Introduction
Increasing evidence has shown that heat stress (HS;
namely, the heat overload induced by high temperatures and high
humidity) negatively affects thermoregulation, limiting sweat
evaporation and thus leading to high body temperatures and a series
of heat stress responses (1). It
has been suggested that the cardiovascular system is primarily
targeted by exposure to HS and it is the most easily injured
(2), which may result in the
development of a series of heat-related illnesses, including heat
cramps, heat syncope, heat exhaustion, heat stroke and even death
(3). Mitochondria are abundantly
distributed in cardiomyocytes due to the high energy demands of the
heart (4), and mitochondria are
vulnerable to various types of stress stimuli (3). The impairment of multiple aspects of
mitochondrial function has been reported to be one of the principal
events occuring in cardiomyocytes subjected to stress, including
the increased permeability of the mitochondrial membrane, decreased
membrane potential and the increased release of cytochrome c
and apoptosis-inducing factor (AIF) from the nucleus into the
cytoplasm (5). Furthermore,
previous research has indicated that HS reduces blood flow, which
leads to a lower oxygen supply and cellular hypoxia; hypoxia in
turn results in the excessive production of reactive oxygen species
(ROS) and oxidative stress (6).
ROS, including superoxide and hydrogen peroxide, are capable of
destroying the structure and function of the cellular membrane, and
of damaging the mitochondria, eventually leading to cell death
(7). Hence, targeting
mitochondrial injury and the metabolism of ROS may confer
cytoprotective effects on heat-stressed cardiomyocytes (exposed to
high temperatures and humidity), and may thus provide a therapeutic
strategy for cardiovascular diseases induced by exposure to HS.
To date, we have already elucidated one of the
mechanisms responsible for the development of HS-related diseases;
namely, the angiotensin II signaling pathway, which is involved in
HS-induced oxidative stress and in the apoptosis of cardiomyocytes
(8). Furthermore, we have proved
in a previous study that a drug, geranylgeranylacetone, is
effective at promoting the expression of heat shock protein 70
(Hsp70) (9). Hsp70 has been
reported to confer effective cytoprotection against various types
of stress stimuli (10).
Metallothionein (MT) is also considered an endogenous
cytoprotective molecule, mainly participating in the protection of
the cardiovascular system (11).
MT has also been reported to be involved in metal homeostasis and
detoxification, radical scavenging and in maintaining the integrity
of membrane structures (12).
However, the protective effects exerted by MT on cardiomyocytes
under conditions of HS remain to be elucidated. It is widely
accepted that vitamin E (VE) is an effective antioxidant (13). In the present study, we
hypothesized that VE may exert a synergistic effect with MT, both
of which participate in the maintenance of mitochondrial function
and ROS levels under conditions of HS.
The tropical marine climate of Southern China, which
is usually hot, wet and rainy, results in excessive heat and
humidity which may account for the high morbidity associated with
cardiovascular diseases in this region. However, little is known
regarding therapeutic approaches which confer cytoprotective
effects on cardiomyocytes under conditions of HS. Thus, in the
present study, we aimed to examine the effects of VE on
cardiomyocytes under conditions of HS. For this purpose, mice were
housed in an artificial environment in order to mimic conditions of
HS. Pre-treatment with VE increased the expression of MT under
conditions of HS. The restoration of mitochondrial function was
indicated by the upregulation of peroxisome proliferator-activated
receptor-γ coactivator-1α (PGC-1α) and nuclear respiratory factor 1
(NRF-1), mitochondrial transcription factor A (TFAM), and increased
adenosine triphosphate (ATP) levels. Moreover, conditions of HS
increased the production of ROS and led to oxidative stress, and
these effects were counteracted by pre-treatment with VE. The
decrease in oxidative stress following treatment with VE was
evidenced by the increased levels of antioxidant enzymes
[superoxide dismutase (SOD) and glutathione (GSH)], and by the
decreased levels of markers of oxidative injury [malondialdehyde
(MDA) and lactate dehydrogenase (LDH)]. Taken together, these
findings demonstrate that pre-treatment with VE confers
cytoprotective effects on cardiomyocytes, possibly by increasing MT
expression under conditions of HS. Our study provides evidence that
VE may be used for the prevention and treatment of cardiovascular
diseases induced by exposure to HS.
Materials and methods
Animals
Eight-week-old BALB/c mice, weighing 30–35 g, were
purchased from the Laboratory Animal Center of Guangdong Province
(Foshan, China). The mice were housed in a pathogen-free
environment at a constant temperature of 20.0±2°C under a 12-h day
and night cycle and fed a routine diet with free access to water.
All animal experimental procedures were performed according to the
guidelines of the National Health and Medical Research Council for
the Care and Use of Animals for Experimental Purposes in China.
This study was approved by the Ethics Committee of Guangzhou
General Hospital of Guangzhou Military Command (Guangzhou, China).
All efforts were made to minimize suffering and all the mice
survived in this study without developing any infections. The mice
were euthanized by an intraperitoneal injection of pentobarbital
sodium (100 mg/kg, Merck KGaA, Darmstadt, Germany).
Establishment of the model of HS and
experimental grouping
In order to mimic HS, a hot chamber was used to
create an environment with a designated room temperature
(40.0±0.05°C) and relative humidity (60±5%). Normal temperature and
humidity (NTH) conditions were designated as a room temperature of
24.0±1°C and relative humidity of 45±5%. A total of 40 mice was
randomly allocated to 1 of the following 4 groups: i) 10 mice
treated with the vehicle were housed under conditions of NTH; ii)
10 mice treated with VE were housed under conditions of NTH; iii)
10 mice treated with the vehicle were housed under conditions of HS
for 4 h per day; and iv) 10 mice treated with VE were housed under
conditions of HS for 4 h per day. VE (Novartis Co., Ltd., Tokyo,
Japan) dissolved in ethyl alcohol was administered orally (500
mg/kg) using feeding needles, as previously reported (14), 2 h prior to the initiation of the
experiment. The equivalent volume of ethyl alcohol was used as the
vehicle, which was also administered orally using feeding needles.
This experiment was conducted over a 4-week period.
Measurement of ATP levels
The mice were treated as described above and
sacrificed under deep anesthesia. The mouse hearts were quickly
excised and frozen in liquid nitrogen in preparation for subsequent
use. The ATP levels in the heart tissues were measured using an ATP
assay kit (Beyotime, Shanghai, China) according to the
manufacturer's instructions. Briefly, heart tissues were subjected
to ATP Detection Lysis buffer, followed by centrifugation at 13,000
× g for 5 min, and the supernatant was added to the substrate
solution. The relative light units were measured using a GloMax™ 96
microplate luminometer (Promega, Madison, WI, USA). A standard
curve was used to calculate the ATP concentration.
Ferric reducing/antioxidant power (FRAP)
assay
A working FRAP solution was prepared by mixing 10
volumes of 300 mM acetate buffer (pH 3.6) with 1 volume of 10 mM
tripyridyltriazine (TPTZ) solution and 1volume of 20 mM
FeCl3 solution. TPTZ was dissolved in 40 mM hydrochloric
acid. Mouse cardiomyocytes were isolated as previouslsy described
(15). Briefly, the BALB/c mice
were sacrificed under deep anesthesia and their hearts were quickly
excised. Within 3 min, the heart tissues were cut into chunks,
digested with collagenase type II (Worthington, Lakewood, NJ, USA)
and protease type XIV (Sigma-Aldrich, St. Louis, MO, USA), and
gently aspirated with a transfer pipette for facilitating the cell
dissociation. The dissociated tissue explants were placed in
minimal essential medium (MEM; HyClone, Logan, UT, USA) containing
12 mM NaHCO3, 10% fetal bovine serum (FBS; HyClone) and
1% penicillin-streptomycin (HyClone) at 37°C with 5% CO2
for cellular migration and confluence. The isolated cardiomyocytes
were also cultured in MEM supplemented with 10% FBS and 1%
penicillin-streptomycin. For the acquisition of samples, the
cultured cardiomyocytes were lysed in RIPA buffer (Sigma-Aldrich)
instead of the medium, followed by repetitive shaking on ice for 10
min. The mixtures were collected as FRAP samples. For the
acquisition of serum, the blood was drawn from heart artery before
sacrifice, and allowed to stand for 1 h at 37°C for coagulation,
followed by centrifugation at 3,000 × g for 10 min. The supernatant
was regarded as serum samples. The samples from both cardiomyocytes
and serum were subjected to FRAP assay. Subsequently, 150 µl
of FeSO4 solution (0, 0.2, 0.4 0.6, 0.8 and 1.0 mM) was
mixed with 4.5 ml FRAP working solution for reaction at 37°C for 10
min, and the absorbance at 593 nm was recorded for drawing a
standard curve. Ten microliters of the samples were also mixed with
FRAP working solution for reaction at 37°C for 10 min, and the
absorbance at 593 nm was recorded. The samples isolated from the
mice housed under NTH conditions without VE pre-treatment served as
the controls. The corresponding concentration of FeSO4
(µM) was set as the antioxidant potential, which was read
from standard curve according to the absorbance of FeSO4
that was identical with samples.
Evaluation of ROS production
In order to evaluate ROS production, the mice were
treated as described above for 4 weeks and sacrificed under deep
anesthesia. The mouse hearts were rapidly excised and frozen in
liquid nitrogen. The levels of ROS in the tissues were quantified
using electron spin resonance spectroscopy with
4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl
(4-hydroxy-TEMPO.7). All measurements were performed in 3 parallel
runs.
Determination of oxidative stress
A biochemical analysis kit (Jiancheng Biotechnology
Co., Nanjing, China) was used to measure the activity of SOD, GSH,
MDA and LDH according to the manufacturer's instructions. Briefly,
the heart tissues were washed in cold 0.9% NaCl solution
repetitively, to wipe out blood residue, followed by drying with
filter paper and weighing. Subsequently, the heart tissues were
transferred to a beaker, and 0.9% NaCl solution was added again in
a weight-to-volume ratio of 1:9. The heart tissues were then cut
into sections and homogenized, followed by centrifugation at 2,000
× g for 15 min. The supernatant was aspirated and subjected to
respective analysis kits for SOD (550 nm), GSH (420 nm), MDA (532
nm) and LDH (450 nm) using a Luminometer (Promega).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The left ventricular tissue was isolated along the
interventricular septum. RNA was extracted from the left
ventricular tissue using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. Total RNA (5
µg) was reverse transcribed into cDNA using M-MLV reverse
transcriptase (Clontech, Palo Alto, CA, USA) according to the
manufacturer's instructions. The SsoFast EvaGreen Supermix
(Bio-Rad, Hercules, CA, USA) was used in order to perform RT-qPCR
and GAPDH served as the internal control. The following PCR
conditions were used: 94°C for 4 min; 35 cycles of 94°C for 20 sec,
55°C for 30 sec, and 72°C for 20 sec with 2 sec for plate reading;
and a melting curve from 65 to 95°C. The experiments were performed
independently at least 3 times. The fold expression was calculated
using the 2−ΔΔCt method. The primer sequences are listed
in Table I.
 | Table IList of primer sequences used for
RT-qPCR. |
Table I
List of primer sequences used for
RT-qPCR.
| Gene | Sense | Antisense |
|---|
| PGC-1α |
5′-GATGTCAGTGACCTCGATGCA-3′ |
5′-CAGCAAGTTGGCCTCATTTTC-3′ |
| NRF-1 |
5′-CCACATTACAGGGCGGTGAA-3′ |
5′-AGTGGCTCCCTGTTGCATCT-3′ |
| TFAM |
5′-CGCCCTAGTAATATCGATCC-3′ |
5′-ATGTTAATCGCTGGAATTGC-3′ |
| MT |
5′-GTGTCCACTCCTGACCAGTATCCTT-3′ |
5′-TCACAGCAGCCAGCATCTCTTCCAT-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-GGGGTCGTTGATGGCAACA-3′ |
Western blot analysis
The proteins were extracted from the left
ventricular tissue using RIPA buffer (Sigma-Aldrich) and the
protein concentration was measured using the Bradford method. A
total of 20 µg protein was isolated by 12% SDS-PAGE followed
by electroblotting onto a nitrocellulose (NC) membrane (Amersham,
Little Chalfont, UK). Subsequently, non-specific binding was
blocked with 2% skim milk in Tris-buffered saline (TBS) at room
temperature for 1 h. Subsequently, the NC membrane was incubated
with primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) diluted in the blocking buffer overnight at 4°C, including
rabbit anti-MT (sc-11377), rabbit anti-PGC-1α (sc-13067), goat
anti-NRF-1 (sc-30911), rabbit anti-TFAM (sc-28200) and goat
anti-GAPDH (sc-48166), which was followed by incubation with
horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa
Cruz Biotechnology), including rabbit anti-goat IgG (sc-2768) and
goat anti-rabbit IgG (sc-2004). The proteins were visualized using
4-chloro-1-naphthol (4-CN). Densitometric analysis was performed
using Image Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
All experiments were performed in triplicate and the
data are presented as the means ± standard deviation (SD). The
statistical differences between 2 groups were determined using a
Student's t-test, and were analyzed by one-way ANOVA within
multiple groups. A p-value <0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS software version 11.5 (SPSS Inc., Chicago, IL,
USA).
Results
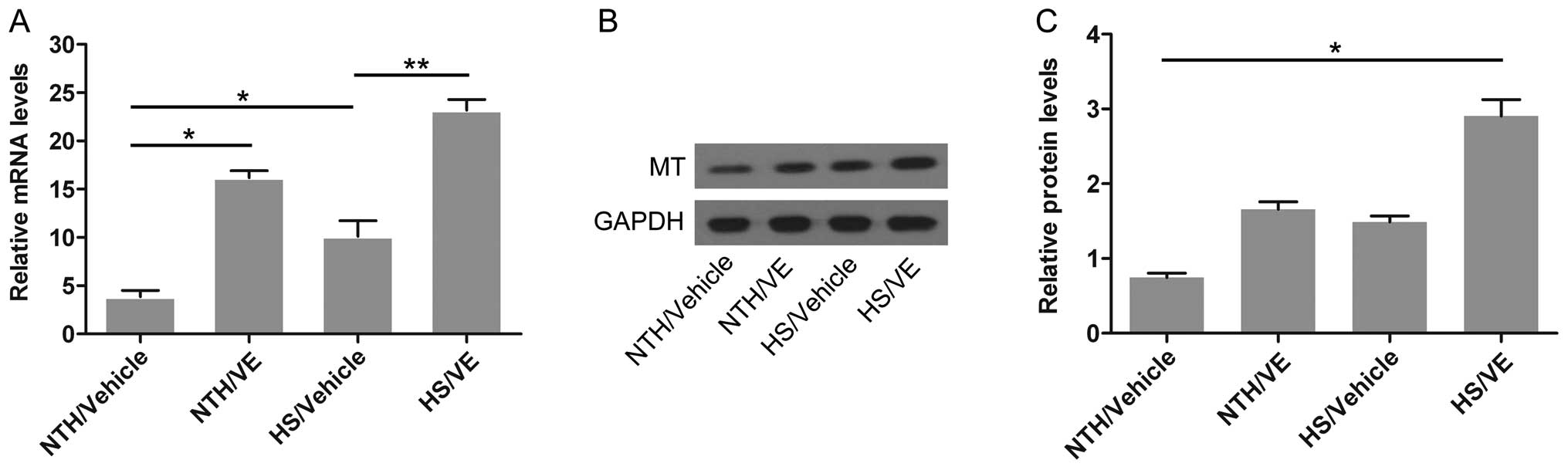
Pre-treatment with VE increases the mRNA
and protein expression of MT in cardiomyocytes under conditions of
HS
MT has been previously reported to play a
cytoprotective role, particularly in cardiomyocytes (16,17). Thus, in this study, to determine
whether MT also participates in the stress response of
cardiomyocytes exposed to high temperatures and humidity, the mRNA
and protein levels of MT in mouse left ventricular tissue were
measured by RT-qPCR and western blot analysis, respectively. As
shown in Fig. 1A, the mRNA
expression of MT was slightly increased (p=0.0318; 16.2±0.71-fold)
by pretreatment with VE under conditions of NTH compared with the
control group (NTH/vehicle; 3.9±0.65-fold). In the absence of
pre-treatment with VE, MT expression was also slightly increased
(p=0.0219; 10.1±1.64-fold) in the cardiomyoctes under conditions of
HS. Notably, pre-treatment with VE significantly upregulated the
mRNA expression of MT (p=0.0038; 23.2±1.11-fold) in the
cardiomyocytes under conditions of HS compared with the NTH/vehicle
group. The above-mentioned results suggested that VE promoted the
transcription of the MT gene in the mouse cardiomyocytes,
particularly under conditions of HS. As shown in Fig. 1B and C, pre-treatment with VE
significantly increased the protein expression of MT under
conditions of HS (p=0.0375; 2.9±0.22-fold) compared with the other
groups, implying that the translation of the MT gene was also
enhanced by pre-treatment with VE in the mouse cardiomyocytes under
conditions of HS.
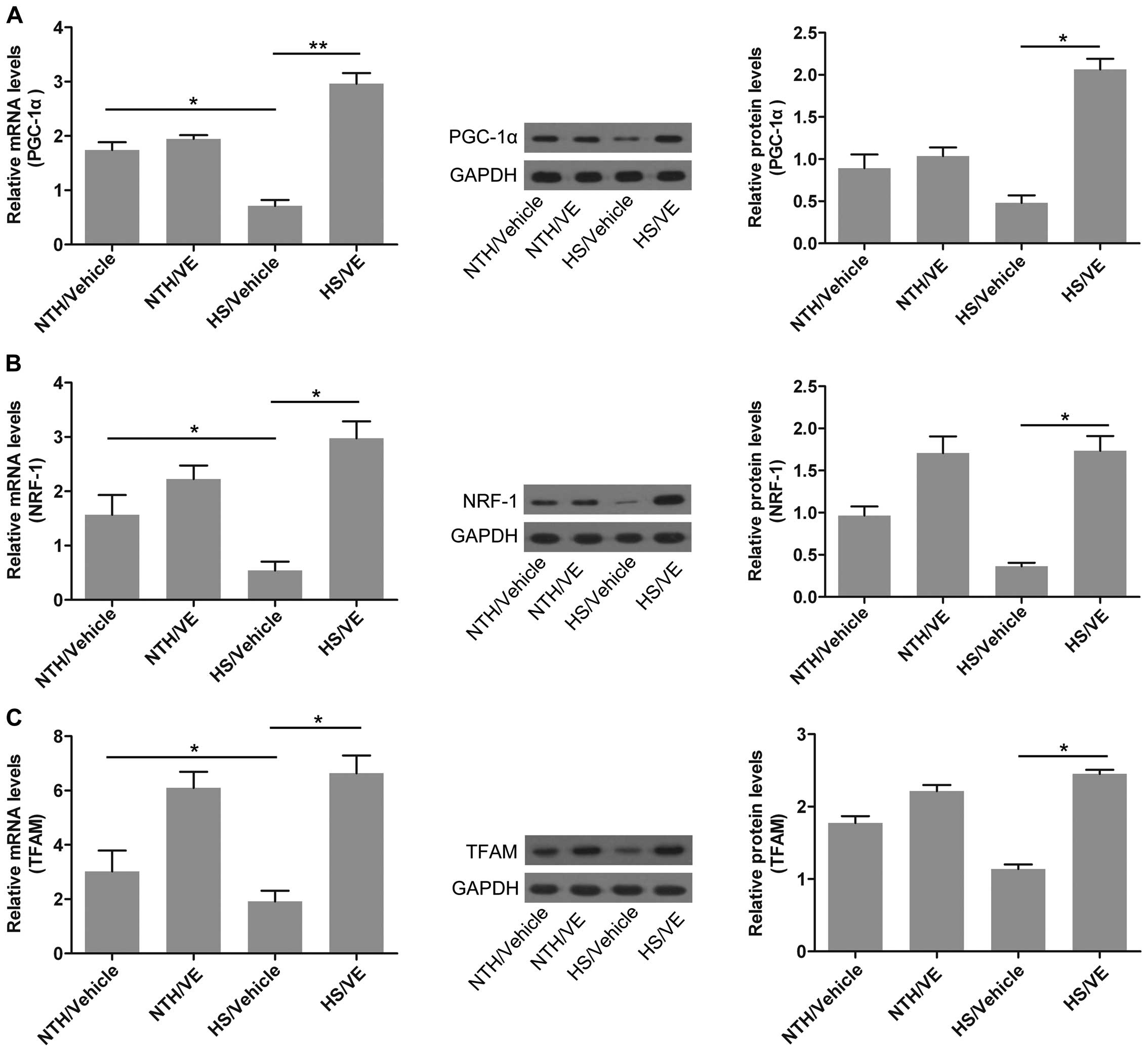
Pre-treatment with VE restores
mitochondrial function in cardiomyocytes under conditions of
HS
The impairment of multiple aspects of mitochondrial
function has been reported to be one of the principal events
occuring in cardiomyocytes exposed to HS (3). An effective method of restoring
mitochondrial function is urgently required. In this study, to
determine whether VE promotes the recovery of mitochondrial
function in cardiomyocytes under conditions of HS, the expression
of genes involved in the regulation of mitochondrial function was
measured in mouse left ventricular tissue by RT-qPCR and western
blot analysis. The mRNA expression of PGC-1α, a transcriptional
activating factor which participates in mitochondrial synthesis
(18), was significantly reduced
in the cardiomyocytes isolated from mice housed under conditions of
HS (p=0.0385); however, its expression was significantly increased
by pre-treatment with VE (p=0.0061; Fig. 2A). The mRNA expression of NRF-1, a
transcription factor regulating the transcription and replication
of mitochondrial DNA (19), was
decreased under conditions of HS (p=0.0313), suggesting the
suppression of mitochondrial function; however, pre-treatment with
VE significantly increased the expression of NRF-1 in the
cardiomyocytes under conditions of HS (p=0.0254; Fig. 2B). The mRNA expression of TFAM,
which plays a role in mitochondrial transcription and replication
(20), was also significantly
reduced in the cardiomyocytes under conditions of HS (p=0.0246),
and significantly increased by pre-treatment with VE (p=0.0369;
Fig. 2C). The protein expression
of these 3 genes also confirmed the above-mentioned alterations
(Fig. 2A–C). These data suggest
that pre-treatment with VE restores mitochondrial function in
cardiomyocytes under conditions of HS.
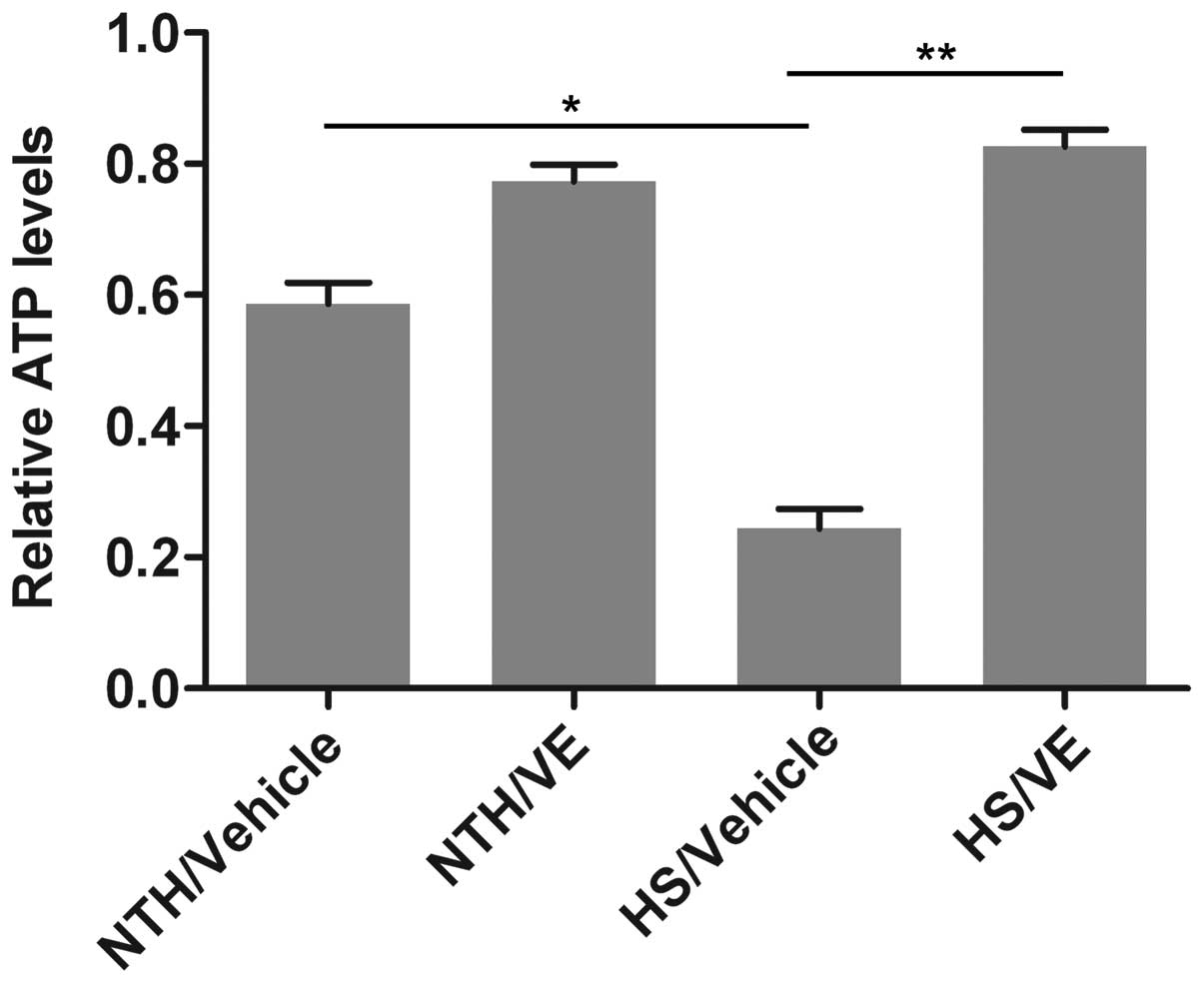
Pre-treatment with VE increases ATP
levels in mouse heart tissue under conditions of HS
To further confirm the effects of VE on
mitochondrial function in mouse heart tissue under conditions of
HS, the levels of ATP, an indicator of mitochondrial function, were
measured using an ATP assay kit. As shown in Fig. 3, the ATP levels were significantly
reduced in the mouse heart tissue under conditions of HS
(p=0.0273), indicating that the exposure to HS impaired
mitochondrial function. By contrast, pre-treatment with VE
increased the ATP levels in the mouse heart tissue under conditions
of HS (p=0.0063), which demonstrated that the restoration of
mitochondrial function had occurred.
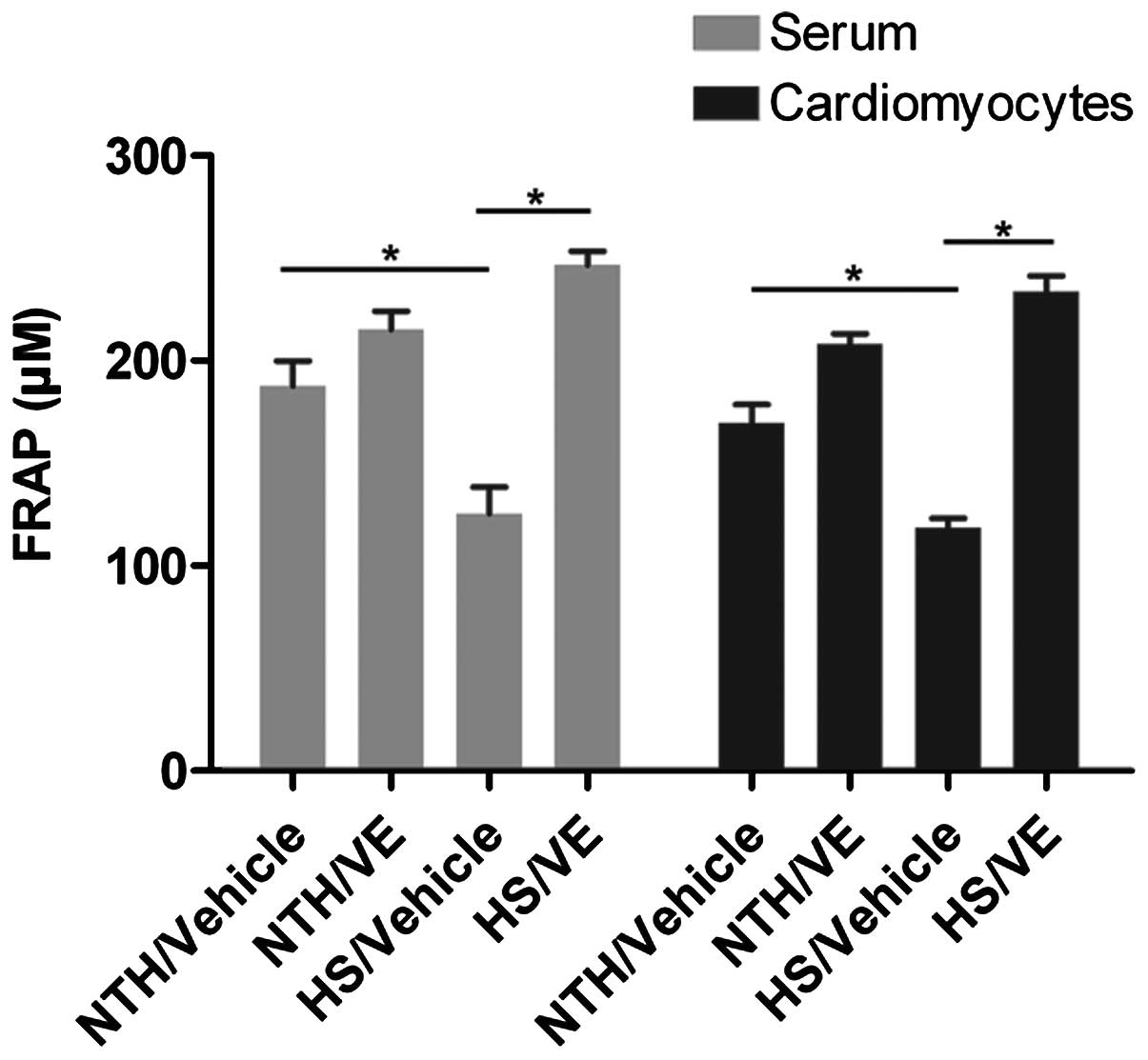
Antioxidant capacity is reduced under
conditions of HS, but these effects are reversed by pre-treatment
with VE
Oxidative stress is another event induced by
exposure to HS and anti-oxidative capacity is an indicator of
oxidative stress. In order to determine the effects of
pre-treatment with VE on the antioxidant capacity in serum and
cardiomyocytes obtained from mice exposed to conditions of HS, a
FRAP assay was performed. The results revealed that the antioxidant
capacity was significantly decreased under conditions of HS
compared with normal conditions (the NTH/vehicle group; p=0.0517 in
serum and 0.0580 in cardiomyocytes, respectively; Fig. 4), indicating that HS conditions
promote oxidative stress. However, pre-treatment with VE
significantly increased the antioxidant capacity in both the serum
and the cardiomyocytes (p=0.0171 in serum and 0.0159 in
cardiomyocytes, respectively; Fig.
4), implying that VE may attenuate oxidative stress induced by
exposure to HS.
Pre-treatment with VE decreases the
production of ROS induced by exposure to HS
The level of ROS is another index of oxidative
stress. The decreased antioxidant capacity may be attributed to the
accumulation of ROS. In this study, to examine the effects of
pre-treatment with VE on the production of ROS, the ROS levels in
the mouse heart tissues were measured using electron spin resonance
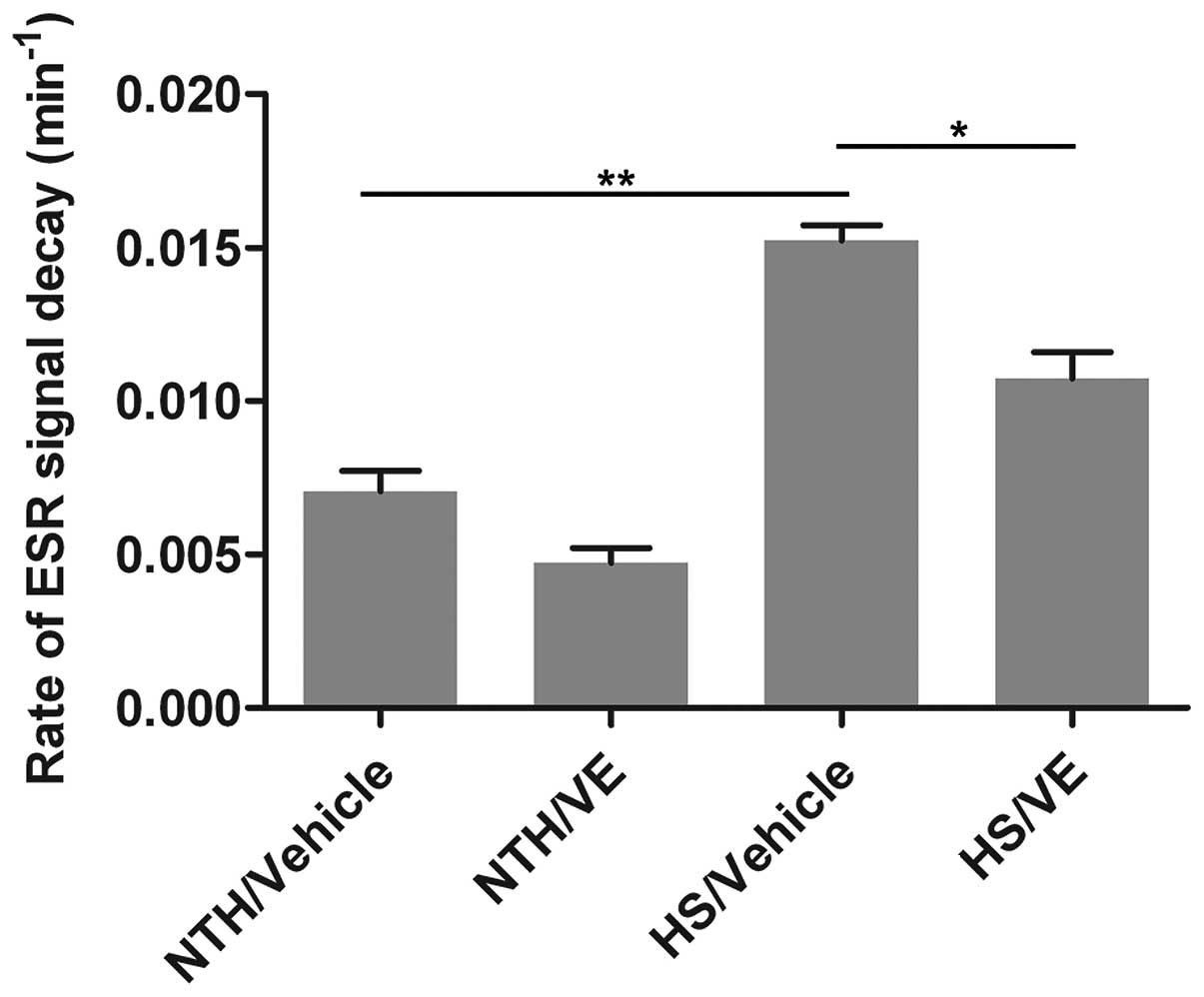
spectroscopy. As shown in Fig. 5,
compared with the NTH/vehicle group, HS induced a significant
increase in the production of ROS (p=0.0042). Moreover, the
pre-treatment with VE significantly decreased the production of ROS
(p=0.0417; Fig. 5) compared with
the HS/vehicle group. These results suggest that pre-treatment with
VE reduces the production of ROS.
Pre-treatment with VE attenuates
oxidative stress induced by exposure to HS
In order to examine the extent of oxidative stress
induced by exposure to HS more specifically, we examined the
effects of VE on the levels of antioxidant enzymes and markers of
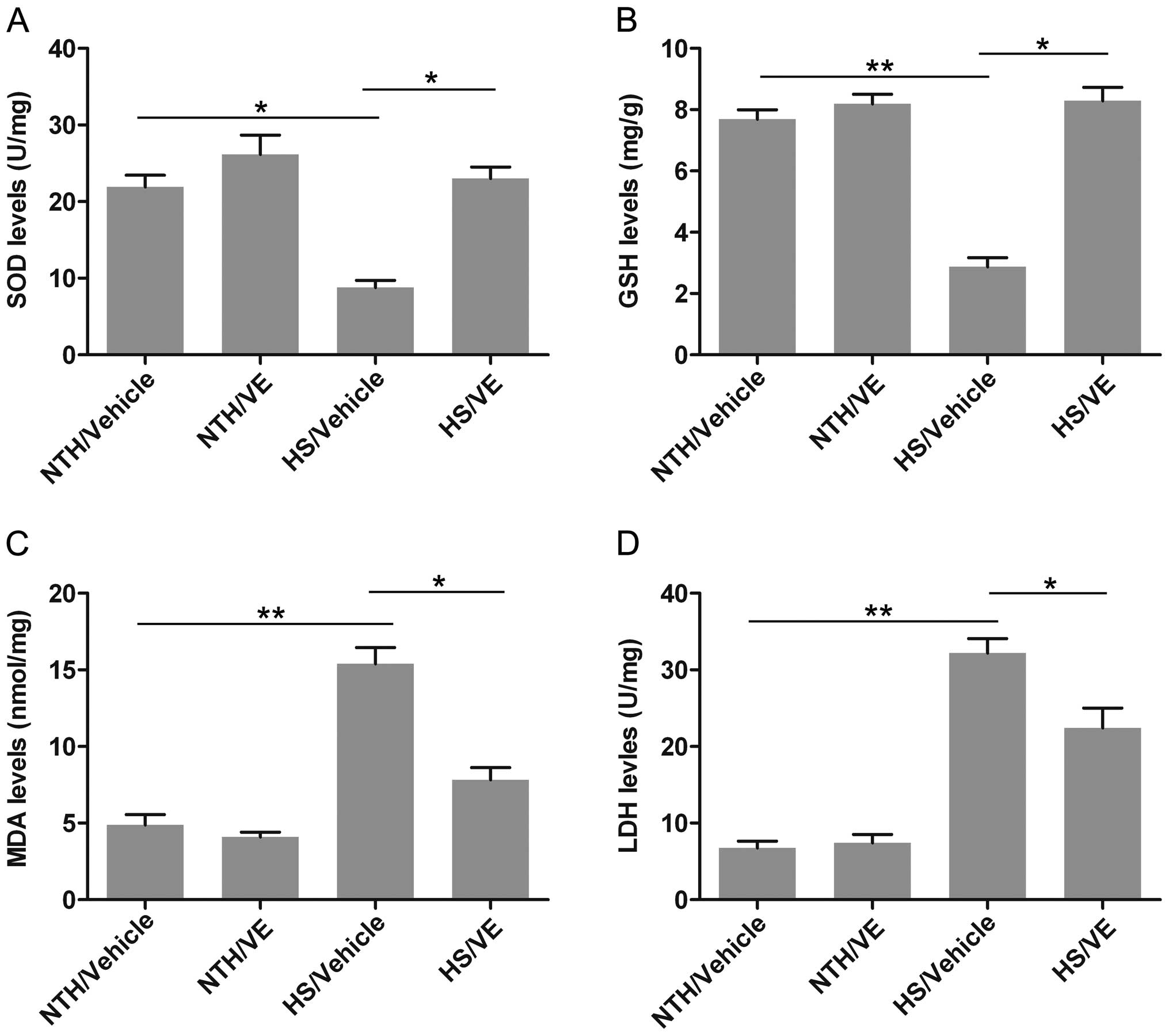
oxidative injury, namely SOD, GSH, MDA and LDH. The level of SOD, a
powerful antioxidant which targets ROS, was significantly decreased
under conditions of HS (p=0.0134; Fig. 6A); however, pre-treatment with VE
significantly increased SOD levels (p=0.0259). Similarly, the level
of GSH, a common antioxidant, was also significantly reduced in the
HS/vehicle group (p=0.0028; Fig.
6B), whereas pre-treatment with VE significantly increased the
GSH level (p=0.0147). The level MDA, an important marker of lipid
peroxidation associated with oxidative stress, was significantly
increased under conditions of HS compared with the NTH/vehicle
group (p=0.0034; Fig. 6C); this
effect was inhibited by pre-treatment with VE (p=0.0267). We also
observed that exposure to HS significantly increased the release of
LDH (p=0.0043; Fig. 6D), whereas
pre-treatment with VE reduced the LDH level (p=0.0412). Taken
together, these results demonstrate that the oxidative stress
induced by exposure to HS may be attenuated by pre-treatment with
VE.
Discussion
In the present study, we demonstrate that
pre-treatment with VE can prevent mitochondrial dysfunction and
oxidative stress in cardiomyocytes induced by exposure to HS,
possibly by increasing the expression of MT. Mitochondrial
dysfunction and oxidative stress are the two principal events
induced by exposure to HS (21,22), which mainly target the
cardiovascular system (2,23). However, effective methods for the
prevention and treatment of cardiovascular diseases caused by
exposure to HS are lacking. This study provides evidence that
pre-treatment with VE has potential therapeutic applications as it
is capable of counteracting the effects of exposure to HS. However,
the application of VE in the prevention of HS warrants further
investigation.
In our previous studies, one of the underlying
mechanisms responsible for heat stress (due to high temperatures
and humidity), as well as a potential drug capable of protecting
cardiomyocytes from HS were explored. More specifically, HS-induced
oxidative stress and the apoptosis of cardiomyocytes were
attributed to the renin-angiotensin system (8). Subsequently, it was proven that
geranylgeranylacetone suppressed the apoptosis of cardiomyocytes
exposed to HS, mainly due to the enhanced expression of Hsp70;
however, it had no effects on oxidative stress (9). Cell apoptosis is the third event
induced by HS (24), in addition
to mitochondrial dysfunction and oxidative stress mentioned in the
present study. In fact, mitochondrial dysfunction consists of a
series of events, including the release of cytochrome c and
AIF from the mitochondrial nucleus into the cytoplasm, which leads
to the insufficient supply of energy and subsequent cellular
apoptosis (25). This process is
termed mitochondria-mediated cell apoptosis. Hence, mitochondrial
dysfunction and cell apoptosis are closely interrelated. Decreased
cell survival induced by cell apoptosis has also been demonstrated
to contribute to HS-induced cardiovascular diseases.
Currently, it is widely accepted that ROS are inter-
and intra-cellular second messengers, participating in
intracellular signal transmission (26,27). However, the excessive production
of ROS may exert negative effects, leading to oxidative stress,
gene silencing, cellular dysfunction and ultimately, apoptosis or
necrosis. ROS are a type of oxidant found in organisms. Hence, a
balance between oxidation and antioxidation is crucial for normal
metabolism, cellular function, as well as correct responses to
various types of stimuli (28).
Above all, the levels of antioxidants within cells, the
cytomembrane, and the cytoplasm may be increased and mobilized to
neutralize the excessive formation of ROS (29,30). It has been previously reported
that VE is an effective antioxidant; Shirpoor et al
investigated the role of VE in diabetes-induced oxidative stress,
and found that as an antioxidant, VE exerts a potent protective
effect (31). In addition,
Takahashi et al reported that VE supplementation attenuated
oxidative stress in postmenopausal women (32). Moreover, in the study by Sakr
et al, VE was proven to effectively attenuate oxidative
stress in a rat model of hypoxia (33). However, the effects of VE on
cardiovascular diseases induced by exposure to HS are not yet fully
understood. In this study, our results suggest that exposure to HS
may induce oxidative stress, as indicated by the overproduction of
ROS and the decreased antioxidant capacity. Pre-treatment with VE
was also demonstrated to attenuate oxidative stress induced by
exposure to HS.
MT belongs to a group of intracellular,
non-enzymatic proteins (6–7 kDa) that is distinguished among
different species (11), and it
is characterized by the binding of metal ions, such as
Zn2+, Cd2+, Cu2+ and
Hg2+ (34).
Ubiquitously expressed MT has been reported to participate in a
number of regulatory activities in both normal and cancer cells,
such as in the homeostasis of zinc ions, the detoxification of
heavy metals, proliferation, apoptosis and cytoprotection against
oxidative damage (35–39). In the present study, the
expression of MT was increased by pre-treatment with VE, suggesting
that MT participates in the protection of mouse cardiomyocytes
against HS.
In conclusion, in this study, we demonstrated that
the oral administration of VE increased the expression of MT, which
was associated with the restoration of mitochondrial function and
reduced oxidative stress. Thus, we provide evidence for the
possible application of VE in the prevention and treatment of
cardiomyocytes under conditions of HS. However, further
investigations are warranted in order to provide more concrete
evidence that the increased expression of MT directly contributes
to the cytoprotective effects of VE, as well as to elucidate the
precise mechanisms responsible for these effects.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81200633) and the China
Postdoctoral Science Foundation (2013M532160).
Abbreviations:
|
HS
|
heat stress
|
|
MT
|
metallothionein
|
|
VE
|
vitamin E
|
|
ROS
|
reactive oxygen species
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor-gamma coactivator-1α
|
|
NRF-1
|
nuclear respiratory factor 1
|
|
TFAM
|
mitochondrial transcription factor
A
|
|
SOD
|
superoxide dismutase
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chien KR: Genomic circuits and the
integrative biology of cardiac diseases. Nature. 407:227–232. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian L, Song X, Ren H, Gong J and Cheng S:
Mitochondrial mechanism of heat stress-induced injury in rat
cardiomyocyte. Cell Stress Chaperones. 9:281–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carreira RS, Lee P and Gottlieb RA:
Mitochondrial therapeutics for cardioprotection. Curr Pharm Des.
17:2017–2035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kyrychenko V, Poláková E, Janíček R and
Shirokova N: Mitochondrial dysfunctions during progression of
dystrophic cardiomyopathy. Cell Calcium. 58:186–195. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hall DM, Buettner GR, Oberley LW, Xu L,
Matthes RD and Gisolfi CV: Mechanisms of circulatory and intestinal
barrier dysfunction during whole body hyperthermia. Am J Physiol
Heart Circ Physiol. 280:H509–H521. 2001.PubMed/NCBI
|
|
7
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Yuan B, Dong W, Yang B, Yang Y,
Lin X and Gong G: Humid heat exposure induced oxidative stress and
apoptosis in cardiomyocytes through the angiotensin II signaling
pathway. Heart Vessels. 30:396–405. 2015. View Article : Google Scholar
|
|
9
|
Wang X, Yuan B, Dong W, Yang B, Yang Y,
Lin X and Gong G: Induction of heat-shock protein 70 expression by
geranylgeranylacetone shows cytoprotective effects in
cardiomyocytes of mice under humid heat stress. PLoS One.
9:e935362014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jäättelä M, Wissing D, Kokholm K, Kallunki
T and Egeblad M: Hsp70 exerts its anti-apoptotic function
downstream of caspase-3-like proteases. EMBO J. 17:6124–6134. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coyle P, Philcox JC, Carey LC and Rofe AM:
Metallothionein: the multipurpose protein. Cell Mol Life Sci.
59:627–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukase Y, Tsugami H, Nakamura Y, Ohba K
and Ohta H: The role of metallothionein and metal transporter on
cadmium transport from mother to fetus. Yakugaku Zasshi.
134:801–804. 2014.In Japanese. View Article : Google Scholar
|
|
13
|
Zhao XL, Li YK, Cao SJ, Hu JH, Wang WH,
Hao RJ, Gui LS and Zan LS: Protective effects of ascorbic acid and
vitamin E on antioxidant enzyme activity of freeze-thawed semen of
Qinchuan bulls. Genet Mol Res. 14:2572–2581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng L: Mice brain tissue injury induced
by diisononyl phthalate exposure and the protective application of
vitamin E. J Biochem Mol Toxicol. 29:311–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Das A, Xi L and Kukreja RC:
Phosphodiesterase-5 inhibitor sildenafil preconditions adult
cardiac myocytes against necrosis and apoptosis. Essential role of
nitric oxide signaling. J Biol Chem. 280:12944–12955. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye G, Metreveli NS, Ren J and Epstein PN:
Metallothionein prevents diabetes-induced deficits in
cardiomyocytes by inhibiting reactive oxygen species production.
Diabetes. 52:777–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GW, Klein JB and Kang YJ:
Metallothionein inhibits doxorubicin-induced mitochondrial
cytochrome c release and caspase-3 activation in cardiomyocytes. J
Pharmacol Exp Ther. 298:461–468. 2001.PubMed/NCBI
|
|
18
|
Liang H and Ward WF: PGC-1alpha: a key
regulator of energy metabolism. Adv Physiol Educ. 30:145–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gleyzer N, Vercauteren K and Scarpulla RC:
Control of mitochondrial transcription specificity factors (TFB1M
and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and
PGC-1 family coactivators. Mol Cell Biol. 25:1354–1366. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda M, Ide T, Fujino T, Arai S, Saku K,
Kakino T, Tyynismaa H, Yamasaki T, Yamada K, Kang D, et al:
Overexpression of TFAM or twinkle increases mtDNA copy number and
facilitates cardioprotection associated with limited mitochondrial
oxidative stress. PLoS One. 10:e01196872015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slimen IB, Najar T, Ghram A, Dabbebi H,
Ben Mrad M and Abdrabbah M: Reactive oxygen species, heat stress
and oxidative-induced mitochondrial damage. A review. Int J
Hyperthermia. 30:513–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Jiao H, Song Z, Zhao J, Wang X
and Lin H: Heat stress impairs mitochondria functions and induces
oxidative injury in broiler chickens. J Anim Sci. 93:2144–2153.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steenland K: Epidemiology of occupation
and coronary heart disease: research agenda. Am J Ind Med.
30:495–499. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Havenith G, Luttikholt VG and Vrijkotte
TG: The relative influence of body characteristics on humid heat
stress response. Eur J Appl Physiol Occup Physiol. 70:270–279.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lane RK, Hilsabeck T and Rea SL: The role
of mitochondrial dysfunction in age-related diseases. Biochim
Biophys Acta. 1847:1387–1400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
San Martín A, Du P, Dikalova A, Lassègue
B, Aleman M, Góngora MC, Brown K, Joseph G, Harrison DG, Taylor WR,
et al: Reactive oxygen species-selective regulation of aortic
inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart
Circ Physiol. 292:H2073–H2082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lassègue B and Griendling KK: Reactive
oxygen species in hypertension; An update. Am J Hypertens.
17:852–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halliwell B and Cross CE: Oxygen-derived
species: their relation to human disease and environmental stress.
Environ Health Perspect. 102(Suppl 10): 5–12. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davies KJ: Oxidative stress: the paradox
of aerobic life. Biochem Soc Symp. 61:1–31. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shirpoor A, Norouzi L, Nemati S and Khadem
Ansari MH: Protective effect of vitamin E against diabetes-induced
oxidized LDL and aorta cell wall proliferation in rat. Iran Biomed
J. 19:117–123. 2015.PubMed/NCBI
|
|
32
|
Takahashi M, Miyashita M, Park JH,
Kawanishi N, Bae SR, Nakamura Y, Sakamoto S and Suzuki K:
Low-volume exercise training and vitamin E supplementation
attenuates oxidative stress in postmenopausal women. J Nutr Sci
Vitaminol (Tokyo). 59:375–383. 2013. View Article : Google Scholar
|
|
33
|
Sakr HF, Abbas AM and El Samanoudy AZ:
Effect of vitamin E on cerebral cortical oxidative stress and
brain-derived neurotrophic factor gene expression induced by
hypoxia and exercise in rats. J Physiol Pharmacol. 66:191–202.
2015.PubMed/NCBI
|
|
34
|
Dziegiel P: Expression of metallothioneins
in tumor cells. Pol J Pathol. 55:3–12. 2004.PubMed/NCBI
|
|
35
|
Dziegiel P, Salwa-Zurawska W, Zurawski J,
Wojnar A and Zabel M: Prognostic significance of augmented
metallothionein (MT) expression correlated with Ki-67 antigen
expression in selected soft tissue sarcomas. Histol Histopathol.
20:83–89. 2005.
|
|
36
|
Pula B, Domoslawski P, Podhorska-Okolow M
and Dziegiel P: Role of metallothioneins in benign and malignant
thyroid lesions. Thyroid Res. 5:262012. View Article : Google Scholar
|
|
37
|
Surowiak P, Matkowski R, Materna V,
Györffy B, Wojnar A, Pudelko M, Dziegiel P, Kornafel J and Zabel M:
Elevated metallothionein (MT) expression in invasive ductal breast
cancers predicts tamoxifen resistance. Histol Histopathol.
20:1037–1044. 2005.PubMed/NCBI
|
|
38
|
Thirumoorthy N, Shyam Sunder A,
Manisenthil Kumar K, Senthil Kumar M, Ganesh G and Chatterjee M: A
review of metallothionein isoforms and their role in
pathophysiology. World J Surg Oncol. 9:542011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wojnar A, Pula B, Piotrowska A, Jethon A,
Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M and Dziegiel P:
Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2
proteins in invasive ductal breast carcinoma. Anticancer Res.
31:3027–3033. 2011.PubMed/NCBI
|