Introduction
Skeletal muscle accounts for 40% of body weight in
humans, and 75% of infused glucose is cleared by skeletal muscle
(1,2); thus, skeletal muscle serves as the
major site of insulin-dependent glucose uptake. Due to the key role
played by skeletal muscle in glucose homeostasis, deleterious
factors which provoke reductions in glucose uptake by skeletal
muscle, described as skeletal muscle insulin resistance, may lead
to decreases in the disposal rate of serum glucose (3).
The etiology of impaired insulin signaling in obese
individuals is multifactorial and appears to be associated with at
least two major events: a state of chronic, low-grade inflammation
and the accumulation of intramyocellular lipids (4). Under physiological conditions, serum
free fatty acids (FFAs) are an important fuel source for skeletal
muscle, and 98–99% of FFAs bind to bovine serum albumin (5). Therefore, physiological
concentrations of FFAs are in the µmol/l range (5). Constantly elevated serum levels of
FFAs result in the transportation of FFAs into skeletal muscle
cells, and subsequently stored as triglyceride. Whenever the
accumulation of triglycerides and/or hydrolysis of triglycerides
exceeds the oxidation capacity, incomplete lipid metabolic
products, such as acyl-CoA, diacylglycerol and ceramide, are
generated. These products inhibit the activation of critical
molecules involved in insulin signaling, such as protein kinase B
(AKT) and insulin receptor substrate (IRS)1 (6), reduce insulin sensitivity, leading
to insulin resistance in skeletal muscle. Previous studies of
muscle from diabetic rodents and human subjects demonstrated that
pharmacological agents, such as 5-aminoimidazole-4-carboxamide
riboside (AICAR) (7), metformin
(8), thiazolidinediones (TZD)
(9,10) and ciliary neurotrophic factor
(11), increase muscle glucose
uptake, and that this response is maintained.
Astragaloside IV, a
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosylcycloastragenol
purified from Astragalus membranaceus (Fisch.) Bunge, is one
of the most widely used plant-derived drugs in traditional Chinese
medicine for diabetes therapy (10). The saponin astragaloside IV has
been reported to exert various pharmacological effects;
astragaloside IV inhibited hepatic glycogen phosphorylase (GP) and
glucose-6-phosphatase (G6P) activities thereby decreasing serum
glucose levels in diabetic mice (12), attenuated lipolysis and reduced
insulin resistance induced by tumor necrosis factor α (TNFα) in
3T3-L1 adipocytes (13), improved
the symptoms of metabolic syndrome in fructose-fed rats (14), prevented human cardiovascular
pathological changes and protected against cardiovascular injury in
rats (15–18) improved renal function (19–22), reduced the progression of
peripheral neuropathy (23), and
attenuated inflammatory responses by suppressing the nuclear
factor-κB (NF-κB) pathway (24–26). Xu et al (27) reported that other astragalosides,
astragaloside II and isoastragaloside I, elevated serum levels of
adiponectin and alleviated insulin resistance and glucose
intolerance in obese mice. Our research team has previously found
that astragaloside IV decreases serum FFA and glucose levels in
mice fed a high-fat diet, and improves insulin sensitivity
(13). In order to examine the
mechanisms through which astragaloside IV decreases serum glucose
concentrations, we selected the major organ responsible for glucose
clearance, skeletal muscle, for in vitro study. Thus, we
found that astragaloside IV increases basal and insulin-stimulated
glucose uptake in C2C12 myotubes through the IRS1/AKT pathway and
suppresses the palmitate-induced activation of the inhibitory κB
kinase (IKK)/inhibitor-κBα (IκBα) pathway.
Materials and methods
Materials
Astragaloside IV, palmitate and protease inhibitor
cocktail were purchased from Sigma (St. Louis, MO, USA). Dulbecco's
modified Eagle's medium (DMEM) and horse serum were purchased from
Gibco Life Technologies (Grand Island, NY, USA). Fetal bovine serum
(FBS) was obtained from PAA Laboratories, Pasching, Austria). Fatty
acid-free bovine serum albumin (BSA) was purchased from Calbiochem
(San Diego, CA, USA). Horseradish peroxidase-conjugated goat
anti-rabbit and rabbit anti-mouse IgG were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Phosphorylated
(p)-IRS1 [tyrosine (Y)612] was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). An antibody against
GAPDH was obtained from Novus Biologicals (Littleton, CO, USA).
Antibodies against p-AKT [serine (Ser)473 and threonine (Thr)308],
AKT, p-insulin receptor β (IRβ), glucose transporter 4 (GLUT4),
p-IKKα/IKKβ and IκBα were purchased from Cell Signaling Technology,
Inc. 2-Deoxy-D-[1,2-3H(N)]-glucose (radiolabeled 2-DG)
was purchased from Amersham (Buckinghamshire, UK), and purified
human insulin was obtained from Eli Lilly (Indianapolis, IN, USA).
SYBR® Premix Ex Taq™ was purchased from Takara Bio,
Dalian, China). SuperScript III was purchased from Invitrogen
(Carlsbad, CA, USA).
Cell culture
The mouse myoblast cell line C2C12 was obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in DMEM supplemented with 10% FBS. When the cells
reached 70% confluence, the medium was replaced with
differentiation medium containing DMEM and 2% horse serum, which
was replaced every other day. After 4 days, the differentiated
C2C12 cells had fused into myotubes. Lipid-containing media were
prepared by the conjugation of FFA with FFA-free BSA, according to
a method previously described by Itani et al (6) with some modifications. Briefly,
palmitate was dissolved in 0.1 N NaOH and diluted in DMEM
containing 2% (wt/vol) fatty acid-free BSA. The myotubes were
incubated for 16 h in serum-free DMEM containing 2% BSA in either
the presence or absence of palmitate. The cells were then incubated
with 100 nM insulin for 30 min. Following incubation, RNA and total
proteins were extracted from the myotubes as described below.
Astragaloside IV was added 2 h prior to palmitate exposure.
2-DG transport assay
Glucose uptake into the C2C12 myotubes was measured
using radiolabeled 2-DG, according to a method previously described
by Perrini et al (28)
with some modifications. Prior to performing the glucose transport
assays, the cells were washed twice with HEPES-buffered saline
(HBS). The glucose transport assays were performed by incubating
the cells with HBS containing radiolabeled 2-DG (0.5 µCi/ml)
at room temperature. The cells with prior exposure to insulin (see
below) were incubated in glucose uptake assay medium supplemented
with insulin. After 30 min, 2-DG uptake was terminated by three
rapid washes with ice-cold phosphate-buffered saline (PBS) +
glucose. The cells were lysed in 0.1 N NaOH, and then 3H
was counted using a liquid scintillation spectrophotometer (Beckman
Instruments, Fullerton, CA, USA).
Measurement of mRNA levels
Total RNA was isolated using TRIzol reagent. The
total RNA isolated by this method is undegraded and free of protein
and DNA contamination. In order to perform amplification, the
following sense and antisense primer sequences were used for
amplification: GLUT4, 5′-GTGACTGGAACACTGGTCCTA-3′ and
5′-CCAGCCACGTTGCATTGTAG-3′; Toll-like receptor 4 (TLR4),
5′-TCTTCTCCTGCCTGACACCA-3′ and 5′-TCTTCTCCTGCCTGACACCA-3′; monocyte
chemotactic protein 1 (MCP-1), 5′-GGCCTGTTGTTCACAGTTGC-3′ and
5′-AGCCGACTCATTGGGATCAT-3′; tumor necrosis factor (TNF)α,
5′-CACAAGATGCTGGGACAGTGA-3′ and 5′-TCCTTGATGGTGGTGCATGA-3′; and
interleukin-6 (IL-6), 5′-CAGCCACTGCCTTCCCTACT-3′ and
5′-CAGTGCATCATCGCTGTTCAT-3′. Preliminary experiments were performed
with various amounts of cDNA to determine the non-saturating
conditions of PCR amplification for all the genes studied.
Therefore, under these conditions, the relative quantification of
mRNA was assessed by the RT-PCR method used in this study. The
expression of specific mRNAs are presented relative to the
expression of the control gene [adenine phosphoribosyltransferase
(APRT), 5′-CGGCAAGATCGACTACATCG-3′ and
5′-CCAGCTCAGCCTTCCCATAC-3′].
Subcellular fractionation
The subcellular fractionation of the C2C12 cells was
performed as described by Tortorella and Pilch (29) with some modifications. The C2C12
cells were washed three times in ice-cold PBS, pH 7.4 (in mM: 137
NaCl, 2.7 KCl, 10 Na2HPO4 and 1.8
KH2PO4) and then the cells were sheared
through a 22-gauge needle 15 times in HES buffer [255 mM sucrose, 4
mM disodium EDTA, 20 mM HEPES pH 7.4, 10 µM leupeptin, 1
µM pepstatin, 1 µM aprotinin, 1 mM
phenylmethylsulfonyl fluoride (PMSF) and 5 mM benzamidine]. The
homogenate was centrifuged at 19,000 × g for 20 min. The pellet was
saved and fractionated further in order to extract the following
fractions: crude 'plasma membrane' (PM) fraction (P1) and crude
nuclear (N)/endoplasmic reticulum (ER) fraction. In order to obtain
the PM fraction, the pellet was resuspended in HES, layered onto a
1.12 M sucrose cushion in 20 mM HEPES and 1 mM disodium EDTA, and
centrifuged at 100,000 × g for 1 h. The sucrose cushion interface
was collected and pelleted at 40,000 × g for 20 min. This
PM-containing pellet (P1) was resuspended in PBS plus protease
inhibitors. All centrifugations were performed at 4 °C by a Beckman
Ultraspeed centrifuge (Beckman Coulter, Brea, CA, USA). GLUT4 was
detected by western blot analysis as described below.
Western blot analysis
The C2C12 cells grown on six-well plates were washed
with ice-cold PBS and then scraped into homogenizing buffer
containing 50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1%
Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM
Na3PO4, 100 mM NaF, 2 mM
Na3VO4, 10 µg/ml leupeptin, 10
µg/ml aprotinin, and 1 mM PMSF. The lysates were centrifuged
at 13,000 rpm for 30 min. Soluble proteins were quantitated using
the bicinchoninic acid kit (Pierce Chemical Co., Rockford, IL, USA)
with BSA as the standard and adjusted to 2 µg/µl.
Aliquots of homogenate were solubilized in Laemmli sample buffer
and the protein was subjected to SDS-PAGE. Proteins were
transferred to nitrocellulose membranes (Whatman; GE Healthcare,
Piscataway, NJ, USA). The membranes were blocked in a solution of
Tris-buffered saline containing 5% nonfat dry milk. Protein was
visualized using the SuperSignal® West Pico
chemiluminescent substrate (Pierce Chemical Co.) assay.
Statistical analysis
Data are presented as the means ± SEM. The
significance between groups was determined using either an unpaired
two-tailed Student's t-test or one-way ANOVA as appropriate. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
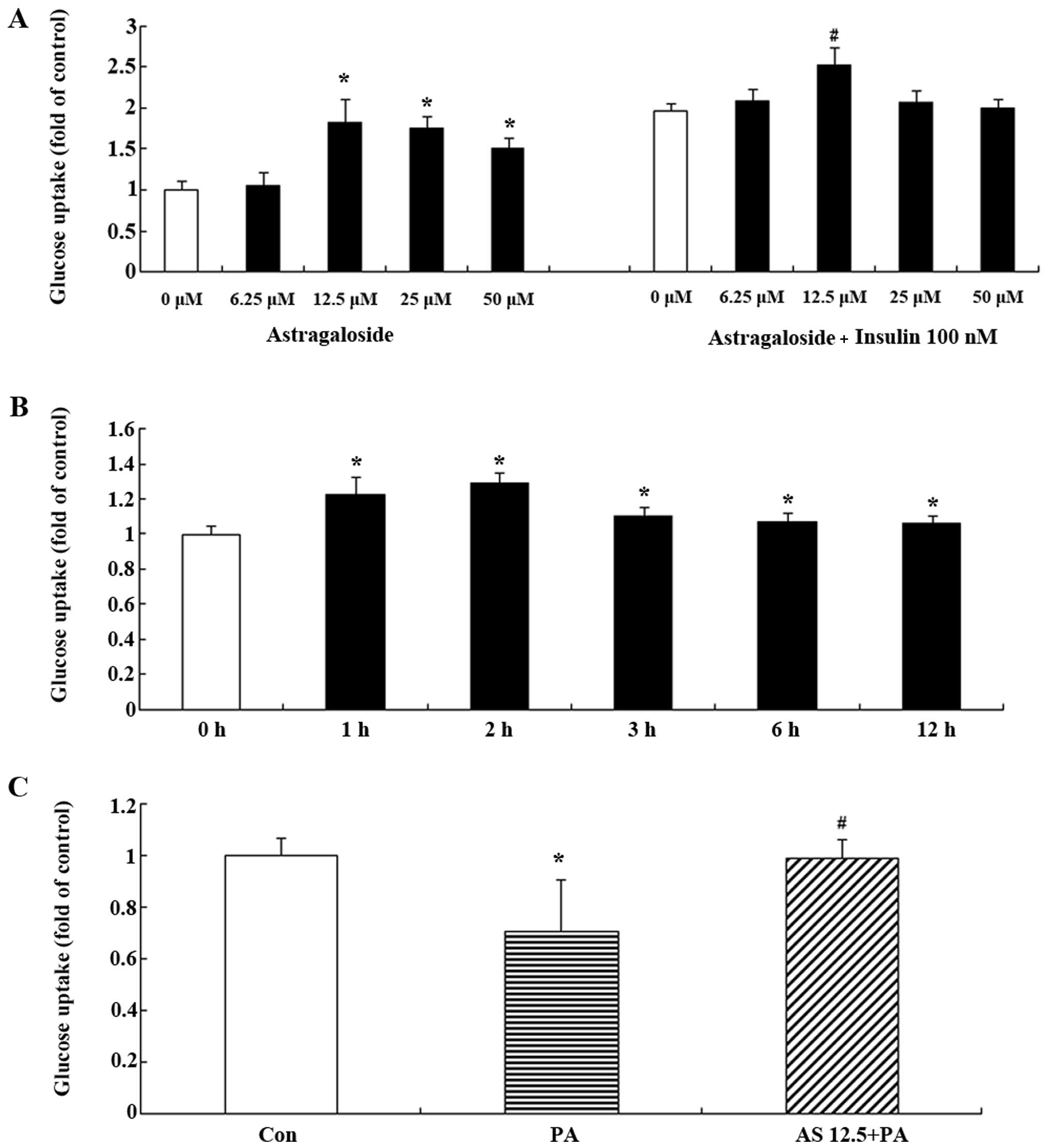
Astragaloside IV increases basal and
insulin-stimulated glucose uptake in C2C12 myotubes
In order to examine the effect of astragaloside IV
on glucose uptake in C2C12 myotubes in the presence or absence of
insulin, differentiated C2C12 myotubes were serum starved overnight
in 0.2% BSA + DMEM, and were then treated with astragaloside IV at
concentrations ranging from 6.25 µM to 50 µM for 12
h. Astragaloside IV increased glucose uptake in the C2C12 myotubes
in a dose-dependent manner with the most significant increase at
12.5 µM (Fig. 1A). In the
basal state, glucose uptake was elevated with increasing
concentrations of astragaloside IV up to 50 µM (Fig. 1A); however, in the
insulin-stimulated C2C12 myotubes, glucose uptake was significantly
increased only at a concentration of 12.5 µM astragaloside
IV (Fig. 1A). We then treated the
C2C12 myotubes with 12.5 µM astragaloside IV for different
periods of time. The C2C12 myotubes showed a significant increase
in glucose uptake after 1 h, and the most significant effect was
observed at 2 h, and was sustained until 12 h (Fig. 1B). Astragaloside IV increased
glucose uptake in C2C12 myotubes in a dose- and time-dependent
manner.
Astragaloside IV ameliorates
palmitate-induced insulin resistance in C2C12 myotubes
In a previous in vitro experiment,
insulin-stimulated glucose uptake was decreased in C2C12 myotubes
exposed to palmitate for 16 h (30). In the present study, we determined
the effect of astragaloside IV on glucose uptake reduced by
palmitate in C2C12 myotubes. The C2C12 myotubes were treated with
12.5 µM astragaloside IV and serum starved for 2 h prior to
incubation with 0.75 mM palmitate for 16 h. A 16-h incubation
period with 0.75 mM palmitate decreased the insulin-stimulated
uptake of 2-DG (P<0.05 vs. insulin-stimulated cells incubated
with BSA alone) (Fig. 1C).
However, pre-treatment with astragaloside IV restored glucose
uptake in the palmitate-exposed C2C12 myotubes (P<0.05)
(Fig. 1C).
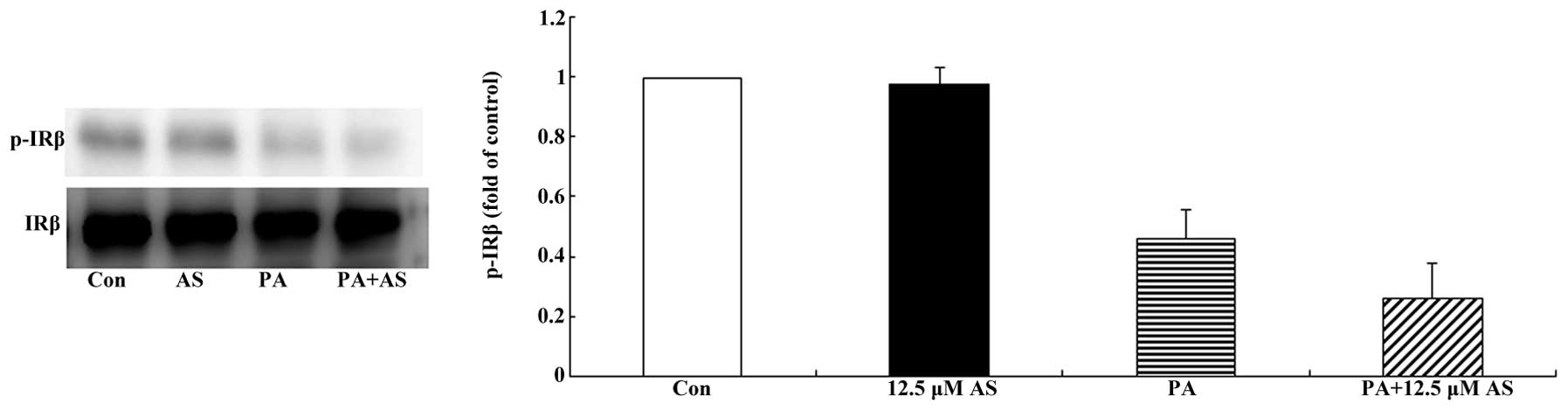
Effect of astragaloside IV on the
phosphorylation of IRβ in the insulin-stimulated state
The subcellular localization of IRβ was evaluated by
subcellular fractionation followed by western blot analysis. As
shown in Fig. 2, astragaloside IV
had no significant effect on the protein expression or the
phosphorylation of IRβ in the presence or absence of palmitate.
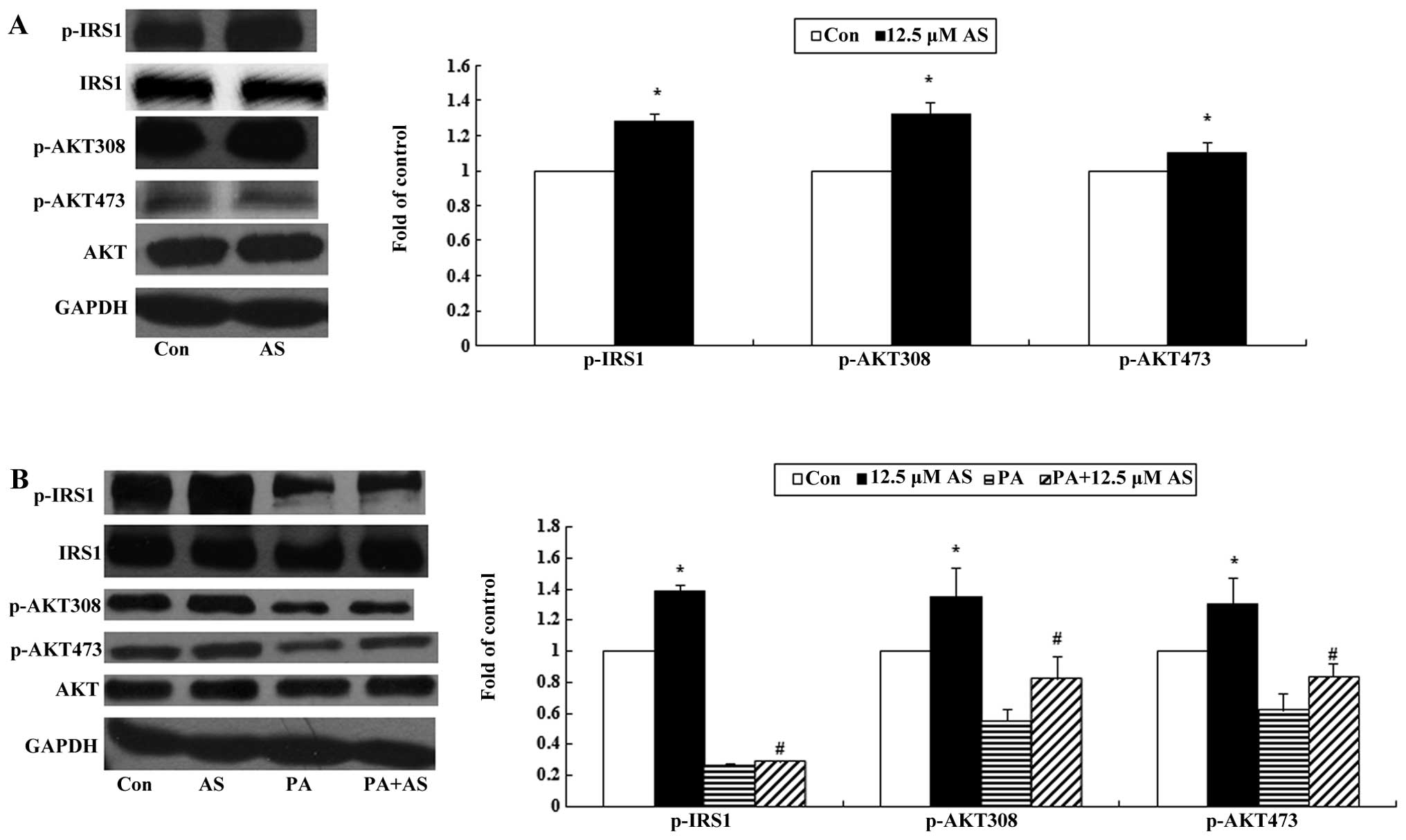
Effect of astragaloside IV on the
phosphorylation of IRS1 and AKT in the basal state
The proteins, IRS1 and AKT, play important roles in
insulin signaling, and the phosphorylation of IRS1 and AKT may
promote glucose uptake in skeletal muscle (31). In this experiment, we found that
astragaloside IV significantly enhanced the phosphorylation of
IRS1(Y612) as well as AKT (Ser473) and (Thr308) (P<0.05;
Fig. 3A).
Effect of astragaloside IV on the
phosphorylation of IRS1 and AKT in the insulin-stimulated
state
As expected, astragaloside IV significantly
increased the insulin-stimulated phosphorylation of IRS1(Y612) and
AKT (Ser473) and (Thr308) (P<0.05; Fig. 3B) in the C2C12 myotubes. Palmitate
exposure reduced the levels of total protein as well as the
phosphorylation of IRS1 and AKT. Pre-treatment with astragaloside
IV ameliorated the reduced phosphorylation levels; however, it had
no effect on the protein expression of IRS1 and AKT (P<0.05;
Fig. 3B).
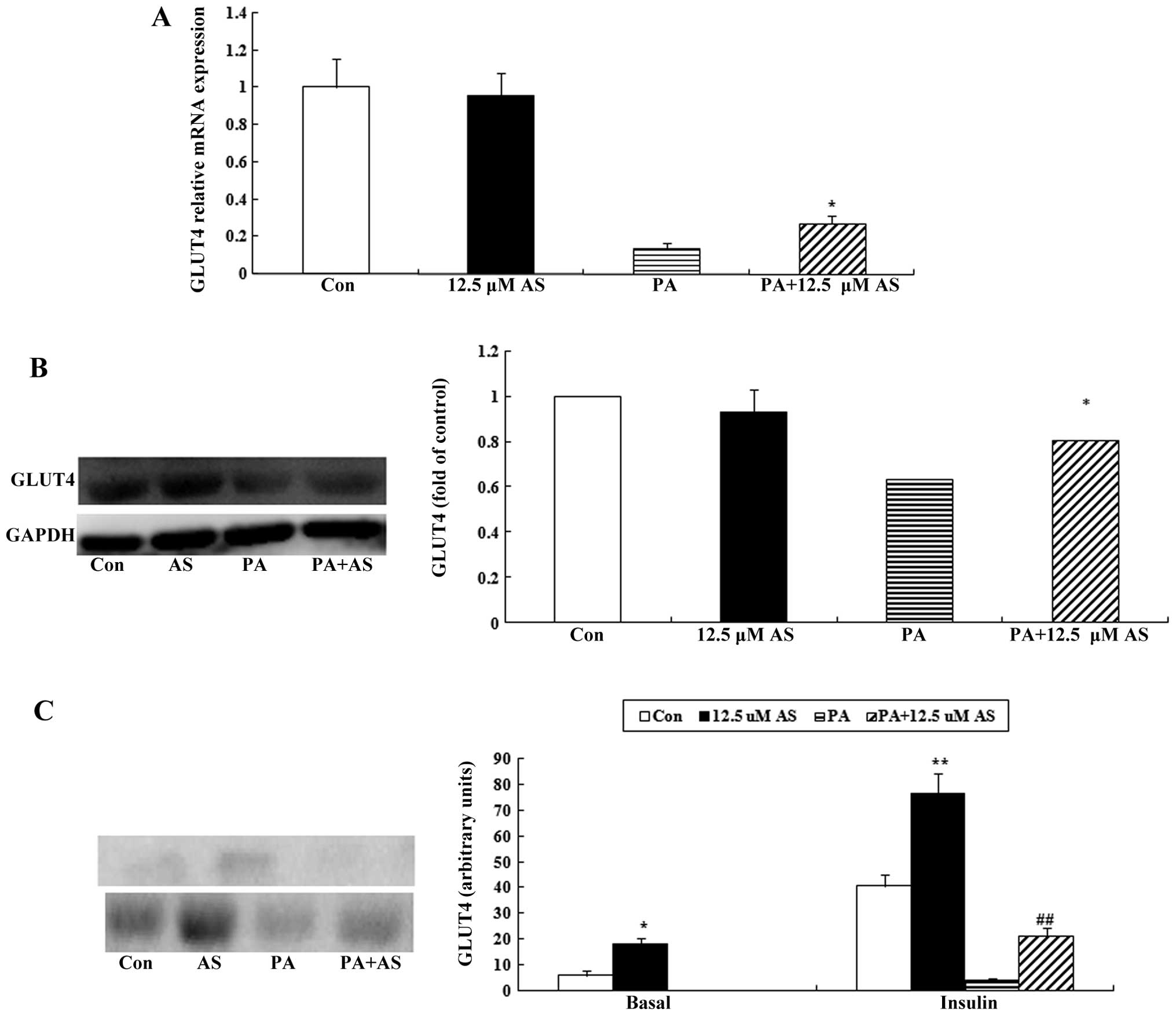
Effect of astragaloside IV on the mRNA
and protein expression of GLUT4
The elevated mRNA and protein expression of GLUT4 is
associated with increased cell surface levels of GLUT4 (32). Thus, we examined the mRNA and
protein expression of GLUT4 in the C2C12 myotubes in the presence
or absence of palmitate. Astragaloside IV had no direct effect on
the mRNA and protein expression of GLUT4 in the C2C12 myotubes
(Fig. 4A and B; P<0.05).
Exposure to palmitate for 16 h caused a marked reduction in the
mRNA and protein levels of GLUT4 in the C2C12 myotubes; however,
pretreatment with astragaloside IV attenuated the deleterious
effect of palmitate (Fig. 4A and
B; P<0.05).
Astragaloside IV enhances the basal and
insulin-stimulated translocation of GLUT4, and partly attenuates
the palmitate-induced decrease in the insulin-stimulated
translocation of GLUT4
The subcellular localization of GLUT4 was evaluated
by subcellular fractionation followed by western blot analysis. As
shown in Fig. 4C, the surface
levels of basal GLUT4 were increased by astragaloside IV
(P<0.05), whereas basal GLUT4 surface levels were not detected
in the palmitate-exposed group. Insulin stimulation resulted in a
marked increase in the GLUT4 levels at the cell surface in the
C2C12 myotubes (Fig. 4C).
Exposure to palmitate decreased the insulin-stimulated
translocation of GLUT4, and this effect was partly antagonized by
astragaloside IV (P<0.05; Fig.
4C).
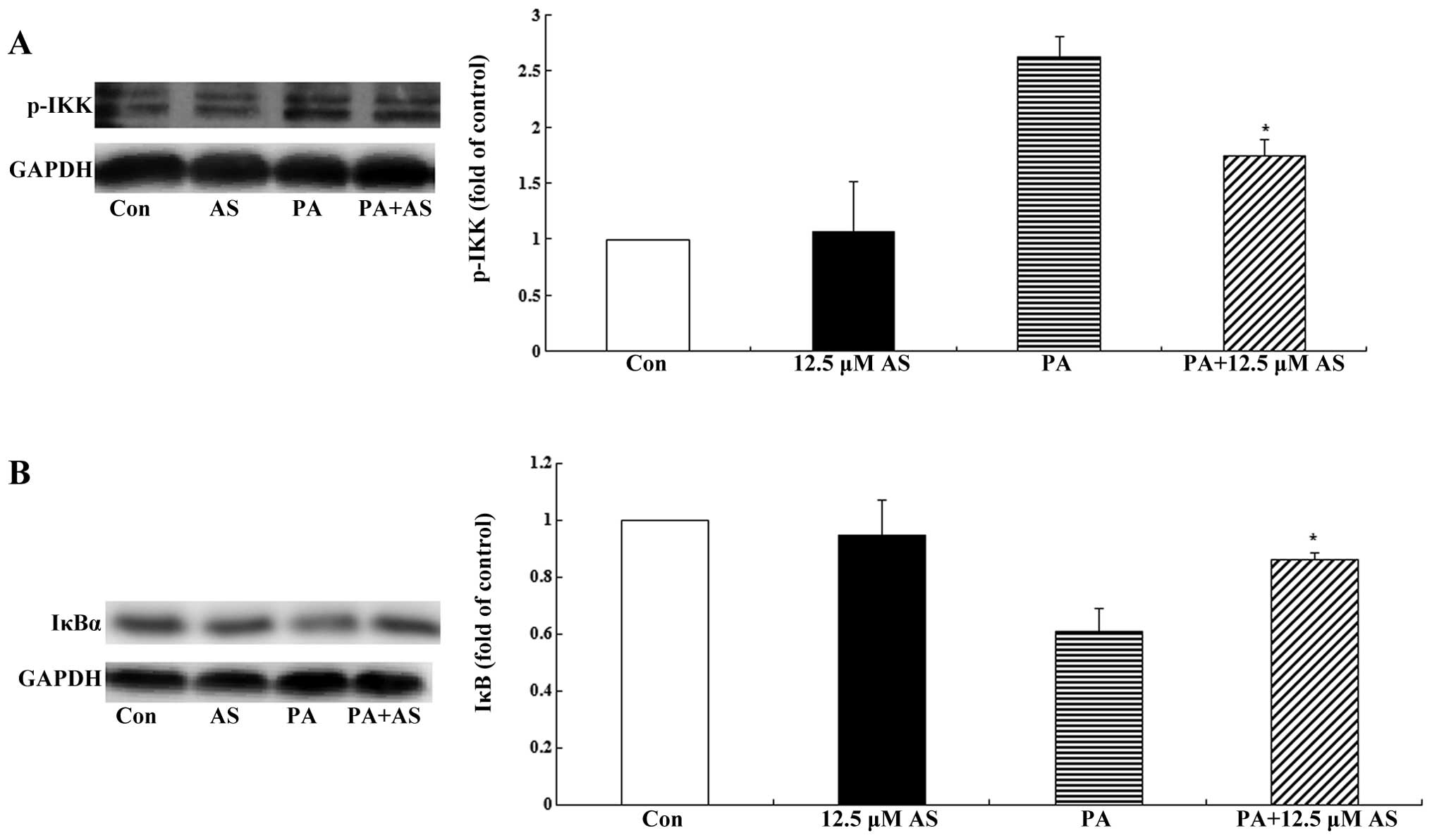
Astragaloside IV suppresses the
activation of the IKK/IκBα pathway
It is well known that the IKK/IκBα/NF-κB pathway
plays an important role in the regulation of inflammatory factors,
and IKK and IκBα are key proteins in this pathway. In the basal
state, NF-κB binds to its inhibitor IκBα to form an inactivated
complex in the cytosol. Exposure to palmitate initiates the
IKK/IκBα/NF-κB cascade, thus activating IKK, which then
phosphorylates IκBα, causing the release of IκBα from NF-κB. NF-κB
then translocates to the nucleus where it promotes the expression
of various inflammatory factors (45). In order to determine the effect of
astragaloside IV on the IKK/IκBα/NF-κB cascade, we detected the
levels of p-IKK and IκBα using western blot analysis. The four-day
differentiated C2C12 myotubes were incubated with astragaloside IV
(12.5 µM, 2 h) prior to stimulation with and without 0.75 mM
palmitate for 1.5 h. As shown in Fig.
5A, palmitate activated IKK (as demonstrated by the increased
level of p-IKK), which lead to the degradation of IκBα (as
demonstrated by the decreased level of IκBα) (Fig. 5B). Pre-treatment with
astragaloside IV for 2 h suppressed the activation of IKK and the
degradation of IκBα (Fig. 5;
P<0.05); this is indicates the inhibitory effect of
astragaloside IV on the IKK/IκBα/NF-κB cascade.
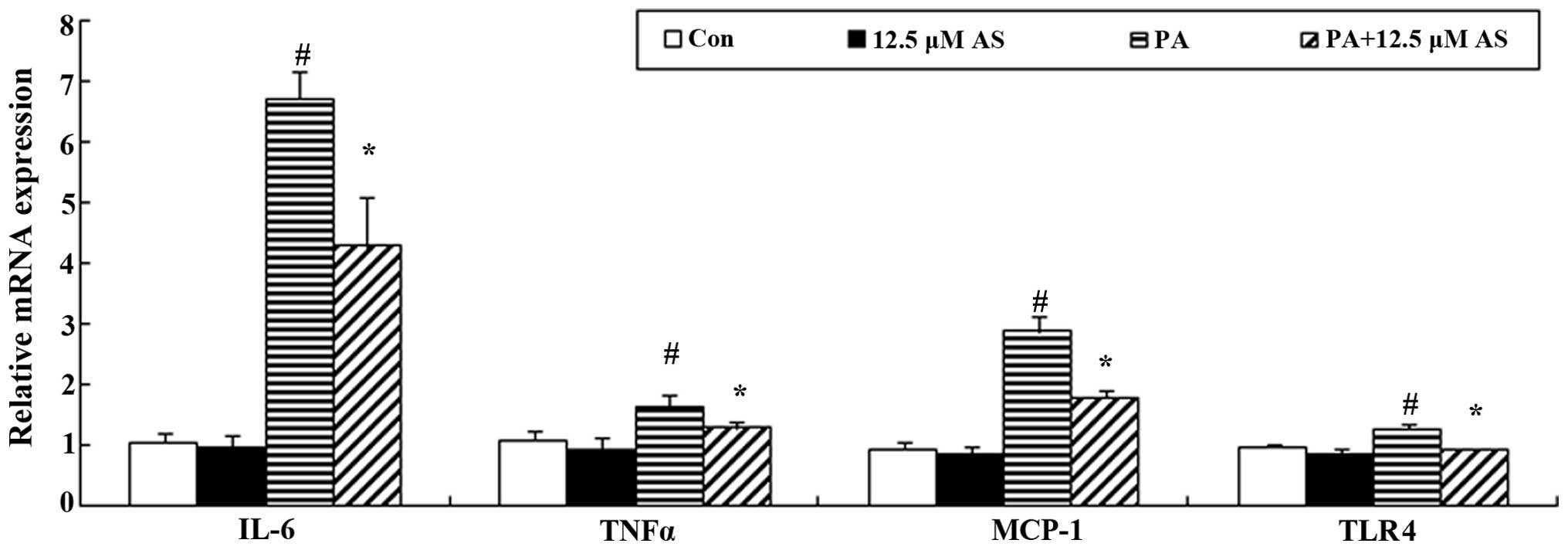
Astragaloside IV decreases the mRNA
expression of MCP-1, IL-6 and TNFα
In vitro, the exposure of skeletal muscle
cells to palmitate and subsequent incubation also induced the
expression of proinflammatory factors such as IL-6 and TNFα.
Following a 4-h incubation with 0.75 mM palmitate, the C2C12
myotubes displayed significantly elevated mRNA expression of MCP-1,
IL-6 and TNFα (Fig. 6). Similar
to the inhibitory effect of astragaloside IV on the IKK/IκBα/NF-κB
cascade, pre-treatment with astragaloside IV for 2 h suppressed the
palmitate-induced increase in the mRNA expression of MCP-1, IL-6
and TNFα (P<0.05) (Fig.
6).
Effect of astragaloside IV on the mRNA
expression of TLR4
TLR4, a receptor of palmitate, is involved in
FFA-induced insulin resistance. Through binding to TLR4, palmitate
increases the expression of inflammatory factors by activating the
IKK/IκBα/NF-κB cascade, with subsequent feedback regulation of the
mRNA expression of TLR4 (45).
The C2C12 myotubes incubated with 0.75 mM palmitate for 16 h
exhibited markedly increased mRNA expression of TLR4 (Fig. 6). Pre-treatment with astragaloside
IV downregulated the mRNA level of TLR4 induced by palmitate
(P<0.05) (Fig. 6).
Discussion
The classic pathway of insulin signaling involving
the trans-location of GLUT4 (33,34) to the cell surface is triggered by
the autophosphorylation of the insulin receptor on multiple
tyrosine residues following insulin binding. This results in the
tyrosine phosphorylation of a family of IRS proteins and the
activation of a complex network of downstream molecules, including
phosphatidylinositol 3-kinase (PI3K) and the serine/threonine
kinase AKT. Skeletal muscle is the most important tissue involved
in insulin-stimulated glucose disposal, and insulin resistance in
skeletal muscle is a major defect in most obese phenotypes
(35).
The present study demonstrated that astragaloside IV
regulates glucose uptake and delineates the proximal signaling
events mediating this response. We demonstrated that astragaloside
IV increased basal glucose uptake in the C2C12 myotubes in a dose-
and time-dependent manner; the highest rate of glucose uptake was
observed at a concentration of 12.5 µM of astragaloside IV
and a treatment period of 2 h produced the most significant effect
on glucose uptake. Astragaloside IV also increased
insulin-stimulated glucose uptake, which revealed that it is
capable of enhancing insulin sensitivity in C2C12 myotubes. We
found that astragaloside IV increased glucose uptake in the C2C12
myotubes through the phosphorylation of IRS1 and AKT in the basal
state. Astragaloside IV also increased the insulin-stimulated
phosphorylation of IRS1 and AKT, thereby displaying a synergistic
interaction with insulin. We also examined the phosphorylation of
IRβ and found that astragaloside IV did not activate IRβ, which
suggests that astragaloside IV activated IRS1 and AKT independently
of IRβ.
Randle et al (36), described the glucose fatty-acid
cycle, which is a metabolic pathway linking fat and carbohydrate
metabolism. In vivo, fatty acid metabolism not only provides
ATP, but also decreases glucose consumption. Elevated fatty acid
uptake always follows increased fatty acid oxidation, which leads
to reduced glucose consumption and insulin tolerance. Abnormal
fatty acid metabolism exists in obese and diabetic patients due to
elevated basal lipolysis and the impaired ability of insulin to
mediate the conversion of serum FFA into triglyceride (37). Insulin-stimulated glucose
transport significantly decreased in skeletal muscle obtained from
patients with type 2 diabetes (38) and obesity (39). The translocation of GLUT4 was
reduced by 90% in the skeletal muscle of type 2 diabetic patients
(40). In this study,
astragaloside IV enhanced the basal and insulin-stimulated
translocation of GLUT4. The C2C12 myotubes exposed to palmitate
exhibited a marked decrease in the mRNA and protein expression of
GLUT4 as well as a decrease in the translocation of GLUT4, whereas
pre-treatment with astragaloside IV partly attenuated the
deleterious effects of palmitate.
Our results indicated that palmitate inhibited the
activation of IRS1 and AKT in the C2C12 myotubes, reduced the
insulin-stimulated translocation of GLUT4, and finally decreased
glucose uptake. Pre-treatment with astragaloside IV ameliorated the
palmitate-induced decrease in the phosphorylation of IRS1 and AKT,
and partly attenuated the palmitate-induced decrease in the
insulin-stimulated translocation of GLUT4. Astragaloside IV
displayed a significant effect on insulin resistance induced by
palmitate in skeletal muscle.
Previous research has demonstrated that type 2
diabetes and obesity are associated with significant increases in
inflammatory factors (41), and
macrophage infiltration in adipose tissue (42,43), which demonstrates the close
association between the immune system and insulin resistance
(42,44–46). The IKK/IκBα pathway is closely
associated with insulin resistance (45,47); it links inflammation to insulin
resistance. TLRs are innate immune receptors; the immune response
to invading microorganisms is mediated through the activation of
TLRs (48). Increasing evidence
has demonstrated that saturated fatty acids activate TLR4 signaling
which activates the NF-κB pathway (49,50), and play roles in insulin
resistance (47,51). Reyna et al (52) recognized the importance of TLR4
after examining insulin resistant skeletal muscle obtained from
obese and type 2 diabetic subjects. Mice lacking TLR4 have been
shown to be partially protected against fatty acid-induced insulin
resistance in skeletal muscle (49,53,54). The activation of the IKK/IκBα
pathway increases the expression of IL-6 and TNFα, which reduces
insulin resistance in skeletal muscle (48,54). IL-6 may be the most critical
factor involved in the modulation of insulin resistance (55,56); a previous study found that the
concentration of IL-6 was 2-3-fold higher in obese subjects without
type 2 diabetes in comparison with lean subjects (55). However, Sell et al
(57) suggested that MCP-1 was
the most important among a number of cytokines, and that
physiological concentrations of MCP-1 may lead to insulin
resistance.
Our results confirmed that palmitate may bind to
TLR4, thus activating IKK, which then phosphorylates IκBα, causing
the release of IκBα from NF-κB. NF-κB then translocates to the
nucleus where it promotes the expression of inflammatory factors,
such as MCP-1, IL-6 and TNFα. These inflammatory factors inhibit
the activation of insulin pathway proteins, finally decreasing
insulin sensitivity. The pre-treatment of the palmitate-exposed
C2C12 myotubes with astragaloside IV decreased the mRNA expression
of TLR4, suppressed the activation of IKK and the degradation of
IκBα, reduced NF-κB activation (data not shown), and thus reduced
the mRNA expression of MCP-1, IL-6 and TNFα, consequently reducing
the insulin resistance induced by palmitate. These findings are
limited by the absence of data regarding NF-κB due to a lack of
funding and we plan to investigate this aspect further in the
future.
In conclusion, astragaloside IV increased glucose
uptake in C2C12 myotubes through the IRS1/AKT pathway. Moreover,
astragaloside IV suppressed the the palmitate-induced activation of
the IKK/IκBα pathway and reduced the secretion of inflammatory
factors. These results may explain, at least in part, the
antidiabetic and insulin-sensitizing effects of astragaloside IV
and A. membranaceus.
Abbreviations:
|
AS
|
astragaloside IV
|
|
PA
|
palmitate
|
|
IRS1
|
insulin receptor substrate 1
|
|
AKT
|
protein kinase B
|
|
GLUT4
|
glucose transporter 4
|
|
IRβ
|
insulin receptor β
|
|
MCP-1
|
monocyte chemotactic protein 1
|
|
TLR4
|
Toll-like receptor 4
|
|
IL-6
|
interleukin-6
|
|
TNFα
|
tumor necrosis factor α
|
|
IκBα
|
inhibitor κBα
|
|
IKK
|
inhibitory κB kinase
|
References
|
1
|
DeFronzo RA, Gunnarsson R, Björkman O,
Olsson M and Wahren J: Effects of insulin on peripheral and
splanchnic glucose metabolism in noninsulin-dependent (type II)
diabetes mellitus. J Clin Invest. 76:149–155. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiens B, Roemen TH and van der Vusse GJ:
Muscular long-chain fatty acid content during graded exercise in
humans. Am J Physiol. 276:E352–E357. 1999.PubMed/NCBI
|
|
3
|
Pirola L, Bonnafous S, Johnston AM,
Chaussade C, Portis F and Van Obberghen E: Phosphoinositide
3-kinase-mediated reduction of Insulin receptor substrate-1/2
protein expression via different mechanisms contributes to the
insulin-induced desensitization of its signaling pathways in L6
muscle cells. J Biol Chem. 278:15641–15651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boden G: Fatty acid-induced inflammation
and insulin resistance in skeletal muscle and liver. Curr Diab Rep.
6:177–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdul-Ghani MA, Muller FL, Liu Y, Chavez
AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M,
et al: Deleterious action of FA metabolites on ATP synthesis:
possible link between lipotoxicity, mitochondrial dysfunction, and
insulin resistance. Am J Physiol Endocrinol Metab. 295:E678–E685.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itani SI, Zhou Q, Pories WJ, MacDonald KG
and Dohm GL: Involvement of protein kinase C in human skeletal
muscle insulin resistance and obesity. Diabetes. 49:1353–1358.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koistinen HA, Galuska D, Chibalin AV, Yang
J, Zierath JR, Holman GD and Wallberg-Henriksson H:
5-amino-imidazole carboxamide riboside increases glucose transport
and cell-surface GLUT4 content in skeletal muscle from subjects
with type 2 diabetes. Diabetes. 52:1066–1072. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu W, Tang S, Shi J, Yin W, Cao S, Bu R,
Zhu D and Bi Y: Metformin attenuates palmitic acid-induced insulin
resistance in L6 cells through the AMP-activated protein
kinase/sterol regulatory element-binding protein-1c pathway. Int J
Mol Med. 35:1734–1740. 2015.PubMed/NCBI
|
|
9
|
Karlsson HK, Hällsten K, Björnholm M,
Tsuchida H, Chibalin AV, Virtanen KA, Heinonen OJ, Lönnqvist F,
Nuutila P and Zierath JR: Effects of metformin and rosiglitazone
treatment on insulin signaling and glucose uptake in patients with
newly diagnosed type 2 diabetes: a randomized controlled study.
Diabetes. 54:1459–1467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meshkani R, Sadeghi A, Taheripak G,
Zarghooni M, Gerayesh-Nejad S and Bakhtiyari S: Rosiglitazone, a
PPARγ agonist, ameliorates palmitate-induced insulin resistance and
apoptosis in skeletal muscle cells. Cell Biochem Funct. 32:683–691.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinberg GR, Watt MJ, Ernst M, Birnbaum
MJ, Kemp BE and Jørgensen SB: Ciliary neurotrophic factor
stimulates muscle glucose uptake by a PI3-kinase-dependent pathway
that is impaired with obesity. Diabetes. 58:829–839. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv L, Wu SY, Wang GF, Zhang JJ, Pang JX,
Liu ZQ, Xu W, Wu SG and Rao JJ: Effect of astragaloside IV on
hepatic glucose-regulating enzymes in diabetic mice induced by a
high-fat diet and streptozotocin. Phytother Res. 24:219–224.
2010.
|
|
13
|
Jiang B, Yang Y, Jin H, Shang W, Zhou L,
Qian L and Chen M: Astragaloside IV attenuates lipolysis and
improves insulin resistance induced by TNFalpha in 3T3-L1
adipocytes. Phytother Res. 22:1434–1439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang N, Wang XH, Mao SL and Zhao F:
Astragaloside IV improves metabolic syndrome and endothelium
dysfunction in fructose-fed rats. Molecules. 16:3896–3907. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li HB, Ge YK, Zhang L and Zheng XX:
Astragaloside IV improved barrier dysfunction induced by acute high
glucose in human umbilical vein endothelial cells. Life Sci.
79:1186–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li ZP and Cao Q: Effects of astragaloside
IV on myocardial calcium transport and cardiac function in ischemic
rats. Acta Pharmacol Sin. 23:898–904. 2002.PubMed/NCBI
|
|
17
|
Zhang S, Tang F, Yang Y, Lu M, Luan A,
Zhang J, Yang J and Wang H: Astragaloside IV protects against
isoproterenol-induced cardiac hypertrophy by regulating
NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One.
10:e01187592015. View Article : Google Scholar
|
|
18
|
Lu M, Tang F, Zhang J, Luan A, Mei M, Xu
C, Zhang S, Wang H and Maslov LN: Astragaloside IV attenuates
injury caused by myocardial ischemia/reperfusion in rats via
regulation of Toll-like receptor 4/nuclear factor-κB signaling
pathway. Phytother Res. 29:599–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Gui D, Chen Y, Mou L, Liu Y and
Huang J: Astragaloside IV improves high glucose-induced podocyte
adhesion dysfunction via alpha3beta1 integrin upregulation and
integrin-linked kinase inhibition. Biochem Pharmacol. 76:796–804.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai P, Yong G, Dingkun G, Qiuyu Z, Kaiyuan
Z and Shanyan L: Aqueous extract of Astragali Radix induces human
natriuresis through enhancement of renal response to atrial
natriuretic peptide. J Ethnopharmacol. 116:413–421. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu WS, Li S, Guo WW, Chen LL and Li YS:
Effects of Astragaloside IV on diabetic nephropathy in rats. Genet
Mol Res. 14:5427–5434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Zhang Y, Sun S, Shen J, Qiu J, Yin
X, Yin H and Jiang S: Inhibitory effects of astragaloside IV on
diabetic peripheral neuropathy in rats. Can J Physiol Pharmacol.
84:579–587. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang WJ, Hufnagl P, Binder BR and Wojta
J: Antiinflammatory activity of Astragaloside IV is mediated by
inhibition of NF-kappaB activation and adhesion molecule
expression. Thromb Haemost. 90:904–914. 2003.PubMed/NCBI
|
|
25
|
Zhao P, Wang Y, Zeng S, Lu J, Jiang TM and
Li YM: Protective effect of astragaloside IV on
lipopolysaccharide-induced cardiac dysfunction via downregulation
of inflammatory signaling in mice. Immunopharmacol Immunotoxicol.
37:428–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang WJ and Frei B: Astragaloside IV
inhibits NF-κB activation and inflammatory gene expression in
LPS-treated mice. Mediators Inflamm. 2015:2743142015. View Article : Google Scholar
|
|
27
|
Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte
PM, Wang Y, Wu D, Chu W, Qin G and Lam KS: Selective elevation of
adiponectin production by the natural compounds derived from a
medicinal herb alleviates insulin resistance and glucose
intolerance in obese mice. Endocrinology. 150:625–633. 2009.
View Article : Google Scholar :
|
|
28
|
Perrini S, Natalicchio A, Laviola L,
Belsanti G, Montrone C, Cignarelli A, Minielli V, Grano M, De
Pergola G, Giorgino R and Giorgino F: Dehydroepiandrosterone
stimulates glucose uptake in human and murine adipocytes by
inducing GLUT1 and GLUT4 translocation to the plasma membrane.
Diabetes. 53:41–52. 2004. View Article : Google Scholar
|
|
29
|
Tortorella LL and Pilch PF: C2C12 myocytes
lack an insulin-responsive vesicular compartment despite
dexamethasone-induced GLUT4 expression. Am J Physiol Endocrinol
Metab. 283:E514–E524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jové M, Planavila A, Sánchez RM, Merlos M,
Laguna JC and Vázquez-Carrera M: Palmitate induces tumor necrosis
factor-alpha expression in C2C12 skeletal muscle cells by a
mechanism involving protein kinase C and nuclear factor-kappaB
activation. Endocrinology. 147:552–561. 2006. View Article : Google Scholar
|
|
31
|
Griffin ME, Marcucci MJ, Cline GW, Bell K,
Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF and Shulman GI:
Free fatty acid-induced insulin resistance is associated with
activation of protein kinase C theta and alterations in the insulin
signaling cascade. Diabetes. 48:1270–1274. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tabandeh MR, Jafari H, Hosseini SA and
Hashemitabar M: Ginsenoside Rb1 stimulates adiponectin signaling in
C2C12 muscle cells through up-regulation of AdipoR1 and AdipoR2
proteins. Pharm Biol. 53:125–132. 2015. View Article : Google Scholar
|
|
33
|
Bryant NJ, Govers R and James DE:
Regulated transport of the glucose transporter GLUT4. Nat Rev Mol
Cell Biol. 3:267–277. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Gorman DJ, Karlsson HK, McQuaid S,
Yousif O, Rahman Y, Gasparro D, Glund S, Chibalin AV, Zierath JR
and Nolan JJ: Exercise training increases insulin-stimulated
glucose disposal and GLUT4 (SLC2A4) protein content in patients
with type 2 diabetes. Diabetologia. 49:2983–2992. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leonard BL, Watson RN, Loomes KM, Phillips
AR and Cooper GJ: Insulin resistance in the Zucker diabetic fatty
rat: a metabolic characterisation of obese and lean phenotypes.
Acta Diabetol. 42:162–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Randle PJ, Garland PB, Hales CN and
Newsholme EA: The glucose fatty-acid cycle. Its role in insulin
sensitivity and the metabolic disturbances of diabetes mellitus.
Lancet. 1:785–789. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McGarry JD: Banting lecture 2001:
dysregulation of fatty acid metabolism in the etiology of type 2
diabetes. Diabetes. 51:7–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krook A, Björnholm M, Galuska D, Jiang XJ,
Fahlman R, Myers MG Jr, Wallberg-Henriksson H and Zierath JR:
Characterization of signal transduction and glucose transport in
skeletal muscle from type 2 diabetic patients. Diabetes.
49:284–292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ,
Flickinger EG, Meelheim D, Fushiki T, Atkinson SM, Elton CW and
Caro JF: An in vitro human muscle preparation suitable for
metabolic studies. Decreased insulin stimulation of glucose
transport in muscle from morbidly obese and diabetic subjects. J
Clin Invest. 82:486–494. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ryder JW, Yang J, Galuska D, Rincón J,
Björnholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H,
Zierath JR and Holman GD: Use of a novel impermeable biotinylated
photo-labeling reagent to assess insulin- and hypoxia-stimulated
cell surface GLUT4 content in skeletal muscle from type 2 diabetic
patients. Diabetes. 49:647–654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Festa A, D'Agostino R Jr, Tracy RP and
Haffner SM; Insulin Resistance Atherosclerosis Study: Elevated
levels of acute-phase proteins and plasminogen activator
inhibitor-1 predict the development of type 2 diabetes: the insulin
resistance atherosclerosis study. Diabetes. 51:1131–1137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kanda H, Tateya S, Tamori Y, Kotani K,
Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K and
Kasuga M: MCP-1 contributes to macrophage infiltration into adipose
tissue, insulin resistance, and hepatic steatosis in obesity. J
Clin Invest. 116:1494–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sinha S, Perdomo G, Brown NF and O'Doherty
RM: Fatty acid-induced insulin resistance in L6 myotubes is
prevented by inhibition of activation and nuclear localization of
nuclear factor kappa B. J Biol Chem. 279:41294–41301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wada J and Makino H: Innate immunity in
diabetes and diabetic nephropathy. Nat Rev Nephrol. 12:13–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan M, Konstantopoulos N, Lee J, Hansen
L, Li ZW, Karin M and Shoelson SE: Reversal of obesity- and
diet-induced insulin resistance with salicylates or targeted
disruption of Ikkbeta. Science. 293:1673–1677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar
|
|
49
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty
acid-induced insulin resistance. J Clin Invest. 116:3015–3025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hwang D: Modulation of the expression of
cyclooxygenase-2 by fatty acids mediated through toll-like receptor
4-derived signaling pathways. FASEB J. 15:2556–2564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sriwijitkamol A, Christ-Roberts C, Berria
R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ and Musi N:
Reduced skeletal muscle inhibitor of kappaB beta content is
associated with insulin resistance in subjects with type 2
diabetes: Reversal by exercise training. Diabetes. 55:760–767.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reyna SM, Ghosh S, Tantiwong P, Meka CS,
Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK,
Sriwijitkamol A and Musi N: Elevated toll-like receptor 4
expression and signaling in muscle from insulin-resistant subjects.
Diabetes. 57:2595–2602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Radin MS, Sinha S, Bhatt BA, Dedousis N
and O'Doherty RM: Inhibition or deletion of the lipopolysaccharide
receptor Toll-like receptor-4 confers partial protection against
lipid-induced insulin resistance in rodent skeletal muscle.
Diabetologia. 51:336–346. 2008. View Article : Google Scholar
|
|
54
|
Tsukumo DM, Carvalho-Filho MA, Carvalheira
JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi
R, Velloso LA and Saad MJ: Loss-of-function mutation in Toll-like
receptor 4 prevents diet-induced obesity and insulin resistance.
Diabetes. 56:1986–1998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kern PA, Ranganathan S, Li C, Wood L and
Ranganathan G: Adipose tissue tumor necrosis factor and
interleukin-6 expression in human obesity and insulin resistance.
Am J Physiol Endocrinol Metab. 280:E745–E751. 2001.PubMed/NCBI
|
|
56
|
Pickup JC, Mattock MB, Chusney GD and Burt
D: NIDDM as a disease of the innate immune system: association of
acute-phase reactants and interleukin-6 with metabolic syndrome X.
Diabetologia. 40:1286–1292. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sell H, Dietze-Schroeder D, Kaiser U and
Eckel J: Monocyte chemotactic protein-1 is a potential player in
the negative cross-talk between adipose tissue and skeletal muscle.
Endocrinology. 147:2458–2467. 2006. View Article : Google Scholar : PubMed/NCBI
|