Introduction
Gastric cancer (GC), one of the most frequent
malignancies, remains the second leading cause of cancer-related
mortality worldwide (the third in males and the fifth in females),
despite great advances in therapeutic regimens and improved
surgical outcomes (1). Based on
Globocan 2012, GC accounts for nearly 952,000 new cases annually in
worldwide (2). Of all GC cases,
90% are malignant and 95% are comprised of gastric adenocarcinoma
(3). More than 70% of new cases
and deaths related to GC are occurred in developing countries
(4). In China in particular, this
cancer has the second highest incidence among commonly diagnosed
cancers (5). Due to a lack of
early specific symptoms, the diagnosis of GC is often delayed,
leading to cancer cell invasion into the muscularis propria
(6). Consistent with other types
of cancer, the development of GC is a multiple-stage process in
which the accumulation of molecular changes lead to malignant
phenotypes with aggressive characteristics (7). Therefore, the detection of the
abnormal expression of molecular markers may be a promising
approach for the early diagnosis and prognosis of patients with
GC.
The human zinc finger of the cerebellum (ZIC) family
genes, comprised of 5 members (ZIC1, ZIC2, ZIC3, ZIC4 and ZIC5)
which are vertebrate homologues of the Drosophila odd-paired
(OPA) gene, are structurally similar to each other, implying that
ZICs share some, but not all, functions (8). ZIC genes encode zinc-finger
transcription factors, each composed of 5
C2H2 zinc-finger domains, which have highest
sequence homology to Drosophila OPA (9). Functionally, ZIC genes play crucial
roles in a wide array of developmental systems, including the
central nervous system (CNS), muscle and skeletal development
(10). The ZIC proteins are
mainly expressed in the developing or mature CNS in a
spatiotemporally restricted manner, but none contain a canonical
nuclear localization signal. As zinc finger transcription factors,
these proteins can bind to the GC-rich sequence in target genes
(11). With the similar zinc
finger domains, the ZIC family members have also been shown to
interact with the Gli family proteins in both an antagonistic and
synergistic manner (12). In
recent years, growing evidence has indicated that ZICs may be
involved in the pathological events of various diseases, including
cancer. For example, ZIC1 was found to participate in the
progression of human medulloblastoma (13), thyroid cancer (14), GC (15–17), colorectal cancer (18), endometrial cancer (19) and mesenchymal neoplasms (20); ZIC2 may function as an oncogene in
small cell lung carcinoma (21),
pancreatic ductal adenocarcinoma (22), epithelial ovarian cancer (23) and cervical cancer (24); genome-wide analysis of CpG island
methylation in bladder cancer identified ZIC4 as a pTa-specific
prognostic marker (25). However,
the roles of the ZIC family members in GC have not yet been fully
elucidated. A comparison of their expression levels and clinical
significance in GC is required.
These observations led us to investigate the
expression profiles of ZIC genes and proteins in GC, and to
determine their clinical implications. We first detected the mRNA
and protein expression levels of ZIC1, ZIC2, ZIC3, ZIC4 and ZIC5 by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis, respectively, using 60 pairs
of human GC and matched normal mucosa tissues. The expression
pattern and subcellular localization of ZIC1 in 160 pairs of human
GC and matched normal mucosa tissues were then examined by
immunohistochemistry. Moreover, the associations of ZIC1 expression
with various clinicopathological characteristics and patient
prognosis were also evaluated.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of
Huai'an First People's Hospital of Nanjing Medical University and
Lianshui Third People's Hospital, Huai'an, China. Written informed
consent was also obtained from all study participants for the use
of their samples. All specimens were handled anonymously according
to the ethical and legal standards.
Patients and tissue samples
For RT-qPCR and western blot analysis, a total of 60
fresh GC and matched normal mucosa specimens were obtained from 60
patients with GC (48 males and 12 females; median age, 58 years;
range, 28–82 years), who underwent surgical resection, at the
Department of Gastroenterology of Huai'an First People's Hospital
from January 2009 to December 2010. All specimens were stored at
−80°C until use to detect the relative expression levels of ZIC1,
ZIC2, ZIC3, ZIC4 and ZIC5 genes and proteins.
For immnohistochemistry, a total of 160
paraffin-embedded GC and matched normal mucosa specimens, in
addition to the 60 cases mentioned above, were obtained from 160
patients with GC (108 males and 52 females; median age, 58 years;
range, 28–86 years) at the Department of Gastroenterology of
Huai'an First People's Hospital from January 2005 to December
2010.
None of the patients had received any radiotherapy
or chemotherapy prior to surgery, and the samples were classified
according to the World Health Organization (WHO) Pathological
Classification of Tumors. Of the 160 cases, 58 (36.25%) were well
or moderately differentiated tumor tissues and 102 (63.75%) were
poorly differentiated or undifferentiated tumor tissues.
Histologically, there were 10 cases of papillary adenocarcinoma, 92
cases of tubular adenocarcinoma, 50 cases of mucinous
adenocarcinoma and 8 cases of signet-ring cell carcinoma. There
were 61 cases of intestinal histological type and 99 cases of
diffuse histological type according to the Lauren classification.
According to the 2002 tumor-node-metastases (TNM) classification
system, 16 cases were TNM stage I, 40 cases were stage II, 42 cases
were stage III and 62 cases were stage IV. The detailed information
on the clinicopathological characteristics of all 160 patients with
GC are shown in Table I.
 | Table IAssociations of ZIC1 expression with
various clinicopathological characteristics of the 160 patients
with GC. |
Table I
Associations of ZIC1 expression with
various clinicopathological characteristics of the 160 patients
with GC.
| Clinical
characteristics | Case no. (%) | ZIC1 expression
| P-value |
|---|
| High (n, %) | Low (n, %) |
|---|
| Age (years) | | | | |
| <58 | 50 (31.25) | 25 (50.00) | 25 (50.00) | NS |
| ≥58 | 110 (68.75) | 50 (45.45) | 60 (54.55) | |
| Gender | | | | |
| Male | 108 (67.50) | 52 (48.15) | 56 (51.85) | NS |
| Female | 52 (32.50) | 23 (44.23) | 29 (55.77) | |
| Tumor size (cm) | | | | |
| <4 | 58 (36.25) | 25 (43.10) | 33 (56.90) | NS |
| ≥4 | 102 (63.75) | 50 (49.02) | 52 (50.98) | |
| Lauren
classification | | | | |
| Diffuse type | 99 (61.88) | 45 (45.45) | 54 (54.55) | NS |
| Intestinal type | 61 (38.12) | 30 (49.18) | 31 (50.82) | |
| Differentiation | | | | |
| Well or
moderate | 58 (36.25) | 29 (50.00) | 29 (50.00) | NS |
| Poor | 102 (63.75) | 46 (45.10) | 56 (54.90) | |
| Lymph node
metastasis | | | | |
| Negative | 100 (62.50) | 60 (60.00) | 40 (40.00) | 0.006 |
| Positive | 60 (37.50) | 15 (25.00) | 45 (75.00) | |
| TNM stage | | | | |
| I | 16 (10.00) | 16 (100.00) | 0 (0.00) | <0.001 |
| II | 40 (25.00) | 37 (92.50) | 3 (7.50) | |
| III | 42 (26.25) | 22 (52.38) | 20 (47.62) | |
| IV | 62 (38.75) | 0 (0.00) | 62 (100.00) | |
| Depth of
invasion | | | | |
| Mucosa or
submucosa | 26 (16.25) | 24 (92.31) | 2 (7.69) | 0.01 |
| Muscularis or
subserosa | 20 (12.50) | 12 (60.00) | 8 (40.00) | |
| Serosa | 82 (51.25) | 37 (45.12) | 45 (54.88) | |
| Adjacent
structure | 32 (20.00) | 2 (6.25) | 30 (93.75) | |
All the 160 patients with GC were given a follow-up
exam ranging from 3 to 6 years. Patients who died from diseases
other than GC or from unexpected events were excluded from the case
collection in this study. For the analysis of survival and
follow-up data, the date of surgery was used to represent the
beginning of the follow-up period. Overall survival was an endpoint
which was calculated as the amount of time between the date of
surgery and the date of death, regardless of the cause.
Disease-free survival was defined as the time from randomization
until recurrence of the tumor or death from any cause. Surviving
patients were censored on March 31, 2013.
RT-qPCR
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). A total of 2 µg RNA was
reverse transcribed using the SuperScript II RNase-Reverse
Transcriptase system (Invitrogen). GAPDH was used as an internal
control. cDNA was then subjected to quantitative (real-time) PCR
(qPCR) using primers specific for ZIC1, ZIC2, ZIC3, ZIC4, ZIC5 and
GAPDH. PCR primers were designed according to the previous study by
Aruga et al (26) as
follows: ZIC1 forward, 5′-GGCCCGGAGCAGAGTAAT-3′ and reverse,
5′-AGCCCTCAAACTCGCACTT-3′ (229 bp, 26 cycles); ZIC2 forward,
5′-CCCTTCAAGGCCAAATACAA-3′ and reverse, 5′-TGCATGTGCTTCTTCCTGTC-3′
(218 bp, 26 cycles); ZIC3 forward, 5′-GCAAGTCTTTCAAGGCGAAG-3′ and
reverse, 5′-CATGCATGTGCTTCTTACGG-3′ (225 bp, 28 cycles); ZIC4
forward, 5′-GCCCTTCAAAGCCAAAT ACA-3′ and reverse,
5′-GCCCTCGAACTCGCATC-3′ (172 bp, 28 cycles); ZIC5 forward,
5′-TCTGCTTCTGGGAGGACTGT-3′ and reverse, 5′-GGGAATGTTTCTTCCGATCA-3′
(252 bp, 28 cycles); and GAPDH forward, 5′-GAAGGTGAAGGTCGGAGT-3′
and reverse, 5′-GAAGATGGTGATGGGATTTC-3′ (226 bp, 28 cycles). The
PCR cycling conditions were as follows: 94°C for 4 min, followed by
40 cycles of 95°C for 1 min, 60°C for 1 min and 72°C for 1 min. The
SYBR Premix Ex Taq™ kit (Takara Bio, Inc., Otsu, Shiga, Japan) was
used to measure the amplified DNA, and qPCR was performed using an
iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA).
The amount of ZICs relative to GAPDH was calculated as the average
2−ΔCt, where ΔCt = Ct−CtGAPDH.
Western blot analysis
The GC and matched normal mucosa tissues were added
to 1 ml of 100 mmol/l Tris-HCl (pH 7.5), 100 mmol/l NaCl, 0.5%
sodium deoxycholate, 1 mmol/l ethylenediaminetetraacetic acid, 1%
Nonidet P-40, and 0.1% sodium dodecyl sulfate and protease
inhibitor. Lysates (100 µg) were resolved on an SDS-PAGE gel
and transferred to PVDF membranes (Millipore, Bedford, MA, USA).
After blocking in 5% milk with TBST, the memberanes were inclubated
with ZIC1 (1:500, ab134951), ZIC2 (1:500, ab150404), ZIC3 (1:500,
ab136431), ZIC4 (1:500, ab199284), ZIC5 (1:500, ab42483) and GAPDH
(1:1000, ab181602) (all from Abcam, Cambridge, UK) antibodies at
room temperature overnight. Subsequently, secondary antibodies
coupled to horseradish peroxidase (HRP) were visualized using a
chemiluminescence with Las-4000 Imaging system (Fujifilm, Tokyo,
Japan). The relative expression levels of proteins were quantified
and normalized to GAPDH.
Immunohistochemistry
The subcellular localization and expression pattern
of ZIC1 protein were examined by immunohistochemistry using
4-µm-thick formalin-fixed paraffin embedded sections of GC
and matched normal mucosa tissues. The tissue sections were dewaxed
in xylene, rehydrated in alcohol, immersed in 3% hydrogen peroxide
for 10 min, and the slides were then treated with antigen in 0.01
mol/l sodium citrate buffer in a microwave for 20 min before
incubating overnight at 4°C with ZIC1 primary antibody [ZIC1
(1:500, ab134951; Abcam)] in 3% bovine serum albumin (BSA).
Subsequently, the sections were washed in 50 mM Tris buffer at pH
7.6 3 times, and the biotinylated link antibody (Dako Corp.,
Carpinteria, CA, USA) and streptavidin-peroxidase conjugate were
applied sequentially for 15 min each. The Dako liquid
3,3′-diaminobenzidine (DAB) substrate-chromogen solution was used
as the chromogen, and hematoxylin was used as the nuclear
counterstain. Finally, the tissue sections were counterstained with
hemalum, before being dehydrated, cleared and mounted. Non-specific
binding was assessed using a non-immune rabbit serum (1:1,000) in
3% BSA in the place of the primary antibody.
To evaluate the results of immunohistochemical
staining, the stained sections were reviewed by 2 independent
pathologists blinded the clinicopathological characteristics of the
patients with GC. They randomly selected 5 visual fields for each
section and counted the number of total tumor cells and positive
cells, and recorded the staining intensity. The semi-quantitative
method was used to determine the staining intensity of the samples
according to the staining intensity and the percentage of
positively stained cells. The staining intensity was classified
into 4 grades: 0, none; 1, poor; 2, moderate and 3, strong. The
percentage of positive cells (P) was scored as follows: 0, 0%; 1,
1–25%; 2, 26–50%; 3, 50–75%; and 4, >75%. The total
immunoreactive score (IRS) was calculated as the staining intensity
score multiplied by the percentage of positively stained cells. IRS
≤2.09 (median value of IRS in GC tissues) was defined as a low
expression, and IRS >2.09 was defined as a high expression.
Statistical analysis
All statistical analyses were performed using SPSS
software version 11.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Continuous variables were expressed as the means ± SD. The
differences of ZIC expression, at the mRNA and protein level,
between the GC tissues and matched normal mucosa were analyzed by
an paired-t test. The associations of ZIC1 expression with various
clinicopathological characteristics of the patients with GC were
analyzed by Fisher's exact test for any 2×2 tables and Pearson's
χ2 test for non-2×2 tables. The survival analysis was
estimated using the Kaplan-Meier method and data were compared
using the log-rank test. Multivariate analysis was performed using
the Cox proportional hazard model. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ZIC1–5 in human GC
tissues
We first detected the expression of the 5 ZIC genes
(ZIC1–5) in 60 fresh GC tissue and matched normal mucosa samples by
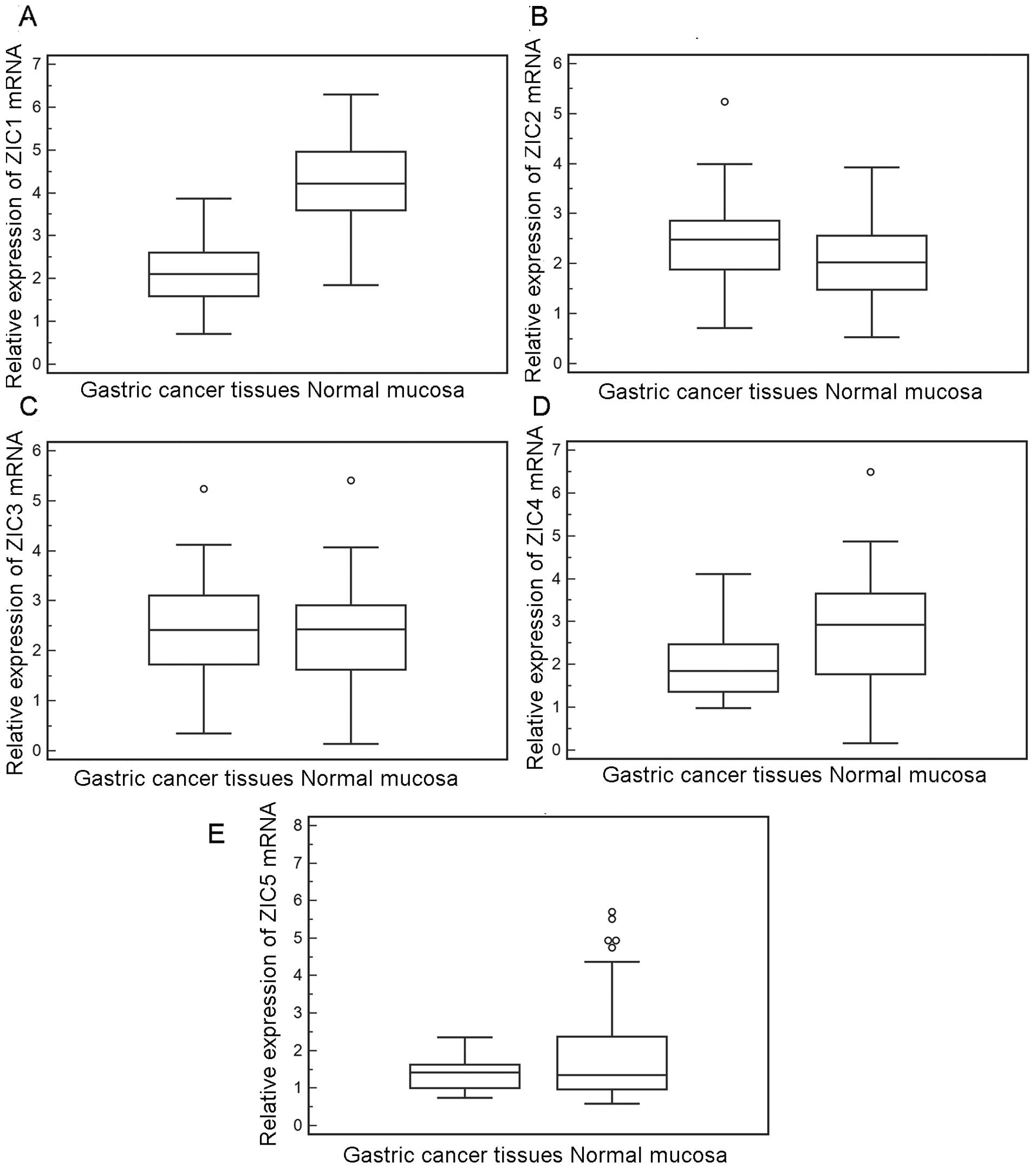
RT-qPCR. As shown in Fig. 1, the
relative mRNA expression level of ZIC1 was significantly decreased
in the GC tissues compared to the matched normal mucosa tissues (GC
vs. normal, 2.15±0.69 vs. 4.28±0.95; P<0.001); however, the
expression levels of ZIC2 (GC vs. normal, 2.42±0.84 vs. 2.08±0.74;
P>0.05), ZIC3 (GC vs. normal, 2.28±1.13 vs. 2.24±1.13,
P>0.05), ZIC4 (GC vs. normal, 2.07±0.86 vs. 2.63±1.46,
P>0.05) and ZIC5 (GC vs. normal, 1.38±0.39 vs. 1.98±1.53,
P>0.05) exhibited no significant differences between the cancer
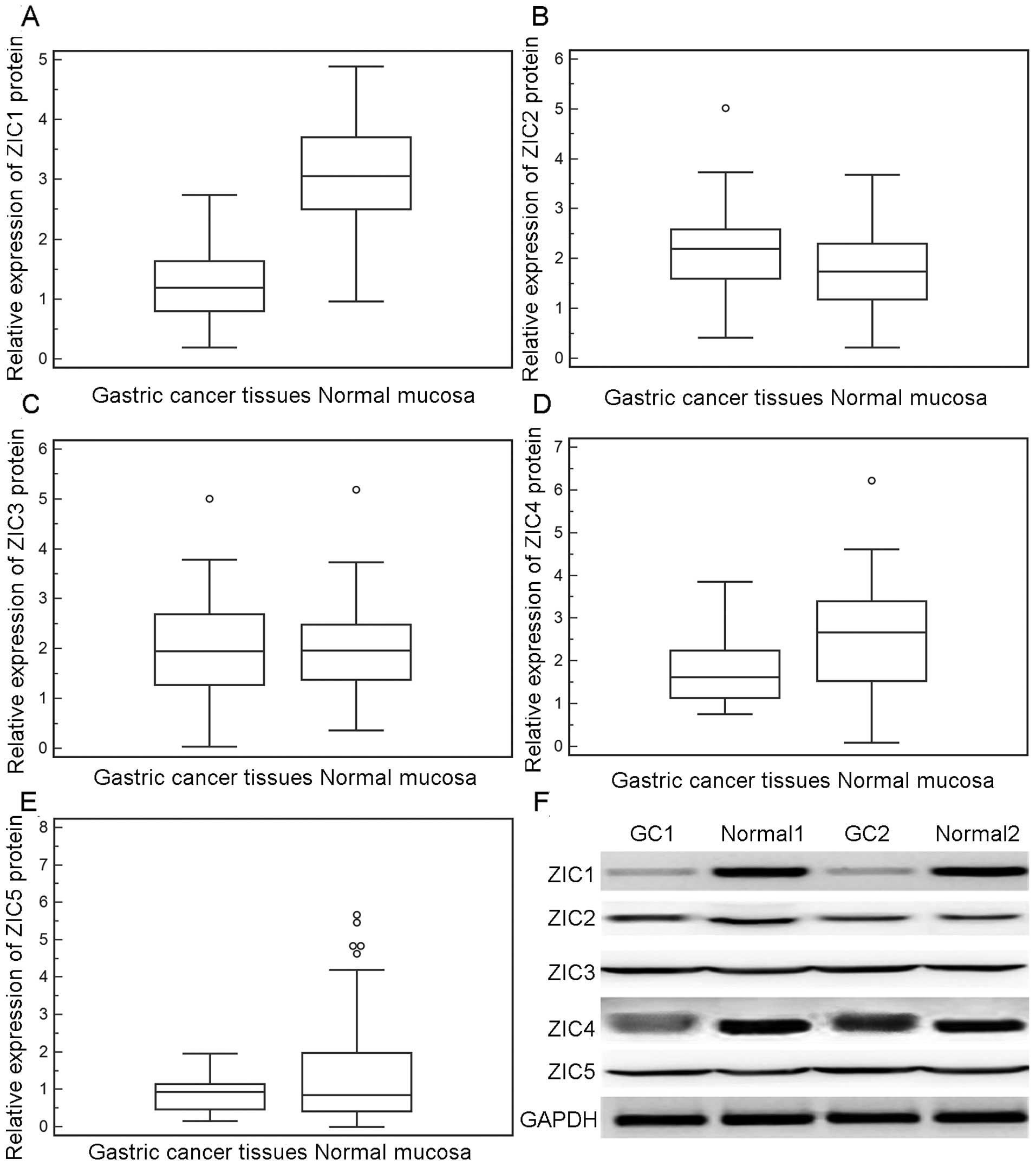
and normal tissue samples, in line with the findings of western
blot analysis (Fig. 2). Notably,
in the 60 fresh normal mucosa samples, ZIC1 mRNA and protein levels
were 1.02- to 5.35-fold and 1.5- to 3.2-fold higher, respectively,
than those in the GC tissues.
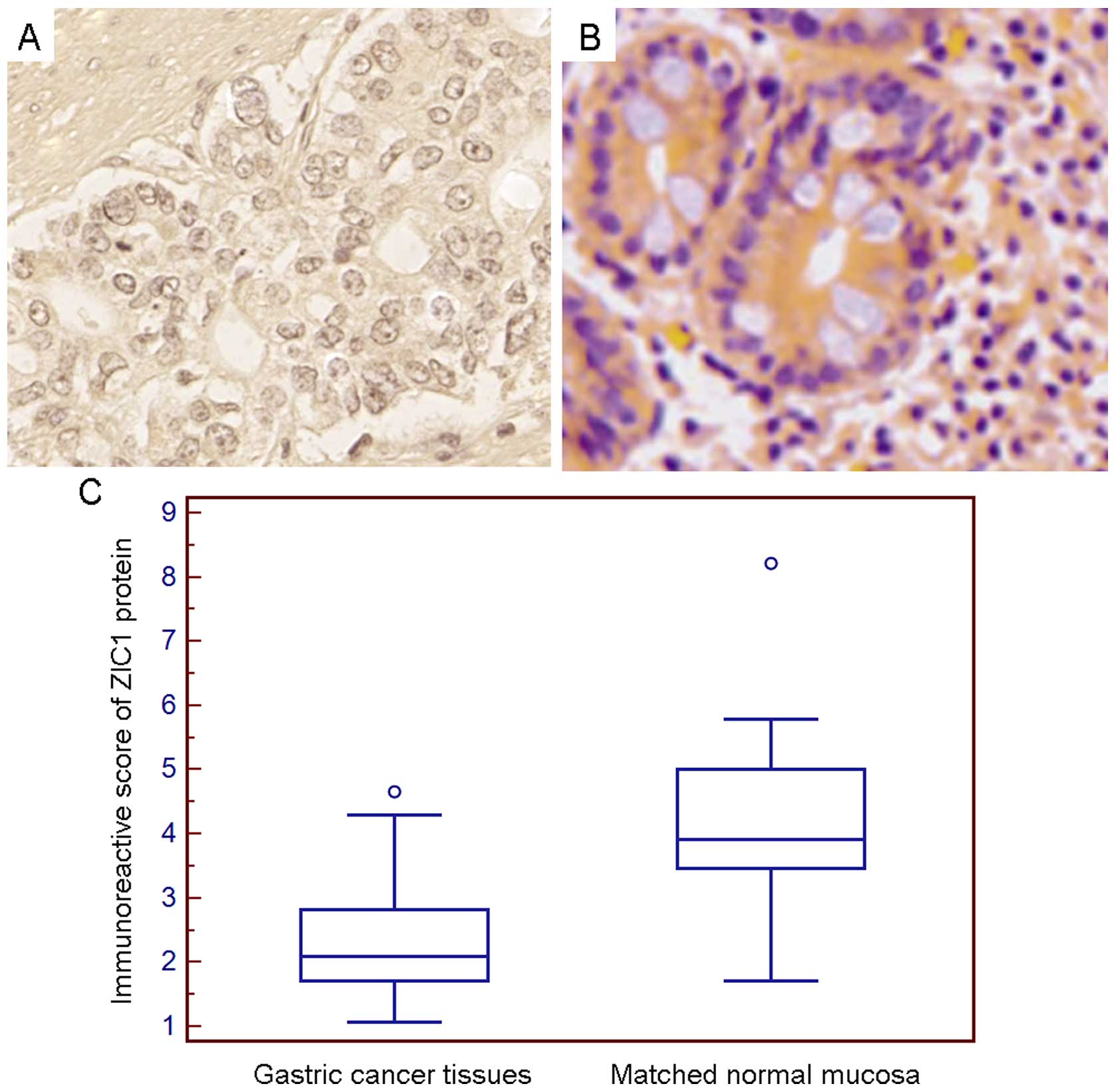
Moreover, immunohistochemistry revealed that the
positive staining for ZIC1 protein in the GC tissues and matched
normal mucosa tissue samples was predominantly localized in the
cell cytoplasm (Fig. 3A). There
was a significant difference in ZIC1 expression between the GC and
matched normal mucosa samples from the same patient (GC vs. normal,
2.33±0.81 vs. 4.20±1.09, P<0.001; Fig. 3C).
Downregulation of ZIC1 is associated with
the aggressive progression of human GC
To assess the association between ZIC1 expression
and various clinicopathological characteristics, the median value
(2.09) of ZIC1 expression in GC tissues was used as a cut-off point
for dividing all 160 GC patients into the ZIC1-low and ZIC1-high
groups. GC patients with an IRS of ZIC1 exceeding the median value
were deemed to be in the ZIC1-high group; all other tissues were
considered to be in the ZIC1-low group. Of the 160 GC patients, 75
(46.88%) displayed a high expression of ZIC1 and 85 (53.12%)
exhibited a low expression of ZIC1. As shown in Table I, the downregulation of ZIC1
(ZIC1-low) was more frequently observed in the GC tissues with
positive lymph node metastasis (P=0.006), an advanced TNM stage
(P<0.001) and a great depth of invasion (P=0.01). However, there
was no significant relation observed between ZIC1 and other
clinicopathological characteristics such as age, gender, tumor
size, lauren classification, and tumor differentiation (all
P>0.05; Table I).
Downregulation of ZIC1 is associated with
a poor prognosis in human GC
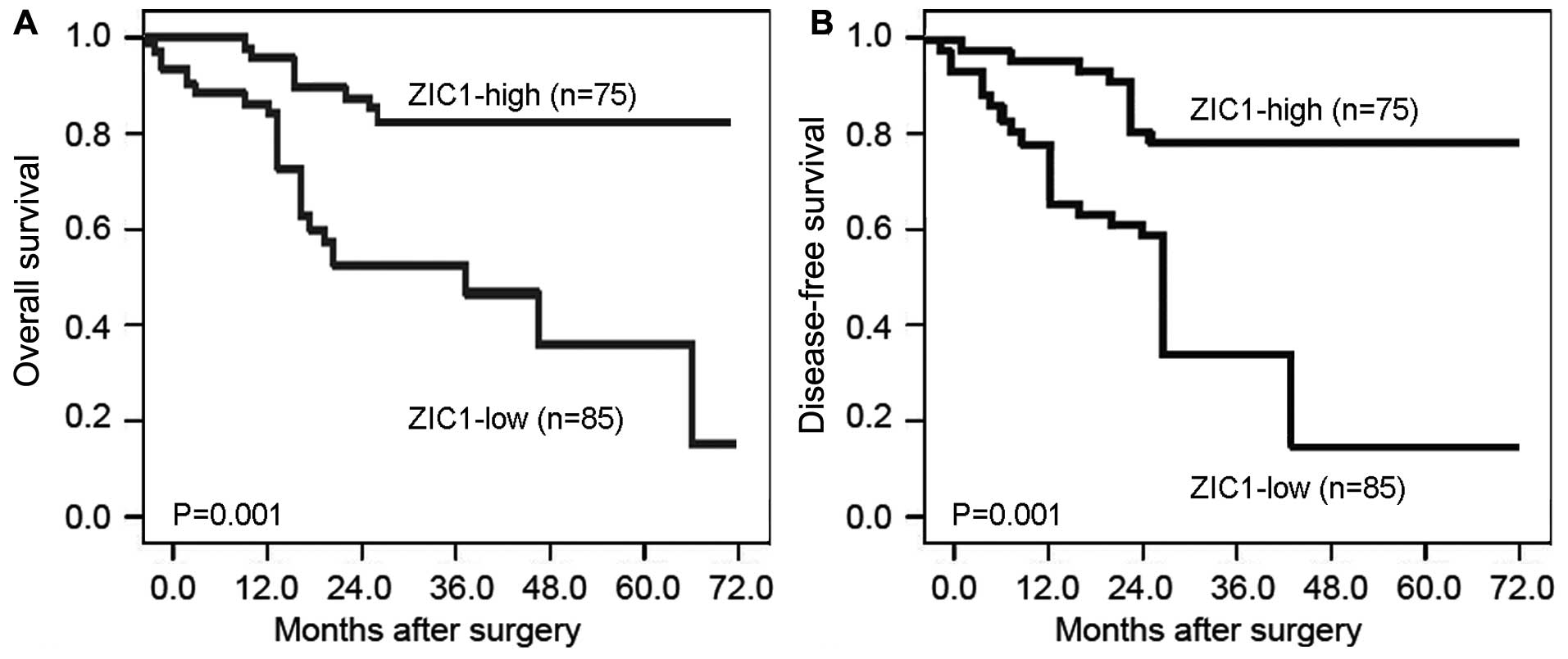
Kaplan-Meier analysis revealed that the GC patients
with a low ZIC1 expression had both a shorter overall survival and
disease-free survival than those with a high ZIC1 expression (both
P=0.001, log-rank test; Fig. 4).
To determine whether ZIC1 expression is an independent risk factor
for prognosis, the Cox proportional hazard regression model was
used. As shown in Table II,
univariate analysis revealed that positive lymph node metastasis
(both P=0.01), an advanced TNM stage (both P<0.001), a great
depth of invasion (P=0.02 and 0.03, respectively) and a low ZIC1
expression (both P=0.001) were significantly associated with a poor
overall survival and disease-free survival. Furthermore,
multivariate analysis revealed that the status of lymph node
metastasis [for overall survival, hazard ratio (HR)=4.658, 95%
CI=0.913–9.621, P=0.01; for disease-free survival, HR=4.126, 95%
CI=0.893–9.282, P=0.01], TNM stage (for overall survival,
HR=10.039, 95% CI=1.925–20.791, P<0.001; for disease-free
survival, HR=9.629, 95% CI=1.902–19.391, P<0.001), depth of
invasion (for overall survival, HR=3.939, 95% CI=0.825–8.791,
P=0.02; for disease-free survival, HR=3.027, 95% CI=0.628–7.128,
P=0.03) and ZIC1 expression (for overall survival, HR=4.928, 95%
CI=0.936–10.362, P=0.01; for disease-free survival, HR=4.068, 95%
CI=0.882–8.968, P=0.02) were independent prognostic markers for
predicting poor prognosis in patients with GC.
 | Table IIPrognostic value of ZIC1 expression
for the overall survival and disease-free survival of patients with
GC by multivariate analysis with Cox regression. |
Table II
Prognostic value of ZIC1 expression
for the overall survival and disease-free survival of patients with
GC by multivariate analysis with Cox regression.
|
Characteristics | Overall survival
| Disease-free
survival
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.005
(0.101–2.008) | NS | 0.798
(0.122–1.689) | NS |
| Gender | 1.379
(0.303–2.743) | NS | 1.199
(0.336–2.306) | NS |
| Tumor size | 0.462
(0.202–1.039) | NS | 0.729
(0.308–1.692) | NS |
| Lauren
classification | 2.232
(0.566–4.676) | NS | 1.928
(0.416–4.086) | NS |
|
Differentiation | 1.019
(0.272–2.069) | NS | 1.012
(0.262–2.016) | NS |
| Lymph node
metastasis | 4.658
(0.913–9.621) | 0.01 | 4.126
(0.893–9.282) | 0.01 |
| TNM stage | 10.039
(1.925–20.791) | <0.001 | 9.629
(1.902–19.391) | <0.001 |
| Depth of
invasion | 3.939
(0.825–8.791) | 0.02 | 3.027
(0.628–7.128) | 0.03 |
| ZIC1
expression | 4.928
(0.936–10.362) | 0.01 | 4.068
(0.882–8.968) | 0.02 |
Discussion
Human GC represents a devastating disease without
early symptoms, and it is associated with a rapid progression and
poor prognosis, leading to an increasing mortality rate. Thus, it
is extremely necessary to identify novel and efficient biomarkers
for the early diagnosis and prognosis of this cancer. In the
present study, we detected the mRNA and protein expression levels
of ZIC1, ZIC2, ZIC3, ZIC4 and ZIC5 in human GC tissues and adjacent
normal mucosa tissue sampoles, but found that only ZIC1 had a
differential expression between the cancer and normal tissues. In
addition, our immunohistochemical analysis verified the
downregulation of ZIC1 protein in GC tissues, and showed that it
significantly correlated with lymph node metastasis, a higher TNM
stage and a deeper invasion. Moreover, Kaplan-Meier curve analysis
demonstrated that the GC patients with a low ZIC1 expression had a
poorer prognosis than those with a high ZIC1 expression. ZIC1
expression was identified as an independent factor of the overall
and disease-free survivals in GC patients by multivariate Cox
analysis. These results suggested that ZIC1 may serve as a
biomarker to predict the progression and prognosis of GC
patients.
As one of the 5 ZIC family genes, ZIC1 is located on
chromosome 3q24 and encodes a C2H2-type zinc
finger transcription factor which is noted for its involvement in
various biological processes, including early patterning,
neurogenesis, dorsal neural tube development, myogenesis and
left-right axis establishment by regulating cell growth,
proliferation and differentiation (27). Under physiological conditions, the
expression of ZIC1 has only been found in neural tissues, and is
restricted to the cerebellum (28). In recent years, growing evidence
has indicatd that ZIC1 may play a critical role in the progression
of several types of cancer, due to its involvement in
fibroproliferative processes and its regulatory effects on several
members of the Wnt and Notch signaling pathways (29). Gan et al (18) reported that ZIC1 expression was
markedly down-regulated in primary colorectal cancer tissues as
compated to adjacent non-tumor tissues through promoter
hypermethylation, and acted as a tumor suppressor to inhibit cell
proliferation and to induce apoptosis by interacting with the MAPK
and PI3K/Akt pathways, as well as the Bcl-xl/Bad/caspase-3 cascade.
Qiang et al (14)
indicated that ZIC1 was frequently downregulated by promoter
hypermethylation in both primary thyroid cancer tissues and thyroid
cancer cell lines, and demonstrated that ZIC1 hypermethylation was
significantly associated with lymph node metastasis in patients
with papillary thyroid cancer, and was also identified as a
putative tumor suppressor by modulating major signaling pathways
and the transcription factor FOXO3a. By contrast, Brill et
al (30) revealed that ZIC1
was overexpressed in all 5 subtypes of liposarcoma compared with
normal fat and in liposarcoma cell lines compared with
adipose-derived stem cells, and may function as an oncogene to
promote proliferation and invasion, and to suppress apoptosis in
dedifferentiated and myxoid/round cell liposarcoma cell lines.
These previous findings suggest that ZIC1 may contribute to cancer
progression in a cancer-specific manner.
As regards GC, Wang et al (15) in 2009 firstly found that ZIC1
expression was distinctly decreased in primary GC tissues in
comparison with non-tumor adjacent gastric tissues through promoter
hypermethylation, and revealed that the ectopic expression of ZIC1
led to the growth inhibition of GC cells through the induction of
S-phase cell cycle arrest; In 2012, the same research group
indicated that the overexpression of ZIC1 could result in the
inactivation of the Shh, PI3K and MAPK signaling pathways, as well
as in the regulation of multiple downstream targets which may be
essential for the development and progression of GC (16); In 2015, Chen et al
(17) also identified ZIC1
promoter hypermethylation in plasma DNA as a potential biomarker
for GC and intraepithelial neoplasia. Consistently, our data
verified the downregulation of ZIC1, at both the mRNA and protein
level in GC tissues, by RT-qPCR, western blot analysis and
immunohistochemistry, respectively. In addition, the ZIC1
expression data obtained from immunohistochemical detection were
analyzed for correlations with clinicopathological characteristics.
As a result, we found that a low ZIC1 expression significantly
correlated with lymph node metastasis, a deeper invasion, and a
higher TNM stage, suggesting that ZIC1 downregulation may affect
the invasion, metastasis and progression of GC, and may indicate
the aggressive behavior of cancer. Apart from the results mentioned
above, Kaplan-Meier curves proved that the patients with a low ZIC1
expression presented with poorer overall and disease-free survivals
than the patients with a high ZIC1 expression. Multivariate Cox
regression analysis confirmed that ZIC1 expression, along with
lymph node metastasis, TNM stage and depth of invasion, was an
independent prognostic factor of GC prognosis. To the best of our
knowledge, this is first study to confirm the clinical significance
of ZIC1 in GC based on a large cohort of clinical samples.
In conclusion, among the human ZIC family genes,
ZIC1 dysregulation, but not that of ZIC2, ZIC3, ZIC4 and ZIC5, may
play a crucial role in GC progression. ZIC1 may serve as a novel
molecular marker to predict the progression, survival and relapse
of patients with GC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colquhoun A, Arnold M, Ferlay J, Goodman
KJ, Forman D and Soerjomataram I: Global patterns of cardia and
non-cardia gastric cancer incidence in 2012. Gut. 64:1881–1888.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian X, Hu J, Zhao J and Chen H: ATP
citrate lyase expression is associated with advanced stage and
prognosis in gastric adenocarcinoma. Int J Clin Exp Med.
8:7855–7860. 2015.PubMed/NCBI
|
|
4
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He ZX and Li B: Recent progress in genetic
and epigenetic profile of diffuse gastric cancer. Cancer Transl
Med. 1:80–93. 2015. View Article : Google Scholar
|
|
6
|
Hashimoto T, Arai K, Yamashita Y, Iwasaki
Y and Hishima T: Characteristics of intramural metastasis in
gastric cancer. Gastric Cancer. 16:537–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanda M and Kodera Y: Recent advances in
the molecular diagnostics of gastric cancer. World J Gastroenterol.
21:9838–9852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali RG, Bellchambers HM and Arkell RM:
Zinc fingers of the cerebellum (Zic): Transcription factors and
co-factors. Int J Biochem Cell Biol. 44:2065–2068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houtmeyers R, Souopgui J, Tejpar S and
Arkell R: The ZIC gene family encodes multi-functional proteins
essential for patterning and morphogenesis. Cell Mol Life Sci.
70:3791–3811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bataller L, Wade DF, Fuller GN, Rosenfeld
MR and Dalmau J: Cerebellar degeneration and autoimmunity to
zinc-finger proteins of the cerebellum. Neurology. 59:1985–1987.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakurai H, Kikuchi K, Tsuchiya T, Kanazawa
H and Tsuda M: Developmentally and regionally regulated alterations
of octamer- and GC-box-binding activities during the postnatal
development of mouse cerebellum. Brain Res Dev Brain Res.
61:161–168. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aruga J, Nagai T, Tokuyama T, Hayashizaki
Y, Okazaki Y, Chapman VM and Mikoshiba K: The mouse zic gene
family. Homologues of the Drosophila pair-rule gene odd-paired. J
Biol Chem. 271:1043–1047. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lacroix J, Schlund F, Leuchs B, Adolph K,
Sturm D, Bender S, Hielscher T, Pfister SM, Witt O, Rommelaere J,
et al: Oncolytic effects of parvovirus H-1 in medulloblastoma are
associated with repression of master regulators of early
neurogenesis. Int J Cancer. 134:703–716. 2014. View Article : Google Scholar :
|
|
14
|
Qiang W, Zhao Y, Yang Q, Liu W, Guan H, Lv
S, Ji M, Shi B and Hou P: ZIC1 is a putative tumor suppressor in
thyroid cancer by modulating major signaling pathways and
transcription factor FOXO3a. J Clin Endocrinol Metab.
99:E1163–E1172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang LJ, Jin HC, Wang X, Lam EK, Zhang JB,
Liu X, Chan FK, Si JM and Sung JJ: ZIC1 is downregulated through
promoter hypermethylation in gastric cancer. Biochem Biophys Res
Commun. 379:959–963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong J, Chen S, Xue M, Du Q, Cai J, Jin
H, Si J and Wang L: ZIC1 modulates cell-cycle distributions and
cell migration through regulation of sonic hedgehog, PI(3)K and
MAPK signaling pathways in gastric cancer. BMC Cancer. 12:2902012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Lin Z, Xue M, Si J and Chen S:
Zic1 promoter hypermethylation in plasma DNA is a potential
biomarker for gastric cancer and intraepithelial neoplasia. PLoS
One. 10:e01339062015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan L, Chen S, Zhong J, Wang X, Lam EK,
Liu X, Zhang J, Zhou T, Yu J, Si J, et al: ZIC1 is downregulated
through promoter hypermethylation, and functions as a tumor
suppressor gene in colorectal cancer. PLoS One. 6:e169162011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS,
Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, et al: Identification of
molecular markers and signaling pathway in endometrial cancer in
Hong Kong Chinese women by genome-wide gene expression profiling.
Oncogene. 26:1971–1982. 2007. View Article : Google Scholar
|
|
20
|
Pourebrahim R, Van Dam K, Bauters M, De
Wever I, Sciot R, Cassiman JJ and Tejpar S: ZIC1 gene expression is
controlled by DNA and histone methylation in mesenchymal
proliferations. FEBS Lett. 581:5122–5126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vural B, Chen LC, Saip P, Chen YT, Ustuner
Z, Gonen M, Simpson AJ, Old LJ, Ozbek U and Gure AO: Frequency of
SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients
with small cell lung carcinoma and their correlation with clinical
parameters. Cancer. 103:2575–2583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inaguma S, Ito H, Riku M, Ikeda H and
Kasai K: Addiction of pancreatic cancer cells to zinc-finger
transcription factor ZIC2. Oncotarget. 6:28257–28268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marchini S, Poynor E, Barakat RR, Clivio
L, Cinquini M, Fruscio R, Porcu L, Bussani C, D'Incalci M, Erba E,
et al: The zinc finger gene ZIC2 has features of an oncogene and
its overexpression correlates strongly with the clinical course of
epithelial ovarian cancer. Clin Cancer Res. 18:4313–4324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan DW, Liu VW, Leung LY, Yao KM, Chan
KK, Cheung AN and Ngan HY: Zic2 synergistically enhances hedgehog
signalling through nuclear retention of Gli1 in cervical cancer
cells. J Pathol. 225:525–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kandimalla R, van Tilborg AA, Kompier LC,
Stumpel DJ, Stam RW, Bangma CH and Zwarthoff EC: Genome-wide
analysis of CpG island methylation in bladder cancer identified
TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers. Eur
Urol. 61:1245–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aruga J, Nozaki Y, Hatayama M, Odaka YS
and Yokota N: Expression of ZIC family genes in meningiomas and
other brain tumors. BMC Cancer. 10:792010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Degreef I, De Smet L, Sciot R, Cassiman JJ
and Tejpar S: Immunohistochemical evidence for Zic1 coexpression
with beta-catenin in the myofibroblast of Dupuytren disease. Scand
J Plast Reconstr Surg Hand Surg. 43:36–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabater L, Bataller L, Suárez-Calvet M,
Saiz A, Dalmau J and Graus F: ZIC antibodies in paraneoplastic
cerebellar degeneration and small cell lung cancer. J Neuroimmunol.
201–202:163–165. 2008. View Article : Google Scholar
|
|
29
|
Merzdorf CS: Emerging roles for zic genes
in early development. Dev Dyn. 236:922–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brill E, Gobble R, Angeles C,
Lagos-Quintana M, Crago A, Laxa B, Decarolis P, Zhang L, Antonescu
C, Socci ND, et al: ZIC1 overexpression is oncogenic in
liposarcoma. Cancer Res. 70:6891–6901. 2010. View Article : Google Scholar : PubMed/NCBI
|