Introduction
Breast cancer (BC) is the most common malignant
disease affecting women. Almost every eighth woman will suffer from
this disease during her life (1).
Multimodal therapy comprising surgery, radiotherapy, chemotherapy,
endocrine therapy and targeted therapy is the standard approach for
the treatment of BC (2,3). Advances in our understanding of the
crucial pathways and factors involved in the development and
progression of BC have led to the establishment of predictive
factors and targeted therapies for BC (4). However, targeted therapies are
mostly effective specifically towards a single factor or pathway
and acquired resistance during therapy is a phenomenon often
observed in the clinic (5,6).
Oncogene switching or the reactivation of signaling pathways by
upstream activators leading to the reactivation of downstream
signaling pathways counteract all efforts to prevent tumor growth
and progression in such a setting. Therefore, a simultaneous
targeting of multiple effectors and pathways by a single agent
represents a promising therapeutic approach.
Heat shock protein (HSP)90 is a key modulator of
post-translational protein folding and has emerged as a target in
anticancer therapy in recent years. HSP90 is ubiquitously
expressed, but is highly active only in tumor tissue. HSP90 belongs
to the family of chaperones and ensures the maturation of a wide
range of effectors, so-called client proteins (CPs), which are
involved in cell growth, differentiation and survival (7,8).
In BC in particular, HSP90 is a very promising therapeutic target,
as human epidermal growth factor receptor 2 (HER2), estrogen
receptor (ER) and progesterone receptor (PR) are known CPs of HSP90
(9). Furthermore, the
phosphatidyl-inositol pathway that is regulated by AKT (AKR mouse
thymoma kinase), a CP of HSP90, has been shown to be involved in
the resistance of BC to chemotherapy (10). In a previous study using a
xenograft model, the inhibition of HSP90 was shown to be associated
with the modulation of angiogenesis (11) and HSP90 expression has been
reported to correlate with a poor outcome in BC (12).
The function of HSP90 is dependent upon ATP. The
natural ansamycin antibiotic, geldanamycin, binds to the ATP
binding site at the N-terminus of HSP90, inhibiting the ATPase
activity of HSP90, which leads to the degradation of the CP
(13); therefore, many oncogenic
signals can be blocked simultaneously by the inhibition of HSP90
(14). The well-known
semi-synthetic derivate, 17-allylamino-17-demethoxy-geldanamycin
(17-AAG), has undergone phase II/III clinical trials (26). However, the lack of solubility in
physiological fluids makes formulation for clinical delivery a
challenge and limits its clinical use. In an aim to couneract this
problem, new water-soluble geldanamycin derivates have been
developed, such as
17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) and
17-[2-(Pyrrolidin-1-yl)ethyl]amino-17-demethoxygeldanamycin
(17-AEPGA) (15–18).
In the present study, we compared the growth
inhibitory effects of the water-soluble HSP90 inhibitors, 17-DMAG
and 17-AEPGA, to those of 17-AAG (not water soluble) on 3 different
human BC cell lines expressing clinically relevant targets (MCF-7,
SKBR-3 and MDA-MB-231) in vitro. Furthermore, we examined
the effects of HSP90 inhibition at the molecular level focusing on
known BC survival and progression factors, such as HER2, epidermal
growth factor receptor 1 (EGFR1) and insulin-like growth factor
type 1 receptor (IGF1R). In addition, other heat shock proteins,
such as HSP70 and HSP27, relevant to HSP90 inhibition (19,20), were analysed.
Materials and methods
Compounds
For in vitro experiments, 17-DMAG (cat. no.
ant-dgl) and 17-AEPGA (cat. no. ant-egl-1) (both from InvivoGen,
San Diego, CA, USA) were dissolved in water to a stock
concentration of 1 mM and used at final concentrations ranging from
0.1 to 10 µM. 17-AAG (cat. no. ant-agl; InvivoGen) was
dissolved in DMSO to a stock concentration of 1 mM and the same
final concentrations (0.1–10 µM) were used. The stock
solutions were stored at −20°C.
Cell lines
The established human BC cell lines, MCF-7, SKBR-3
and MDA-MB-231, were obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640
medium (cat. no. 72400-021) supplemented with 10% fetal calf serum
(cat. no. 100500-064), 1% vol/vol penicillin/streptomycin (cat. no.
15140-122) (all from Gibco, Carlsbad, CA, USA) and 1% vol/vol
gentamycin (10 mg/ml) (cat. no. AG A2712; Biochrom AG, Cambridge,
UK). The cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2. For cell line authentication, a short
tandem repeat (STR) DNA profile is used by the cell bank, and the
cells were passaged <6 months following receipt in our
laboratory.
The MCF-7 BC cell line was cultured from a
69-year-old Caucasian woman in 1979 and is characterized by the
presence of ER and PR and the lack of HER2 overexpression. The
SKBR-3 BC cell line was derived from a 43-year-old Caucasian woman
at Memorial Sloan-Kettering Cancer Center in 1970, and it is
characterized by HER2 overexpression and the lack of ER and PR
expression. The MDA-MB-231 BC cell line was obtained from a
51-year-old Caucasian woman in 1973, by Dr R. Calleau at the
University of Texas MD Anderson Cancer Center and has no expression
of PR, ER or HER2.
Evaluation of cell doubling time and
determination of 50% cell growth inhibition (GI50)
The anti-proliferative activity of all 3 tested
HSP90 inhibitors was determined in vitro using the 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide
colorimetric method, also known as the MTT reduction assay. This
assay is based on the ability of viable cells to reduce
MTT-tetrazolium salt into MTT-formazan by the mitochondrial enzyme
succinate-dehydrogenase.
Before initiating the proliferation assay, the cell
doubling time of each tested cell line was evaluated by MTT assay.
Subsequently, the MCF-7, SKBR-3 and MDA-MB-231 cancer cells were
cultured in 96-well microtiter plates and seeded at pre-determined
densities. Ensuring cell confluence rates of >60%, fresh medium
with or without each HSP90 inhibitors was added at various
concentrations for 1, 2 or 3 doubling times. Subsequently, 12.5 ml
of an MTT solution in medium (5 mg/ml MTT; Sigma Chemical Co., St.
Louis, MO, USA) was added for 3 h. The medium was removed and the
MTT-formazan crystals were solubilised by the addition of DMSO (100
ml/well). The absorbance was determined at 562 nm. The drug
concentration inhibiting 50% (IC50) growth compared to
the untreated cells was calculated.
Cell proliferation assay
The MCF-7, SKBR-3 and MDA-MB-231 cancer cells were
harvested by trypsinization, counted and 100 µl of cell
suspension was filled in each well of 96-well plates at
concentrations of 10×103 cells/well. Ensuring cell
confluence rates of >60%, fresh medium with or without 17-AAG,
17-DMAG and 17-AEPGA was added at concentrations from 0.1 to 10
µM for 48 or 72 h. MTT assay was performed as described
above. The results were expressed as a percentage of the control.
The absorbance of the control (cell culture without any treatment)
corresponds to 100% MTT reduction. Six independent experiments were
performed for each cell line and the data are presented as the
means ± SD.
Western blot analysis
To verify the effects of HSP90 inhibition at the
molecular level, particularly at the protein level, western blot
analyses of all 3 human BC cell lines following treatment with
17-AAG, 17-AEPGA and 17-DMAG were performed.
For western blot analysis, 5 ml of cell suspension
with a density of 0.6×106 cells/ml was used to fill the
dishes 6 cm in diameter followed by incubation overnight until
>60% confluence was reached. The cells were then treated with
17-AAG, 17-AEPGA or 17-DMAG at concentrations of 1 and 5 µM
for 24 h. After 24 h, the cells were washed with Dulbecco's
phosphate-buffered saline (DPBS) (cat. no. 14190; Gibco) and
harvested with EDTA (cat. no. L2113; Biochrom AG). The cells were
then lysed in a solution comprising lysis buffer, protein inhibitor
and benzonase. The protein content was determined using the BCA
protein assay kit (Pierce/Thermo Fisher Scientific Inc., Rockford,
IL, USA) and equal amounts of protein (30 µg/volume) were
loaded onto a 12% acrylamide gel containing H2O, 30%
acrylamide/Bis (Bio-Rad Laboratories Inc., Hercules, CA, USA), 1.5
M Tris buffer pH 8.8, 10% SDS (Gibco), 10% APS and TEMED (Bio-Rad
Laboratories Inc.). For protein separation, SDS-PAGE was used,
followed by the transfer onto a nitrocellulose membrane [Trans-Blot
Transfer Medium, Pure Nitrocellulose Membrane (0.45 µm);
Bio-Rad Laboratories Inc.]. The blots were then incubated with
antibodies and chemiluminescence was detected with SuperSignal West
Dura Extended Duration Substrate (Pierce/Thermo Fisher Scientific
Inc.). The following antibodies were used: anti-EGFR1 (Cat. no.
8083), anti-HER2 (Cat. no. 2242), anti-IGF1R (Cat. no. 3027),
anti-β-catenin (Cat. no. 8480) (all from Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-HSP90 (Cat. no. ADI-SPA-831),
anti-HSP70 (Cat. no. ADI-SPA-820) (both from Stressgen, San Diego,
CA, USA), anti-HSP27 (Cat. no. 2402; Cell Signaling Technology,
Inc.), anti-poly(ADP-ribose) polymerase (PARP) (Cat. no. sc-8007;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-cleaved PARP
(Cat. no. 5625), anti-caspase-3 (Cat. no. 9662) and anti-cleaved
caspase-3 (Cat. no. 9661) (all from Cell Signaling Technology,
Inc.). Actin (Cat. no. A5441; Sigma Chemical Co.) was used as
loading control.
Statistical analysis
QuickCals calculator for scientists (GraphPad
Software, Inc., La Jolla, CA, USA) was used for statistical
analysis. The Student's t-test was used to compare the means. To
determine the GI50 values a 5-parameter logistic
regression model was carried out using R software (The R Foundation
for Statistical Computing, Vienna, Austria). Differences were
considered statistically significant with two-tailed P-values
<0.05.
Results
Cell doubling time
To determine a test confluence in which active cell
proliferation is permitted during the experiments and also a
sufficient confluence for the addition of the drug, the cell
doubling time of each cancer cell line was first evaluated using
different initial seeding densities and by following the cell
growth for 7 days (data not shown). The doubling times for the
MCF-7, SKBR-3 and MDA-MB-231 cancer cells were found to be 29±6,
19±4 and 23±7 h, respectively. These data are consistent with the
reported doubling times of the tested cells in the literature
(21). To evaluate the dose- and
time-dependent effects of HSP90 inhibitors on the tested cancer
cell lines, each cell line was exposed to the drugs for 48 and 72
h.
Cell proliferation assay and
GI50 value
Table I summarizes
the results of MTT assay with all 3 tested cell lines and the 3
HSP90 inhibitors. We evaluated the required GI50
concentration for each cell line by MTT assay. All 3 tested BC cell
lines were exposed to the specific HSP90 inhibitors for 48 and 72
h, ensuring full coverage of at least 2 cell doubling times. The
sensitivity to the HSP90 inhibitors differed in all cell lines and
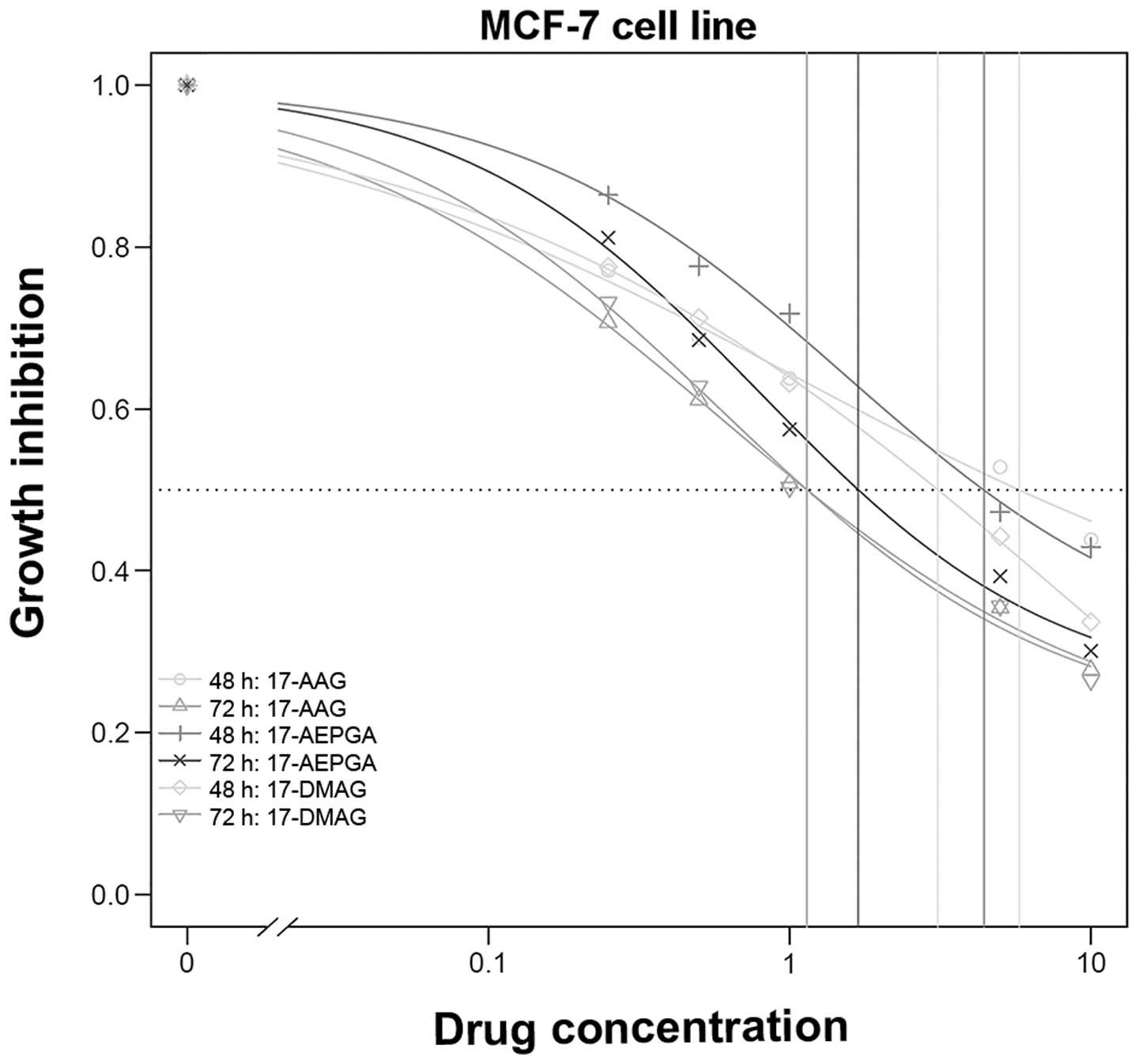
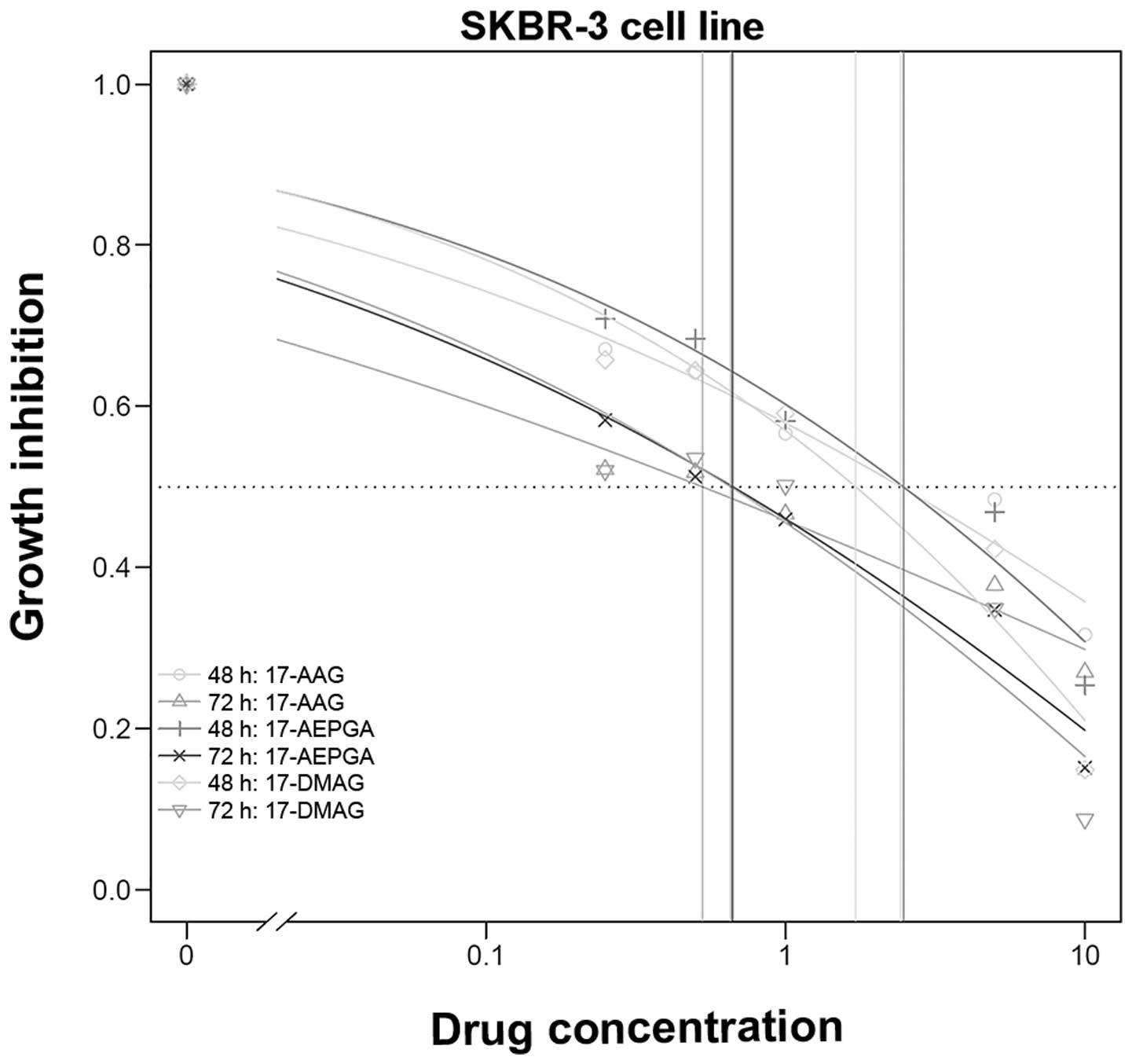
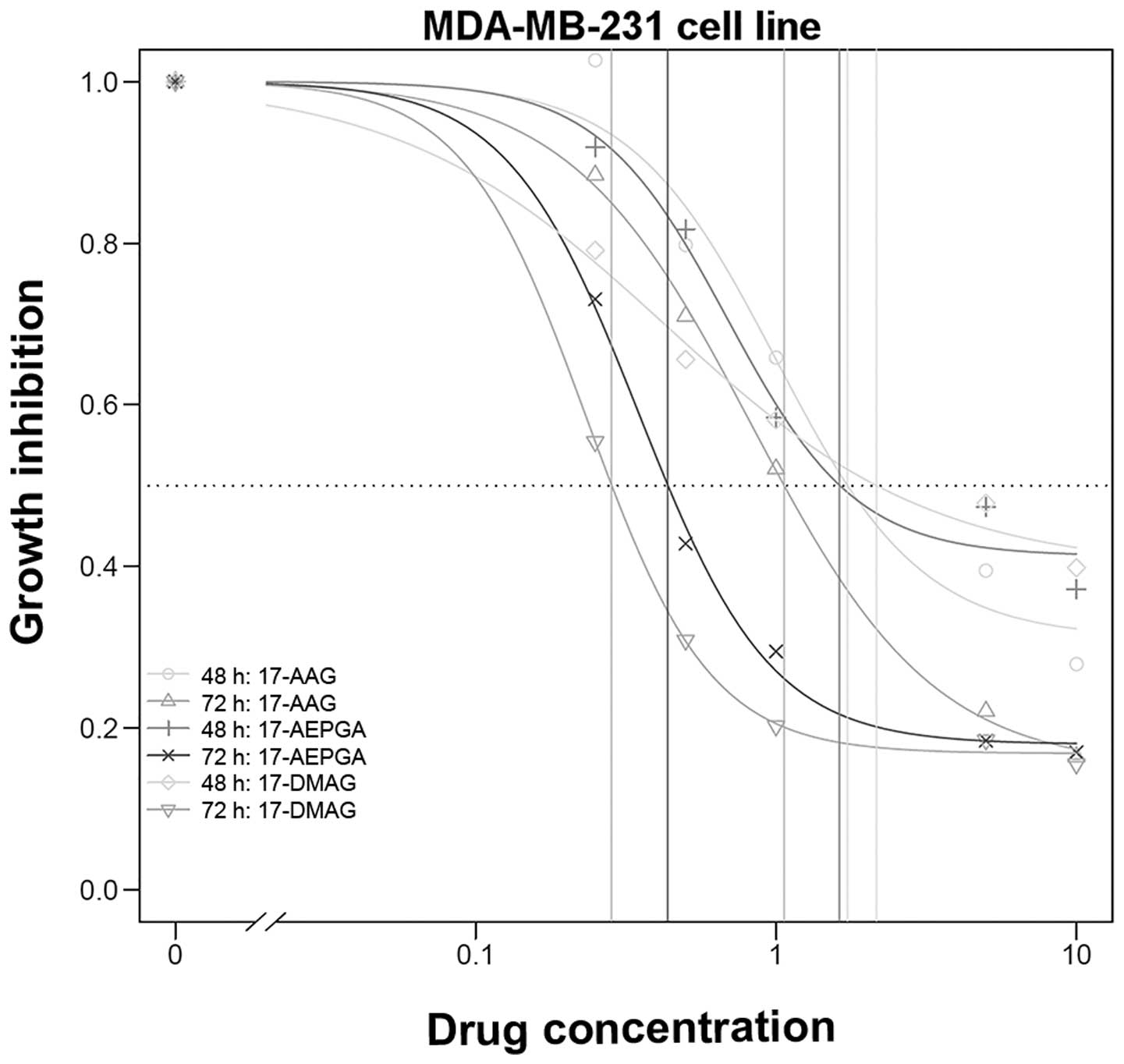
a dose- and time-dependent effect was evident (Table I and Figs. 1Figure 2–3).
 | Table ICalculated GI50 values for
all 3 inhibitors in all 3 cell lines tested. |
Table I
Calculated GI50 values for
all 3 inhibitors in all 3 cell lines tested.
| Cell line | 17-AAG 48 h
(µM) | SE | 17-AEPGA 48 h
(µM) | SE | P-valuea | 17-DMAG 48 h
(µM) | SE | P-valuea |
|---|
| MCF-7 | 5.77 | 22.56 | 4.40 | 4.43 | 0.95 | 3.11 | 10.13 | 0.93 |
| SKBR-3 | 2.43 | 12.51 | 2.47 | 6.28 | 1.00 | 1.71 | 2.17 | 0.96 |
| MDA-MB-231 | 1.73 | 0.45 | 1.63 | 0.43 | 0.88 | 2.16 | 1.76 | 0.70 |
| Cell line | 17-AAG 72 h
(µM) | SE | 17-AEPGA 72 h
(µM) | SE | P-valuea | 17-DMAG 72 h
(µM) | SE | P-valuea |
|---|
| MCF-7 | 1.14 | 0.69 | 1.69 | 0.82 | 0.54 | 1.14 | 0.52 | 1.00 |
| SKBR-3 | 0.53 | 2.07 | 0.67 | 1.89 | 0.96 | 0.66 | 1.04 | 0.96 |
| MDA-MB-231 | 1.06 | 0.18 | 0.44 | 0.38 | 0.002 | 0.28 | 0.02 | 0.0001 |
In the MCF-7 cells, the required GI50 of
17-AAG decreased >50% when the cells were exposed for 72 h
compared to 48 h. The same trend was observed with 17-AEPGA and
17-DMAG in the MCF-7 cells (Table
I); however the decrease did not reach statistical significance
with all 3 tested derivates. No significant difference was observed
when comparing the efficacy of 17-AAG, 17-DMAG and 17-AEPGA.
Fig. 1 presents the proliferation
assay results for the MCF-7 cells.
In the SKBR-3 cells as well, no significant
time-dependent difference was observed for 17-AAG, 17-AEPGA and
17-DMAG (Table I and Fig. 2). In addition, in terms of
efficacy, no significant differences between water-soluble 17-AEPGA
and 17-DMAG compared to non-water-soluble 17-AAG were observed.
Fig. 2 presents the results of
the proliferation assay for the SKBR-3 cells.
In the MDA-MB-231 cells, a clear time-dependent
effect was observed for 17-AEPGA (P=0.01); the other inhibitors did
not exhibit a significant difference. Furthermore, most significant
differences in the efficacy of 17-AEPGA and 17-DMAG compared to
that of 17-AAG were observed in the MDA-MB-231 cell line. Highly
significant differences following 72 h of exposure were observed
between 17-AEPGA and 17-DMAG compared to 17-AAG, resulting in a
GI50 ≤1 µM; no differences were observed at 48 h
of exposure. The MDA-MB-231 cells were found to be the most
sensitive among all 3 tested cell lines (Table I and Fig. 3).
Inhibition of tumor progression and
survival factors
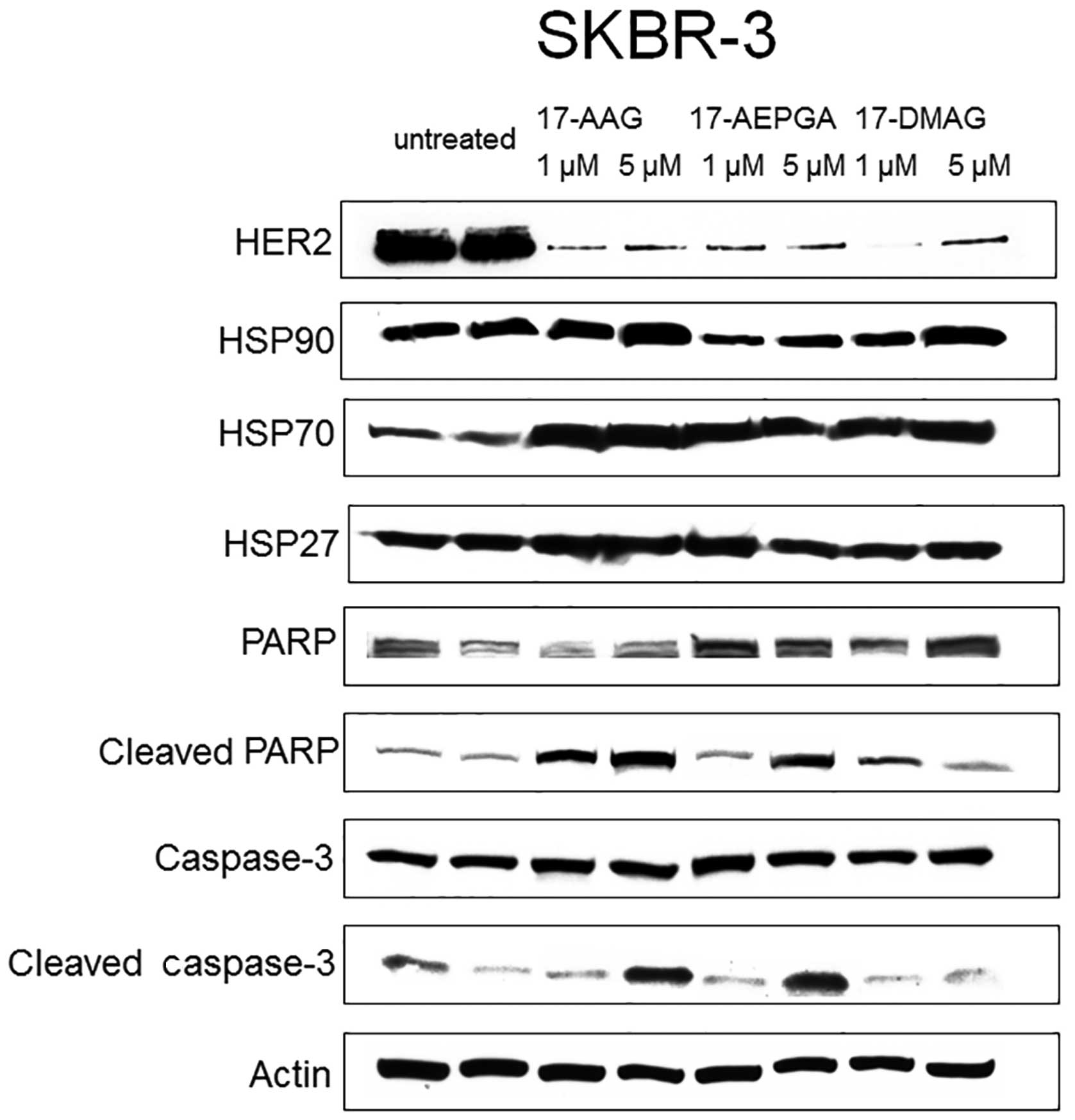
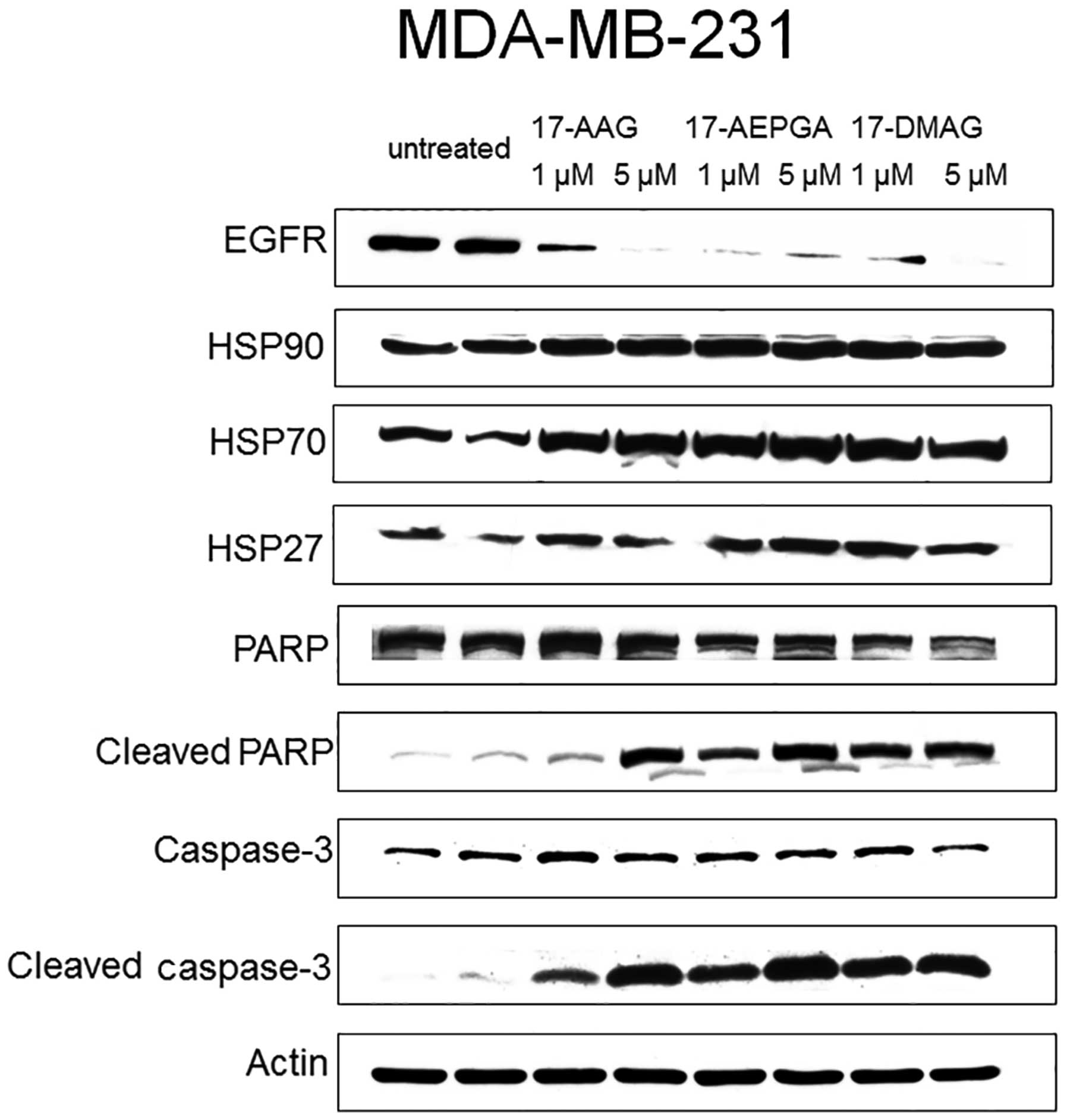
All 3 cell lines were examined in terms of HER2,
IGF1R and EGFR1 expression. However, HER2 was most highly expressed
in the SKBR-3 cells, IGF1R was most highly expressed in the MCF-7
cells and EGFR1 was most highly expressed in the MDA-MB-231 cells.
For western blot analysis, the highest expression of each factor in
each cell line was analysed only.
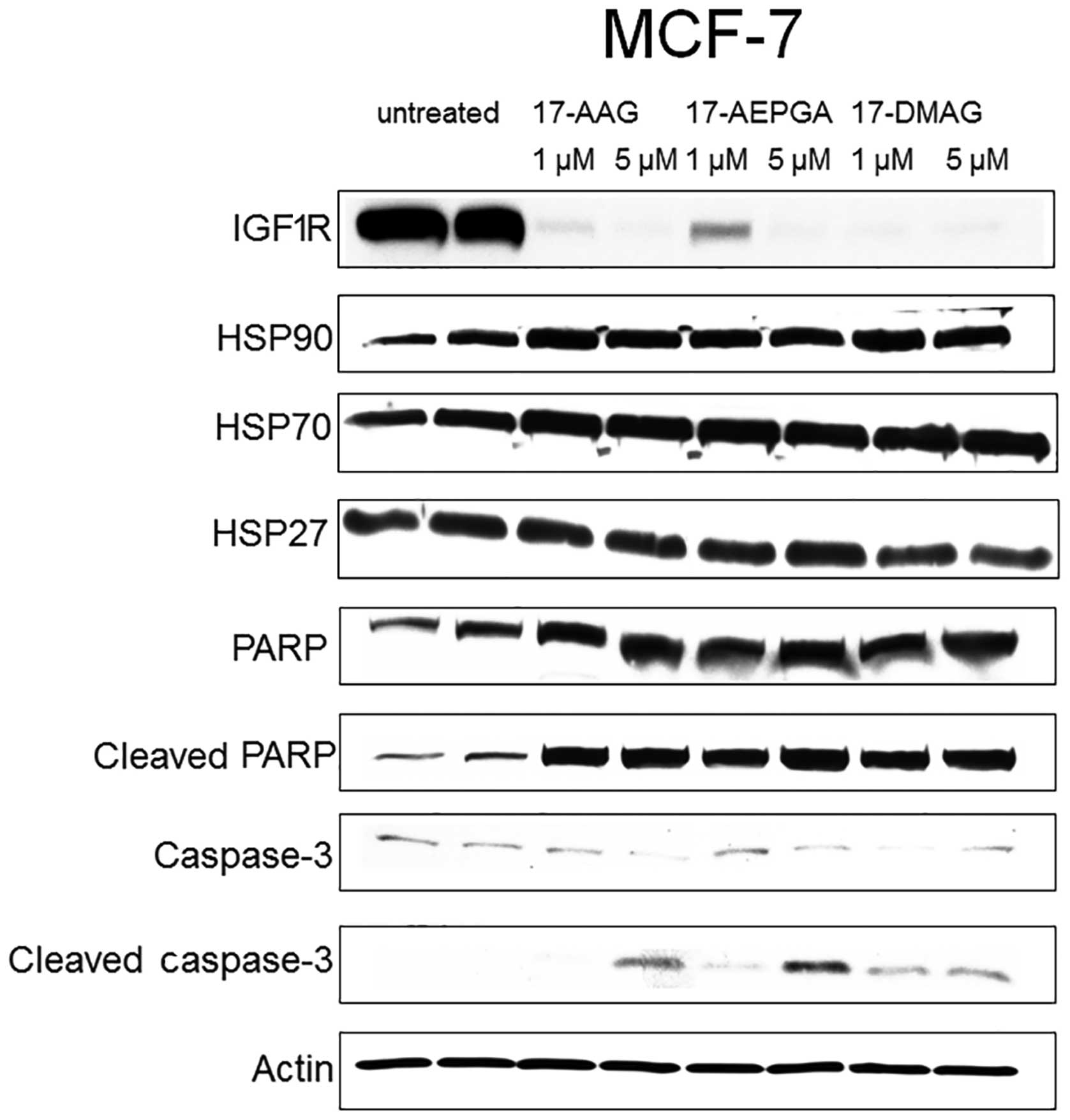
Only 24 h of exposure to 17-AAG, 17-AEPGA and
17-DMAG resulted in the downregulation of the specific CP in all 3
tested cell lines (Figs.
4Figure 5–6). This effect was already evident
following exposure to the drugs at 1 µM, suggesting that
HSP90 inhibition leads to the rapid impairment of
post-translational processes within the cell. The expression of
HER2 in the SKBR-3 cells, that of IGF1R in the MCF-7 cells and that
of EGFR1 in the MDA-MB-231 cells was almost not detectable
following treatment with the 3 tested HSP90 inhibitors in all 3 BC
cell lines, as shown by western blot analysis (Figs. 4Figure 5–6). By contrast, the expression of HER2,
EGFR1 and IGF1R was not affected in the untreated cells (Figs. 4Figure 5–6). Parallel to the inhibition of the
expression of CPs in the treated cells, apoptosis was also already
present following exposure to the drugs at 1 µM in all 3
cell lines (Figs. 4Figure 5–6). Apoptosis was evaluated by the
cleavage of caspase-3 and PARP. The blots in Figs. 4Figure 5–6 demonstrate that caspase-3 and PARP
cleavage products were detected in all 3 cell lines following
treatment with 17-AAG, 17-AEPGA and 17-DMAG. By contrast, in the
untreated cells, cleavage products were not significantly
detectable. HSP90 protein itself was not affected by HSP90
inhibition, as 17-AAG, 17-AEPGA and 17-DMAG resulted only in
functional HSP90 inhibition. As a hallmark of successful HSP90
inhibition, HSP70 expression was upregulated in all 3 cell lines.
The expression of HSP27, a small chaperone that is known to
correlate with a poor BC outcome and more aggressive breast tumors,
was also analysed. However, no significant differences in HSP27
expression were observed in any cell line (Figs. 4Figure 5–6).
Discussion
Since the introduction of molecular profiling and
targeted therapy, the concept of neoadjuvant and adjuvant therapy
in BC has changed dramatically. In particular, prognostic and
predictive factors have been identified on the basis of molecular
genetics. ER and HER2 overexpression in BC are a paradigm for
targeted therapy (4). The
targeted therapy approach however, is severely limited by acquired
resistance in the clinic. Such resistance is often mediated by the
cross-linkage between the different signaling pathways that are
crucial for the survival of BC cells and are dependent upon
proteins, including kinases, growth receptors and hormones.
All these factors have a common pivotal point when
passing through the post-translational modification process. To
attain the functional active mature protein structure, all these
factors require HSP90 (22).
HSP90, as an ubiquitously expressed chaperone, represents an
important therapeutic target in the treatment of BC (23).
Apart from the dependency of the CP upon HSP90, the
selective inhibition of HSP90 in tumor cells alone due to much the
higher activity of HSP90 in tumor cells represents an additional
attribute of this promising target in oncology (24). The natural ansamycin antibiotic,
geldanamycin, belongs to the group of HSP90 inhibitors; however, it
cannot be used clinically due to unacceptable hepatotoxicity.
However, its well-known semi-synthetic derivate, 17-AAG, is
currently undergoing phase II/III clinical trials in BC and other
malignancies (https://clinicaltrials.gov/ct2/results?term=Tanespimycin+&Search=Search).
Tanespimycin (17-AAG derivate) has demonstrated promising antitumor
activity and tolerability in a phase II trial in patients with
HER2-positive metastatic breast cancer, when administered in
combination with trastuzumab (Herceptin®) in patients
whose disease progressed following treatment with trastuzumab
alone. Although the overall noted antitumor activity was only
moderate, the present study represents the first of its kind,
addressing tumor regression in solid tumors by HSP90 inhibition
(25,26). As regards other cancer types, such
as multiple myeloma, tanespimycin has also shown promising results
in a phase Ib dose-escalating trial in combination with bortezomib
(27). Based on these findings,
tanespimycin has been granted orphan drug status in the US and
Europe.
Another formulation of the 17-AAG-based HSP90
inhibitor was shown to have antitumor effects in gastrointestinal
and stromal tumors (GIST) and non-small cell lung cancer (NSCLC)
(retaspimycin) (28). However,
most of the clinical trials with geldanamycin-based HSP90
inhibitors have been carried out with 17-AAG formulations,
resulting in difficulties in the delivery of the compound in the
clinic due to the lack of water-solubility in physiological fluids.
Thus, the water-soluble derivatives, 17-DMAG and 17-AEPGA, were
developed. 17-DMAG has been tested in some phase I studies (29–31);
to the best of our knowledge, no clinical study with 17-AEPGA has
been published to date.
We have been able to demonstrate that in
vitro, the water-soluble geldanamycin derivates, 17-DMAG and
17-AEPGA, are equally effective or superior compared to 17-AAG. The
calculated GI50 value was comparable to that of 17-AAG
in all 3 cell lines and was significantly lower in the MDA-MB-31
cells (Table I). Our
GI50 calculations are consistent with the reported
values in the literature (15,16).
Treatment of all 3 tested human BC cell lines for 48
h with 17-DMAG, 17-AEPGA and 17-AAG demonstrated a significant
anti-proliferative effect (Figs.
1Figure 2–3). Prolongation of the treatment period
up to 72 h resulted in a decrease in the required GI50
(Table I). A possible explanation
for this finding may be that only proteins that are in the process
of post-translational modification are inhibited and redirected to
proteosomal degradation by HSP90 inhibition. By contrast, already
pre-existing functional mature proteins are not affected by HSP90
inhibition. Consequently, not all signaling pathways crucial for
cell survival are affected simultaneously so that crosslinks
between the different signaling pathways may act complementary in
the initial phase, but are severely hampered when particular
amounts of protein and or numbers of signaling pathways are
downregulated within the cancer cell. In addition, in our
experiments, the cells were not cell cycle-synchronized, so that
treatment for 72 h, assuring two cell cycle doubling times of
exposure to HSP90 inhibitors, ensured that all cells were affected
by HSP90 inhibition. Although the anti-proliferative effects become
evident with a delay, the detrimental alterations at the molecular
level were evident as soon as 24 h and at very low concentrations
as shown by the results of western blot analysis (Figs. 4Figure 5–6).
In BC cells, tyrosine kinases, such as IGF1R, EGFR1
and HER2 comprise a major oncogene family being responsible for
tumor progression and growth. The molecular profiling revealed that
all 3 cell lines expressed IGF1R, HER2 and EGFR1. For western blot
analysis, however, the experiments were limited to the most
significantly expressed tyrosine kinase. IGF1R was most highly
expressed in the MCF-7 cells, EGFR1 was most highly expressed in
the MDA-MB-231 cells and HER2 was most highly expressed in the
SKBR-3 BC cells. Although in the proliferation assay a significant
inhibition of proliferation was observed in the MCF-7, SKBR-3 and
MDA-MB-231 cells following 48 h of exposure at higher
concentrations, the results of western blot analysis, however,
demonstrated that at the molecular level, significant alterations
in the expression profiles of the tested CPs already began to take
effect at the concentration of 1 µM for each tested
inhibitor. Furthermore, apoptosis was also already present at the
concentration of 1 µM in all cell lines, as proven by
caspase-3 and PARP assay (Figs.
4Figure 5–6).
As a hallmark of successful HSP90 inhibition, HSP70
was equally upregulated in all 3 cell lines following treatment.
Despite our encouraging results with the water-soluble compounds,
the upregulation of HSP70 remains a challenging problem for the
application of HSP90 inhibitors in the clinic. HSP70 functions as a
chaperone itself. In the multi-HSP90 chaperon complex, HSP70
recruits substrate-. However with its anti-apoptotic and
cytoprotective properties, HSP70 promotes cancer cell survival and
is also active in the absence of HSP90. Furthermore, HSP70 has also
been shown to correlate with malignant transformation and growth
(32). Sun et al demonstrated that in triple negative BC,
HSP70 significantly correlates with tumor aggressiveness in terms
of metastatic potential (33). In addition, HSP70 is also known to
interact with the ligand-binding domain of steroid hormone
receptors and chaperoning their maturation, as well as the
translocation of these receptors from the cytosol to the nucleus
(34). This function of HSP70 could be fatal and boost
hormone-sensitive tumor cells. These findings indicate that the
utilization of HSP90 inhibitors as single agents in the treatment
of BC is inherent with the danger of possible switching to a more
aggressive tumor biology.
HSP27 is also known to be overexpressed in BC and
has been associated with decreased survival, lymph node positivity
and resistance to chemotherapy (35). Recently,
trastuzumab-resistant BC cells were found to overexpress HSP27. We
found that HSP27 expression was not affected by HSP90 inhibition.
However, it remains an additional target in the treatment of BC, as
studies using have siRNA demonstrated encouraging results in terms
of the apoptosis and chemosensitivity of HSP27-overexpressing BC
cells (36).
In conclusion, our data suggest that HSP90 may be a
promising target in the treatment of BC and the newly available
water-soluble derivates have equal or superior anti-proliferative
effects to those of 17-AAG. The downregulation of CPs and the
induction of apoptosis was observed following treatment with
17-AAG, 17-AEPGA and 17-DMAG from the concentration of 1 µM.
HSP27 expression was not affected, but HSP70 was upregulated by all
3 tested HSP90 inhibitors. The new water-soluble derivate may help
to overcome the difficulties associated with the delivery of the
drugs in the clinic. Further studies are required to test the
bioavailability of the new water-soluble derivates in vivo.
Combination studies with HSP90 inhibitors and other
target-therapies and or chemotherapy are essential to address the
utility of HSP90 inhibition in BC treatment as a single
administration is afflicted with molecular alterations that may be
fatal. Further studies and research are warranted to find
strategies to counteract HSP70 and HSP27 in BC.
References
|
1
|
Sainsbury R: The development of endocrine
therapy for women with breast cancer. Cancer Treat Rev. 39:507–517.
2013. View Article : Google Scholar
|
|
2
|
Mittendorf EA, Wu Y, Scaltriti M,
Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H,
Eksambi S, et al: Loss of HER2 amplification following
trastuzumab-based neoadjuvant systemic therapy and survival
outcomes. Clin Cancer Res. 15:7381–7388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scaltriti M, Eichhorn PJ, Cortés J,
Prudkin L, Aura C, Jiménez J, Chandarlapaty S, Serra V, Prat A,
Ibrahim YH, et al: Cyclin E amplification/overexpression is a
mechanism of trastuzumab resistance in HER2+ breast
cancer patients. Proc Natl Acad Sci USA. 108:3761–3766. 2011.
View Article : Google Scholar
|
|
4
|
Burrows F, Zhang H and Kamal A: Hsp90
activation and cell cycle regulation. Cell Cycle. 3:1530–1536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zajac M, Gomez G, Benitez J and
Martínez-Delgado B: Molecular signature of response and potential
pathways related to resistance to the HSP90 inhibitor, 17AAG, in
breast cancer. BMC Med Genomics. 3:442010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basso AD, Solit DB, Munster PN and Rosen
N: Ansamycin antibiotics inhibit Akt activation and cyclin D
expression in breast cancer cells that overexpress HER2. Oncogene.
21:1159–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song CH, Park SY, Eom KY, Kim JH, Kim SW,
Kim JS and Kim IA: Potential prognostic value of heat-shock protein
90 in the presence of phosphatidylinositol-3-kinase overexpression
or loss of PTEN, in invasive breast cancers. Breast Cancer Res.
12:R202010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prodromou C and Pearl LH: Structure and
functional relationships of Hsp90. Curr Cancer Drug Targets.
3:301–323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Powers MV and Workman P: Targeting of
multiple signalling pathways by heat shock protein 90 molecular
chaperone inhibitors. Endocr Relat Cancer. 13(Suppl 1): S125–S135.
2006. View Article : Google Scholar
|
|
11
|
Hollingshead M, Alley M, Burger AM, Borgel
S, Pacula-Cox C, Fiebig HH and Sausville EA: In vivo antitumor
efficacy of 17-DMAG
(17-dimethylaminoethylamino-17-demethoxygeldanamycin
hydrochloride), a water-soluble geldanamycin derivative. Cancer
Chemother Pharmacol. 56:115–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith V, Sausville EA, Camalier RF, Fiebig
HH and Burger AM: Comparison of
17-dimethylaminoethylamino-17-demethoxygeldanamycin (17DMAG) and
17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: Effects on
Hsp90 and client proteins in melanoma models. Cancer Chemother
Pharmacol. 56:126–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yerushalmi R, Gelmon KA, Leung S, Gao D,
Cheang M, Pollak M, Turashvili G, Gilks BC and Kennecke H:
Insulin-like growth factor receptor (IGF-1R) in breast cancer
subtypes. Breast Cancer Res Treat. 132:131–142. 2012. View Article : Google Scholar
|
|
15
|
Sieuwerts AM, Klijn JG, Peters HA and
Foekens JA: The MTT tetrazolium salt assay scrutinized: how to use
this assay reliably to measure metabolic activity of cell cultures
in vitro for the assessment of growth characteristics, IC50-values
and cell survival. Eur J Clin Chem Clin Biochem. 33:813–823.
1995.PubMed/NCBI
|
|
16
|
Jhaveri K, Taldone T, Modi S and Chiosis
G: Advances in the clinical development of heat shock protein 90
(Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823:742–755.
2012. View Article : Google Scholar :
|
|
17
|
Miyata Y, Nakamoto H and Neckers L: The
therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des.
19:347–365. 2013. View Article : Google Scholar
|
|
18
|
Ochel HJ, Eichhorn K and Gademann G:
Geldanamycin: The prototype of a class of antitumor drugs targeting
the heat shock protein 90 family of molecular chaperones. Cell
Stress Chaperones. 6:105–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modi S, Stopeck AT, Gordon MS, Mendelson
D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, et al:
Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is
safe and active in trastuzumab-refractory HER-2 overexpressing
breast cancer: A phase I dose-escalation study. J Clin Oncol.
25:5410–5417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Modi S, Stopeck A, Linden H, Solit D,
Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME,
Sugarman S, et al: HSP90 inhibition is effective in breast cancer:
a phase II trial of tanespimycin (17-AAG) plus trastuzumab in
patients with HER2-positive metastatic breast cancer progressing on
trastuzumab. Clin Cancer Res. 17:5132–5139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richardson PG, Chanan-Khan AA, Lonial S,
Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M
and Anderson KC: Tanespimycin and bortezomib combination treatment
in patients with relapsed or relapsed and refractory multiple
myeloma: Results of a phase 1/2 study. Br J Haematol. 153:729–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wagner AJ, Chugh R, Rosen LS, Morgan JA,
George S, Gordon M, Dunbar J, Normant E, Grayzel D and Demetri GD:
A phase I study of the HSP90 inhibitor retaspimycin hydrochloride
(IPI-504) in patients with gastrointestinal stromal tumors or
soft-tissue sarcomas. Clin Cancer Res. 19:6020–6029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramanathan RK, Egorin MJ, Erlichman C,
Remick SC, Ramalingam SS, Naret C, Holleran JL, TenEyck CJ, Ivy SP
and Belani CP: Phase I pharmacokinetic and pharmacodynamic study of
17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor
of heat-shock protein 90, in patients with advanced solid tumors. J
Clin Oncol. 28:1520–1526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jhaveri K, Miller K, Rosen L, Schneider B,
Chap L, Hannah A, Zhong Z, Ma W, Hudis C and Modi S: A phase I
dose-escalation trial of trastuzumab and alvespimycin hydrochloride
(KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin
Cancer Res. 18:5090–5098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pacey S, Wilson RH, Walton M, Eatock MM,
Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U,
Roels B, et al: A phase I study of the heat shock protein 90
inhibitor alvespimycin (17-DMAG) given intravenously to patients
with advanced solid tumors. Clin Cancer Res. 17:1561–1570. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mosser DD, Caron AW, Bourget L,
Denis-Larose C and Massie B: Role of the human heat shock protein
hsp70 in protection against stress-induced apoptosis. Mol Cell
Biol. 17:5317–5327. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo
Y and Guo Y: Identification of metastasis-related proteins and
their clinical relevance to triple-negative human breast cancer.
Clin Cancer Res. 14:7050–7059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daniel S, Bradley G, Longshaw VM, Söti C,
Csermely P and Blatch GL: Nuclear translocation of the
phosphoprotein Hop (Hsp70/Hsp90 organizing protein) occurs under
heat shock, and its proposed nuclear localization signal is
involved in Hsp90 binding. Biochim Biophys Acta. 1783:1003–1014.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansen RK, Parra I, Lemieux P, Oesterreich
S, Hilsenbeck SG and Fuqua SA: Hsp27 overexpression inhibits
doxorubicin-induced apoptosis in human breast cancer cells. Breast
Cancer Res Treat. 56:187–196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang SH, Kang KW, Kim KH, Kwon B, Kim SK,
Lee HY, Kong SY, Lee ES, Jang SG and Yoo BC: Upregulated HSP27 in
human breast cancer cells reduces Herceptin susceptibility by
increasing Her2 protein stability. BMC Cancer. 8:2862008.
View Article : Google Scholar : PubMed/NCBI
|