Introduction
Integrins are the main receptors that connect the
cytoskeleton to the extracellular matrix (ECM), and an intimate
relationship exists between integrins and mechanical strain.
Integrins act as mechanoreceptors in bone and transduce mechanical
signals into biochemical responses within bone cells (1,2).
Integrin-β1 has been reported to be expressed on the
surface of osteoblasts (3).
Mechanical strain may cause the redistribution of integrin-β1 on
the osteosarcoma cell surface, and integrin-β1 antibodies inhibit
the activity of mechanosensitive ion channels (4). The expression of an
osteoblast-specific dominant negative form of integrin-β1 resulted
in reduced bone mass with increased cortical porosity in the long
bones of mice (5). Fluid flow
shear stress or mechanical tensile strain were demonstrated to
upregulate integrin-β1 expression in osteoblasts, and induce cell
proliferation or differentiation (6–8).
These studies demonstrated that integrin-β1 mediates the impact of
mechanical strain on the proliferation and the differentiation of
osteoblasts. A recent study of ours revealed that integrin-β1
mediates osteoblastic differentiation and ECM formation which was
enhanced by mechanical tensile strain (9).
It has been previously demonstrated by researchers
that Wnt/β-catenin signaling is required for mechanotransduction in
bone (10). It has been found
that mechanical strain and fluid shear stress induce the nuclear
translocation of β-catenin in osteoblasts and periodontal ligament
cells, and activate the β-catenin signal pathway (11–13). In the developing chick embryo,
integrin-β1 was demonstrated to regulate cell shape and tissue
morphogenesis indirectly by regulating Wnt and Notch signaling
(14). Integrin-α3β1, acting in
coordination with c-Met, regulated the expression of Wnt 7b
transcripts expressed in developing papilla (15). Thus, we hypothesized that
integrin-β1 regulated Wnt/β-catenin signaling in response to the
mechanical stimulation of osteoblasts.
In the present study, we aimed to examine this
hypothesis by stimulating MC3T3-E1 cells with mechanical strain in
order to explore the involvement of integrin-β1 in β-catenin
signaling in response to the mechanical stimulation of
osteoblasts.
Materials and methods
Cell culture
MC3T3-E1 cells were provided by the School of Basic
Medicine of Peking Union Medical College (Beijing, China). The
MC3T3-E1 mouse pre-osteoblastic cell line has been shown to
differentiate into osteoblasts and osteocytes (16,17). The cells were maintained in
α-minimal essential medium (α-MEM; Invitrogen, San Diego, CA, USA)
containing 10% fetal calf serum and 1% penicillin-streptomycin.
Application of mechanical strain to
cultured cells
Mechanical tensile strain was generated by a
specially designed four-point bending device (provided by the
Institute of Medical Equipment, Academy of Military Medical
Sciences, Tianjin, China) as previously described (18,19). The four-point bending device has
been shown to produce homogenous, predominantly uniaxial strains of
the cell culture substrate so that every cell is subjected to the
same deformation (20,21). The cells were seeded at a density
of 2×104/cm2 in the cell culture dishes and
cultivated until they reached 80% confluence. For 1 h/day, the cell
cultures were subjected to mechanical strain of 2,500 microstrain
(µε) at 0.5 Hz for 3 days. Unstrained (control) cultures
were incubated under the same conditions for the maximum period of
mechanical strain application.
RNA interference (RNAi) targeted against
integrin-β1
The specific small interfering RNA (siRNA) targeting
mouse integrin-β1 was purchased from Invitrogen (Table I). At 60–70% confluence, the
MC3T3-E1 cells were transfected with integrin-β1 Stealth siRNA
(si-Itgβ1) or negative control siRNA using Lipofectamine 2000
(Invitrogen), according to the manufacturer's instructions.
Mechanical strain was applied 48 h after transfection.
 | Table IsiRNA sequences of integrin-β1. |
Table I
siRNA sequences of integrin-β1.
| Description | Type | Sequence | Quantity |
|---|
|
Itgβ1-MSS205553 | RNA |
UAGAAAUGUUGGAACACUUUCGUCC | 10 nmol |
| RNA |
GGACGAAAGUGUUCCAACAUUUCUA | 10 nmol |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
LS reagent (Invitrogen) according to the manufacturer's
instructions. One microgram of total RNA was reverse transcribed to
complementary DNA (cDNA) using the SuperScript III reverse
transcriptase kit (Invitrogen). qPCR was performed with an ABI
StepOne Real-Time PCR machine in a 48-well format using the Fast
SYBR-Green Master Mix kit (both from Applied Biosystems, Foster
City, CA, USA). The primer sequences for the PCR reactions are
listed in Table II. Triplicate
samples were used for these experiments. The amplification reaction
included a denaturation step at 94°C for 3 min followed by 40
cycles of 94°C for 15 sec, and annealing and extension at each
annealing temperature for 30 sec. The PCR products were normalized
for the amount of GAPDH in the same sample, which was also
standardized on a dilution curve from the sample.
 | Table IIPrimer sequences used in RT-qPCR. |
Table II
Primer sequences used in RT-qPCR.
| Gene | | Primer sequences
(5′-3′) |
|---|
| Itgβ1 | Forward: |
GCAACGCATATCTGGAAACT |
| Reverse: |
CAAAGTGAAACCCAGCATCC |
| Runx2 | Forward: |
AGTAGCCAGGTTCAACGAT |
| Reverse: |
GGAGGATTTGTGAAGACTGTT |
| OCN | Forward: |
AGTCTGACAAAGCCTTCA |
| Reverse: |
AAGCAGGGTTAAGCTCACA |
| GAPDH | Forward: |
TGCACCACCAACTGCTTAGC |
| Reverse: |
GGCATGGACTGTGGTCATGAG |
Western blot analysis of integrin-β1,
phosphorylated (p-) glycogen synthase kinase-3β (GSK-3β) and
β-catenin
Following trypsinization and centrifugation, the
cell lysates were harvested with RIPA lysis medium containing
protease inhibitors (Tianjin Weike Biotechnology Co., Ltd., Tianjin
China). The protein content of the cell lysates was quantified
using the Bradford method. Equal amounts of protein were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electrotransferred onto polyvinylidene difluoride
(PVDF) membranes (Millipore, Bedford, MA, USA). After blocking with
5% skim milk (Solarbio, Beijing, China), the membranes were
incubated overnight with the primary antibodies [integrin-β1
(sc-374430; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
p-GSK-3β (sc-11757; Santa Cruz Biotechnology, Inc.), β-catenin
(BM1575; Wuhan Boster Bio-engineering Co., Ltd., Wuhan, China], and
then incubated with secondary antibody conjugated with horseradish
peroxidase. The immunoreactive bands were visualized using an
Enhanced Chemiluminescence Detection kit (Santa Cruz Biotechnology,
Inc.). The optical density of the protein bands was determined
using a Gel Doc 2000 system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The expression of GAPDH was used as a loading control and
the data were normalized against those of the corresponding GAPDH.
The results are expressed relative to the control.
Determination of alkaline phosphatase
(ALP) activity
The cells were harvested and lysed by brief
sonication in lysis buffer (10 mmol/l HEPES, 250 mmol/l sucrose, 5
mmol/l Tris-HCl, 0.1% Triton X-100, pH 7.5). ALP activity in the
cellular fraction was measured using a fluorometric detection kit
(Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China)
according to the manufacturer's instructions. The ALP activity of
each sample was normalized to the protein concentration.
Fluorescent immunolocalisation of
β-catenin
For this experiment, the cells were fixed in 4%
paraformaldehyde for 10 min, and incubated in phosphate-buffered
saline (PBS) containing 10% fetal calf serum/0.1% Triton X-100
(v/v) for 20 min to block non-specific binding and to facilitate
access to intracellular epitopes. Following incubation overnight at
4°C with β-catenin antibody (1:500 dilution; Santa Cruz
Biotechnology, Inc.), the cells were incubated with FITC-conjugated
goat anti-rabbit secondary antibody (1:100; Sigma-Aldrich, St.
Louis, MO, USA) for 60 min in the dark at room temperature. The
cells were mounted in PBS containing 100 µg propidium iodide
(PI) to stain the nuclei. The cells were washed three times in PBS
following each incubation. Images were captured with a laser
scanning confocal microscope (LSCM; FV500; Olympus, Tokyo, Japan),
and analyzed using Image-Pro Plus 6.2 software (Media Cybernetics
Inc., Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
for bone morphogenetic proteins (BMPs)
BMP ELISA kits (Wuhan Boster Bio-engineering Co.,
Ltd.) were used in order to detect BMP-2 and BMP-4 levels in the
culture medium. The culture medium was collected following exposure
to mechanical strain, and the samples were placed into microtiter
plates coated with BMP-2 or BMP-4 antibody and incubated for 1.5 h
at room temperature. After washing, horseradish
peroxidase-conjugated streptavidin was added to the plates to
catalyze the conversion of a chromogenic substrate
(tetramethylbenzidine) to a colored solution. The absorbance was
measured at 450 nm on a Microplate Reader (Model 680; Bio-Rad
Laboratories, Inc.). The results are presented as the percentage of
activity change, compared with the control.
Statistical analysis
The data are presented as the means ± standard
deviation, and analyzed by SPSS 10.0 software (SPSS, Inc., Chicago,
IL, USA) using one-way analysis of variance (ANOVA). A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
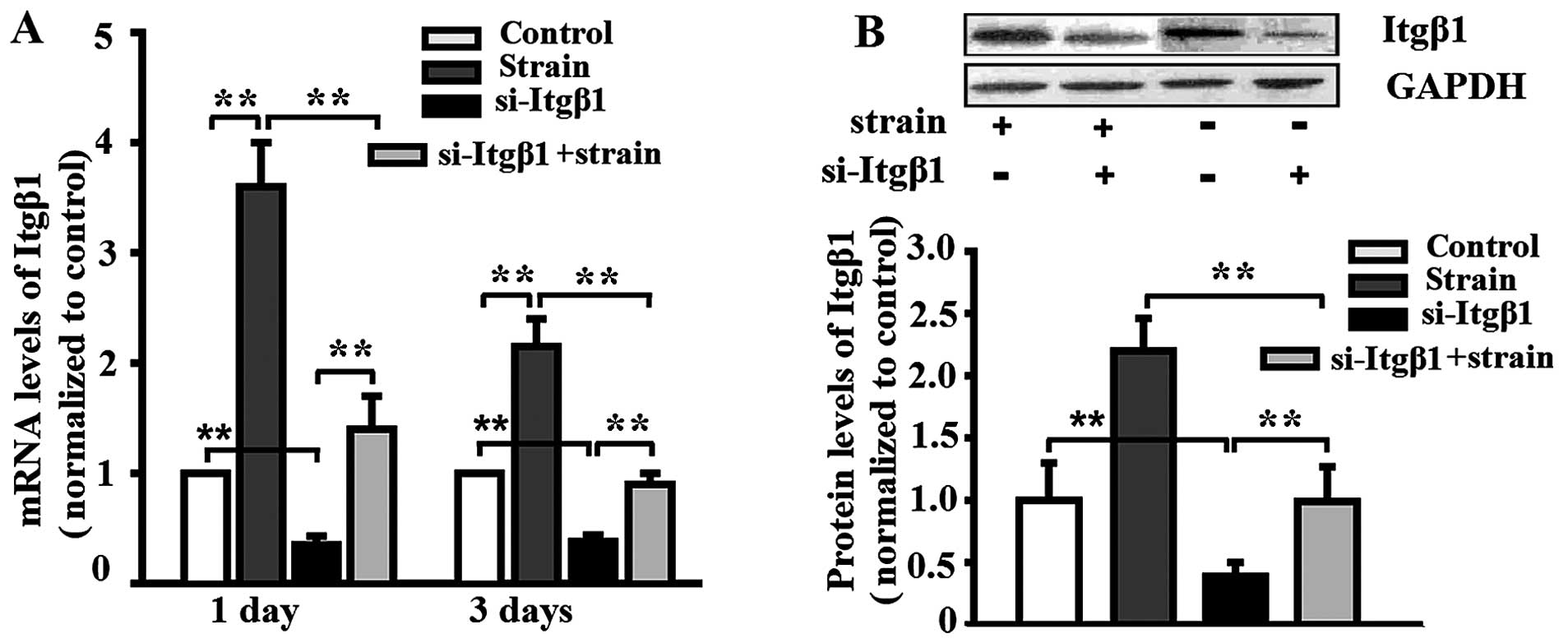
Mechanical strain enhances integrin-β1
expression
The MC3T3-E1 cells were pre-treated with si-Itgβ1
and then subjected to mechanical strain for 3 days. Mechanical
strain enhanced the mRNA and the protein expression of integrin-β1,
and the knockdown of integrin-β1 attenuated the enhancement
(Fig. 1). The mRNA and the
protein expression of integrin-β1 in the MC3T3-E1 cells which were
not stimulated by the application of mechanical strain was also
reduced by si-Itgβ1 (Fig. 1).
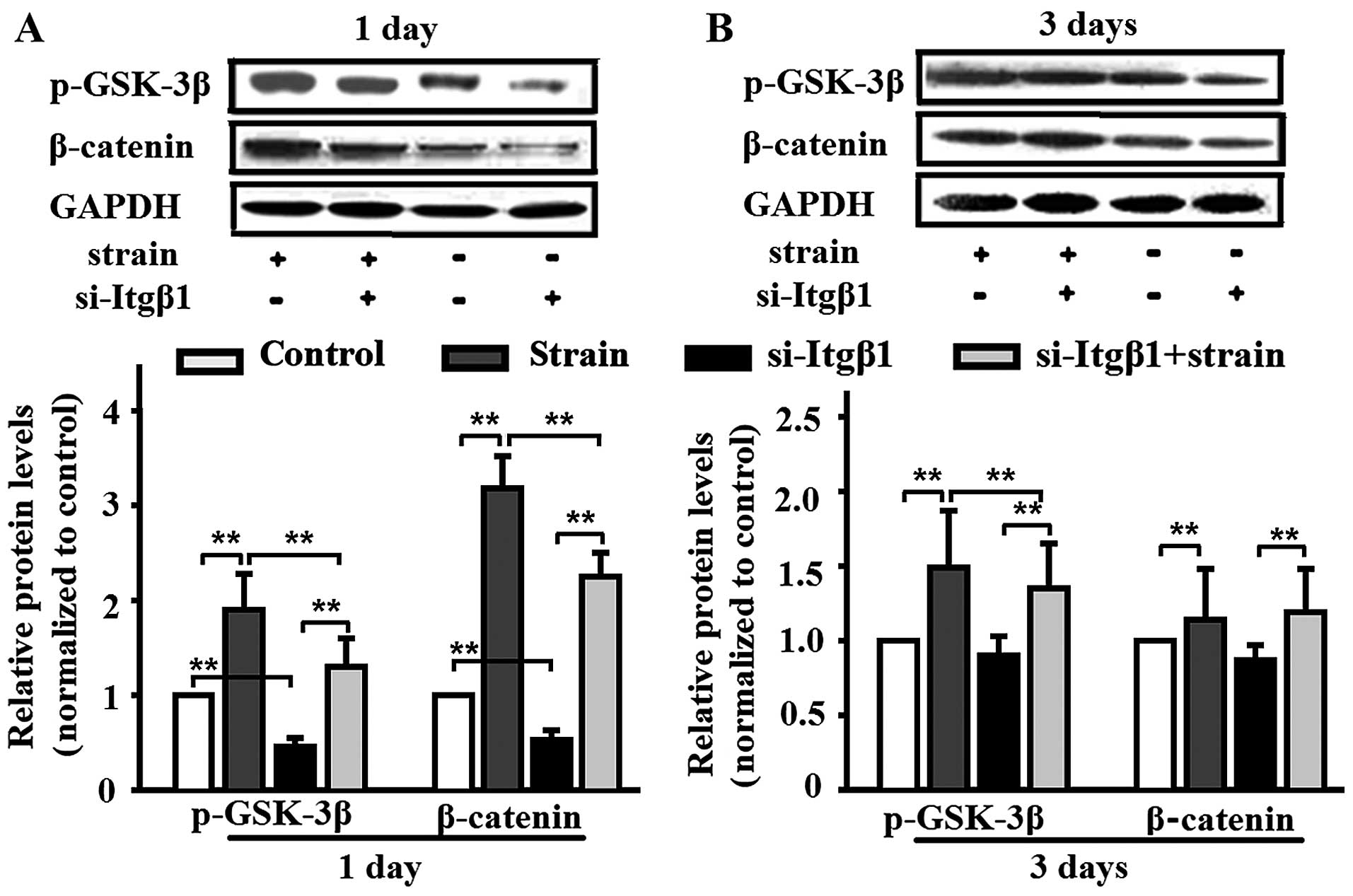
Mechanical strain activates the β-catenin
signal pathway, and this effect is inhibited by integrin-β1
knockdown
Mechanical strain enhanced the protein expression of
β-catenin, and pre-treatment with si-Itgβ1 decreased the protein
levels of β-catenin (Fig. 2).
Similar to β-catenin, mechanical strain increased the protein level
of p-GSK-3β, and the increase was inhibited by integrin-β1
knockdown (Fig. 2). At the same
time, si-Itgβ1 also reduced the protein levels in the unstrained
MC3T3-E1 cells on day 1 (Fig.
2A). Under normal conditions, activated GSK-3β phosphorylates
β-catenin, targeting it for degradation. The inactivation of GSK-3β
by phosphorylation leads to β-catenin accumulation and subsequent
translocation into the nucleus, where it modulates gene
transcription, and subsequently activates the β-catenin signal
pathway (22–24).
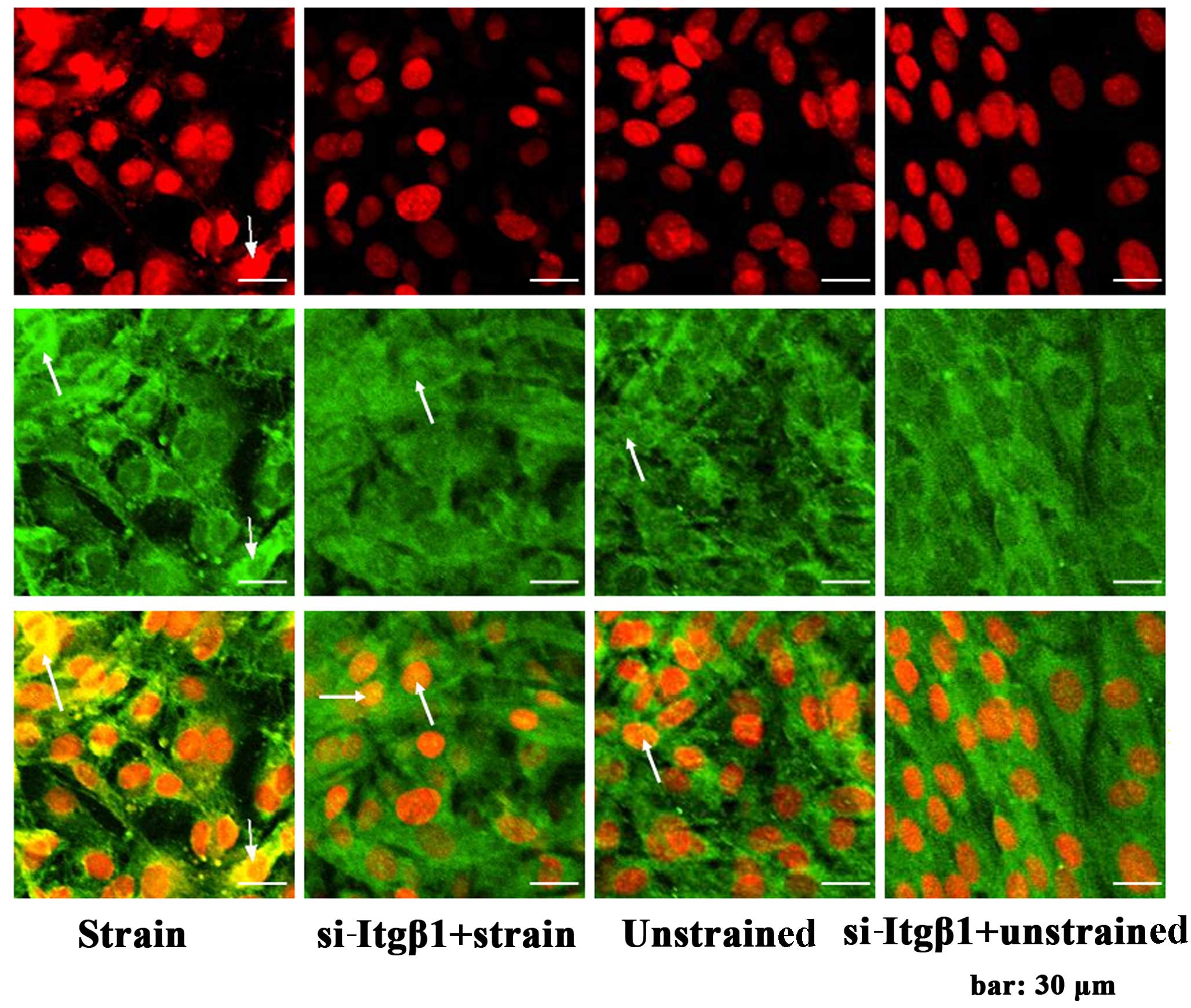
Additionally, in this study, mechanical strain
induced β-catenin translocation into the nuclei of the MC3T3-E1
cells, and si-Itgβ1 hampered the translocation (Fig. 3). The results confirmed that the
application of mechanical strain activated the β-catenin signal
pathway, and that the activation was inhibited by integrin-β1
knockdown.
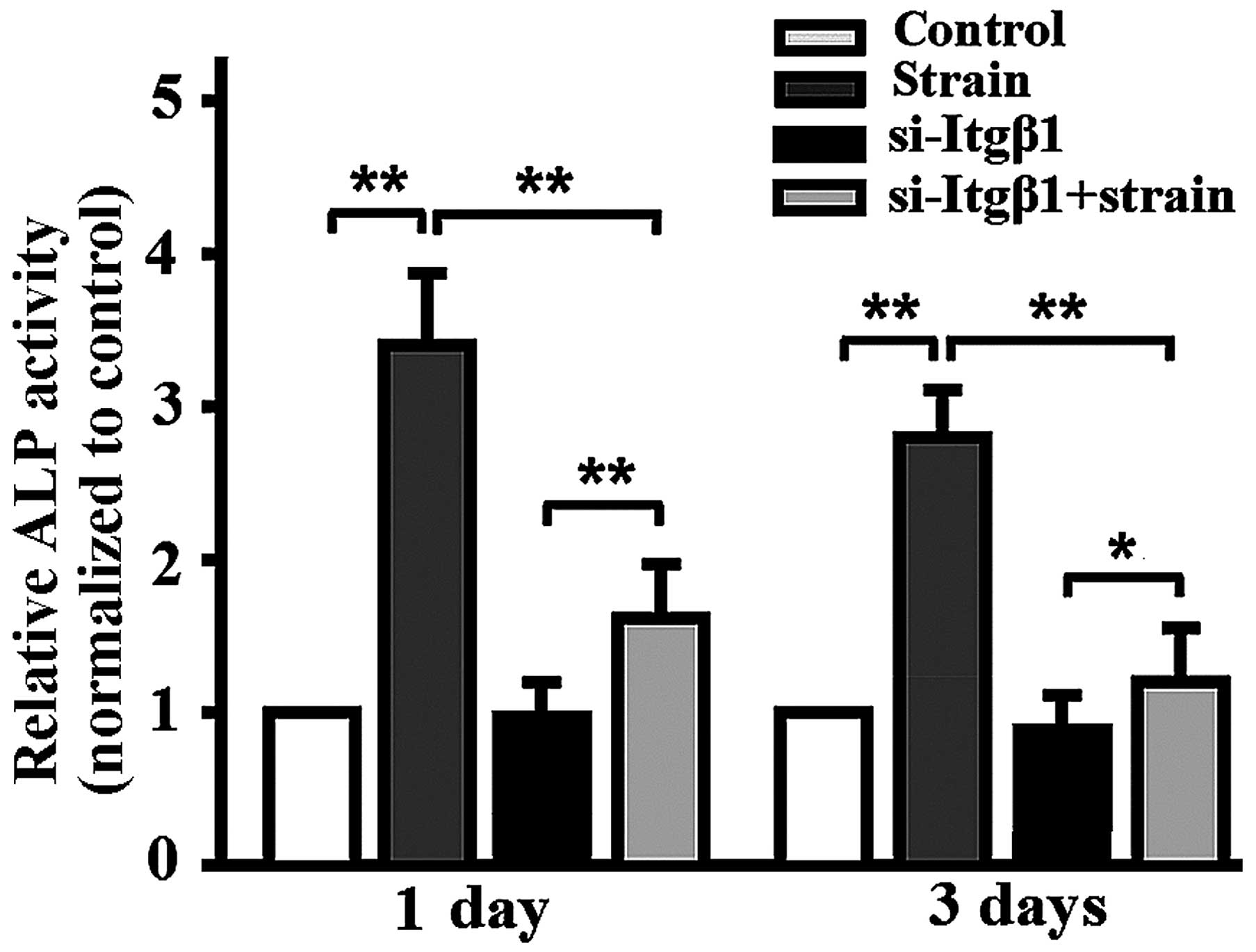
Mechanical strain promotes osteoblastic
differentiation, which is attenuated by integrin-β1 RNAi
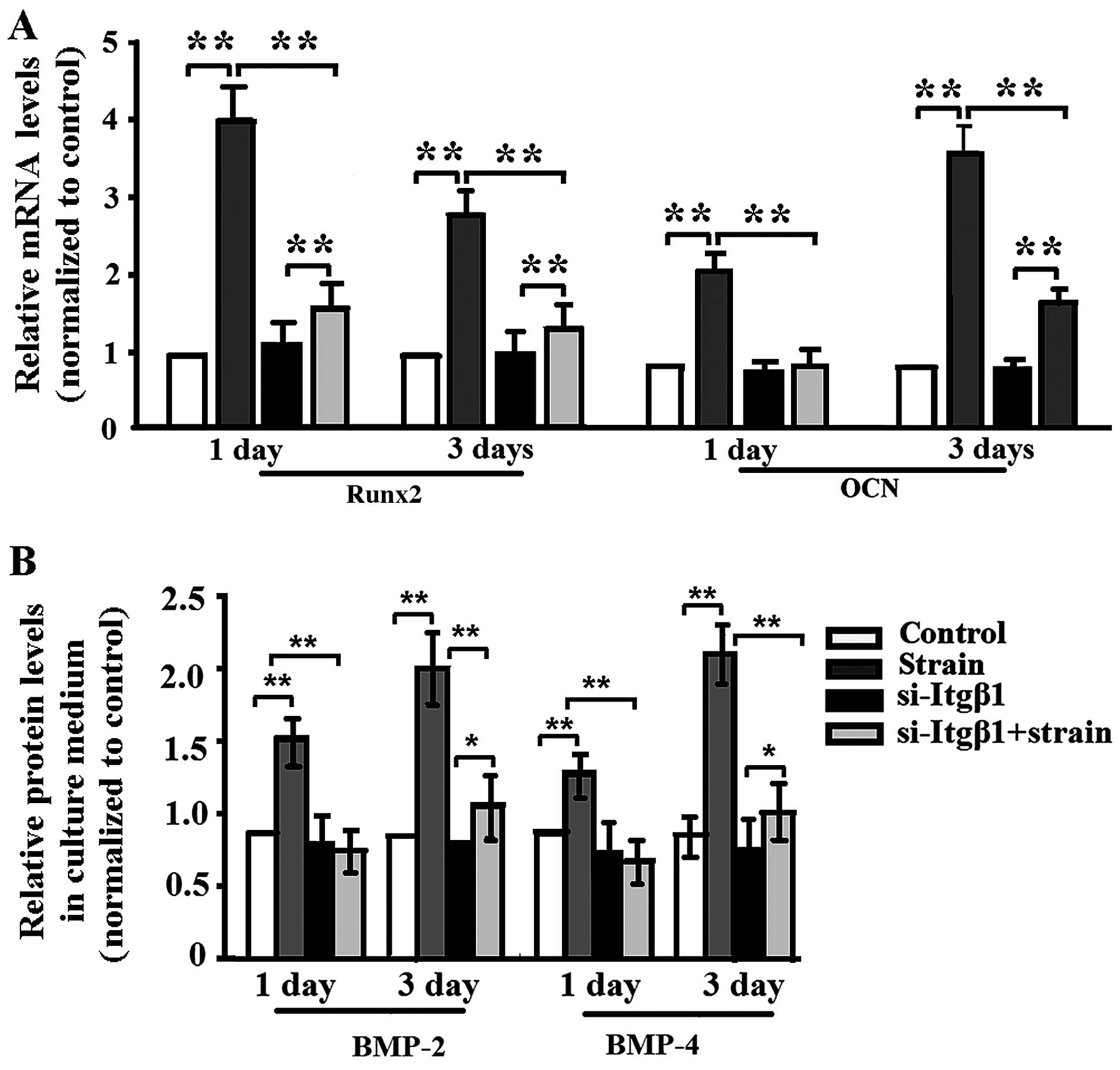
Mechanical strain enhanced ALP activity (Fig. 4), the mRNA expression of
runt-related transcriptional factor 2 (Runx2) and osteocalcin (OCN)
in the MC3T3-E1 cells (Fig. 5A),
the protein levels of BMP-2/4 (Fig.
5B), and si-Itgβ1 lowered ALP activity, the mRNA expression of
Runx3 and OCN and the protein levels of BMP-2/4 in the mechanically
strained MC3T3-E1 cells (Figs. 4
and 5). When the cells were not
mechanically strained, si-Itgβ1 had no effect on ALP activity, the
mRNA expression of Runx2 or OCN, and the protein levels of BMP-2/4
(Figs. 4 and 5).
ALP is a marker of early osteogenic differentiation
(25), Runx2 is the critical
transcription factor that regulates osteoblast differentiation
(26), and OCN is a late marker
of differentiation corresponding with matrix deposition and
mineralization (27). BMP-2/4 are
also markers of osteoblastic differentiation (28,29). Thus, the results indicated that
the application of mechanical strain promoted osteoblastic
differentiation which was attenuated by integrin-β1 RNAi.
Discussion
Mechanical loading is a potent regulator of bone
remodeling and maintenance of bone mass, and many signaling
pathways are involved in the response of bone cells to mechanical
strain (30,31). However, the characterization of
the cellular and molecular events linking loading and bone response
remains incomplete.
As an important member of the integrin family,
integrin-β1 is also mechanoresponsive, as it mediates the response
of bone to mechanical strain. Mechanical stretching upregulated the
expression of integrin-β1 in osteosarcoma cells (4). Fluid shear stress increased the
expression of integrin-β1 in C57BL/6J mouse osteoblasts (32). In addition, integrin-β1 was
required for focal adhesion kinase-independent activation of MAP
kinase induced by mechanical stress (33).
Wnt/β-catenin signaling has been shown to be
important in the osteogenic differentiation of mesenchymal stem
cells and bone formation/development, as well as in the mechanical
response of osteoblasts (22,23). Mechanical strain induces bone
formation through the activation of Wnt/β-catenin pathways.
Consistent with β-catenin activation, osteoblasts respond to
mechanical loading with the increased expression of Wnt/β-catenin
target genes (11,34). However, to date, the role of
integrin-β1 in the regulation of Wnt/β-catenin signaling in
osteoblasts subjected to mechanical strain remains poorly
understood.
Our previous studies demonstrated that mechanical
tensile strain at a frequency of 0.5 Hz and intensities of
2,000–3,000 µε for 1 h/day promoted the osteoblastic
differentiation of MC3T3-E1 cells (increased bone ECM
proteins/genes such as collagen I, OCN and BMPs) (35–37), suggesting that mechanical tensile
strain promoted osteoblast ECM formation. Thus, in this study, we
selected 0.5 Hz at 2,500 µε mechanical strain for 1
h/day.
In the present study, integrin-β1 knockdown reduced
the relative protein levels of p-GSK-3β and β-catenin, which were
enhanced following exposure to mechanical strain. The application
of mechanical strain to the MC3T3-E1 cells also caused β-catenin
accumulation in the cytoplasm and subsequent translocation into the
nucleus, and si-Itgβ1 inhibited the accumulation and translocation
of β-catenin.
GSK-3β and β-catenin are important components of the
Wnt/β-catenin signaling pathway. GSK-3β normally phosphorylates
β-catenin, targeting it for degradation. After phosphorylation,
p-GSK-3β is inactivated, which leads to β-catenin accumulation in
the cytoplasm and subsequent translocation into the nucleus, where
it modulates gene transcription (22–24). The present study demonstrated that
mechanical strain activated the β-catenin signal pathway which was
inhibited by integrin-β1 knockdown.
ALP is widely used as a marker of osteogenic
differentiation, with increasing enzymatic activity associated with
an osteoblastic phenotype (38).
Runx2, OCN and BMP-2/4 are also markers of osteogenic
differentiation (26–29). In this study, the application of
mechanical strain promoted osteoblastic differentiation which was
attenuated by integrin-β1 knockdown. Thus, integrin-β1 knockdown
inhibited Wnt/β-catenin signal transduction in osteoblasts, in
response to mechanical strain.
si-Itgβ1 reduced the protein levels of β-catenin and
p-GSK-3β in the unstrained MC3T3-E1 cells after 1 day although it
had no effect on osteoblastic differentiation. Future studies are
warranted into the associations among integrin-β1, β-catenin
signaling and osteoblastic differentiation.
In conclusion, the knockdown of integrin-β1
inhibited the activation of the β-catenin signal pathway and
osteoblastic differentiation induced by the application of
mechanical strain at 2,500 µε, which indicated that
mechanical strain promoted osteoblastic differentiation through
integrin-β1-mediated β-catenin signaling.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 11372351,
11432016 and 31370943).
References
|
1
|
Pommerenke H, Schmidt C, Dürr F, Nebe B,
Lüthen F, Muller P and Rychly J: The mode of mechanical integrin
stressing controls intracellular signaling in osteoblasts. J Bone
Miner Res. 17:603–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shyy JY and Chien S: Role of integrins in
endothelial mechanosensing of shear stress. Circ Res. 91:769–775.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gohel AR, Hand AR and Gronowicz GA:
Immunogold localization of beta 1-integrin in bone: effect of
glucocorticoids and insulin-like growth factor I on integrins and
osteocyte formation. J Histochem Cytochem. 43:1085–1096. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carvalho RS, Scott JE and Yen EH: The
effects of mechanical stimulation on the distribution of beta 1
integrin and expression of beta 1-integrin mRNA in TE-85 human
osteosarcoma cells. Arch Oral Biol. 40:257–264. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zimmerman D, Jin F, Leboy P, Hardy S and
Damsky C: Impaired bone formation in transgenic mice resulting from
altered integrin function in osteoblasts. Dev Biol. 220:2–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapur S, Baylink DJ and Lau KH: Fluid flow
shear stress stimulates human osteoblast proliferation and
differentiation through multiple interacting and competing signal
transduction pathways. Bone. 32:241–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh CR, Chiu JJ, Lee CI, Lee PL, Shih YT,
Sun JS, Chien S and Cheng CK: Estrogen augments shear
stress-induced signaling and gene expression in osteoblast-like
cells via estrogen receptor-mediated expression of beta1-integrin.
J Bone Miner Res. 25:627–639. 2010. View Article : Google Scholar
|
|
8
|
Yan YX, Gong YW, Guo Y, Lv Q, Guo C,
Zhuang Y, Zhang Y, Li R and Zhang XZ: Mechanical strain regulates
osteoblast proliferation through integrin-mediated ERK activation.
PLoS One. 7:e357092012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng Q, Guo Y, Liu Y, Li R, Zhang X, Liu
L, Wang Y, Zhang X and Zou X: Integrin-β1, not integrin-β5,
mediates osteoblastic differentiation and ECM formation promoted by
mechanical tensile strain. Biol Res. 48:252015. View Article : Google Scholar
|
|
10
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P,
Brown EL, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Case N, Ma M, Sen B, Xie Z, Gross TS and
Rubin J: Beta-catenin levels influence rapid mechanical responses
in osteoblasts. J Biol Chem. 283:29196–29205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norvell SM, Alvarez M, Bidwell JP and
Pavalko FM: Fluid shear stress induces beta-catenin signaling in
osteoblasts. Calcif Tissue Int. 75:396–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Premaraj S, Souza I and Premaraj T:
Mechanical loading activates β-catenin signaling in periodontal
ligament cells. Angle Orthod. 81:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rallis C, Pinchin SM and Ish-Horowicz D:
Cell-autonomous integrin control of Wnt and Notch signalling during
somitogenesis. Development. 137:3591–3601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Chattopadhyay N, Qin S, Szekeres C,
Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner
JH and Kreidberg JA: Coordinate integrin and c-Met signaling
regulate Wnt gene expression during epithelial morphogenesis.
Development. 136:843–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SA, Das S, Lee H, Kim JH, Kim SJ, Song
YM, Kim IS, Hwang SJ and Byun KM: A surface plasmon resonance
sensor for quantitative analysis of mineralization of osteoblast
cells. Proceedings of IEEE Sensors. Jan;2010. View Article : Google Scholar
|
|
17
|
Gan Q, Lu X, Yuan Y, Qian J, Zhou H, Lu X,
Shi J and Liu C: A magnetic, reversible pH-responsive nanogated
ensemble based on Fe3O4 nanoparticles-capped
mesoporous silica. Biomaterials. 32:1932–1942. 2011. View Article : Google Scholar
|
|
18
|
Tang LL, Wang YL, Pan J and Cai SX: The
effect of step-wise increased stretching on rat calvarial
osteoblast collagen production. J Biomech. 37:157–161. 2004.
View Article : Google Scholar
|
|
19
|
Wang L, Zhang XZ, Guo Y, Chen XZ, Li RX,
Liu L, Shi CH, Guo C and Zhang Y: Involvement of BMPs/Smad
signaling pathway in mechanical response in osteoblasts. Cell
Physiol Biochem. 26:1093–1102. 2010. View Article : Google Scholar
|
|
20
|
Bottlang M, Simnacher M, Schmitt H, Brand
RA and Claes L: A cell strain system for small homogeneous strain
applications. Biomed Tech (Berl). 42:305–309. 1997. View Article : Google Scholar
|
|
21
|
Owan I, Burr DB, Turner CH, Qiu J, Tu Y,
Onyia JE and Duncan RL: Mechanotransduction in bone: osteoblasts
are more responsive to fluid forces than mechanical strain. Am J
Physiol. 273:C810–C815. 1997.PubMed/NCBI
|
|
22
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/β-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hill TP, Später D, Taketo MM, Birchmeier W
and Hartmann C: Canonical Wnt/β-catenin signaling prevents
osteoblasts from differentiating into chondrocytes. Dev Cell.
8:727–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett
JR and Mills GB: Phosphorylation and inactivation of glycogen
synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA.
97:11960–11965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beck GR Jr, Zerler B and Moran E:
Phosphate is a specific signal for induction of osteopontin gene
expression. Proc Natl Acad Sci USA. 97:8352–8357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu M, Hesse E, Morvan F, Zhang JP, Correa
D, Rowe GC, Kiviranta R, Neff L, Philbrick WM, Horne WC and Baron
R: Zfp521 antagonizes Runx2, delays osteoblast differentiation in
vitro, and promotes bone formation in vivo. Bone. 44:528–536. 2009.
View Article : Google Scholar :
|
|
27
|
Mahalingam CD, Datta T, Patil RV, Kreider
J, Bonfil RD, Kirkwood KL, Goldstein SA, Abou-Samra AB and Datta
NS: Mitogen-activated protein kinase phosphatase 1 regulates bone
mass, osteoblast gene expression, and responsiveness to parathyroid
hormone. J Endocrinol. 211:145–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang WG, Kim EJ and Koh JT: Tunicamycin
negatively regulates BMP2-induced osteoblast differentiation
through CREBH expression in MC3T3E1 cells. BMB Rep. 44:735–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan M and Cao X: BMP signaling in skeletal
development. Biochem Biophys Res Commun. 328:651–657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liedert A, Kaspar D, Blakytny R, Claes L
and Ignatius A: Signal transduction pathways involved in
mechanotransduction in bone cells. Biochem Biophys Res Commun.
349:1–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Papachroni KK, Karatzas DN, Papavassiliou
KA, Basdra EK and Papavassiliou AG: Mechanotransduction in
osteoblast regulation and bone disease. Trends Mol Med. 15:208–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau KH, Kapur S, Kesavan C and Baylink DJ:
Up-regulation of the Wnt, estrogen receptor, insulin-like growth
factor-I, and bone morphogenetic protein pathways in C57BL/6J
osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes
to the differential anabolic response to fluid shear. J Biol Chem.
281:9576–9588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lal H, Verma SK, Smith M, Guleria RS, Lu
G, Foster DM and Dostal DE: Stretch-induced MAP kinase activation
in cardiac myocytes: differential regulation through β1-integrin
and focal adhesion kinase. J Mol Cell Cardiol. 43:137–147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Armstrong VJ, Muzylak M, Sunters A, Zaman
G, Saxon LK, Price JS and Lanyon LE: Wnt/β-catenin signaling is a
component of osteoblastic bone cell early responses to load-bearing
and requires estrogen receptor alpha. J Biol Chem. 282:20715–20727.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan YX, Song M, Guo C, Guo Y, Gong YW, Li
RX and Zhang XZ: The effects of substrate-streching strajn on the
BMP-2 mRNA expression in three kinds of mouse cell lines. Chin J
Gerontol. 30:109–112. 2010.
|
|
36
|
Gong YW, Yan YX, Zhang Y, Zhang XZ and Guo
Y: The effect of substrate-stretching strain on the expression of
Runx2 in mouse osteoblasts. Chin J Osteoporos. 17:185–188.
2011.
|
|
37
|
Liu L, Guo Y, Wan ZM, Shi CH, Li JY, Li
RX, Hao QX, Li H and Zhang XZ: Effects of phytoestrogen a-ZAL and
mechanical stimulation on proliferation, osteoblastic
differentiation, and OPG/RANKL expression in MC3T3-E1
pre-osteoblasts. Cell Mol Bioeng. 5:427–439. 2012. View Article : Google Scholar
|
|
38
|
Aubin JE, Liu F, Malaval L and Gupta AK:
Osteoblast and chondroblast differentiation. Bone. 17(Suppl 2):
77S–83S. 1995. View Article : Google Scholar : PubMed/NCBI
|