Introduction
Colorectal cancer (CRC) is a major cause of cancer
morbidity and mortality. Nearly 150,000 US residents are diagnosed
annually with CRC, and approximately one-third of patients with CRC
succumb to the disease (1). The
lifetime risk of CRC in the US is 6%, and the average age at
diagnosis is 66 years (2).
Primary CRC originates from epithelial cells that line the
gastrointestinal tract (3).
During progression to metastasis, cancer cells are thought to
acquire a mesenchymal phenotype, which allows them to leave the
site of the primary tumor, invade surrounding tissues, and migrate
to distant organs. After seeding, these cells switch back to an
epithelial phenotype and proliferate to form metastases (4). The processes by which cells switch
between the epithelial and mesenchymal phenotypes are known as
epithelial-to-mesenchymal transition (EMT) and its counterpart,
mesenchymal-to-epithelial transition (MET) (5). However, the molecular mechanisms
responsible for EMT in CRC are not yet fully understood.
The steroid hydroxylase cytochrome P450, family 7,
subfamily B, polypeptide 1 (CYP7B1), a member of the cytochrome
P450 enzyme family, has attracted increasing attention over the
years due to its multiple reported roles for key events in cellular
physiology (6–14). CYP7B1 is widely expressed in
tissues of human and other species and metabolizes several steroids
involved in hormonal signaling and other processes. Substrates for
CYP7B1 include 5a-androstane-3b, 17b-diol (3b-Adiol), an estrogen
receptor (ER) agonist and dehydroepiandrosterone (DHEA), an
essential precursor for androgens and estrogens (13–18). CYP7B1 expression is diminished in
ER+ tumors and is predictive of overall survival in
breast cancer (19,20). However, its role in CRC is not yet
fully understood.
MicroRNAs (miRNAs or miRs), are small non-coding
RNAs which are 21–25 nt in length, and are widely expressed in
eukaryotic cells, functioning as post-translational regulators
(21). Due to their wide variety
of target genes, miRNAs affect a number of biological pathways,
including cell proliferation, development and differentiation. The
deregulation of miRNAs facilitates cancer development by
upregulating oncogenes or silencing tumor suppressor genes
(22). miRNAs have been
demonstrated to regulate the expression levels of major
cancer-related genes and hence may be useful in the treatment of
cancer (23,24).
In this study, we found that miR-17 not only
promoted EMT, but also promoted the formation of a stem cell-like
population in colon cancer DLD1 cells. Our results revealed that
miR-17 degrdade CYP7B1 mRNA expression in DLD1 cells. In addition,
we found that the silencing of CYB7B1 promoted EMT and the
formation of a stem cell-like population in the colon cancer cells.
Thus, our findings suggest that miR-17 induces EMT consistent with
the cancer stem cell phenotype by regulating CYP7B1 expression in
colon cancer.
Materials and methods
Cell culture and tissues samples
DLD1 cells were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with fetal bovine serum (FBS) (both from HyClone, Ogden, UT, USA)
and penicillin-streptomycin. Normal and tumor tissues were obtained
from 6 patients were recruited from Shandong Provincial Qianfoshan
Hospital, Jinan, China. The tissues were obtained during colon
cancer surgery. Normal tissues were adjacent to the tumor tissues.
None of the patients received any anti-cancer treatment prior to
surgery. The use of human tissue samples was carried out in
accordance with internationally recognised guidelines as well as
local and national regulations. This study was approved by the
Ethics Committee of Shandong Provincial Qianfoshan Hospital and all
patients provided written informed consent prior to obtaining the
samples.
Plasmids and transfection
The shRNA plasmids were obtained from Tiangen
(Beijing, China). Both scramble control sequence and interference
sequence (shRNA) of CYP7B1 were designed and synthesized to build
the recombinant plasmids; Pre-miR-17 and control miR (purchased
from Ambion, Inc. Austin, TX, USA). The cells were cultured in
serum-free medium without antibiotics and then separately
transfected with shCYP7B1/scramble plasmids or Pre-miR-17/control
miR using transfection reagent (Lipofectamine 2000; Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's
instructions.
Western blot analysis
The tissues or cells were homogenized in RIPA lysis
buffer containing PMSF and centrifuged at 200 × g for 10 min.
Protein lysates were separated by electrophoresis and transferred
onto PVDF membranes, and the blots were blocked with 5% non-fat
milk for 1 h and incubated overnight at 4°C with primary antibodies
against CYP7B1 (ab175889; 1:500), vimentin (ab92547; 1:500), SNAIL
(ab82846; 1:500), transforming growth factor beta 1 (TGFB1;
ab92486; 1:500), zinc finger E-box binding homeobox (ZEB)1
(ab203829; 1:500), ZEB2 (ab138222; 1:500), Twist (ab50581; 1:500),
β-catenin (ab32572; 1:500), Notch1 (ab8925; 1:500), β-actin
(ab8227; 1:500) and CD44 (ab157107; 1:500) (all from Abcam,
Cambridge, MA, USA). After washing, the blots were incubated with
secondary antibodies (anti-rabbit secondary antibodies; ab6721;
1:10,000; Abcam). The protein bands were visualized by
chemiluminescence and exposed to the Odyssey™ Infrared Imaging
system (Gene Company, Lincoln, NE, USA).
Sphere growth analysis
The DLD1 cells transfected with shCYP7B1/scramble
plasmids (1×103) in serum-free DMEM/1 mM Na-pyruvate
were seeded on 0.5% agar pre-coated 6-well plates. After 1 week,
half the medium was exchanged with serum-free medium every third
day. Single spheres were selected and counted under a
stereomicroscope (Olympus, Tokyo, Japan).
In vitro migration and invasion
assays
The DLD1 cells transfected as indicated above
(1×105) were placed into the upper compartment of the
Transwell insert (Costar, Cambridge, MA, USA). For invasion assay,
the two compartments were separated by a porous filter (8 µm
pore) coated with Matrigel (BD Biosciences, San Jose, CA, USA). For
migration assay, the filter membranes was not coated with Matrigel.
The chambers were incubated for 24 h at 37°C, and the filters were
then fixed in methanol and stained with hematoxylin. Quantification
of the migration and invasion assays were performed by counting the
number of cells at the lower surface of the filters.
Bioinformatics analysis
Potential targets of miRNAs were identified by a
combined approach based on the commonly used web tool for
bioinformatics algorithms miRanda (http://www.microrna.org/microrna/home.do).
Quantitative (real-time) polymerase chain
reaction (qPCR) for miR-17
qPCR for miR-17 was performed using the total RNA
kit I (Omega Bio-Tek, Norcross, GA, USA) and the miRcute miRNA qPCR
detection kit (Tiangen). U6 miRNA was used as a housekeeping
control. Small RNA was purified and enriched with the miRcute miRNA
Isolation kit (Tiangen). miRNAs were prolonged by Escherichia
coli poly(A) polymerase, and reverse transcription was
performed with the miRcute miRNA First-Strand cDNA Synthesis kit
and real-time PCR with the miRcute miRNA qPCR detection kit
(Tiangen). Forward primers and reverse primers were provided by
Tiangen. The universal reverse primer was provided in the miRcute
miRNA qPCR detection kit.
Reverse transcription-quantitative PCR
(RT-qPCR) for CYP7B1
Total RNA was isolated from cells or tissues using
TRIzol reagent (Invitrogen). cDNA was synthesized from 1 µg
of total RNA in a 20 µl reverse transcription (RT) system
followed by PCR amplification in a 50 µl PCR system
performed using an RT-PCR kit (Promega, Madison, WI, USA). The
housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
was used as an RNA loading control. The PCR primer sequences are as
follows: CYP7B1 forward, 5′-CAATCCATGCAGTCACCTTC-3′ and reverse,
5′-TGCCTAGAGAAAAACAGAAAGACA-3′; and GAPDH forward,
5′-ATTCAACGGCACAGTCAAGG-3′ and reverse, 5′-GCAGAAGGGGCGGAGATGA-3′.
PCR was conducted according to the manufacturer's instructions and
the PCR products were analyzed by agarose gel electrophoresis. Gels
were photographed and the densities of the bands were determined
with a computerized image analysis system (Alpha Innotech, San
Leandro, CA, USA). The area of each band was calculated as the
integrated density value (IDV). Real-time PCR for CYP7B1 was
performed with a Power SYBR-Green PCR Master Mix (Applied
Biosystems, Carlsbad, CA, USA) according to the manufacturer's
instructions.
Immunofluorescence staining
The cells transfected as indicated above were fixed
with paraformaldehyde and permeabilized in Triton X-100 (Beijing
Solarbio Biological Technology Co., Ltd., Beijing, China). After
blocking, anti-CYP7B1 antibody (ab175889; Abcam) was added followed
by incubation overnight at 4°C. After washing with
phosphate-buffered saline (PBS), fluorescence-conjugated
anti-rabbit secondary antibodies (ab6721; 1:10,000; Abcam) were
added, and the coverslips were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen-Molecular Probes,
Eugene, OR, USA) for visualization of the nuclei. Microscopic
analysis was observed under a Zeiss LSM-510 confocal microscope
(Carl Zeiss, Jena, Germany).
Wound healing assay
The cells transfected as indicated above were seeded
into a 24-well plate in DMEM containing 10% FBS and cultured to 90%
confluence. The cell monolayer was subjected to a mechanical
scratch wound using a sterile pipette tip. After washing with PBS,
the cells were further incubated in DMEM without FBS for different
periods of time. Digitized images of the wound area were captured
using a IX71 fluorescence microscope (Olympus).
Statistical analysis
The results are shown as the means ± SEM. The
Student's t-test was used to perform comparisons between two
groups. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Silencing of CYP7B1 promotes EMT in colon
cancer cells
In an attempt to examine CYP7B1 expression between
colon cancer tissues and adjacent normal tissues, we performed
western blot analysis using the cancer tissues and normal tissues.
Protein was isolated from 6 pairs of colon cancer tissues and
normal tissues (patient nos. 1–6). We found that CYP7B1 protein
expression was significantly decreased in the cancer tissues
compared with the adjacent normal tissues (Fig. 1A). This suggests that CYP7B1 may
be a tumor suppressor gene in colon cancer.
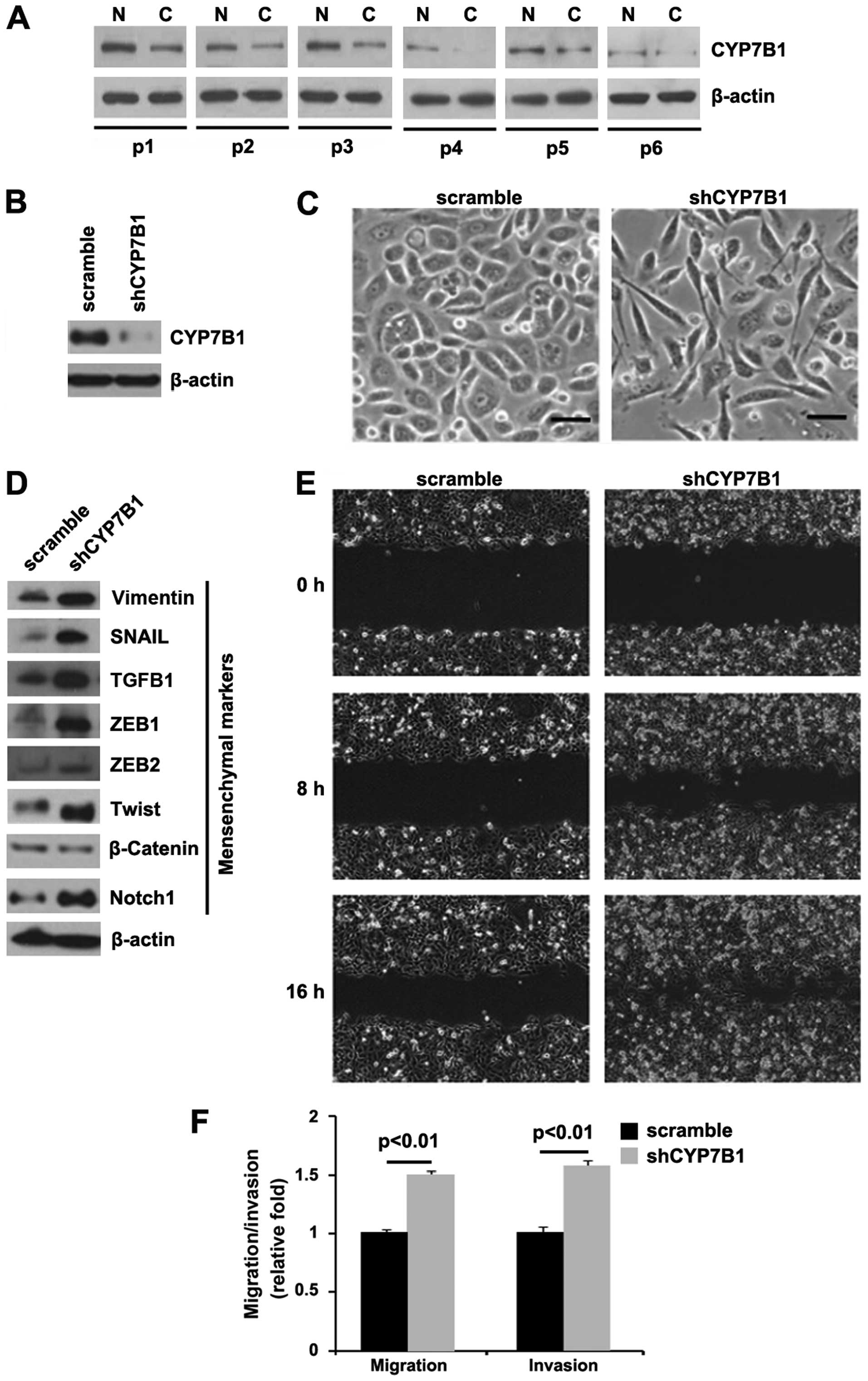 | Figure 1Silencing of CYP7B1 promotes
epithelial-mesenchymal transition (EMT) in colon cancer DLD1 cells.
(A) Western blot analysis for CYP7B1 in colon cancer tissues (C)
and adjacent normal tissues (N). Patients were numbered no. 1–6.
All the 6 patients were diagnosed with colon cancer. β-actin was
used as a loading control, n=6. (B) Western blot analysis for
CYP7B1 in DLD1 cells transfected with shCYP7B1 plasmid or the
scramble plasmid. β-actin was used as a loading control, n=3
experiments. (C) DLD1 cells were transfected as indicated. Cells
were then photographed following transfection, n=3 experiments. (D)
Western blot analysis for vimentin, SNAIL, TGFB1, ZEB1, ZEB2,
Twist, β-catenin and Notch1 in DLD1 cells transfected with shCYP7B1
plasmid or scramble plasmid. β-actin was used as a loading control,
n=3 experiments. (E) Wound-healing assays for DLD1 cells
transfected with shCYP7B1 plasmid or scramble plasmid. The cell
layer was photographed following transfection, n=3 experiments. (F)
Invasion and migration assays for DLD1 cells transfected as
indicated, n=3 experiments. |
In order to determine the role of CYP7B1 in colon
cancer, we transfected the DLD1 cells with shCYP7B1 plasmid and
western blot analysis was then performed. We found that CYP7B1
protein expression was significantly decreased in the cells
transfected with the shCYP7B1 plasmid (Fig. 1B) and the silencing of CYP7B1 led
to significant changes in DLD1 cell morphology (EMT, change in
phenotype from a cobblestone-like to a spindle-like morphology)
(Fig. 1C).
To further verify that the changes in cell
morphology were caused by EMT, the expression levels of mesenchymal
markers were compared in the DLD1 cells transfected with the
shCYP7B1 plasmid and the cells transfected with the scramble
plasmid. The results revealed that the expression of the
mesenchymal markers (vimentin, SNAIL, TGFB1, ZEB1, ZEB2, Twist and
Notch1) was induced by the silencing of CYP7B1 in the DLD1 cells
(Fig. 1D).
EMT can result in increased cell invasion and
migration (25–27). Thus, we hypothesized that shCYP7B1
may also affect the invasion and migration ability of the DLD1
cells. To confirm this hypothesis, we performed cell invasion and
migration assays, and would healing assay. We found that the
silencing of CYP7B1 enhanced the migration (Fig. 1E and F) and invasion (Fig. 1F) ability of the cells.
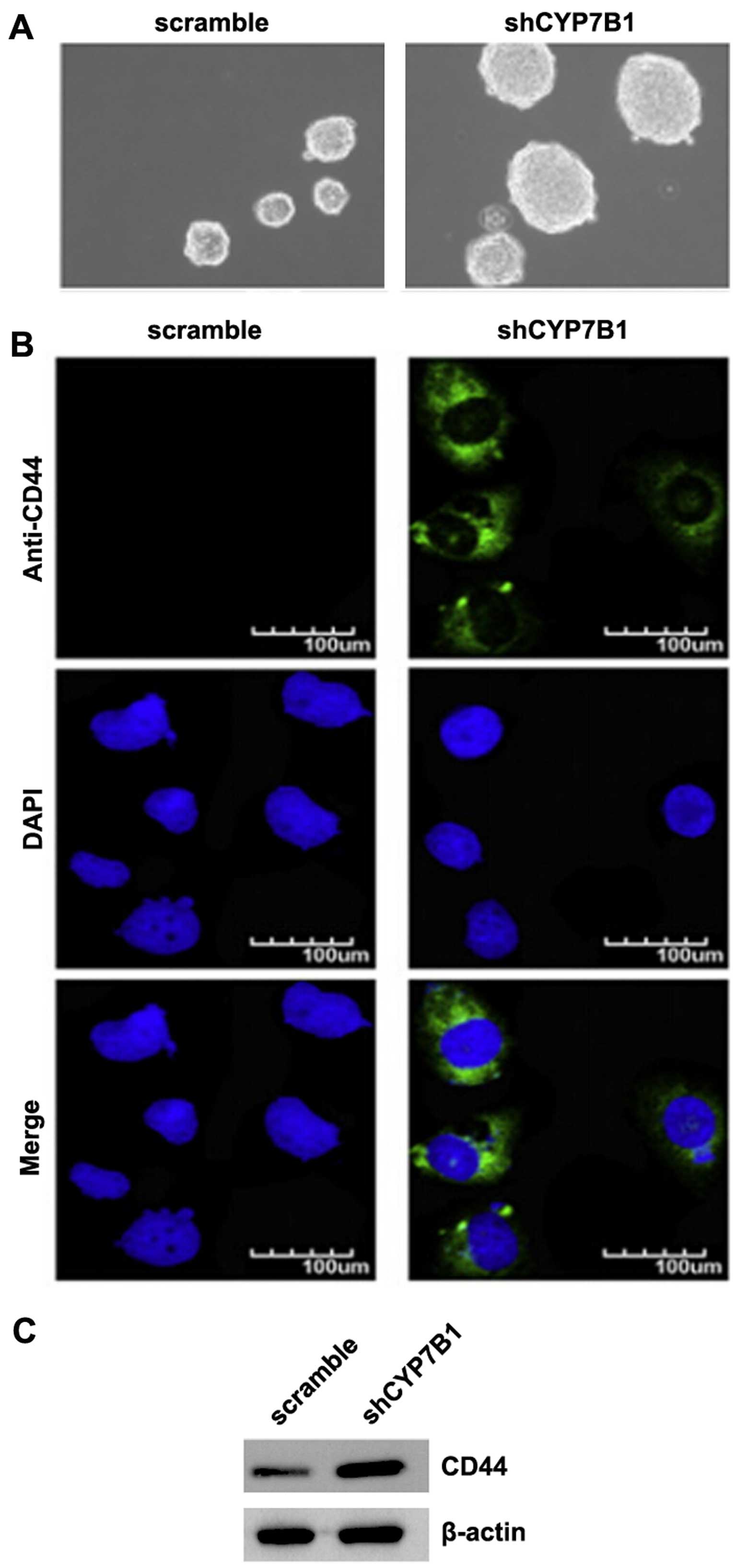
Silencing of CYP7B1 promotes the
formation of a stem cell-like population in colon cancer cells
EMT not only confers tumor cells with a distinct
advantage for metastatic dissemination, but it also provides those
cells with cancer stem cell-like characters for proliferation and
drug resistance (28–31). To determine whether colon cancer
cells with an EMT phenotype have stem-like cell characteristics,
sphere forming assay was conducted to assess the capacity of cancer
stem cells (CSCs) or CSC-like cell self-renewal in this study. We
found that the formation of spheres was increased by the silencing
of CYP7B1 in the DLD1 cells (Fig.
2A). CD44 is a robust marker and is of functional importance
for colorectal CSCs for cancer initiation (32). We also performed
immunofluorescence staining to determine whether CD44 was affected
by the silencing of CYP7B1 in the cells. The results revealed that
CD44 protein was significantly increased by the silencing of CYP7B1
in the DLD1 cells (Fig. 2B).
Consistent with the results of immunofluorescence staining, the
results of western blot analysis demonstrated that CD44 protein
expression was increased by the silencing of CYP7B1 in the cells
(Fig. 2C).
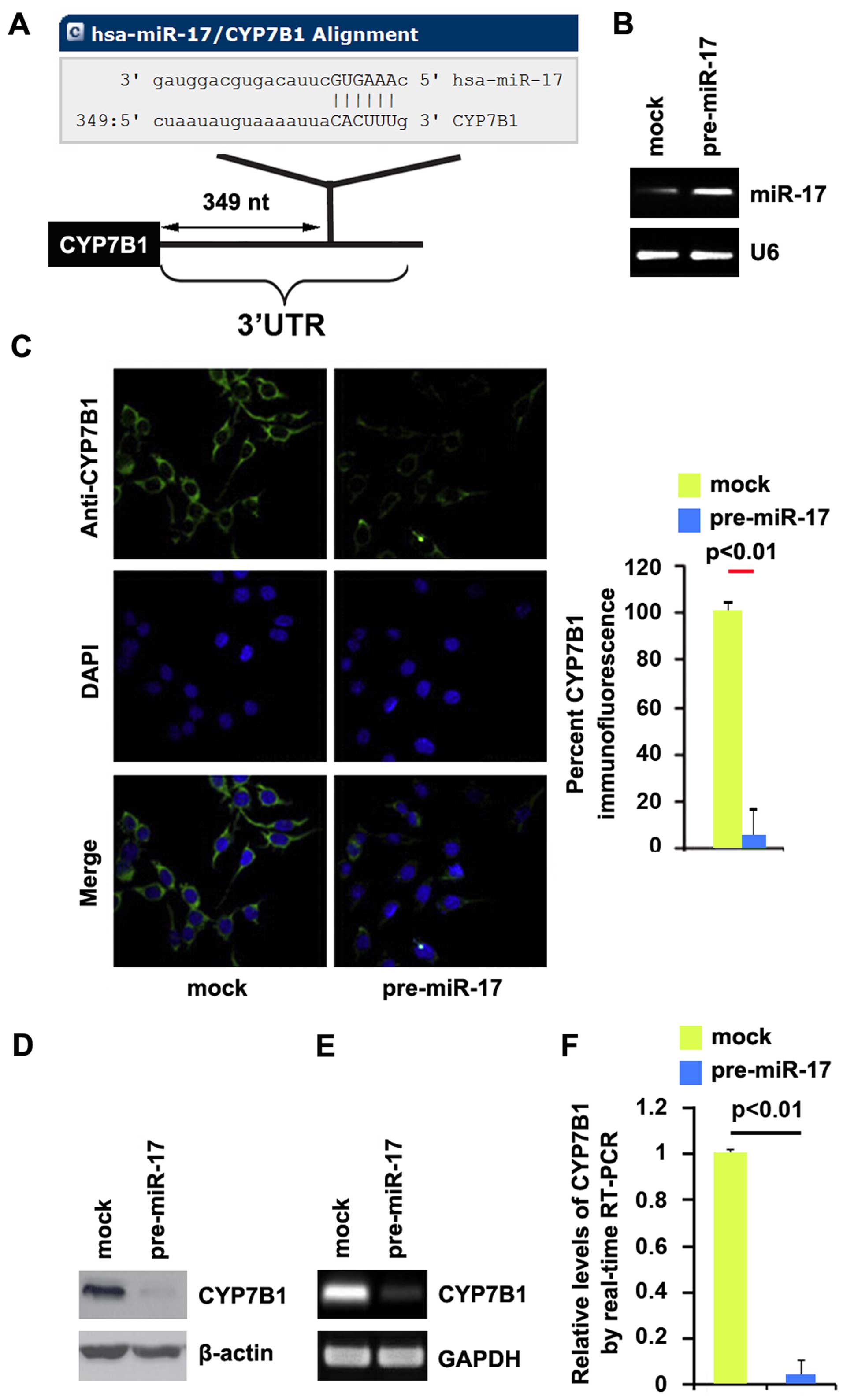
miR-17 degrades CYP7B1 in colon cancer
cells
Having demonstrated that the silencing of CYP7B1
promotes EMT and the formation of a stem cell-like population in
colon cancer cells, we then wished to determine the mechanisms
regulating CYP7B1 expression in the disease. miRNAs are small
regulatory non-coding RNAs of 21–25 nucleotides in length. miRNAs
are generated from their precursor transcripts by a series of
processing steps. Mature miRNAs mediate mRNA degradation or
suppress mRNA translation by binding to the 3′ untranslated region
(3′UTR) of target mRNAs (33). To
further confirm whether CYP7B1 can be regulated by miRNAs, we used
the commonly used prediction algorithm, miRanda (http://www.microrna.org/microrna/home.do), to analyze
the 3′UTR of CYP7B1. A dozen miRNAs were found by the algorithm.
However, we focused on miR-17, as miR-17 expression has been
confirmed to be significantly higher in CRC tissues than in normal
tissues (34). However, its role
in CRC has not yet been fully elucidated. The target sites on the
3′UTR of CYP7B1 are shown in Fig.
3A. We reasoned that miR-17 may downregulate CYP7B1 expression
by targeting its 3′UTR in colon cancer. In an attempt to determine
the role of miR-17 in regulating CYP7B1 expression in colon cancer
cells, the DLD1 cells were transfected with pre-miR-17 or control
miR. Following transfection, miR-17 expression was detected by qPCR
and the results revealed that miR-17 expression was significantly
increased by transfection of the cells with pre-miR-17 (Fig. 3B). Subsequently, we performed
immunofluorescence staining in the DLD1 cells transfected with
pre-miR-17 or control miR. The results revealed that CYP7B1 protein
expression was evidently suppressed in the cells transfected with
pre-miR-17 (Fig. 3C). We then
performed RT-qPCR and western blot analysis to detect CYP7B1
expression in the DLD1 cells transfected with pre-miR-17 or control
miR. The results revealed that the CYP7B1 protein (Fig. 3D) and mRNA (Fig. 3E) expression levels were
significantly downregulated in the cells transfected with
pre-miR-17. Consistent with the results of RT-qPCR, qPCR
demonstrated that CYP7B1 mRNA expression was decreased in the DLD1
cells transfected with pre-miR-17, compared with the control
miR-transfected cells (Fig. 3F).
All the data demonstrated that miR-17 degraded CYP7B1 in colon
cancer cells.
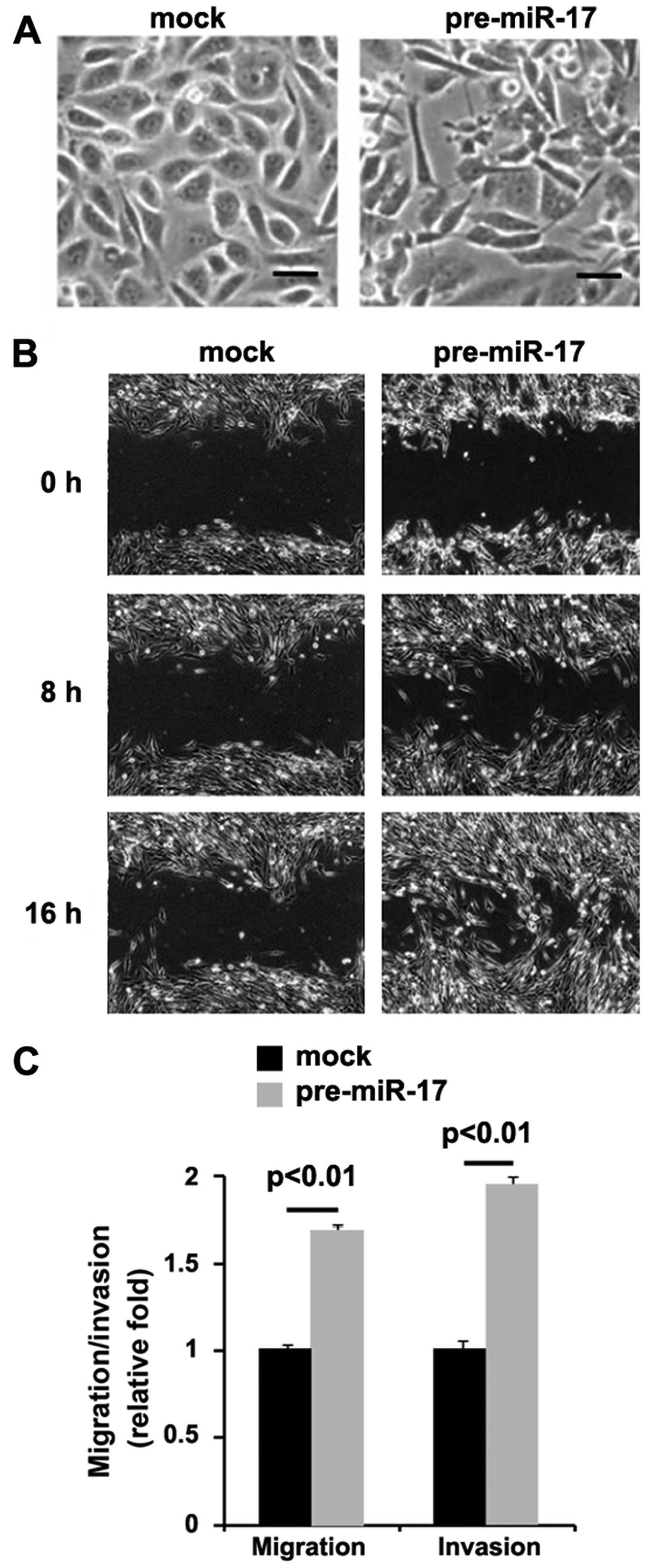
miR-17 promotes EMT in colon cancer
cells
In order to determine the role of miR-17 in colon
cancer, we transfected the DLD1 cells with pre-miR-17. We found
that the overexpression of miR-17 led to significant changes in
DLD1 cell morphology (EMT, change in phenotype from a
cobblestone-like to a spindle-like morphology) (Fig. 4A). To further verify that the
changes in cell morphology were caused by EMT, we performed
invasion and migration assays, and would healing assay. We found
that the overexpression of miR-17 enhanced the migration (Fig. 4B and C) and invasion (Fig. 4C) ability of the cells.
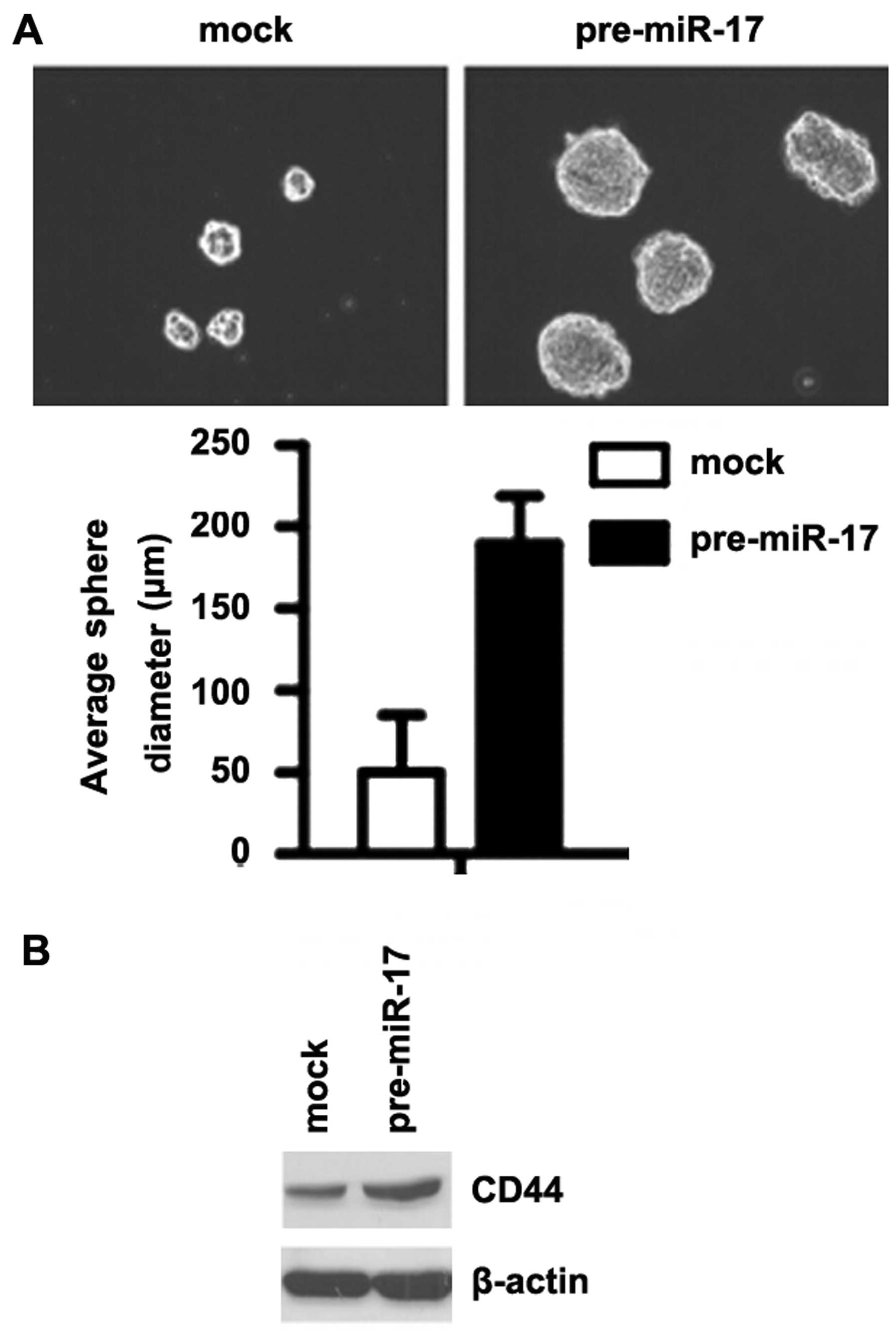
miR-17 promotes the formation of a stem
cell-like population in colon cancer cells
To determine whether miR-17 promotes the development
of stem-like cell characteristics, we performed sphere-forming
assay to assess the capacity of CSC or CSC-like cell self-renewal
in this study. We found that formation of spheres was increased by
the overexpression of miR-17 in the DLD1 cells (Fig. 5A). We also performed western blot
analysis to determine whether CD44 is affected by miR-17 in the
cells. The results revealed that CD44 protein expression was
significantly increased by the overexpression of miR-17 in the DLD1
cells (Fig. 5B).
Discussion
Mounting evidence suggests that the deregulation of
miRNAs is involved in colon cancer pathogenesis, microsatellite
stability status, therapeutic outcome and patient prognosis
(35–37). miR-17 expression has been
confirmed to be significantly higher in CRC tissues than in normal
tissues (34,38). However, its role in CRC has not
yet been fully elucidated. In line with previous reports, we found
that miR-17 not only promoted the EMT phenotype, but also promoted
the formation of a stem cell-like population in colon cancer DLD1
cells. The results further confirmed that miR-17 is an oncogene in
colon cancer. There is evidence to support that high levels of
miR-17-92 cluster inhibit tumor growth and metastasis in
vivo (39). However, Yu et
al reported that the miR-17-92 cluster and its paralogs were
significantly elevated in patients with colon cancer, and the
increased expression of miR-17 was associated with a poor survival
(40), further supporting our
findings that miR-17 is an oncogene in colon cancer.
Previous studies have demonstrated that CYP7B1 plays
an important role in cancer development and progression (20,41,42). The overexpression of CYP7B1 has
been detected in prostatic adenocarcinoma (41). However, CYP7B1 expression is
dimished in ER+ tumors and is predictive of a poor
overall survival in breast cancer (20). We found that CYP7B1 protein
expression was decreased in colon cancer tissues and that the
silencing of CYP7B1 promoted the EMT phenotype and the formation of
a stem cell-like population. In addition, CYP7B1 mRNA was degraded
by miR-17 in colon cancer cells. We aim to further determine
whether the expression of miR-17 inversely correlates with CYP7B1
expression in colorectal tumors in future studies.
In conclusion, miR-17-mediated CYP7B1 regulation in
colon cancer cells demonstrated in this study has potential basic
and clinical implications. On the one hand, miR-17 is a powerful
oncogene by promoting EMT and the formation of a stem cell-like
population in human colon cancer cells and the pharmacological
suppression of miR-17 may represent a promising therapeutic
strategy. On the other hand, CYP7B1 is a tumor suppressor gene and
the overexpression of miR-17 can downregulate its expression. Our
data lay the foundations for future research into the role of
CYP7B1 in CRC and in other types of cancer.
Acknowledgments
This study was supported by grants from the Shandong
Natural Science Foundation (no. ZR2011HQ054).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hawk ET and Levin B: Colorectal cancer
prevention. J Clin Oncol. 23:378–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Z, Martin KO, Javitt NB and Chiang JY:
Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs
and gene CYP7B1. J Lipid Res. 40:2195–2203. 1999.PubMed/NCBI
|
|
7
|
Sulcová J and Stárka L: Characterisation
of microsomal dehydroepiandrosterone 7-hydroxylase from rat liver.
Steroids. 12:113–126. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Norlin M and Wikvall K: Biochemical
characterization of the 7alpha-hydroxylase activities towards
27-hydroxycholesterol and dehydroepiandrosterone in pig liver
microsomes. Biochim Biophys Acta. 1390:269–281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shoda J, Toll A, Axelson M, Pieper F,
Wikvall K and Sjövall J: Formation of 7 alpha- and 7
beta-hydroxylated bile acid precursors from 27-hydroxycholesterol
in human liver microsomes and mitochondria. Hepatology. 17:395–403.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weihua Z, Lathe R, Warner M and Gustafsson
JA: An endocrine pathway in the prostate, ERbeta, AR,
5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate
growth. Proc Natl Acad Sci USA. 99:13589–13594. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Norlin M: Expression of key enzymes in
bile acid biosynthesis during development: CYP7B1-mediated
activities show tissue-specific differences. J Lipid Res.
43:721–731. 2002.PubMed/NCBI
|
|
12
|
Martin C, Ross M, Chapman KE, Andrew R,
Bollina P, Seckl JR and Habib FK: CYP7B generates a selective
estrogen receptor beta agonist in human prostate. J Clin Endocrinol
Metab. 89:2928–2935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rose KA, Stapleton G, Dott K, Kieny MP,
Best R, Schwarz M, Russell DW, Björkhem I, Seckl J and Lathe R:
Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of
neurosteroids 7alpha-hydroxy dehydroepiandrosterone and
7alpha-hydroxy pregnenolone. Proc Natl Acad Sci USA. 94:4925–4930.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dulos J, Verbraak E, Bagchus WM, Boots AM
and Kaptein A: Severity of murine collagen-induced arthritis
correlates with increased CYP7B activity: Enhancement of
dehydroepiandrosterone metabolism by interleukin-1beta. Arthritis
Rheum. 50:3346–3353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rainey WE, Rehman KS and Carr BR: The
human fetal adrenal: Making adrenal androgens for placental
estrogens. Semin Reprod Med. 22:327–336. 2004. View Article : Google Scholar
|
|
16
|
Kim SB, Hill M, Kwak YT, Hampl R, Jo DH
and Morfin R: Neurosteroids: Cerebrospinal fluid levels for
Alzheimer's disease and vascular dementia diagnostics. J Clin
Endocrinol Metab. 88:5199–5206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katyare SS, Modi HR and Patel MA:
Dehydroepiandrosterone treatment alters lipid/phospholipid profiles
of rat brain and liver mitochondria. Curr Neurovasc Res. 3:273–279.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer D and Forstner K: Impact of
dehydroepiandrosterone on hepatocarcinogenesis in the rat (Review).
Int J Oncol. 25:1021–1030. 2004.PubMed/NCBI
|
|
19
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q, Ishikawa T, Sirianni R, Tang H,
McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA,
et al: 27-Hydroxycholesterol promotes cell-autonomous, ER-positive
breast cancer growth. Cell Rep. 5:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson DM, Anderson KM, Chang CL,
Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM,
Liou J, Bassel-Duby R and Olson EN: A micropeptide encoded by a
putative long noncoding RNA regulates muscle performance. Cell.
160:595–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi JJ: New hope for a microRNA therapy
for liver cancer. Cell. 137:990–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kota J, Chivukula RR, O'Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng
GQ, Wan XX, He QY, Li JH, Qu JQ, et al: Activation of EGFR promotes
squamous carcinoma SCC10A cell migration and invasion via inducing
EMT-like phenotype change and MMP-9-mediated degradation of
E-cadherin. J Cell Biochem. 112:2508–2517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung H, Lee KP, Park SJ, Park JH, Jang YS,
Choi SY, Jung JG, Jo K, Park DY, Yoon JH, et al: TMPRSS4 promotes
invasion, migration and metastasis of human tumor cells by
facilitating an epithelial-mesenchymal transition. Oncogene.
27:2635–2647. 2008. View Article : Google Scholar
|
|
27
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X,
Jin H, Cahuzac N, Mehrpour M, Lu Y and Chen Q: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Diosdado B, van de Wiel MA, Terhaar Sive
Droste JS, Mongera S, Postma C, Meijerink WJ, Carvalho B and Meijer
GA: MiR-17-92 cluster is associated with 13q gain and c-myc
expression during colorectal adenoma to adenocarcinoma progression.
Br J Cancer. 101:707–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Monzo M, Navarro A, Bandres E, Artells R,
Moreno I, Gel B, Ibeas R, Moreno J, Martinez F, Diaz T, et al:
Overlapping expression of microRNAs in human embryonic colon and
colorectal cancer. Cell Res. 18:823–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lanza G, Ferracin M, Gafà R, Veronese A,
Spizzo R, Pichiorri F, Liu CG, Calin GA, Croce CM and Negrini M:
mRNA/microRNA gene expression profile in microsatellite unstable
colorectal cancer. Mol Cancer. 6:542007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
39
|
Jiang H, Wang P, Wang Q, Wang B, Mu J,
Zhuang X, Zhang L, Yan J, Miller D and Zhang HG: Quantitatively
controlling expression of miR-17-92 determines colon tumor
progression in a mouse tumor model. Am J Pathol. 184:1355–1368.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu G, Tang JQ, Tian ML, Li H, Wang X, Wu
T, Zhu J, Huang SJ and Wan YL: Prognostic values of the miR-17-92
cluster and its paralogs in colon cancer. J Surg Oncol.
106:232–237. 2012. View Article : Google Scholar
|
|
41
|
Olsson M, Gustafsson O, Skogastierna C,
Tolf A, Rietz BD, Morfin R, Rane A and Ekström L: Regulation and
expression of human CYP7B1 in prostate: Overexpression of CYP7B1
during progression of prostatic adenocarcinoma. Prostate.
67:1439–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang W and Norlin M: Regulation of steroid
hydroxylase CYP7B1 by androgens and estrogens in prostate cancer
LNCaP cells. Biochem Biophys Res Commun. 344:540–546. 2006.
View Article : Google Scholar : PubMed/NCBI
|