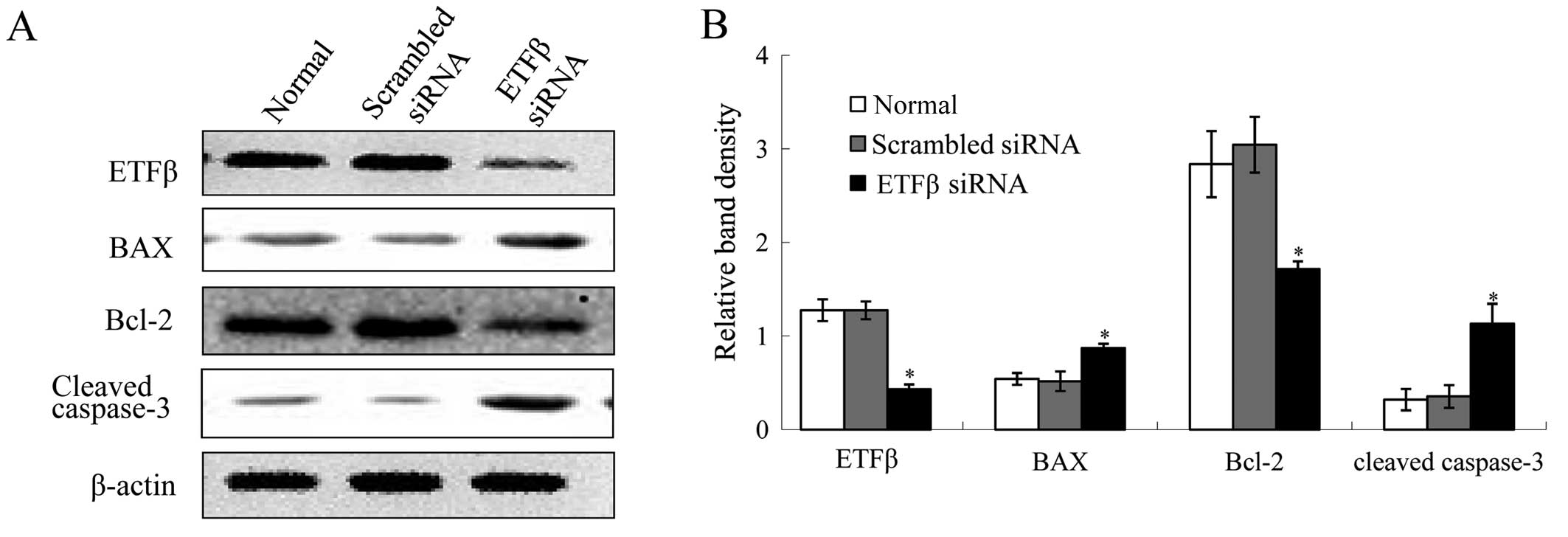
Knockdown of ETFβ results in NRK-52E cell
apoptosis
In order to better understand the functional role of
ETFβ in TEC apoptosis, we knocked down ETFβ in NRK-52E cells. As
shown in Fig. 6A and B, knockdown
of ETFβ reduced the protein expression levels of ETFβ
significantly, which elevated expressions of BAX and cleaved
caspase-3. There was also decreased expression of Bcl-2. Moreover,
in ETFβ siRNA treated cells, inhibiton of ETFβ expression caused
increased apoptosis of cells compared with normal cells or cells
transfected with scrambled siRNA (Fig. 6C).
Discussion
Although tubular atrophy and tubulointerstitial
fibrosis associated with tubular apoptosis are the most convincing
evidence for the progression of DN (16), the mechanisms underlying this
progression were poorly understood. In the present study, we noted
excessive apoptosis, which was associated with progressive renal
injury in diabetic patients and rats. To our surprise, we
discovered that a significant decrease in ETFβ was associated with
an increase in TEC apoptosis and progressive renal injury in the
diabetic patients and rats, which suggests a negative link between
ETFβ expression and TEC apoptosis. Importantly, downregulation of
ETFβ by employing siRNA resulted in significant apoptosis of
NRK-52E cells. Therefore, we suggest that decreased expression of
ETFβ is closely related to apoptosis in TECs.
Tubular cell apoptosis occurs earlier than the onset
of kidney fibrosis, and results in matrix accumulation in the
tubulointerstitium (17).
Previous research has shown that apoptosis is one of the
pathological features of progressive kidney injury in patients with
diabetes (18). Oxidative stress
and mitochondrial dysfunction have been reported to contribute to
tubular cell apoptosis (19,20), but the mechanisms mediating
excessive apoptosis during the course of DN have not been fully
explored. Downregulation of ETFβ associated with type 2 diabetes
was noted using a quantitative proteomic approach and mouse
pancreatic islets (21). In the
present study, we discovered that decreased ETFβ resulted in
tubular cell apoptosis, which is a cause of DN.
In the present study, we found that decreased ETFβ
expression was accompied by tubular cell apoptosis both in human
samples and rat kidney samples. The elevated BAX/Bcl-2 ratio in
diabetic OLETF rats activates subsequent apoptotic signaling and
cleaves pro-caspase-3 to the active form (22). Cleaved caspase-3 expression is
considered a hallmark of apoptosis (23), and our data demonstrated eleveated
cleaved caspase-3 expression in the kidney cortices of 56-week-old
diabetic rats, which was consistent with decreased expression of
ETFβ and increased TUNEL-positive stainings.
The suppression of ETFβ in TECs may be a result of
the diabetic conditions of high glucose and dyslipidemia; in fatty
acid β-oxidation, amino acid oxidation, and choline metabolism, ETF
is positioned at a key metabolic branch point, as it accepts
electrons from at least nine dehydrogenases and transfers them to
the membrane-bound respiratory chain (24). According to functional and
structural research of ETFβ, the absence of the ETFβ subunit is a
result of its role as a 'fixed' electron carrier, but it should be
flexible to adapt different structural dehydrogenases upstream
(8). However, the mechanism by
which ETFβ is regulated in cases of diabetes is still unclear and
requires further investigation. Previous study has demonstrated
that elevation of upstream dehydrogenase by peroxisome
proliferator-activated receptor δ attenuated apoptosis induced by
fatty acids in a β-cell line (25). Moreover, the expression of ETF is
significantly downregulated in human vein endothelial cells when
apoptosis is induced by digoxin (26).
The results of our present study demonstrated that
decreased expression of ETFβ was associated with TEC apoptosis,
suggesting that ETFβ plays a protective role in TEC apoptosis. In
the tubular regions, the mitochondrial β-oxidation pathway is much
better developed than in glomerular regions (27). Therefore, as the electron receptor
for the first reaction of fatty acid β-oxidation, ETFβ deficiency
in the tubular cells may cause decreased energy supply (28). Also, since the physiological
upstream acceptors for the electrons are missing, decreased
expression of ETFβ may result in generation of excessive ROS
(29). Oxidative stress induced
by ROS has been suggested to be a common mediator in apoptosis
(30,31) and particularly in DN, a state in
which oxidative stress is increased by high glucose and
dyslipidemia (32).
In conclusion, for the first time to the best of our
knowledge, the present study provided evidence that ETFβ expression
is decreased in kidneys of diabetics, and this is associated with
TEC apoptosis and renal injury. Moreover, downregulation of ETFβ by
siRNA induces apoptosis of renal tubular cells. In conclusion,
decreased expression of ETFβ in DN is associated with TEC apoptosis
during the progression of diabetes.
Acknowledgments
The present study was supported by a Project of
International Collaboration in Science and Technology Grant, China
(grant no. 2011DFA31860), the National Major Scientific and
Technological Special Project for 'Significant New Drugs
Development' during the Twelfth Five-year Plan Period (no.
2012ZX09103201-014) and the National Natural Science Foundation of
China (grant no. 81173422 and 81130066). We gratefully acknowledge
Frank J. Burczynski for a critical reading and language revision of
the manuscript. We also thank Nissi S. Wang for assistance with
editing the manuscript.
References
|
1
|
Shaheen FA and Al-Khader AA: Epidemiology
and causes of end stage renal disease (ESRD). Saudi J Kidney Dis
Transpl. 6:277–281. 2005.
|
|
2
|
Okamura DM, Pasichnyk K, Lopez-Guisa JM,
Collins S, Hsu DK, Liu FT and Eddy AA: Galectin-3 preserves renal
tubules and modulates extracellular matrix remodeling in
progressive fibrosis. Am J Physiol Renal Physiol. 300:F245–F253.
2011. View Article : Google Scholar :
|
|
3
|
Futrakul N and Futrakul P: Renal
microvascular and tubular injuries in type II diabetic nephropathy.
Kidney Int. 74:390author reply 390–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beyenbach KW: Kidneys sans glomeruli. Am J
Physiol Renal Physiol. 286:F811–F827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishizaki K, Schauer N, Larson TR, Graham
IA, Fernie AR and Leaver CJ: The mitochondrial electron transfer
flavoprotein complex is essential for survival of Arabidopsis in
extended darkness. Plant J. 47:751–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Covington MD and Schnellmann RG: Chronic
high glucose downregulates mitochondrial calpain 10 and contributes
to renal cell death and diabetes-induced renal injury. Kidney Int.
81:391–400. 2012. View Article : Google Scholar
|
|
7
|
Ruzicka FJ and Beinert H: A new
iron-sulfur flavoprotein of the respiratory chain. A component of
the fatty acid beta oxidation pathway. J Biol Chem. 252:8440–8445.
1977.PubMed/NCBI
|
|
8
|
Toogood HS, Leys D and Scrutton NS:
Dynamics driving function: new insights from electron transferring
flavoproteins and partner complexes. FEBS J. 274:5481–5504. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Frerman FE and Kim JJ: Structure
of electron transfer flavoprotein-ubiquinone oxidoreductase and
electron transfer to the mitochondrial ubiquinone pool. Proc Natl
Acad Sci USA. 103:16212–16217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodman SI, Binard RJ, Woontner MR and
Frerman FE: Glutaric acidemia type II: gene structure and mutations
of the electron transfer flavoprotein:ubiquinone oxidoreductase
(ETF:QO) gene. Mol Genet Metab. 77:86–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawano K, Hirashima T, Mori S, Saitoh Y,
Kurosumi M and Natori T: Spontaneous long-term hyperglycemic rat
with diabetic complications. Otsuka Long-Evans Tokushima Fatty
(OLETF) strain. Diabetes. 41:1422–1428. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao TT, Zhang HJ, Lu XG, Huang XR, Zhang
WK, Wang H, Lan HY and Li P: Chaihuang-Yishen granule inhibits
diabetic kidney disease in rats through blocking TGF-β/Smad3
signaling. PLoS One. 9:e908072014. View Article : Google Scholar
|
|
13
|
Huang XR, Chung AC, Zhou L, Wang XJ and
Lan HY: Latent TGF-beta1 protects against crescentic
glomerulonephritis. J Am Soc Nephrol. 19:233–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelsen S, He X and Chade AR: Early
superoxide scavenging accelerates renal microvascular rarefaction
and damage in the stenotic kidney. Am J Physiol Renal Physiol.
303:F576–F583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White KE and Bilous RW: Type 2 diabetic
patients with nephropathy show structural-functional relationships
that are similar to type 1 disease. J Am Soc Nephrol. 11:1667–1673.
2000.PubMed/NCBI
|
|
17
|
Docherty NG, O'Sullivan OE, Healy DA,
Fitzpatrick JM and Watson RW: Evidence that inhibition of tubular
cell apoptosis protects against renal damage and development of
fibrosis following ureteric obstruction. Am J Physiol Renal
Physiol. 290:F4–F13. 2006. View Article : Google Scholar
|
|
18
|
Kumar D, Robertson S and Burns KD:
Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem.
259:67–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verzola D, Bertolotto MB, Villaggio B,
Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT,
Garibotto G and Deferrari G: Oxidative stress mediates apoptotic
changes induced by hyperglycemia in human tubular kidney cells. J
Am Soc Nephrol. 15(Suppl 1): S85–S87. 2004. View Article : Google Scholar
|
|
20
|
Chen JF, Liu H, Ni HF, Lv LL, Zhang MH,
Zhang AH, Tang RN, Chen PS and Liu BC: Improved mitochondrial
function underlies the protective effect of pirfenidone against
tubulointerstitial fibrosis in 5/6 nephrectomized rats. PLoS One.
8:e835932013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu H, Yang Y, Allister EM, Wijesekara N
and Wheeler MB: The identification of potential factors associated
with the development of type 2 diabetes: a quantitative proteomics
approach. Mol Cell Proteomics. 7:1434–1451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan J and Horvitz HR: A first insight
into the molecular mechanisms of apoptosis. Cell. 116(Suppl 2):
S53–S56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huppertz B, Frank HG and Kaufmann P: The
apoptosis cascade - morphological and immunohistochemical methods
for its visualization. Anat Embryol (Berl). 200:1–18. 1999.
View Article : Google Scholar
|
|
24
|
Roberts DL, Frerman FE and Kim JJ:
Three-dimensional structure of human electron transfer flavoprotein
to 2.1-A resolution. Proc Natl Acad Sci USA. 93:14355–14360. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan J, Jiang L, Lü Q, Ke L, Li X and Tong
N: Activation of PPARdelta up-regulates fatty acid oxidation and
energy uncoupling genes of mitochondria and reduces
palmitate-induced apoptosis in pancreatic beta-cells. Biochem
Biophys Res Commun. 391:1567–1572. 2010. View Article : Google Scholar
|
|
26
|
Qiu J, Gao HQ, Liang Y, Yu H and Zhou RH:
Comparative proteomics analysis reveals role of heat shock protein
60 in digoxin-induced toxicity in human endothelial cells. Biochim
Biophys Acta. 1784:1857–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bastin J, Djouadi F, Geloso JP and
Merlet-Benichou C: Postnatal development of oxidative enzymes in
various rat nephron segments: effect of weaning on different diets.
Am J Physiol. 259:F895–F901. 1990.PubMed/NCBI
|
|
28
|
Hirokawa S, Shimanuki T, Kitajima H,
Nishimori Y and Shimosaka M: Knockdown of electron transfer
flavoprotein β subunit reduced TGF-β-induced α-SMA mRNA expression
but not COL1A1 in fibroblast-populated three-dimensional collagen
gel cultures. J Dermatol Sci. 68:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosca MG, Vazquez EJ, Chen Q, Kerner J,
Kern TS and Hoppel CL: Oxidation of fatty acids is the source of
increased mitochondrial reactive oxygen species production in
kidney cortical tubules in early diabetes. Diabetes. 61:2074–2083.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buttke TM and Sandstrom PA: Oxidative
stress as a mediator of apoptosis. Immunol Today. 15:7–10. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jee SH, Kim HJ and Lee J: Obesity, insulin
resistance and cancer risk. Yonsei Med J. 46:449–455. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horie K, Miyata T, Maeda K, Miyata S,
Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum
JL and Kurokawa K: Immunohistochemical colocalization of
glycoxidation products and lipid peroxidation products in diabetic
renal glomerular lesions. Implication for glycoxidative stress in
the pathogenesis of diabetic nephropathy. J Clin Invest.
100:2995–3004. 1997. View Article : Google Scholar
|