Introduction
It has been well documented that prenatal skin
wounds heal rapidly and without scar formation. Although the
molecular and cellular network underlying this phenomenon is not
yet fully understood, increasing evidence suggests that this
phenomenon may be associated with an attenuated prenatal immune
response (1–3). Anecdotal clinical evidence has
suggested that an attenuated immune response can still be present
at a very early postnatal period of life, and it may be associated
with excellent wound healing as seen during digital distal phalanx
regeneration in mice and infants (4). Furthermore, cleft lip reconstructive
surgery performed during the first postnatal week ensures a quick
and almost scarless healing process (5). Primary human keratinocytes and
fibroblasts isolated from residual tissues following cleft lip
surgery have provided a unique opportunity to compare the most
important structural components participating in tissue repair and
regeneration, in newborns and older children (6).
Epithelial-mesenchymal interactions (EMIs) are well
known to play important roles during the course of many
physiological and pathological processes including the repair of
skin and mucous membranes. For instance, EMIs are responsible for
the morphogenesis of acral type epidermis (7). Moreover, EMIs initiate a sequence of
events leading to several developmental steps determining the fate
of epithelial and mesenchymal cells and resulting in the
development of skin appendages such as hair follicles and nails
(8). The development and
maintenance of tissue architecture depends upon a chain of EMIs
that are controlled by several factors such as bone morphogenetic
protein (BMP)-3, Sonic hedgehog and members of the WNT family
(8,9). Mesenchymal cells can even determine
the type of arising appendages as was demonstrated in 1963 in a
series of elegant experiments using avian embryos (10). These data suggest that EMIs are
important for the formation of cutaneous appendages and underscore
the formative potential of dermal fibroblasts.
Fibroblasts and endothelial cells are principal
cellular components of granulation tissue. It is important that
sufficient wound granulation occurs to replace lost tissue before
re-epithelisation from wound edges and hair follicles takes place
(11). Activated fibroblasts
start to synthesize components of the extracellular matrix (ECM)
and organize them into mechanically supportive structures. They are
also frequently transformed to α smooth muscle actin
(αSMA)-expressing cells, the myofibroblasts (MFs) (12–14). MFs generate remarkable contractile
force leading to wound contraction and they also produce various
bioactive growth factors, cytokines and chemokines (13). On the other hand, they also
release multiple proteases responsible for dynamic remodeling and
turnover of ECM components. The presence of activated fibroblasts
and/or MFs is not strictly restricted to wound healing. They are
also a hallmark of tumors. In this scenario, cancer-associated
fibroblasts (CAFs) also significantly influence the biological
properties of tumors including local aggressivity and the formation
of distant metastases (15).
Compared with normal fibroblasts, CAFs differ significantly in the
expression of almost 600 genes as determined by whole genome
transcriptional analysis (16).
Furthermore, CAFs are able to influence the differentiation pattern
of normal keratinocytes including the induction of
epithelial-mesenchymal transition in vitro (17,18). Factors produced by activated
fibroblasts, namely insulin-like growth factor (IGF)-2, BMP-4,
interleukin (IL)-6, IL-8, chemokine (C-X-C motif) ligand 1 (CXCL1),
fibroblast growth factor 7 (FGF-7), leptin, nerve growth factor
(NGF) and transforming growth factor-β (TGF-β), can influence the
epithelial and other cell types at the wound and cancer site,
respectively (16,19,20). Using a similar repertoire of
signaling cascades, the final biological outcome is remarkably
different in wounds and tumors.
It is evident that the age-dependent clinical
presentation of scars following cleft lip reconstructive surgery
calls for a better understanding of the basic biological processes
underlying the fibrotic and regenerative capacities of higher
organisms. Hence, the present study is focused on a functional and
phenotypic comparison of fibroblasts and keratinocytes isolated
from newborns and adults. To complete the series of experiments, we
further studied the EMIs of these cells in matching and
non-matching combinations in vitro.
Materials and methods
Isolation of cells
Residual skin from cleft lip reconstructive surgery
in 3 newborns and aesthetic surgery waste material in 3 adults was
used to isolate keratinocytes and fibroblasts. These samples were
obtained at the Ear, Nose and Throat (ENT) Department of the Second
Faculty of Medicine and the Department of Plastic Surgery of the
Third Faculty of Medicine, at Charles University in Prague. All
samples were acquired under Local Ethics Committee approval
together with explicit written informed consent from the patients
or their legal representatives in the case of minors. The
processing of skin samples and the isolation of cell populations
was described by Krejčí et al (6). Fibroblasts were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with
antibiotics (penicillin 100 U/ml and streptomycin 100 μg/ml)
and 10% fetal bovine serum (FBS) (all from Biochrom GmbH, Berlin,
Germany) and cultured at 37°C and 5% CO2 in a humidified
incubator. Keratinocytes were cultured on mitomycin C-treated
(Sigma-Aldrich, Prague, Czech Republic) 3T3 feeder cells in
keratinocyte medium DMEM + F12 3:1 with 10% FBS, supplemented with
0.4 μg/ml hydrocortisone, 10−10 M cholera toxin,
10 ng/ml epidermal growth factor (all from Sigma-Aldrich) and 0.12
U/ml insulin Actrapid (Novo Nordisk, Bagsværd, Denmark) under the
same conditions.
Multipotency assessment of neonatal
fibroblasts (NFs) and adult fibroblasts (AFs)
The adipogenic, chondrogenic and osteogenic
potential of NFs and AFs was tested using a commercial Human
Mesenchymal Stem Cell Functional Identification kit (R&D
Systems, Minneapolis, MN, USA). Briefly, 1,000 cells/cm2
on fibronectin-coated coverslips were applied and cultured for 3
consecutive days in DMEM supplemented with 5% FBS. The cells were
cultured for a total of three weeks according to the manufacturer's
instructions. The differentiation status of the tested cells was
examined using antibodies provided in the kit.
Migration of fibroblasts
NFs and AFs were also used to perform a migration
assay. Briefly, the cells were inoculated in 6-well plates
(Corning, Rochester, NY, USA). Each well contained 7 isolated 10
μl-drops in a regular geometrical arrangement providing a
constant distance between neigh-boring drops at baseline in all
cases. Each inoculum contained 5,000 cells. Three biological
replicates were performed to achieve consistency. After complete
cell attachment, 2 ml of DMEM was added to each well (6 h after
inoculation) and the cells were then cultured for the next 7 days.
The culture medium was changed on day 3 and 5. The growth of cells
was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (21); blue formazan was
dissolved in 2 ml of dimethylsulfoxide (DMSO; Sigma-Aldrich) and
the absorbance of 200 μl of blue colored solution was
measured at 570 nm using the microplate reader EL800 (Bio-Tek,
Winooski, VT, USA). After the visualization of the cells by
formazan production at the end of the MTT assay,
photo-documentation was performed and the extension of single drops
was acquired and analyzed using the graphical software ImageJ.
Migration of keratinocytes
For the migration test, neonatal keratinocytes (NKs)
and adult keratinocytes (AKs) were used. Briefly, keratinocytes
were inoculated at a density of 25,000 cells/cm2 on the
coverslips placed in an 8-well dish Nunc (Thermo Fisher Scientific,
Rochester, NY, USA) on a mitomycin C-treated feeder layer (3T3
cells, 30,000 cells/cm2) and further cultured in
keratinocyte growth medium to full confluence. Uniform circular
defects were made using the 8 mm biopsy punch (Kai Medical, Seki
City, Japan). The size of single defects was photo-documented after
24, 48 and 96 h. In parallel to decreasing the size of the defect,
the phenotype of migrating keratinocytes was analyzed
immunocytochemically.
Co-culture of fibroblasts and
keratinocytes
Mutual interactions of fibroblasts and keratinocytes
were studied in co-cultures using the insert system. The cells were
always co-cultured in DMEM. To study the influence of keratinocytes
on fibroblast migration, NFs or AFs were inoculated in the same
manner as mentioned above. After their adhesion, collagen inserts
(Corning) were placed into the wells and NKs or AKs were inoculated
at a density of 40,000 cells/cm2. Regarding the faster
growth of fibroblasts influenced by keratinocytes, the experiment
was evaluated by the MTT assay after 5 days. To study the influence
of keratinocytes on fibroblast phenotype, NFs or AFs were
inoculated on coverslips at a density of 1,000 cells/cm2
and after adhesion, collagen inserts with AKs or NKs were placed
into the wells. The cells were co-cultured for 7 days and the
culture medium was changed every 2 days. Selected markers
(vimentin, nestin, SMA and fibronectin) (Table I) were detected using
immunocytochemistry. Finally, the influence of fibroblasts on
keratinocyte phenotype was studied as well. NKs or AKs were seeded
on coverslips, as described in the migration experiment and placed
in 6-well plates. Twenty-four hours later, Falcon PET inserts
(Corning) were put into the wells and AFs or NFs were inoculated at
a density of 5,000 cells/insert. The cells were co-cultured under
the same conditions as mentioned above. The phenotype of
keratinocytes was detected by immunocytochemistry (keratin 8, 14
and 19) (Table I).
 | Table IAntibodies used for
immunocytochemical analysis. |
Table I
Antibodies used for
immunocytochemical analysis.
| Primary
antibody | Supplier
(location) | Secondary
antibody/fluorochrome | Supplier
(location) |
|---|
| Wide spectrum
cytokeratin/P | Abcam,
Cambridge
(Cambridge, UK) | Swine
anti-rabbit/FITC | Dako
(Glostrup, Denmark) |
Cytokeratin
8/P
Nestin/P |
Sigma-Aldrich
(Prague, Czech Republic) | | |
| Fibronectin/P | | | |
| High molecular
weight cytokeratin/M | Dako
(Glostrup, Denmark) | Goat
anti-mouse/TRITC |
Sigma-Aldrich
(Prague, Czech Republic) |
| Cytokeratin
19/M | | | |
| Smooth muscle
actin/M | | | |
| Vimentin/M | | | |
| Cytokeratin
14/M |
Sigma-Aldrich
(Prague, Czech Republic) | | |
Immunocytochemistry (ICC)
The cells grown on coverslips were fixed with 2%
(w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 10
min, then washed with PBS and permeabilized by 0.05% Triton X-100
(Sigma-Aldrich) in PBS. The non-specific binding of immunoglobulins
via Fc receptors was blocked by 3.3% non-immune porcine serum
(Dako, Glostrup, Denmark). The panel of antibodies used is
presented in Table I (antibody
dilution respected the supplier's instructions). The specificity of
binding of the secondary antibodies was tested by isotype controls.
Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(DAPI; Sigma-Aldrich). The stained coverslips were mounted in
Vectashield (Vector Laboratories, Peterborough, UK) and analyzed
using an Eclipse 90i fluorescence microscope (Nikon, Prague, Czech
Republic) equipped with a Cool-1300Q CCD camera (Vosskühler GmbH,
Osnabrück, Germany) and LUCIA 5.1 computer-assisted image analysis
system (Laboratory Imaging, Prague, Czech Republic).
Polymerase chain reaction (PCR)
analysis
RNA from subconfluent cultures of NFs and AFs was
isolated using an RNeasy micro kit (Qiagen, Gaithersburg, MD, USA).
DNase I (Qiagen) was applied in RNA solution to properly remove
genomic DNA, and the purification procedure was repeated. Reverse
transcription was performed with 1 μg of total RNA and an
AccuScript High Fidelity First Strand cDNA Synthesis kit
(Stratagene, San Diego, CA, USA) according to the manufacturer's
instructions. For negative control, the same reverse transcription
reaction without reverse transcriptase was performed. The PCR
reaction was performed with REDTaq ReadyMix (Sigma-Aldrich). All
primers are listed in Table
II.
 | Table IIPrimers for the genes of epidermal
neural crest stem cell molecular signature. |
Table II
Primers for the genes of epidermal
neural crest stem cell molecular signature.
| Primer name | Sequence | Primer name | Sequence |
|---|
| Hs PCBP4_F |
ctcctgcaaatggtggaaat | Hs GAPDH_R |
tgtggtcatgagtccttcca |
| Hs PCBP4_R |
ctgaccctggacagagaagc | Hs VARS2_F |
gtctacctccatgccatcgt |
| Hs CALR_F |
tctcagttccggcaagttct | Hs VARS2_R |
gaagtggcggtaacccagta |
| Hs CALR_R |
tctgagtctccgtgcatgtc | Hs CRMP1_F |
ggcggtggagtacaacatct |
| Hs PYGO2_F |
ctctgtcccaacgatttgct | Hs CRMP1_R |
cacaggaccgtcatacatgc |
| Hs PYGO2_R |
aagctgttggcatctggagt | Hs CRYAB_F |
ttcttcggagagcacctgtt |
| Hs H1FX_F |
gcgttgtccccatctaagaa | Hs CRYAB_R |
ttttccatgcacctcaatca |
| Hs H1FX_R |
agcttgaaggaaccgttgg | Hs MSX2_F |
gtctccagcctgcccttc |
| Hs ETS 1_F |
ggaggaccagtcgtggtaaa | Hs MSX2_R |
gtggcatagagtcccacagg |
| Hs ETS 1_R |
tttgaattcccagccatctc | Hs MYO10_F |
cgcaacaaccaggatacctt |
| Hs PEG10_F |
tcccactacctgatgcacaa | Hs MYO10_R |
tccgcttctccagtttctgt |
| Hs PEG10_R |
atctacctggtggtggcttg | Hs THOP1_F |
agccttctgtgcatcgactt |
| Hs VDAC1_F |
ctcagccaacactgagacca | Hs THOP1_R |
tccttgaggatagcgcagtt |
| Hs VCAC1_R |
cagccctcgtaacctagcac | Hs UBE4b_F |
agcctctggtggagcaagta |
| Hs SELH_F |
tagccgagaagcgagagaag | Hs UBE4b_R |
ttaaggatttcggcaaccac |
| Hs SELH_R |
gccccttcttaatcccagtc | Hs TSEN15_F |
ggagatgccacccaagttta |
| Hs AGPAT6_F |
gagtcctggaacctgctgag | Hs TSEN15_R |
tggcagcataaatccatcag |
| Hs AGPAT6_R |
gccaccatttcttggtctgt | Hs ADAM12_F |
cagtttcacggaaacccact |
| Hs GAPGD_F |
cgagatccctccaaaatcaa | Hs ADAM12_R |
gcctctgaaactctcggttg |
Transcriptome analysis
Total RNA was isolated using an RNeasy micro kit
(Qiagen) according to the manufacturer's instructions. The quality
and concentration of RNA were measured with a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The
RNA integrity was analyzed with an Agilent Bioanalyzer 2100
(Agilent, Santa Clara, CA, USA). Only samples with intact RNA
profiles were used for expression profiling analyses (RIN
>9).
Illumina HumanHT-12 v4 Expression BeadChips
(Illumina, San Diego, CA, USA) were used for the microarray
analysis following the standard protocol. In brief, 200 ng RNA was
amplified with Illumina TotalPrep RNA Amplification kit (Ambion,
Austin, TX, USA) and 750 ng of labeled RNA was hybridized on the
chip according to the manufacturer's instructions. The analysis was
performed in two biological replicates per group. The raw data were
preprocessed using GenomeStudio software (version 1.9.0.24624;
Illumina) and the limma package (22) of the Bioconductor (23), as previously described (24): the transcription profiles were
background corrected using a normal-exponential model, quantile
normalized and variance stabilized using base 2 logarithmic
transformation.
The moderated t-test was used to detect transcripts
differentially expressed between the sample groups [within limma;
(21)]. False discovery rates
values were used to select significantly differentially transcribed
genes (FDR <0.05). The transcription data are Minimum
Information About a Microarray Experiment (MIAME)-compliant and
have been deposited in the ArrayExpress database. Gene set
enrichment analysis (GSEA) and determination of gene functions were
performed using Enrichr web service (25).
Statistical analysis
Statistical analysis was performed using PAST
(version 3.12) free scientific analysis software kindly provided by
Dr Ø. Hammer, University of Oslo, Norway. Individual data sets
describing size of AF and NF covered areas were first tested for
normality of distribution (Shapiro-Wilk test). To assess the
equality of variances for both groups, Levene's test for
homoscedasticity was used. Confirming these two assumptions,
independent (two-tailed) t-test was performed to compare the size
of AF- and NF-covered areas, respectively with the null hypothesis
that the AF and NF covered areas are equal, alpha level 0.001.
Thus, if the P-value is less than the chosen alpha level, then the
null hypothesis is rejected and there is evidence that the data
tested are unequal.
Results
Differentiation potential of NFs and
AFs
Both NF as well as AF RNA profiles were consistent
with the molecular signature of epidermal neural crest stem cells
(26) (Fig. 1). However, these two cell types
significantly differed in the differentiation potential. The
adipogenic differentiation of NFs was confirmed by the adipocyte
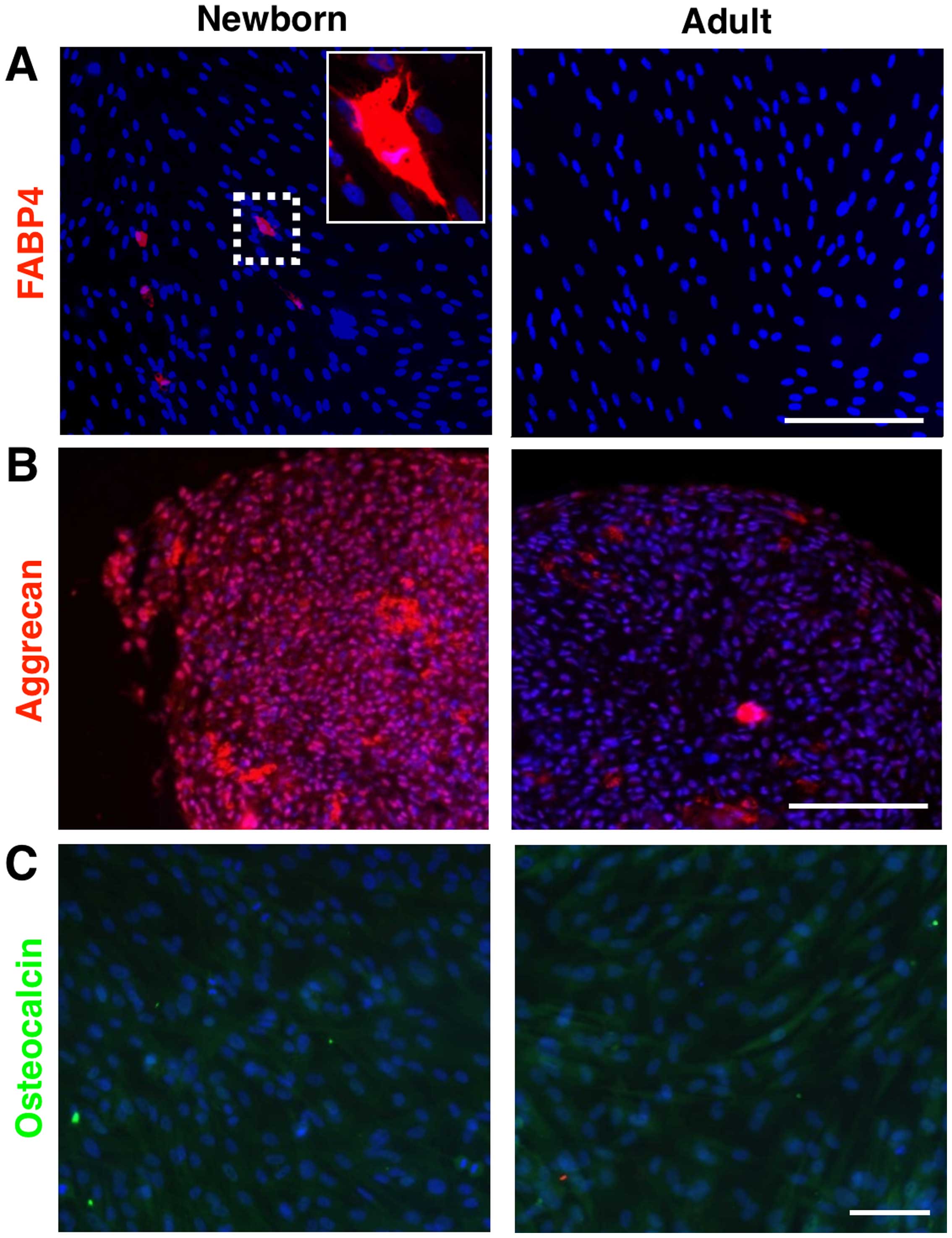
marker FABP4 (Fig. 2A) and also
by the content of lipid droplets (oil red positive staining, image
not shown). Similarly, we succeeded in demonstrating the
chondrogenic differentiation of NF (Fig. 2B), which was verified by the
detection of aggrecan. However, we failed in the case of osteogenic
differentiation in NFs (Fig. 2C).
On the contrary, the adult counterparts (AFs) were not capable of
differentiating into any of these three lineages (Fig. 2).
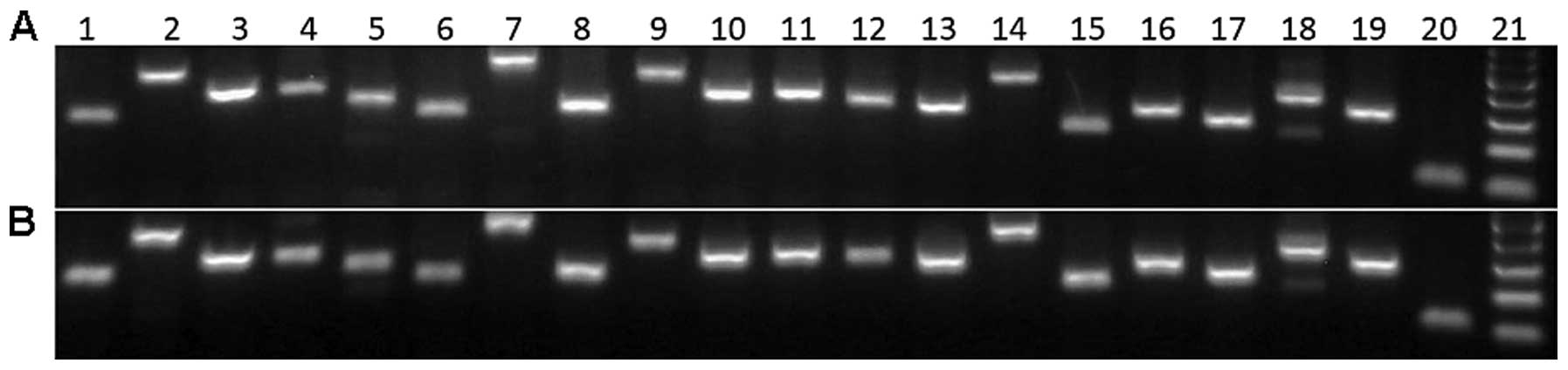 | Figure 1(A) Neonatal and (B) adult
fibroblasts express markers of epidermal neural crest stem cells.
1, CRYAB; 2, PEG10; 3, AGPAT6; 4, UBE4B; 5, H1FX; 6, CRMP1; 7,
MYO10; 8, SELH; 9, ETS1; 10, VDAC1, 11, TSEN15; 12, MSX2; 13,
VARS2; 14, ADAM12; 15, CALR; 16, PYGO2; 17, THOP1; 18, PCBP4; 19,
GAPDH; 20, 18S RNA; 21, 100 bp ladder. |
MTT assay and migration of
fibroblasts
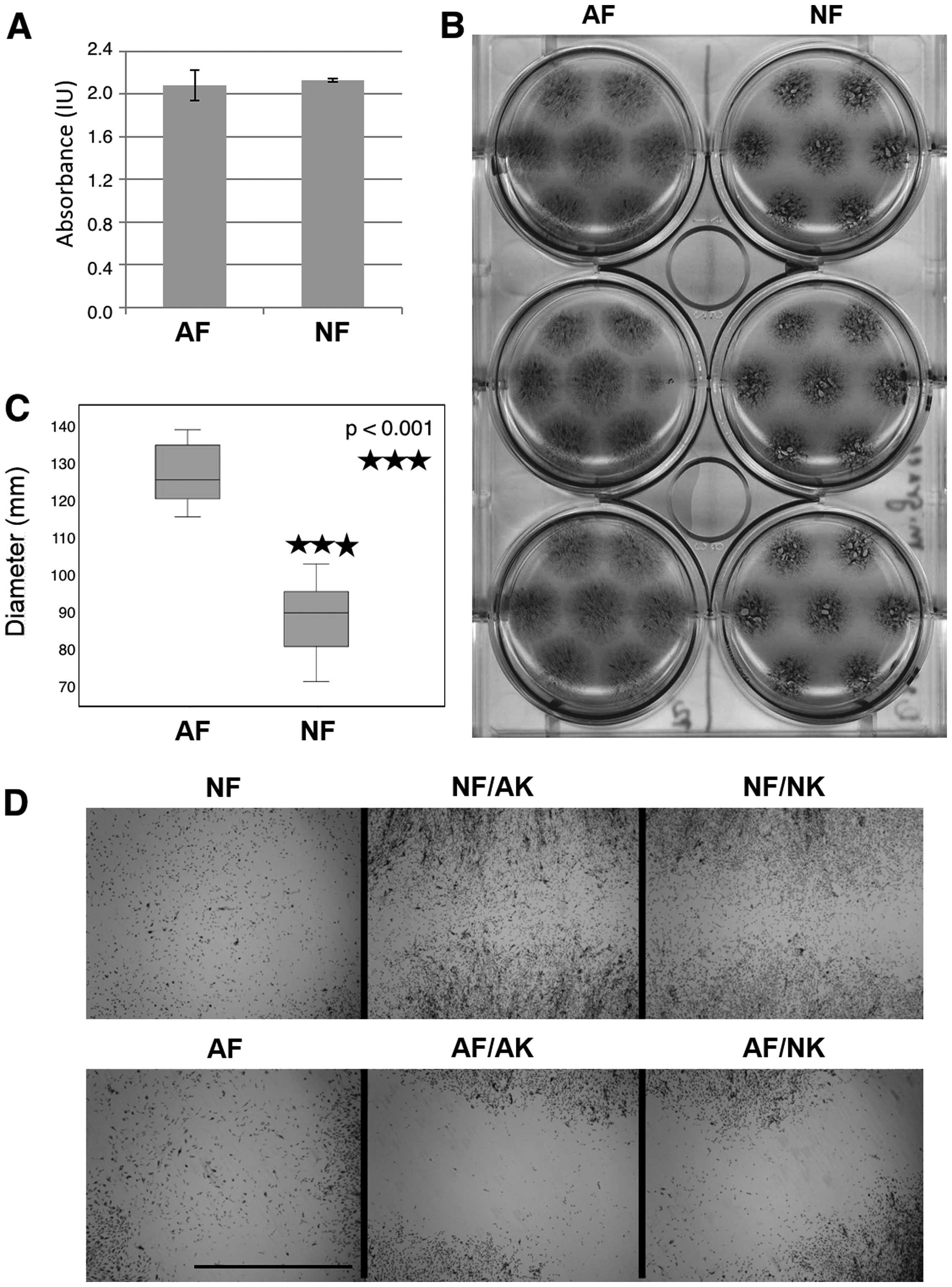
Surprisingly, the results of the MTT assay (Fig. 3A) indicated that the final
metabolic activity of the NF and AF cultures were quite similar at
the endpoint. When keratinocytes were added to the culture system,
the growth of AFs as well as NFs increased approximately 1.7 times
(as assessed by the MTT assay). Notable differences between the
stimulation by NKs and AKs were not found (data not shown).
Cell migration was assessed on inoculum spreading
visualized during the MTT assay at the same time. Regardless of the
comparable metabolic activity of AFs and NFs, these cells differed
in their migration potential. From the original 10 μl drop
inoculum, AFs were able to reach the size of the final circular
spot of 12 mm in diameter that was somewhat higher than in NFs (8.8
mm) (Fig. 3B and C). Both types
of keratinocytes in Transwell co-culture further increased the
migration of NFs in the same manner, while in AFs the situation
remained the same. These results are clearly illustrated in
Fig. 3D, where the space between
the borders of neighboring growth is shown.
Migration of keratinocytes
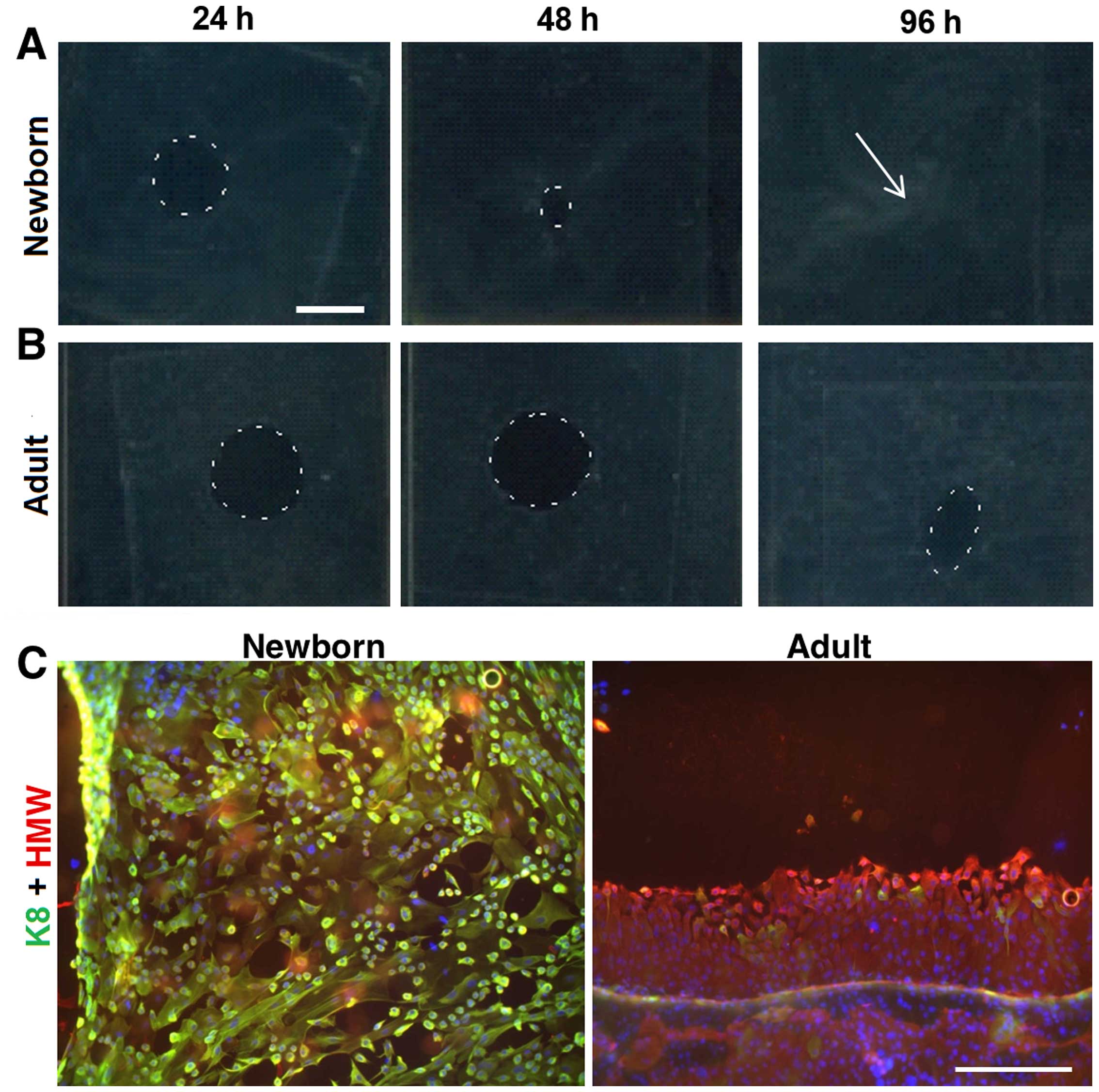
The migration potential of NKs and AKs was studied
on biopsy punch-made round defects (8 mm) in confluent monolayers
of NKs and AKs, respectively. We observed remarkable differences
between both keratinocyte types. NKs were able to heal the defect
completely within 96 h (Fig. 4A)
whereas nearly one third of the defect area in AKs remained
unclosed at this time point (Fig.
4B). NKs colonizing the defect were uniformly very small (<5
μm), rounded and practically all cells expressed keratin 8.
On the contrary, AKs created highly organized sheets and the very
small keratinocytes were localized only at the leading edge.
Moreover, all AKs were negative for keratin 8 (Fig. 4C).
Expression of αSMA and production of
fibronectin
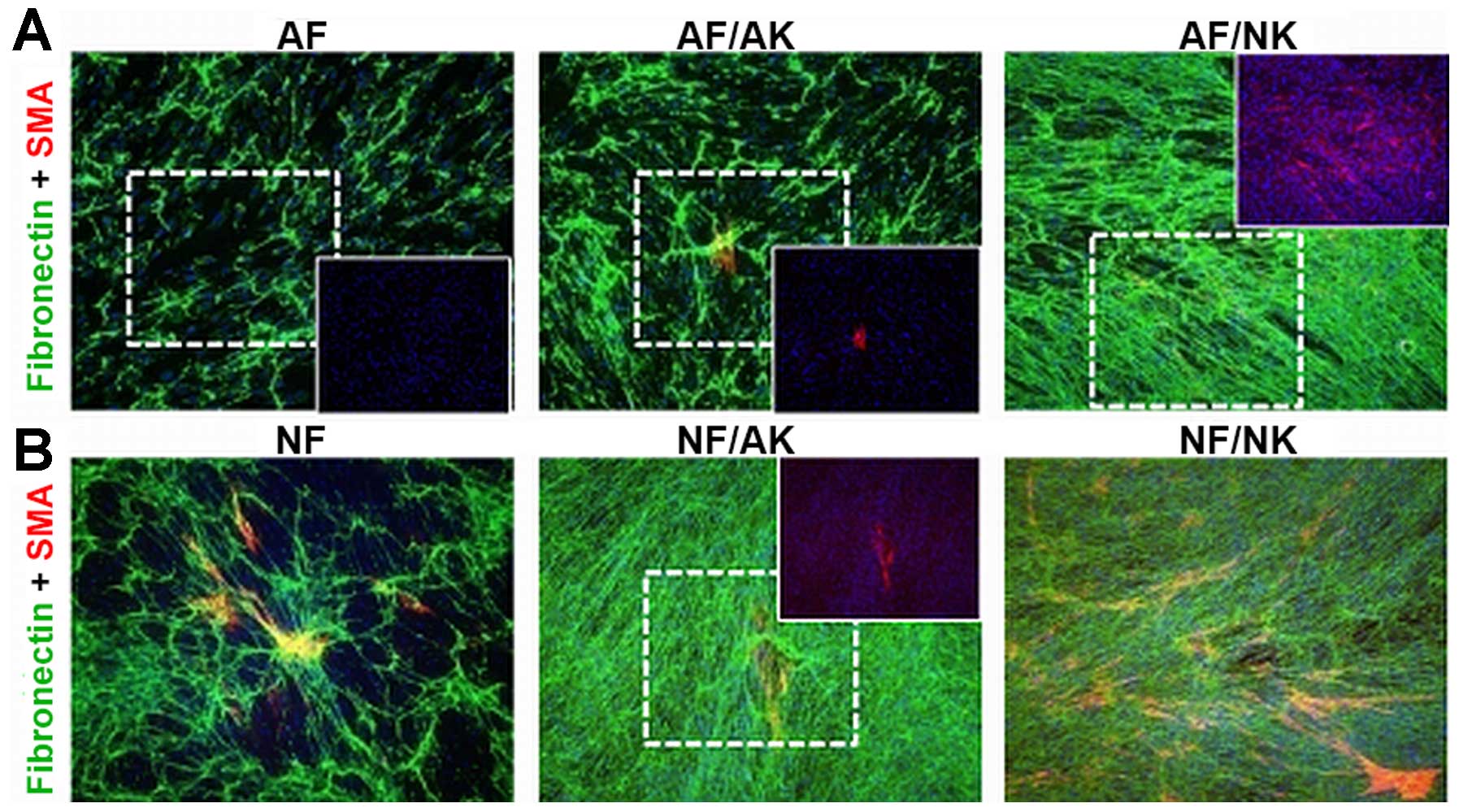
Both AFs as well as NFs produced fibronectin rich
ECM and its production was further enhanced in co-cultures with
keratinocytes. While NKs stimulated AFs and NFs to the same degree,
AKs increased the production of fibronectin, mainly in NFs
(Fig. 5). No MFs (αSMA-positive)
were detected in AF cultures (Fig.
5A); however, numerous MFs were observed in the case of NFs
(Fig. 5B). The presence of AKs in
co-culture resulted in a slight increase in the number of MFs in AF
and NF cultures, respectively. By contrast, the presence of NKs in
co-cultures generated a remarkable increment in the number of MFs
in both AF and NF cultures, suggesting a higher frequency of
transition to MFs (Fig. 5).
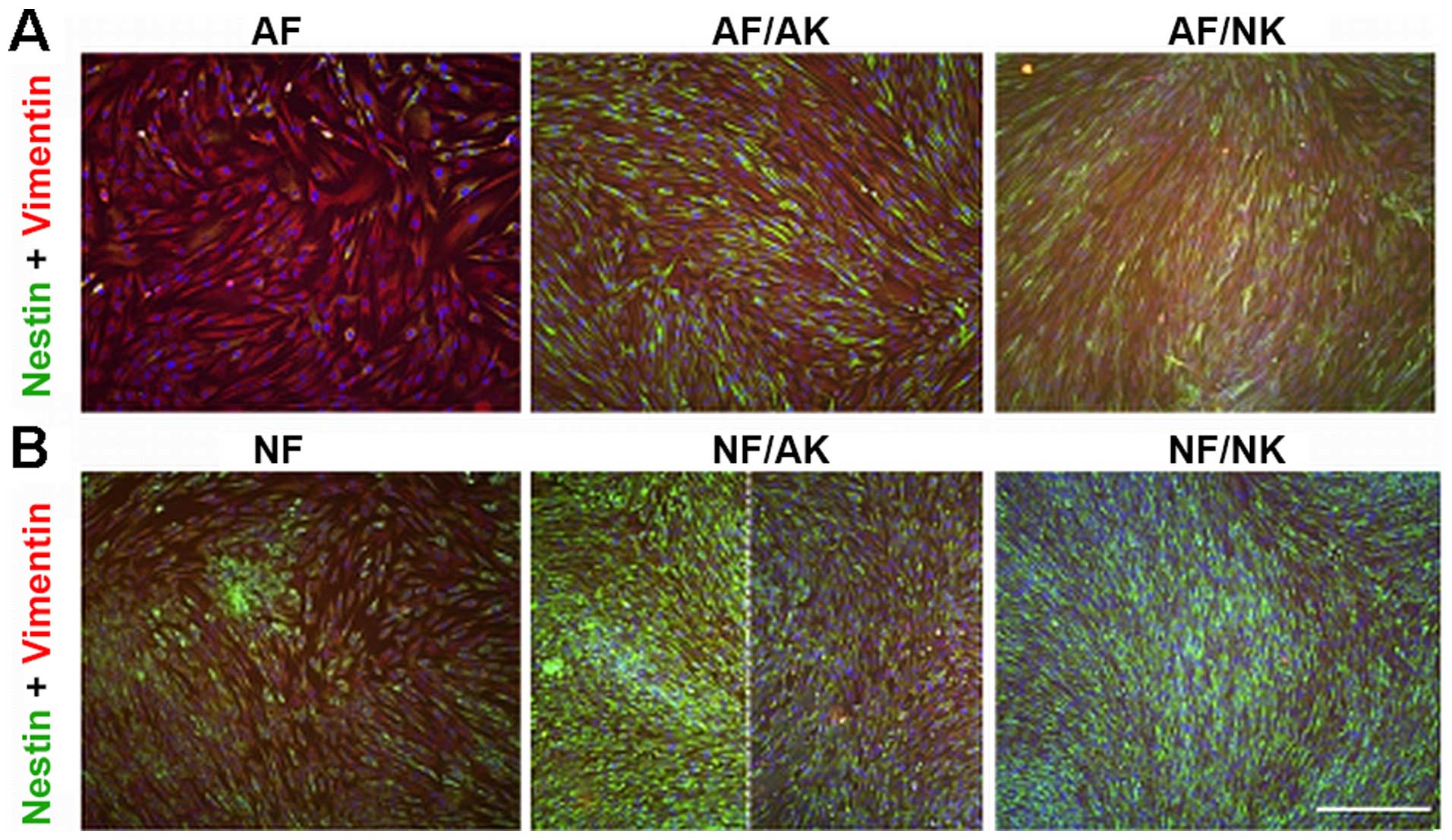
Expression of nestin
Nestin-positive fibroblasts were very rare in the
culture of normal AFs (Fig. 6A).
Strikingly, the majority of NFs were positive for this intermediate
filament (Fig. 6B). Both AKs and
NKs in co-culture stimulated the expression of nestin in both AF
and NF cultures (Fig. 6). While
the increase in nestin positivity was uniformly distributed over
the population of AFs (Fig. 6A),
the nestin-positive NFs were detected only locally in the case of
AKs (Fig. 6B).
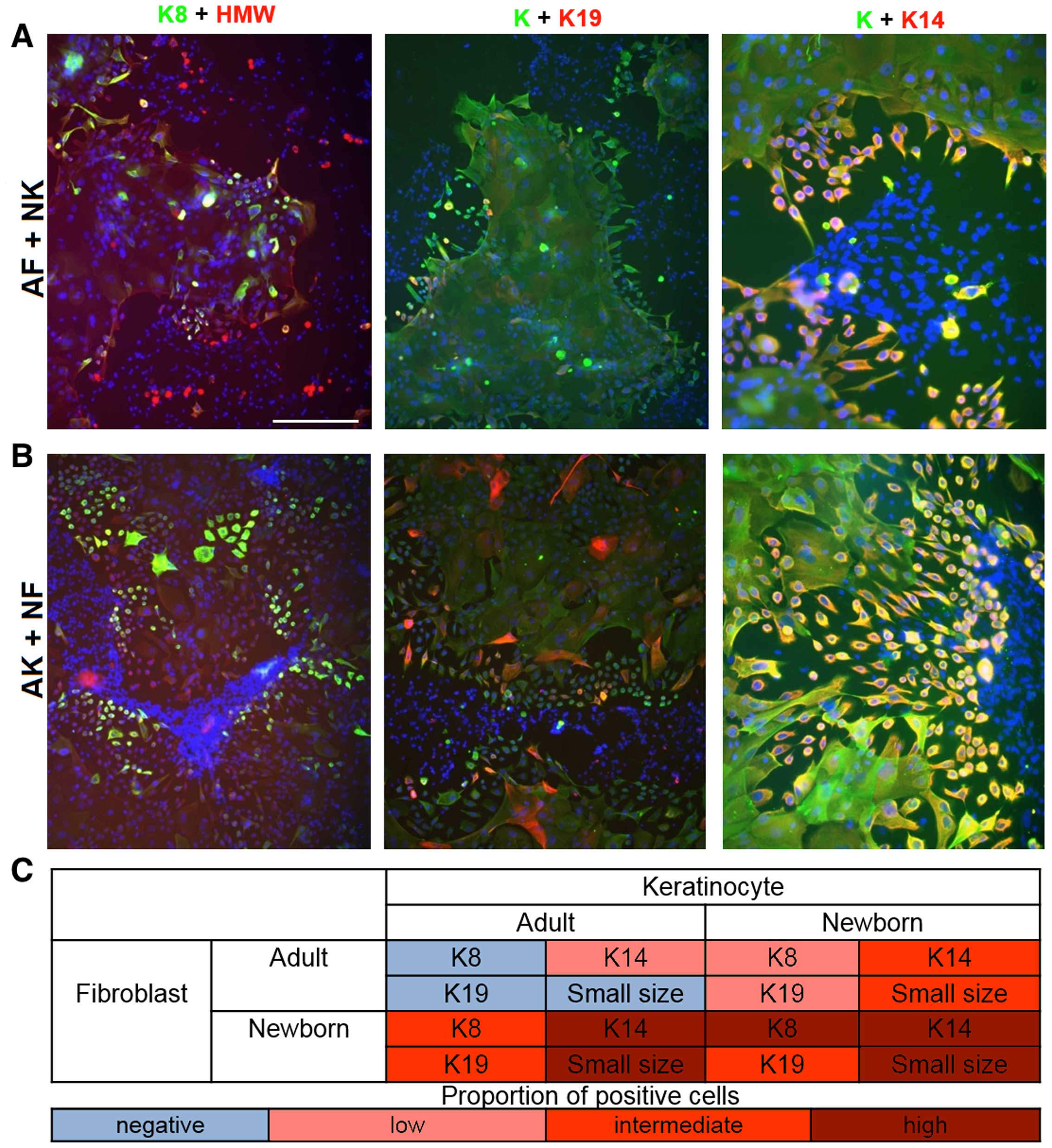
ICC of co-cultures of keratinocytes and
fibroblasts (AF/AK, AF/NK, NF/NK and NF/AK)
At day 7, individual keratinocyte colonies were
still isolated in all experimental settings. Small keratinocytes
were present at their peripheries. In the co-culture of NFs with
NKs (NF/NK), small keratinocytes frequently expressed keratin 8 and
19 (image not shown). Furthermore all cells were positive for
keratin 14. The occurrence of small, rounded keratinocytes on the
periphery of colonies was significantly rarer when NKs were
cultured with AFs (Fig. 7A) and
the expression of the keratins studied in NKs decreased.
Strikingly, NFs strongly influenced the phenotype of AKs (Fig. 7B). This NF/AK experimental setting
typically resulted in a significantly higher number of small
keratinocytes exhibiting triple positivity K8/19/14 than seen in
co-cultures of AF/AK (image not shown) and even in co-cultures of
AF/NK (Fig. 7). These small
keratinocytes in AK/NF type co-cultures were devoid of typical
intercellular bridges.
Comparison of expression profiles of NFs
and AFs fibroblasts
In this study using microarray technology expression
profiles of cultivated NFs and AFs were compared. Interestingly,
the expression analysis revealed the existence of 1,509
differentially-regulated genes (FDR <0.05).
Growth factors, ILs and other extracellular factors,
together with ECM-related genes were considered the most important
group of genes with the potential to influence the investigated
phenotype of cells. Thus, we performed GSEA of the group of
extracellular factors upregulated in NFs (FDR <0.2) with the aim
of determining their specific biological role and prospective
agents that may be responsible for paracrine stimulation. The
following genes from those upregulated in NFs are known to
positively regulate cell division (GO:0051781: FGF5, TGFB2, MDK,
TGFB3, IL1B, VEGFB, FGF1, PGF and VEGFA), cell growth (GO:0001558:
TGFB2, IGFBP5, IGFBP4, IGFBP2, IGFBP7, NGF, KAZALD1, CTGF, CXCL16,
VEGFA, GDF10, IL-6, TGFB3, INHBB, FGF1 and BMP6) and stimulate cell
chemotaxis (GO:0060326: CXCL6, TGFB2, IL-6, IL1B, VEGFB, CXCL1,
CXCL14, CXCL16 and VEGFA). Arguably, the observed differences in
the expression of genes, which stimulate cells responsible for the
acute phase of the inflammatory response, could be the reason why
stimulation by NFs improves tissue regeneration (Table III).
 | Table IIIGSEA (GO Biological processes) of the
group of extracellular factors upregulated in NFs (FDR
<0.2). |
Table III
GSEA (GO Biological processes) of the
group of extracellular factors upregulated in NFs (FDR
<0.2).
| Term | Overlap | P-value | Adjusted
P-value | Z-score | Combined score |
|---|
| Positive regulation
of cell division (GO:0051781) | 9/120 | 2.5E-10 | 3.1E-07 | −2.25 | 33.67 |
| Positive regulation
of MAPK cascade (GO:0043410) | 12/395 | 3.9E-09 | 1.7E-06 | −2.46 | 32.72 |
| Regulation of cell
division (GO:0051302) | 10/234 | 4.1E-09 | 1.7E-06 | −2.39 | 31.75 |
| Growth
(GO:0040007) | 10/329 | 9.3E-08 | 1.9E-05 | −2.41 | 26.11 |
| Regulation of cell
growth (GO:0001558) | 10/322 | 7.7E-08 | 1.9E-05 | −2.34 | 25.42 |
| Cell chemotaxis
(GO:0060326) | 8/155 | 4.3E-08 | 1.3E-05 | −2.25 | 25.24 |
| Chemotaxis
(GO:0006935) | 9/263 | 1.7E-07 | 2.6E-05 | −2.39 | 25.18 |
| Taxis
(GO:0042330) | 9/263 | 1.7E-07 | 2.6E-05 | −2.39 | 25.16 |
| Positive regulation
of peptidyl-tyrosine phosphorylation (GO:0050731) | 7/139 | 3.8E-07 | 5.0E-05 | −2.27 | 22.48 |
| Response to
corticosteroid (GO:0031960) | 7/140 | 4.0E-07 | 5.0E-05 | −2.24 | 22.20 |
Overall, NFs and AF differ in the expression of
genes that influence ECM organization (GO:0030198 and GO:0022617),
morphogenesis (GO:0009887 and GO:0048598), cell adhesion
(GO:0030155), epithelial cell proliferation (GO:0050678),
angiogenesis (GO:0001525) and ossification (GO:0030278), as well as
positively regulate cell motility and migration (GO:2000147 and
GO:0030335) (see Table IV for
full details). The products of the deregulated genes localize
primarily to the ECM (GO:0031012), extracellular space
(GO:0005615), and cell surface (GO:0009986). The changes also occur
in adherent (GO:0005912) and anchoring junctions (GO:0070161). From
the molecular function perspective, these products are mainly
responsible for binding of glycosaminoglycans (GO:0005539), sulfur
compounds (GO:1901681), collagen (GO:0005518), heparin
(GO:0008201), IGF (GO:0005520), cell adhesion molecules
(GO:0050839) as well as ECM structural constitution (GO:0005201),
growth factor activity (GO:0008083) and many other functions
related to extracellular processes.
 | Table IVGSEA (GO terms) of the genes
deregulated between NFs and AFs (FDR <0.05). |
Table IV
GSEA (GO terms) of the genes
deregulated between NFs and AFs (FDR <0.05).
| Term | Overlap | P-value | Adjusted
P-value | Z-score | Combined
score |
|---|
| GO: Biological
processes | | | | | |
| Extracellular
matrix organization (GO:0030198) | 91/359 | 2.6E-18 | 6.7E-15 | −2.38 | 77.69 |
| Extracellular
structure organization (GO:0043062) | 91/360 | 3.0E-18 | 6.7E-15 | −2.38 | 77.68 |
| Organ morphogenesis
(GO:0009887) | 79/405 | 6.0E-11 | 9.0E-08 | −2.36 | 38.26 |
| Embryonic
morphogenesis (GO:0048598) | 76/403 | 5.4E-10 | 6.1E-07 | −2.43 | 34.73 |
| Regulation of cell
adhesion (GO:0030155) | 66/336 | 1.9E-09 | 1.7E-06 | −2.45 | 32.62 |
| Regulation of
epithelial cell proliferation (GO:0050678) | 53/258 | 2.1E-08 | 1.2E-05 | −2.45 | 27.83 |
| Angiogenesis
(GO:0001525) | 51/236 | 9.2E-09 | 6.9E-06 | −2.30 | 27.33 |
| Embryonic organ
morphogenesis (GO:0048562) | 34/121 | 1.4E-08 | 9.1E-06 | −2.19 | 25.48 |
| Positive regulation
of cell motility (GO:2000147) | 54/287 | 1.9E-07 | 6.6E-05 | −2.46 | 23.67 |
| Positive regulation
of cell migration (GO:0030335) | 53/280 | 2.1E-07 | 6.7E-05 | −2.45 | 23.58 |
| GO: Cellular
component | | | | | |
| Extracellular
matrix (GO:0031012) | 90/348 | 1.8E-18 | 8.9E-16 | −2.25 | 78.03 |
| Extracellular space
(GO:0005615) | 163/1120 | 2.3E-11 | 5.6E-09 | −2.19 | 41.63 |
| Extracellular
matrix part (GO:0044420) | 37/127 | 1.7E-09 | 2.7E-07 | −2.12 | 31.97 |
| Proteinaceous
extracellular matrix (GO:0005578) | 53/239 | 2.6E-09 | 3.2E-07 | −2.12 | 31.64 |
| Cell surface
(GO:0009986) | 72/437 | 2.4E-07 | 2.4E-05 | −2.25 | 23.95 |
| Extracellular
region (GO:0005576) | 183/1585 | 7.2E-06 | 2.9E-04 | −2.43 | 19.80 |
| Adherens junction
(GO:0005912) | 64/405 | 3.9E-06 | 2.7E-04 | −2.25 | 18.47 |
| Anchoring junction
(GO:0070161) | 65/419 | 5.6E-06 | 2.8E-04 | −2.22 | 18.14 |
| Cell-substrate
adherens junction (GO:0005924) | 58/358 | 5.7E-06 | 2.8E-04 | −2.21 | 18.06 |
| Extracellular
vesicular exosome (GO:0070062) | 291/2717 | 2.9E-06 | 2.4E-04 | −2.15 | 17.93 |
| GO: Molecular
function | | | | | |
| Glycosaminoglycan
binding (GO:0005539) | 40/190 | 4.9E-07 | 1.7E-04 | −2.36 | 20.55 |
| Sulfur compound
binding (GO:1901681) | 42/206 | 5.4E-07 | 1.7E-04 | −2.32 | 20.16 |
| Extracellular
matrix structural constituent (GO:0005201) | 23/68 | 1.7E-07 | 1.6E-04 | −2.22 | 19.40 |
| Collagen binding
(GO:0005518) | 20/62 | 2.0E-06 | 4.6E-04 | −2.22 | 17.05 |
| Growth factor
activity (GO:0008083) | 33/163 | 9.9E-06 | 1.5E-03 | −2.34 | 15.18 |
| Heparin binding
(GO:0008201) | 30/140 | 9.6E-06 | 1.5E-03 | −2.23 | 14.45 |
| Insulin-like growth
factor binding (GO:0005520) | 11/27 | 7.6E-05 | 8.7E-03 | −2.95 | 13.97 |
| Cell adhesion
molecule binding (GO:0050839) | 32/168 | 3.8E-05 | 5.0E-03 | −2.29 | 12.11 |
| Fibronectin binding
(GO:0001968) | 10/26 | 2.3E-04 | 1.8E-02 | −2.78 | 11.16 |
| RNA polymerase II
core promoter proximal region sequence-specific DNA binding
transcription factor activity (GO:0000982) | 35/204 | 1.1E-04 | 1.0E-02 | −2.29 | 10.53 |
Discussion
This study illustrates significant functional and
morphological differences between adult and newborn fibroblasts and
keratinocytes and thus harmonizes with our previous observations
(6). In this context, NFs express
nestin and they are also frequently positive for αSMA. This makes
them distinct from AFs. Nestin-positive fibroblasts were also
described in vivo in fetal human skin (27). Our successful in vitro
differentiation of NFs into adipocytes and chondrocytes is in
agreement with similar observations reported by others (28). This remarkable plasticity of NFs
is later lost during life and thus, not seen in AFs. The high
frequency of spontaneous transformation of NFs to MFs is most
likely related to wound contraction, a key step of proper wound
closure (14). Besides this, NKs
were able to heal standardized experimental in vitro wounds
in a significantly shorter time than AKs. When we focused on the
fibroblast-keratinocyte interactions in the co-culture, NFs (not
seen in the co-culture with AFs) induced the presence of numerous
small keratinocytes on the periphery of the AK colonies. These
small peripheral AKs lacked intercellular contacts and all were
positive for keratin 14 (marker of basal layer), K8 and K19
(markers of simple epithelia), thus indicating the poor
differentiation level of the cells (29). Of note, keratin 19 is present in
the fetal epidermis, but not in adult interfollicular epidermal
keratinocytes (30). Keratin 8 is
typically paired with keratin 18 and is temporarily present in the
developing epidermis and malignant tumors (31). Furthermore, these small
keratinocytes were observed earlier in fetal/neonatal epidermis of
human and porcine origin, respectively (6,32).
Fibroblasts isolated from epidermal carcinomas and dermatofibroma
revealed a similar effect to AKs in the co-culture (17,19,33). Similarly, melanoma cells and
neural crest stem cells isolated from hair follicles induced the
presence of small cells at the periphery of AK colonies (34). The seemingly malignant phenotype
of these small keratinocytes does not imply that the cells
underwent malignant transformation. Interestingly, these
experiments revealed remarkable similarities between wound repair
and tumor growth as already postulated by Dvorak and later by other
authors (13,35,36).
As elucidated elsewhere (19), even on the protein level it has
been shown that pro-inflammatory factors such as IL-6, IL-8 and
CXCL1, produced by CAFs, influence the phenotype of keratinocytes.
Although the effector molecules acting on epidermal cells are
similar in the case of CAFs and NF, the final effect is not
identical. NFs differ from AFs in the expression of several genes
related to ECM structure and organization. However, both types of
fibroblasts (NFs and AFs) produce selected structural
glycoproteins, e.g. fibronectin, to a comparable extent. However,
the genome-wide analysis also revealed differentially-expressed
genes positively regulating cell division and proliferation, and
genes for chemotaxis. The products of upregulated chemotactic
genes, such as IL1B, IL-6, CXCL1, CXCL6, CXCL14, CXCL16, TGFB2,
VEGFA and VEGFB, are involved in the acute phase of the
inflammatory response. The observed differences in their expression
herein, may also be responsible for remarkably efficient wound
healing in the short postnatal period. Indeed, inflammation during
the course of prenatal and neonatal healing is attenuated with
diminished production of IL-6, IL-8 and CXCL1 by NFs (37). In this study, we also found 51
differentially-regulated genes associated with angiogenesis, a
process that is of utmost importance to wound healing. Taken
together, these data suggest that a large number of expressed genes
involved in tissue repair and regeneration differ between AFs and
NFs. Clarifying differences in the expression profiles of NFs and
AFs will allow us to better understand the excellent results of lip
cleft surgery in neonates reported by clinicians (5).
However, at this point in time it still remains
speculative whether the course of ageing reflects the difference
between neonatal and adult cells. Therefore, we can only
hypothesize whether the mechanism is genetic or epigenetic. The
moment of birth represents a sharp threshold requiring prompt
adaptation of the newborn organism to exist independently from the
maternal body in the outer environment. However, clinical evidence
demonstrates that the improved and almost scarless prenatal healing
in mammals (including humans) persists also during the early
postnatal period of life (38,39). The age of the cell donor thus
represents a very important parameter influencing the behavior of
cells in vitro and it also affects the course of wound
healing after grafting. An observed antifibrotic effect of NFs
(40) as well as expression
profiles of human fibroblasts performed by us and others (41) support these findings.
We conclude that phenotype and functional
properties, including EMI, of NFs and NKs differ from those seen in
adult cells. These differences can, in addition to other factors,
participate in the successful and almost scarless healing during
the neonatal period. From this point of view, this study brings new
data demonstrating the functional consequences that can help us to
better understand the molecular basis of differences between
neonatal and adult wound healing. Further studies deciphering
particular mechanisms responsible for these differences are needed,
because this knowledge may be of great clinical significance in
wound healing management.
Acknowledgments
The authors are grateful to Marie Jindráková and
Radana Kavková for excellent technical assistance. This study was
supported by the Grant Agency of the Czech Republic
(P304-13-20293S), the Charles University (project of Specific
University Research, PRVOUK-27 and UNCE 204013) and by LQ1604 NPU
II provided by MEYS and CZ.1.05/1.1.00/02.0109 BIOCEV provided by
ERDF and MEYS. This publication is also in part a result of the
project implementation: 'The Equipment for Metabolomic and Cell
Analyses' (no. CZ.1.05/2.1.00/19.0400, supported by the Research
and Development for Innovations Operational Programme (RDIOP)
co-financed by the European Regional Development Fund and the state
budget of the Czech Republic). The fellowship of Rosana Mateu in
Prague was supported by Marie Curie Training Network project
GLYCOPHARM (contract no. 317297). The study was also supported in
part by the Agency for Science and Research (APVV-14-0731 and
APVV-0408-12) and the Grant Agency of the Ministry of Education,
Science, Research and Sport of the Slovak Republic (VEGA-1/0404/15
and VEGA-1/0048/15). The authors are also grateful to Dr Ø. Hammer
and co-workers (42) for PAST for
free access to statistical software package.
References
|
1
|
Liechty KW, Adzick NS and Crombleholme TM:
Diminished interleukin 6 (IL-6) production during scarless human
fetal wound repair. Cytokine. 12:671–676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bukovsky A, Caudle MR, Carson RJ, Gaytán
F, Huleihel M, Kruse A, Schatten H and Telleria CM: Immune
physiology in tissue regeneration and aging, tumor growth, and
regenerative medicine. Aging (Albany NY). 1:157–181. 2009.
View Article : Google Scholar
|
|
3
|
Walraven M, Talhout W, Beelen RH, van
Egmond M and Ulrich MM: Healthy human second-trimester fetal skin
is deficient in leukocytes and associated homing chemokines. Wound
Repair Regen. 24:533–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han M, Yang X, Lee J, Allan CH and Muneoka
K: Development and regeneration of the neonatal digit tip in mice.
Dev Biol. 315:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borsky J, Veleminska J, Jurovčík M, Kozak
J, Hechtova D, Tvrdek M, Cerny M, Kabelka Z, Fajstavr J, Janota J,
et al: Successful early neonatal repair of cleft lip within first 8
days of life. Int J Pediatr Otorhinolaryngol. 76:1616–1626. 2012.
View Article : Google Scholar
|
|
6
|
Krejčí E, Kodet O, Szabo P, Borský J,
Smetana K Jr, Grim M and Dvořánková B: In vitro differences of
neonatal and later postnatal keratinocytes and dermal fibroblasts.
Physiol Res. 64:561–569. 2015.
|
|
7
|
Yamaguchi Y, Itami S, Tarutani M, Hosokawa
K, Miura H and Yoshikawa K: Regulation of keratin 9 in
nonpalmoplantar keratinocytes by palmoplantar fibroblasts through
epithelial-mesenchymal interactions. J Invest Dermatol.
112:483–488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biggs LC and Mikkola ML: Early inductive
events in ectodermal appendage morphogenesis. Semin Cell Dev Biol.
25–26:11–21. 2014. View Article : Google Scholar
|
|
9
|
Mikkola ML and Millar SE: The mammary bud
as a skin appendage: unique and shared aspects of development. J
Mammary Gland Biol Neoplasia. 11:187–203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rawles ME: Tissue interactions in scale
and feather development as studied in dermal-epidermal
recombinations. J Embryol Exp Morphol. 11:765–789. 1963.PubMed/NCBI
|
|
11
|
Cohen BH, Lewis LA and Resnik SS: Would
healing: a brief review. Int J Dermatol. 14:722–726. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon YB, Kim HW, Roh DH, Yoon SY, Baek RM,
Kim JY, Kweon H, Lee KG, Park YH and Lee JH: Topical application of
epidermal growth factor accelerates wound healing by myofibroblast
proliferation and collagen synthesis in rat. J Vet Sci. 7:105–109.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smetana K Jr, Szabo P, Gál P, André S,
Gabius HJ, Kodet O and Dvořánková B: Emerging role of tissue
lectins as microenvironmental effectors in tumors and wounds.
Histol Histopathol. 30:293–309. 2015.
|
|
14
|
Dvořánková B, Szabo P, Lacina L, Gal P,
Uhrova J, Zima T, Kaltner H, André S, Gabius HJ, Sykova E and
Smetana K Jr: Human galectins induce conversion of dermal
fibroblasts into myofibroblasts and production of extracellular
matrix: potential application in tissue engineering and wound
repair. Cells Tissues Organs. 194:469–480. 2011. View Article : Google Scholar
|
|
15
|
Smetana K Jr, Dvořánková B, Szabo P,
Strnad H and Koláø M: Role of stromal fibroblasts in cancer
originated from squamous epithelia. Dermal Fibroblasts:
Histological Perspectives, Characterization and Role in Disease.
Bai X: Nova Sciences; Publishers, New York: pp. 83–94. 2013
|
|
16
|
Strnad H, Lacina L, Kolár M, Cada Z, Vlcek
C, Dvoránková B, Betka J, Plzák J, Chovanec M, Sáchová J, et al:
Head and neck squamous cancer stromal fibroblasts produce growth
factors influencing phenotype of normal human keratinocytes.
Histochem Cell Biol. 133:201–211. 2010. View Article : Google Scholar
|
|
17
|
Lacina L, Smetana K Jr, Dvoránková B,
Pytlík R, Kideryová L, Kucerová L, Plzáková Z, Stork J, Gabius HJ
and André S: Stromal fibroblasts from basal cell carcinoma affect
phenotype of normal keratinocytes. Br J Dermatol. 156:819–829.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacina L, Dvoránkova B, Smetana K Jr,
Chovanec M, Plzák J, Tachezy R, Kideryová L, Kucerová L, Cada Z,
Boucek J, et al: Marker profiling of normal keratinocytes
identifies the stroma from squamous cell carcinoma of the oral
cavity as a modulatory microenvironment in co-culture. Int J Radiat
Biol. 83:837–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kolář M, Szabo P, Dvořánková B, Lacina L,
Gabius HJ, Strnad H, Sáchová J, Vlček C, Plzák J, Chovanec M, et
al: Upregulation of IL-6, IL-8 and CXCL-1 production in dermal
fibroblasts by normal/malignant epithelial cells in vitro:
immunohistochemical and transcriptomic analyses. Biol Cell.
104:738–751. 2012. View Article : Google Scholar
|
|
20
|
Szabo P, Valach J, Smetana K Jr and
Dvořánková B: Comparative analysis of production of IL-8 and CXCL-1
by normal and cancer stromal fibroblasts. Folia Biol. 59:134–147.
2013.
|
|
21
|
Ferrari M, Fornasiero MC and Isetta AM:
MTT colorimetric assay for testing macrophage cytotoxic activity in
vitro. J Immunol Methods. 131:165–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smyth GK: Linear models and empirical
Bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:e32004.
|
|
23
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valach J, Fík Z, Strnad H, Chovanec M,
Plzák J, Cada Z, Szabo P, Sáchová J, Hroudová M, Urbanová M, et al:
Smooth muscle actin-expressing stromal fibroblasts in head and neck
squamous cell carcinoma: increased expression of galectin-1 and
induction of poor prognosis factors. Int J Cancer. 131:2499–2508.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z,
Meirelles GV, Clark NR and Ma'ayan A: Enrichr: interactive and
collaborative HTML5 gene list enrichment analysis tool. BMC
Bioinformatics. 14:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu YF, Zhang ZJ and Sieber-Blum M: An
epidermal neural crest stem cell (EPI-NCSC) molecular signature.
Stem Cells. 24:2692–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sellheyer K and Krahl D: Spatiotemporal
expression pattern of neuroepithelial stem cell marker nestin
suggests a role in dermal homeostasis, neovasculogenesis, and tumor
stroma development: a study on embryonic and adult human skin. J Am
Acad Dermatol. 63:93–113. 2010. View Article : Google Scholar
|
|
28
|
Chang Y, Guo K, Li Q, Li C, Guo Z and Li
H: Multiple directional differentiation difference of neonatal rat
fibroblasts from six organs. Cell Physiol Biochem. 39:157–171.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lane EB and McLean WH: Keratins and skin
disorders. J Pathol. 204:355–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan KK, Salgado G, Connolly JE, Chan JK
and Lane EB: Characterization of fetal keratinocytes, showing
enhanced stem cell-like properties: a potential source of cells for
skin reconstruction. Stem Cell Rep. 3:324–338. 2014. View Article : Google Scholar
|
|
31
|
Casanova ML, Bravo A, Martínez-Palacio J,
Fernández-Aceñero MJ, Villanueva C, Larcher F, Conti CJ and Jorcano
JL: Epidermal abnormalities and increased malignancy of skin tumors
in human epidermal keratin 8-expressing transgenic mice. FASEB J.
18:1556–1558. 2004.PubMed/NCBI
|
|
32
|
Klíma J, Motlík J, Gabius H-J and Smetana
K Jr: Phenotypic characterization of porcine interfollicular
keratinocytes separated by elutriation: a technical note. Folia
Biol (Praha). 53:33–36. 2007.
|
|
33
|
Kideryová L, Lacina L, Dvoránková B, Stork
J, Cada Z, Szabo P, André S, Kaltner H, Gabius HJ and Smetana K Jr:
Phenotypic characterization of human keratinocytes in coculture
reveals differential effects of fibroblasts from benign fibrous
histiocytoma (dermatofibroma) as compared to cells from its
malignant form and to normal fibroblasts. J Dermatol Sci. 55:18–26.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kodet O, Lacina L, Krejčí E, Dvořánková B,
Grim M, Štork J, Kodetová D, Vlček Č, Šáchová J, Kolář M, et al:
Melanoma cells influence the differentiation pattern of human
epidermal keratinocytes. Mol Cancer. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lacina L, Plzak J, Kodet O, Szabo P,
Chovanec M, Dvorankova B and Smetana K Jr: Cancer microenvironment:
what can we learn from the stem cell niche. Int J Mol Sci.
16:24094–24110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bermudez DM, Canning DA and Liechty KW:
Age and pro-inflammatory cytokine production: wound-healing
implications for scar-formation and the timing of genital surgery
in boys. J Pediatr Urol. 7:324–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muneoka K, Allan CH, Yang X, Lee J and Han
M: Mammalian regeneration and regenerative medicine. Birth Defects
Res C Embryo Today. 84:265–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi Y, Cox C, Lally K and Li Y: The
strategy and method in modulating finger regeneration. Regen Med.
9:231–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pratsinis H, Armatas A, Dimozi A, Lefaki
M, Vassiliu P and Kletsas D: Paracrine anti-fibrotic effects of
neonatal cells and living cell constructs on young and senescent
human dermal fibroblasts. Wound Repair Regen. 21:842–851. 2013.
View Article : Google Scholar
|
|
41
|
Kalfalah F, Seggewiß S, Walter R, Tigges
J, Moreno-Villanueva M, Bürkle A, Ohse S, Busch H, Boerries M,
Hildebrandt B, et al: Structural chromosome abnormalities,
increased DNA strand breaks and DNA strand break repair deficiency
in dermal fibroblasts from old female human donors. Aging (Albany
NY). 7:110–122. 2015. View Article : Google Scholar
|
|
42
|
Hammer Ø, Harper DAT and Ryan PD: PAST:
Paleontological statistics software package for education and data
analysis. Palaeontol Electronica. 4:9pp2001.
|