Introduction
Chlorhexidine (CHX) is considered the gold standard
in the antiseptic treatment of the oral cavity, due to its high
antibactericidal capability (1,2),
its inhibitory effects on glycosidic and proteolytic (3) and matrix metalloproteinase
activities (4), and its reducing
effecfts on the leucocyte concentration (to basal levels) and on
pro-inflamatory cytokines (5).
CHX is an antimicrobial agent that belongs to the group of
N5 derivatives of 1:6-bis-biguanidohexane (6,7)
and is also effective in the treatment of non-bacterial oral
infections. CHX binds to negatively charged sites on the bacterial
surface wall through electrostatic forces. Such an interaction
affects the membrane structure and causes the leakage of
intracellular bacterial components (1,8,9).
With the use of CHX mouth-rinse formulations, there
is an immediate bactericidal effect due to cytoplasmic
precipitation. The bacteriostatic effect is further induced by the
adsorption and prolonged release of CHX from oral surfaces
(1,10). This antiplaque formation effect
and lack of systemic toxicity (5)
render CHX a commonly used antiseptic in post-surgical dental
treatment. However, recent studies have demonstrated that CHX
exerts potent cytotoxic effects on human periodontal tissues, such
as gingival fibroblasts (11,12), gingival epithelial cells (13), periodontal ligament cells
(14), cultured alveolar bone
cell (15) and on osteoblastic
cells (7). It also reduces
gingival fibroblast adhesion to fibronectin (16) and prevents fibroblast attachment
to root surfaces; thus, it can interfere with periodontal treatment
and regeneration (7). Yet it is
difficult to compare all the published results, as they refer to
the different commercial mouth rinsing fluids containing CHX, each
one containing different concentrations of this active chemical
agent. Some of these mouth rinsing fluids also contain alcohol,
which can influence cell proliferation and morphology.
Our previous study indicated that an alcohol
concentration of 10% does not inhibit fibroblast proliferation and
the presence of alcohol in mouth rinsing fluids containing 0.10%
CHX has no deleterious effects on healing capacity (17). On the contrary, it helps stimulate
wound healing (11). In addition,
the culture media used in in vitro experiments differ [fetal
bovine serum (FBS) or calf bovine serum]. Usually, experiments for
evaluating the cytotoxicity of antiseptics are carried out in cell
culture medium containing 10% FBS, which is similar to the
composition of artificial wound fluid (18); however, FBS has an attenuating
effect against CHX-induced cytotoxicity (8). Although a number of studies have
demonstrated the cytotoxicity of CHX (12,13,16), none of the observations lasted for
>24 h and none of the studies used the short-cut video to
demonstrate the results. Moreover, in this study, we used the
PANsys3000 system to examine the effects of CHX on human gingival
fibroblasts (HGFs) cultured without FBS. PANsys3000 is a highly
automated cell-culture system that is used for in vitro cell
culture and for the analysis of diverse cell lines in conditions
similar to those observed in vivo. This system enables the
culture of various cells and the usage of diverse culture media at
the same time, using the cell culture conditions of choice and
constant microscopic observation. Simultaneously, in our study, we
applied the xCELLigance real-time cell analysis (RTCA) system as a
non-invasive and label-free approach to assess cell proliferation
in real-time on a cell culture level.
Materials and methods
Cell culture
All experiments were conducted using a human
gingival fibroblast (HGF) cell line (reference no. P10866;
Innoprot, Biscay, Spain). Gingival fibroblasts were transferred in
aseptic conditions from freezing medium [Dulbecco's modified
Eagle's medium (DMEM)/F12 (1:1), 10% FBS, 10% dimethyl sulfoxide
(DMSO) (all from Gibco, Grand Island, NY, USA)], to a 90-mm sterile
petri dish (Sarstedt, Nuembrecht, Germany) containing 10 ml of
growth medium with the following composition: DMEM/F12 (1:1)
medium, 10% FBS, antibiotics (penicillin 100 µg/ml and
streptomycin 100 µg/ml) and 2 mmol/l L-glutamine (all from
Gibco). The cells were grown in aseptic conditions, in an incubator
at 37°C with 5% CO2 and 100% humidity. The cells were
cultured until 90% confluent. At this point, they were washed with
phosphate-buffered saline (PBS) and trypsinized with trypsin/EDTA
solution (0.25% trypsin containing 0.01% EDTA). After 5 min of
incubation, complete growth medium was added, and the cell
suspension was transferred to petri dishes.
Stimulation of gingival fibroblasts with
CHX
To evaluate the effecs of CHX on fibroblasts, the
cells were grown in regular culture medium for 24 h. The medium was
then replaced with appropriate CHX dilutions. The practical
dilution was obtained by dissolving commercially available CHX
solution [Curasept ADS 220 (0.2% CHX)] in FBS-free medium. The
final dilutions of CHX in the FBS-free medium were as follows:
0.002, 0.01, 0.02, 0.04 and 0.2%. The cells were stimulated with
CHX for 15 min and the solutions were then replaced with regular
growth medium and the cells were grown under standard conditions
for 48 h.
Analysis of cell growth and
morphology
Cell growth and morphology were assayed using
PANsys3000. PANsys 3000 (Systech GmbH, Augsburg, Germany) is a
multi-chamber fully automated cell culture system used for in
vitro experiments simulating in vivo conditions. It
allows the culture of different cell types and several components
simultaneously with a variety of culture conditions and continuous
microscopic observations. The parameter defined as the cell index
(CI) represents cell growth, measuring the relative change in
electrical impedance in the presence or absence of cells in the
wells. CI is a unitless parameter and is calculated using the
following formula: CI = (Zi−Z0)/15 where
Zi is the impedance during the experiment and
Z0 is the impedance at the beginning of the experiment
(19–21).
The cells were grown prior to the experiment for 24
h in an incubator at 37°C with 5% CO2 and 90% humidity
(ftp.strefa.pl, user: m.wyganowska+kdvision.eu; password: Wyga1,
supplementary 1.avi). Subsequently, the growth media were removed
and replaced with the appropriate CHX dilutions (0.002, 0.01, 0.02,
0.04 and 0.2%) and the cells were resuspended in 1 ml of DMEM
FBS-free medium. The control cells were treated with 1 ml of DMEM
FBS-free medium. The cells were incubated for 15 min at 37°C. After
the CHX solution was removed, the cells were rinsed with Hank's
solution (Cytogen, Wetzlar, Germany) and complete growth medium was
added. Further observations were conducted for the following 48 h.
Images were acquired at 10-min intervals and finally combined into
a video. All of the images were acquired in the same plate region
(region of interest).
Assessment of cell proliferation
rate
Real-time cell analyses (xCELLigence system; Roche
Applied Science, Mannheim, Germany; ACEA Biosciences, San Diego,
CA, USA) were performed to determine the effects of CHX on gingival
fibroblast proliferation. The electronic impedance of the sensor
electrodes was measured to allow the monitoring and detection of
physiologic changes of the cells on the electrodes. The voltage
applied to the electrodes during real-time cell analysis was
approximately 20 mV root mean square. The impedance measured
between electrodes in a well depends on electrode geometry, the ion
concentration in the well, and whether the cells are attached to
the electrodes. In the presence of cells, cells attached to the
electrode sensor surfaces act as insulators, and thereby alter the
local ion environment at the electrode-solution interface, leading
to increased impedance. Thus, the larger the value of electrode
impedance, the larger the number of cells growing on the
electrodes.
During the cell proliferation measurements, the
cells were passaged after reaching confluency and were trypsinized
with 0.25% trypsin. After seeding 200 µl of the cell
suspensions into the wells (10,000 cells/well) of the E-plate 96
(ACEA Biosciences), the HGFs were kept in culture to obtain the CI
value of approximately 2. Subsequently, the cells were treated with
the appropriate dilutions of CHX and released from the metallic
alloy material of the electrodes and monitored every 15 min for 48
h. The control plate contained cells not stimulated with CHX, but
with the replacement of the growth medium with FBS-free medium and
were then cultured in complete culture medium.
Cell cycle analysis
The cells were seeded in 60-mm culture dishes at a
density of 5×105 cells/dish and allowed to adhere
overnight. Following 15 min of incubation with CHX at dilutions
(0.002, 0.004, 0.01, 0.02, 0.04 and 0.2%), the cells were washed
twice with PBS and the solutions were then replaced with regular
growth medium, and the cells were grown under standard conditions
for 48 h. Subsequently, the cells were trypsinized (trypsin;
Cytogen) and fixed with ice-cold 70% ethanol at −20°C for 24 h.
Subsequently, the cells were centrifuged, washed once with PBS, and
then incubated with RNAse A (50 µg/ml in PBS) for 30 min.
Following centrifugation at 100 rpm for 10 min at 4°C, the
supernatant with RNAse A was removed and intracellular DNA was
labeled with 0.5 ml of cold propidium iodide (PI) solution (0.1%
Triton X-100, 0.1 mM EDTA, 50 µg/ml PI in PBS) on ice for 30
min in the dark. Cell cycle distribution was measured using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). For
each experiment, 10,000 cells were examined. The fluorescence of PI
was excited using an argon laser (488 nm). The emission of red
fluorescence of PI was detected in the FL3 channel (>650 nm) All
data were collected and analyzed using CellQuest Pro software
(v.5.2.1) (Becton-Dickinson, Franklin Lakes, NJ, USA). The
distribution of cells in the cell cycle
(G0/G1, S and G2/M) and apoptosis
were calculated using the ModFit LT program for cell cycle analysis
(Verity Software House Inc., Topsham, ME, USA).
Statistical analysis
Statistical analysis was performed using Statistica
v.10 (StatSoft, Inc., Tulsa, UK). The Shapiro-Wilk test was used
for the normality test of continuous variables. The mean ± standard
deviation was used to describe the results of the experiments. The
parametric test one-way ANOVA with the multiple comparison Tukey's
post-test were applied. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
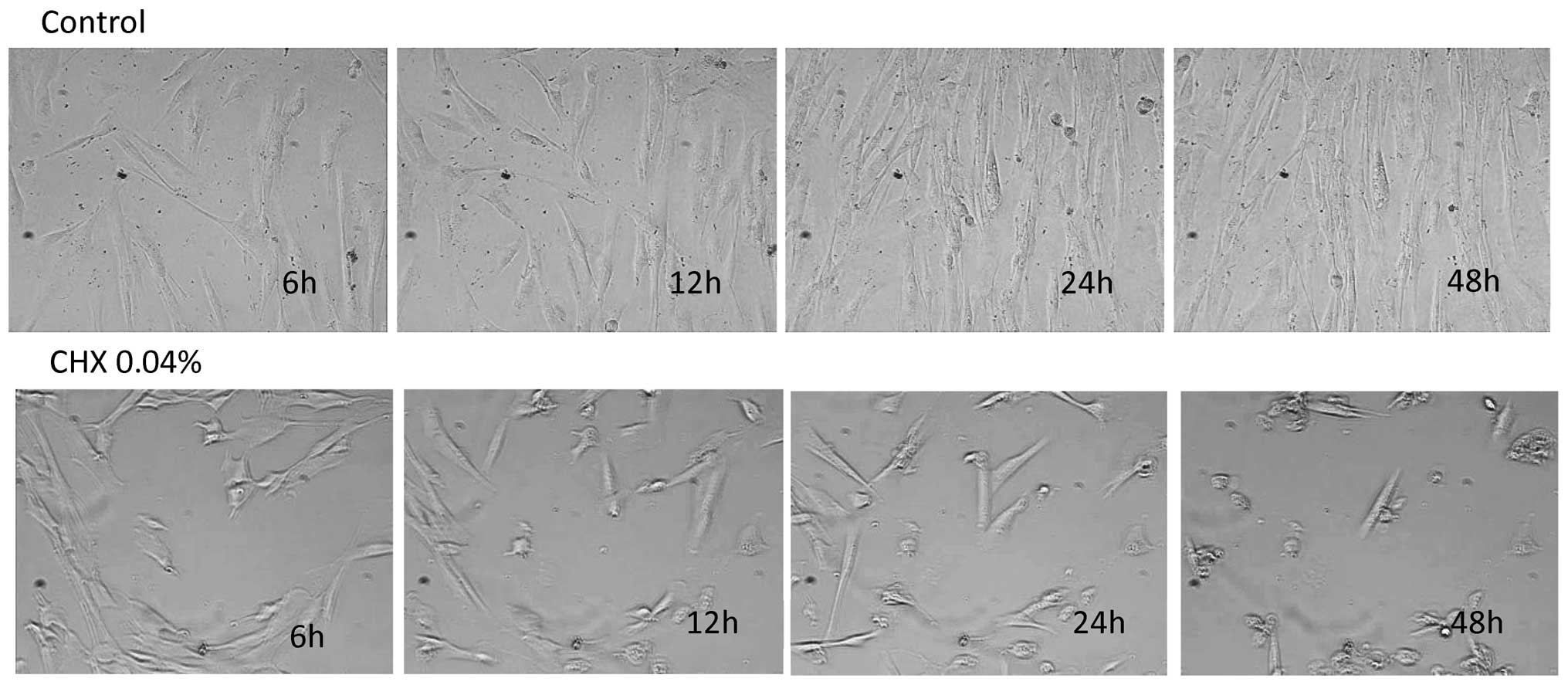
Cell growth and morphology
In the control group, fibroblast morphology did not
vary significantly during the duration of the experiment. During
the 48 h of culture after DMEM stimulation, the gingival
fibroblasts formed a confluent layer with lamellipodia and
spreading of the cellular matrix (Fig. 1, top panel) (ftp.strefa.pl, user: m.wyganowska+kdvision.eu; password: Wyga1,
supplementary 2.avi). Morphologically, no significant difference
was observed between the control cells and the CHX
0.002%-stimulated cells. Both groups exhibited a characteristic
spindle-shaped fibroblast morphology. In the cells stimulated with
CHX at the concentration of 0.01 and 0.02%, a decrease in cell
proliferation and a decrease in the number of cell divisions were
noted (ftp.strefa.pl, user: m.wyganowska+kdvision.eu; password: Wyga1,
supplementary 3.avi). There were no significant changes observed in
the morphology of the fibroblasts between both groups. In the cells
stimulated with CHX at the concentration of either 0.04 or 0.2%, a
progressive inhibition of cell growth and division was observed
(ftp.strefa.pl, user: m.wyganowska+kdvision.eu; password: Wyga1,
supplementary 4.avi). The growth inhibition was accompanied by the
appearance of the small round-shaped cells (Fig. 1, bottom panel).
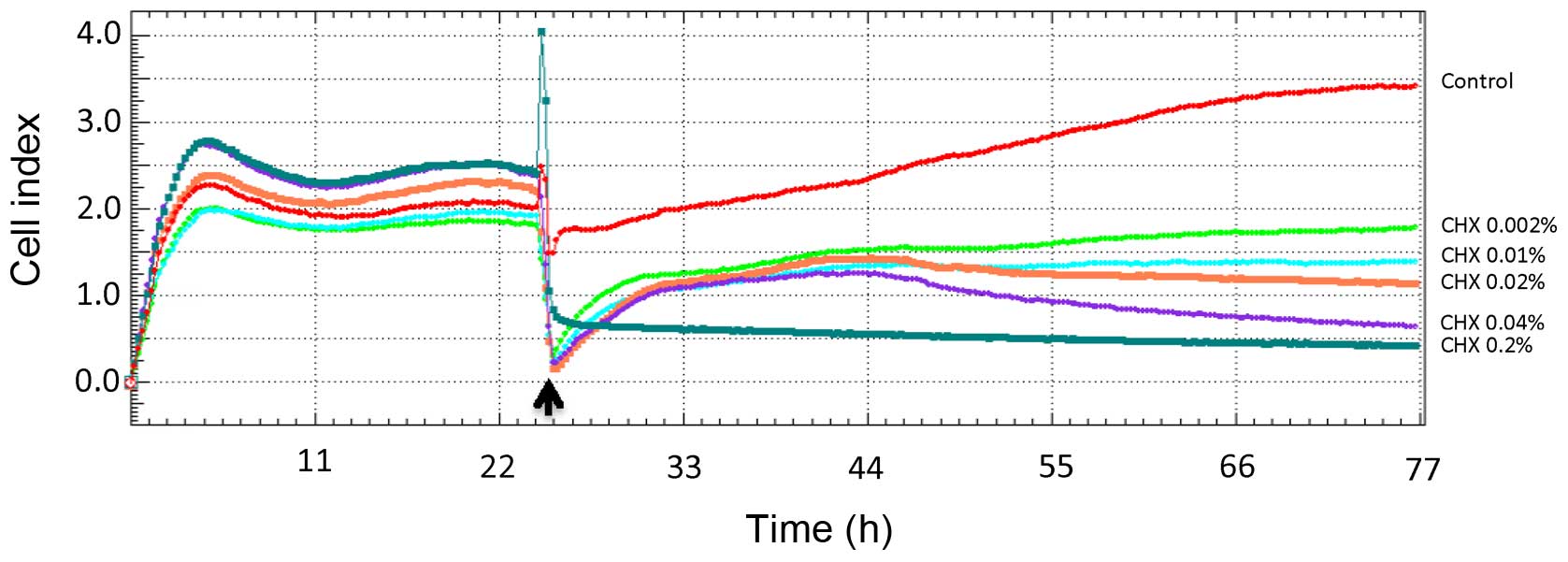
Cell proliferation rate
Cell proliferation assays were performed using the
xCELLigence system. After seeding the HGFs into the wells, the mean
impedance change (n=5) was measured. Impedance was recorded every
15 min. To improve the clarity of the presentation of the results,
only 4 post-CHX stimulation read-outs were analyzed: at 12, 24, 36
and 48 h. No stimulated HGFs obtained a CI value of approximately 2
after 24 h of culture (Fig. 2).
The control cells (treated with DMEM FBS-free medium) exhibited a
significant increase in the cell index, which at 12 h after
stimulation attained a value of 1.5±0.5 and increased to 2.6±0.6
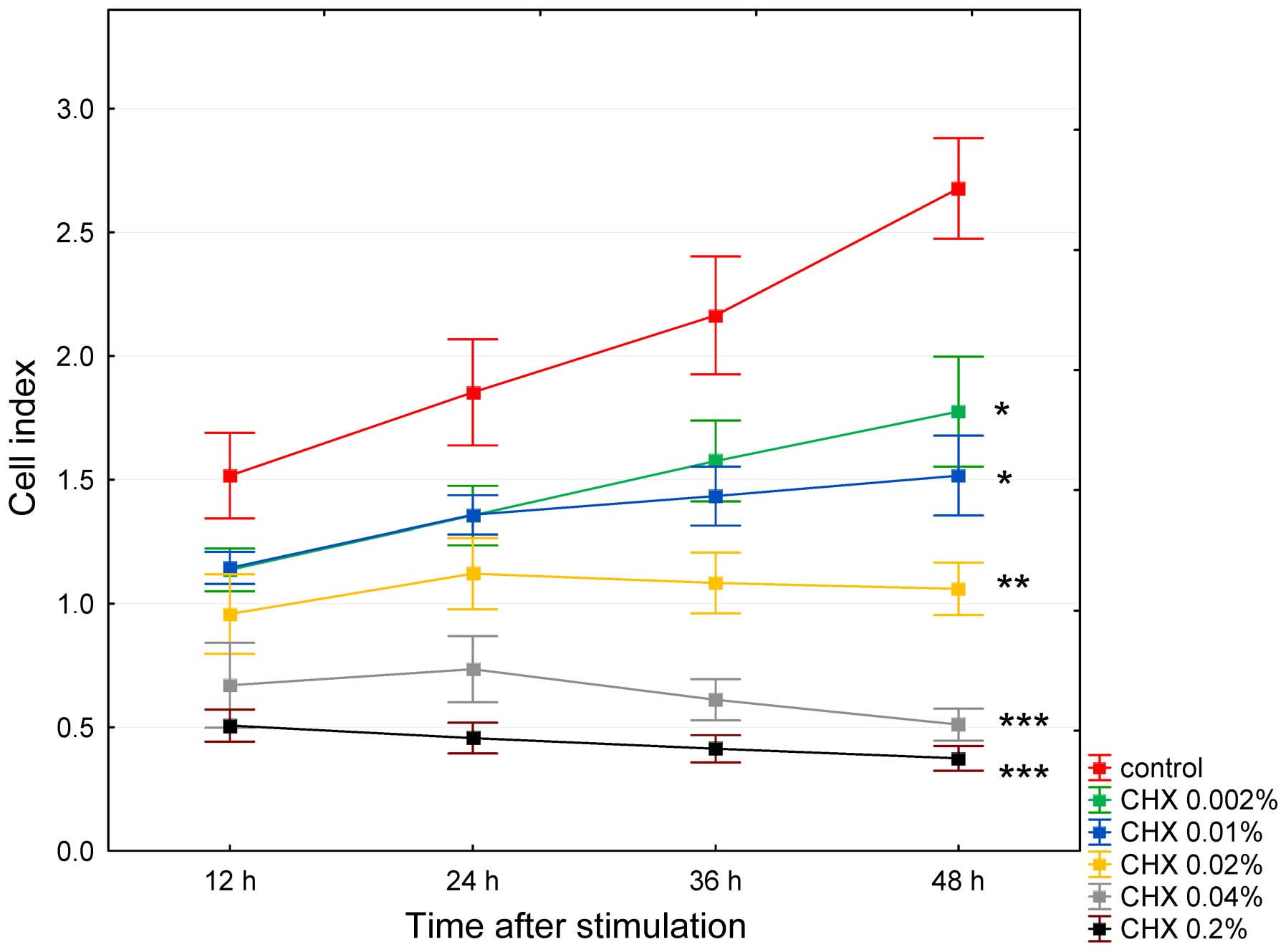
after 48 h of incubation (p=0.003). The anti-proliferative
concentration- and time-dependent effects of CHX on the HGFs are
shown in Fig. 3. The cells
stimulated with CHX at the concentration of 0.002% exhibited a
significant increase in the CI value at 48 h (p<0.05), albeit
significantly lower (p<0.05) than the control group. At higher
CHX concentrations, the effects were less pronounced at the
concentration of 0.01%, almost leveled off at the concentration of
0.02%, and were reversed at the concentrations of 0.04 and 0.2%,
with insignificant increase in CI values (p>0.05) at the
concentrations of 0.02 and 0.04% observed at 24 h as shown in
Fig. 3.
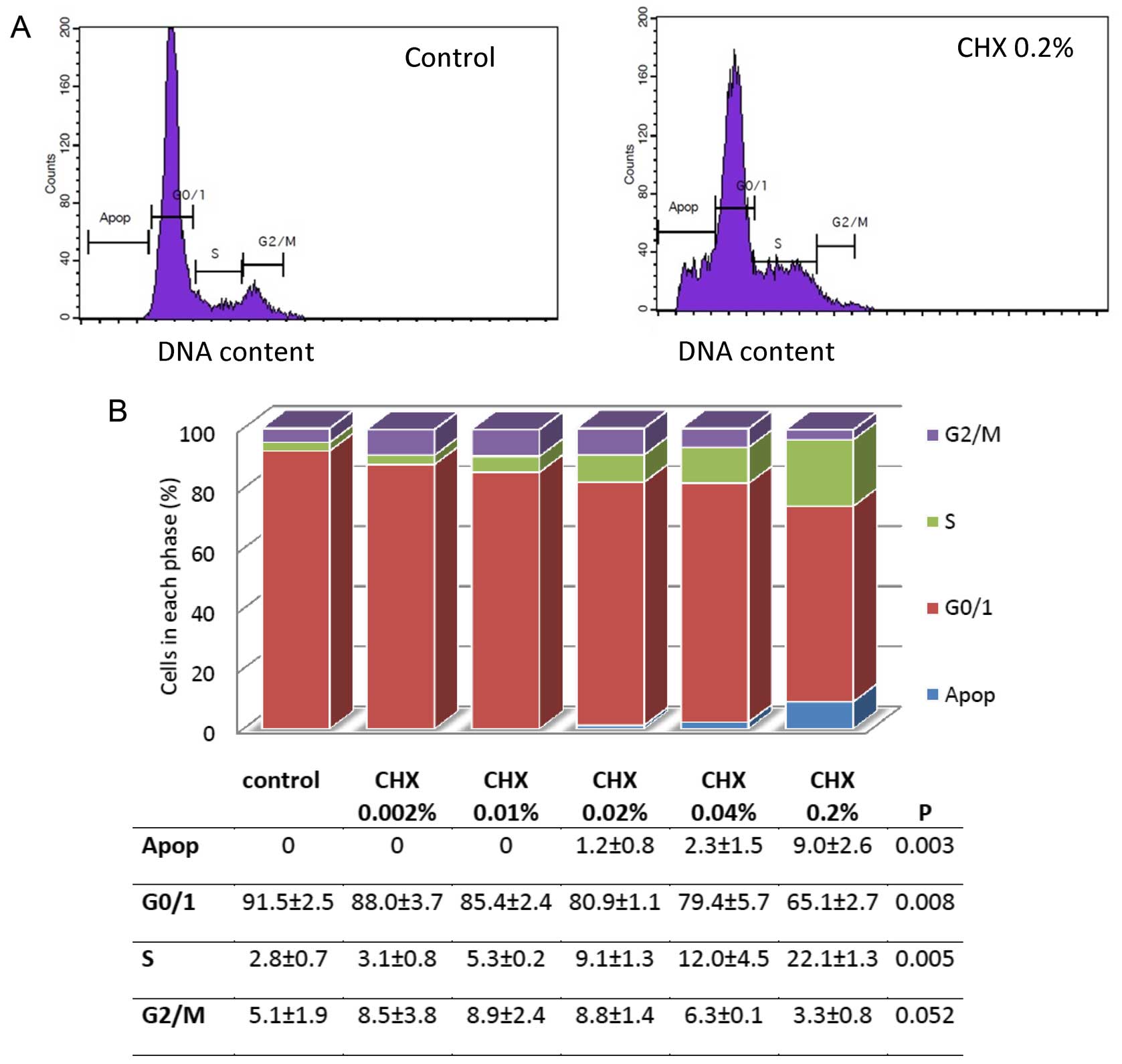
Cell cycle analysis
Flow cytometry was used to examine the changes in
the cell cycle of the HGFs that were either not stimulated, or
stimulated with CHX. The separation of the cells into apoptotic and
the G0/G1, S or G2/M phases was
based upon linear fluorescence intensity after staining with PI.
Representative profiles are shown in Fig. 4A. A decrease in the percentage of
cells in the G0/G1 phase and a buildup of
cells in the S phase was observed. This process was
concentration-dependent. The HGFs not stimulated with CHX had
91.5±2.5% of cells in the G0/G1 phase,
whereas the cells stimulated with 0.002, 0.01, 0.02, 0.04 or 0.2%
CHX had 88.0±3.7, 85.4±2.4, 80.9±1.1, 79.4±5.7 and 65.1±2.7% of
cells in the G0/G1 phase, respectively
(Fig. 4B). In the control group,
the percentage of cells in the S phase was 2.8±0.7%. Following
stimulation with CHX, a concentration-dependent increase in the
percentage of cells in the S phase was observed; the cells
stimulated with the highest concentration of CHX had 22.1±1.3% of
cells in the S phase (Fig. 4B).
No apoptosis was observed either in the unstimulated or in the
cells stimulated with 0.002 or 0.01% CHX. With the increasing CHX
concentration, a significant enhancement in the percentage of cells
undergoing apoptosis was detected, with the highest concentration
corresponding to 9.0±2.6% of apoptotic cells (Fig. 4B).
Discussion
CHX has been widely utilized as a wound antiseptic
and oral antimicrobial rinse. There have been numerous reports on
its safety as an oral rinse; however, its effects on wound healing
have been contradictory. It has been suggested that the direct
application of CHX during regenerative periodontal therapy could
have severe toxic effects on gingival fibroblasts, endothelial
cells and alveolar osteoblasts, thus negatively interfering with
the early healing phase (7).
In a previous study using an infected animal wound
healing model with the polymer drug delivery system of PDGF, the
use of hydrophilic protein promoting healing and CHX, a hydrophobic
antimicrobial agent, effectively inhibited the proliferation of
bacteria without exhibiting cytotoxicity to mammalian cells
(22). However, CHX has been
shown to induce an inflammatory reaction (23), tissue necrosis (24), and to retard the granulation of
tissue formation and wound healing (25). Some other studies have established
that CHX inhibits cell growth, proliferation and collagen synthesis
in human osteoblasts (7,26) and human alveolar bone cells
(15).
The comparison of the results from studies on the
effects of CHX on periodontal tissue is complicated and practically
impossible due to the different research methodologies applied by
different authors; in particular, the duration of cell exposure,
the CHX concentrations and the media used. Therefore, the most
important aim of this study was to use the methods (PANsys 3000,
xCELLigence) that allow us to observe the effects of CHX on cell
lines in conditions similar to those observed in vivo and in
real-time, and to deliver the most reliable results.
For this purpose, all experiments were conducted
without medium containing fetal bovine, which is usually used for
the similarity to the artificial wound fluid. It has been indicated
that FBS has an attenuating effect against CHX-induced
cytotoxicity, which results in a higher cell survival rate
(8).
There are a number of different suggestions in the
literature (1,7,24,27) for the duration of cell exposure to
CHX during in vitro experiments. Due to the slow release of
CHX from the tooth surface and soft tissue following application,
it maintains its antimicrobial activity in the oral cavity for
extended periods. During this time, the oral tissues are exposed to
progressively lower concentrations of CHX. Furthermore, the
periodontal pocket is a specific environment in which the gingival
crevicular fluid is replaced approximately 40 times/h (28) and is usually penetrated by mouth
rinse only to approximately 4% of its depth during mouth rinsing.
Therefore, we decided to expose gingival fibroblasts to a CHX
dilution for longer periods of time than the standard time of oral
rinsing, but shorter than the expected release time from soft
tissue.
In this study, during the constant microscopic
observation of cell morphology and growth in conditions similar to
those observed in vivo, we observed that either cell
morphology or growth did not exhibit any changes in comparison with
the control group following stimulation with 0.002% CHX. In the
cells stimulated with CHX at the concentrations of 0.01 and 0.02% a
decrease in both the dynamics of cell proliferation and the number
of cell divisions was noted, although only in the final hours of
observation. There were no significant changes observed in
fibroblast morphology between these two groups. In the cells
stimulated with 0.04 or 0.2% CHX, the progressive inhibition of
growth and cell division was observed, which was most significant
at 32 h with CHX at 0.04%, and at 16 h with CHX at 0.2%. At these
time points, only small round-shaped cells were observed. These
results are different from the ones presented in the literature.
Giannelli et al (7) used
the concentration of CHX similar to ours; however, following
long-term treatment (5 and 15 min), the authors observed massive
cell death with any concentration used (using the calorimetric
method and confocal microscopy). The results were established 4 h
after exposure. Even following short-term treatment (1 min) with
higher concentrations of CHX (0.03–0.12%) a significant reduction
in cell viability was observed.
The only comparable results of fibroblast morphology
were achieved for fibroblast stimulated with 0.002% CHX, even if
the time of exposure differed. In our study it was after 15 min of
treatment, after 1 min of treatment in the study by Giannelli et
al (7), 24 h in the study by
Dogan et al (1) and 1 h in
the study by Pucher and Daniel (27). Faria et al (25), using CHX at the concentration of
0.001% observed many morphological changes in the fibroblasts, and
the cells were completely destroyed from the concentration of
0.004%. These effects were observed using a scanning electron
microscope directly following short-term stimulation. The
discrepancies between these results may be due to the heterogeneity
of human and animal (murine) fibroblasts, and the different
investigation methods used in the different studies (1,27).
On the other hand, in the study by Ros-Llor and
Lopez-Jornet (29), they did not
report any genotoxic effects against oral mucosa cells resulting
from mouth rinse containing CHX. The study evaluated DNA damage,
cytokinetic defects, proliferative potential and cell death caused
by the frequent use of triclosan, CHX and essential oils in ethanol
solutions. No nuclear abnormalities in exfoliated cells, collected
from cheeks with a cytobrush, were observed (29).
Our data concur with those obtained by other authors
(1,12,30), and confirmed the
anti-proliferative effects of CHX on HGFs in in vitro
conditions. The in vitro cytotoxicity of CHX occurred in a
concentration and time-dependent manner. Moreover, Mariotti and
Rumpf (12) postulated that CHX,
in concentrations which have little effect on cellular
proliferation, can significantly reduce both collagen and
non-collagen protein production by HGFs in vitro.
In this study, we used the RTCA technique
(xCELLigance RTCA system) to provide real-time data concerning the
way that CHX alters the behavior of fibroblasts. The xCELLigance
RTCA platform is highly accurate for monitoring cell behavior and
it correlates very well with conventional adhesion, proliferation
and migration assays. This non-invasive and label-free platform is
being used as a robust system to measure the toxicological response
to nanoparticles and novel compounds (31,32). Having exposed the HGFs to CHX, we
were able to demonstrate a significant decrease in CI, which
correlated with a decrease in cell proliferation.
Due to the fact that the xCELLigance system is an
impedance-based platform, the changes in the CI value can also be
interpreted as morphological changes in the cells. The decrease in
the CI value associated with the highest CHX concentrations used
could result from both the diminished cell proliferation rate and
cell morphological changes, namely the decrease in the number of
cell divisions and the appearance of small round-shaped cells.
Many cytotoxic agents modulate the intricate balance
between cell proliferation and cell death (33). Cell death occurs through a
spectrum of morphological and biochemical pathways culminating in
apoptosis, necrosis or autophagy. Reduced viability often results
from diminished cell proliferation or cell cycle arrest. The
suggested mechanisms underlying CHX-induced cytotoxicity are
connected with the inhibition of collagen synthesis (12,26), the inhibition of protein synthesis
(14,27) or the induction of reactive oxygen
species (ROS) (34). Faria et
al (24,25) found that only at the concentration
of 0.00025% CHX there was no sign of apoptosis and necrosis. The
increase in the number of apoptotic cells was
concentration-dependent, but starting from 0.004% CHX there was no
difference compared with the control group. The higher
concentration of CHX induced cell necrosis. Chang et al
(14) used 5% CHX and indicated
that it was cytotoxic to periodontal ligament cells at the
concentration of 0.0001% or greater, and inhibited protein
synthesis at the 0.005% concentration. The protein synthesis was
almost completely inhibited by the concentration of >0.05%, as
was the mitochondrial activity of the human periodontal ligament
cells, which was completely inhibited by 0.125% CHX. CHX may also
induce cell death by apoptosis and necrosis via endoplasmic
reticulum stress (25). Based on
studies conducted on human osteoblastic and murine endothelial
cells and fibroblasts, Gianelli et al (7) suggested that CHX exerts toxic
through the induction of apoptotic and auto/necrotic cell death and
involves the reduction of mitochondrial membrane potential, an
increase in intracellular Ca2+ levels and oxidative
stress.
Our results suggest that CHX induced cell cycle
arrest at the S phase. Both the number of cells at the
G0/G1 and G2/M phase decreased,
while the number of cells at the S phase increased. Hidalgo et
al (8) observed that CHX
exerted an inhibitory concentration-dependent effect on DNA
synthesis from the concentration as low as 0.0001% in dermal
fibroblasts. In this study, the changes in the cell cycle were
observed at the concentration of 0.04% (minimum). Looking at this
discrepancy, one can speculate that CHX exhibits a different degree
of cytotoxicity towards different cell types. However, this
difference may also be due to the different times of cell exposure
to CHX. Hidalgo et al (8)
incubated cells with CHX for 3, 6, 8 and 24 h, whereas we incubated
the cells with CHX for 15 min. These could also be reasons for
differences in DNA synthesis observed in our study. Hidalgo et
al (8) used
5-bromodeoxy-uridine (BrdU), a thymidine analogue that is
incorporated into the cells during the DNA synthetic phase of
replicating cells (during the S phase of the cell cycle). They
observed a significant decrease in BRdU incorporation that occurred
at the concentration of 0.0001% CHX, which reflects the decrease in
the number of cells in the S phase. We observed the
concentration-dependent accumulation of cells in the S phase
together with a decrease of cells in the G2/M phase
following stimulation with CHX, which indicates that these cells do
not seem to re-enter the cell cycle. It cannot be excluded that CHX
is able to modify cell culture conditions so that quiescent S phase
cells appear. Thus, the accumulation of inactive cells in the S
phase would accompany the decreasing frequency of BrdU-positive
cells. However, further research is warranted to confirm these
findings.
Cell cycle arrest is often followed by resumed entry
into the cell cycle or cell demise via apoptosis. Our results
suggest that cells were arrested in the S phase to repair the
CHX-induced DNA damage, and that some of the damage was not
repaired causing the cells to undergo apoptosis.
In our study, we did not detect any apoptotic
symptoms in the CHX-stimulated cells at the concentration below
0.01%. The percentage of apoptotic cells increased to 9.0% of cells
at the highest concentration. The number of apoptotic cells was
assessed based on the percentage of sub-G1 (<2N DNA) fraction in
HGFs, the internucleosomal DNA fragmentation being one of the
hallmarks of apoptosis. As DNA oligomers are extracted during cell
staining, apoptotic cells can be identified on DNA content
frequency histograms, as cells with fractional sub-G1 DNA content.
However, the sub-G1 DNA content cannot be used as the sole marker
of apoptotic cells, as DNA fragmentation to the oligo- or
mono-nucleosomal-size fragments does not always take place during
apoptosis (35).
To summarize, in conditions similar to those
observed in vivo, the low CHX concentration has a different
effect on gingival fibroblasts than the high concentration.
However, even this low concentration has a greater influence on
cells than the untreated controls. The low CHX concentration has
minimal cytotoxicity, as it decreases proliferation without
inducing morphological changes and apoptosis.
These findings suggest a different clinical protocol
for patients with improper oral hygiene and patients after surgical
treatment. The low CHX concentration can have antimicrobial
activity and does not influence wound healing. It was found that
0.004% CHX in toothpaste inhibits bacterial colonization and growth
on an enamel surface; however, even this low concentration of CHX
was higher than the minimal concentration needed for the
elimination of Streptoccocus mutans (36). The minimal inhibitory
concentration (MIC) of CHX on periodontal pathogens is 0.0012%. In
addition, the penetration of CHX into the biofilm seems to be
easier at lower concentrations. The compact matrix inhibits the
diffusion of solutes, such as CHX into the biofilm. It is possible
that conformational changes in biofilm structure, such as the
opening up of the water channel, could assist in the diffusion of
CHX into deeper layers. It was observed after using CHX at the
concentration of 0.05%, but not at the concentration of 0.2%
(37). Our previous clinical
study also confirmed the effectiveness of a low CHX concentration
(0.04%) in he subgingival irrigation in patients treated for
chronic periodontal disease (38).
In conclusion, the aim of this study was to evaluate
the effects of different concentrations of CHX on HGFs. The low
concentration (0.002%) of CHX does not interfere with the
proliferation and morphology of gingival fibroblasts. The higher
concentration (≥0.04%) of CHX inhibits cell proliferation and, to a
certain extent, affects cell morphology. Thus, the application of
CHX in the post-surgical antiseptic treatment of the oral cavity
should be limited.
Acknowledgments
This study was supported by the Poznan University of
Medical Sciences research grant (no. 50201-044105190-06466).
References
|
1
|
Dogan S, Günay H, Leyhausen G and Geurtsen
W: Effects of low-concentrated chlorhexidine on growth of
Streptococcus sobrinus and primary human gingival fibroblasts. Clin
Oral Investig. 7:212–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salem AM, Adams D, Newman HN and Rawle LW:
Antimicrobial properties of 2 aliphatic amines and chlorhexidine in
vitro and in saliva. J Clin Periodontol. 14:44–47. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beighton D, Decker J and Homer KA: Effects
of chlorhexidine on proteolytic and glycosidic enzyme activities of
dental plaque bacteria. J Clin Periodontol. 18:85–89. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gendron R, Grenier D, Sorsa T and Mayrand
D: Inhibition of the activities of matrix metalloproteinases 2, 8,
and 9 by chlorhexidine. Clin Diagn Lab Immunol. 6:437–439.
1999.PubMed/NCBI
|
|
5
|
Houri-Haddad Y, Halabi A and Soskolne WA:
Inflammatory response to chlorhexidine, minocycline HCl and
doxycycline HCl in an in vivo mouse model. J Clin Periodontol.
35:783–788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cronan CA, Potempa J, Travis J and Mayo
JA: Inhibition of Porphyromonas gingivalis proteinases (gingipains)
by chlorhexidine: Synergistic effect of Zn(II). Oral Microbiol
Immunol. 21:212–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gianelli M, Chellini F, Margheri M,
Tonelli P and Tani A: Effect of chlorohexidine digluconate on
different cell types: A molecular and ultrastructural
investigation. Toxicol In Vitro. 2:308–317. 2008. View Article : Google Scholar
|
|
8
|
Hidalgo E and Dominguez C: Mechanisms
underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro.
15:271–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koontongkaew S and Jitpukdeebodintra S:
Interaction of chlorhexidine with cytoplasmic membranes of
Streptococcus mutans GS-5. Caries Res. 29:413–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonesvol P: Oral pharmacology of
chlorohexidine. J Clin Periodontol. 4:49–65. 1997. View Article : Google Scholar
|
|
11
|
Boisnic S, Ben Slama L, Branchet-Gumila
MC, Watts M and d'Arros G: Wound healing effect of Eludril in a
model of human gingival mucosa. Rev Stomatol Chir Maxillofac.
107:431–435. 2006.In French. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mariotti AJ and Rumpf DA:
Chlorhexidine-induced changes to human gingival fibroblast collagen
and non-collagen protein production. J Periodontol. 70:1443–1448.
1999. View Article : Google Scholar
|
|
13
|
Babich H, Wurzburger BJ, Rubin YL,
Sinensky MC and Blau L: An in vitro study on the cytotoxicity of
chlorhexidine digluconate to human gingival cells. Cell Biol
Toxicol. 11:79–88. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang YC, Huang FM, Tai KW and Chou MY:
The effect of sodium hypochlorite and chlorhexidine on cultured
human periodontal ligament cells. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 92:446–450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cabral CT and Fernandes MH: In vitro
comparison of chlorhexidine and povidone-iodine on the long-term
proliferation and functional activity of human alveolar bone cells.
Clin Oral Investig. 11:155–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cline NV and Layman DL: The effects of
chlorhexidine on the attachment and growth of cultured human
periodontal cells. J Periodontol. 63:598–602. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wyganowska-Swiatkowska M, Urbaniak P,
Szkaradkiewicz A, Jankun J and Kotwicka M: Effects of
chlorhexidine, essential oils and herbal medicines (Salvia,
Chamomile, Calendule) on human fibroblast in vitro. Cent Eur J
Immunol. In Press.
|
|
18
|
Campbell KE, Keast D, Woodbury G and
Houghton P: Wear time in two hydrocolloid dressing using a novel
in-vivo model. Wounds. 15:40–48. 2003.
|
|
19
|
Marlina S, Shu MH, AbuBakar S and Zandi K:
Development of a real-time cell analysing (RTCA) method as a fast
and accurate screen for the selection of chikungunya virus
replication inhibitors. Parasit Vectors. 8:5792015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urcan E, Haertel U, Styllou M, Hickel R,
Scherthan H and Reichl FX: Real-time xCELLigence impedance analysis
of the cytotoxicity of dental composite components on human
gingival fibroblasts. Dent Mater. 26:51–58. 2010. View Article : Google Scholar
|
|
21
|
Xing JZ, Zhu L, Gabos S and Xie L:
Microelectronic cell sensor assay for detection of cytotoxicity and
prediction of acute toxicity. Toxicol In Vitro. 20:995–1004. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang B, Zhang G and Brey EM: Dual
delivery of chlorhexidine and platelet-derived growth factor-BB for
enhanced wound healing and infection control. Acta Biomater.
9:4976–4984. 2013. View Article : Google Scholar
|
|
23
|
Onçağ O, Hoşgör M, Hilmioğlu S, Zekioğlu
O, Eronat C and Burhanoğlu D: Comparison of antibacterial and toxic
effects of various root canal irrigants. Int Endod J. 36:423–432.
2003. View Article : Google Scholar
|
|
24
|
Faria G, Celes MR, De Rossi A, Silva LA,
Silva JS and Rossi MA: Chlorhexidine-induced apoptosis or necrosis
in L929 fibroblasts to cultured 1929 fibroblasts. J Endod.
33:715–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faria G, Cardoso CR, Larson RE, Silva JS
and Rossi MA: Chlorhexidine-induced apoptosis or necrosis in L929
fibroblasts: A role for endoplasmic reticulum stress. Toxicol Appl
Pharmacol. 234:256–265. 2009. View Article : Google Scholar
|
|
26
|
Lee TH, Hu CC, Lee SS, Chou MY and Chang
YC: Cytotoxicity of chlorhexidine on human osteoblastic cells is
related to intracellular glutathione levels. Int Endod J.
43:430–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pucher JJ and Daniel JC: The effects of
chlorhexidine digluconate on human fibroblasts in vitro. J
Periodontol. 63:526–532. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goodson JM: Pharmacokinetic principles
controlling efficacy of oral therapy. J Dent Res. 68:1625–1632.
1989.
|
|
29
|
Ros-Llor I and Lopez-Jornet P: Cytogenetic
analysis of oral mucosa cells, induced by chlorhexidine, essential
oils in ethanolic solution and triclosan mouthwashes. Environ Res.
132:140–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsourounakis I, Palaiologou-Gallis AA,
Stoute D, Maney P and Lallier TE: Effect of essential oil and
chlorhexidine mouthwashes on gingival fibroblast survival and
migration. J Periodontol. 84:1211–1220. 2013. View Article : Google Scholar
|
|
31
|
Ramis G, Martínez-Alarcón L, Quereda JJ,
Mendonça L, Majado MJ, Gomez-Coelho K, Mrowiec A, Herrero-Medrano
JM, Abellaneda JM, Pallares FJ, et al: Optimization of cytotoxicity
assay by real-time, impedance-based cell analysis. Biomed
Microdevices. 15:985–995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Quereda JJ, Martínez-Alarcón L, Mendoça L,
Majado MJ, Herrero-Medrano JM, Pallarés FJ, Ríos A, Ramírez P,
Muñoz A and Ramis G: Validation of xCELLigence real-time cell
analyzer to assess compatibility in xenotransplantation with
pig-to-baboon model. Transplant Proc. 42:3239–3243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Müller G and Kramer A: Biocompatibility
index of antiseptic agents by parallel assessment of antimicrobial
activity and cellular cytotoxicity. J Antimicrob Chemother.
61:1281–1287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeung SY, Huang CS, Chan CP, Lin CP, Lin
HN, Lee PH, Jia HW, Huang SK, Jeng JH and Chang MC: Antioxidant and
pro-oxidant properties of chlorhexidine and its interaction with
calcium hydroxide solutions. Int Endod J. 40:837–844. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Darzynkiewicz Z, Bedner E and Traganos F:
Difficulties and pitfalls in analysis of apoptosis. Methods Cell
Biol. 63:527–546. 2001. View Article : Google Scholar
|
|
36
|
Zampatti O, Roques C and Michel G: An in
vitro mouth model to test antiplaque agents: Preliminary studies
using a toothpaste containing chlorhexidine. Caries Res. 28:35–42.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hope CK and Wilson M: Analysis of the
effects of chlorhexidine on oral biofilm vitality and structure
based on viability profiling and an indicator of membrane
integrity. Antimicrob Agents Chemother. 48:1461–1468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wyganowska-Swiatkowska M, Jaskula J and
Wieckowska B: Professional irrigation in periodontal treatment.
Polish J Eviront Stud. 16:316–319. 2008.
|