Introduction
Hematopoietic stem cells (HSCs) are defined by their
ability to generate all cells of the hematopoietic system. The stem
cell niche provides a specific microenvironment for HSCs to reside,
and is responsible for their fate in terms of quiescence,
self-renewal and differentiation (1). Recent studies have clarified the
role of the marrow microenvironment in the pathogenesis of
hematologic tumors. It has been reported that the identification of
target molecules can be exploited to eradicate the leukemic stem
cells from the niche (2,3). Modern hematopoietic stem/progenitor
cell (HSPCs) culture systems that closely mimic marrow physiology,
can provide an experimental tool with which to understand the
niche-mediated regulation of HSCs, under both physiological and
diseased conditions (4). The
latter may potentially help to design and develop novel therapeutic
strategies to target the HSC niche. In addition, during the past
three decades, HSC transplantation has become a well-established
treatment for hematologic malignancies and non-malignant disorders.
To improve the clinical outcome of autologous and allogeneic HSC
transplantation, many study groups are focusing on the ex
vivo expansion of HSCs, particularly for those cases in which
the graft is of limited size, such as HSCs from cord blood
(5–7). However, the expansion of HSCs in
vitro is difficult to be achieved due to known stem cell
characteristics. Although the contribution of conventional
hematopoietic culture systems to the knowledge of human HSC biology
is unquestionable, these existing HSC culture systems cannot meet
the requirements of the clinical application. Therefore it is
necessary to constantly improve ex vivo experimental systems
to closely resemble their in vivo counterparts.
HSCs are regulated by intrinsic mechanisms and
extrinsic signals mediated via specialized microenvironments known
as 'niches'. The self-renewal and differentiation ability of HSCs
are regulated by two major elements: endothelial and vascular
regulatory elements. In addition, the osteoblastic niche localized
at the inner surface of the bone cavity has been recognized as the
main regulator of HSC fate and serves as a reservoir for long-term
HSC storage in a quiescent state (8,9).
The deletion of the gene Dicer expressed specifically in
osteo-progenitors and immature osteoblasts (OBs), has been shown to
affect hematopoiesis, indicating the involvement of a precise cell
group within osteo-lineage cells in HSC maintenance (10). Taichman et al demonstrated
that the in vitro culture of human bone marrow
CD34+ cells with human OBs supported a 3-4-fold
expansion of long-term culture-initiating cells in vitro
(11). On the other hand, the
vascular niche predominantly consists of the bone marrow sinusoidal
endothelial cells, which are a part of the vascular system, and
perivascular cells which surround the bone marrow vasculature,
known as mesenchymal stem cells or mesenchymal stromal cells (MSCs)
(12–15). Bone marrow-derived stromal cells
(BMSCs), from which OBs differentiate, possess the properties and
functions of niche cells: namely CXC chemokine ligand 12
(CXCL12)-abundant reticular cells (CAR cells) (16) and Nestin+ MSCs. It has
been reported that purified HSCs specifically home to
Nestin+ MSCs in the bone marrow of irradiated mice and
Nestin+ cell depletion results in a significantly
compromised homing process (17).
BMSCs have been shown to regulate the proliferation of HSCs rather
than quiescence and support HSC maintenance and engraftment
(17). Furthermore, BMSCs are
characterized by their multi-differentiation potential. Our
previous study demonstrated that OBs differentiated from BMSCs
supported the maintenance and multipotency of HSPCs from umbilical
cord blood in a 2D-culture system (18), suggesting that MSCs and OBs are
suitable candidates with wich to build a novel HSP culture
system.
In vitro HSC research is commonly carried out
by culturing cells as monolayers using conventional tissue culture
techniques. Although the contribution of conventional hematopoietic
culture systems to the knowledge of human HSC biology is
unquestionable, they lacked the three-dimensional (3D)
architecture, thus failing to mimic the in vivo HSC niche,
described as a three-dimensional microenvironment within the
subendosteal region of bone marrow (1–3).
In recent years, in vitro 3D-cultures of HSPCs have been
shown to obviously be superior to bi-dimensional (2D) culture,
which consists of HSPCs plus either sole MSCs or OBs, or both
(10,19,20). These findings imply that a 3D
architecture is important to mimic physiological conditions ex
vivo.
Bone marrow is located in both long bones (e.g.,
femur) and flat bones (e.g., calvaria); however, bio-derived bone
scaffolds (BDBS) which are made from human femurs can preserve the
natural spongy architecture of trabecular bones and more closely
mimic the HSC niche in vivo. In our previous study, we
utilized OBs and BDBS to create a 3D culture system, which was
primarily demonstrated to support the maintenance and expansion of
HSPCs in vitro, and this system was obviously superior to 2D
culture systems (21). Taking
into consideration the deep understanding of the HSC niche, in the
present study, we used a mixture of MSCs and OBs differentiated
from BMSCs and BDBS to improve our 3D-Mix culture system and to
explore their synergized function on the BDBS, illustrating that
its features can more closely mimic those of the HSC niche. Our
data demonstrate that the 3D-Mix culture system has some features
more similar to those of the HSC niche in supporting the
maintenance and expansion of HSPCs in vitro.
Materials and methods
Preparation of BDBS
The BDBS characterized with respect to natural
porosity, pore size and minerals were made from the human skeleton
and were manufactured by the Division of Stem Cell and Tissue
Engineering (Laboratory of Biotecherapy, Sichuan University,
Chengdu, China). The preparation of BDBS involved a process through
which a series of physical and chemical procedures were performed
to drastically wipe off the main antigens, such as cells and
lipoproteins, apart from bone morphogenetic protein, collagen and
salinity (22), which included
degreasing, partial deproteinization, decalcification and extensive
washing with distilled water. Finally, the BDBS were lyophilized
and sterilized by 60Co gamma-ray irradiation
(20–25×103 Gy) before being stored at 4°C. The human
BDBS were cut into sections (1.0×0.5×0.5 cm) in order to fit into
wells of 24-well plates, and soaked in neonatal bovine serum for 6
h. They were then soaked in Dulbecco's modified Eagle's medium
(DMEM) with 10% neonatal bovine serum again for 12 h before being
used. The morphology of the BDBS was characterized by scanning
electron microscopy.
Isolation and culture of human BMSCs
Heparinized human bone marrow cells were obtained
from the posterior iliac crest of healthy volunteers with informed
consent in accordance with the Declaration of Helsinki and
following the approval of the Institutional Review Board of Sichuan
Provincial Hospital. Bone marrow mononuclear cells (MNCs) were
isolated after Ficoll-Hypaque (Sigma Diagnostics, St. Louis, MO,
USA) gradient centrifugation at 400 × g for 30 min and plated in 25
cm2 cell culture flasks with expansion medium containing
L-DMEM (Gibco Life Technologies, Paisley, UK), 10% fetal bovine
serum (FBS; Gibco-BRL, Grand Island, NY, USA), 100 U/ml penicillin,
100 µg/ml streptomycin, 0.29 mg/ml L-glutamine and 3 mg/ml
HEPES buffer (R&D Systems, Minneapolis, MN, USA) at 37°C. After
72 h, the non-adherent cells were discarded, and half medium change
was performed twice a week. When the adherent MSCs grew to >80%
confluence, they were detached with 0.05% trypsin/0.53 mmol/l EDTA
(Sigma Diagnostics), and inoculated at 5×103
cells/cm2 in 3 or 4 fresh tissue culture flasks.
Induction of differentiation of human
BMSCs into OBs
When the MSCs at passage 3 achieved close to 60%
confluence, the L-DMEM medium was discarded and replaced with
osteogenic medium (F12 medium containing 10% FBS, 10−8
mol/l dexamethasone, 50 µg/ml ascorbic acid and 10 mmol/l
β-sodium glycerol phosphate) to induce MSC differentiation into
OBs. After 10 days of half changing the medium daily, the F12
medium was removed and was replaced with L-DMEM medium again. The
osteogenic differentiation of the MSCs was assessed by alkaline
phosphatase (ALP) staining, Alizarin Red S staining and by the
analyses of the expression of type I collagen and osteocalcin.
ALP staining
ALP staining was performed using the Gomori modified
calcium-cobalt method, as previously described (23). Briefly, the cells were fixed with
a solution of 95% alcohol for 10 min, and then incubated with ALP
at 37°C for 4 to 6 h. Subsequently, the cells were stained with
solutions of 2% cobalt nitrate and 1% ammonium sulfide. After being
air-dried, the cells on slides were mounted and observed under a
light microscope (Olympus BX51, Olympus, Tokyo, Japan). Cells that
were positive for ALP were stained a brown or tan color.
Alizarin Red S staining
Alizarin Red S staining was used to detect any
calcified nodules in the cells, as previously described (24). The cells were fixed with 90%
ethanol at room temperature for 1 h and stained with 40 mmol/l
Alizarin Red S (Sigma) (pH 4.2) for 30 min. The mineralized nodules
were observed under a light microscope (Olympus BX51, Olympus).
Immunohistochemistry
The expression levels of type I collagen and
osteocalcin were detected by immunohistochemistry. The cells on
slides were fixed with cold acetone for 30 min at room temperature
and incubated with blocking solution for 30 min at 37°C.
Subsequently, the slides were incubated with primary antibodies
specific for type I collagen (Cat. no. MAB8887, Millipore,
Temecula, CA, USA) and osteocalcin (cat. no. sc-74495, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. The
following day, the cells were rinsed with PBS and incubated at 37°C
for 40 min in the dark with fluorescein isothiocyanate (FITC,
green)- or rhodamine (red)-conjugated secondary antibodies
(Molecular Probes, Carlsbad, CA, USA). Furthermore, the nuclei were
stained with 5 mg/ml DAPI (blue). The stained slides were observed
under a laser confocal microscope (Olympus Fluoview FV500,
Olympus).
Establishment of ex vivo hematopoietic
culture systems (3D and 2D)
The 3D-culture system was established as follows: i)
The OBs differentiated from the BMSCs for 10 days and the BMSCs at
passage 3 were harvested and mixed at a ratio of 1:1 to act as seed
cells. ii) Bio-derived bone blocks were soaked in L-DMEM for 2 days
and then in FBS for 2 h before seeding the cells to facilitate cell
adherence. iii) The mixture of BMSCs and OBs (1:1) was suspended
into 8×106 cells/ml in 20 µl fresh L-DMEM and
carefully dripped into prepared bone blocks to avoid overflow
followed by being incubation at 37°C with 5% CO2 in an
incubator for 4 h to allow cell adherence. The bone blocks were
then completely immersed in the L-DMEM medium supplemented with 10%
FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml
L-glutamine and 3 mg/ml HEPES buffer. They were then grown to
confluence for 7–10 days and treated with 60Co gamma
irradiation for 10 min (25 Gy). A 3D-culture system with only MSCs
or OBs differentiated from BMSCs as seed cells was designed as the
experimental control group, respectively. In addition, an
irradiated (25 Gy) mixture of cells (5×104 cells) was
seeded and cultured in a 24-well plate to build the 2D system. In
total, we used 4 culture systems (2D-Mix, 3D-OB, 3D-MSC and
3D-Mix).
Cultivation of purified CD34+
cells from human umbilical cord blood (UCB) in the 3D or 2D culture
system
Human UCB cells were obtained from mothers at the
end of full-term deliveries at Sichuan Provincial People's Hospital
after obtaining written informed consent and the approval of the
Institutional Review Board of Sichuan Provincial Hospital. The UCB
samples was incubated with 0.5% (w/v) methyl cellulose dissolved in
Hanks' solution at room temperature for 30 min to sedimentate the
UCB red blood cells before the MNCs were collected by Ficoll-Paque
centrifugation and the CD34+ cells were then enriched
from the MNCs by using immune-magnetic beads (EasySep CD34 positive
selection kit) and suspended in 20 µl Myelo-Cult H5100
medium (both from StemCell Technologies Inc., Vancouver, BC,
Canada) followed by being seeded into the 3D or 2D culture system
and cultured with Myelo-Cult medium containing 1 µM freshly
dissolved hydrocortisone without exogenous cytokines. After 2 weeks
of co-culture, the bone blocks were vigorously washed 3 times with
phosphate-buffered saline (PBS) to collect the non-adherent cells,
and then enzymatically dissociated with 0.125% trypsin (0.78 mmol/l
EDTA) followed by being pipetted and centrifuged at 300 × g for 15
min to harvest the adherent cells. Cells in the 2D culture system
were also collected by trypsin digestion and centrifugation after 2
weeks of co-culture. The CD34+ cells from each culture
system were subjected to various phenotypic and functional assays
as described below.
Hoechst 33342/propidium iodide (PI)
fluorescent staining
After 2 weeks of HSPC culture, the CD34+
cells were washed off and the bone blocks were transferred to a
24-well culture plate followed by the addition of Hoechst (10
µg/ml). The 24-well culture plate was incubated at 37°C for
10 min and then PI (10 µl/ml) was added. After 20 min of
incubation at 4°C, the 24-well plate was taken out of the freezer
before the bone blocks were observed under a fluorescence
microscope (Olympus IX71, Olympus) to identify the viable/dead
cells attached on the trabecular bone. The cells in the 2D culture
system were also stained and observed under a fluorescence
microscope as a control.
Quantitative PCR (qPCR)
qPCR was performed for the detection of the mRNA
expression of cyclin-dependent kinase inhibitor 1A (CDKN1A, p21)
using DNA-binding SYBR-Green dye (Applied Biosystems, Foster City,
CA, USA) for the detection of the PCR products. The primers used
were as follows: p21 forward, 5′-GGAAGACCATGTGGACCTGT-3′ and
reverse, 5′-GGCGTTTGGAGTGGTAGAAA-3′; β-actin (internal reference)
forward, 5′-GCAAGCAGGAGTATGACGAG-3′ and reverse,
5′-CAAATAAAGCCATGCCAATC-3′, which were purchased from Applied
Biosystems. The cycling conditions were as follows: an initial
denaturation at 95°C for 15 min, followed by 50 cycles of
denaturation at 94°C for 15 sec, annealing at 55°C for 15 sec, and
extension at 72°C for 15 sec. The β-actin gene was used as a
reference. The assay was replicated in 3 independent
experiments.
Flow cytometry (FCM)
To assess the percentage of HSPCs remaining in the
2D or 3D culture system before and after each culture, FCM was
performed to analyze the immunophenotypes of CD34 and CD38.
Briefly, a total of 5×105 test cells was suspended in 50
µl PBS and stained with 10 µg/ml of purified mouse
anti-human CD34/CD38 antibody (FcR) (Cat. no. 550760;
Becton-Dickinson, Mountain View, CA, USA) at 4°C for 30 min. The
replicate sample incubated with isotype-matched antibodies was used
as a control. Dead cells were eliminated by staining with 7AAD.
After the cells were washed twice in the same buffer, a minimum of
2×104 events of each sample was analyzed on a FACS cell
sorter using Cell Quest software (Becton-Dickinson).
Colony-forming unit (CFU) assay and
morphological examination
The colony-forming assay was performed to assess the
colony-forming ability of the cells. Briefly, the CD34+
cells from the initial UCB samples or the cultured cells were
incubated in methylcellulose medium with recombinant cytokines
(MethoCult GF+ H4435; StemCell Technologies Inc.) at
2×103 in 35-mm tissue culture dishes (Costar, Lowell,
MA, USA). The dishes were incubated at 37°C in a humidified
atmosphere with 50 ml/l CO2 in air. All cultures were
carried out in triplicate. After 14 days of culture, colonies
belonging to burst-forming unit-erythroid (BFU-E), colony-forming
unit-granulocyte/macrophage (CFU-GM), colony-forming
unit-macrophage (CFU-M) and colony forming
unit-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM)
consisting of ≥50 cells were scored under an inverted microscope
(Olympus IX81, Olympus). To assess the accuracy of in situ
identification, individual colonies were selected and stained with
Wright's staining (Merck, Darmstadt, Germany) for the morphological
identification of cells.
Long-term culture initiating cell
(LTC-IC) assay
A modified LTC-IC assay was performed as previously
described (25). Briefly,
irradiated (80 Gy) mouse bone marrow stromal cells (M2-10B4,
American Type Culture Collection, Rockville, MD, USA) were seeded
at 105 cells/well in 96-well flat-bottomed plates as a
feeder layer. CD34+ cell subpopulations purified from
UCB or those isolated from cultured cells by sorting with a
FACSVantage (Becton-Dickinson) were seeded at a limiting dilution
on the feeder layer in serum-containing medium. For each
evaluation, at least 3 cell concentrations were used with 24
replicates per concentration. The plates were incubated at 37°C, 5%
CO2 with weekly half medium exchanges. After 5 weeks of
culture, the cells were harvested and transferred to
methylcellulose medium with recombinant cytokines (MethoCultGFt
H4434V; StemCell Technologies Inc.) for colony-forming assays.
After 14 days of culture, colonies with >50 cells were counted
to assess LTC-IC activities and we calculate the frequency of
LTC-IC according to the manufacturer's instructions (StemCell
Technologies Inc.).
NOD/SCID repopulating cell (SRC)
assay
Human cord blood CD34+ cells were
cultured for 14 days in 4 culture systems (2D-Mix, 3D-OB, 3D-MSC
and 3D-Mix). The cells were harvested, suspended in 1 ml α-MEM and
injected intravenously via the tail vein into 8-week-old,
sub-lethally-irradiated (2.5 Gy) NOD/SCID mice (n=40; Central
Institute for Experimental Animals, Institutes for Biological
Sciences, Shanghai, China). This animal experiment was approved by
the Institutional Animal Care and Use Committee of the Sichuan
Provincial People's Hospital. Each mouse received cells equivalent
to 104 CD34+ cells together with irradiated
(15 Gy using a 60Co γ-irradiator) non-repopulating
CD34− cells as accessory cells. The recipients were
sacrificed by carbon dioxide asphyxiation 8 weeks following
transplantation, and bone marrow MNCs were harvested by Ficoll
density gradient centrifugation and stained with FITC-labeled
anti-human CD45 antibody to analyze for the presence of human
CD45+ cells by FCM. Mice were considered positive for
human HSC engraftment when at least 0.1% CD45+ human
cells were detected in the mouse bone marrow cells. The SRC
frequency was calculated based on the Poission distribution using
the equation Pi = e(−N) × (Ni/i!) at P0, as previously described
(26,27). PCR analysis using human specific
17α-satellite gene expression (17α-satellite,
5′-ACGGGATAACTGCACCTAAC-3′; 5′-CCATAGGAGGGTTCAACTCT-3′) was
performed to confirm the FCM results.
Enzyme-linked immunosorbent assay
(ELISA)
After 2 weeks of HSPC culture, the media from 4
culture systems were clarified at 3,500 rpm for 5 min, and the
supernatants were subjected to quantitative ELISA for the secreted
fibronectin, collagen IV, vitronectin and laminin using
commercially available fibronectin, collagen IV, vitronectin and
laminin ELISA kits (RayBiotech Inc., Norcross, GA, USA) according
to the manufacturer's instructions.
Western blot analysis
The MSCs and OBs in the 4 culture systems were
harvested, washed twice in cold 1X PBS (Gibco, Invitrogen), and
subsequently lysed in ice-cold RIPA lysis buffer [50 mmol/l
Tris-HCl (pH 8.0), 150 mmol/l NaCl, 0.1% SDS, 1% NP-40, 0.25%
sodium deoxycholate and 1 mmol/l EDTA] with freshly added protease
inhibitor cocktail (Calbiochem, Shanghai, China). After 30 min of
lysis on ice, the cell lysates were cleared by centrifugation at
12,000 × g, and the protein concentration was measured using the
BCA Protein assay kit (Pierce, Pittsburgh, PA, USA). Equal amounts
of the protein samples were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and blotted
onto PVDF membranes (GE Healthcare Biosciences, Piscataway, NJ,
USA). The membranes were blocked with PBS-T containing 5% non-fat
dry milk and incubated with anti-angiopoietin-1 (cat. no. ab76956;
Abcam, Cambridge, MA), anti-osteopontin (cat. no. sc-10591; Santa
Cruz Biotechnology, Inc.), anti-stem cell factor (SCF; cat. no.
ab52603), CXCL12 (cat. no. ab25117), RUNX2 (cat. no. ab76956) (all
3 from Abcam, Cambridge, MA, USA) and anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (cat. no. sc-5565; Santa Cruz
Biotechnology, Inc.) antibodies. Membrane-bound first-step
antibodies were reacted with horseradish peroxidase
(HRP)-conjugated secondary antibody (Sigma). The bands were
visualized with enhanced chemiluminescence (Millipore, Billerica,
MA, USA). Protein bands were quantified using ImageJ software 1.43
(NIH, Bethesda, MD, USA) and normalized to the levels of GAPDH
(loading control).
Statistical analysis
The data were presented as the means and standard
error. Statistical comparisons were performed using the two-sided
Student's t-test. Values of p<0.05 and p<0.01 were considered
to indicate statistically significant differences.
Results
HSCs derived from 3D culture system
exhibit a morphological characterization similar to the HSC
niche
It is now clear that MSCs and OBs are two crucial
components of the HSC niche in adult bone marrow. Thus, we selected
a mixture of MSCs and OB-like cells differentiated from MSCs to
mimic the HSC niche and to build an HSC in vitro culture
system in our study. Our primary MSCs were derived from the bone
marrow of healthy adults, and were then differentiated into OB-like
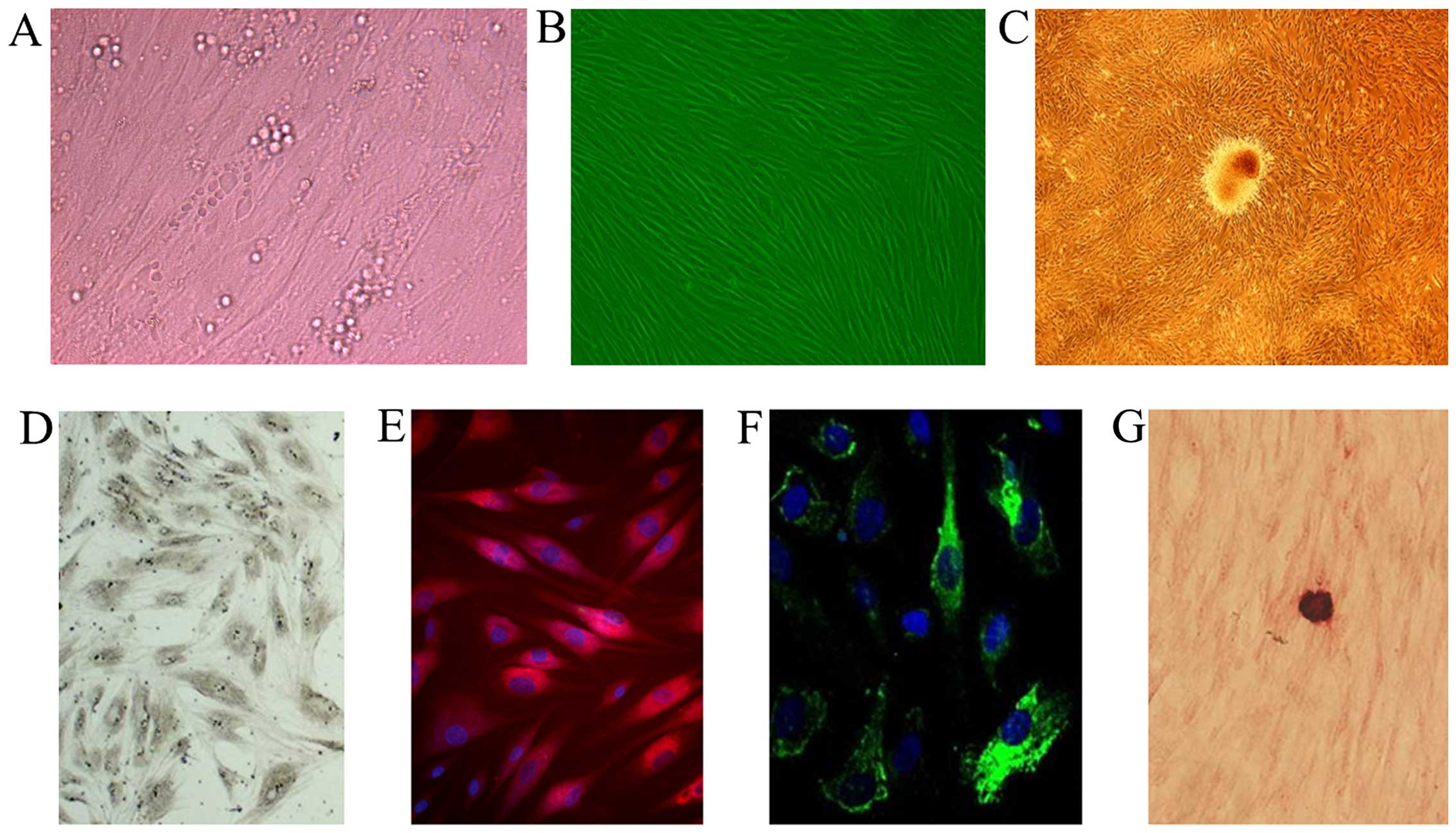
cells by culture in osteogenic medium for 10 days (Fig. 1B and C). We then performed
morphological and histological analyses of the MSCs differentiated
into OBs. Our results revealed that the OB-like cells
differentiated from the MSCs were a heterogeneous population, as
evidenced by asynchronous differentiation and the dynamic
expression of osteogenic markers, including ALP, type I collagen,
osteocalcin and calcium nodules (Fig.
1D–G).
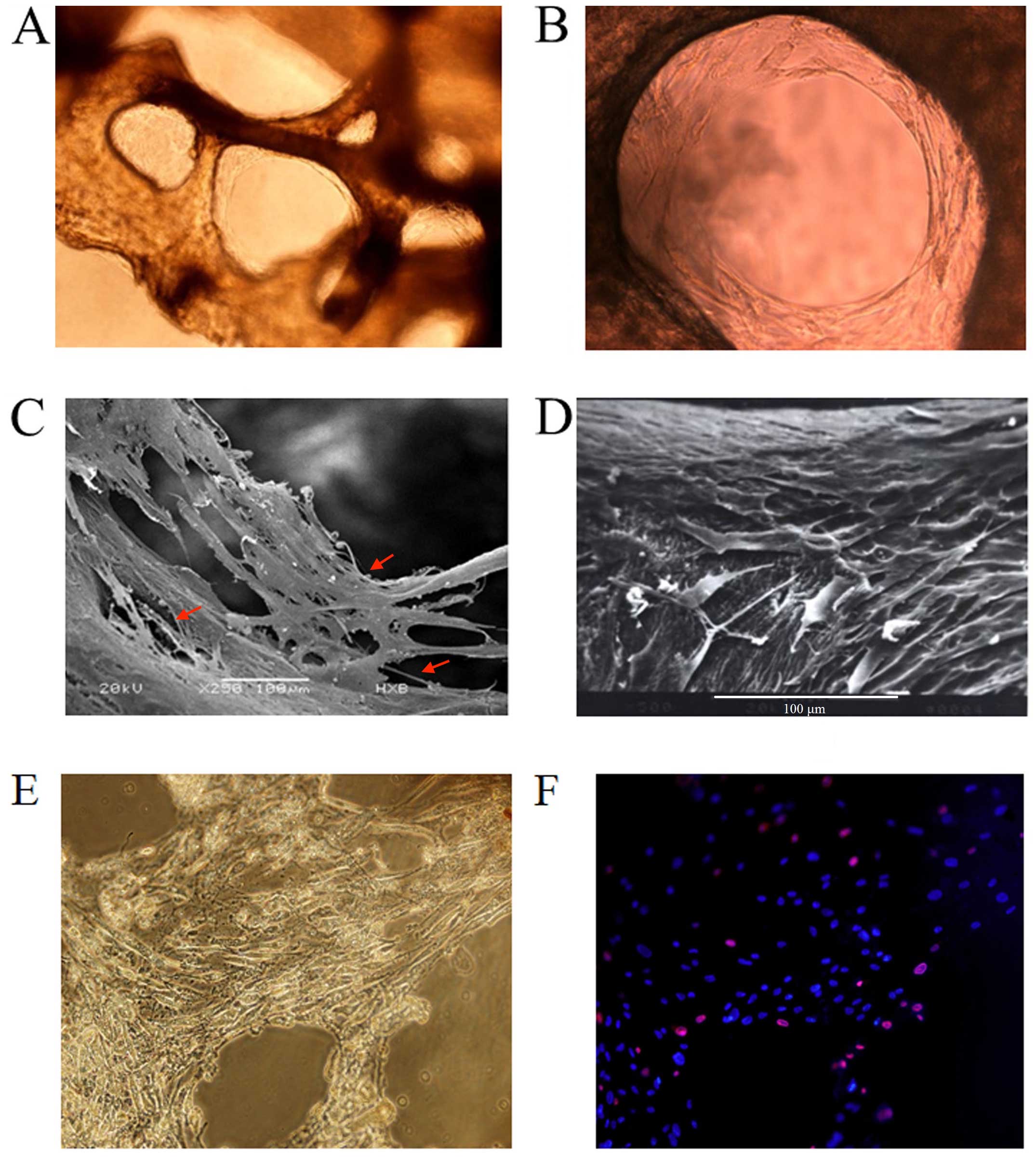
Since the BDBS material was derived from human long
bone (femurs) where adult hematopoietic tissues are mainly located,
they thus supplied a spatial structure very similar to the HSC
niche. Our study demonstrated an excellent biocompatibility of the
bio-derived bone with human BMSCs and OBs differentiated from MSCs
in vitro. After seeding the BMSCs and OBs differentiated
from MSCs into the bio-derived bone for 7–10 days, the cells grew
along with the inner surfaces of bone trabecular pores and formed a
stable fibrous 3D structure. As shown by scanning electron and
inverted microscopy, the cells tightly attached to the surface of
the trabecular bone and secreted an amount of extracellular matrix
(ECM) components which appeared elliptical with pseudopodal
extensions and filled the intertrabecular cavity of cancellous bone
(Fig. 2A–D). These features
indicated a 'biomimetic HSC niche' with BDBS as a 3D scaffold, and
a mixture of MSCs and OB-like cells as HSCs. Compared to the 3D
culture system, the mixture of MSCs and OB-like cells in the 2D
system was flat in shape and produced a lower amount of ECM
components (Fig. 1A).
In order to determine whether the 3D-Mix system is
able to supply a long-term culture of HSC in vitro, the
frequency of live and dead feeder cells (MSCs and OB-like cells) on
trabecular bone of BDBS after 2 weeks of HSPC culture was
investigated by Hoechst 33342/PI fluorescent staining (Fig. 2E–F). It was shown that feeder cell
death was 8.1% in the 3D-Mix culture system, whereas feeder cell
death in the 2D-Mix culture system reached 17.3% after 2 weeks
(data not shown).
3D-Mix of MSCs and OB differentiated from
MSCs provide a microenvironment closely similar to the HSC
niche
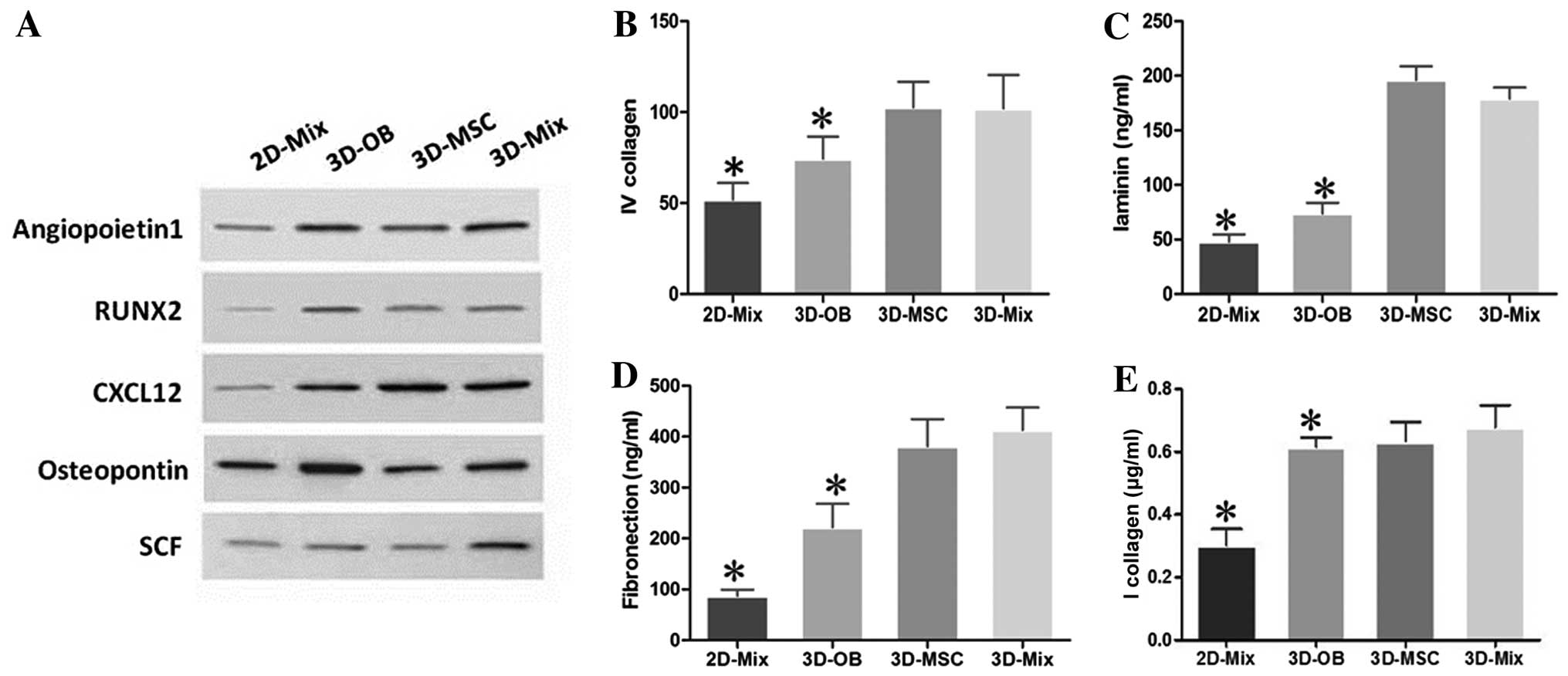
It is known that the bone marrow niche plays key
roles in the self-renewal, survival, and maintenance of HSCs by
expressing many crucial molecules, including chemokines, growth
factors, cell-surface and adhesion molecules (28). Thus, in this study, we examined
the protein expression of angiopoietin-1, RUNX2, CXCL12,
osteopontin and SCF, which are genes that control HSC properties,
in our 2D and 3D culture system. The results of western blot
analysis revealed that all these genes were highly expressed in the
3D-Mix, despite the fact that the expression of individual genes
was slightly lower than or close to that of the other culture
systems, such as the expression of osteopontin in the 3D-OB (was
higher), and that of CXCL12 in the 3D-MSC (was higher) systems.
These findings implied that the interactions of human BMSCs and OBs
in the 3D-Mix may contribute to a more comprehensive and balanced
expression of cytokines, which likely more closely resembles the
physiological state of the HSC niche and is more beneficial to HSPC
in vitro culture compared with the other culture systems
(Fig. 3A).
The HSC microenvironment is enriched with ECM
proteins, such as fibronectin, collagen IV, vitronectin and
laminin, which are an essential part of the HSC niche (29). Thus, in this study, the culture
systems were subjected to ELISA to determine the presence of these
molecules. It was observed that all these ECM molecules were highly
expressed at the protein level in the 3D-Mix, although the levels
of individual ECM molecules, such as laminin and collagen IV were
slightly lower than or similar to those in the 3D-MSC system
(Fig. 3B–E). These findings
indicated that the interactions of human BMSCs and OBs in the
3D-Mix provided most comprehensive ECM protein amounts to support
and modulate HSPCs in vitro, compared to the other 3
systems.
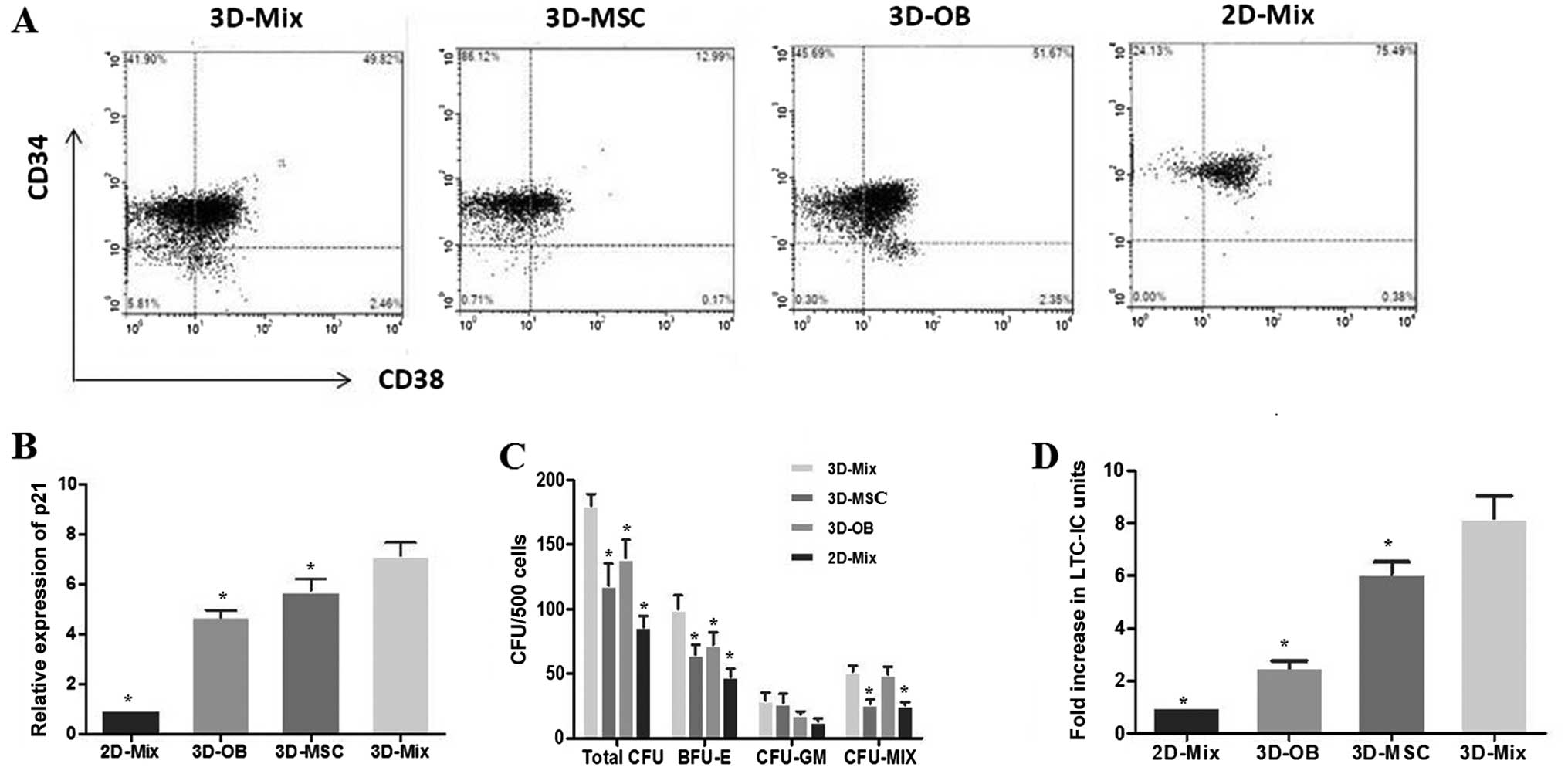
Abundant growth of CD34+ cells
in the 3D-Mix system
It is known that CD34 positivity is used as the most
common immune phenotype for human HSPCs, and
CD34+CD38− cells are regarded as the more
primitive cell subpopulation. After 2 weeks of HSPC culture in the
3D or 2D system, the cells were harvested and subjected to FCM to
count the number of CD34+ cells,
CD34+CD38− cells representing long-term
repopulating cells (LTRCs) and CD34+CD38+
cells representing short-term repopulating cells (STRCs) in these
systems (Fig. 4A). It was
observed that the levels of expansion in the total CD34+
cell numbers in the 3D-Mix were significantly higher (p<0.05),
compared to the other 3 systems (3D-MSC, 3D-OB and 2D-Mix). The
yield of CD34+CD38− cells in the 3D-Mix was
significantly higher than that in the 3D-OB and 2D-Mix, although
there was no statistically significant difference compared with the
3D-MSC system. Moreover, the expansion of
CD34+CD38+ cells in the 3D-Mix was also
significantly improved when compared to that in the 3D-MSC and
2D-Mix (p<0.05), although the number of
CD34+CD38+ cells in the 3D-Mix was only
slightly higher than that of the 3D-OB system (Table I). Overall, these findings
indicated that the 3D-Mix culture system had the advantages of both
the 3D-OB and 3D-MSC systems, in that it did not only expand the
total number of CD34+ haematopoietic cells, but also
maintained these primitive cells in vitro.
 | Table IUmbilical cord blood HSC expansion
over 7 days of culture. |
Table I
Umbilical cord blood HSC expansion
over 7 days of culture.
| Group | 3D-Mix | 3D-MSC | 3D-OB | 2D-Mix |
|---|
| CD34+
cells | 14.86±3.74 | 7.69±1.67a | 11.52±4.58a | 2.63±1.17a |
|
CD34+CD38−
cells | 5.42±1.07 | 4.96±1.44 | 1.52±0.53a | 0.57±0.16a |
|
CD34+CD38+
cells | 12.65±4.42 | 6.36±2.07a | 10.87±5.33 | 2.09±1.35a |
A large proportion of HSCs is known to be in a
quiescent state in the bone marrow niche (9). It was not apparent whether HSCs in
this state enter the cell cycle at all. In this study, in order to
address this issue, HSPCs from the culture systems were subjected
to qPCR assay to determine the expression of p21, which is an
essential regulator of the quiescence of HSCs (30). Our results revealed that p21
expression in the HSCs from the 3D-Mix system was significantly
higher than that in the HSCs from the other culture systems,
particularly in comparison with the 3D-OB and 2D-Mix systems,
indicating that a large percentage of HSCs from the 3D-Mix was
maintained in the G0 stage of the cell cycle. This suggested that
the 3D-Mix possessed the ability to foster a large pool of
quiescent HSCs, which is a critical niche characteristic in
vivo (Fig. 4B).
3D-Mix system provides superior support
for active multi-lineage hematopoiesis
Cultured CD34+ cells from the systems
were also subjected to in vitro CFU assays to evaluate the
proliferation and differentiation potential of hematopoietic
progenitor cells. The CD34+ cells were sorted after 2
weeks of culture to avoid contamination with other cells and we
performed methylcellulose assay to determine their potential clonal
growth as, CFU-GEMM (termed CFU-MIX), CFU-GM and BFU-E. Colony
formation assays revealed a significantly higher output of
progenitors from the 3D-Mix compared to the other 3 culture systems
(p<0.001). The distribution of CFUs was analyzed using an
inverted microscope to observe the colony morphology, showing a
significantly higher number of BFU-E in the 3D-Mix compared to the
other 3 culture systems. Moreover, the frequencies of CFU-GM and
CFU-MIX in the 3D-Mix were similar to those in the 3D-MSC and 3D-OB
systems, respectively (Fig.
4C).
In parallel to the experiment on committed
progenitors, we also examined the effects of the 3D-Mix on more
primitive progenitors, as measured using the in vitro LTC-IC
assay. CD34+ cells generated in the 4 culture systems
were subjected to in vitro LTC-IC assay to determine whether
they could preserve the ability to sustain long-term hematopoiesis.
Following the initial 14-day culture period in the 4 culture
systems, the output of CD34+ cells was plated on M2-10B4
for 5 weeks and then cultured in methylcellulose to analyze the
LTC-IC-derived colony-forming cells. It was observed that the
LTC-IC frequency in the 3D-Mix system was higher than that of the
other 3 systems (Fig. 4D).
Human cord blood CD34+ cells were
cultured for 14 days in the 4 culture systems. The cells were
harvested, washed and injected into sublethally irradiated (2.5 Gy)
NOD/SCID mice; each mouse received cells equivalent to
104 starting CD34+ cells. At 8 weeks
post-cell transplantation, bone marrow cells were harvested from
the recipients and subjected to FCM with an anti-human CD45
antibody. Mice were considered positive for human HSC engraftment
when at least 0.1% CD45+ human cells were detected in
the mouse bone marrow cells. Bone marrow cells from positive mice
were further analyzed by FCM using antibodies for human CD33 and
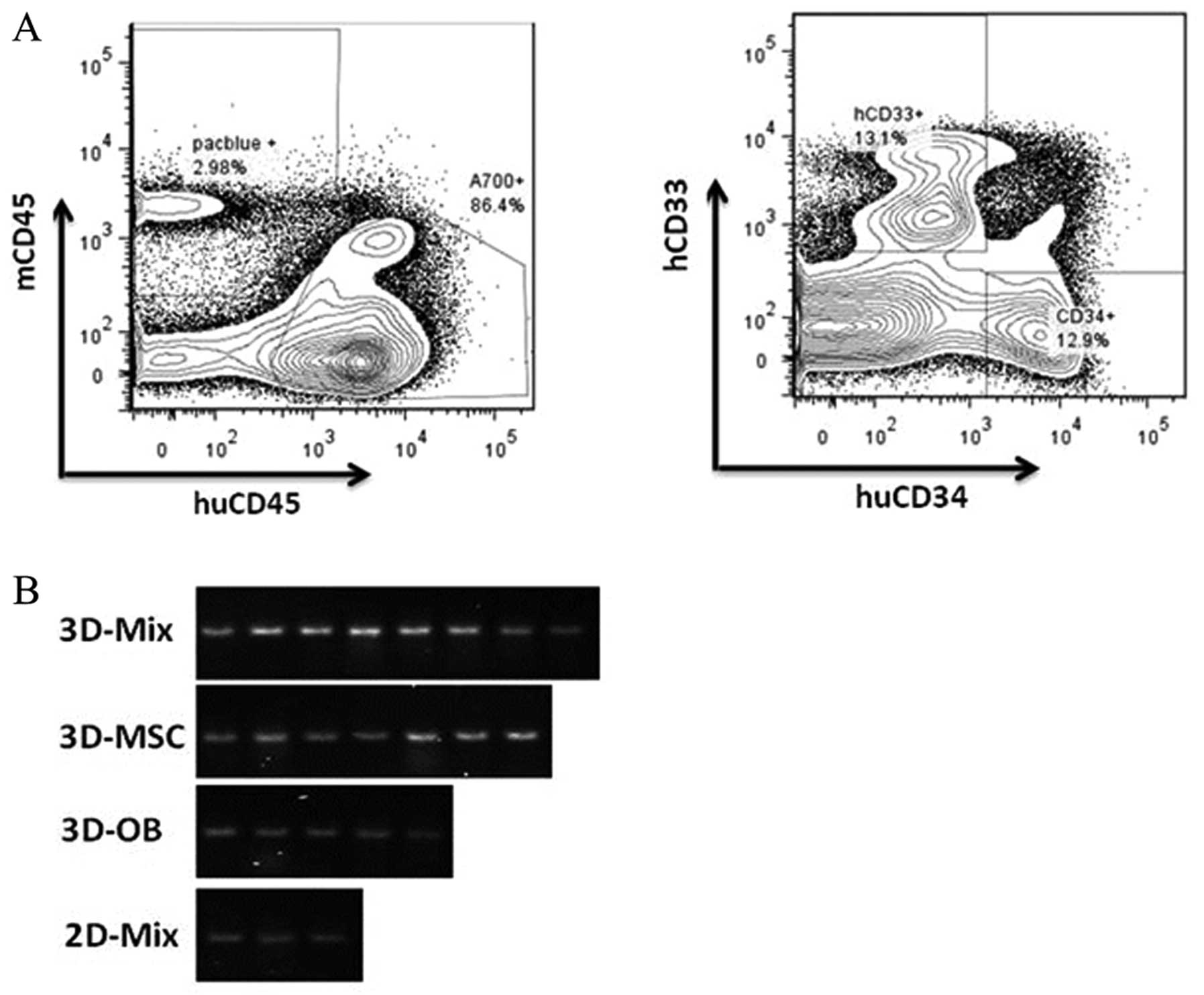
CD34 antigens (Fig. 5A). We
calculated the SRC concentration using the Poisson probability at
P0. In our experiments, the positivity of human HSC engraftment in
the 3D-Mix system were found to be the highest out of the 4 culture
systems and cord blood CD34+ cells cultured in the
3D-Mix system were observed to have significantly higher SRC
frequencies than the cells cultured in the other 3 systems
(p<0.01; Table II).
Furthermore, PCR analysis of 17α-satellite gene expression revealed
the presence of human hematopoietic cells in the bone marrow of the
NOD/SCID mice (Fig. 5B).
 | Table IIExpansion of SRCs in the 3D-Mix
culture system. |
Table II
Expansion of SRCs in the 3D-Mix
culture system.
| Culture system | Positive/total | % Positive | SRC frequency |
|---|
| 3D-Mix | 8/10 | 80 | 1 in 5832 |
| 3D-MSC | 7/10 | 70a | 1 in 7901a |
| 3D-OB | 5/10 | 50a | 1 in 10745a |
| 2D-Mix | 3/10 | 30a | 1 in 16227a |
Discussion
An ideal HSPC culture system should possess both the
characteristics of the long-term maintenance of the HSC pool and
the continuousy promoting effects on the proliferation of the
hematopoietic progenitor pool. However, the method with which to
achieve this apparently contradictory and dynamic process in
vitro continues to be an intriguing issue in HSPC culture
research (1). To date, there is
still not a suitable HSPC culture system to provide a suitable
platform with which to study cellular biology and to be used in
clinical practice by helping to achieve steady-state hematopoiesis
in vivo. Although cytokine-driven culture which is
supplemented with cytokines, such as fetal liver tyrosine kinase-3
ligand (FLT3-L), SCF, interleukin-3 (IL-3) and thrombopoietin (TPO)
has been demonstrated to effectively promote expansion, it is
difficult to maintain long-term HSPC culture due to the rapid onset
of differentiation and the rapid loss of multipotency in culture,
which thus renders this system insufficient for the clinical
applications of these cells. By contrast, HSCs efficiently
self-renew in their natural microenvironment (known as the HSC
niche) in the bone marrow (31).
Recently, studies have indicated that the HSC niche is responsible
for HSC fate and have clarified the important regulatory component
of the HSC niche (32–34). Therefore, closely mimicking the
bone marrow niche ex vivo is considered to be an effective
means of developing novel culture strategies to specifically
regulate the balance of HSPC self-renewal and proliferation.
Studies have proven that this method is very effective (19,35,36). With the in-depth understanding of
the complex HSC niche, we further improved the HSPC culture
protocol based on our previous study, which was demonstrated to
initially have both properties of maintaining a certain number of
the stem cell pool and continuously promoting the proliferation of
progenitors, compared with other traditional culture systems. The
HSC niche is an in vivo regulatory microenvironment where
HSCs reside and maintain their capability of self-renewal and
multipotency, and is composed of all types of cellular and humoral
factors. OBs and BMSCs have long been revealed to be the two major
crucial components of the HSC niche in bone marrow to support the
maintenance, proliferation and differentiation of HSCs. OBs are
derived from the MSCs in bone marrow. Namely, when MSCs commit to
osteogenesis, they differentiate into osteoprogenitors that
progress into pre-osteoblasts, OBs, and finally into osteocytes
that mineralize and form bone (37). Actually, the osteoblastic niche
has been proven to include osteoprogenitor cells, quiescent bone
lining cells and active OBs (12). Sacchetti et al demonstrated
that human CD45−CD146+ osteoprogenitor cells
were able to transfer hematopoietic activity to an ectopic site
(38). It was found that the
deletion of OBs under the control of collagen a1 type I promoter
led to a decrease in the numbers of HSCs (39). That was why we selected the OBs
differentiated from human BMSCs to act as a candidate of feeder
cells in this study, instead of directly using a mature OB cell
line. Our experiments of the induction of the differentiation of
human BMSCs to OBs indicated that the MSCs that differentiated into
OBs were a heterogeneous population at various stages of
differentiation, rather than just fully mature OBs. MSCs express
high levels of HSC maintenance factor transcripts, including
CXCL12, angiopoietin-1, SCF, IL-7 and osteopontin. Méndez-Ferrer
et al identified a nestin-expressing MSC population
(nestin+ MSC) that is closely associated with putative
HSCs. The depletion of nestin+ MSCs by the inducible
expression of diphtheriatoxin receptor in nestin-expressing cells
caused the mobilization of 50% of HSCs to the spleen (40). In addition, in another study, the
homing of transplanted progenitor cells into MSC-depleted
recipients was shown to be reduced by 90% (41). Recently, Omatsu et al found
that CAR cells are important in supporting the proliferation of
HSPCs. Further data confirmed that CAR cells which express
osteogenic and adipogenic genes are a form of osteogenic-adipo
progenitor derived from MSCs (42). Therefore, in this study, we
selected chose human BMSCs as the other candidate of feeder cells
in our culture system. In order to more closely mimic a multi-cell
state in the HSC niche to produce synergistic control of the
balance between quiescence, self-renewal and proliferation in the
HSPC culture system, we co-cultured BMSCs and OBs differentiated
from MSCs without any cytokines in this study to create a novel
culture system to support the maintenance and proliferation of
HSPCs in vitro.
In order to mimic the physical conditions of the HSC
niche, many groups have tested different materials as scaffolds to
build a 3D culture system, which include natural materials, such as
cellulose porous microspheres and collagen carriers, or synthetic
materials, such as macroporous PEG hydrogels, porous biomatrix,
porous polyvinyl formal resin, polyethylene terephthalate,
colloidal crystals and porous gelatin microspheres (13–15,43,44). In this study, we adopted
bio-derived bone made from human femurs as a system scaffold which
preserves the natural spongy architecture of trabecular bone to
more closely mimic the HSC niche in vivo. This study
demonstrated that the BDBS had good biocompatibility and more
importantly, partially reserved calcium, phosphonium and other ECM
proteins, which have been proven to promote the osteogenic
differentiation of MSCs in vitro. It is known that 3D
cultures allow the reconstruction of the complex tissue
architecture, thus providing a better platform with which to study
cellular biology. There is evidence to indicate that 3D culture
systems are superior to traditional 2D systems as regards the
maintenance and expansion of HSPCs, which proves that the spatial
architecture is one of the most important physiological conditions
in the stem cell niche and influences the biological behavior of
HSCs (37,45–47). The natural scale and pore size of
bio-derived bone as a scaffold are close to the natural
architecture of the HSC niche in vivo. In this study, the
mixture of human BMSCs and OBs differentiated from MSCs attached to
this natural scaffold very well (70–80% attachment) (data not
shown). These two cell populations were observed to grow in the
porous network of trabecular bone and form a meshwork-like
structure, while the HSCs embedded and grew in the intercellular
spaces. 3D-MSC and 3D-OB systems are used as controls apart from
the traditional 2D culture system (10,19,20). We carried out extensive analyses
of specialized microenvironments that were recognized by
hematopoietic CD34+ cells in the 3D culture system. The
HSC niche is known to express many crucial molecules, which include
chemokines, growth factors, cell-surface and adhesion molecules,
and plays key roles in HSC self-renewal, survival and maintenance
(28). Furthermore, the majority
of the secreted factors which have been proposed to control HSC
fate, such as angiopoietin-1, RUNX2, CXCL12, osteopontin and SCF
are supplied by MSCs and OBs in the HSC niche (48). We found that the interactions of
human BMSCs and OBs in the 3D-Mix system may contribute to a more
comprehensive and balanced expression of cytokines, which more
closely mimics the physiological state of the HSC niche and is more
beneficial to HSPC culture in vitro compared with the other
culture systems. The HSC microenvironment is enriched with ECM
proteins, such as fibronectin, collagen IV, vitronectin and
laminin, which are an essential part of the HSC niche (29). Thus, in this study, the culture
systems were subjected to ELISA to determine the presence of these
molecules. High levels of ECM molecules were found to be secreted
by the MSCs and OBs differentiated from MSCs and deposited on the
surface of the two cell populations, which means that they
themselves were capable of adding a physiologically relevant
dimension to the culture system. In addition, our data indicated
that the interactions of human BMSCs and OBs in the 3D-Mix system
provided a comprehensive amount of ECM proteins to support and
modulate HSPC culture in vitro.
In the present study, phenotypic analysis of
expanded cells by FCM revealed that the proportion of
CD34+CD38− cells representing LTRC in the
3D-Mix system was significantly higher than that in the 3D-OB
system, although there was no statistically significant difference
in the yield of CD34+CD38+ cells (which
represent STRCs) between them, which implies that MSCs play an
important role in maintaining the quiescence HSCs in vitro.
On the other hand, although half of the OBs in the 3D-OB system was
replaced by human BMSCs in the 3D-Mix system, the number of
CD34+CD38+ cells between the two culture
systems exhibited no obvious difference, which implies that the
interaction of MSCs with OBs also has an effect on promoting HPC
proliferation in vitro. We also found that the yield of
CD34+CD38+ cells from the 3D-Mix culture
system was significantly higher than that from the 3D-MSC system,
although the number of CD34+CD38− cells was
slightly lower than that from the 3D-MSC system, indicating tht OBs
differentiated from human BMSCs play a critical role in improving
the proliferation of HSPCs in vitro. On the other hand,
although half of the MSCs in the 3D-MSC system was replaced by OBs
in the 3D-Mix system, the number of
CD34+CD38− cells in the 3D-Mix was close to
that in the 3D-MSC system, which implies that the interaction of
OBs with MSCs also has the effect of promoting HSC self-renewal
in vitro.
PCR analysis of p21, which is an essential regulator
of the quiescence of HSCs, also confirmed the FCM results, which
showed that p21 expression in the HSCs from the 3D-Mix system was
significantly higher than that in the cells from the 3D-OB system
and very close to that in the cells from the 3D-MSC system,
indicating that a large percentage of HSCs from the 3D-Mix system
was maintained in the G0 stage of the cell cycle. It is known that
UCB LTC-ICs are present among the CD34+CD38−
cell fraction and LTC-IC assays exhibit multilineage
differentiation ability and major proliferative potential, which is
regarded as a functional measure of self-renewal (47). Our results demonstrated that the
frequency of LTC-ICs from the 3D-Mix system was statistically
higher than that of the other 3 culture systems, particularly the
3D-OB and 2D-Mix systems, which demonstrated that the 3D-Mix system
contained a higher number of more primitive hematopoietic cells
ex vivo compared with the other 3 systems, possessing the
important ability of fostering a large pool of quiescent HSCs,
which is a critical niche characteristic in vivo. In the SRC
assay, which indicated the reconstituting ability of these cultured
cells, the difference in the percentage of chimerism of human
CD45+ cells among bone marrow cells of mice transplanted
with cultured cells strongly suggested that the 3D-Mix more
effectively supported ex vivo-generated HSCs with the
ability to sustain and reconstitute long-term human hematopoiesis
in vivo, compared with the other 3 systems.
In conclusion, our data demonstrate that the 3D
culture system, established by the co-culture of BMSCs and OBs
differentiated from MSCs on human BDBS, is a more comprehensive and
balanced system, which not only continuously and effectively
promotes the self-renewal and ex vivo expansion of HSPCs,
but also maintains a large pool of primitive HPCs with superior
phenotypic and functional attributes. Compared with the other 3
culture systems, the 3D-Mix culture system has some features which
are more similar to those of the HSC niche, namely as regards the
maintenance and expansion of HSPCs in vitro.
References
|
1
|
Vunjak-Novakovic G and Scadden DT:
Biomimetic platforms for human stem cell research. Cell Stem Cell.
8:252–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konopleva MY and Jordan CT: Leukemia stem
cells and microenvironment: Biology and therapeutic targeting. J
Clin Oncol. 29:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konopleva M, Tabe Y, Zeng Z and Andreeff
M: Therapeutic targeting of microenvironmental interactions in
leukemia: Mechanisms and approaches. Drug Resist Updat. 12:103–113.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma MB, Limaye LS and Kale VP:
Mimicking the functional hematopoietic stem cell niche in vitro:
recapitulation of marrow physiology by hydrogel-based
three-dimensional cultures of mesenchymal stromal cells.
Haematologica. 97:651–660. 2012. View Article : Google Scholar :
|
|
5
|
Bird GA, Polsky A, Estes P, Hanlon T,
Hamilton H, Morton JJ, Gutman J, Jimeno A, Turner BC and Refaeli Y:
Expansion of human and murine hematopoietic stem and progenitor
cells ex vivo without genetic modification using MYC and Bcl-2
fusion proteins. PLoS One. 9:e1055252014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soufizomorrod M, Soleimani M, Hajifathali
A, Mohammadi MM and Abroun S: Expansion of CD133 umbilical cord
blood derived hematopoietic stem cells on a biocompatible
microwells. Int J Hematol Oncol Stem Cell Res. 7:92013.
|
|
7
|
Bari S, Chu PP, Lim A, Fan X, Gay FP,
Bunte RM, Lim TK, Li S, Chiu GN and Hwang WY: Protective role of
functionalized single walled carbon nanotubes enhance ex vivo
expansion of hematopoietic stem and progenitor cells in human
umbilical cord blood. Nanomedicine (Lond). 9:1304–1316. 2013.
|
|
8
|
Zhang J, Niu C, Ye L, Huang H, He X, Tong
WG, Ross J, Haug J, Johnson T, Feng JQ, et al: Identification of
the haematopoietic stem cell niche and control of the niche size.
Nature. 425:836–841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arai F and Suda T: Maintenance of
quiescent hematopoietic stem cells in the osteoblastic niche. Ann
NY Acad Sci. 1106:41–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Maggio N, Piccinini E, Jaworski M,
Trumpp A, Wendt DJ and Martin I: Toward modeling the bone marrow
niche using scaffold-based 3D culture systems. Biomaterials.
32:321–329. 2011. View Article : Google Scholar
|
|
11
|
Taichman RS, Reilly MJ and Emerson SG:
Human osteoblasts support human hematopoietic progenitor cells in
vitro bone marrow cultures. Blood. 87:518–524. 1996.PubMed/NCBI
|
|
12
|
Wu JY, Scadden DT and Kronenberg HM: Role
of the osteoblast lineage in the bone marrow hematopoietic niches.
J Bone Miner Res. 24:759–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagley J, Rosenzweig M, Marks DF and
Pykett MJ: Extended culture of multipotent hematopoietic
progenitors without cytokine augmentation in a novel
three-dimensional device. Exp Hematol. 27:496–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Ma T, Kniss DA, Yang ST and Lasky
LC: Human cord cell hematopoiesis in three-dimensional nonwoven
fibrous matrices: In vitro simulation of the marrow
microenvironment. J Hematother Stem Cell Res. 10:355–368. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Chai C, Jiang XS, Teoh S-H and
Leong KW: Co-culture of umbilical cord blood CD34þ cells with human
mesenchymal stem cells. Tissue Eng. 12:2161–2170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugiyama T, Kohara H, Noda M and Nagasawa
T: Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4
chemokine signaling in bone marrow stromal cell niches. Immunity.
25:977–988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Liu T, Meng W and Zhi W:
Osteoblasts differentiated from human marrow bone mesenchymal stem
cells support hematopoietic stem/progenitor cells from umbilical
cord blood. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 14:552–556. 2006.In
Chinese. PubMed/NCBI
|
|
19
|
Raic A, Rödling L, Kalbacher H and
Lee-Thedieck C: Biomimetic macroporous PEG hydrogels as 3D
scaffolds for the multiplication of human hematopoietic stem and
progenitor cells. Biomaterials. 35:929–940. 2014. View Article : Google Scholar
|
|
20
|
Miyoshi H, Ohshima N and Sato C: Three -
dimensional culture of mouse bone marrow cells on stroma formed
within a porous scaffold: Influence of scaffold shape and
cryopreservation of the stromal layer on expansion of
haematopoietic progenitor cells. J Tissue Eng Regen Med. 7:32–38.
2013. View Article : Google Scholar
|
|
21
|
Huang XB, Liu T, Meng WT, Deng L, Zhi W
and Zhu HL: Imitating human hematopoietic niche with osteoblasts
derived from human marrow mesenchymal stem cells to sustain
stem/progenitor cells proliferation. Chin J Hematol. 27:795–800.
2006.In Chinese.
|
|
22
|
Xie H, Yang F, Deng L, Luo J, Qin T, Li X,
Zhou GQ and Yang Z: The performance of a bone-derived scaffold
material in the repair of critical bone defects in a rhesus monkey
model. Biomaterials. 28:3314–3324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun F, Li X, Yang C, Lv P, Li G and Xu H:
A role for PERK in the mechanism underlying fluoride-induced bone
turnover. Toxicology. 325:52–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang YC, Zhu HM, Cai JQ, Huang YZ, Xu J,
Zhou Y, Chen XH, Li XQ, Yang ZM and Deng L: Hypoxia inhibits the
spontaneous calcification of bone marrow-derived mesenchymal stem
cells. J Cell Biochem. 113:1407–1415. 2012. View Article : Google Scholar
|
|
25
|
Verfaillie CM: Direct contact between
human primitive hematopoietic progenitors and bone marrow stroma is
not required for long-term in vitro hematopoiesis. Blood.
79:2821–2826. 1992.PubMed/NCBI
|
|
26
|
Chen J, Astle CM and Harrison DE:
Development and aging of primitive hematopoietic stem cells in
BALB/cBy mice. Exp Hematol. 27:928–935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Ellison FM, Eckhaus MA, Smith AL,
Keyvanfar K, Calado RT and Young NS: Minor antigen h60-mediated
aplastic anemia is ameliorated by immunosuppression and the
infusion of regulatory T cells. J Immunol. 178:4159–4168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendelson A and Frenette PS: Hematopoietic
stem cell niche maintenance during homeostasis and regeneration.
Nat Med. 20:833–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muth CA, Steinl C, Klein G and
Lee-Thedieck C: Regulation of hematopoietic stem cell behavior by
the nanostructured presentation of extracellular matrix components.
PLoS One. 8:e547782013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng T, Rodrigues N, Shen H, Yang Y-g,
Dombkowski D, Sykes M and Scadden DT: Hematopoietic stem cell
quiescence maintained by p21cip1/waf1. Science. 287:1804–1808.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taichman RS: Blood and bone: Two tissues
whose fates are intertwined to create the hematopoietic stem-cell
niche. Blood. 105:2631–2639. 2005. View Article : Google Scholar
|
|
32
|
Catlin SN, Busque L, Gale RE, Guttorp P
and Abkowitz JL: The replication rate of human hematopoietic stem
cells in vivo. Blood. 117:4460–4466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH,
Terhorst C and Morrison SJ: SLAM family receptors distinguish
hematopoietic stem and progenitor cells and reveal endothelial
niches for stem cells. Cell. 121:1109–1121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osawa M, Hanada K-i, Hamada H and Nakauchi
H: Long-term lymphohematopoietic reconstitution by a single
CD34-low/negative hematopoietic stem cell. Science. 273:242–245.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raynaud CM, Butler JM, Halabi NM, Ahmad
FS, Ahmed B, Rafii S and Rafii A: Endothelial cells provide a niche
for placental hematopoietic stem/progenitor cell expansion through
broad transcriptomic modification. Stem Cell Res (Amst).
11:1074–1090. 2013. View Article : Google Scholar
|
|
36
|
de Barros AP, Takiya CM, Garzoni LR,
Leal-Ferreira ML, Dutra HS, Chiarini LB, Meirelles MN, Borojevic R
and Rossi MI: Osteoblasts and bone marrow mesenchymal stromal cells
control hematopoietic stem cell migration and proliferation in 3D
in vitro model. PLoS One. 5:e90932010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bianco P: Bone and the hematopoietic
niche: A tale of two stem cells. Blood. 117:5281–5288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sacchetti B, Funari A, Michienzi S, Di
Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG,
Riminucci M and Bianco P: Self-renewing osteoprogenitors in bone
marrow sinusoids can organize a hematopoietic microenvironment.
Cell. 131:324–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Visnjic D, Kalajzic Z, Rowe DW, Katavic V,
Lorenzo J and Aguila HL: Hematopoiesis is severely altered in mice
with an induced osteoblast deficiency. Blood. 103:3258–3264. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Méndez-Ferrer S, Lucas D, Battista M and
Frenette PS: Haematopoietic stem cell release is regulated by
circadian oscillations. Nature. 452:442–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anthony BA and Link DC: Regulation of
hematopoietic stem cells by bone marrow stromal cells. Trends
Immunol. 35:32–37. 2014. View Article : Google Scholar :
|
|
42
|
Omatsu Y, Sugiyama T, Kohara H, Kondoh G,
Fujii N, Kohno K and Nagasawa T: The essential functions of
adipo-osteogenic progenitors as the hematopoietic stem and
progenitor cell niche. Immunity. 33:387–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tun T, Miyoshi H, Aung T, Takahashi S,
Shimizu R, Kuroha T, Yamamoto M and Ohshima N: Effect of growth
factors on ex vivo bone marrow cell expansion using three -
dimensional matrix support. Artif Organs. 26:333–339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nichols JE, Cortiella J, Lee J, Niles JA,
Cuddihy M, Wang S, Bielitzki J, Cantu A, Mlcak R, Valdivia E, et
al: In vitro analog of human bone marrow from 3D scaffolds with
biomimetic inverted colloidal crystal geometry. Biomaterials.
30:1071–1079. 2009. View Article : Google Scholar :
|
|
45
|
Chitteti BR, Bethel M, Voytik-Harbin SL,
Kacena MA and Srour EF: In vitro construction of 2D and 3D
simulations of the murine hematopoietic niche. Stem Cell Niche.
Springer; pp. 43–56. 2013, View Article : Google Scholar
|
|
46
|
Hirabayashi Y, Hatta Y, Takeuchi J, Tsuboi
I, Harada T, Ono K, Glomm WR, Yasuda M and Aizawa S: Novel
three-dimensional long-term bone marrow culture system using
polymer particles with grafted epoxy-polymer-chains supports the
proliferation and differentiation of hematopoietic stem cells. Exp
Biol Med. 236:1342–1350. 2011. View Article : Google Scholar
|
|
47
|
Tang Y, Chen J and Young NS: Expansion of
haematopoietic stem cells from normal donors and bone marrow
failure patients by recombinant hoxb4. Br J Haematol. 144:603–612.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Asada N and Katayama Y: Regulation of
hematopoiesis in endosteal microenvironments. Int J Hematol.
99:679–684. 2014. View Article : Google Scholar : PubMed/NCBI
|