Introduction
Allergic inflammation is accompanied by the
coordinated expression of a number of genes and proteins that
initiate, sustain, and propagate immune responses and tissue
remodeling (1). Mast cells are
immune cells of hematopoietic origin that contribute to host
defense mechanisms through the receptor-mediated release of
inflammatory mediators (2), as
well as the inflammatory reactions associated with allergic
disorders such as asthma and anaphylaxis (3,4).
IgE-mediated degranulation and cytokine production by mast cells
were shown to be reduced by piperine through the inhibition of Lyn,
p38, extracellular signal-regulated kinase (ERK), and Ras
phosphorylation (5). FcεRI-MCs
release TNF-α and interleukin-6 (IL-6), which trigger anaphylaxis
and mediate the symptoms and tissue effects of chronic atopic
disorders (6). Mast cells were
shown to be increased in the asthmatic airways, followed by IL-6
release (7).
IL-6 is a pleiotropic cytokine involved in the
regulation of inflammatory and immunological responses, acute phase
protein production, and hematopoiesis (8). Serum sIL-6R levels are increased in
asthma patients (9), indicating
the likely involvement of IL-6 in the pathogenesis of allergic
inflammation. Pre-incubation of mast cells with IL-6 can
significantly upregulate the IgE-mediated histamine release
(10). Such observations
indicated that IL-6 has a close relationship with mast
cell-mediated inflammation. Regulation of inflammatory responses is
ensured by coordinated control of the gene expression in
participating immune system and tissue cells. A class of short
single-stranded RNA molecules termed microRNAs (miRNAs or miRs)
have been demonstrated to be involved in the regulation of
inflammatory responses (11).
miRNAs constitute a large family of small non-coding
RNAs that have emerged as key post-transcriptional regulators in a
wide variety of organisms (12).
Previous findings demonstrated the associations of miRNAs with many
types of inflammatory state. Upregulation of miRNA-221 has been
detected in lung biopsy specimens in an OVA-induced murine asthma
model, whereas the inhibition of miR-221 reduced airway
inflammation (13).
miR-223 was first identified bioinformatically and
subsequently characterized in the hematopoietic system, where it is
specifically expressed in the myeloid compartment (14,15). miR-223 is fairly specific to the
hematopoietic lineage, where it limits inflammation and prevents
collateral damage during infection (16). Previous studies have reported that
miR-223 can regulate neutrophil activity and inflammation (17). The downregulation of miR-223 was
shown to promote IL-6 and IL-1β expression in macrophages (18). It is clear that miR-223 is
important in inhibiting the development of inflammatory cells;
however, miR-223 function in mast cells remains unclear. The
present study aimed to examine the relationship between miR-223 and
inflammation in mast cells, and determine the underlying molecular
mechanisms.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco-BRL (Grand Island, NY,
USA). LY294002 and Cell Counting Kit-8 (CCK-8) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The mouse IL-6 immunoassay kit
was obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
Specific monoclonal antibodies against AKT (#9272) and
phosphorylated-AKT (p-AKT; #4060) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Specific polyclonal
antibodies against insulin-like growth factor-1 receptor (IGF1R;
sc-713) and GAPDH (sc-25778) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The secondary antibody, goat
anti-rabbit IgG-HRP (sc-2004) was also obtained from Santa Cruz
Biotechnology, Inc.
Cell culture and transfection
Mast cells purchased from the Shanghai Institute of
Cell Library (Shanghai, China) were cultured in DMEM supplemented
with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin at
37°C in 5% CO2. The cells were transfected with miR-223
lentiviral vector or miR-223 inhibitor using the Lipofectamine 2000
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) followed
by 48 h of culture. Supernatants and cells were then rinsed with
ice-cold PBS and cells were collected for subsequent analysis.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from mast cells using TRIzol
reagent (Invitrogen Life Technologies). Total RNA (200 ng) from
each specimen was used for primer-specific reverse transcription
(RT). RT was performed using the TaqMan MicroRNA Reverse
Transcription kit, according to the manufacturer's instructions
(Applied Biosystems Life Technologies, Foster City, CA, USA). The
cDNA samples were used for quantitative PCR, according the TaqMan
MicroRNA assay kit manufacturer instructions (Applied Biosystems
Life Technologies). Data were analyzed by the 2−ΔΔCt
method.
Enzyme-linked immunosorbent assay
(ELISA)
IL-6 amounts were measured using ELISA kits from
R&D Systems, Inc., according to the manufacturer's
instructions. IL-6 levels were expressed as a ratio of IL-6 amounts
to cell number.
Microarray analysis
To identify the possible changes in the signaling
pathways following miR-223 expression, the cells were divided into
the blank control and miR-223 knockdown groups and subjected to
microarray analysis (Kangchen Bio-tech Inc., Shanghai, China), as
previously described (19).
Luciferase reporter assay
Mast cells were seeded in 24-well tissue culture
plates the day prior to transfection. miR-223-3p mimics (mimics NC
as control group) and IGFR-1-3′UTR vector in luciferase reporter
vector were transiently co-transfected into cells. After 48 h, the
cells were lysed and luciferase activity was detected with the Dual
Luciferase Reporter Assay kit (Promega Corp., Madison, WI, USA) to
validate the role of miRNA target gene in inhibiting
translation.
Western blotting
Total protein (50 µg) from each sample was
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and transferred onto nitrocellulose membranes. The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature (20°C) and probed with the corresponding primary
antibodies overnight at 4°C. After three washes with TBST, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody at room temperature (20°C) for 2 h. Proteins
were detected using the enhanced chemiluminescence detection system
(Bio-Rad, Hercules, CA, USA). Bands were captured by the Image Lab
system (Bio-Rad) and their densities were analyzed after
normalization by GAPDH levels.
Statistical analysis
Statistical analysis was performed using the SPSS
software (SPSS 20.0; Chicago, IL, USA). Data were presented as mean
± standard deviation (SD) from at least three independent
experiments. The Student's t-test was used to analyze the
differences between two groups. P<0.05 was considered
statistically significant.
Results
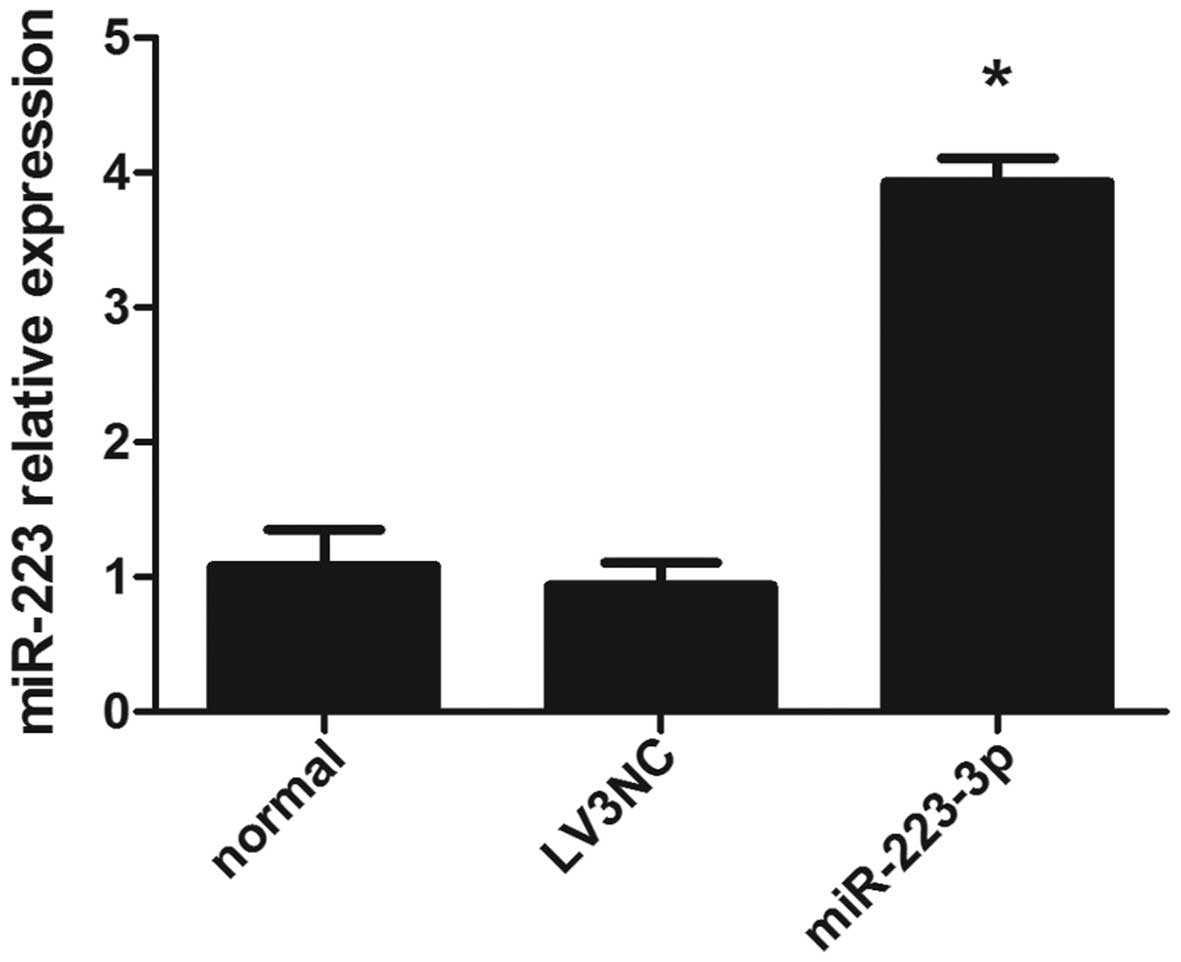
Overexpression of miR-223 in mast
cells
To assess the effect of miR-223 on IL-6 expression
in mast cells, the cells were transfected with miR-223 lentiviral
vector and miR-223 expression was detected by RT-qPCR. As expected,
miR-223 levels were higher following transfection with lentiviral
vector into mast cells, compared with the negative control and
blank control group (Fig. 1).
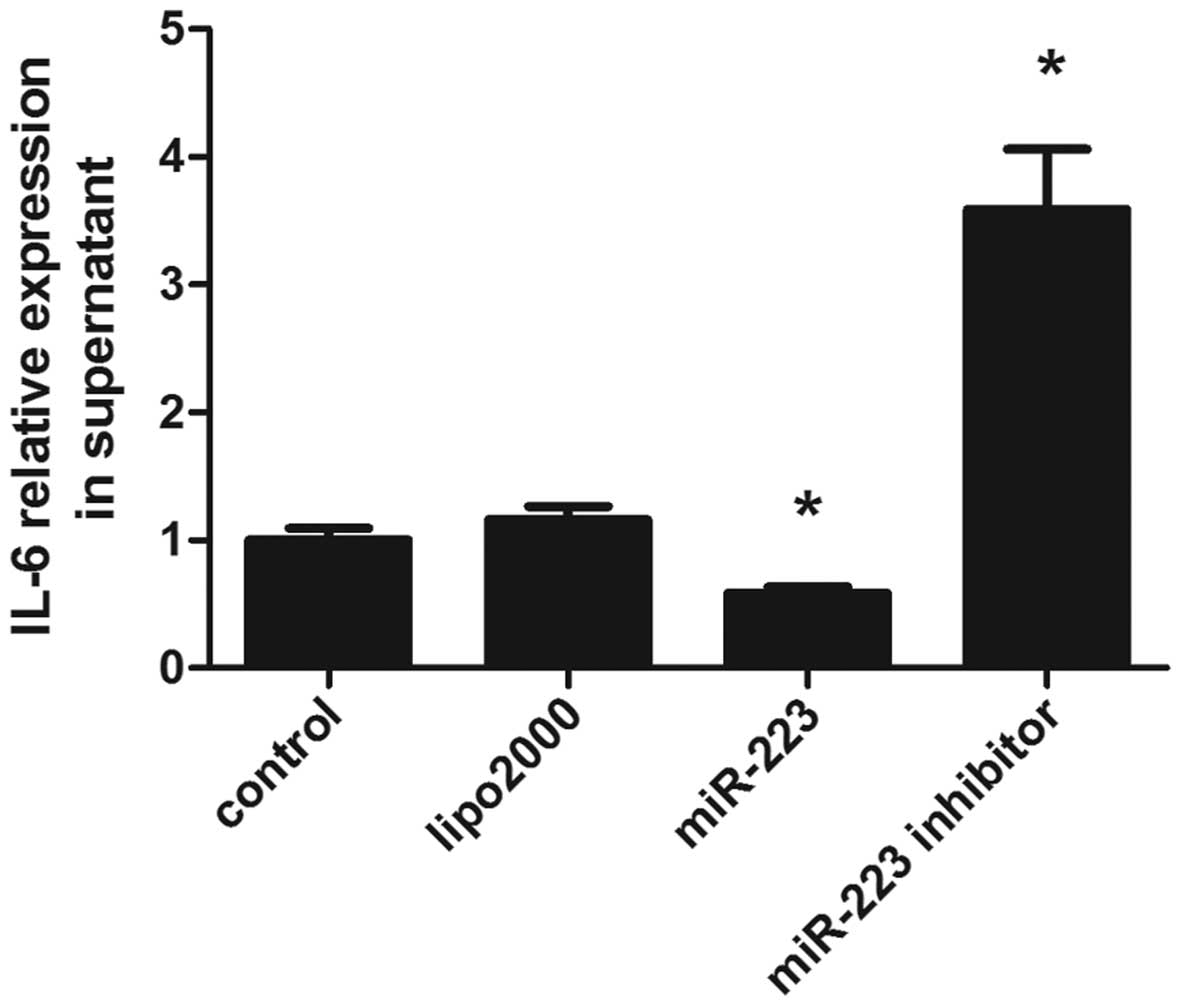
Overexpression of miR-223 inhibits IL-6
secretion
IL-6 is a multifunctional cytokine produced by a
wide variety of cells and plays vital roles in immunological
responses, hematopoiesis, host defense, and acute phase reaction
(20). The involvement of miR-223
has been demonstrated in various types of cancer, inflammatory
diseases, autoimmune diseases and other pathological processes
(21). Following the transfection
of miR-223 lentiviral vector into mast cells, the cells were
cultured for 48 h, and the supernatants were collected for IL-6
detection by ELISA. Overexpression of miR-223 suppressed IL-6
secretion, while miR-223 downregulation resulted in increased IL-6
levels (Fig. 2).
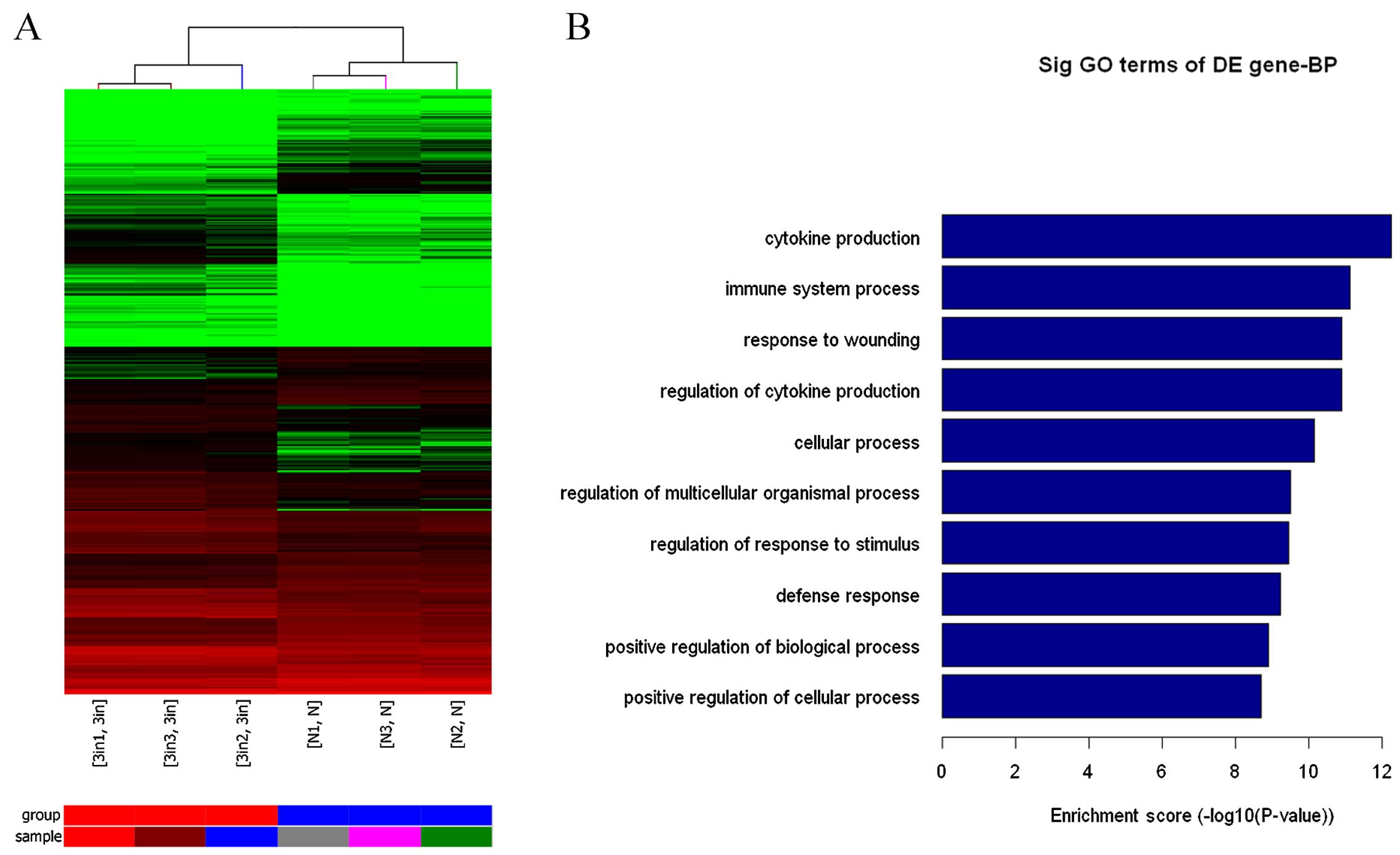
Changes in signaling pathways in mast
cells differentially expressing miR-223
After transfection of miR-223 lentiviral vector into
mast cells, miR-223 markedly altered the phosphatidylinositol
3-kinase (PI3K)-AKT signaling pathway as assessed by gene chip,
bioinformatics analysis and pathway analysis. Conversely, this
pathway was activated by miR-223 downregulation. These findings
indicate that the main function of miR-223 may be associated with
cytokine secretion in mast cells. According to the gene chip and
pathway analysis, miR-223 regulated IL-6 secretion via IGF1R/PI3K
signaling in mast cells (Fig.
3).
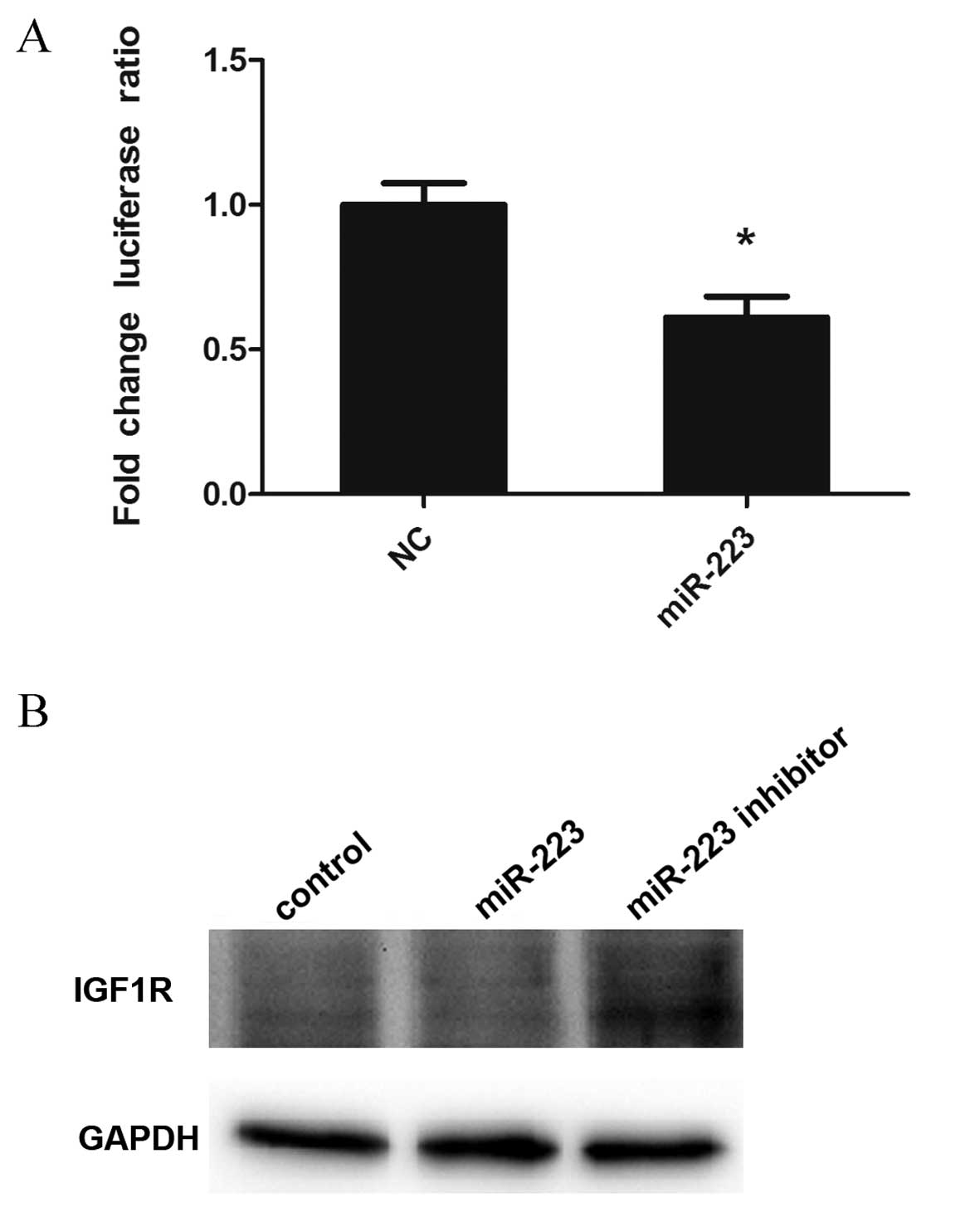
Luciferase reporter assay and western
blot analysis confirm IGF1R as a target gene of miR-223
According to our gene chip data and bioinformatics
analysis, IGF1R was a target gene of miR-223 in mast cells. To
assess whether miR-223 directly affects IGF1R, luciferase reporter
technology was used. As shown in Fig.
4A, IGF1R was confirmed as a target gene of miR-223 in mast
cells. In addition, IGF1R levels were detected in experimental and
control groups by western blotting. The results showed reduced
IGF1R expression in cells overexpressing miR-223, whereas the
downregulation of miR-223 produced the opposite effects (Fig. 4B).
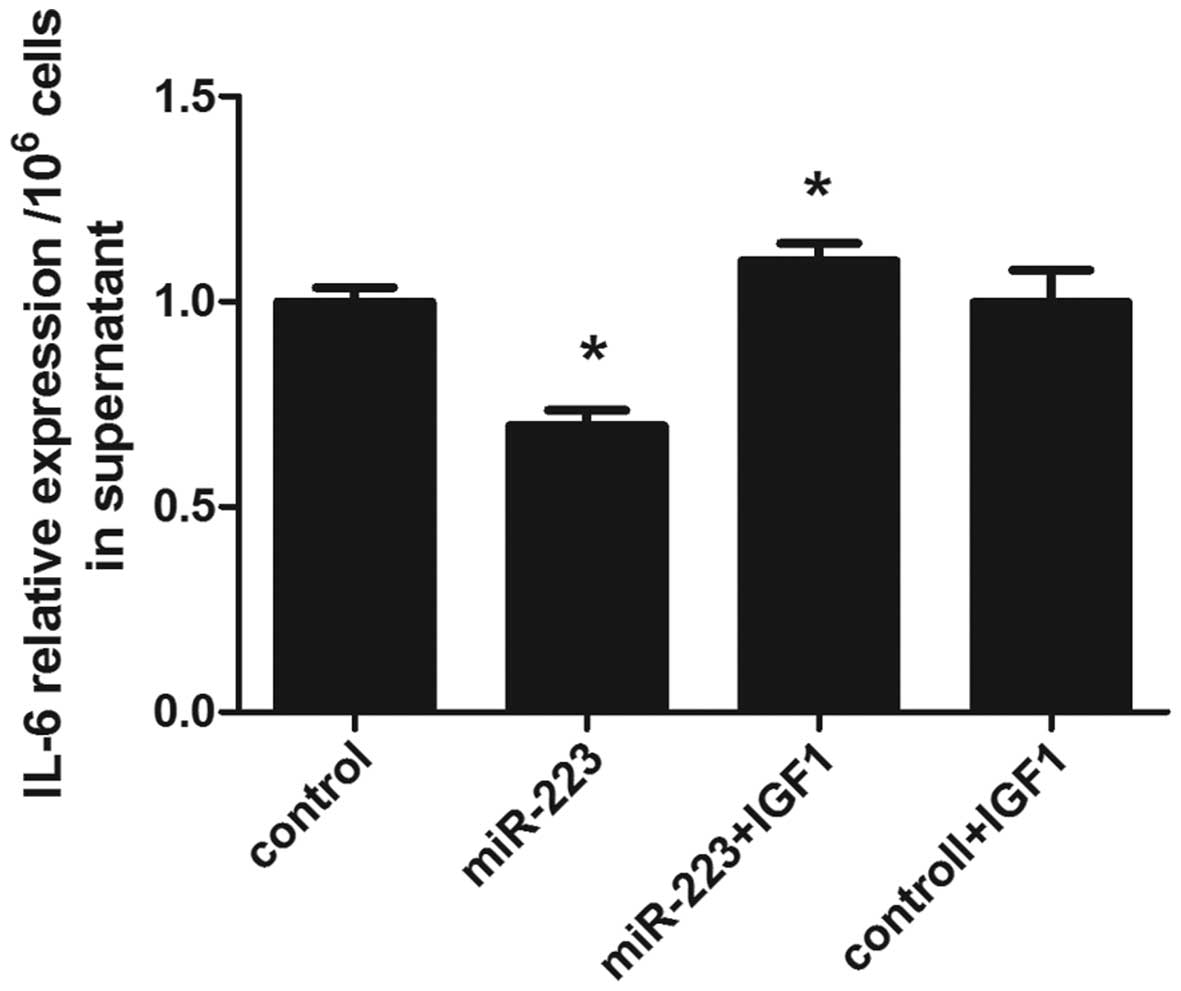
IGF-1 promotes IL-6 secretion following
the upregulation of miR-223 in mast cells
Luciferase reporter gene and western blot analysis
confirmed IGF1R as a target gene of miR-223. Thus, mast cells were
transfected with insulin-like growth factor-1 (IGF1), and IL-6
secretion was subsequently assessed using ELISA. It was found that
incubation with IGF-1 reversed the IL-6 concentration decrease
caused by miR-223 in mast cells (Fig.
5).
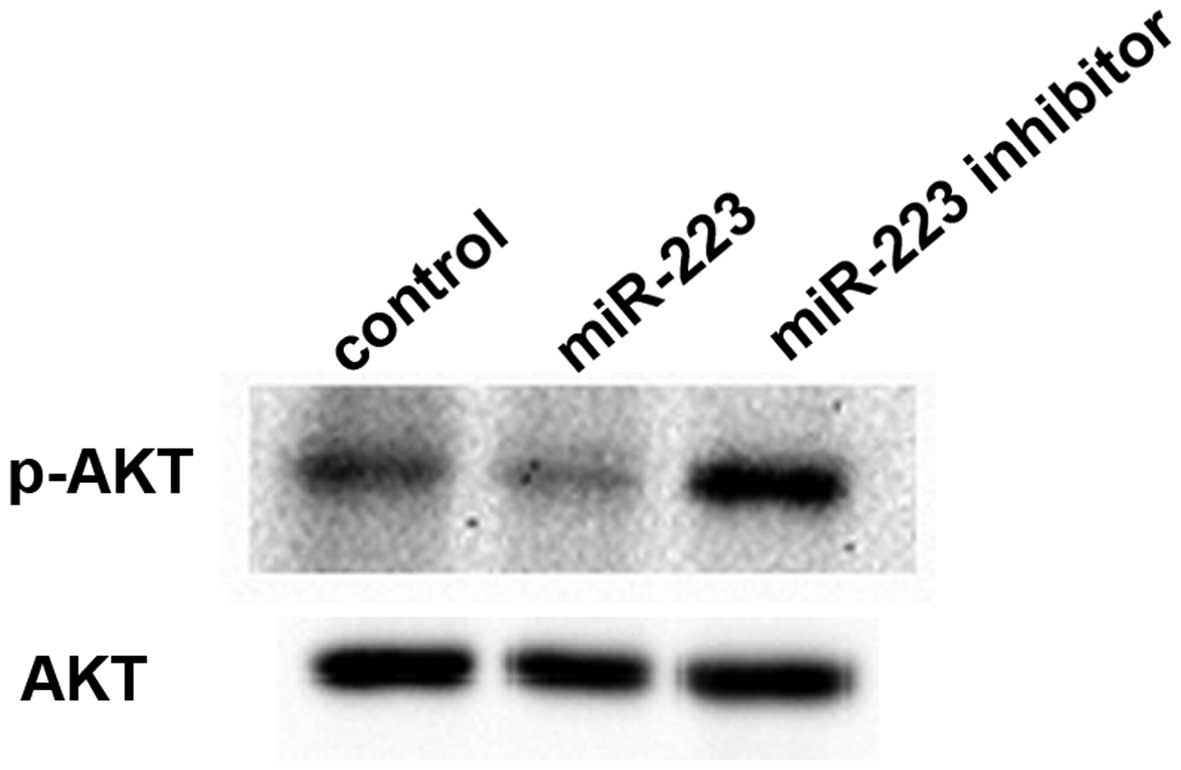
miR-223 inhibits the PI3K-AKT signaling
pathway in mast cells
Since miR-223 suppressed IGF1R expression, we
assessed whether the IGF1R-mediated downstream signaling pathway
was also affected by miR-223. Gene chip and pathway analysis
predicted that PI3K-AKT signaling was negatively regulated by
miR-223 in mast cells. The expression levels of AKT, an essential
protein kinase in the PI3K-AKT pathway downstream of IGF1R, and its
phosphorylated form p-AKT were evaluated by western blotting. We
found that total AKT protein levels were similar in the three
groups. However, phosphorylated AKT protein levels were reduced
after the downregulation of miR-223 and increased after the miR-223
upregulation compared with the control group (Fig. 6).
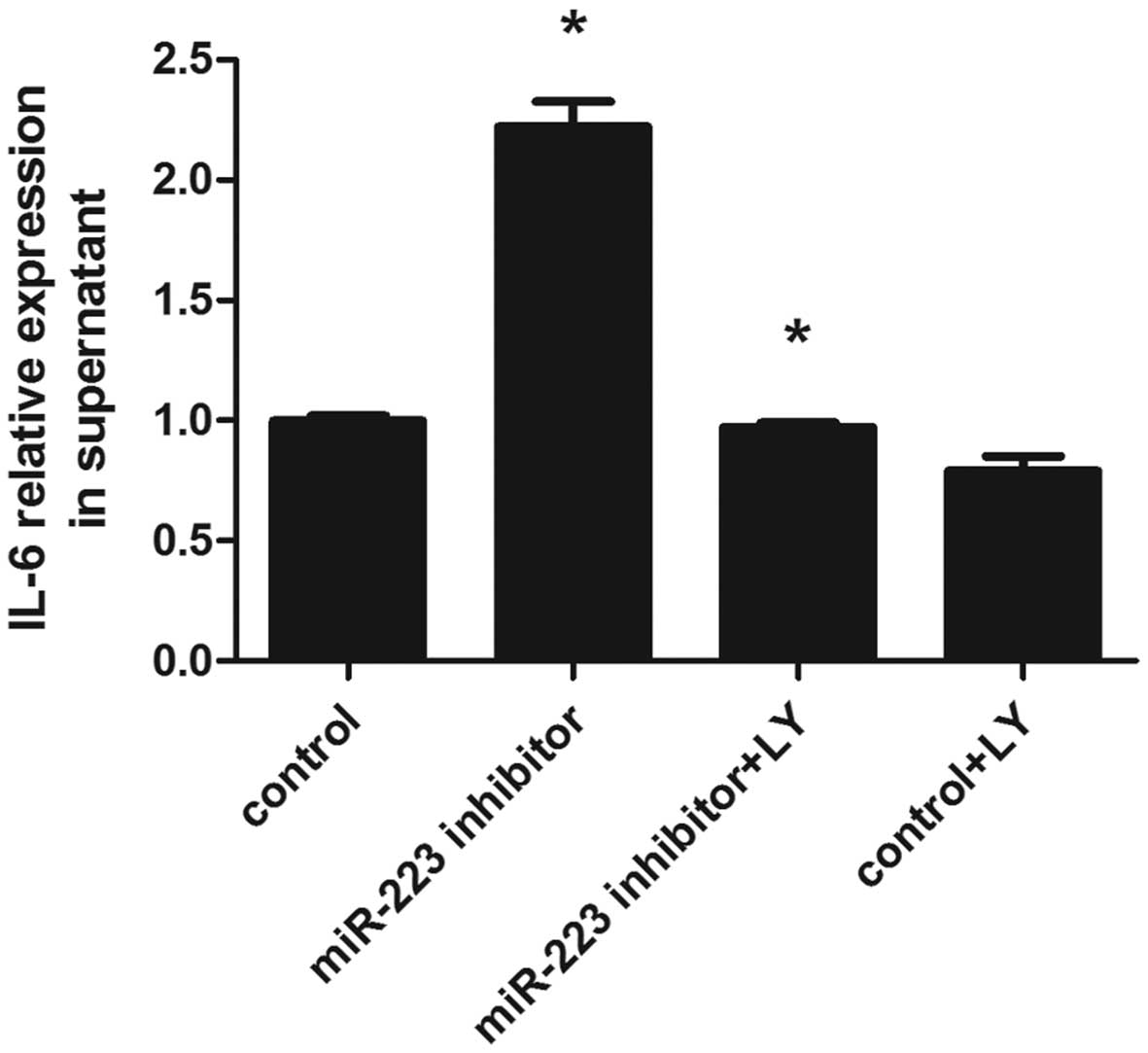
Specific PI3K-inhibitor LY294002
decreases IL-6 concentration
PI3K activity is essential for the differentiation
of mast cells, as well as their long-term survival and function
(22). PI3K inhibitors, including
wortmannin and LY294002, have been widely reported to inhibit
antigen-mediated degranulation and cytokine production in rodent
and human mast cells (23–25).
To investigate whether the PI3K-AKT signaling pathway plays a role
in IL-6 secretion, the specific PI3K-inhibitor LY294002 was used to
treat control cells and those with silenced miR-223: cells were
incubated with LY294002 for 30 min prior to treatment with LPS for
6 h. Subsequently, IL-6 secretion in the supernatants was detected
by ELISA. As shown in Fig. 7,
LY294002 blocked the PI3K-AKT signaling pathway and reversed IL-6
induction by suppressing miR-223 in mast cells.
Discussion
It has been demonstrated that miR-223 targets
leukemia fusion protein, providing the evidence for a link between
epigenetic silencing of a miRNA locus and the differentiation block
of myeloid precursors (26). In
the present study, miR-223 was identified as an important modulator
of IL-6 secretion in mast cells. Previous studies have reported the
vital role of mast cells in inflammatory reactions. MC-derived
IL-10 was also shown to reduce B-cell responses and antibody
production (27). miR-155
expression enhances FcεRI degranulation and the release of TNFα,
IL-6, and IL-13 in relation to the activity of the PI3K/AKT pathway
in mast cells (28).
Sporothrix schenckii yeasts were shown to induce TNF-α and
IL-6 release, and activate the ERK signaling pathway in mast cells
(29).
In the present study, miR-223 was involved in the
regulation of cytokine secretion in mast cells. The relevant role
of miR-223 in the process was testified by the overexpression and
knockdown experiments. Upregulation of miR-223 in mast cells
resulted in reduced IL-6 secretion, while its downregulation
increased IL-6 expression. From the perspective of inflammatory
responses, miRNAs have recently been shown to be expressed in
immune cells and to target proteins involved in inflammation
regulation, consequently affecting the magnitude of the response
(30). For instance, miR-146a is
involved in the regulation of endothelial cell inflammation via the
modulation of Nox4 expression in a diabetic atherothrombosis model
(31). In addition, miR-155 was
shown to contribute to regulating inflammation of allergic airway
by modulating TH2 responses through the transcription factor PU.1
(32).
Based on previous studies, an increased number of
molecular mechanisms of the miR-223 effect in mast cells have been
revealed (33,34,37). Gene chip and bioinformatics
analysis predicted IGF1R as a target gene of miR-223, and that the
PI3K-AKT signaling pathway was decreased in mast cells.
Subsequently IGF1R was confirmed as a miR-223 target using
luciferase assay reporter. Overexpression of miR-223 in mast cells
resulted in decreased IGF1R and p-AKT protein levels compared to
control cells, while silencing miR-223 in mast cells increased
IGF1R and p-AKT protein expression. It is now recognised that
miRNAs exert their function of fine modulators of gene expression
by controlling translational efficiency of a large number of target
genes (35). It has been shown
that miR-223 targets NLRP3, and can prevent its early translation
in the myeloid lineage (36). In
addition, miR-223 targets FBXW7/hCdc4 expression at the
post-transcriptional level and appears to regulate cell apoptosis,
proliferation, and invasion in gastric cancer (37).
The results of the present study showed that miR-223
may regulate IL-6 levels via IGF1R/PI3K signaling in mast cells.
Similarly, the PI3K inhibitor LY294002 blocked AKT phosphorylation
and reversed the induction of IL-6 secretion caused by miR-223
knockdown in mast cells. When mast cells were incubated with IGF1,
IL-6 levels significantly increased compared with the control
cells. The PI3K-AKT signaling pathway has been previously
demonstrated to have a close relationship with inflammation. This
association has been well studied in various tumors and
inflammatory diseases. Overexpression of miR-223 during the dextran
sulfate sodium (DSS)-induced mouse model of colitis-associated
tumor growth inhibited AKT phosphorylation and IGF-1R expression
(38). The upregulated expression
of WNT5a in PCOS was shown to increase inflammation and oxidative
stress predominantly via the PI3K/AKT signaling pathway (39). B-type natriuretic peptide
post-conditioning also significantly inhibited a TNF-α and IL-6
level increase through the PI3K/AKT signaling pathway (40).
In conclusion, miR-223 decreased IL-6 secretion in
mast cells. Additionally, IGF1R, as a target gene of miR-223, and
the PI3K signaling pathway, are involved in the regulation of mast
cells by miR-223.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81200012, awarded
to F. L.) and the National Natural Science Foundation of China (no.
81370132, awarded to D. Z.).
References
|
1
|
Lu TX and Rothenberg ME: Diagnostic,
functional, and therapeutic roles of microRNA in allergic diseases.
J Allergy Clin Immunol. 132:3–13; quiz 14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galli SJ and Tsai M: Mast cells in allergy
and infection: versatile effector and regulatory cells in innate
and adaptive immunity. Eur J Immunol. 40:1843–1851. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mekori YA and Metcalfe DD: Mast cells in
innate immunity. Immunol Rev. 173:131–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM, Wilson TM and Metcalfe DD: The
mast cell and allergic diseases: role in pathogenesis and
implications for therapy. Clin Exp Allergy. 38:4–18. 2008.
|
|
5
|
Huang J, Zhang T, Han S, Cao J, Chen Q and
Wang S: The inhibitory effect of piperine from Fructus piperis
extract on the degranulation of RBL-2H3 cells. Fitoterapia.
99:218–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sumpter TL, Ho CH, Pleet AR, Tkacheva OA,
Shufesky WJ, Rojas-Canales DM, Morelli AE and Larregina AT:
Autocrine hemokinin-1 functions as an endogenous adjuvant for
IgE-mediated mast cell inflammatory responses. J Allergy Clin
Immunol. 135:1019–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradding P, Roberts JA, Britten KM,
Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH and
Holgate ST: Interleukin-4, -5, and -6 and tumor necrosis
factor-alpha in normal and asthmatic airways: Evidence for the
human mast cell as a source of these cytokines. Am J Respir Cell
Mol Biol. 10:471–480. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina-Holgado E and Molina-Holgado F:
Mending the broken brain: Neuroimmune interactions in neurogenesis.
J Neurochem. 114:1277–1290. 2010.PubMed/NCBI
|
|
9
|
Yokoyama A, Kohno N, Sakai K, Kondo K,
Hirasawa Y and Hiwada K: Circulating levels of soluble
interleukin-6 receptor in patients with bronchial asthma. Am J
Respir Crit Care Med. 156:1688–1691. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanagida M, Fukamachi H, Ohgami K, Kuwaki
T, Ishii H, Uzumaki H, Amano K, Tokiwa T, Mitsui H, Saito H, et al:
Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4,
IL-5, and IL-6 on the survival of cultured human mast cells. Blood.
86:3705–3714. 1995.PubMed/NCBI
|
|
11
|
Rebane A and Akdis CA: MicroRNAs:
Essential players in the regulation of inflammation. J Allergy Clin
Immunol. 132:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayoral RJ, Pipkin ME, Pachkov M, van
Nimwegen E, Rao A and Monticelli S: MicroRNA-221-222 regulate the
cell cycle in mast cells. J Immunol. 182:433–445. 2009. View Article : Google Scholar :
|
|
13
|
Qin HB, Xu B, Mei JJ, Li D, Liu JJ, Zhao
DY and Liu F: Inhibition of miRNA-221 suppresses the airway
inflammation in asthma. Inflammation. 35:1595–1599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar
|
|
15
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haneklaus M, Gerlic M, O'Neill LA and
Masters SL: miR-223: infection, inflammation and cancer. J Intern
Med. 274:215–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnnidis JB, Harris MH, Wheeler RT,
Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD and
Camargo FD: Regulation of progenitor cell proliferation and
granulocyte function by microRNA-223. Nature. 451:1125–1129. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Wang H, Liu Y, Song Y, Lai L, Han
Q, Cao X and Wang Q: Inducible microRNA-223 down-regulation
promotes TLR-triggered IL-6 and IL-1β production in macrophages by
targeting STAT3. PLoS One. 7:e429712012. View Article : Google Scholar
|
|
19
|
Zhou Y, Yang J, Deng H, Xu H, Zhang J, Jin
W, Gao H, Liu F and Zhao D: Respiratory syncytial virus infection
modulates interleukin 8 production in respiratory epithelial cells
through a transcription factor activator protein 1 signaling
pathway. Mol Med Rep. 10:1443–1447. 2014.PubMed/NCBI
|
|
20
|
Akira S and Kishimoto T: IL-6 and NF-IL6
in acute-phase response and viral infection. Immunol Rev.
127:25–50. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taïbi F, Metzinger-Le Meuth V, Massy ZA
and Metzinger L: miR-223: an inflammatory oncomiR enters the
cardiovascular field. Biochim Biophys Acta. 1842:1001–1009. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MS, Rådinger M and Gilfillan AM: The
multiple roles of phosphoinositide 3-kinase in mast cell biology.
Trends Immunol. 29:493–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MS, Kuehn HS, Metcalfe DD and
Gilfillan AM: Activation and function of the mTORC1 pathway in mast
cells. J Immunol. 180:4586–4595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okayama Y, Tkaczyk C, Metcalfe DD and
Gilfillan AM: Comparison of Fc epsilon RI- and Fc gamma RI-mediated
degranulation and TNF-alpha synthesis in human mast cells:
Selective utilization of phosphatidylinositol-3-kinase for Fc gamma
RI-induced degranulation. Eur J Immunol. 33:1450–1459. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tkaczyk C, Beaven MA, Brachman SM,
Metcalfe DD and Gilfillan AM: The phospholipase C gamma 1-dependent
pathway of Fc epsilon RI-mediated mast cell activation is regulated
independently of phosphatidylinositol 3-kinase. J Biol Chem.
278:48474–48484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fazi F, Racanicchi S, Zardo G, Starnes LM,
Mancini M, Travaglini L, Diverio D, Ammatuna E, Cimino G, Lo-Coco
F, et al: Epigenetic silencing of the myelopoiesis regulator
microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 12:457–466.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chacón-Salinas R, Limón-Flores AY,
Chávez-Blanco AD, Gonzalez-Estrada A and Ullrich SE: Mast
cell-derived IL-10 suppresses germinal center formation by
affecting T follicular helper cell function. J Immunol. 186:25–31.
2011. View Article : Google Scholar :
|
|
28
|
Biethahn K, Orinska Z, Vigorito E,
Goyeneche-Patino DA, Mirghomizadeh F, Föger N and Bulfone-Paus S:
miRNA-155 controls mast cell activation by regulating the PI3Kγ
pathway and anaphylaxis in a mouse model. Allergy. 69:752–762.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romo-Lozano Y, Hernández-Hernández F and
Salinas E: Sporothrix schenckii yeasts induce ERK pathway
activation and secretion of IL-6 and TNF-α in rat mast cells, but
no degranulation. Med Mycol. 52:862–868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Neill LA, Sheedy FJ and McCoy CE:
MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat
Rev Immunol. 11:163–175. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang HJ, Huang YL, Shih YY, Wu HY, Peng CT
and Lo WY: MicroRNA-146a decreases high glucose/thrombin-induced
endothelial inflammation by inhibiting NAPDH oxidase 4 expression.
Mediators Inflamm. 2014:3795372014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malmhall C, Alawieh S, Lu Y, Sjöstrand M,
Bossios A, Eldh M and Rådinger M: MicroRNA-155 is essential for
TH2-mediated allergen-induced eosinophilic inflammation
in the lung. J Allergy Clin Immunol. 133:1429–1438.
1438.e1421–1427. 2014. View Article : Google Scholar
|
|
33
|
Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M,
Zhu Y, Zhao Q, Dong YW, Shao K, et al: MicroRNA-223 regulates FOXO1
expression and cell proliferation. FEBS Lett. 586:1038–1043. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Zhao DY, Xu H, Zhou H, Yang QY,
Liu F and Zhou GP: Down-regulation of microRNA-223 promotes
degranulation via the PI3K/Akt pathway by targeting IGF-1R in mast
cells. PLoS One. 10:e01235752015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauernfeind F, Rieger A, Schildberg FA,
Knolle PA, Schmid-Burgk JL and Hornung V: NLRP3 inflammasome
activity is negatively controlled by miR-223. J Immunol.
189:4175–4181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Guo Y, Liang X, Sun M, Wang G, De W
and Wu W: MicroRNA-223 functions as an oncogene in human gastric
cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol.
138:763–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Josse C, Bouznad N, Geurts P, Irrthum A,
Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V and Oury C:
Identification of a microRNA landscape targeting the PI3K/Akt
signaling pathway in inflammation-induced colorectal
carcinogenesis. Am J Physiol Gastrointest Liver Physiol.
306:G229–G243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li
M, Liu N, Liu P and Qiao J: Up-regulated expression of WNT5a
increases inflammation and oxidative stress via PI3K/AKT/NF-κB
signaling in the granulosa cells of PCOS patients. J Clin
Endocrinol Metab. 100:201–211. 2014. View Article : Google Scholar
|
|
40
|
Hu G, Huang X, Zhang K, Jiang H and Hu X:
Anti-inflammatory effect of B-type natriuretic peptide
postconditioning during myocardial ischemia-reperfusion:
involvement of PI3K/Akt signaling pathway. Inflammation.
37:1669–1674. 2014. View Article : Google Scholar : PubMed/NCBI
|