Introduction
Orthotopic liver transplantation is the only
currently available treatment for severe liver failure; however,
the use of this technique is limited by organ shortages (1). Hepatocyte transplantation has become
an attractive alternative approach to treating liver diseases
(2). However, the isolation of
sufficient numbers of transplantable hepatocytes is restricted by
the small number of marginal donor organs (3). In addition, adult human hepatocytes
have poor proliferative potential that is likely to be insufficient
for effectively repopulating the host liver (4). The search for greater cell
resources, which may be used to replace primary hepatocytes, has
prompted investigations into the generation of hepatocytes from
stem cells of extrahepatic origin, owing to their ready
availability and unrestricted ability to propagate and
differentiate (5). Mesenchymal
stem cells (MSCs), which are found in various tissues and organs,
have become an important resource for regenerative medicine, as
they are readily available cells that proliferate in vitro
and have the capacity for multiple lineage differentiation
(6). Previous studies using rats,
mice and humans have confirmed that bone marrow-derived MSCs
(BM-MSCs) as well as MSCs derived from umbilical cord blood or
adipose tissue may differentiate into hepatocyte-like cells under
selective growth conditions in vitro, which suggests that
adult stem cell-based therapies may provide alternative therapeutic
approaches for the treatment of liver diseases (7–9).
Considerable efforts over the last decade have been
dedicated to the evaluation of culture conditions in order to
obtain differentiated hepatocytes from stem cells. The sequential
addition of liver-specific factors into culture systems in a
time-dependent manner that mimics the secretion pattern during
liver embryogenesis is important for the differentiation of stem
cells into hepatocytes (10).
Advances in induction technology have enabled the in vitro
differentiation of MSCs into hepatocyte-like cells using a
two-dimensional (2D) culture system (11). However, the 2D culture method has
limitations in terms of controlling stem cell differentiation
pathways, resulting in low differentiation efficiency.
Three-dimensional (3D) culture systems have been shown to promote
enhanced cellular structure and function in many types of cells and
tissues, including mammary epithelial cells, MSCs as well as neural
and hepatic cells (12–14). MSCs cultured as 3D spheroids in
suspension are characterized by enhanced levels of differentiation
and higher degrees of maturity of MSC-derived hepatocytes compared
with traditional adherent monolayer cultures (15–17).
Bio-scaffolds derived from decellularized
organ/tissue matrix have been used for the differentiation of stem
cells due to the preserved extracellular matrix (ECM) components,
which include numerous chemical and biophysical cues for
differentiation (18,19). In addition, previous findings have
shown that the differentiation of stem/progenitor cells is lineage
restricted by the tissue-specific biomatrix scaffold (18,19). Therefore, a decellularized liver
may potentially be used as a tool for stem cell differentiation and
maturation, and eventually be used to engineer autologous liver
grafts. Previous studies have demonstrated that the differentiation
of stem cells derived from different tissues into hepatocyte-like
cells is more efficient in a decellularized liver biomatrix
(20,21).
As interactions between stem cells and the ECM are
required for inducing lineage-specific differentiation and
maintaining the biological functions of hepatocyte-like cells by
providing a composite set of chemical and structural signals, in
the present study we employed both 3D spheroid and decellularized
liver scaffold (DLS) culture systems to promote hepatocyte
maturation of the hepatocyte-like cells. This combination is a
novel method whereby rat BM-MSCs self-aggregated into spheroids in
3D culture plates and were then implanted into the DLS.
Materials and methods
Animals
Male Bama miniature pigs (Guangxi, China) weighing
10–12.5 kg were obtained from the Animal Experiment Center of
Sichuan University (Chengdu, China), and the whole liver was
harvested. The animals were maintained under a 12-h light/dark
cycle with free access to standard laboratory food and water. All
experimental protocols were approved by the Animal Experiment
Center of Sichuan University. All animals were cared for in
accordance with the requirements of the Laboratory Animal Welfare
Act and amendments.
Six livers were isolated from male Bama miniature
pigs for perfusion decellularization. The surgeries were performed
under ketamine (6 mg/kg body weight, administered IP; Kelun,
Chengdu, China) and xylazine (10 mg/kg IP; Kelun) anesthesia. Under
deep anesthesia, a laparotomy was performed and the liver was
exposed. After systemic heparinization through the inferior vena
cava, the hepatogastric ligament was carefully dissected. The
proximal PV was catheterized. The hepatic artery and common bile
duct were ligated and transected. All perihepatic ligaments were
severed. Simultaneously, the liver was slowly perfused with 2
liters of deionized water containing 0.1% EDTA (Kelun) through a
cannula in the PV, and the SHIVC was transected, allowing outflow
of the perfusate. Following blanching, the liver was stored at
−80°C overnight.
Evaluation of decellularized porcine
liver
We used our previously established decellularization
protocol to obtain liver scaffolds (22). The liver was perfused with 1%
Triton X-100 (Amresco, Solon, OH, USA) for 3 h and then by 1% SDS
(Promega, San Luis Obispo, CA, USA) in deionized water at a rate of
200 ml/min for 6 h after thawing. This was followed by 3 h of
perfusion with 1% Triton X-100 to remove residual SDS.
Subsequently, the liver was washed with 20 liters of distilled
water to remove residual detergent, followed by infusion of 40
liters of phosphate-buffered saline (PBS) at 200 ml/min. To
determine whether collagen I (1:1,000, mouse polyclonal IgG,
GTX26308; GeneTex, Irvine, CA, USA); collagen IV (1:100, rabbit
polyclonal IgG, bs-4595R; BIOSS, Beijing, China); laminin (1:1,000,
mouse polyclonal IgG, GTX11574) and fibronectin (1:100, rabbit
polyclonal IgG, GTX72724) (both from GeneTex) were retained in the
decellularized matrices, the liver ECM samples were sectioned and
stained by immunohistochemistry with the indicated antibodies and
dilutions. Briefly, paraffin sections were rehydrated, incubated in
antigen retrieval solution, and stained using antibodies to
fibronectin, laminin, and collagen I and IV. Images of the stained
slides were captured using an upright microscope (BX51; Olympus,
Tokyo, Japan). Sulfated glycosaminoglycans (GAGs) were quantified
using the Blyscan GAG assay kit (Biocolor, Carrickfergus, UK).
Histological analysis
Normal fresh liver (n=6 of each group),
decellularized liver matrix, and recellularized liver samples were
fixed in 4% paraformaldehyde at room temperature for 24 h. They
were dehydrated using a graded ethanol series, immersed in xylene,
and embedded in paraffin. The ECM samples were cut into 5-μm
sections and stained with hema-toxylin and eosin (H&E).
Sections were mounted in mounting media containing
4′,6-diamidino-2-phenylindole (DAPI; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) to confirm the extent of
decellularization.
Scanning electron microscopy (SEM)
Following decellularization, the samples were fixed
in 2.5% glutaraldehyde for at least 12 h at room temperature. The
samples were then briefly rinsed in deionized water, dehydrated via
a graded ethanol series, and dried in a critical point dryer (HCP2;
Hitachi, Tokyo, Japan). Finally, the samples were sputter-coated
with gold prior to SEM imaging. Electron micrographs of liver
cross-sections were obtained at 5.0 kV and ×1,000 magnification
using a Hitachi S-4800 scanning electron microscope (Hitachi).
Cultivation of rat BM-MSCs
Commercial rat BM-MSCs (Cyagen Biosciences,
Guangzhou, China) at passages 6-8 (P6-8) were used in the following
experiments. Sprague-Dawley rat MSC growth medium (no. RASMX-90011;
Cyagen Biosciences) was used for cell culture. The medium was
replaced at least every 2–3 days.
Hepatic differentiation of BM-MSCs in
vitro
To induce hepatic differentiation, serum-free
Iscove's modified Dulbecco's medium (IMDM; HyClone, Beijing, China)
supplemented with a combination of growth factors described
previously was used to induce the differentiation of BM-MSCs
(7). Prior to the two-step
induction protocol, the cells were serum-deprived for two days in
IMDM supplemented with 20 ng/ml epidermal growth factor (EGF) and
10 ng/ml basic fibroblast growth factor (bFGF). The induction
protocol was as follows: i) the BM-MSCs were treated with
differentiation medium consisting of IMDM supplemented with 20
ng/ml hepatocyte growth factor (HGF), 10 ng/ml bFGF, and 0.61 g/l
nicotinamide (all from Sigma-Aldrich, St. Louis, MO, USA) for 7
days; ii) all groups were induced with maturation medium, which
consisted of IMDM supplemented with 20 ng/ml oncostatin M, 1 mmol/l
dexamethasone, and 50 mg/ml insulin-transferrin-selenium premix
(all from Sigma-Aldrich) for 2 weeks.
Formation and characterization of 3D
BM-MSC spheroids Formation of BM-MSC spheroids
For spheroid cultures, the harvested BM-MSCs were
suspended in 10 ml serum-free medium at 1×106 cells/ml
and inoculated into glass spheroid dishes (13×8×4 cm) and were
surface siliconized with Sigmacote (Sigma-Aldrich). The spheroid
dishes were incubated with continuous rocking at 10 rpm using the
Rocker system (introduced by Mayo Clinic, Rochester, MN, USA) to
induce spheroid formation, as previously described (23). After aggregation, 100 μl
aliquots were removed from the spheroid dishes to determine the
number, diameter, and total volume (cell mass) of the spheroids
using a Multisizer 3 (560-μm aperture; Beckman Coulter,
Fullerton, CA, USA).
Cell viability assay
The viability of BM-MSC spheroids was evaluated
using the FluoroQuench fluorescent viability stain (One Lambda,
Canoga Park, CA, USA). The samples were imaged using a DFC 495
fluorescence microscope (Leica, Wetzlar, Germany).
5-Ethynyl-2′-deoxyuridine (EdU)
staining
For EdU staining (cat. no. C10130-1; RiboBio,
Guangzhou, China), BM-MSC spheroids were added to the respective
culture flasks and cultured with 0.1% EdU overnight. EdU was probed
using Apollo staining (RiboBio) thereafter.
Cell seeding
Four culturing methods for differentiation [single
cell (2D), spheroids (3D), 2D + DLS and 3D + DLS] were studied.
After the decellularization procedure, the decellularized livers
were cut into discs of 8×8×3-mm3 and placed into 24-well
plates for lyophilization, followed by sterilization with gamma
irradiation (1,000 rad) for 2 h. Prior to cell seeding, the discs
were incubated in culture medium at 37°C overnight. After the
medium was aspirated, a cell suspension (100 μl) of
harvested BM-MSCs or BM-MSC spheroids was pipetted onto the center
of the disc. The cells were allowed to settle and attach to the
disc scaffold for 4 h. Subsequently, 2 ml medium from stage one of
the induction protocol was added slowly. Monolayer cells were
cultured in normal 6-well plates as the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To harvest the differentiated cells or spheroids
cultured in the DLS, the bio-scaffolds were washed twice with
phosphate-buffered solution (PBS), chopped (cut into sections by
opthalmic scissors) and digested with 1 mg/ml collagenase type II
(Gibco, Grand Island, NY, USA) for 25 min at 37°C. After filtration
through a 200-μm mesh screen and repeated pipetting, the
cells were washed twice with PBS. Total RNA was extracted from the
differentiated cells of all groups using TRIzol solution (cat. no.
15596-026; Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. cDNA was synthesized from 1 μg
total RNA using random primers and PrimeScript reverse
transcriptase (part of iScript cDNA synthesis kit; cat. no.
170-8890; Bio-Rad, Hercules, CA, USA). Quantitative PCR reactions
for the indicated genes were performed using an iScript cDNA
synthesis kit and a fluorescent temperature cycler (C1000 Thermal
Cycler; Bio-Rad). The primers sequences are listed in Table I.
 | Table IRT-qPCR primer sequences. |
Table I
RT-qPCR primer sequences.
| Gene | GenBank Accesion
No. | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| HNF1β | NM_013103 |
AATCCCAGCAAGGAAGAGAG |
ACCAGTTGTAGACACGGACC |
| HNF6 | NM_022671 |
CCTGGAGCAAACTCAAGTCC |
CCGTGTTCTTGCTCTTTCC |
| TAT | NM_012668 |
GGCACCTTCAGAAGATTTTG |
GCCAGTGGTTCGTATTTGC |
| FOXA1 | NM_012742 |
GGTTCGGAGTTGAAGTCTCC |
GGGGTGGTTAAAGGAGTAGTG |
| CK19 | NM_199498 |
GCCTACCTGAAGAAGAACCAC |
CAATGCCTGGTGTGGAATC |
| AFP | NM_012493 |
GCTGACAACATGGAGGAATG |
TGAGTACAGCCTGGAGGTTC |
| ALB | NM_134326 |
GGCACCAAGTGTTGTACCCT |
AGCACACACAGACGGTTCAG |
| ARG1 | NM_017134 |
CAACACTCCGCTGACAACC |
CAGATATGCAGGGGGTCAC |
| CYP1A1 | NM_012540 |
AGCTAATCAAAGAGCACTACAGG |
CCTTATCATCTGAGAGCTGG |
| CYP1A2 | NM_012541 |
GAGAAGGTGATGCTCTTCGG |
ATGCAGGAGGATGGCTAAGA |
Immunofluorescence staining
The retrieved samples were embedded in optimum
cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek,
Torrance, CA, USA) and frozen. The 4-μm frozen sections were
fixed in 4% paraformaldehyde in PBS for 10 min at room temperature.
In order to detect cytoplasmic proteins, the sections were
permeabilized with 0.1% Triton X-100 for 10 min at room
temperature, 5% goat serum and 1% bovine serum albumin which was
used for blocking. The sections were incubated with the following
primary antibodies overnight at 4°C: alpha fetoprotein (AFP;
AF5134; Affinity, Cambridge, UK), albumin (ALB; ab8940; Abcam,
Cambridge, UK) and cytokeratin 19 (CK19; AF0192; Affinity).
Following incubation with the primary antibodies, the cells were
washed with PBS and then incubated with fluorescence-conjugated
secondary anti-goat IgG or anti-chicken IgG (Abcam) for 1 h at room
temperature. After nuclear staining with DAPI, the slides were
mounted and analyzed with a fluorescence microscope (Leica DMI
6000; Leica, Mannheim, Germany).
Hepatocyte-specific function assays
Albumin and urea production
The conditioned media from the differentiated
BM-MSCs of all groups were collected on day 21 and stored at −20°C
until used for assaying. The albumin level was tested using an
ELISA kit (Rat Albumin ELISA Quantitation set, E110-125; Bethyl
Laboratories, Inc., Montgomery, TX, USA), according to the
manufacturer's instructions. The urea concentration was measured
using the QuantiChrom Urea assay kit (DIUR-500; Bioassay Systems
LLC, Hayward, CA, USA), and absorbance was measured using a Sunrise
micro-plate reader (MQX 200; BioTek, Winooski, VT, USA). All values
were normalized to the number of cells.
Periodic acid-Schiff (PAS)
staining
Glycogen storage in the induced BM-MSCs of all
groups was determined using a PAS kit (Jiancheng, Nanjing, China)
according to the manufacturer's instructions.
Statistical analysis
All data were analyzed using SPSS statistical
software (version 17.0). Data are presented as the means ± SEM.
One-way analysis of variance (ANOVA) for multiple comparisons was
performed to compare datasets. Dunnett's analysis was performed to
compare the two groups' datasets. A p-value <0.05 was considered
to indicate a statistically significant difference.
Results
Characterization of decellularized
porcine liver
Whole-organ decellularization was achieved through
portal perfusion, using sodium dodecyl sulfate (SDS) and Triton
X-100. This treatment effectively lyses cell membranes, disrupts
intracellular organelles, and removes cellular debris from the
tissue. After decellularization, the porcine liver parenchyma
became semi-transparent, and the acellular scaffold retained the
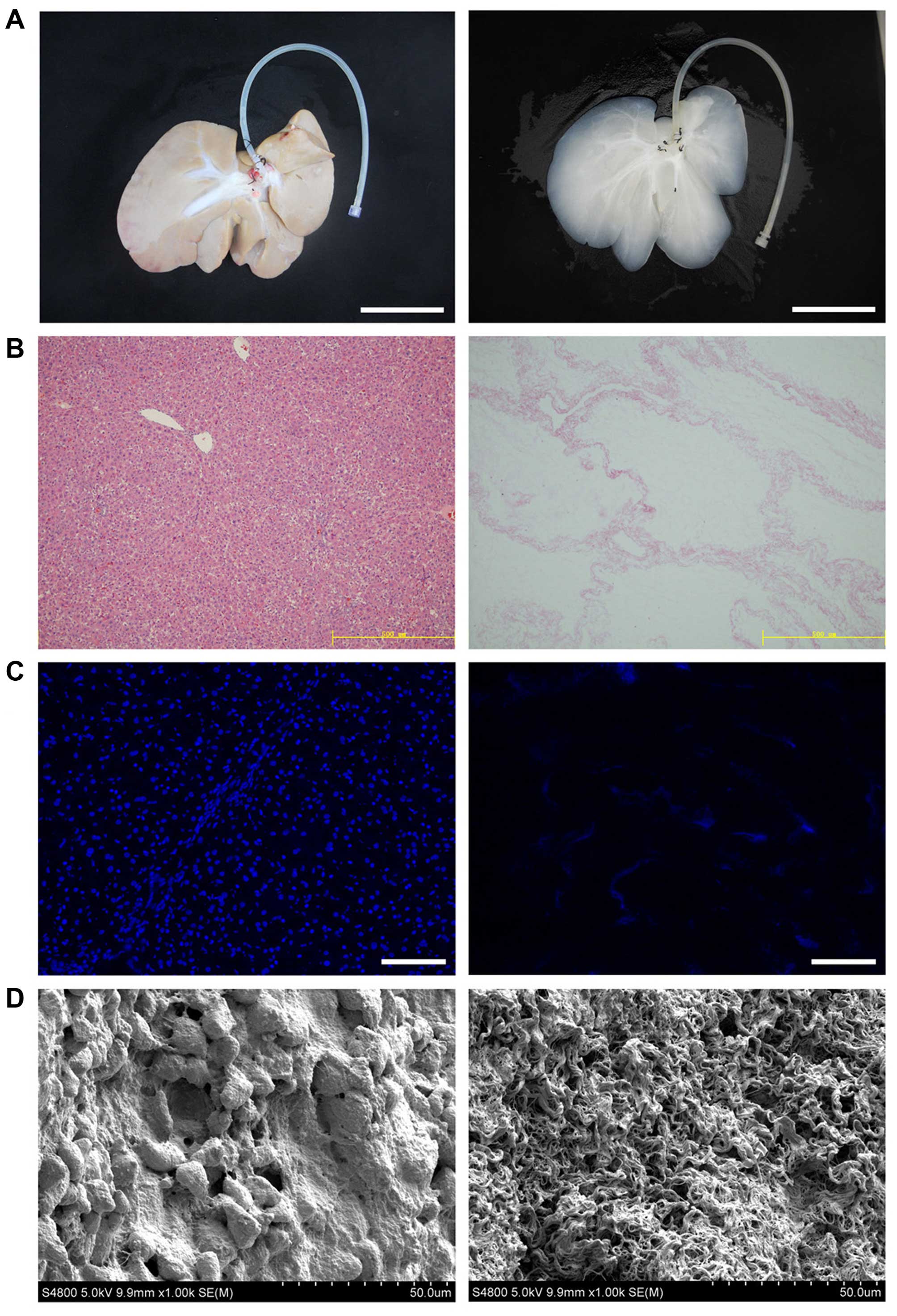
gross appearance and size of the liver (Fig. 1A). H&E staining revealed the
presence of pink staining, which is typical of collagen, whereas
the blue staining typical of cellular nuclear material was not
observed (Fig. 1B). The lack of
DAPI staining in the biomatrix confirmed the absence of cell nuclei
(Fig. 1C). We examined
decellularized tissue sections by SEM in order to evaluate whether
the ultrastructure of the bio-scaffold was preserved after
decellularization (Fig. 1D).
Reticular collagen fibers, which provide support for the hepatic
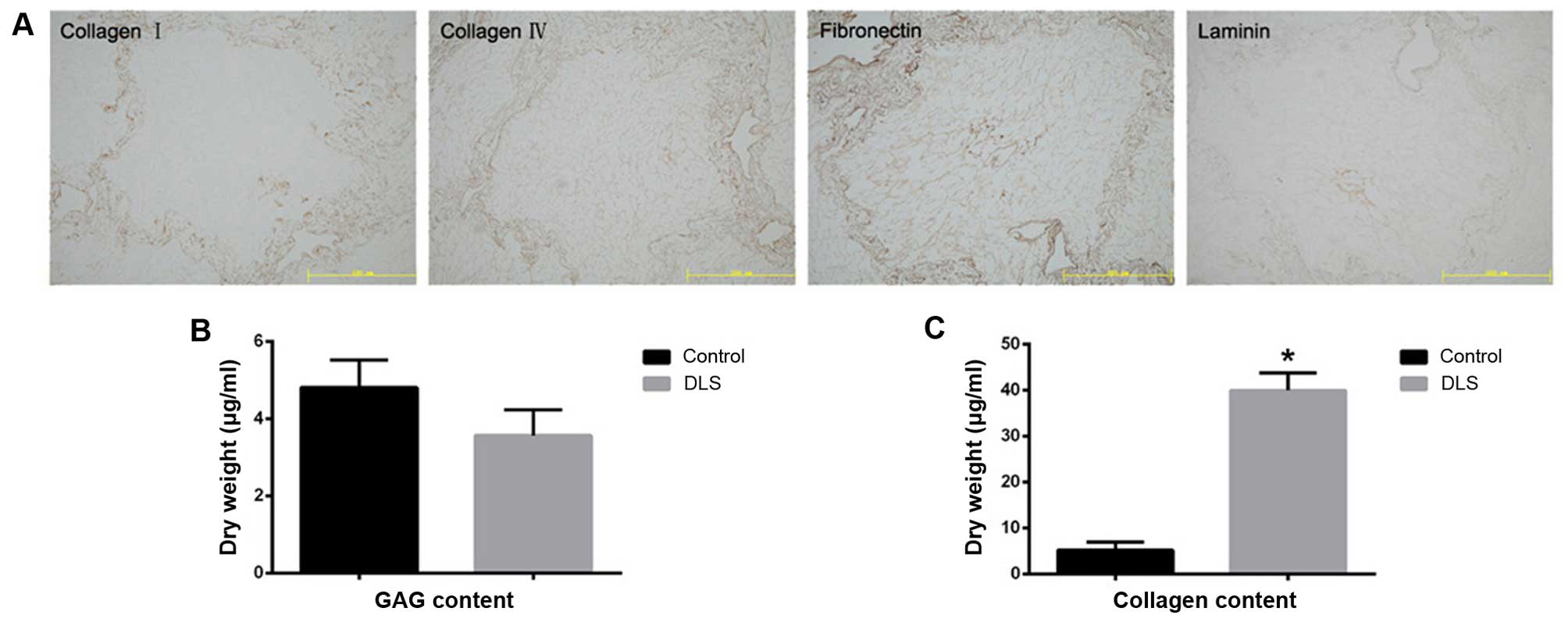
tissue, are readily apparent. Immunostaining of the four ECM
proteins, namely collagen type I, collagen type IV, fibronectin,
and laminin, indicated that the structural components and basement
membrane composition of the ECM had been retained (Fig. 2A). There was also a reduction in
sulfated GAG content in the decellularized liver tissue (Fig. 2B). The collagen content in the
decellularized liver tissue was noted to be significantly higher
(p<0.05) than that found in the fresh liver tissue, which may be
explained by the removal of cellular material (Fig. 2C).
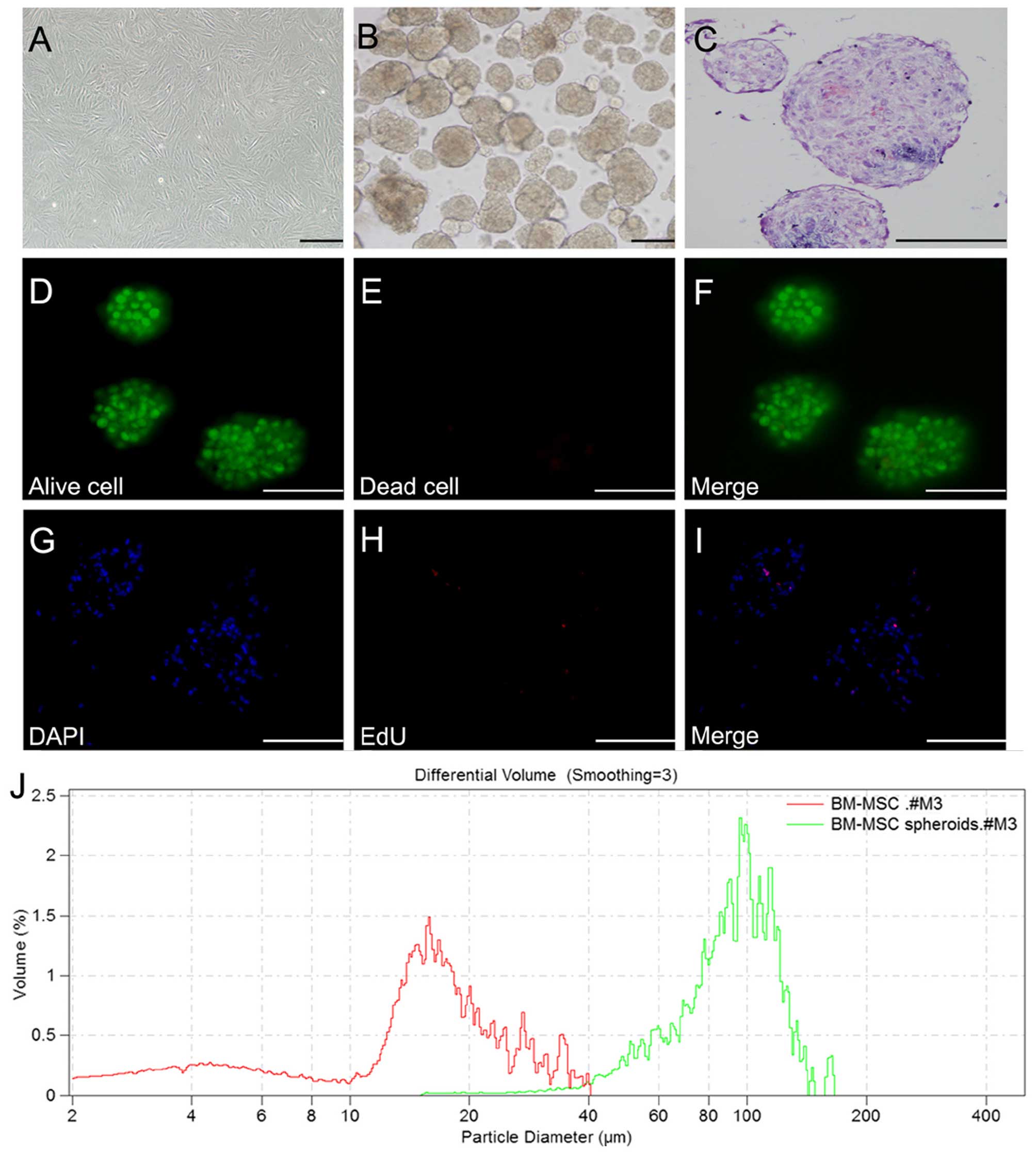
3D spheroid formation
P6-8 BM-MSCs were harvested in order to examine the
formation of 3D spheroids (Fig.
3A). As previously described (24), under optimized cell number and
growth conditions, BM-MSC aggregates were allowed to form through
the forced aggregation method for 2 days (Fig. 3B). In addition, BM-MSC
differentiation to the hepatic cell lineage was maintained in
monolayer cell culture at a similar cell concentrations. H&E
staining revealed that the cells in spheroids closely adhered to
each other and were compacted (Fig.
3C). The Live/Dead staining assay revealed that cells in the 3D
spheroids maintained high viability, whereas individual cells not
in clusters were no longer viable (Fig. 3D–F). In order to assess cell
proliferation within the BM-MSC spheroids, samples were collected
for an EdU assay on day 2. EdU staining of the BM-MSC spheroids
demonstrated the presence of proliferating cells within the
spheroids on day 2. However, EdU-positive cells comprised only a
small number of the cells within spheroids, indicating that only a
small percentage of the cells (<5%) was actively proliferating
in 3D culture (Fig. 3G–I). The
spheroids were found to have an average diameter of 100 μm
(Fig. 3J).
Hepatic gene and protein expression of
MSC-derived cells
To determine whether the 3D spheroid culture in the
DLS promoted hepatocyte maturation of the hepatocyte-like cells,
the transcription levels of various genes associated with hepatic
development were examined. A comparison of gene expression during
the hepatic differentiation of BM-MSCs within the 2D culture, 2D +
DLS culture, 3D culture and 3D + DLS culture was performed using
RT-qPCR. The transcription levels of various genes associated with
hepatic development, namely hepatocyte nuclear factor 1β
(HNF1β), hepatocyte nuclear factor 6 (HNF6),
AFP, CK19, ALB, tyrosine aminotransferase
(TAT), forkhead box A1 (FOXA1), arginase 1
(ARG1), and the members of the cytochrome P450 subunits
cytochrome P450, family 1, subfamily A, members 1 and 2
(CYP1A1 and CYP1A2) were examined. The expression
levels of these genes were similar in the 3D and 2D + DLS groups,
and levels in both were clearly higher than in traditional 2D
culture. We found that the relative gene expression levels of
differentiating BM-MSCs within the 3D + DLS group after 3 weeks of
hepatic induction were significantly higher than the other groups
(p<0.05), which indicated that 3D spheroid culture in the DLS
provided a preferable external environment for differentiation
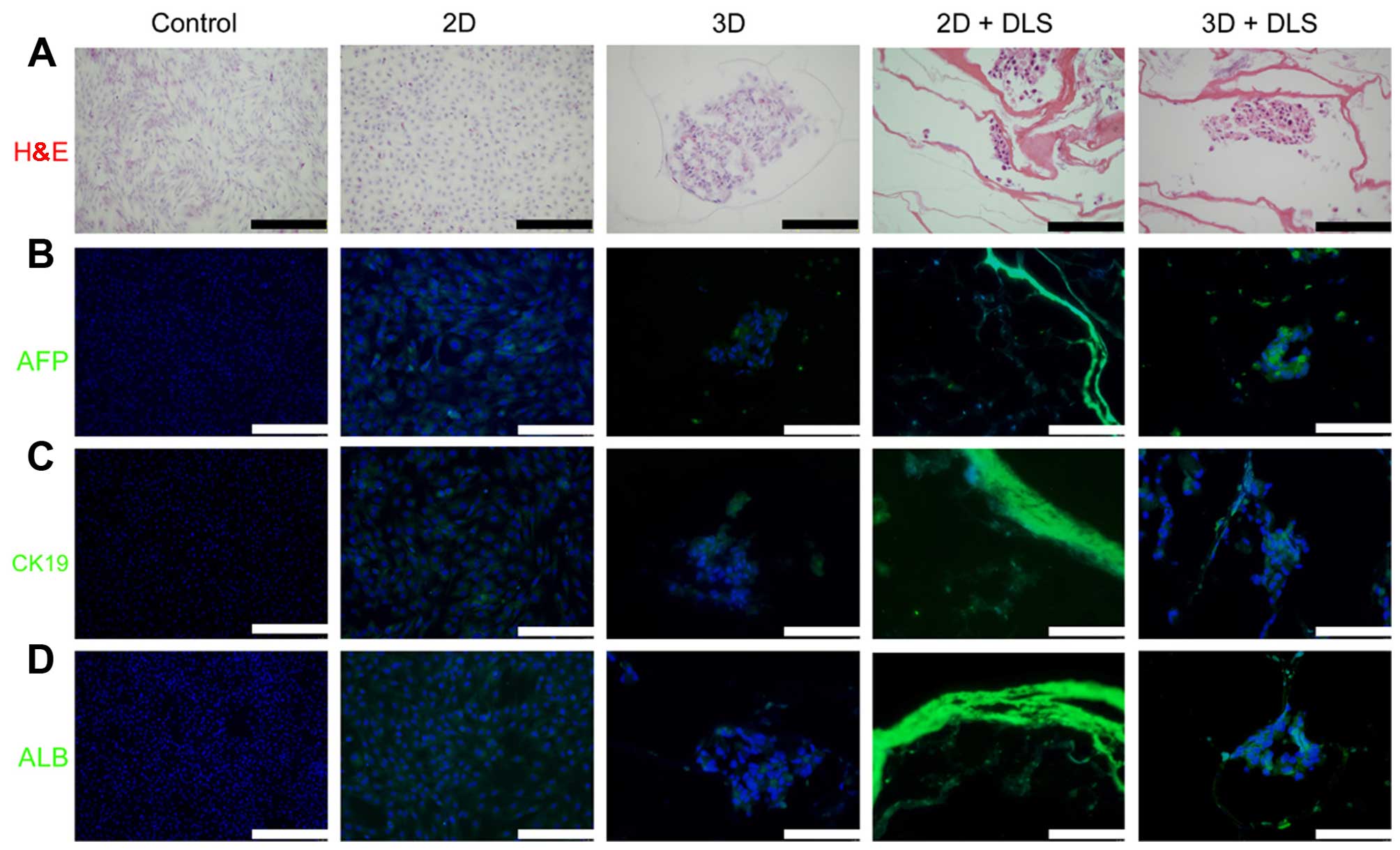
(Fig. 4). Immunofluorescence
analysis was then performed to reveal the expression of various
hepatic progenitor protein markers, namely AFP, CK19 and ALB
(Fig. 5). The high AFP and CK19
expression observed in the cells in the 3D + DLS group may
represent the phenotype of hepatic progenitors, which is consistent
with the RT-qPCR results.
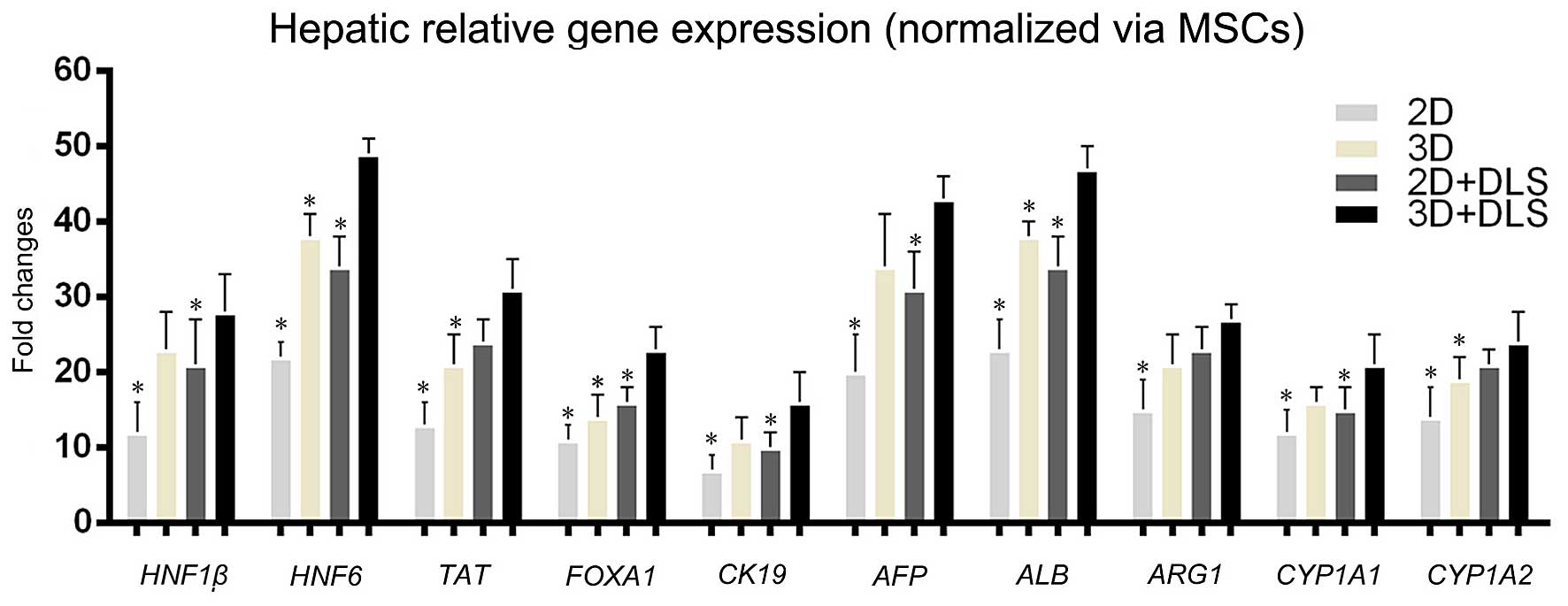 | Figure 4Hepatic-related gene expression
analysis measured by RT-qPCR. Comparison of gene transcription
levels after 3 weeks of hepatic induction among all the groups.
Hepatocyte nuclear factor 1β (HNF1β), hepatocyte nuclear
factor 6 (HNF6), alpha fetoprotein (AFP), cytokeratin
19 (CK19), albumin (ALB), tyrosine aminotransferase
(TAT), forkhead box A1 (FOXA1), arginase 1
(ARG1), and the members of the cytochrome P450 subunits,
family 1, subfamily A, members 1 and 2 (CYP1A1 and
CYP1A2) were examined. Statistically significant differences
relative to levels of undifferentiated mesenchymal stem cells
(MSCs) using the two-dimensional (2D) approach, which were
arbitrarily set to 1.0, are indicated. *p<0.05 vs.
three-dimensional (3D) + decellularized liver scaffold (DLS)
group. |
Functional analysis of differentiated
BM-MSCs
We evaluated a number of hepatic functions in the
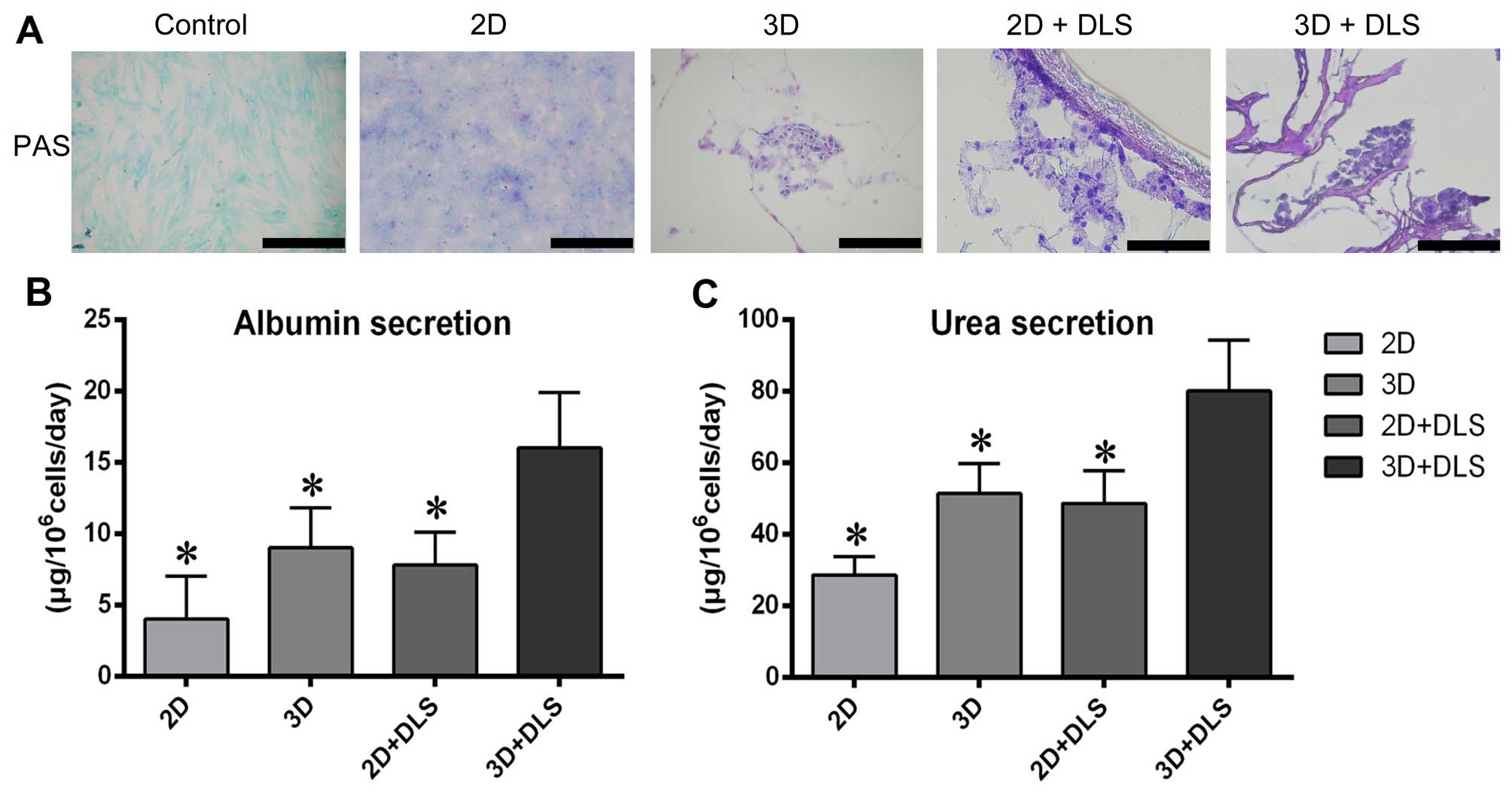
differentiated BM-MSCs in all groups. PAS staining revealed that
after hepatic differentiation, the 3D + DLS group had the highest
glycogen storage capability (Fig.
6A). To evaluate metabolic activity, we quantified ALB
secretion and urea production by the differentiated BM-MSCs in all
four groups after 3 weeks of hepatic induction. The cumulative ALB
level of the differentiated cells in the 3D + DLS group was
significantly higher than in the other groups (p<0.05) (Fig. 6B). The urea concentration in the
media produced by the differentiated cells in the 3D + DLS group
was markedly higher than that in the 2D culture media over the same
period of time (p<0.05) (Fig.
6C). Taken together, these findings clearly demonstrate the
superiority of the 3D spheroid culture on the DLS in terms of
supporting the hepatic differentiation of BM-MSCs.
Discussion
Functional hepatocytes may be exploited for clinical
and scientific applications. The generation of stem cell-derived
hepatocytes holds considerable promise for future clinical
applications. However, there remain some obstacles to obtaining
abundant supplies of hepatocyte-like cells. In the present study, a
natural 3D scaffold from decellularized liver matrix was used to
optimize the differentiation of MSC spheroids to functional
hepatocytes. Our data demonstrated that the 3D biomatrix scaffold
as well as spheroid culture promotes the induction of
lineage-specific differentiation of MSCs into hepatocyte-like
cells, which is consistent with the findings of other studies
(15–17,20,21). Moreover, we suggest that the
combination of cell-cell interactions and ECM plays a positive role
in the differentiation of MSCs to hepatocyte-like cells.
3D spheroid cultures are useful for maintaining
primary hepatocyte functions, which may be enhanced by effective
cell-cell interactions. In our experiments, 3D culture spheroids
were generated from BM-MSCs using a novel rocking culture system.
It is known that the size of the spheroid is important for
generating spheroids with high viability, and spheroids up to 100
μm in diameter are not oxygen limited (25). To prevent necrosis of the core
caused by a deficiency of oxygen and nutrients, the diameter of the
spheroid was controlled by the cell seeding density, culture time
and the rocking speed. Previous 3D spheroid culture systems often
involved a cell-accumulation technique in microwells with certain
types of cells (15,16). However, these culture systems
experienced difficulties in terms of producing sufficient numbers
of MSC spheroids. Our Rocker system is therefore a convenient
device that forms abundant spheroids from single MSCs.
Compared to the 2D culture system, the aggregate
culture yielded more hepatocyte-like cells using the same culture
volume. Moreover, it promoted higher transcript levels of
hepatocyte-specific genes and more mature functions in the final
stages of differentiation. This may have been due to cell-cell
interactions, which are observed in the native environment of
hepatocytes. In addition, the functional polarity of cells in
spheroids has been shown to help in enhancing and stabilizing the
differentiated functions of hepatocytes (26). Whether similar positive effects of
3D culture on hepatocytes are involved in enhancing the
differentiation of stem cells certainly warrants further
investigation. Taken together, these findings indicate that the 3D
culture provides more suitable conditions for producing
hepatocyte-like cells than the mono-layer culture.
Whole-organ decellularization is an attractive
technique for the preparation of a natural biomatrix scaffold. To
date, the potential application of this technique has been
demonstrated successfully for a number of organs, including the
heart, lung, liver, kidney and bladder (27). A decellularized native
liver-derived bioscaffold may provide a suitable environment for
differentiation by providing not only a 3D structure but also by
maintaining bioactive molecules. Thus, decellularized organs may
potentially be used as a tool for stem cell differentiation and
maturation to eventually engineer autologous liver grafts for
transplantation, as previously described (20,21). Triton X-100 is usually used to
solubilize cellular membranes, and SDS is used to clear the
remaining nuclear remnants from the matrix. It has been noted
previously that the order of detergents used in the
decellularization procedure has an effect on retaining the ECM
(28). In the present study, we
utilized a unique protocol based on a Triton-SDS-Triton perfusion
to prepare the decellularized liver, creating a translucent liver
matrix within a relatively short period of time in which the porous
architecture and partial ECM of the original organ was
preserved.
The composition and concentration of ECM proteins
are important for cell attachment, growth and differentiation
(19). Although it has been
reported that the liver biomatrix scaffold exhibited independent
inductive potential for the differentiation of MSCs into cells of
hepatic lineage, hepatic growth factors and cytokines were adopted
in the differentiation protocol of the seeded cells in all groups
in order to achieve maximal hepatic induction (20). When compared with the 2D culture
system, the extensive analyses of synthetic and metabolic functions
demonstrated that the MSCs cultured in the DLS exhibited more
abundant and stable functions. The DLS generated in this study is
capable of efficiently promoting the hepatic differentiation of
MSCs, implying high efficiency in mass transfer. These results
suggest that the hepatic maturation of the differentiated BM-MSCs
was higher in the DLS than in monolayer cultures.
3D cell culture systems are thought to more closely
resemble the physiological tissue environment by enabling greater
cell-cell and cell-matrix interactions than conventional monolayer
culture techniques. 3D spheroid cultures and 3D DLS cultures
provide different functional supports for MSC differentiation. To
the best of our knowledge, this study is the first to use a
combination of these two culture techniques to successfully
generate functional hepatocyte-like cells from MSCs. The
upregulation of hepatic-enriched transcription factors
(HNF1β, HNF6 and FOXA1), hepatic progenitor
marker proteins (AFP and CK19), liver-associated
enzymes (TAT and ARG1), plasma protein (ALB),
and conjugating enzymes (CYP1A1 and CYP1A2) was
observed in the MSC spheroids cultured on DLSs, and the expression
levels were significantly higher than in the other groups.
Moreover, the protein expression and hepatic-specific functions
confirmed the hepatic differentiation of the MSC spheroids in the
DLS culture. These results suggest that this culture combination
promotes the hepatic differentiation of murine MSCs into high
yields of mature hepatocytes.
In conclusion, in this study we discussed the in
vitro production of functional hepatocytes from BM-MSC
spheroids on DLSs. Our findings may have future applications in
stem cell-based liver regenerative medicine for the treatment of
liver injuries and the establishment of a bioartificial liver.
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
BM-MSCs
|
bone marrow-derived MSCs
|
|
2-D
|
two-dimensional
|
|
3-D
|
three-dimensional
|
|
ECM
|
extracellular matrix
|
|
DLS
|
decellularized liver scaffold
|
|
H&E
|
hematoxylin and eosin
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
GAGs
|
glycosaminoglycans
|
|
SEM
|
scanning electron microscopy
|
|
IMDM
|
Iscove's modified Dulbecco's
medium
|
|
EGF
|
epidermal growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
HGF
|
hepatocyte growth factor
|
|
EdU
|
5-ethynyl-2′-deoxyuridine
|
|
RT-qPCR
|
reverse transcription
quantitative-polymerase chain reaction
|
|
OCT
|
optimum cutting temperature
|
|
PBS
|
phosphate-buffered solution
|
|
AFP
|
alpha fetoprotein
|
|
ALB
|
albumin
|
|
CK19
|
cytokeratin 19
|
|
PAS
|
periodic acid-Schiff
|
|
SDS
|
sodium dodecyl sulfate
|
|
HNF1β
|
hepatocyte nuclear factor 1β
|
|
HNF6
|
hepatocyte nuclear factor 6
|
|
TAT
|
tyrosine aminotransferase
|
|
FOXA1
|
Forkhead box A1
|
|
ARG1
|
arginase 1
|
Acknowledgments
The present study received funding from the National
Natural Scientific Foundations of China (no. 81200315), and the
Sichuan Province Science and Technology Support Project (no.
2013SZ0080).
References
|
1
|
Brown RS Jr: Live donors in liver
transplantation. Gastroenterology. 134:1802–1813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vosough M, Moslem M, Pournasr B and
Baharvand H: Cell-based therapeutics for liver disorders. Br Med
Bull. 100:157–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nussler A, Konig S, Ott M, Sokal E, Christ
B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M, et
al: Present status and perspectives of cell-based therapies for
liver diseases. J Hepatol. 45:144–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zamule SM, Coslo DM, Chen F and Omiecinski
CJ: Differentiation of human embryonic stem cells along a hepatic
lineage. Chem Biol Interact. 190:62–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shafritz DA, Oertel M, Menthena A,
Nierhoff D and Dabeva MD: Liver stem cells and prospects for liver
reconstitution by transplanted cells. Hepatology. 43(Suppl 1):
S89–S98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz RE, Reyes M, Koodie L, Jiang Y,
Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS and Verfaillie CM:
Multipotent adult progenitor cells from bone marrow differentiate
into functional hepatocyte-like cells. J Clin Invest.
109:1291–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang
SH, Yang IH, Park HK, Han H and Kim H: In vitro differentiation of
human umbilical cord blood-derived mesenchymal stem cells into
hepatocyte-like cells. Biochem Biophys Res Commun. 330:1153–1161.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lange C, Bassler P, Lioznov MV, Bruns H,
Kluth D, Zander AR and Fiegel HC: Hepatocytic gene expression in
cultured rat mesenchymal stem cells. Transplant Proc. 37:276–279.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chivu M, Dima SO, Stancu CI, Dobrea C,
Uscatescu V, Necula LG, Bleotu C, Tanase C, Albulescu R, Ardeleanu
C and Popescu I: In vitro hepatic differentiation of human bone
marrow mesenchymal stem cells under differential exposure to
liver-specific factors. Transl Res. 154:122–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee KD, Kuo TKC, Whang-Peng J, Chung YF,
Lin CT, Chou SH, Chen JR, Chen YP and Lee OK: In vitro hepatic
differentiation of human mesenchymal stem cells. Hepatology.
40:1275–1284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong JZ, Sarrazin S, Cassio D, Gauthier F
and Alvarez F: Application of spheroid culture to human hepatocytes
and maintenance of their differentiation. Biol Cell. 81:77–81.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Guo G, Li L, Chen F, Bao J, Shi YJ
and Bu H: Three-dimensional spheroid culture of human umbilical
cord mesenchymal stem cells promotes cell yield and stemness
maintenance. Cell Tissue Res. 360:297–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frith JE, Thomson B and Genever PG:
Dynamic three-dimensional culture methods enhance mesenchymal stem
cell properties and increase therapeutic potential. Tissue Eng Part
C Methods. 16:735–749. 2010. View Article : Google Scholar
|
|
15
|
Subramanian K, Owens DJ, O'Brien TD,
Verfaillie CM and Hu WS: Enhanced differentiation of adult bone
marrow-derived stem cells to liver lineage in aggregate culture.
Tissue Eng Part A. 17:2331–2341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subramanian K, Owens DJ, Raju R, Firpo M,
O'Brien TD, Verfaillie CM and Hu WS: Spheroid culture for enhanced
differentiation of human embryonic stem cells to hepatocyte-like
cells. Stem Cells Dev. 23:124–131. 2014. View Article : Google Scholar :
|
|
17
|
Takayama K, Kawabata K, Nagamoto Y,
Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa
T, Furue MK and Mizuguchi H: 3D spheroid culture of
hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing.
Biomaterials. 34:1781–1789. 2013. View Article : Google Scholar
|
|
18
|
Ng SL, Narayanan K, Gao S and Wan AC:
Lineage restricted progenitors for the repopulation of
decellularized heart. Biomaterials. 32:7571–7580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Cui CB, Yamauchi M, Miguez P,
Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier
C, et al: Lineage restriction of human hepatic stem cells to mature
fates is made efficient by tissue-specific biomatrix scaffolds.
Hepatology. 53:293–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji R, Zhang N, You N, Li Q, Liu W, Jiang
N, Liu J, Zhang H, Wang D, Tao K and Dou K: The differentiation of
MSCs into functional hepatocyte-like cells in a liver biomatrix
scaffold and their transplantation into liver-fibrotic mice.
Biomaterials. 33:8995–9008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang WC, Cheng YH, Yen MH, Chang Y, Yang
VW and Lee OK: Cryo-chemical decellularization of the whole liver
for mesenchymal stem cells-based functional hepatic tissue
engineering. Biomaterials. 35:3607–3617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Bao J, Zhou YJ, Wang YJ, Du ZG, Shi
YJ, Li L and Bu H: Optimizing perfusion-decellularization methods
of porcine livers for clinical-scale whole-organ bioengineering.
Biomed Res Int. 2015:7854742015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao J, Fisher JE, Lillegard JB, Wang W,
Amiot B, Yu Y, Dietz AB, Nahmias Y and Nyberg SL: Serum-free medium
and mesenchymal stromal cells enhance functionality and stabilize
integrity of rat hepatocyte spheroids. Cell Transplant. 22:299–308.
2013. View Article : Google Scholar :
|
|
24
|
Cheng NC, Wang S and Young TH: The
influence of spheroid formation of human adipose-derived stem cells
on chitosan films on stemness and differentiation capabilities.
Biomaterials. 33:1748–1758. 2012. View Article : Google Scholar
|
|
25
|
Glicklis R, Merchuk JC and Cohen S:
Modeling mass transfer in hepatocyte spheroids via cell viability,
spheroid size, and hepatocellular functions. Biotechnol Bioeng.
86:672–680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haouzi D, Baghdiguian S, Granier G, Travo
P, Mangeat P and Hibner U: Three-dimensional polarization
sensitizes hepatocytes to Fas/CD95 apoptotic signalling. J Cell
Sci. 118:2763–2773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crapo PM, Gilbert TW and Badylak SF: An
overview of tissue and whole organ decellularization processes.
Biomaterials. 32:3233–3243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabetkish S1, Kajbafzadeh AM, Sabetkish N,
Khorramirouz R, Akbarzadeh A, Seyedian SL, Pasalar P, Orangian S,
Beigi RS, Aryan Z, et al: Whole-organ tissue engineering:
decellularization and recellularization of three-dimensional matrix
liver scaffolds. J Biomed Mater Res A. 103:1498–1508. 2015.
View Article : Google Scholar
|