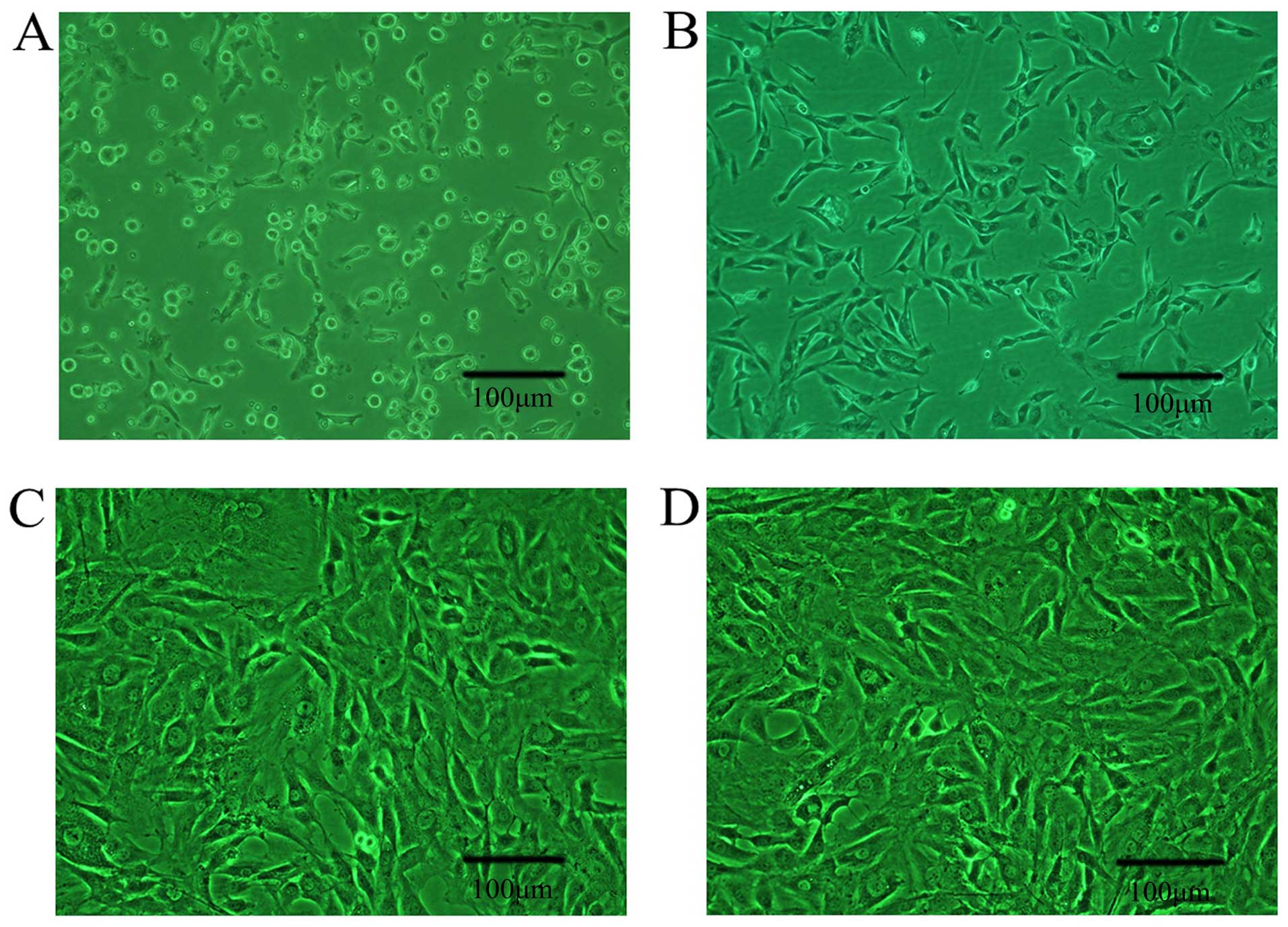
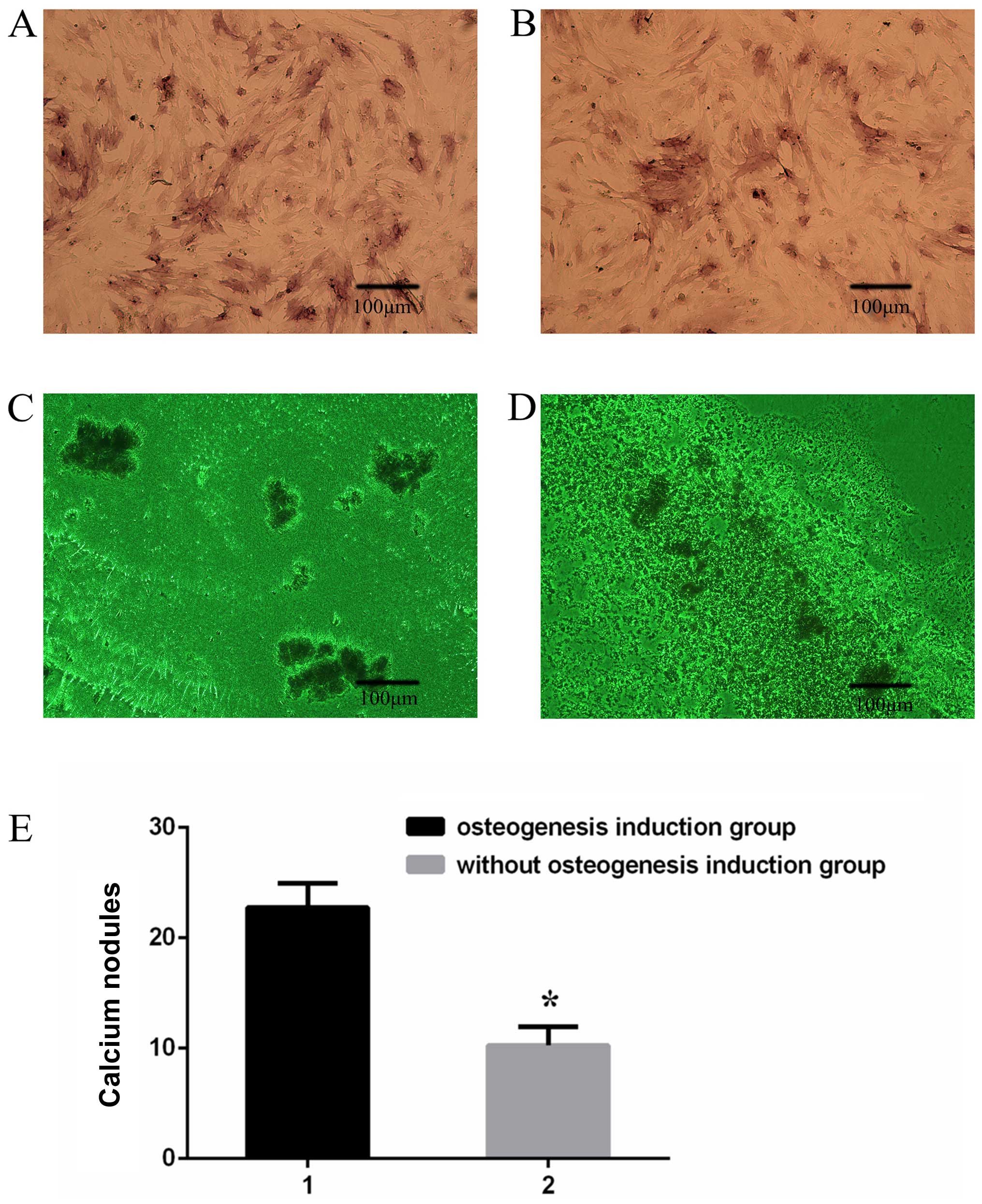
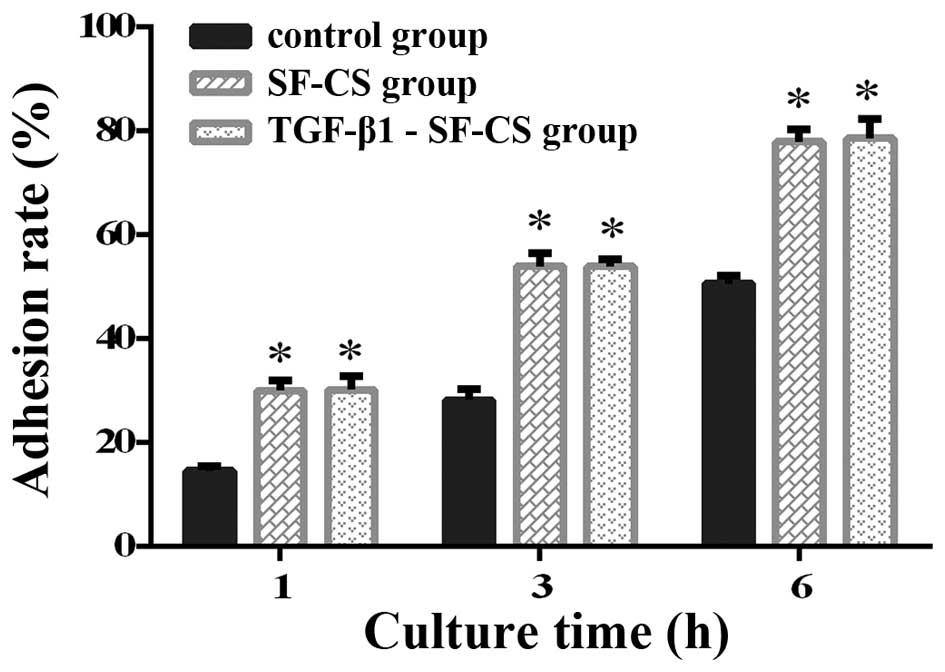
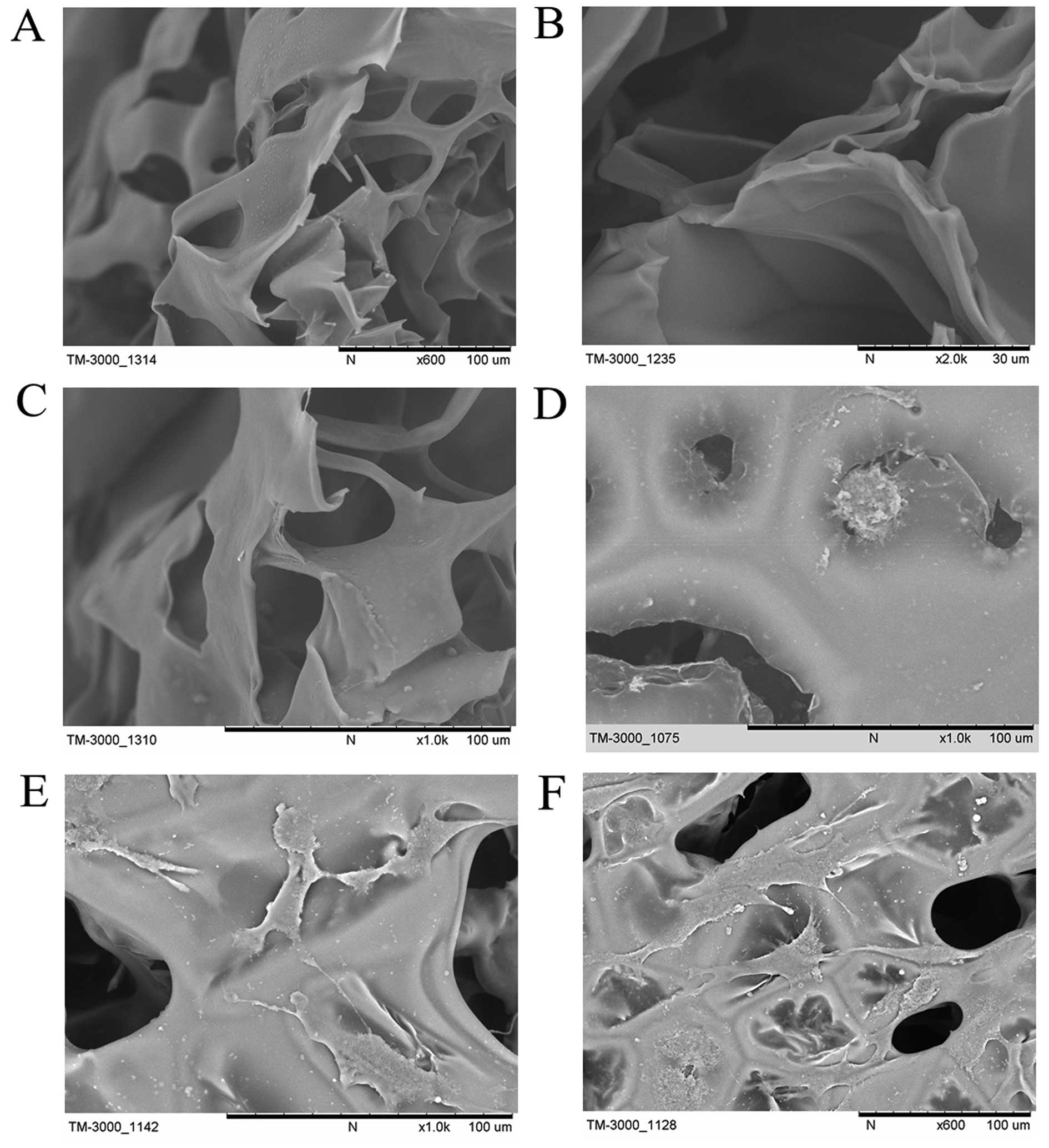
|
1
|
Vacanti JP and Langer R: Tissue
engineering: the design and fabrication of living replacement
devices for surgical reconstruction and transplantation. Lancet.
354(Suppl 1): SI32–SI34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheller EL, Krebsbach PH and Kohn DH:
Tissue engineering: state of the art in oral rehabilitation. J Oral
Rehabil. 36:368–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hankenson KD, Dishowitz M, Gray C and
Schenker M: Angiogenesis in bone regeneration. Injury. 42:556–561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andrew JG, Hoyland J, Andrew SM, Freemont
AJ and Marsh D: Demonstration of TGF-beta 1 mRNA by in situ
hybridization in normal human fracture healing. Calcif Tissue Int.
52:74–78. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baylink DJ, Finkelman RD and Mohan S:
Growth factors to stimulate bone formation. J Bone Miner Res.
8(Suppl 2): S565–S572. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonewald LF and Mundy GR: Role of
transforming growth factor-beta in bone remodeling. Clin Orthop
Relat Res. 250:261–276. 1990.PubMed/NCBI
|
|
8
|
Connelly JT, Wilson CG and Levenston ME:
Characterization of proteoglycan production and processing by
chondrocytes and BMSCs in tissue engineered constructs.
Osteoarthritis Cartilage. 16:1092–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mouw JK, Connelly JT, Wilson CG, Michael
KE and Levenston ME: Dynamic compression regulates the expression
and synthesis of chondrocyte-specific matrix molecules in bone
marrow stromal cells. Stem Cells. 25:655–663. 2007. View Article : Google Scholar
|
|
10
|
Johnstone B, Hering TM, Caplan AI,
Goldberg VM and Yoo JU: In vitro chondrogenesis of bone
marrow-derived mesenchymal progenitor cells. Exp Cell Res.
238:265–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy CM, O'Brien FJ, Little DG and
Schindeler A: Cell-scaffold interactions in the bone tissue
engineering triad. Eur Cell Mater. 26:120–132. 2013.PubMed/NCBI
|
|
12
|
Roberts AB and Sporn MB: Physiological
actions and clinical applications of transforming growth
factor-beta (TGF-beta). Growth Factors. 8:1–9. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cashman JD, Eaves AC, Raines EW, Ross R
and Eaves CJ: Mechanisms that regulate the cell cycle status of
very primitive hematopoietic cells in long-term human marrow
cultures. I. Stimulatory role of a variety of mesenchymal cell
activators and inhibitory role of TGF-beta. Blood. 75:96–101.
1990.PubMed/NCBI
|
|
15
|
Spinella-Jaegle S, Roman-Roman S, Faucheu
C, Dunn FW, Kawai S, Galléa S, Stiot V, Blanchet AM, Courtois B,
Baron R and Rawadi G: Opposite effects of bone morphogenetic
protein-2 and transforming growth factor-beta1 on osteoblast
differentiation. Bone. 29:323–330. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SJ, Lee KY, Ha WS and Park SY:
Structural changes and their effect on mechanical properties of
silk fibroin/chitosan blends. J Appl Polym Sci. 74:2571–2575. 1999.
View Article : Google Scholar
|
|
17
|
El Sayed K, Marzahn U, John T, Hoyer M,
Zreiqat H, Witthuhn A, Kohl B, Haisch A and Schulze-Tanzil G:
PGA-associated heterotopic chondrocyte cocultures: implications of
nasoseptal and auricular chondrocytes in articular cartilage
repair. J Tissue Eng Regen Med. 7:61–72. 2013. View Article : Google Scholar
|
|
18
|
De Santis R, Gloria A, Russo T, D'Amora U,
Zeppetelli S, Dionigi C, Sytcheva A, Herrmannsdörfer T, Dediu V and
Ambrosio L: A basic approach toward the development of
nanocomposite magnetic scaffolds for advanced bone tissue
engineering. J Appl Polym Sci. 122:3599–3605. 2011. View Article : Google Scholar
|
|
19
|
Tong S, Xu DP, Liu ZM and Wang XK:
Construction and in vitro characterization of three-dimensional
silk fibroinchitosan scaffolds. Dent Mater J. 34:475–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gobin AS, Froude VE and Mathur AB:
Structural and mechanical characteristics of silk fibroin and
chitosan blend scaffolds for tissue regeneration. J Biomed Mater
Res A. 74:465–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whang K, Tsai DC, Nam EK, Aitken M,
Sprague SM, Patel PK and Healy KE: Ectopic bone formation via
rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J
Biomed Mater Res. 42:491–499. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uebersax L, Merkle HP and Meinel L:
Insulin-like growth factor I releasing silk fibroin scaffolds
induce chondrogenic differentiation of human mesenchymal stem
cells. J Control Release. 127:12–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Vepari C, Jin HJ, Kim HJ and Kaplan
DL: Electrospun silk-BMP-2 scaffolds for bone tissue engineering.
Biomaterials. 27:3115–3124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hosseinkhani H, Hosseinkhani M,
Khademhosseini A and Kobayashi H: Bone regeneration through
controlled release of bone morphogenetic protein-2 from 3-D tissue
engineered nano-scaffold. J Control Release. 117:380–386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luginbuehl V, Wenk E, Koch A, Gander B,
Merkle HP and Meinel L: Insulin-like growth factor I-releasing
alginate-tricalciumphosphate composites for bone regeneration.
Pharm Res. 22:940–950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung YI, Ahn KM, Jeon SH, Lee SY, Lee JH
and Tae G: Enhanced bone regeneration with BMP-2 loaded functional
nanoparticle-hydrogel complex. J Control Release. 121:91–99. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B, Brown KV, Wenke JC and Guelcher SA:
Sustained release of vancomycin from polyurethane scaffolds
inhibits infection of bone wounds in a rat femoral segmental defect
model. J Control Release. 145:221–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Long JL and Tranquillo RT: Elastic fiber
production in cardiovascular tissue-equivalents. Matrix Biol.
22:339–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi J, Wang A, Sen S, Wang Y, Kim HJ,
Mitts TF and Hinek A: Insulin induces production of new elastin in
cultures of human aortic smooth muscle cells. Am J Pathol.
180:715–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim UJ, Park J, Kim HJ, Wada M and Kaplan
DL: Three-dimensional aqueous-derived biomaterial scaffolds from
silk fibroin. Biomaterials. 26:2775–2785. 2005. View Article : Google Scholar
|
|
31
|
Li C, Vepari C, Jin HJ, Kim HJ and Kaplan
DL: Electrospun silk-BMP-2 scaffolds for bone tissue engineering.
Biomaterials. 27:3115–3124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chou CH, Cheng WT, Lin CC, Chang CH, Tsai
CC and Lin FH: TGF-beta1 immobilized tri-co-polymer for articular
cartilage tissue engineering. J Biomed Mater Res B Appl Biomater.
77:338–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sohier J, Moroni L, van Blitterswijk C, de
Groot K and Bezemer JM: Critical factors in the design of growth
factor releasing scaffolds for cartilage tissue engineering. Expert
Opin Drug Deliv. 5:543–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lyons F, Partap S and O'Brien FJ: Part 1:
scaffolds and surfaces. Technol Health Care. 16:305–317.
2008.PubMed/NCBI
|
|
35
|
Mooney DJ, Baldwin DF, Suh NP, Vacanti JP
and Langer R: Novel approach to fabricate porous sponges of poly
(D,L-lactic-co-glycolic acid) without the use of organic solvents.
Biomaterials. 17:1417–1422. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung HJ and Park TG: Surface engineered
and drug releasing pre-fabricated scaffolds for tissue engineering.
Adv Drug Deliv Rev. 59:249–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raman R, Sasisekharan V and Sasisekharan
R: Structural insights into biological roles of
protein-glycosaminoglycan interactions. Chem Biol. 12:267–277.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gama CI, Tully SE, Sotogaku N, Clark PM,
Rawat M, Vaidehi N, Goddard WA III, Nishi A and Hsieh-Wilson LC:
Sulfation patterns of glycosaminoglycans encode molecular
recognition and activity. Nat Chem Biol. 2:467–473. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hempel U, Hintze V, Möller S,
Schnabelrauch M, Scharnweber D and Dieter P: Artificial
extracellular matrices composed of collagen I and sulfated
hyaluronan with adsorbed transforming growth factor β1 promote
collagen synthesis of human mesenchymal stromal cells. Acta
Biomater. 8:659–666. 2012. View Article : Google Scholar
|
|
40
|
Ker EDF, Chu B, Phillippi JA, Gharaibeh B,
Huard J, Weiss LE and Campbell PG: Engineering spatial control of
multiple differentiation fates within a stem cell population.
Biomaterials. 32:3413–3422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen YH, Shoichet MS and Radisic M:
Vascular endothelial growth factor immobilized in collagen scaffold
promotes penetration and proliferation of endothelial cells. Acta
Biomater. 4:477–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oh SH, Kim TH and Lee JH: Creating growth
factor gradients in three dimensional porous matrix by
centrifugation and surface immobilization. Biomaterials.
32:8254–8260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anderson SM, Chen TT, Iruela-Arispe ML and
Segura T: The phosphorylation of vascular endothelial growth factor
receptor-2 (VEGFR-2) by engineered surfaces with electrostatically
or covalently immobilized VEGF. Biomaterials. 30:4618–4628. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martin TA, Caliari SR, Williford PD,
Harley BA and Bailey RC: The generation of biomolecular patterns in
highly porous collagen-GAG scaffolds using direct photolithography.
Biomaterials. 32:3949–3957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weadock K, Olson RM and Silver FH:
Evaluation of collagen crosslinking techniques. Biomater Med
Devices Artif Organs. 11:293–318. 1983–1984. View Article : Google Scholar
|
|
46
|
Hortensius RA and Harley BA: The use of
bioinspired alterations in the glycosaminoglycan content of
collagen-GAG scaffolds to regulate cell activity. Biomaterials.
34:7645–7652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haines AH: Non-equivalence of D- and
L-trehalose in stabilising alkaline phosphatase against
freeze-drying and thermal stress. Is chiral recognition involved?
Org Biomol Chem. 4:702–706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kreilgaard L, Jones LS, Randolph TW,
Frokjaer S, Flink JM, Manning MC and Carpenter JF: Effect of Tween
20 on freeze-thawing- and agitation-induced aggregation of
recombinant human factor XIII. J Pharm Sci. 87:1597–1603. 1998.
View Article : Google Scholar
|
|
49
|
Pikal-Cleland KA and Carpenter JF:
Lyophilization-induced protein denaturation in phosphate buffer
systems: monomeric and tetrameric beta-galactosidase. J Pharm Sci.
90:1255–1268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Klibanov AM: Enzymatic catalysis in
anhydrous organic solvents. Trends Biochem Sci. 14:141–144. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nwe N, Furuike T and Tamura H: The
mechanical and biological properties of chitosan scaffolds for
tissue regeneration templates are significantly enhanced by
chitosan from Gongronella butleri. Materials (Basel). 2. pp.
374–398. 2009, View Article : Google Scholar
|
|
52
|
Neamnark A, Sanchavanakit N, Pavasant P,
Bunaprasert T, Supaphol P and Rujiravanit R: In vitro
biocompatibility evaluations of hexanoyl chitosan film. Carbohydr
Polym. 68:166–172. 2007. View Article : Google Scholar
|
|
53
|
Kim J, Yang HJ, Cho TH, Lee SE, Park YD,
Kim HM, Kim IS, Seo YK, Hwang SJ and Kim SJ: Enhanced regeneration
of rabbit mandibular defects through a combined treatment of
electrical stimulation and rhBMP-2 application. Med Biol Eng
Comput. 51:1339–1348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui X, Zhang B, Wang Y and Gao Y: Effects
of chitosan-coated pressed calcium sulfate pellet combined with
recombinant human bone morphogenetic protein 2 on restoration of
segmental bone defect. J Craniofac Surg. 19:459–465. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shafiei Z, Bigham AS, Dehghani SN and
Nezhad ST: Fresh cortical autograft versus fresh cortical allograft
effects on experimental bone healing in rabbits: radiological,
histopathological and biomechanical evaluation. Cell Tissue Bank.
10:19–26. 2009. View Article : Google Scholar
|
|
56
|
Draper ER and Goodship AE: A novel
technique for four-point bending of small bone samples with
semi-automatic analysis. J Biomech. 36:1497–1502. 2003. View Article : Google Scholar : PubMed/NCBI
|