Introduction
Esophageal cancer is the eighth most common cancer,
and approximately 25–30 million new cases are confirmed each year.
There are two major histological subtypes of esophageal cancer:
esophageal adenocarcinoma and esophageal squamous cell carcinoma
(1–3). Given the fact that the incidence of
esophageal cancer is rising (4),
it is imperative to assess the effectiveness of chemotherapeutic
drugs for the treatment of this type of cancer, and to elucidate
the mechanisms responsible for its development, in order to select
effective chemotherapeutic regimens.
5-Azacytidine is a common anticancer drug. It is
mainly used in chemotherapy for various types of cancer, including
breast cancer, melanoma and acute myeloid leukemia (5–12).
One of the most important mechanisms responsible for its antitumor
activity previously proposed is that the drug can incorporate into
DNA during DNA replication and inhibit DNA methylation (13). DNA methylation plays an important
role in tumorigenesis and cancer progression (14–17). Aberrant DNA methylation leads to
the inactivation of tumor suppressor genes in the development and
progression of cancers, including esophageal cancer (18–20).
Cadherin 1 (CDH1) and SRY-box containing gene
17 (SOX17) are two members that are aberrantly methylated in
esophageal cancer cells. CDH1 belongs to the cadherin super-family,
and is also known as epithelial cadherin (E-cadherin) or uvomorulin
(21). CDH1, as a tumor
suppressor gene, plays an essential role in maintaining cell
adhesion and adherent junctions in normal tissues (22). The expression of CDH1 is
frequently absent in a variety of epithelial tumors in which normal
intercellular junctions are lost (23–27). SOX17 belongs to the high-mobility
group (HMG)-box transcriptive factor superfamily. It is highly
conserved during evolution and plays an important role in the
formation of embryonic germ layers (28). Frequent methylation of SOX17
promoter was detected in colon, liver, breast and lung cancers
(29–31). The hypermethylation of the
SOX17 promoter and the lower expression of SOX17 has
previously been shown in breast cancer, resulting in cancer cell
proliferation (32). In lung
cancer cells, the promoter of SOX17 is also hypermethylated,
leading to the silencing or the inhibition of SOX17 expression. In
addition, SOX17 promoter methylation is remitted by
5-azacytidine in lung cancer cells (31). The hypermethylation of the
CDH1 and SOX17 promoters and the reduced expression
of CDH1 and SOX17 have been reported to occur in esophageal cancer
(33–35). These data suggest that these two
genes are epigenetically involved in the development and
progression of esophageal cancer.
Based on the facts that 5-azacytidine can affect DNA
methylation and that the promoters of tumor suppressor genes are
usually downregulated by high hypermethylation in esophageal
cancer, in this study, we aimed to examine the effects of treatment
with 5-azacytidine on esophageal cancer cells and to elucidate the
mechanisms responsible for its antitumor activity in esophageal
cancer. Our findings may help clinicians select an effective
chemotherapeutic regimen for the treatment of esophageal cancer,
and may also aid in the prognostic evaluation.
Materials and methods
Cell culture
The EC9706 esophageal cancer cell line was purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Thermo Fisher Scientific,
Waltham, MA, USA) and 100 U/ml penicillin/streptomycin (Invitrogen
Life Technologies, Carlsbad, CA, USA), and were routinely incubated
at 37°C under a 5% CO2 atmosphere.
Treatment with 5-azacytidin
The EC9706 cells were seeded into 6-well culture
plate (NEST Biotechnology, Jiangsu, China) at a density of
106 cells/well and cultured overnight. The cells were
treated with 5-azacytidine (50 µM) (5-aza; Sigma-Aldrich,
St. Louis, MO, USA) for 72 h and dimethyl sulfoxide (DMSO; 1:100)
as the negative control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following treatment with 5-azacytidine for 72 h,
total RNA was isolated from the EC9706 cells using TRIzol reagent
(Life Technologies) according to the manufacturer's instructions.
RNA was reverse transcribed using the PrimeScript® First
Strand cDNA Synthesis kit (Takara, Shiga, Japan) and then amplified
with PCR primers on the iQ5 Real-time Quantitative PCR system
(Bio-Rad, Hercules, CA, USA). The average Ct, from triplicate
assays, was used for further calculations. Relative expression
levels were normalized to the control and actin as the internal
control. The reverse transcribed reaction included approximately 1
µg of cDNA, 0.5 µg of each primer, 20 nmoles of
dNTPs, 4 µl of 5X PCR buffer, 12 units of RiboLock™
Ribonuclease inhibitor and 1 unit of ReverTra Ace polymerase in a
final reaction volume of 20 µl. The PCR cycling conditions
were as follows: 42°C for 60 min; and 72°C for 10 min. The primers
used for PCR were as follows: SOX17 forward,
5′-GGGATACGCCAGTGACGAC-3′ and reverse, 5′-CCTTAGCCCACACCATGAAA-3′;
CDH1 forward, 5′- AATGCCGCCATCGCTTAC-3′ and reverse,
5′-TCAGGCACCTGACCCTTGTA-3′; and actin forward,
5′-GCACCCAGCACAATGAAGA-3′ and reverse,
5′-AATAAAGCCATGCCAATCTCA-3′.
Western blot analysis
Total proteins were mixed by loading buffer and
boiled for 5 min. Equivalent quantities of protein were separated
by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels and
transferred onto polyvinylidene fluoride membranes. The membranes
were then blocked with 10% defatted milk for 1 h at 4°C. This was
followed by the addition of primary antibodies specific to the
SOX17 markers (1:1,000; species raised in, rabbit; specificity,
rat, human and mouse; polyclonal antibody; Ab191699; Abcam,
Cambridge, UK), anti-E-Cadherin antibody IgG (CDH1; 1:1,000;
species raised in, rabbit; specificity, rat, human and mouse;
monoclonal antibody; Ab133597; Abcam), anti-MMP2 antibody IgG
(1:1000; species raised in, rabbit; specificity, rat, mouse, human;
polyclonal antibody; 4022; Cell Signaling Technology, Danvers, MA,
USA) and anti-MMP9 antibody IgG (1:1000; species raised in, rabbit;
specificity, human; polyclonal antibody; 2270; Cell Signaling
Technology). in phosphate-buffered saline (PBS) containing 5% BSA
incubated with the membranes at 4°C overnight. The membranes were
then washed and incubated with the corresponding horseradish
peroxidase (HRP)-conjugated secondary antibody (1:5,000; SC-2004;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h. The
bound secondary antibody was visualized using an enhanced
chemiluminescence (ECL) system (Pierce Biotechnology, Rockford, IL,
USA).
siRNA transfection
siRNAs targeting SOX17 (SOX17 siRNA) and CDH1 (CDH1
siRNA) were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China) and transfection was performed using Lipofectamine RNAiMAX
(Life Technologies) according to the manufacturer's instructions.
The negative control siRNA was also synthesized by Shanghai
GenePharma Co., Ltd. The EC9706 cells were seeded in a 6-well plate
at a density of 106 cells/well and allowed to attach
overnight (16 h). Subsequently, SOX17 siRNA, CDH1 siRNA or NC siRNA
were transfected into the EC9706 cells at a final concentration of
50 nM using Lipofectamine RNAiMAX. The interference efficiency was
detected by RT-qPCR at 48 h post-transfection.
Cell viability assay
The EC9706 cells were seeded into 96-well culture
plates (5,000 cells/well). 5-Azacytidine was added to the wells at
a concentration of 50 µM. Cell viability was assayed using
the Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto,
Japan) at 1–5 days following treatment with 5-azacytidine. The
absorbance was measured at 450 nm using a microplate reader.
Cell colony formation assay
Soft agar assays were performed to detect the
colony-forming ability of the EC9706 cells at 96 h following
treatment with 5-azacytidine (50 µM). The cells were then
resuspended and seeded into 6-well culture plates (1,000
cells/well). On day 7, the cells were washed with PBS twice and
fixed with 75% ethanol for 30 min and stained with 0.2% crystal
violet for visualization and photographing by microscope (Mi 8;
Leica, Wetzlar, Germany).
Apoptosis assay
Cell apoptosis was determined by flow cytometry
using the Annexin V-FITC Apoptosis Detection kit (Sungene, Tianjin,
China). The EC9706 cells were seeded in 6-well culture plates.
5-Azacytidine was added to the wells at the concentration of 50
µM. The cells were harvested 96 h following treatment with
5-azacytidine, washed in PBS and incubated with Annexin V and
propidium iodide (PI) in binding buffer in the dark at room
temperature for 10 min. The stained cells were analyzed using the
BD FASAria Cell Sorter (BD Biosciences, Franklin Lakes, NJ,
USA).
Cell invasion assay
Transwell invasion chambers coated with Matrigel (50
µl/filter) (BD Biosciences) were performed to detect EC9706
cell invasion. The EC9706 cells were transferred on the top of the
chambers (50,000 cells/chamber) in DMEM and the lower chambers were
supplemented with DMEM containing 10% fetal calf serum 96 h
following treatment with 5-azacytidine (50 µM). The cells
were fixed and stained with GenMed crystal violet after 48 h of
culture.
Detection of methylation by
bisulfite-assisted genomic sequencing PCR (BSP)
EC9706 cell genomic DNA was extracted 72 h following
treatment with 5-azacytidine (50 µM). Genomic DNA (1
µg) was modified and purified according to the instructions
provided with the EZ DNA Methylation-Gold™ kit (D5005, Zymo
Research, Shanghai, China). Modified and purified genomic DNA was
amplified using the primers listed in Table I. The PCR products were cloned and
sequenced. The methylation rates of each promoter were analyzed in
the website: http://quma.cdb.riken.jp/.
 | Table IPrimer used for bisulfite-assisted
genomic sequencing PCR (BSP). |
Table I
Primer used for bisulfite-assisted
genomic sequencing PCR (BSP).
| Name | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| CDH1-1 |
TGATTTTAGGTTTTAGTGAG |
CAAACTCACAAATACTTTAC |
| CDH1-2 |
GAATTGTAAAGTATTTGTGAGTTTG |
AATACCTACAACAACAACAACAAC |
| CDH1-3 |
GTTGTTGTTGTTGTTGTAGGTATTT |
CCACTCCCATCACTAAAAAATC |
| SOX17-1 |
AGAGTGAAGGAATATTGGA |
CAAAACTACACCTACCCC |
| SOX17-2 |
GGGGTAGGTGTAGTTTTG |
TACCCAAAACCCCCAACC |
| SOX17-3 |
GGTTGGGGGTTTTGGGTA |
CCCTTCACCTTCATATCC |
| SOX17-4 |
GGATATGAAGGTGAAGGG |
CTACACACCCCTAATTTT |
| SOX17-5 |
GTTTAAAATTAGGGGTGTG |
CTCCCCCCTCAAACTTTA |
Statistical analysis
Values are expressed as the means ± standard error
of 3 independent experiments. The statistical significance of the
differences was calculated using Prism software (version 4.0a;
GraphPad Software, Inc., La Jolla, CA, USA) by one-way analysis of
variance. Values of P<0.05 and P<0.01 were considered to
indicate statistically significant differences.
Results
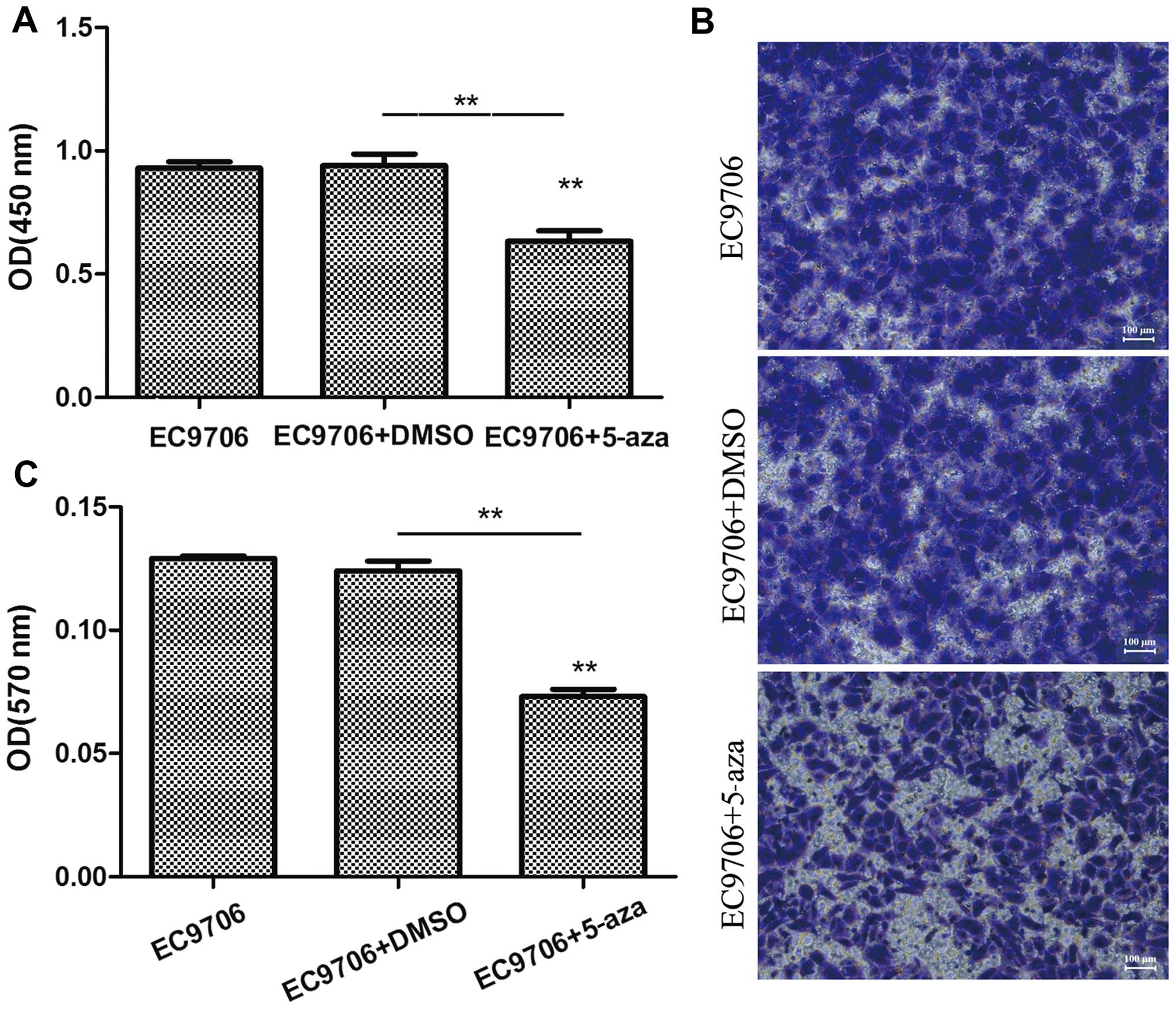
5-Azacytidine suppresses the
proliferation and invasion of EC9706 cells
To examine the effects of 5-azacytidine on the
growth of esophageal cancer cells, the EC9706 cells were treated
with 50 µM 5-azacytidine for 72 h, and CCK-8 cell viability
assay and Transwell assay were then performed to determine the
effects of 5-azacytidine on cell proliferation and invasion. The
results of CCK-8 assay revealed that the viability of the EC9706
cells was markedly suppressed following treatment with
5-azacytidine (Fig. 1A). In
addition, the results of Transwell invasion assay indicated that
the invasive ability of the EC9706 cells was significantly
decreased following treatment with 5-azacytidine (Fig. 1B and C).
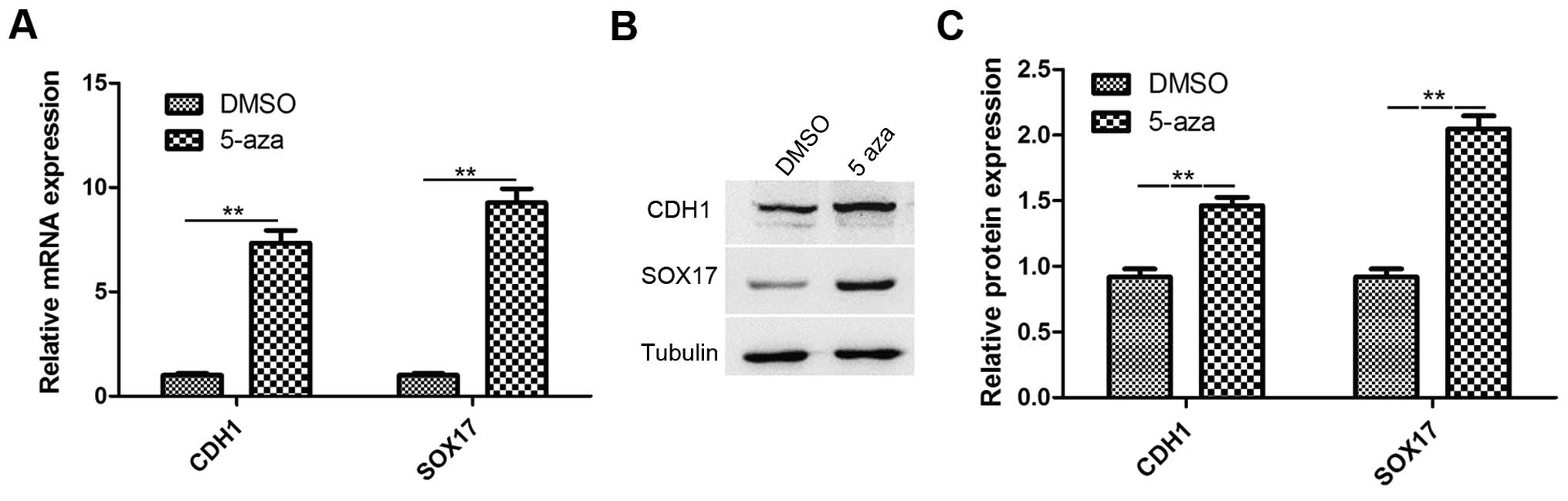
Treatment with 5-azacytidine upregulates
CDH1 and SOX17 expression in EC9706 cells
Studies have reported that CDH1 and SOX17 are
involved in the inhibition of cancer metastasis and growth,
respectively. CDH1 and SOX17 are downregulated in esophageal cancer
(22,32). Therefore, in this study, RT-qPCR
and western blot analysis were performed to analyze the expression
of CDH1 and SOX17 in the EC9706 cells treated with 5-azacytidine
(50 µM) for 72 h. The results revealed that the CDH1
and SOX17 expression levels were significantly increased in
the 5-azacytidine treatment group, in comparison to the DMSO
vehicle control (Fig. 2A). The
results of western blot analysis also revealed that the CDH1 and
SOX17 protein expression levels were significantly upregulated by
5-azacytidine treatment (Fig.
2B).
5-Azacytidine inhibits EC9706 cell
metastasis via the upregulation of CDH1
To further confirm the involvement of CDH1 in the
inhibition of EC9706 cell invasion induced by 5-azacytidine, the
cells were transfected with CDH1 siRNA to knockdown CDH1
expression. The results of western blot analysis revealed that
transfection with CDH1 siRNA suppressed the upregulation of CDH1
that was induced by 5-azacytidine (Fig. 3A). The results of Transwell
invasion assay demonstrated that the inhibitory effects of
5-azacytidine on EC9706 cell invasion were impaired by transfection
with CDH1 siRNA (Fig. 3B).
Western blot analysis also revealed that 5-azacytidine
significantly downregulated the expression of matrix
metalloproteinase (MMP)2 and MMP9. The suppressive effects of
5-azacytidine on the expression of MMP2 and MMP9 were attenuated by
transfection with CDH1 siRNA (Fig.
3C). These results indicate the involvement of CDH1 in the
suppression of EC9706 cell metastasis by 5-azacytidine.
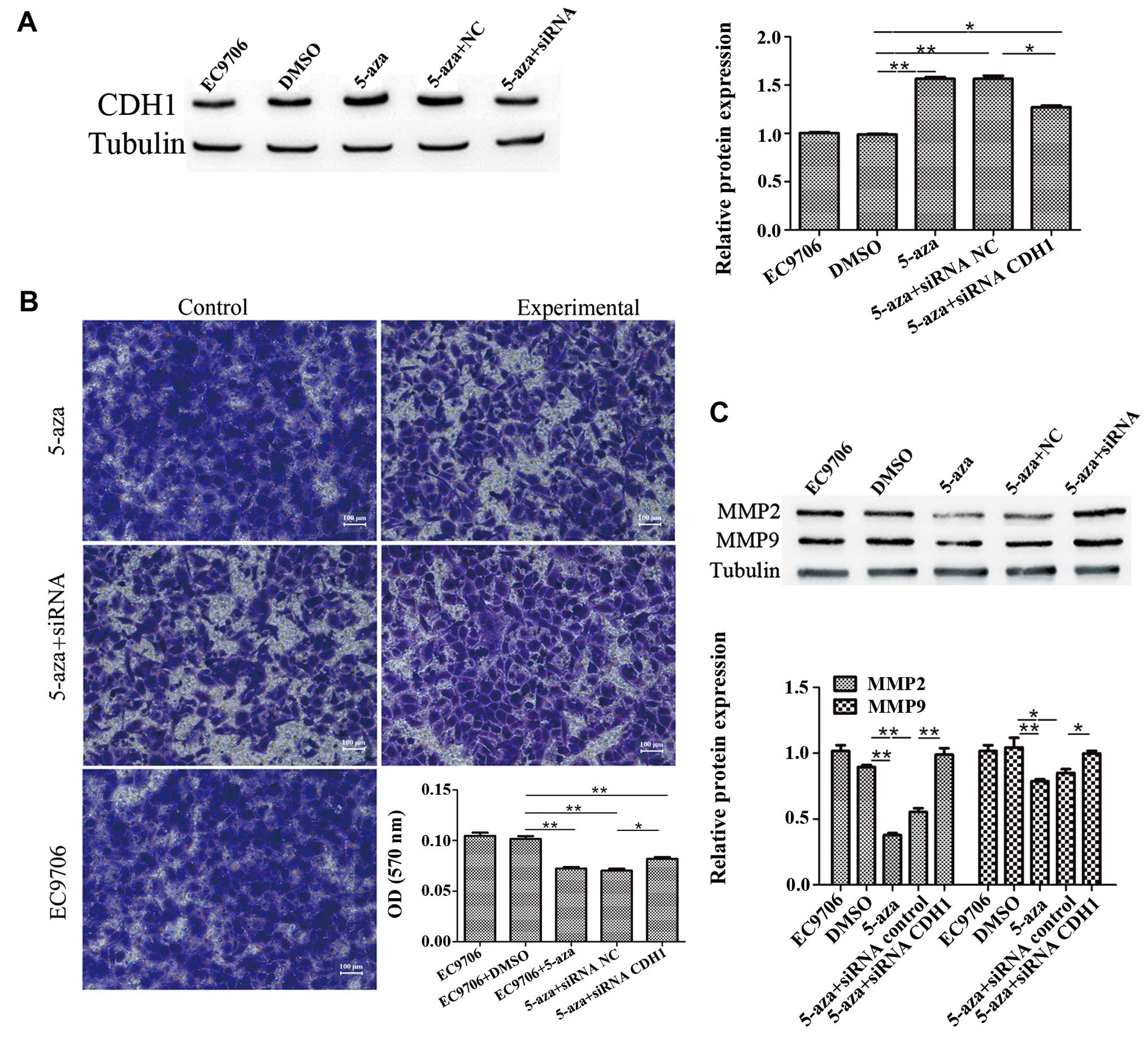 | Figure 35-Azacytidine inhibits EC9706 cell
metastasis via the upregulation of cadherin 1 (CDH1). CDH1 siRNA
attenuated the inhibitory effects of 5-azacytidine on the
metastasis of EC9706 cells. (A) Western blot analysis was used to
detect the expression of CDH1 in EC9706 cells. Compared with the
negative control (DMSO-treated cells), the expression of CDH1 was
increased following treatment with 5-azacytidine
(**P<0.01), whereas the expression of CDH1 was
significantly reduced following CDH1-targeted siRNA transfection
(*P<0.05). (B) Transwell assay was used to detect the
invasion of EC9706 cells. EC9706 cell invasion was significantly
inhibited by 5-azacytidine (**P<0.01), but not by
DMSO, whereas the invasive ability of the cells was increased by
CDH1 siRNA transfection (**P<0.01). The panels
correspond to the following groups: top left panel, DMSO-treated
vehicle control; top right panel, 5-azacytidine-treated cells;
middle left panel, cells treated with 5-azacytidine and transfected
with the control siRNA; middle right panel, cells treated with
5-azacytidine and transfected with CDH1 siRNA; bottom panel,
untreated cells. (C) Western blot analysis was used to detect
matrix metalloproteinase (MMP)2 and MMP9 expression. The results
revealed that the expression of MMP2 and MMP9 was significantly
reduced following treatment with 5-azacytidine in the EC9706 cells
(**P<0.01 and *P<0.05), while the
expression of MMP2 and MMP9 was increased by CDH1 siRNA
transfection (**P<0.01 and *P<0.05).
5-aza, 5-azacytidine. |
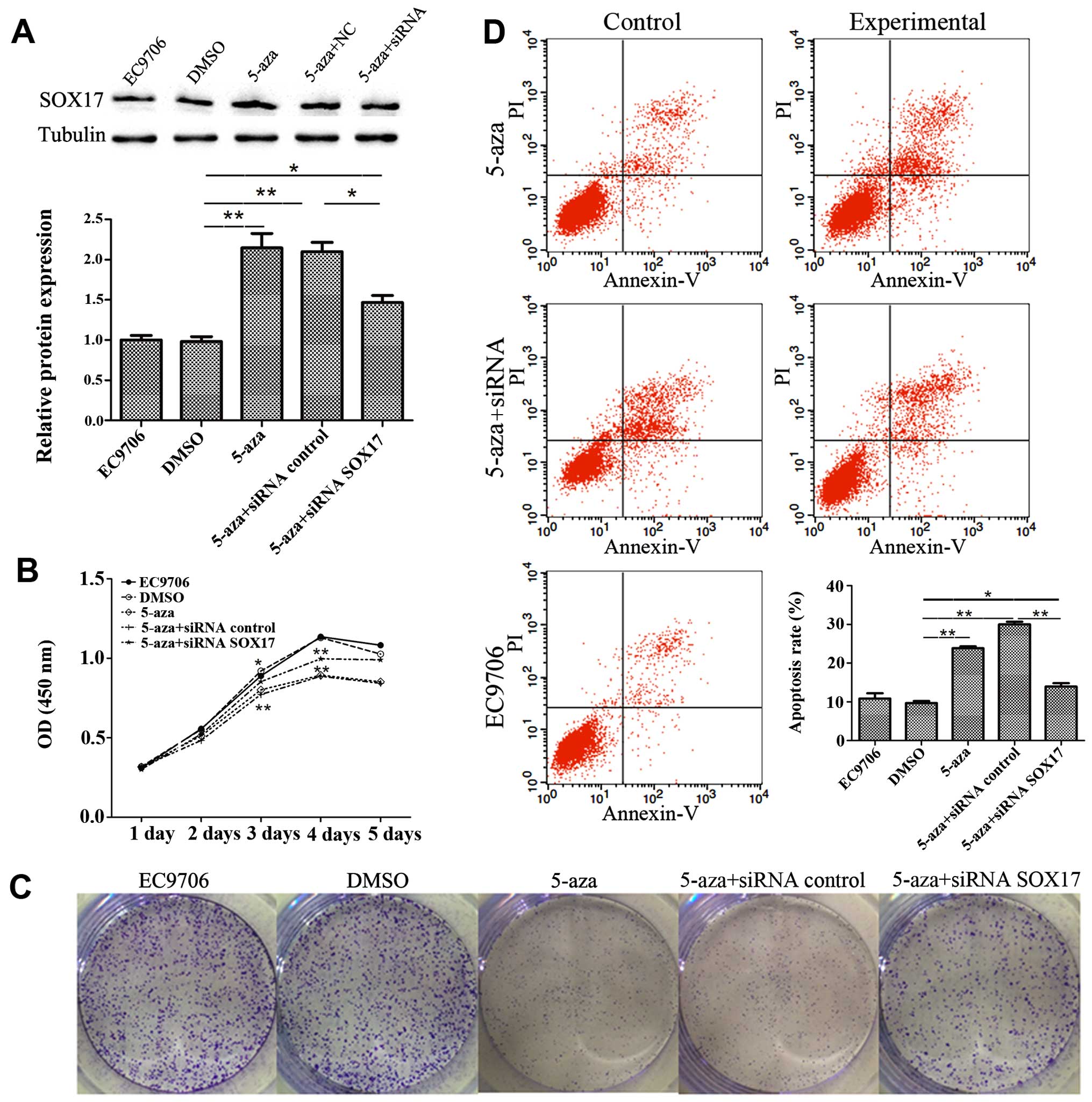
5-Azacytidine inhibits EC9706 cell
proliferation through the upregulation of SOX17
To verify the involvement of SOX17 in the inhibition
of EC9706 cell proliferation by 5-azacytidine, SOX17 siRNA was used
to knockdown SOX17 expression. The results of western blot analysis
revealed that transfection with SOX17 siRNA suppressed SOX17
expression which had been increased by 5-azacytidine (Fig. 4A). CCK8 growth curves proved that
SOX17 siRNA suppressed the 5-azacytidine-induced growth inhibition
(Fig. 4B). This finding was also
supported by the results of colony formation assay (Fig. 4C). Flow cytometry displayed the
effects of 5-azacytidine and SOX17 on cell apoptosis. The apoptotic
rate in the 5-azacytidine-treated EC9706 cells increased by 13.32%
compared to the negative control (DMSO-treated cells). Transfection
with SOX17 siRNA abolished the 5-azacytidine-induced apoptosis of
EC9706 cells (Fig. 4D). These
results indicate the involvement of SOX17 in the inhibition of
EC9706 cell proliferation by 5-azacytidine.
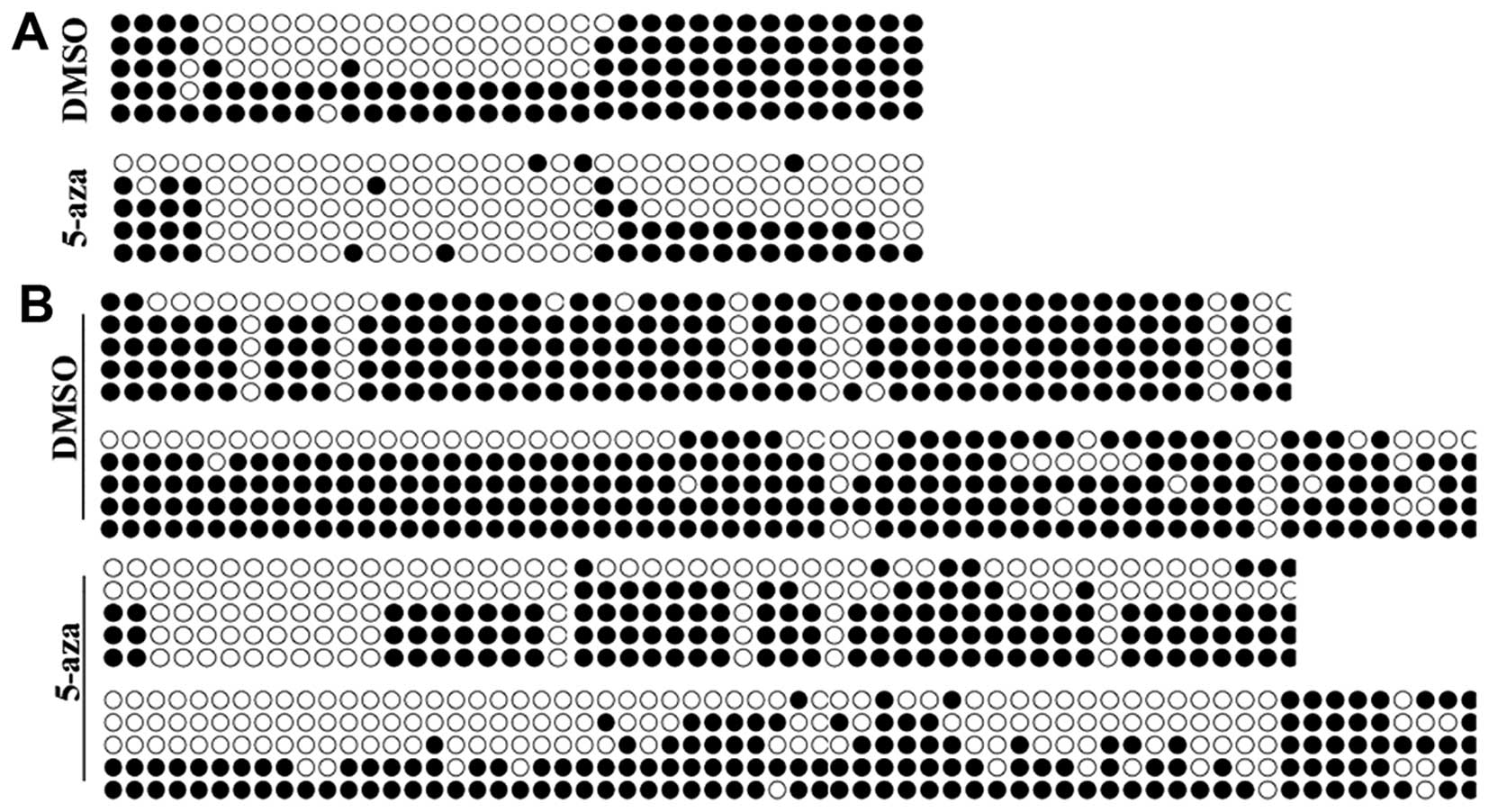
Methylation of CDH1 and SOX17 promoters
is decreased by 5-azacytidine in EC9706 cells
Studies have confirmed that 5-azacytidine is able to
regulate gene expression by decreasing the methylation of their
promoters (14). We hypothesized
that 5-azacytidine upregulates the expression of CDH1 and SOX17 via
their promoter methylation. BSP methylation analysis was performed
to evaluate the methylation of the CDH1 and SOX17
promoters. The results revealed that the promoters of CDH1
and SOX17 were hypermethylated in the EC9706 cells. Yet, the
methylation levels in the EC9706 cell were significantly reduced
following treatment with 5-azacytidine (Fig. 5).
Discussion
Recently, the incidence of esophageal cancer has
gradually increased. Esophageal cancer has become the second cause
of cancer-related mortality in China (36). To date, the most common treatment
for esophageal cancer is surgery, chemotherapy and radiotherapy, in
combination. However, the curative effects of 5-fluorouracil (5-FU)
and cisplatin (DDP), two basic clinical chemotherapeutic drugs for
esophageal cancer, is unsatisfactory in patients with highly
metastatic esophageal cancer (37,38). It has been reported that
epigenetic behaviors, such as DNA methylation, play important roles
in the development and metastasis of esophageal cancer. Previous
studies have found that tumorigenesis and the metastasis of
esophageal cancer result from the downregulation of the expression
of multiple tumor suppressor genes and the abnormal
hypermethylation of their promoters (33–35). Studies have shown that
5-azacytidine, a clinical chemotherapeutic drug used in the
treatment of various types of cancer, inhibits the methylation of
the promoter of multiple genes and affects the expression of these
genes (39,40). In this study, we used EC9706 cells
to assess the anticancer effects of 5-azacytidine in esophageal
cancer.
This study confirmed that the proliferation and
invasion of EC9706 cells were inhibited by 5-azacytidine,
suggesting that 5-azacytidine is effective for the treatment of
esophageal cancer. To investigate the mechanisms of action of
5-azacytidine, we screened numbers of genes that could be related
to the proliferation and metastasis of cancer cells by western blot
analysis. We found that the expression levels of SOX17 and CDH1
were significantly upregulated in the EC9706 cells by
5-azacytidine. Combined with the findings of previous studies, we
hypothesized that SOX17 and CDH1 may participate in the
5-azacytidine-mediated inhibition of cell proliferation and
metastasis. To further confirm our hypothesis, siRNAs were used to
knockdown the expression of SOX17 and CDH1 in the EC9706 cells. We
found that the siRNA-mediated downregulation of CDH1 was greatly
impaired by 5-azacytidine, while the 5-azacytidine-induced
inhibition of EC9706 cell growth and the induction of apoptosis
were significantly attenuated by the siRNA-mediated downregulation
of SOX17. These results support our hypothesis that SOX17 and CDH1
are involved in the 5-azacytidine-induced inhibition of the
proliferation and metastasis of EC9706 cells, respectively.
To examine the mechanisms responsible for the
regulation of CDH1 and SOX17 expression by 5-azacytidine, the
methylation of the SOX17 and CDH1 promoters was
analyzed. Our results revealed that the methylation of the
SOX17 and CDH1 promoters was significantly decreased
by 5-azacytidine treatment in the EC9706 cells. This may be an
important regulatory pattern for 5-azacytidine to regulate SOX17
and CDH1 expression.
In conclusion, the findings of our study confirm
that 5-azacytidine inhibits esophageal cancer cell proliferation
and metastasis by exerting inhibitory effects on the methylation of
SOX17 and CDH1 promoters. Our findings may prove to
be beneficial to clinicians in selecting appropriate
chemotherapeutic regimens and enhancing the therapeutic
effects.
Abbreviations:
|
SOX17
|
SRY-box containing gene 17
|
|
CDH1
|
cadherin-1
|
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81101813) and the Qingdao
Outstanding Health Professional Development Fund (V-YQ2014Y14).
References
|
1
|
Wu C, Kraft P, Zhai K, Chang J, Wang Z, Li
Y, Hu Z, He Z, Jia W, Abnet CC, et al: Genome-wide association
analyses of esophageal squamous cell carcinoma in Chinese identify
multiple susceptibility loci and gene-environment interactions. Nat
Genet. 44:1090–1097. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown LM, Devesa SS and Chow WH: Incidence
of adenocarcinoma of the esophagus among white Americans by sex,
stage, and age. J Natl Cancer Inst. 100:1184–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamangar F, Malekzadeh R, Dawsey SM and
Saidi F: Esophageal cancer in Northeastern Iran: A review. Arch
Iran Med. 10:70–82. 2007.PubMed/NCBI
|
|
4
|
Rubenstein JH and Shaheen NJ:
Epidemiology, diagnosis, and management of esophageal
adenocarcinoma. Gastroenterology. 149:302–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai J, Yang F, Zhang W, Wang Y, Xu J, Song
W, Huang G, Gu J and Guan X: TAp73 and ΔNp73 have opposing roles in
5-aza-2′-deoxycytidine-induced apoptosis in breast cancer cells.
Mol Cells. 37:605–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El Baroudi M, La Sala D, Cinti C and
Capobianco E: Pathway landscapes and epigenetic regulation in
breast cancer and melanoma cell lines. Theor Biol Med Model.
11(Suppl 1): S82014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Momparler RL: Epigenetic therapy of cancer
with 5-aza-2′-deoxycytidine (decitabine). Semin Oncol. 32:443–451.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lemaire M, Momparler LF, Bernstein ML,
Marquez VE and Momparler RL: Enhancement of antineoplastic action
of 5-aza-2′-deoxycytidine by zebularine on L1210 leukemia.
Anticancer Drugs. 16:301–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Momparler RL and Bovenzi V: DNA
methylation and cancer. J Cell Physiol. 183:145–154. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahn JS, Kim YK, Min YH, Cheong JW, Jang
JH, Jung CW, Kim IH, Yoon HJ, Lee HG, Sohn SK, et al: Azacitidine
pre-treatment followed by reduced-intensity stem cell
transplantation in patients with higher-risk myelodysplastic
syndrome. Acta Haematol. 134:40–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maurillo L, Venditti A, Spagnoli A,
Gaidano G, Ferrero D, Oliva E, Lunghi M, D'Arco AM, Levis A,
Pastore D, et al: Azacitidine for the treatment of patients with
acute myeloid leukemia: Report of 82 patients enrolled in an
Italian Compassionate Program. Cancer. 118:1014–1022. 2012.
View Article : Google Scholar
|
|
12
|
Sudan N, Rossetti JM, Shadduck RK, Latsko
J, Lech JA, Kaplan RB, Kennedy M, Gryn JF, Faroun Y and Lister J:
Treatment of acute myelogenous leukemia with outpatient
azacitidine. Cancer. 107:1839–1843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho YH, Yazici H, Wu HC, Terry MB,
Gonzalez K, Qu M, Dalay N and Santella RM: Aberrant promoter
hypermethylation and genomic hypomethylation in tumor, adjacent
normal tissues and blood from breast cancer patients. Anticancer
Res. 30:2489–2496. 2010.PubMed/NCBI
|
|
15
|
Esteller M, Sanchez-Cespedes M, Rosell R,
Sidransky D, Baylin SB and Herman JG: Detection of aberrant
promoter hypermethylation of tumor suppressor genes in serum DNA
from non-small cell lung cancer patients. Cancer Res. 59:67–70.
1999.PubMed/NCBI
|
|
16
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palmisano WA, Divine KK, Saccomanno G,
Gilliland FD, Baylin SB, Herman JG and Belinsky SA: Predicting lung
cancer by detecting aberrant promoter methylation in sputum. Cancer
Res. 60:5954–5958. 2000.PubMed/NCBI
|
|
18
|
Mikhailenko DS and Kushlinskii NE: The
somatic mutations and aberrant methylation as potential genetic
markers ofurinary bladder cancer. Klin Lab Diagn. 61:78–83. 2016.In
Russian. PubMed/NCBI
|
|
19
|
Vaissière T1, Hung RJ, Zaridze D, Moukeria
A, Cuenin C, Fasolo V, Ferro G, Paliwal A, Hainaut P, Brennan P, et
al: Quantitative analysis of DNA methylation profiles in lung
cancer identifies aberrant DNA methylation of specific genes and
its association with gender and cancer risk factors. Cancer Res.
69:243–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaz AM and Grady WM: Epigenetic biomarkers
in esophageal cancer. Cancer Lett. 342:193–199. 2014. View Article : Google Scholar
|
|
21
|
Bussemakers MJ, van Bokhoven A, Mees SG,
Kemler R and Schalken JA: Molecular cloning and characterization of
the human E-cadherin cDNA. Mol Biol Rep. 17:123–128. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Q, Guo Q, Chen L and Liu S:
Clinicopathological significance and potential drug targeting of
CDH1 in lung cancer: A meta-analysis and literature review. Drug
Des Devel Ther. 9:2171–2178. 2015.PubMed/NCBI
|
|
23
|
Karayiannakis AJ, Syrigos KN, Chatzigianni
E, Papanikolaou S, Alexiou D, Kalahanis N, Rosenberg T and
Bastounis E: Aberrant E-cadherin expression associated with loss of
differentiation and advanced stage in human pancreatic cancer.
Anticancer Res. 18:4177–4180. 1998.
|
|
24
|
Zheng Z, Pan J, Chu B, Wong YC, Cheung AL
and Tsao SW: Downregulation and abnormal expression of E-cadherin
and beta-catenin in nasopharyngeal carcinoma: Close association
with advanced disease stage and lymph node metastasis. Hum Pathol.
30:458–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oka H, Shiozaki H, Kobayashi K, Inoue M,
Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S,
Takeichi M, et al: Expression of E-cadherin cell adhesion molecules
in human breast cancer tissues and its relationship to metastasis.
Cancer Res. 53:1696–1701. 1993.PubMed/NCBI
|
|
26
|
Cui H, Wang L, Gong P, Zhao C, Zhang S,
Zhang K, Zhou R, Zhao Z and Fan H: Deregulation between miR-29b/c
and DNMT3A is associated with epigenetic silencing of the CDH1
gene, affecting cell migration and invasion in gastric cancer. PLoS
One. 10:e01239262015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Sun J, Wang B, Ren JC, Su W and
Zhang T: MicroRNA-10b triggers the epithelial-mesenchymal
transition (EMT) of laryngeal carcinoma Hep-2 cells by directly
targeting the E-cadherin. Appl Biochem Biotechnol. 176:33–44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Viotti M, Nowotschin S and Hadjantonakis
AK: SOX17 links gut endoderm morphogenesis and germ layer
segregation. Nat Cell Biol. 16:1146–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Glöckner SC, Guo M, Machida EO,
Wang DH, Easwaran H, Van Neste L, Herman JG, Schuebel KE, Watkins
DN, et al: Epigenetic inactivation of the canonical Wnt antagonist
SRY-box containing gene 17 in colorectal cancer. Cancer Res.
68:2764–2772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia Y, Yang Y, Liu S, Herman JG, Lu F and
Guo M: SOX17 antagonizes WNT/β-catenin signaling pathway in
hepatocellular carcinoma. Epigenetics. 5:743–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin D, Jia Y, Yu Y, Brock MV, Herman JG,
Han C, Su X, Liu Y and Guo M: SOX17 methylation inhibits its
antagonism of Wnt signaling pathway in lung cancer. Discov Med.
14:33–40. 2012.PubMed/NCBI
|
|
32
|
Fu D, Ren C, Tan H, Wei J, Zhu Y, He C,
Shao W and Zhang J: Sox17 promoter methylation in plasma DNA is
associated with poor survival and can be used as a prognostic
factor in breast cancer. Medicine (Baltimore). 94:e6372015.
View Article : Google Scholar
|
|
33
|
Kuo IY, Chang JM, Jiang SS, Chen CH, Chang
IS, Sheu BS, Lu PJ, Chang WL, Lai WW and Wang YC: Prognostic CpG
methylation biomarkers identified by methylation array in
esophageal squamous cell carcinoma patients. Int J Med Sci.
11:779–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Chen Z, Zhao X, Wang J, Ding D, Wang
Z, Tan F, Tan X, Zhou F, Sun J, et al: MicroRNA-25 promotes cell
migration and invasion in esophageal squamous cell carcinoma.
Biochem Biophys Res Commun. 421:640–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee EJ, Lee BB, Han J, Cho EY, Shim YM,
Park J and Kim DH: CpG island hypermethylation of E-cadherin (CDH1)
and integrin alpha4 is associated with recurrence of early stage
esophageal squamous cell carcinoma. Int J Cancer. 123:2073–2079.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leonard GD and Reilly EM: Post-operative
chemotherapy improves disease-free survival, but not overall
survival in people with oesophageal squamous cell carcinoma. Cancer
Treat Rev. 30:473–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen HX and Wang Z: Retrospective study of
adjuvant chemotherapy effects on survival rate after three-field
lymph node dissection for stage IIA esophageal cancer. Asian Pac J
Cancer Prev. 16:5169–5173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yun H, Damm F, Yap D, Schwarzer A,
Chaturvedi A, Jyotsana N, Lübbert M, Bullinger L, Döhner K, Geffers
R, et al: Impact of MLL5 expression on decitabine efficacy and DNA
methylation in acute myeloid leukemia. Haematologica. 99:1456–1464.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Öz S, Raddatz G, Rius M, Blagitko-Dorfs N,
Lübbert M, Maercker C and Lyko F: Quantitative determination of
decitabine incorporation into DNA and its effect on mutation rates
in human cancer cells. Nucleic Acids Res. 42:e1522014. View Article : Google Scholar : PubMed/NCBI
|