Introduction
The clinical manifestations of aging are fine wrinkles, thin and transparent skin, loss of underlying fat leading to hollowed cheeks and eye sockets, dry and itchy skin, lack of sufficient perspiration, hair graying, hair loss or hirsutism, and thinning of the nail plates (1). It is widely accepted that intrinsic aging is primarily caused by accumulated damage due to free radicals and by reactive oxygen species (ROS)-induced damage to critical cellular macromolecules (2). Not only does ROS production increase with age but the ability of human skin cells to repair DNA damage steadily decreases over time (3).
ROS play an important role in skin aging. In the skin, about 1.5–5% of the consumed oxygen is converted into ROS by intrinsic processes (4). ROS are continuously produced as byproducts in the electron transport chain of aerobic metabolism in the mitochondria, and are regarded as the principal cause of intrinsic aging (5). Keratinocytes and fibroblasts are the main producers of mitochondrial ROS in the skin.
Rutin, a quercetin glycoside (6), is a member of the bioflavonoid family also referred to as vitamin P (7). Previous research has identified rutin as an antioxidant with anti-inflammatory, antiallergenic, antiviral, and anticarcinogenic properties, capable of scavenging superoxide radicals (8–13). It has also been demonstrated that rutin is capable of inhibiting human platelet aggregation stimulated by collagen (14), decreasing capillary fragility (15), prolonging activated partial thromboplastin time (16) as well as of exerting anti-thrombotic effects (17).
The present study confirms that rutin reduces skin aging by strengthening dermal density and elasticity through the regulation of enzymes in the extracellular matrix (ECM).
Materials and methods
Cell culture
Human dermal fibroblasts, (HDFs; Lonza, Basel, Switzerland) were cultured in Dulbecco's modified Eagle medium (Gibco/Life Technologies, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin (Gibco/Life Technologies) at 37°C in an atmosphere of 5% CO2. Rutin was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide.
Cell viability assay
HDFs were seeded at a density of 3×103 cells/well in a 96-well plate and incubated for 24 h. Rutin (0–200 µM) was later added to the cells for 24 h to measure the cytotoxicity of rutin. To determine the protective effects against oxidative stress, the cells were pre-treated with rutin (0–100 µM) for 3 h and then exposed to 0.2 mM H2O2 for 24 h. The cytotoxicity of rutin in HDFs was evaluated using a water-soluble tetrazolium salt (WST-1) assay (EZ-Cytox cell viability assay kit; Itsbio, Seoul, Korea). The WST-1 solution was added to cultured cells at a volume equal to 10% of the culture medium, and then the cells were incubated at 37°C for 1 h. Cell viability was evaluated by measuring the absorbance at 450 nm using an iMark microplate reader (Bio-Rad, Hercules, CA, USA).
Cellular senescence analysis
The expression of lysomal galactosidase as a marker for senescent HDFs was determined using the senescence-associated (SA)-β-galactosidase staining kit (BioVision, Milpitas, CA, USA) according to the manufacturer's instructions. HDFs were seeded at a density of 2×105 cells/well in a 60-mm cell culture plate and incubated until they reached 90% confluence. Subsequently, the cells were treated with rutin and H2O2 for 24 h, the medium was removed, and the cells were washed once with phosphate-buffered saline (PBS). A fixing solution (0.5 ml; 4% formaldehyde and 0.5% glutaraldehyde in PBS buffer, pH 7.2) was added to the culture plate for 1 h in order to fix the cells, and 0.5 ml of a staining solution [staining solution 470 µl and 5 µl staining supplement, and 20 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) in 25 µl dimethylformamide] for 24 h. The cells then were treated with 1 ml 70% glycerol, and images were captured using an Olympus IX51 microscope (Tokyo, Japan). The percentage of senescent cells was calculated as the senescence ratio [senescent cells (%) = stained cells/total cells ×100].
Measurement of ROS scavenging activity
Intracellular ROS formation was measured by adding 2′,7′-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich). DCF-DA is non-fluorescent until it is hydrolyzed by intracellular esterases and oxidized into the highly fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. The cells were seeded in 60-mm cell culture plates at a density of 2×105 cells/well and incubated for 24 h. Following incubation, the cells were pre-treated with rutin (0–50 µM) for 3 h, and then exposed to 0.2 mM H2O2 for 24 h. After 24 h, 10 µM DCF-DA was added to the culture media for 30 min, and the medium containing DCF-DA was removed by washing with PBS. The cells were detached from the plates with 1% trypsin-EDTA and washed with PBS, and ROS was detected by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. The purity and concentration of the RNA were evaluated using MaestroNano®, a microspectrophotometer (Maestrogen, Las Vegas, NV, USA). All cDNAs for sensitive and specific miRNA detection were synthesized using the miScript II RT kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The following primers were used: collagen, type I, alpha 1 (COL1A1) COL1A1 forward, 5′-AGGGCCAAGACGAAGACATC-3′ and reverse, 5′-AGATCACGTCATCGCACAACA-3′; matrix metalloproteinase (MMP)1 forward, 5′-TCTGACGTTGATCCCAGAGAGCAG-3′ and reverse, 5′-CAGGGTGACACCAGTGACTGCAC-3′. qPCR was performed using EvaGreen dye (Solis BioDyne, Tartu, Estonia) and LineGene K software (Bioer, Hangzhou, China). The CT value for each gene expressed was normalized to that of β-actin forward, 5′-GGATTCCTATGTGGGCGACGA-3′ and reverse, 5′-CGCTCGGTGAGGATCTTCATG-3′. The 2−ΔΔCt method was used to calculate the relative expression level of each gene (6).
Subjects for clinical evaluation
The study protocols were approved by the Institutional Review Board of GeneCellPharm Incorporated (Cheongju-si, Korea). All subjects were informed of the objective of the study and provided informed consent and agreed to use skin care products during the study. Forty women, aged 30–50 years, were selected for a randomized and double-blind clinical trial (control group, 45.50±5.79 years; experimental group, 45.70±5.34 years). The selection of subjects was based on age, signs of skin aging, and being neither pregnant nor nursing. Subjects who presented with symptoms of itching or erythema, or those who hindered the evaluation process due to excessive drinking or smoking were excluded from the experiment. The subjects were divided into control and experimental groups, each containing 20 subjects. All conditions were the same except for the test material used on the experimental group. The study was conducted for 4 weeks. Biometric parameters were measured three times: prior to application, and 2 and 4 weeks after application. During this study, each subject performed a self-evaluation, and using a scale of severity (0, none; 1, mild; 2, severe; 3, very severe), completed a questionnaire to indicate to what extent they exhibited any skin disorders, such as erythema, itching, scaling, edema and tingling and burning sensations, at each visit. The subjects who presented with skin disorders withdrew from the clinical evaluation process.
In order to determine whether the rutin-containing cream had any adverse effects, the subjects were asked individually about the condition of their skin, and a visual evaluation of skin reactions, such as erythema, itching, scaling, tingling, tightness and prickling or burning sensations was performed at each visit. No adverse effects were reported based on either a visual evaluation or the questionnaire (Table I). To examine dermal density, skin elasticity as well as any improvement in wrinkles around the eye, the subjects were instructed to apply 2 g of test material to the face, including the eye rim, every morning and night for 4 weeks. The subjects and investigators were blinded to the test and control treatments. At each visit, all subjects washed with the cleanser provided and remained quietly in a room with constant temperature (22±1°C) and humidity (45±5%) so that all subjects would be evaluated under the same conditions.
 |
Table I
Abnormal skin reactions reported by subjects.
|
Table I
Abnormal skin reactions reported by subjects.
| Abnormal reaction |
Severity |
Abnormal reaction |
Severity |
| Erythema |
0 |
Tingling |
0 |
| Swelling (edema) |
0 |
Burning |
0 |
| Scaling (epidermis) |
0 |
Tightness |
0 |
| Itching |
0 |
Prickling |
0 |
The cream was prepared by incorporating the ingredients in the 3 phases (A, B, C). The ingredients in the A phase (distilled water, glycerin, 1,3-butylene glycol) were combined and heated until all the components were melted, and the ingredients in the B phase (distilled water, dipotassium phosphate, sodium hydroxylate, rutin) were combined and heated to the same temperature, to ensure homogeneity. The A and B phase ingredients were combined and emulsified using a homo mixer (Tokushu Kika Kogyo Co., Ltd., Japan) at 5,000 rpm for 10 min. The mixture was cooled to 60°C and blended with the homogenized phase C (emulium delta, sepipuls 400) at 5,000 rpm for 10 min. By then, the temperature of the mixture had dropped to 45°C. The mixture was combined and homogenized, while maintaining the pH at 6.2. The cream provided to the experimental group contained 2% (wt %) rutin and the cream provided to the control group was prepared using the same volume of water in place of rutin.
Measurement of skin elasticity
To evaluate the improvement in skin elasticity, a DermaLab USB elasticity probe (Cortex Technology ApS, Hadsund, Denmark) was applied and the results were analyzed using the associated application software (version 1.09). Measurements were obtained using a fixed elasticity probe on the left cheek of each subject. To analyze the elasticity measurements, Young's modulus (E) was calculated as a dose-dependent representation of skin elasticity. The measurements of elasticity were taken three times: prior to application, and 2 and 4 weeks after application. The measurement unit is MPa.
Measurement of dermal density
To evaluate dermal density, a DUB SkinScanner (tpm taberna pro medicum GmbH, Lüeneburg, Germany) was used. Dermal density was measured 3 cm from the left eye, applying a couplant for ultrasonic examination. The analysis range was limited to the area between the dermis and upper panniculus. The measurements were taken three times: prior to application, and 2 and 4 weeks after application.
Measurement of the length and area of crow's feet as well as under-eye wrinkles
To evaluate the extent of improvement in wrinkles, particularly at the eye rim, a Robo skin analyzer CS50 (Inforward Inc., Tokyo, Japan) was used. The facial images were captured from each subject placed in identical positions with equal lighting: on the front, left and right sides of the face. To evaluate improvement, measurements were taken three times: prior to application, and 2 and 4 weeks after application. We analyzed the captured images, matching the facial feature points accurately. The measurement unit of crow's feet length is mm and area is mm2.
Statistical analysis
In cellular efficacy tests, all results are presented as the mean percentages ± standard deviation (SD) of three independent experiments. A P-value <0.05 as determined by the Student's t-test, was considered statistically significant. In clinical efficacy tests, statistical analyses were conducted using SPSS software (version 17.0 for Windows; IBM, Armonk, NY, USA). Paired t-tests were performed in cases of repeated measurements on the same subject. To analyze subject questionnaires, mean values, SDs, and percentages were used. The formula used to measure the percentage change for each skin parameter was percentage change = [(A−B)/B] ×100, where A is defined as the individual value of any parameter at the 2 and 4 week visits, and B represents the zero hour of the assessed parameter.
Results
Cytotoxicity of rutin in HDFs
To determine whether rutin affects the viability of HDFs, cultured HDFs were exposed to concentrations of rutin ranging from 0–200 µM for 24 h. Rutin reduced the cell viability of HDFs by 13.23% at a concentration of 50 µM and by 26.05% at 100 µM (Fig. 1A). The cytotoxicity of rutin increased at concentrations >100 µM, thus, we used 100 µM as the maximum concentration in subsequent experiments (data not shown). To examine the ability of rutin to inhibit cellular damage, we examined cell viability, which increased in a dose-dependent manner, in 0.2 mM H2O2-exposed HDFs (Fig. 1B). H2O2 reduced cell viability to 78.48%; however, following the addition of rutin to the H2O2-exposed cells, the viability of HDFs increased to 85.53, 91.67, 97.50 and 95.04% with the addition of 1, 10, 50 and 100 µM rutin, respectively.
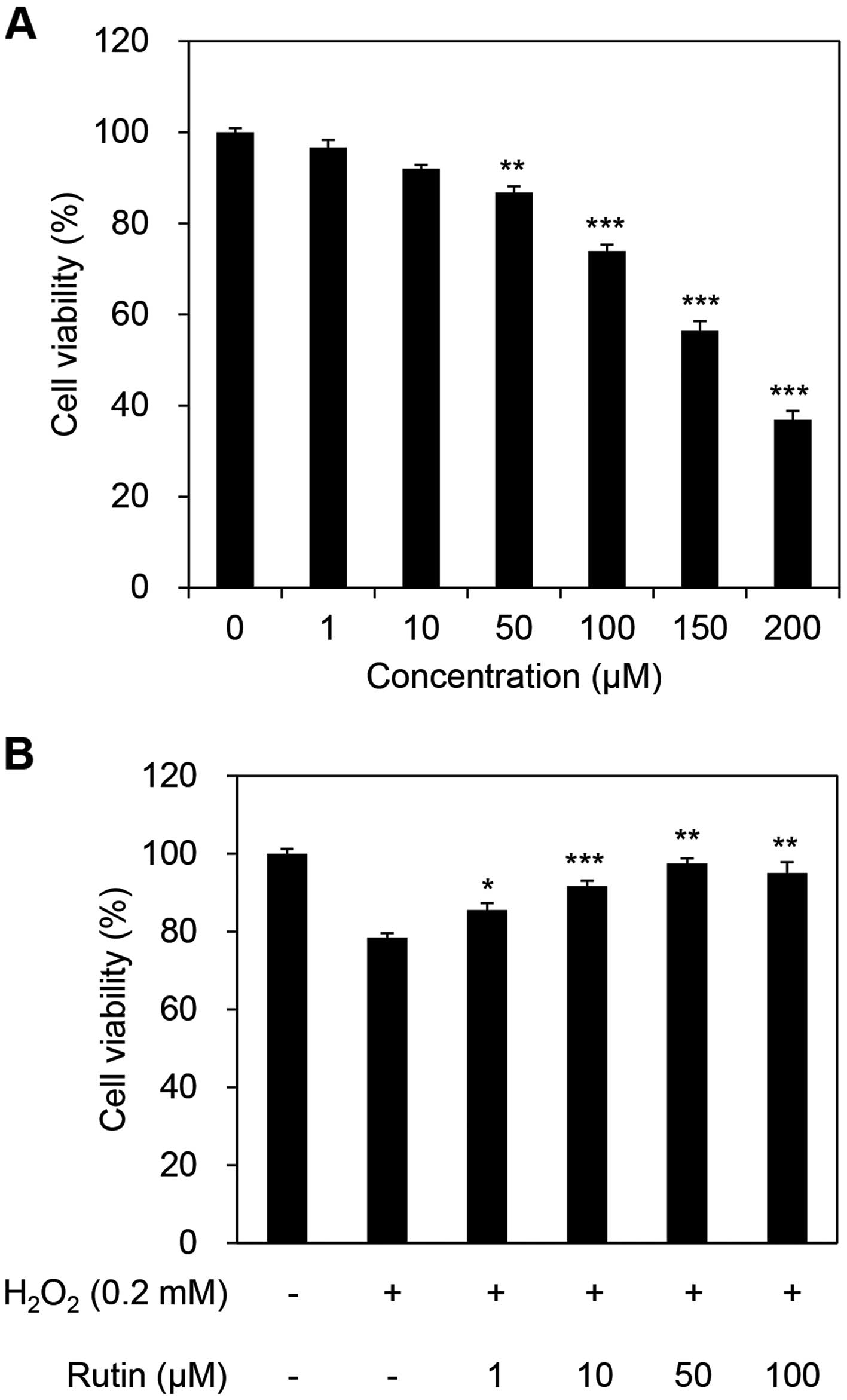 |
Figure 1
Effects of rutin on cell viability in rutin- and H2O2-treated human dermal fibroblasts (HDFs). (A) Measurement of cell cytotoxicity of rutin in HDFs. (B) Inhibitory effects of rutin on H2O2-induced cellular damage response. *P<0.05, **P<0.01 and ***P<0.001 as determined by the Student's t-test.
|
Senescent cell detection assay
Next, we examined the inhibition of senescence by rutin using the SA-β-galactosidase assay. The percentage of senescent cells was 59.80% in the cells exposed to H2O2 only. This ratio decreased in a dose-dependent manner to 49.28, 35.30 and 19.26% with the administration of 1, 10 and 50 µM rutin (Fig. 2). These results indicate that H2O2 acts as a stimulator of senescence and that rutin inhibits H2O2-induced cellular senescence.
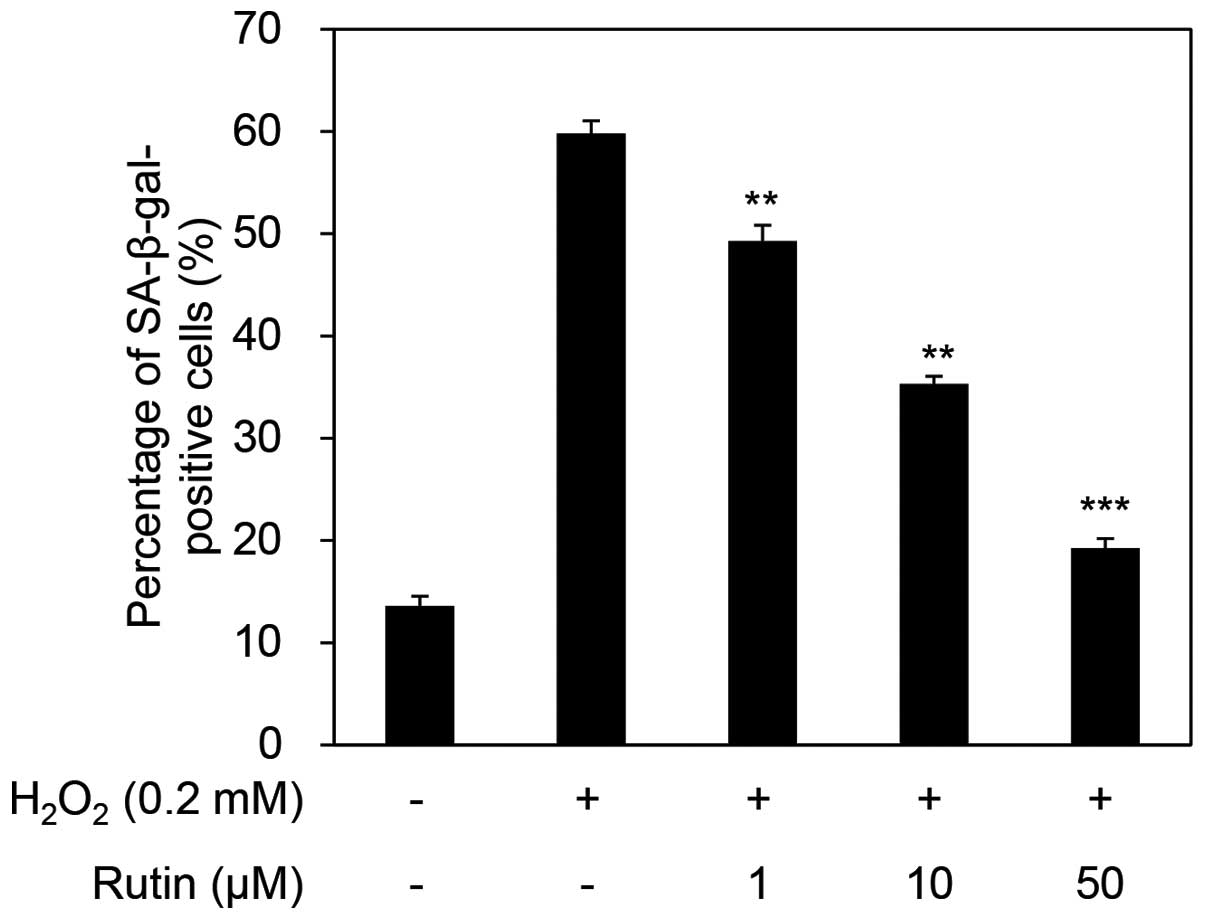 |
Figure 2
Inhibitory effects of rutin on H2O2-induced cellular senescence of human dermal fibroblasts (HDFs). **P<0.01 and ***P<0.001 as determined by the Student's t-test. SA-β-gal, SA-β-galactosidase.
|
Detection of ROS scavenging activity
To determine cellular ROS levels, we performed the DCF-DA assay in H2O2- exposed HDFs. The exposure of HDFs to 0.2 mM H2O2 increased ROS levels >three times that of the control cells. The cells exposed to 0.2 mM H2O2 and treated with rutin exhibited decreased ROS levels in a dose-dependent manner to a relative intensity of 2.6-, 1.9- and 1.6-fold following treatment with 1, 10 and 50 µM rutin (Fig. 3). These results demonstrate that rutin decreases cellular levels of ROS.
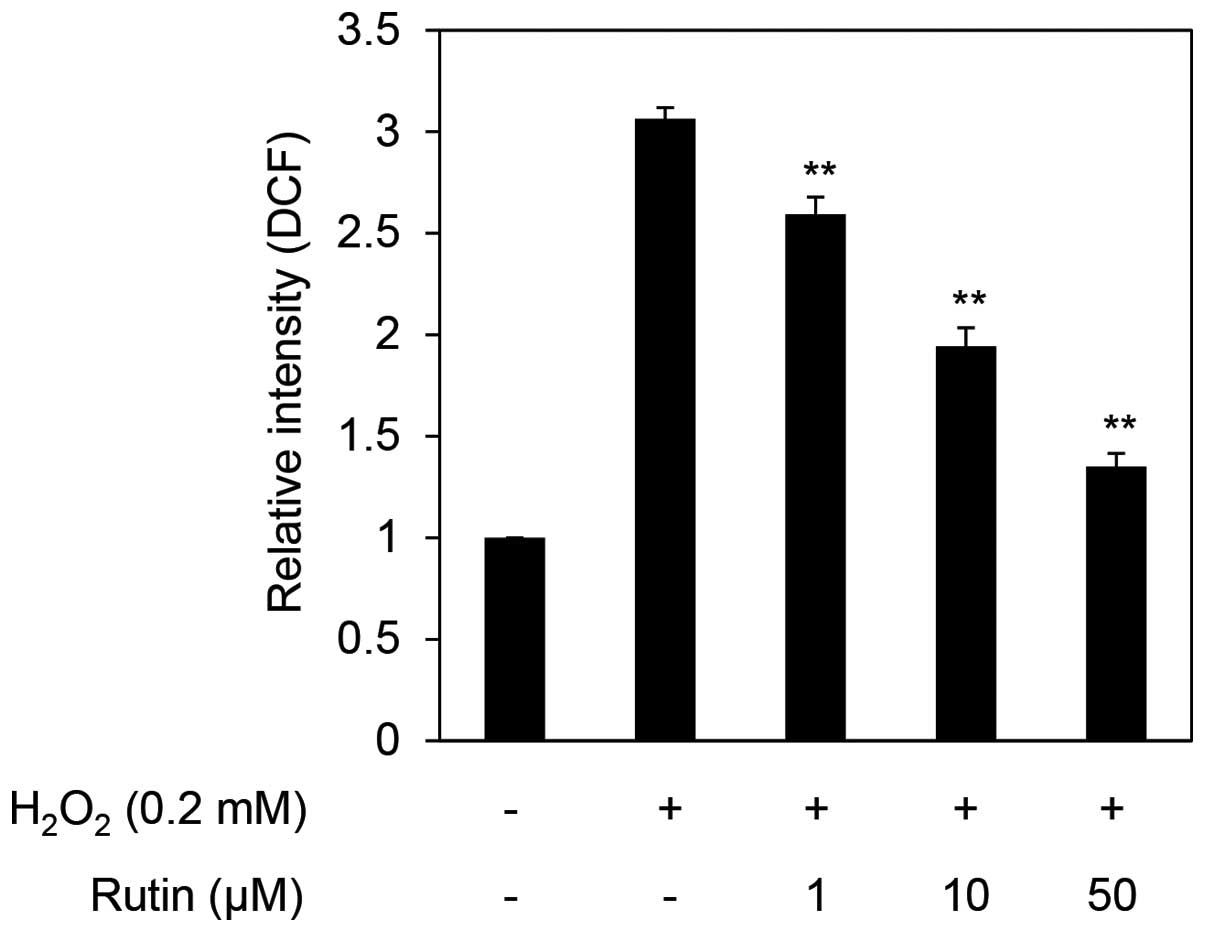 |
Figure 3
Reactive oxygen species (ROS) scavenging effects of rutin on H2O2-treated human dermal fibroblasts (HDFs). **P<0.01 as determined by the Student's t-test.
|
Analysis of mRNA expression levels of COL1A1 and MMP1
ROS are known to be inducers of MMPs, which degrade intracellular substances (18,19). MMPs are divided into three types: collagenase (MMP1 and MMP8), gelatinase (MMP2 and MMP9), and stromelysin (MMP3); additionally, MMP14 and MMP15 have transmembrane domains which determines their substrate specificity (20–24). MMP1, generated in fibroblasts, is highly present in senescent cells and ultimately decreases collagen degradation (25–33). Collagen, the most abundant protein in the dermis, provides structural support; >90% of collagen in the body is type I collagen (34). COL1A1 encodes the major component of type I collagen and its expression level decreased to 0.67 following treatment with H2O2 compared with no treatment. The relative expression of COL1A1 increased to 0.81, 0.90 and 0.97 in a dose-dependent manner with the addition of 1, 10 and 50 µM rutin, respectively, compared with H2O2 alone (Fig. 4A). On the other hand, the expression of MMP1 in the cells exposed to H2O2 increased to 2.6 compared with that in the untreated cells. However, the relative MMP1 expression decreased to 2.06, 1.50 and 1.16 following treatment with 1, 10 and 50 µM rutin, respectively (Fig. 4B).
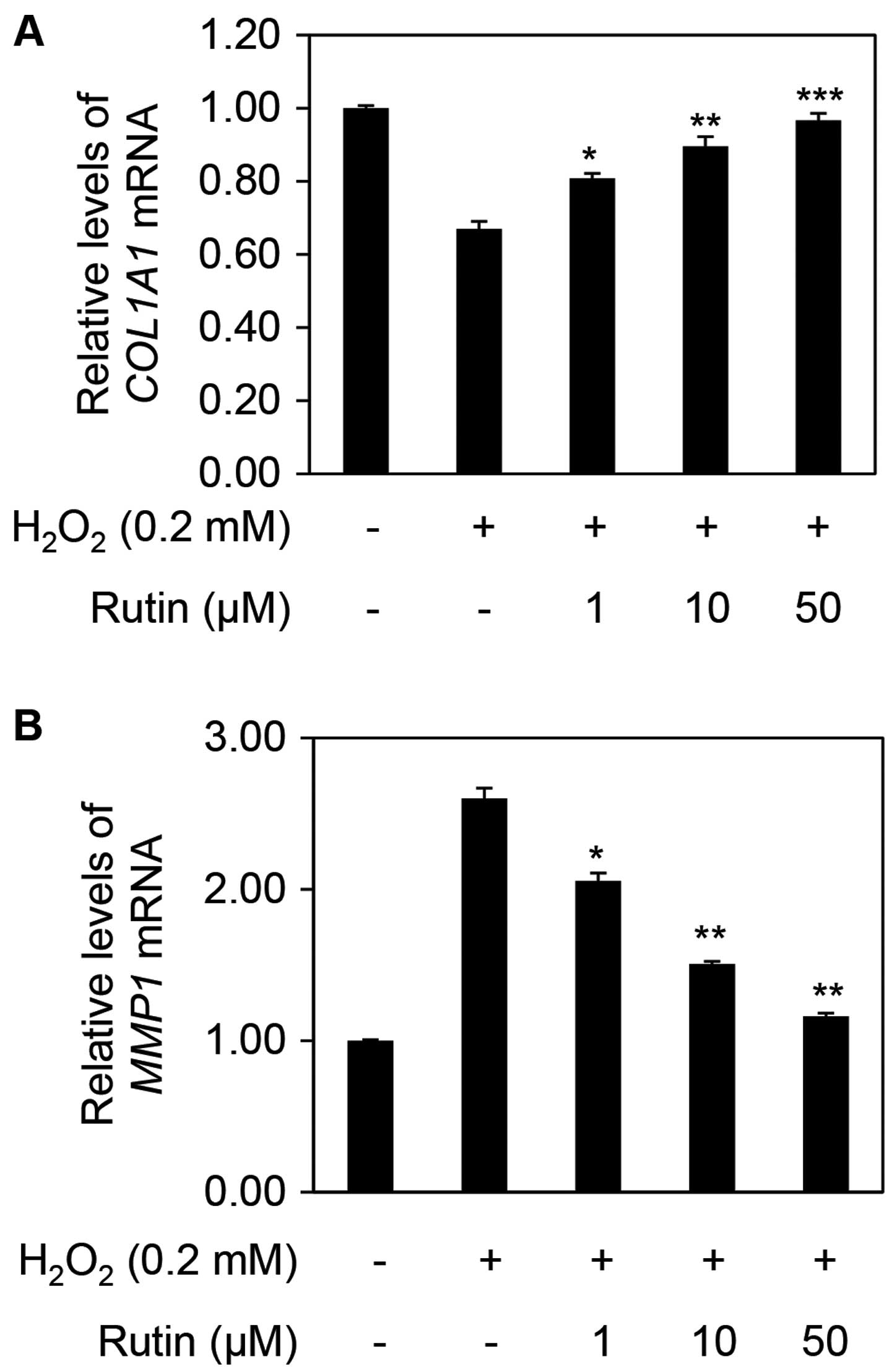 |
Figure 4
Effects of rutin on the mRNA expression of collagen, type I, alpha 1 (COL1A1) and matrix metalloproteinase 1 (MMP1) in human dermal fibroblasts (HDFs). Relative mRNA expression levels of (A) COL1A1 and (B) MMP1 in H2O2- and rutin-treated HDFs. The results are representative of three independent experiments (means ± SD). *P<0.05, **P<0.01 and ***P<0.001 as determined by the Student's t-test.
|
Evaluation of dermal density
As aging occurs, the expression of MMPs is increased which results in the degradation of skin substrate proteins (35). Dermal thickness and density were found to decrease with increasing concentrations of MMPs and degradation of albuminoids and the collagen layer (35). To evaluate the clinical efficacy of rutin on aging skin, we demonstrated the effect of cream containing rutin on dermal density. The measurements obtained for the control cream were dermal densities of 49.88% prior to use, and 49.72% after 2 weeks and 49.45% after 4 weeks of use (Fig. 5). The subjects using rutin-containing cream were found to have a dermal density of 48.79% prior to use, 54.02% after 2 weeks and 58.62% after 4 weeks of application (Fig. 5). Dermal density measurements, represented as percentages, are proportional to density. These experimental data were statistically significant (P<0.05 and P<0.001). To determine the extent of any improvement in skin density, we calculated the improvement as a percentage, based on the value prior to application. Notably, the dermal density improvement rate was −0.30% after 2 weeks and −0.85% after 4 weeks in the control group. On the other hand, in the experimental group, the improvement rate of dermal density was 10.73% after 2 weeks and 20.16% after 4 weeks of use. These results indicate that rutin increases dermal thickness.
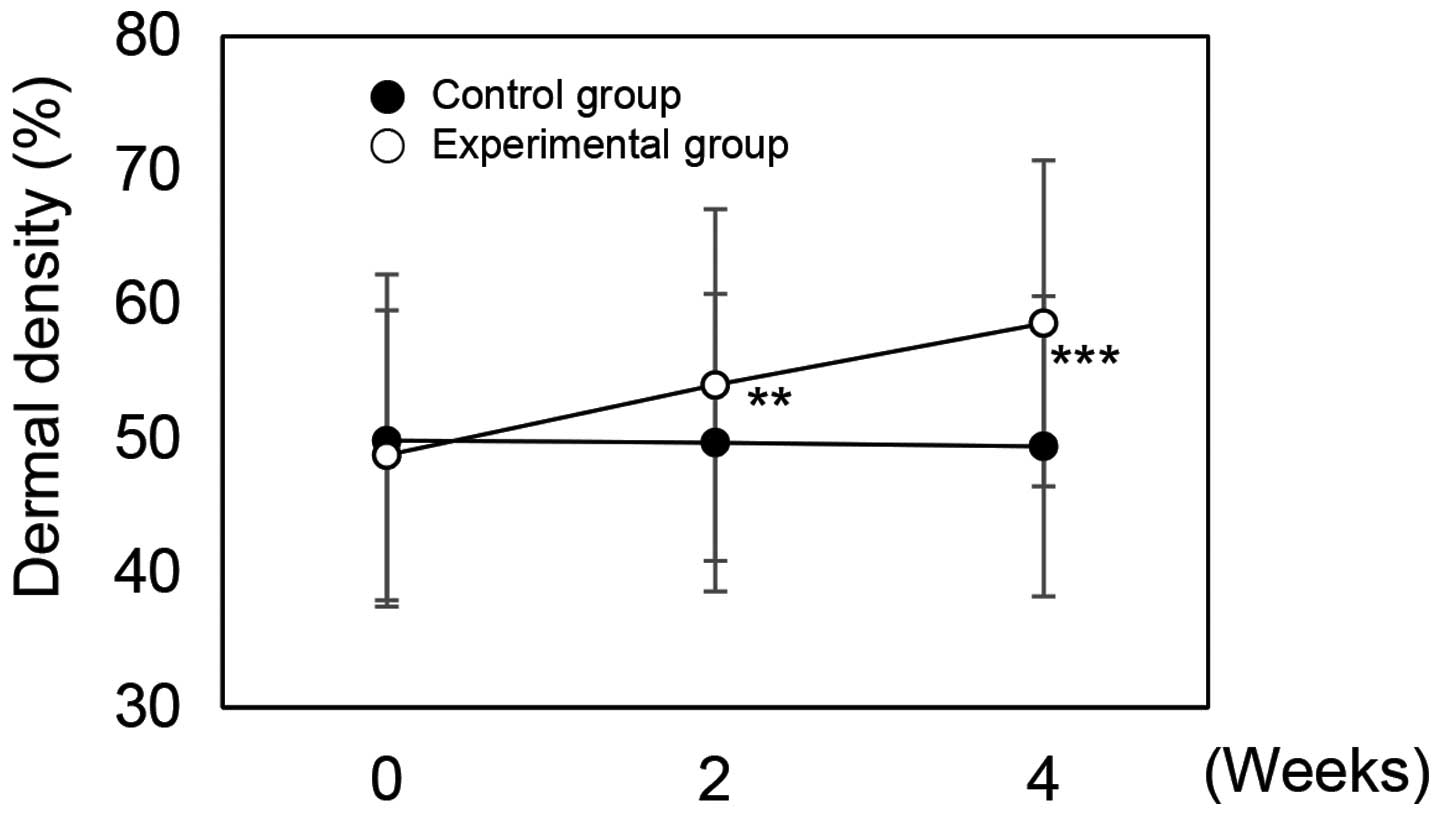 |
Figure 5
Dermal density of skin to which rutin-containing or control cream was applied. **P<0.05 and ***P<0.001 versus the control group at the same time point as determined by the Student's t-test.
|
Evaluation of length and area of crow's feet
Wrinkles, a representative aging phenomenon, occur in response to structural alterations of cells and tissues. Wrinkles arising due to intrinsic aging are formed by decreases in collagen and elasticity, denaturalization of elastic fibers and the stratum corneum, and a loss of skin moisture (36). Wrinkles arising from extrinsic aging are formed by ROS, which damage lipids and proteins in the skin, through the production of inflammatory cytokines (27,37). Previous research has found that ROS reduce collagen and elastin synthesis and are a major cause of wrinkle formation as a result of increased protease activities in various cellular signal transduction systems (38). Thus, we examined whether rutin may potentially be used as an anti-wrinkle ingredient in creams through clinical efficacy experiments.
The length of crow's feet were 63.50 mm prior to application, 64.25 mm after 2 weeks, and 64.95 mm after 4 weeks in the control group (Fig. 6A). In the experimental group, lengths were 68.40 mm prior to application, and 59.20 mm after 2 weeks and 52.05 mm after 4 weeks of application (Fig. 6A). The measured values of the experimental group were statistically significant (P<0.05 and P<0.001). To compare improvements in the length of crow's feet between the control and experimental groups, we analyzed the data over time. In the control group, improvement rates were −1.18% after 2 weeks and −2.28% after 4 weeks application. In the experimental group, improvement rates were 13.45% after 2 weeks and 23.90% after 4 weeks of use. From these results, we confirmed the effects of rutin on wrinkle improvement.
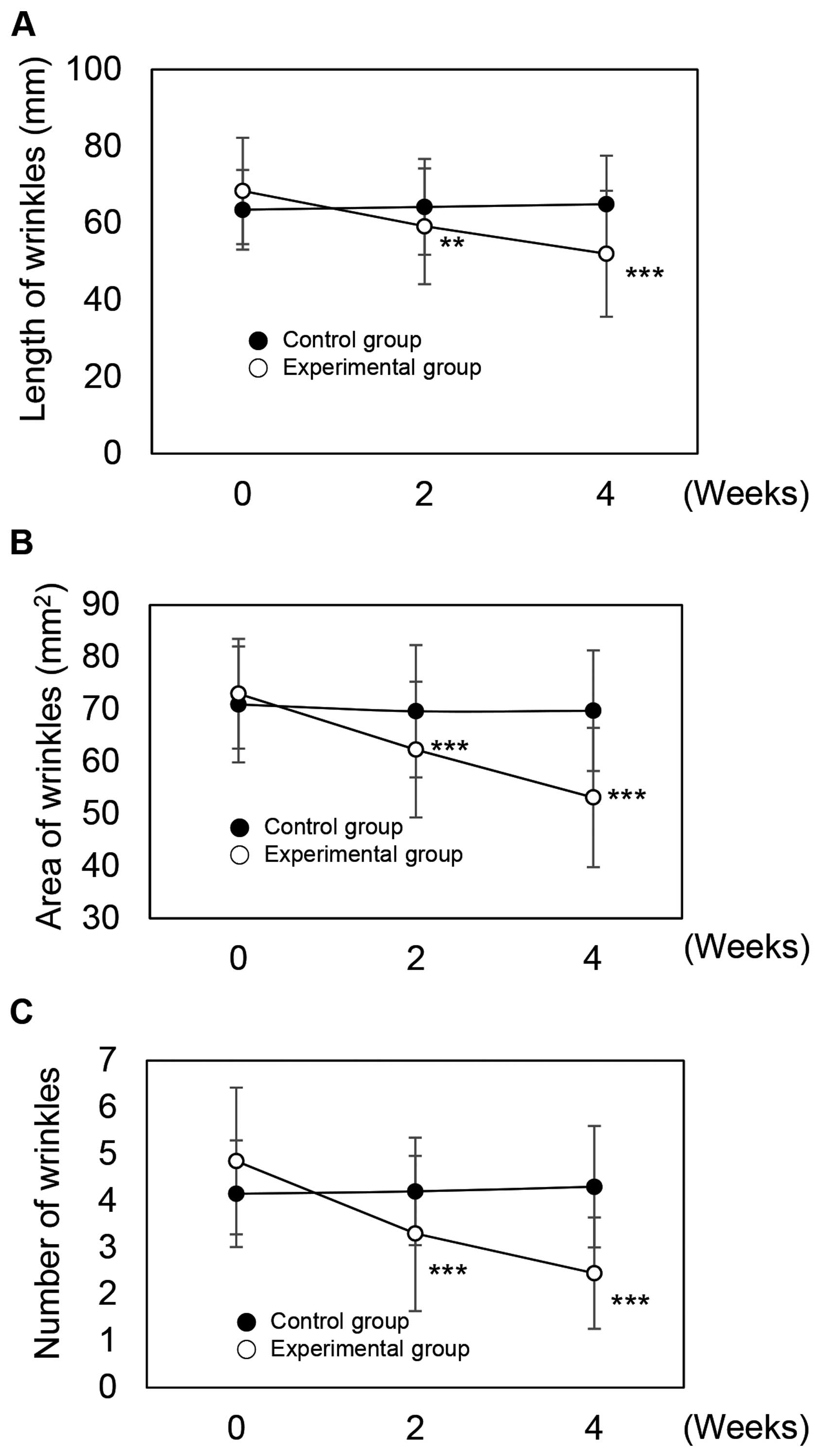 |
Figure 6
Beneficial effects of rutin-containing cream on eye wrinkles. The measurements of the (A) length and (B) area of crow's feet. (C) Measurements of wrinkle number under the eye rim. **P<0.05 and ***P<0.001 versus the control group at the same time point as determined by the Student's t-test.
|
In the control group, the area affected by crow's feet was 70.95 mm2 prior to application, and 69.65 mm2 after 2 weeks and 69.75 mm2 after 4 weeks of use (Fig. 6B). In the experimental group, the area affected by crow's feet was 73.00 mm2 before application, and 62.30 mm2 after 2 weeks and 53.15 mm2 after 4 weeks of application (Fig. 6B). The experimental group results were statistically significant (P<0.001).
The use of rutin-containing cream significantly reduced the areas affected by crow's feet over time. In the control group, the improvement rates were −1.83% after 2 weeks and 1.69% after 4 weeks. The improvement rates in the experimental group were 14.66% after 2 weeks and 27.19% after 4 weeks. From these results, we again confirmed the effects of rutin on wrinkle improvement.
Evaluation of under-eye wrinkles
In the control group, the average number of under-eye wrinkles was 4.15 prior to application, 4.20 after 2 weeks, and 4.30 after 4 weeks (Fig. 6C). In the experimental group, the number of under-eye wrinkles was 4.85 before application, and 3.30 after 2 weeks and 2.45 after 4 weeks application (Fig. 6C). The measured values of the experimental group were statistically significant (P<0.001).
We then assessed the improvement in under-eye wrinkles over time. In the control group, improvements were −1.20% after 2 weeks and −3.61% after 4 weeks application. Improvement rates in the experimental group were 31.96% after 2 weeks and 49.48% after 4 weeks. From these results, we confirmed the effects of rutin on wrinkle improvement.
Evaluation of skin elasticity
The dermis is composed of ECM that contains fibrous proteins such as collagen and elastin. Dermal fibroblasts regulate skin elasticity (39). Factors, such as ROS, ultraviolet (UV) rays or age, cause skin damage, wrinkle formation, and decreased skin elasticity by degrading collagen and elastin (39,40). In this study, we examined the effects of rutin on skin elasticity. In the control group, elasticity was 7.28 before application, and 7.25 after 2 weeks and 7.25 after 4 weeks application. In the experimental group, elasticity was 7.42 before application, and 9.30 after 2 weeks and 10.43 after 4 weeks of use (Fig. 7). The experimental group results were statistically significant (P<0.001). To compare the improvement in skin elasticity between the control and experimental groups, we analyzed the measured data over time. In the control group, improvements were −0.41% after 2 weeks and −0.48% after 4 weeks application. Improvement rates in the experimental group were 25.34% after 2 weeks and 40.50% after 4 weeks. From these results, we confirmed the beneficial effects of rutin on elasticity. All subjects had no adverse effects following a self-evaluation to determine the presence of any skin disorders (Table I).
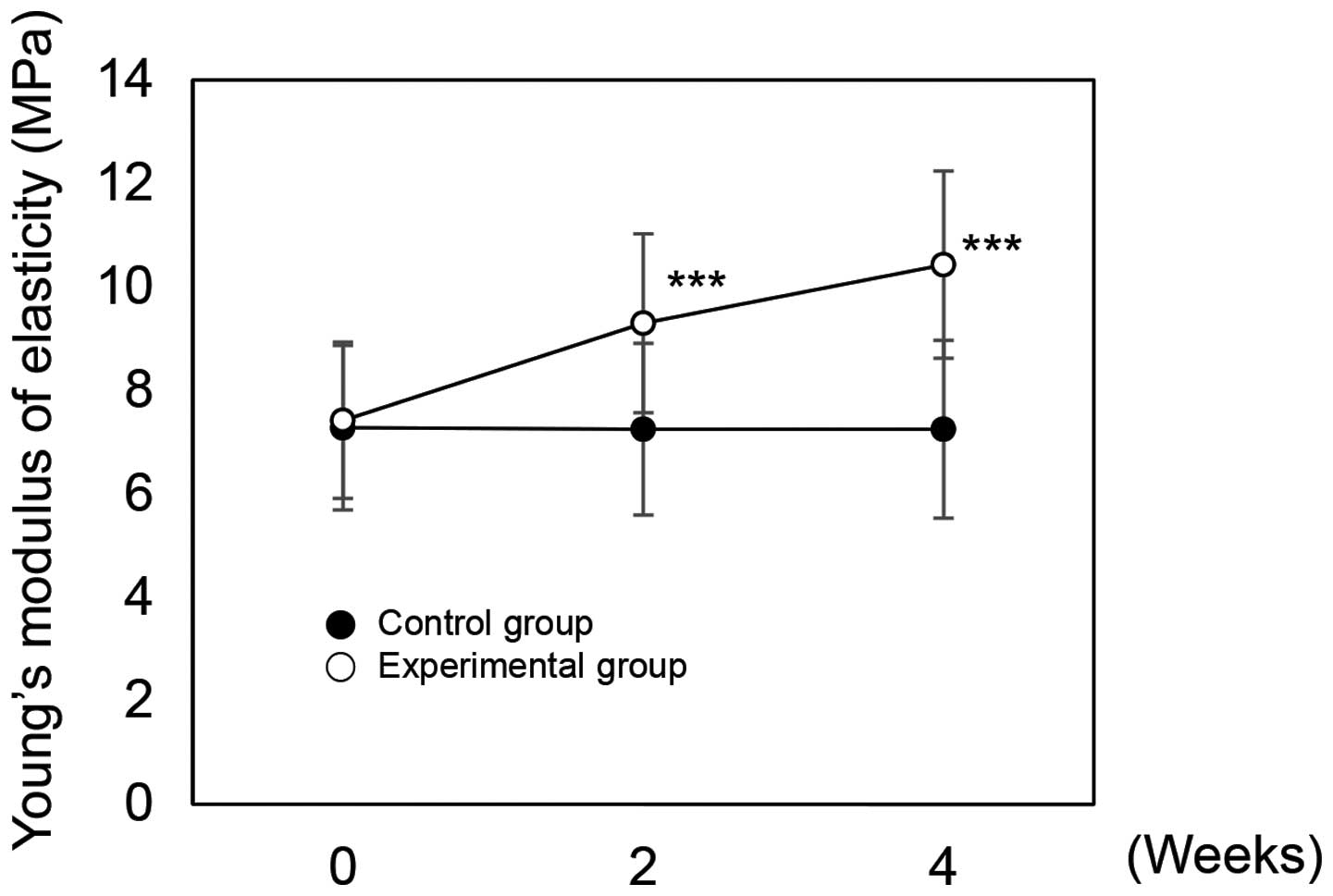 |
Figure 7
Effects on Young's modulus of skin elasticity of rutin-containing cream applied over time. ***P<0.001 versus the control group at the same time point as determined by the Student's t-test.
|
Discussion
The present study examined the antioxidant and anti-aging effects as well as the beneficial effects of rutin on skin barrier function through cellular and clinical efficacy experiments. The results of cellular experiments on HDFs indicated the antioxidant and anti-aging effects of rutin. In HDFs, rutin protects cells against damage induced by H2O2. Furthermore, we identified a ROS scavenging effect of rutin by DCF-DA assay and reduced H2O2-induced cellular senescence. After analyzing the expression of MMP1 and COL1A1, we verified that rutin reduces MMP1 expression and increases COL1A1 expression. Notably, cutaneous elasticity was reported to be the composite result of the amount of elastic fibers and ECM-filling materials (41,42). Additionally, MMPs are known to degrade all components of the ECM, such collagen, elastin, fibronectin, proteoglycans and laminin (34,43,44). Thus, MMP expression not only impairs procollagen synthesis and degrades the ECM, but leads to a loss of elasticity and skin dryness (45,46). Thus, we conducted experiments to determine the clinical efficacy of rutin.
We first manufactured rutin-containing cream. The clinical efficacy tests were conducted with 40 subjects, women between 30–50 years of age, selected for a randomized and double-blind study. The subjects were divided into two groups, experimental and control, and 2% rutin-containing or basic cream were applied, respectively. Dermal density improved 10.73% after 2 weeks and 20.16% after 4 weeks of application of the rutin-containing cream in the experimental group. We also showed that rutin-containing cream improved skin elasticity 25.34% after 2 weeks and 40.50% after 4 weeks of application. Reduced skin elasticity contributes to damaged skin structure and aging, particularly wrinkle formation (47–50). Thus, we investigated the length, area and number of wrinkles around the eyes of the rutin-treated subjects.
The length of crow's feet improved by 13.45% after 2 weeks and 23.90% after 4 weeks. Additionally, the area of crow's feet improved by 14.66% after 2 weeks and 27.19% after 4 weeks. After analyzing the average number of under-eye wrinkles, we demonstrated the effects of rutin on facial wrinkles. The number of under-eye wrinkles improved by 31.96% after 2 weeks and 49.48% after 4 weeks. These results indicate that rutin improves skin dermal density, reduces fine winkles, and enhances elasticity. We suggest that rutin may be used as a major ingredient in anti-aging cosmetics in order to improve skin elasticity and reduce wrinkles.
Acknowledgments
The present study was supported by the KU Research Professor Program (H.-J.C.) of Konkuk University. This study was also supported by grants from the Ministry of Science, ICT and Future Planning (no. 20110028646), the Korean Health Technology R&D Project, Ministry of Health and Welfare (grant no. HN13C0075), and the Ministry of Oceans and Fisheries (no. OF123321) of Republic of Korea.
References
|
1
|
Zouboulis CC and Makrantonaki E: Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 29:3–14. 2011. View Article : Google Scholar
|
|
2
|
Beckman KB and Ames BN: The free radical theory of aging matures. Physiol Rev. 78:547–581. 1998.PubMed/NCBI
|
|
3
|
Pons B, Belmont AS, Masson-Genteuil G, Chapuis V, Oddos T and Sauvaigo S: Age-associated modifications of Base Excision Repair activities in human skin fibroblast extracts. Mech Ageing Dev. 131:661–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poljšak B, Dahmane RG and Godić A: Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 21:33–36. 2012.
|
|
5
|
Farage MA, Miller KW, Elsner P and Maibach HI: Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 30:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KH, Lee KW, Kim DY, Park HH, Kwon IB and Lee HJ: Optimal recovery of high-purity rutin crystals from the whole plant of Fagopyrum esculentum Moench (buckwheat) by extraction, fractionation, and recrystallization. Bioresour Technol. 96:1709–1712. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, de Oliveira Costa Dias G, Morel AF, Nogueira CW and Rocha JB: Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 1107:192–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V and Martín Calero MJ: Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 71:45–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamalakkannan N and Prince PS: Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin Pharmacol Toxicol. 98:97–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bishnoi M, Chopra K and Kulkarni SK: Protective effect of rutin, a polyphenolic flavonoid against haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Fundam Clin Pharmacol. 21:521–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Makino T, Kanemaru M, Okuyama S, Shimizu R, Tanaka H and Mizukami H: Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J Nat Med. 67:881–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Ding Y, Xie D, Hu D, Li P, Li X, Xue W, Jin L and Song B: Design, synthesis, and antiviral activity of novel rutin derivatives containing 1, 4-pentadien-3-one moiety. Eur J Med Chem. 92:732–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perk AA, Shatynska-Mytsyk I, Gerçek YC, Boztaş K, Yazgan M, Fayyaz S and Farooqi AA: Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 14:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheu JR, Hsiao G, Chou PH, Shen MY and Chou DS: Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J Agric Food Chem. 52:4414–4418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
MacLean AL and Brambel CE: Dicumarol and rutin in vascular disorders. Trans Am Ophthalmol Soc. 44:194–213. 1946.
|
|
16
|
Kuntić V, Filipović I and Vujić Z: Effects of rutin and hesperidin and their Al(III) and Cu(II) complexes on in vitro plasma coagulation assays. Molecules. 16:1378–1388. 2011. View Article : Google Scholar
|
|
17
|
Choi JH, Kim DW, Park SE, Lee HJ, Kim KM, Kim KJ, Kim MK, Kim SJ and Kim S: Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J Biosci Bioeng. 120:181–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG and Waxman S: Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 94:2102–2111. 1999.PubMed/NCBI
|
|
19
|
Jabs T: Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 57:231–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woessner JF Jr: Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 5:2145–2154. 1991.PubMed/NCBI
|
|
21
|
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E and Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 370:61–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Will H and Hinzmann B: cDNA sequence and mRNA tissue distribution of a novel human matrix metalloproteinase with a potential transmembrane segment. Eur J Biochem. 231:602–608. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matrisian LM: The matrix-degrading metalloproteinases. BioEssays. 14:455–463. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matrisian LM: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burke EM, Horton WE, Pearson JD, Crow MT and Martin GR: Altered transcriptional regulation of human interstitial collagenase in cultured skin fibroblasts from older donors. Exp Gerontol. 29:37–53. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bizot-Foulon V, Bouchard B, Hornebeck W, Dubertret L and Bertaux B: Uncoordinate expressions of type I and III collagens, collagenase and tissue inhibitor of matrix metalloproteinase 1 along in vitro proliferative life span of human skin fibroblasts. Regulation by all-trans retinoic acid. Cell Biol Int. 19:129–135. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S and Voorhees JJ: Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vincenti MP, White LA, Schroen DJ, Benbow U and Brinckerhoff CE: Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryot Gene Expr. 6:391–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Westermarck J and Kähäri VM: Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 13:781–792. 1999.PubMed/NCBI
|
|
30
|
White LA and Brinckerhoff CE: Two activator protein-1 elements in the matrix metalloproteinase-1 promoter have different effects on transcription and bind Jun D, c-Fos, and Fra-2. Matrix Biol. 14:715–725. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
White LA, Maute C and Brinckerhoff CE: ETS sites in the promoters of the matrix metalloproteinases collagenase (MMP-1) and stromelysin (MMP-3) are auxiliary elements that regulate basal and phorbol-induced transcription. Connect Tissue Res. 36:321–335. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karin M, Liu Z and Zandi E: AP-1 function and regulation. Curr Opin Cell Biol. 9:240–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Angel P, Szabowski A and Schorpp-Kistner M: Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 20:2413–2423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim JY, Kim OK, Lee J, Lee MJ, Kang N and Hwang JK: Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr Res Pract. 8:398–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK and Yager DR: Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 6:127–134. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parrado J, Bougria M, Ayala A, Castaño A and Machado A: Effects of aging on the various steps of protein synthesis: fragmentation of elongation factor 2. Free Radic Biol Med. 26:362–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 138:1462–1470. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berneburg M and Krutmann J: Photoaging-associated large-scale deletions of mitochondrial DNA. Methods Enzymol. 319:366–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rossetti D, Kielmanowicz MG, Vigodman S, Hu YP, Chen N, Nkengne A, Oddos T, Fischer D, Seiberg M and Lin CB: A novel anti-ageing mechanism for retinol: induction of dermal elastin synthesis and elastin fibre formation. Int J Cosmet Sci. 33:62–69. 2011. View Article : Google Scholar
|
|
40
|
Hachiya A, Sriwiriyanont P, Fujimura T, Ohuchi A, Kitahara T, Takema Y, Kitzmiller WJ, Visscher MO, Tsuboi R and Boissy RE: Mechanistic effects of long-term ultraviolet B irradiation induce epidermal and dermal changes in human skin xenografts. Am J Pathol. 174:401–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sherratt MJ: Tissue elasticity and the ageing elastic fibre. Age (Dordr). 31:305–325. 2009. View Article : Google Scholar
|
|
42
|
Tracy LE, Minasian RA and Caterson EJ: Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care (New Rochelle). 5:119–136. 2016. View Article : Google Scholar
|
|
43
|
Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M: Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cho S, Won CH, Lee DH, Lee MJ, Lee S, So SH, Lee SK, Koo BS, Kim NM and Chung JH: Red ginseng root extract mixed with Torilus fructus and Corni fructus improves facial wrinkles and increases type I procollagen synthesis in human skin: a randomized, double-blind, placebo-controlled study. J Med Food. 12:1252–1259. 2009. View Article : Google Scholar
|
|
45
|
Manosroi A, Chutoprapat R, Abe M, Manosroi W and Manosroi J: Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm Biol. 50:208–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kligman LH and Kligman AM: The nature of photoaging: its prevention and repair. Photodermatol. 3:215–227. 1986.PubMed/NCBI
|
|
47
|
Hussain SH, Limthongkul B and Humphreys TR: The biomechanical properties of the skin. Dermatol Surg. 39:193–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roh E, Kim JE, Kwon JY, Park JS, Bode AM, Dong Z and Lee KW: Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit Rev Food Sci Nutr. Jun 26–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA and Voorhees JJ: Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 105:285–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fisher GJ and Voorhees JJ: Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 3:61–68. 1998.PubMed/NCBI
|





















