Introduction
Ischemia and reperfusion injury following cardiac
operations or myocardial infarction is the principal reason for
cardiac failure, morbidity, and mortality (1,2).
However, an effective treatment for preventing myocardial ischemia
and reperfusion injury remains unavailable due to the complicated
underlying mechanism. Under ischemic or hypoxic conditions, the
mitochondria in cardiomyocytes become vulnerable and damaged,
leading to the production of excessive reactive oxygen species
(ROS) (3). An increase in ROS
induces lipid peroxidation, DNA oxidative damage, and cardiac cell
oxidative damage, eventually leading to cell apoptosis (4). Therefore, the identification of a
target capable of scavenging ROS in cardiomyocytes may provide a
novel effective therapy for the management of myocardial ischemia
and reperfusion injury.
Phase II detoxification enzymes and antioxidant
enzymes are the critical components of cellular defensive machinery
against ROS (5). These enzymes
are regulated by the transcription factor nuclear factor erythroid
2-related factor 2 (Nrf2) (6).
The activation of Nrf2-mediated transcription is monitored by
kelch-like ECH-associated protein 1 (Keap1). Under basal
conditions, Keap1 interacts with Nrf2, thus facilitating
ubiquitination and degradation of Nrf2. When Nrf2 is released from
Keap1, Nrf2 protein translocates into the nucleus and binds to the
antioxidant responsive elements to activate the transcription of
antioxidant enzymes including heme oxygenase 1 (HO-1), glutathione
S-transferase (GST), glutamate-cysteine ligase catalytic subunit
(GCLc) and NADPH-quinone oxidoreductase 1 (NQO1) (7). The Keap1-Nrf2 signaling axis is
suggested to play an important role in oxidative damage-related
chronic diseases, including cardiovascular disease (8). Therefore, targeting Keap1-Nrf2 is
considered an important approach for preventing myocardial ischemia
and reperfusion injury.
MicroRNAs (miRNAs or miRs) are endogenous,
single-stranded, small, non-coding RNAs that are capable of
modulating gene expression post-transcriptionally through binding
to the 3′-untranslated region (3′-UTR) of target mRNA, leading to
mRNA degradation and/or translational inhibition (9,10).
Therefore, miRNAs are involved in regulating a number of cellular
processes, including cell proliferation, survival and apoptosis
(11). Mounting evidence
indicates that the aberrant expression of miRNAs occurs in various
human diseases, including myocardial infarction, and miRNAs are of
therapeutic value in the management of myocardial infarction
(12). The Keap1-Nrf2 signaling
axis may be modulated by miRNAs (13). However, research into whether the
Keap1-Nrf2 signaling axis is regulated by specific miRNAs in
cardiomyocytes following myocardial ischemia and reperfusion injury
is limited.
miR-200a has been reported to be involved in
regulating the oxidative stress response (14,15). However, the role of miR-200a in
ischemia and reperfusion-induced oxidative stress in the myocardium
remains poorly understood. In the present study, we aimed to
examine the role of miR-200a in cardiomyocytes under hypoxic
conditions. We showed that miR-200a expression was significantly
downregulated in ischemic myocardial tissues and hypoxic
cardiomyocytes, indicating a potential role for miR-200a in
regulating the survival of cardiomyocytes. Notably, the
overexpression of miR-200a increased cell survival and inhibited
cell apoptosis and ROS production in cardiomyocytes under hypoxic
conditions. Further data showed that miR-200a functioned through
targeting and regulating the Keap1-Nrf2 signaling axis. Taken
together, these findings suggest that targeting the Keap1-Nrf2
signaling axis by miR-200a is a promising therapeutic strategy for
preventing myocardial injury due to ischemia or hypoxia-induced
oxidative stress.
Materials and methods
Cell culture and clinical tissue
preparation
Human adult cardiomyocytes were purchased from
ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in
cardiomyocyte medium (ScienCell Research Laboratories) containing
fetal bovine serum (FBS; Gibco, Rockville, MD, USA) and 1%
penicillin-streptomycin mix (Sigma, St. Louis, MO, USA) as per the
recommended protocols. 293T cells obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS (both
from Gibco) and 1/100 streptomycin-penicillin mix (Sigma). All
cells were maintained in a humidified incubator containing 5%
CO2 at 37°C. The left ventricular myocardial tissues
were obtained from patients with end-stage heart failure due to
ischemic cardiomyopathy at the time of heart transplantation (n=5).
Left ventricular tissues obtained from the non-failing hearts of
brain-dead organ donors (n=5) were used as controls. The myocardial
samples were immediately frozen in liquid nitrogen and stored at
−80°C for use. The study protocol was reviewed and approved by the
Institutional Human Experiment and Ethics Committee of Xianyang
Central Hospital (Xianyang, China) and informed consent was
obtained from all subjects.
Cell transfection and hypoxic
treatment
For overexpression of miR-200a, miR-200a mimics
(GenePharma, Shanghai, China) were used to transfect cells, and
negative control mimics (NC mimics) were used as the control. For
inhibition of miR-200a, a specific inhibitor against miR-200a was
transfected into the cells, and negative control inhibitor (NC
inhibitor) was used as the control. The miR-200a mimics (50 nM) and
miR-200a inhibitor (100 nM) were transfected into cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. The transfection efficiency was
evaluated by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). In order to induce hypoxia, the cells were
placed in a hypoxia chamber containing 94% N2, 1%
O2, and 5% CO2 at 37°C. Cells cultured under
normoxic conditions were taken as control.
RT-qPCR
Total RNA was extracted using an miRNeasy mini kit
(Qiagen, Shanghai, China) according to the manufacturer's
instructions. The cDNA was synthesized using a PrimeScript RT
reagent kit (Takara, Dalian, China). RT-qPCR amplification was
performed using a SYBR Premix Ex Taq GC kit (Takara). The following
PCR cycling conditions were used: template denaturation step at
94°C for 4 min; followed by 30 cycles of 20 sec at 94°C, 30 sec at
55°C, and 20 sec at 72°C; and 72°C for 5 min. The following primers
were used: Keap1 forward, 5′-TACGATGTGGAAACAGAGACGTGGA-3′ and
reverse, 5′-TCAACAGGTACAGTTCTGGTCAATCT-3′; HO-1 forward,
5′-CTGGAGGAGGAGATTGAGCG-3′ and reverse, 5′-ATGGCTGGTGTGTAGGGGAT-3′;
NQO1 forward, 5′-TGATCGTACTGGCTCACTCA-3′ and reverse,
GTCAGTTGAGGTTCTAAGAC-3′; GCLc forward, 5′-TCCAGGTGACATTCCAAGCC-3′
and reverse, 5′-GAAATCACTCCCCAGCGACA-3′; GST forward,
5′-CGGTACTTGCCTGCCTTTG-3′ and reverse, 5′-ATTTGTTTTGCATCCACGGG-3′;
β-actin forward, 5′-TGACGTGGACATCCGCAAAG-3′ and reverse,
5′-CTGGAAGGTGGACA GCGAGG-3′; miR-200a forward, 5′-CGTAACACTGTCTG
GTAACGATGT-3′ and reverse, 5′-TGGTGTCGTGGAGTCG-3′; and U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. Fold-changes relative to control
were calculated by the 2−ΔΔCt method.
Protein preparation and western blot
analysis
The nuclear and cytosolic proteins from cells were
extracted using a Nuclear Extract kit (Beyotime, Haimen, China)
according to the manufacturer's instructions. Briefly, the cells
were lysed in cytosolic protein extraction reagent followed by
centrifugation at 12,000 rpm (4°C) for 5 min. The supernatants
containing cytosolic proteins were collected. The remaining
precipitation was lysed by nuclear protein extraction reagent
followed by centrifugation at 12,000 rpm (4°C) for 10 min. The
supernatants containing nuclear proteins were collected. The
protein concentration was measured using a BCA kit (Beyotime).
Protein extractions were separated by 12.5% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were
then transferred to a polyvinylidene difluoride membrane
(Millipore, Boston, MA, USA). The membrane was then blocked and
probed with primary antibodies at 4°C overnight. Subsequently, the
membrane was incubated with horseradish peroxidase conjugated
secondary antibodies (1:2,000; Beyotime) and detected using
chemiluminescence. The following primary antibodies, which were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA), were used in this experiment: anti-Keap1 (sc-33569),
anti-Nrf2 (sc-13032), anti-β-actin (sc-130656) and anti-Lamin B
(sc-6216). The signal intensity of the western blots was analyzed
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockille, MD, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was detected by the MTT assay.
Briefly, the cells were seeded into 96-well plates at a density of
1×104 cells/well and cultured overnight. The cells were
transfected with miR-200a mimics or miR-200a inhibitor and then
subjected to hypoxic conditions for 48 h. Thereafter, 20 μl
of MTT stock solution (Sigma) was added to each well and incubated
for 4 h prior to adding 200 μl of dimethyl sulfoxide/well
(Sigma). After the formazan crystals dissolved, absorbance was
measured at 490 nm using a microplate spectrophotometer (Bio-Tek
Instruments, Winooski, VT, USA).
Lactate dehydrogenase (LDH) assay
Cell survival was measured using an LDH assay.
Briefly, the cells treated with miR-200a mimics or miR-200a
inhibitor were subjected to hypoxia for 48 h. The cells were
harvested and lysed using 0.2% Triton X-100 followed by
centrifugation at 10,000 × g for 10 min at 4°C. The supernatants
were collected and analyzed using an LDH assay kit (Beyotime). The
OD value at 490 nm was determined using a microplate reader
(Bio-Tek Instruments). The lysis ratio was calculated according to
the following formula: (experimental release-spontaneous
release)/(maximum release-spontaneous release) ×100%.
Cell apoptosis assay
Cell apoptosis was evaluated using a caspase-3
activity assay. Briefly, the cells were lysed in ice-cold lysis
buffer. Following centrifugation at 10,000 × g for 1 min at 4°C,
the supernatant was collected, and the protein concentration was
measured. Approximately 100 μg of protein was reacted with 5
μl of 4 mM DEVD-p-nitroanilide (pNA) substrate at 37°C for 2
h. Finally, the levels of pNA release were quantified using a
spectrophotometer (Bio-Tek Instruments).
Measurement of ROS
ROS production was detected using the
2′,7′-dichlorofluorescein diacetate (DCFH-DA) method. Briefly, the
cells were incubated with 50 μM DCFH-DA (Sigma) for 30 min
at 37°C in a dark place. After washing with phosphate buffer
solution, the fluorescence intensity was measured in a fluorescence
spectrophotometer (Bio-Tek Instruments) with an excitation
wavelength of 485 nm and emission wavelength of 530 nm.
Luciferase reporter assay
The potential target genes of miR-200a were
predicted by bioinformatics analysis using Targetscan (http://targetscan.org/) and MicroRNA.org -Targets and Expression (http://www.microrna.org/) databases. The 3′-UTR of
Keap1 was cloned into pmirGLO dual-luciferase vectors (Promega,
Madison, WI, USA) downstream of the luciferase gene. The
pmirGLO-Keap1 3′-UTR constructs or relevant mutants were
co-transfected with miR-200a mimics into 293T cells using
Lipofectamine 2000 (Invitrogen). Following a 48-h incubation
period, luciferase activity was measured using a Dual-Glo
luciferase assay system (Promega).
Rescue assay
For overexpression of Keap1, the open reading frame
of Keap1 cDNA without 3′-UTR was cloned into pcDNA3.0 vectors
(BioVector, Beijing, China). The pcDNA3/Keap1 constructs were
transfected into cells using Lipofectamine 2000 (Invitrogen) at a
final concentration of 0.5 μg/μl. Empty vectors were
used as a control.
Statistical analysis
All experimental data are presented as the means ±
standard deviation. Statistical difference was determined by
one-way analysis of variance (ANOVA) or the Student's t-test (SPSS
software version 11.5; SPSS Inc., Chicago, IL, USA). When ANOVA
showed statistical significance, a post-hoc Bonferroni test was
conducted. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
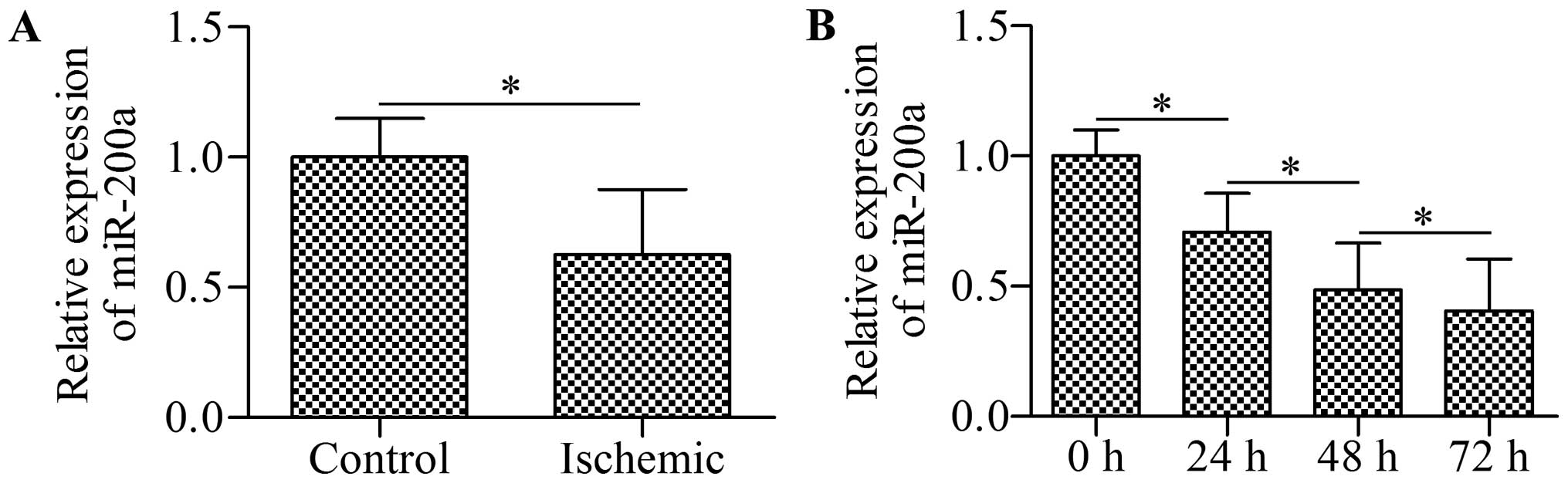
miR-200a is downregulated in ischemic
myocardial tissues and hypoxic cardiomyocytes
To examine the potential role of miR-200a in
ischemic heart disease, we first detected the expression of
miR-200a in clinical myocardial tissues using RT-qPCR. The results
revealed that miR-200a expression was significantly decreased in
the ischemic myocardial tissues compared with that in the
non-failing myocardial tissues (Fig.
1A). Furthermore, an in vitro experiment showed that
miR-200a was downregulated in a time-dependent manner in
cardiomyocytes exposed to hypoxia (Fig. 1B). These results indicate the
important role of miR-200a in ischemic heart disease.
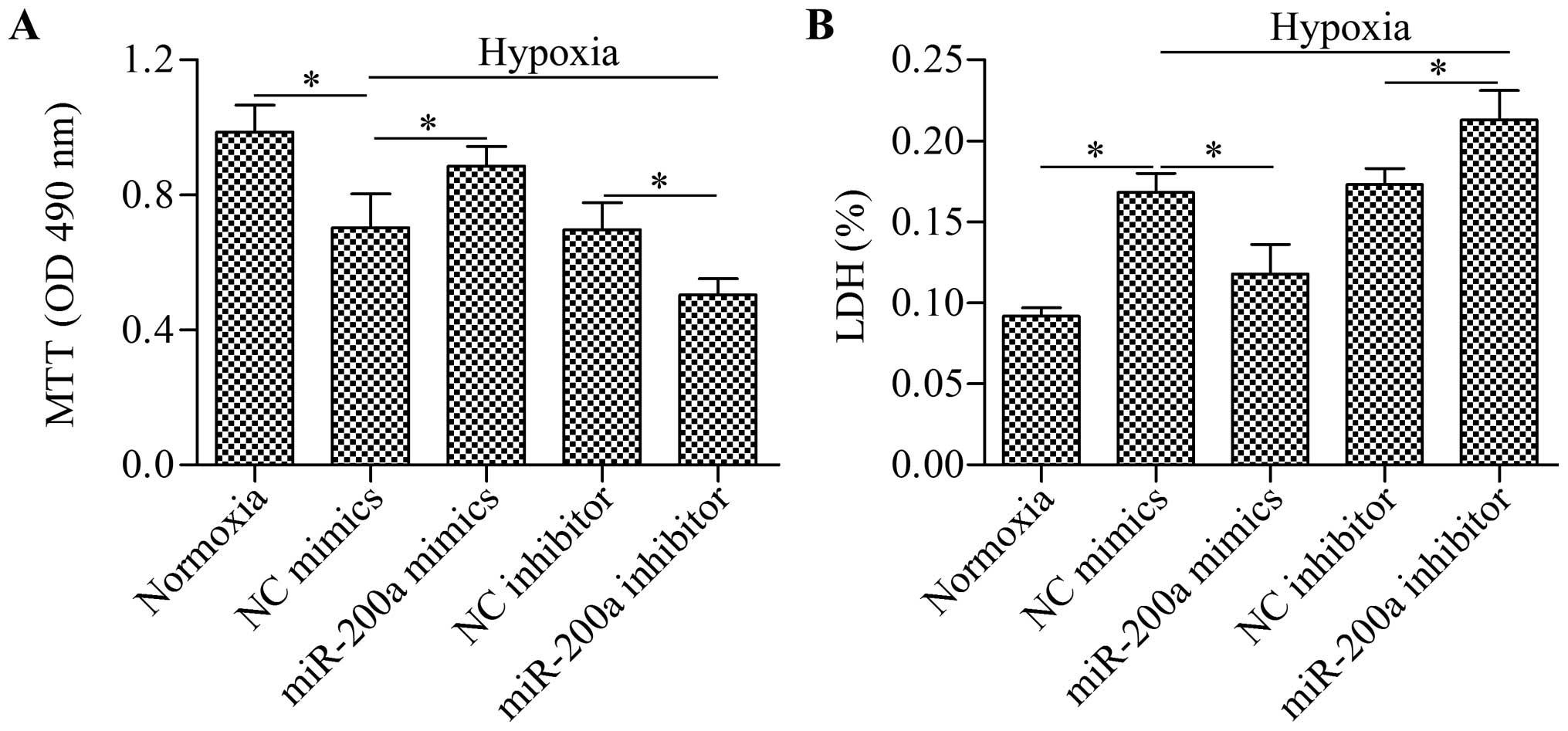
Overexpression of miR-200a rescues cell
viability impaired by hypoxia
To deterine the biological effect of miR-200a on
cell viability, we performed gain-of-function or loss-of-function
experiments using miR-200a mimics or miR-200a inhibitor to
overexpress miR-200a or silence miR-200a. We then detected their
effects on cell viability using MTT and LDH assays. The results
showed that cell viability was significantly impaired by hypoxia,
but this impairment was partially rescued by miR-200a
overexpression (Fig. 2). By
contrast, the silencing of miR-200a by miR-200a inhibitor
significantly augmented the impaired cell viability induced by
hypoxia. These results suggest that overexpression of miR-200a is
beneficial for cardiomyocyte survival.
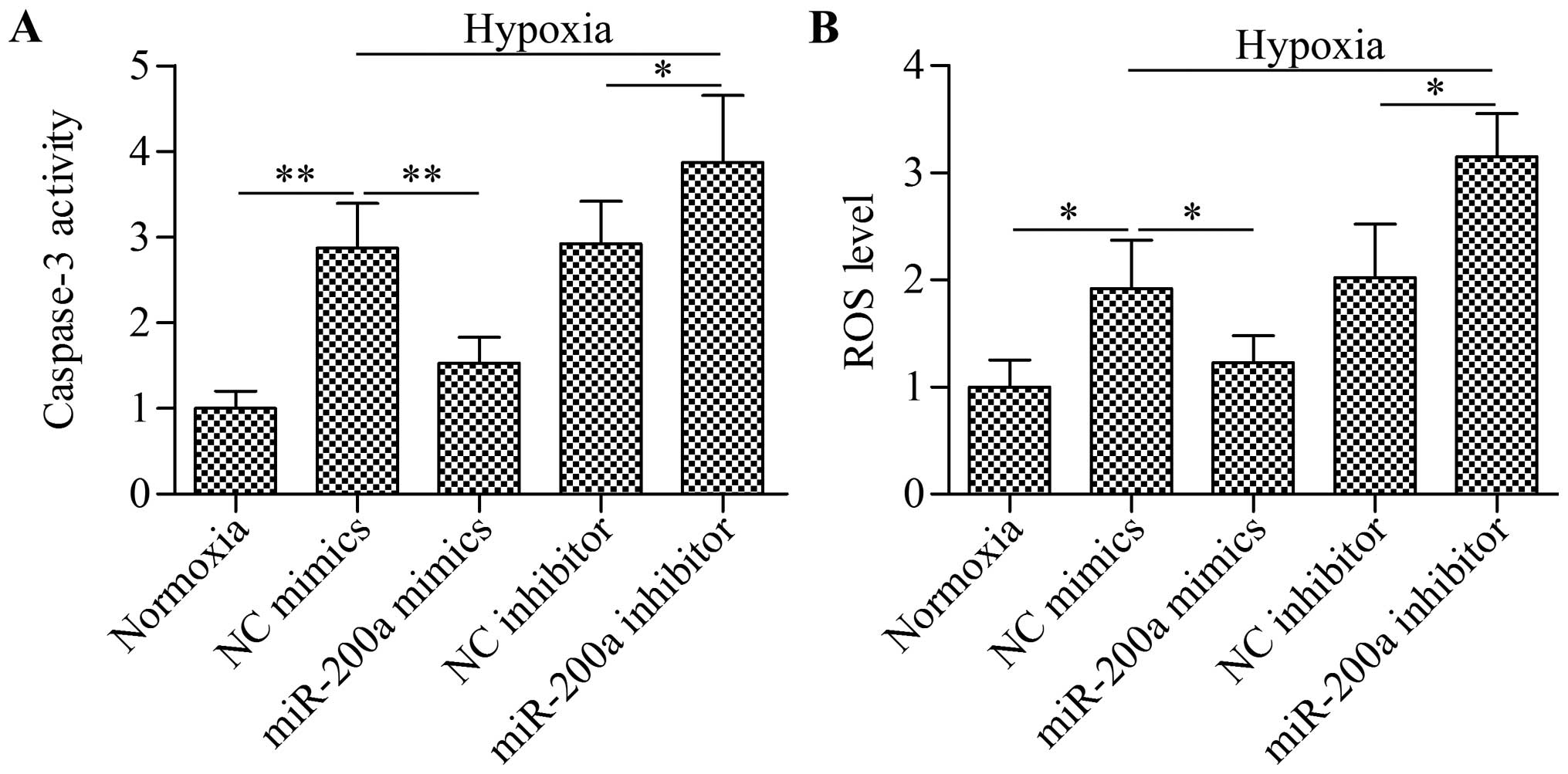
Overexpression of miR-200a decreases cell
apoptosis and ROS production induced by hypoxia
To further verify the biological effect of miR-200a
in regulating cardiomyocyte survival, we evaluated cell apoptosis
using a caspase-3 assay. The results showed that the activity of
caspase-3 was significantly upregulated by hypoxia, which was
significantly reduced by miR-200a overexpression or further
increased by miR-200a silencing (Fig.
3A). Moreover, the excessive ROS levels induced by hypoxia was
significantly decreased by miR-200a overexpression or further
increased by miR-200a silencing (Fig.
3A). These results suggest that overexpression of miR-200a
improved cardiomyocyte survival by reducing cell apoptosis and ROS
production under hypoxic conditions.
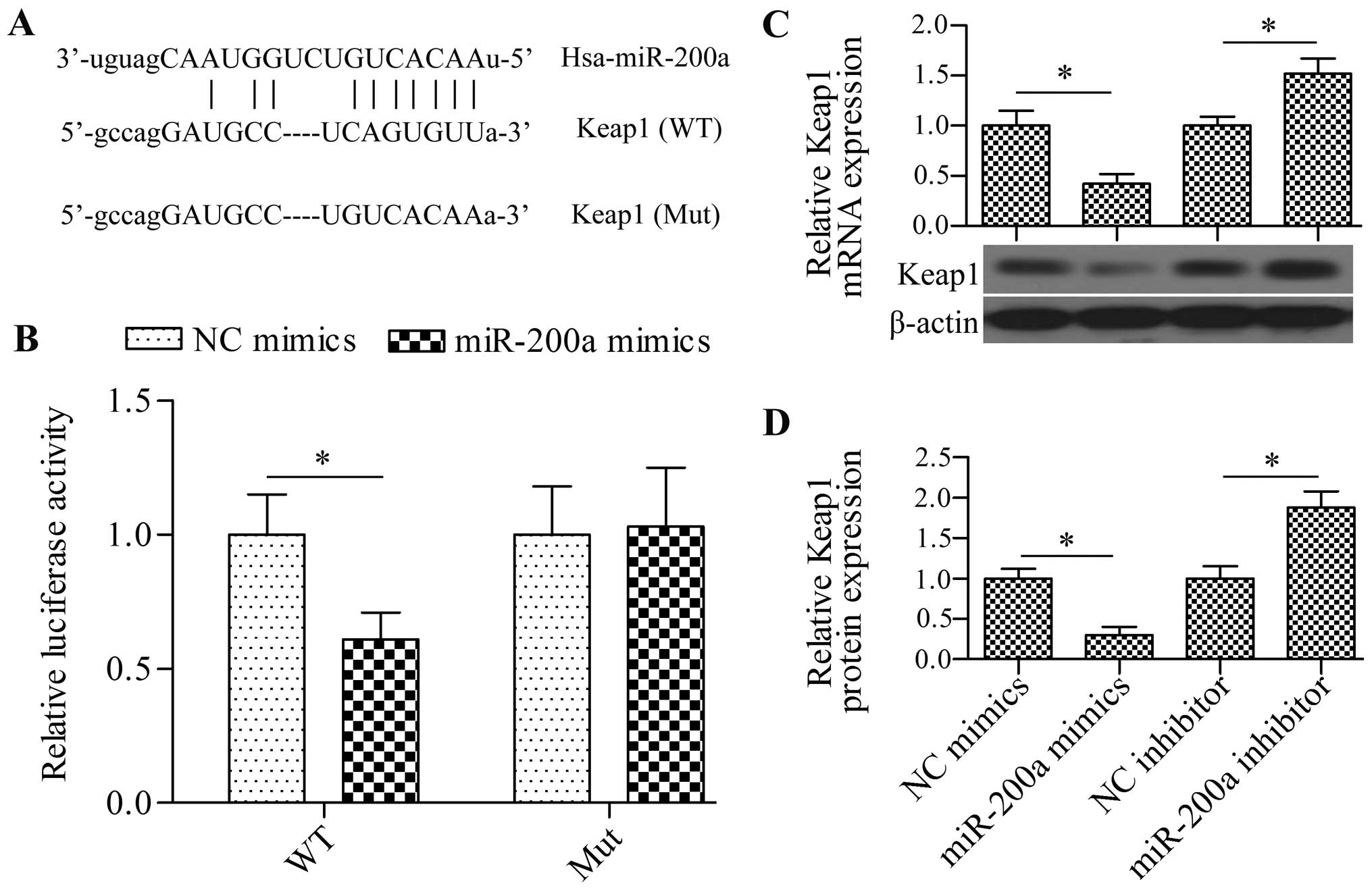
Keap1 is a target of miR-200a
To elucidate the underlying molecular mechanism, we
performed a bioinformatic analysis to predict the possible target
gene of miR-200a. Notably, we found that Keap1 contained a
theoretical miR-200a binding site in the 3′-UTR (Fig. 4A). To verify this prediction, we
cloned the Keap1 3′-UTR into pmirGLO reporter vectors and
co-transfected miR-200a mimics with this vector in 293T cells. The
results showed that miR-200a overexpression significantly decreased
luciferase activity (Fig. 4B). We
also detected the effect of miR-200a on the mutated constructs, in
which miR-200a binding sites were mutated. The results showed that
miR-200a failed to decrease the activity of the luciferase gene
with mutant 3′-UTR (Fig. 4B). We
further detected the direct effect of miR-200a on the mRNA and
protein expression of Keap1. Both RT-qPCR and western blot analysis
revealed that the transfection of cardiomyocytes with miR-200a
mimics significantly reduced the mRNA and protein expression of
Keap1, whereas the transfection of miR-200a inhibitor significantly
increased the expression of Keap1 (Fig. 4C and D). These findings suggest
that miR-200a inhibited Keap1 expression by directly targeting the
3′-UTR.
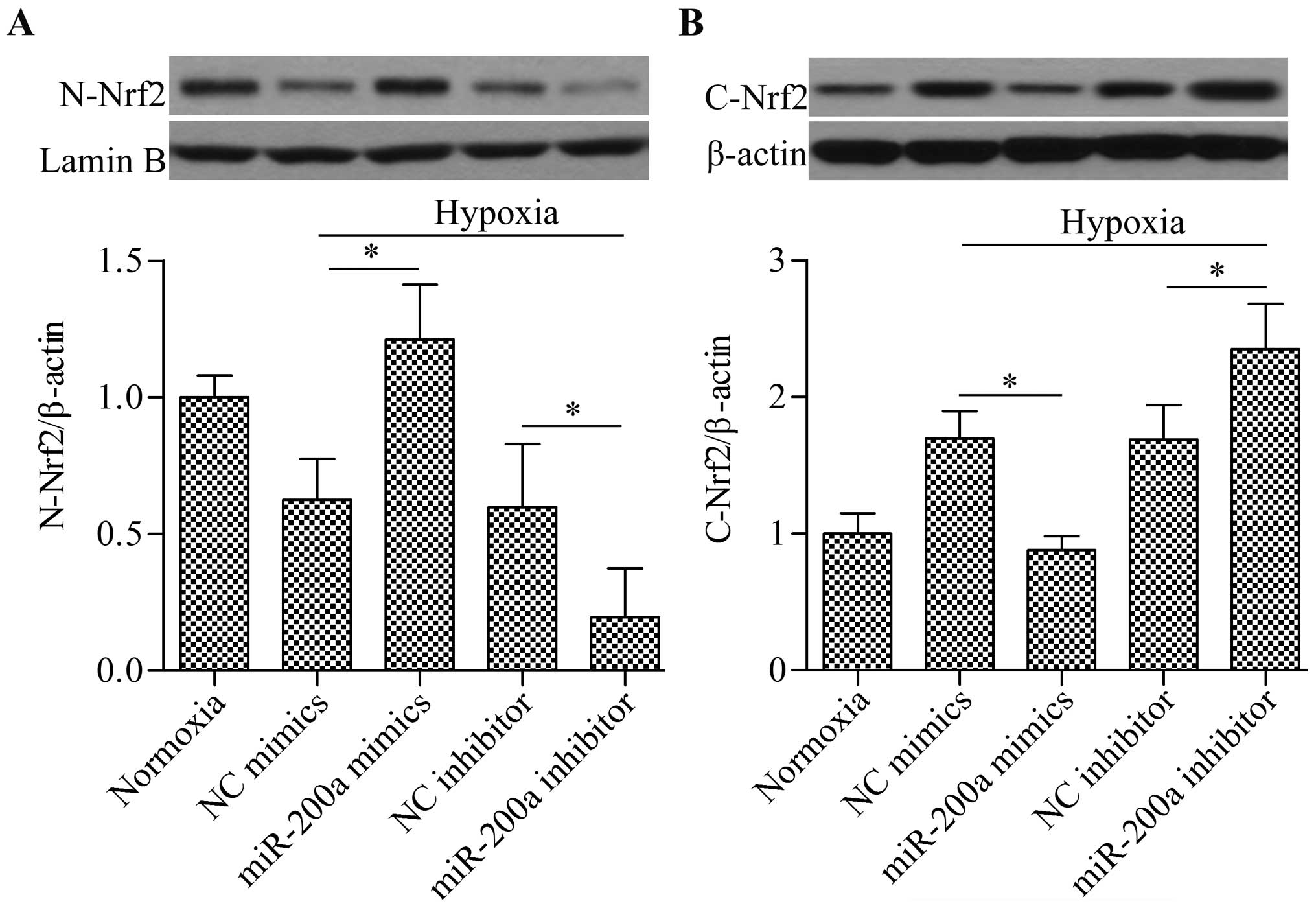
Overexpression of miR-200a upregulates
Nrf2 nuclear translocation
Given the inhibitory effect of miR-200a on Keap1
expression, we speculated that miR-200a may affect Nrf2 nuclear
translocation. To examine this hypothesis, we evaluated nuclear and
cytosolic Nrf2 protein expression by western blot analysis. The
results showed that the overexpression of miR-200a significantly
increased nuclear Nrf2 levels (Fig.
5A), whereas cytosolic Nrf2 levels were decreased (Fig. 5B) in the cells under hypoxic
conditions. Conversely, the transfection of miR-200a inhibitor
showed the opposite effect (Fig.
5). Taken together, these findings suggest that the
overexpression of miR-200a promotes Nrf2 release from Keap1,
leading to the increased accumulation of nuclear Nrf2.
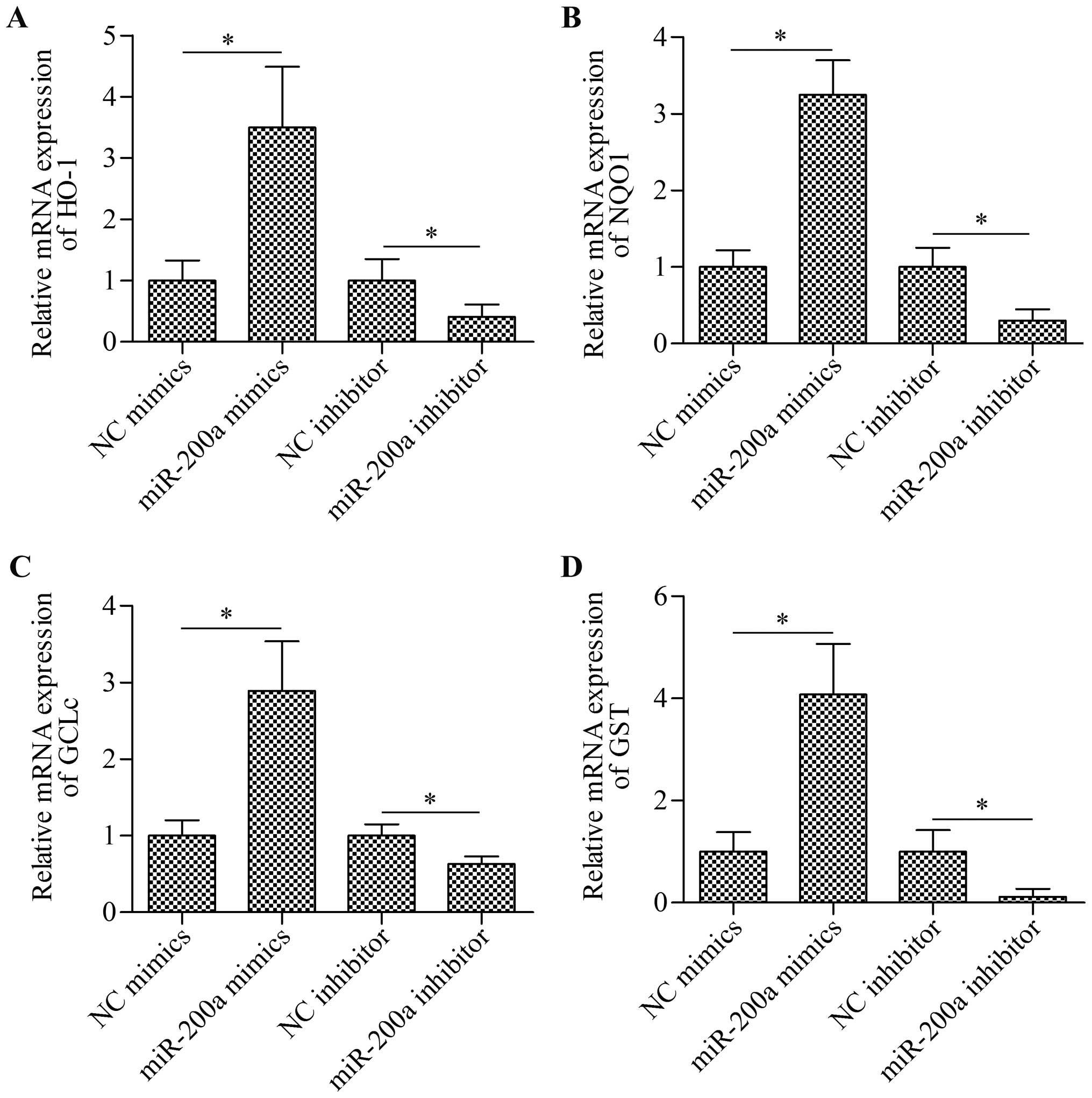
Overexpression of miR-200a increases the
expression of antioxidant genes downstream of the Nrf2 signaling
pathway
To further confirm the effect of miR-200a on the
Nrf2 signaling pathway, we detected the effect of miR-200a on the
expression of antioxidant enzymes, namely HO-1, NQO1, GCLc and GST,
that are transcribed upon Nrf2 activation. The results of RT-qPCR
demonstrated that the transfection of miR-200a mimics significantly
increased the expression of HO-1, NQO1, GCLc and GST, whereas the
transfection of miR-200a inhibitor markedly decreased the
expression of these antioxidant enzymes (Fig. 6). The data indicate that the
overexpression of miR-200a promotes the activation of the Nrf2
signaling pathway.
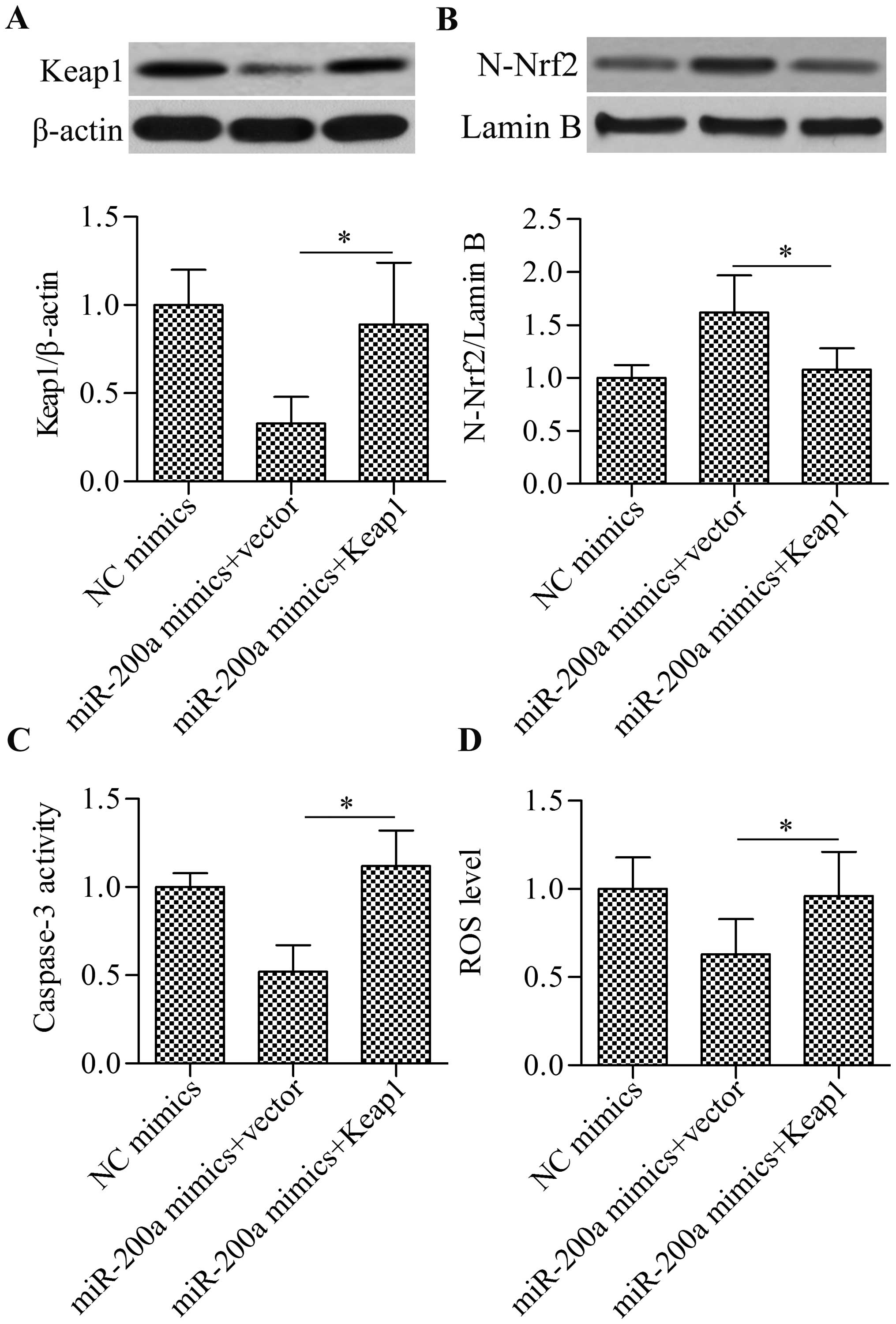
Overexpression of Keap1 reverses the
effect of miR-200a overexpression on Nrf2 signaling pathway
To further verify whether miR-200a regulates the
Nrf2 signaling pathway through Keap1, we performed a rescue
experiment. Cells were co-transfected with miR-200a mimics and
Keap1 overexpressing vectors. Western blot analysis showed that
restoring Keap1 expression (Fig.
7A) significantly blocked the enhancing effect of miR-200a
mimics on nuclear Nrf2 accumulation (Fig. 7B). Furthermore, the restoration of
Keap1 expression blocked the inhibitory effect of miR-200a mimics
on cell apoptosis (Fig. 7C) and
ROS generation (Fig. 7D). Taken
together, these results indicate that miR-200a regulates the Nrf2
signaling pathway, cell apoptosis and ROS levels through Keap1.
Discussion
In recent years, finding ways of overcoming the
hypoxia-induced apoptosis of cardiomyocytes has remained the
principal challenge in the treatment of ischemic heart disease. In
this study, we demonstrated that miR-200a plays an important role
in regulating cardiomyocyte survival under hypoxic conditions. We
delineated that miR-200a directly targeted and inhibited Keap1, the
native regulator of Nrf2. Therefore, inhibiting the expression of
Keap1 with miR-200a released Nrf2, leading to the increased nuclear
translocation of Nrf2 and subsequent activation of antioxidant
genes. During ischemia or hypoxia, oxidative phosphorylation in
mitochondria is severely impaired, leading to excessive ROS
production and oxidative stress. Moreover, prolonged oxidative
stress decreases the antioxidant defense ability of cells (16,17). Therefore, enhancing the
Nrf2-mediated transcription of antioxidant genes in order to induce
the antioxidant defense abilities of cardiomyocytes is a feasible
option. Considering the regulatory effect of miR-200a on the
Keap1-Nrf2 signaling axis, miR-200a may serve as a critical
molecular target for the treatment of ischemic heart disease.
Nrf2 is the master regulator of cellular adaptation
to oxidative stress that regulates a series of antioxidant enzymes
(18,19). The overexpression of Nrf2 has been
demonstrated to protect against oxidative stress and cell apoptosis
following ischemia and reperfusion injury (20,21). Increasing evidence indicates that
Nrf2 is the target of numerous antioxidant reagents through which
antioxidant reagents protect cells against oxidative stress and
cell apoptosis induced by external insults (22–25). The Nrf2 signaling pathway plays an
important role in cardiovascular disease (8). Acute exercise stress may promote
antioxidant mechanisms in the myocardium through the activation of
Nrf2 signaling (26). The
apoptosis of cardiomyocytes induced by exposure to high glucose
levels is inhibited by diallyl trisulfide through Nrf2 signaling
(27). The activation of Nrf2
signaling may effectively attenuate the apoptosis of cardiomyocytes
under ischemic or hypoxic conditions (28–31). The Keap1 protein is the native
regulator of Nrf2 that blocks Nrf2 nuclear import and Nrf2
signaling activation. Thus, the inhibition of Keap1 increases Nrf2
activity (32,33). In this study, we have shown that
the inhibition of Keap1 by miR-200a significantly increased the
nuclear translocation of Nrf2 and subsequent downstream gene
transcription, thus leading to decreased ROS production and cell
apoptosis induced by hypoxia in cardiomyocytes. These findings
further support the important role of Nrf2 signaling in protecting
cardiomyocytes against hypoxia.
The miRNAs represent novel tools which are capable
of interrupting the Keap1-Nrf2 interaction. miR-141 is reported to
target Keap1 and regulate Nrf2 signaling in renal oxidative stress
induced by hyperglycemia (34).
Similarly, Shi et al reported that miR-141 targeted Keap1 to
regulate Nrf2 signaling thereby conferring drug resistance to
5-fluorouracil on hepatocellular carcinoma cells (35). The downregulation of miR-29
promotes Keap1 expression that reduces Nrf2, leading to high
glucose-induced apoptosis (36).
miR-200a is also reported to target Keap1 and regulate the
Keap1-Nrf2 signaling pathway in breast cancer cells (37) and in hepatic stellate cells
(38). However, whether specific
miRNAs target Keap1-Nrf2 in cardiomyocytes has not been well
studied. Herein, for the first time to the best of our knowledge,
we demonstrated that Keap1 was targeted and regulated by miR-200a
in cardiomyocytes using a luciferase reporter assay, RT-qPCR, and
western blot analysis. The overexpression of miR-200a was shown to
activate Nrf2 signaling through inhibiting Keap1, whereas the
restoration of Keap1 expression significantly abrogated the effect
of miR-200a overexpression on Nrf2 activation-mediated cell
protection against hypoxia. Various studies also showed that Nrf2
may be targeted and regulated by specific miRNAs. Higher
erythrocytic miR-144 expression results in decreased Nrf2
expression through targeting the 3′-UTR of Nrf2, leading to
impaired oxidative stress tolerance in erythrocytes (39). Furthermore, miR-28 and miR-93 are
reported to interact with the 3′-UTR of Nrf2 (40,41). These findings support that miRNAs
are promising, novel tools for modulating the Keap1-Nrf2 signaling
pathway.
In the present study, we have demonstrated that
miR-200a is significantly downregulated in ischemic myocardial
tissues and hypoxic cardiomyocytes. Hypoxic/ischemic stress has
been suggested to contribute to the major elements for epigenetic
programming such as DNA methylation (42). Hypermethylation of the promoter
miR-200a has been observed in cancer cells grown under hypoxic
conditions accompanied by a significant decrease in miR-200a
expression (43). These findings
raise the possibility that the promoter of miR-200a may be
hypermethylated by hypoxic/ischemic stress which results in the
decreased expression of miR-200a in ischemic myocardial tissues and
hypoxic cardiomyocytes. Mounting evidence indicates that the
overexpression of miR-200a promotes the pro-survival signaling
pathways and dampens the pro-apoptotic genes (44,45). Notably, the high expression of
miR-200a is reportedly associated with extended survival of
cardiomyocytes, implying that miR-200a is beneficial for
cardiomyocyte survival (46). We
found that the overexpression of miR-200a protected cardiomyocytes
against hypoxia-induced oxidative stress and apoptosis. The
advantageous effect of miR-200a was due to its inhibitory effect on
Keap1 through directly targeting the 3′-UTR of Keap1, leading to
the activation of the Nrf2 signaling pathway. Taken together, these
findings suggest that the overexpression of miR-200a protects
cardiomyocytes against hypoxia-induced apoptosis by modulating the
Keap1-Nrf2 signaling pathway, and this representing a novel
strategy for the treatment of ischemic heart disease.
Abbreviations:
|
Keap1
|
kelch-like ECH-associated protein
1
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
miRNAs
|
microRNAs
|
|
ROS
|
reactive oxygen species
|
|
UTR
|
untranslated region
|
|
HO-1
|
heme oxygenase 1
|
|
GST
|
glutathione S-transferase
|
|
GCLc
|
glutamate-cysteine ligase catalytic
subunit
|
|
NQO1
|
NADPH-quinone oxidoreductase 1
|
References
|
1
|
Hausenloy DJ, Boston-Griffiths E and
Yellon DM: Cardioprotection during cardiac surgery. Cardiovasc Res.
94:253–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scherz-Shouval R and Elazar Z: ROS,
mitochondria and the regulation of autophagy. Trends Cell Biol.
17:422–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glinka YY and Youdim MB: Inhibition of
mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J
Pharmacol. 292:329–332. 1995.PubMed/NCBI
|
|
5
|
Wasserman WW and Fahl WE: Functional
antioxidant responsive elements. Proc Natl Acad Sci USA.
94:5361–5366. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann GE, Bonacasa B, Ishii T and Siow RC:
Targeting the redox sensitive Nrf2-Keap1 defense pathway in
cardiovascular disease: protection afforded by dietary isoflavones.
Curr Opin Pharmacol. 9:139–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boon RA and Dimmeler S: MicroRNAs in
myocardial infarction. Nat Rev Cardiol. 12:135–142. 2015.
View Article : Google Scholar
|
|
13
|
Guo Y, Yu S, Zhang C and Kong AN:
Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med.
88:337–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Quirantes R, Segura-Carretero A, Micol V,
Joven J, Bosch-Barrera J, Del Barco S, Martin-Castillo B, et al:
Metformin lowers the threshold for stress-induced senescence: a
role for the microRNA-200 family and miR-205. Cell Cycle.
11:1235–1246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mateescu B, Batista L, Cardon M, Gruosso
T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P,
Sastre-Garau X and Mechta-Grigoriou F: miR-141 and miR-200a act on
ovarian tumorigenesis by controlling oxidative stress response. Nat
Med. 17:1627–1635. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al Ghouleh I, Khoo NK, Knaus UG,
Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM,
Kelley EE, Bauer PM, et al: Oxidases and peroxidases in
cardiovascular and lung disease: new concepts in reactive oxygen
species signaling. Free Radic Biol Med. 51:1271–1288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becker LB: New concepts in reactive oxygen
species and cardiovascular reperfusion physiology. Cardiovasc Res.
61:461–470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niture SK, Khatri R and Jaiswal AK:
Regulation of Nrf2 - an update. Free Radic Biol Med. 66:36–44.
2014. View Article : Google Scholar
|
|
20
|
Lee LY, Harberg C, Matkowskyj KA, Cook S,
Roenneburg D, Werner S, Johnson J and Foley DP: Overactivation of
the nuclear factor (erythroid-derived 2)-like 2-antioxidant
response element pathway in hepatocytes decreases hepatic
ischemia/reperfusion injury in mice. Liver Transpl. 22:91–102.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammadzadeh M, Halabian R, Gharehbaghian
A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM and Roudkenar
MH: Nrf-2 overexpression in mesenchymal stem cells reduces
oxidative stress-induced apoptosis and cytotoxicity. Cell Stress
Chaperones. 17:553–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito Y, Tsuruma K, Ichihara K, Shimazawa
M and Hara H: Brazilian green propolis water extract up-regulates
the early expression level of HO-1 and accelerates Nrf2 after UVA
irradiation. BMC Complement Altern Med. 15:4212015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv H, Ren H, Wang L, Chen W, Ci X and Lico
A: Lico A enhances Nrf2-mediated defense mechanisms against
t-BHP-induced oxidative stress and cell death via Akt and ERK
activation in RAW 264.7 cells. Oxid Med Cell Longev.
2015:7098452015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang K, Jiang Y, Wang W, Ma J and Chen M:
Escin activates AKT-Nrf2 signaling to protect retinal pigment
epithelium cells from oxidative stress. Biochem Biophys Res Commun.
468:541–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muthusamy VR, Kannan S, Sadhaasivam K,
Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L and
Rajasekaran NS: Acute exercise stress activates Nrf2/ARE signaling
and promotes antioxidant mechanisms in the myocardium. Free Radic
Biol Med. 52:366–376. 2012. View Article : Google Scholar
|
|
27
|
Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH,
Liang HY and Kuo WW: Antioxidant effects of diallyl trisulfide on
high glucose-induced apoptosis are mediated by the
PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J
Cardiol. 168:1286–1297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Sano M, Shinmura K, Tamaki K,
Katsumata Y, Matsuhashi T, Morizane S, Ito H, Hishiki T, Endo J, et
al: 4-hydroxy-2-nonenal protects against cardiac
ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol
Cell Cardiol. 49:576–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng C, Sun Z, Tong G, Yi W, Ma L, Zhao B,
Cheng L, Zhang J, Cao F and Yi D: α-Lipoic acid reduces infarct
size and preserves cardiac function in rat myocardial
ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2
pathway. PLoS One. 8:e583712013. View Article : Google Scholar
|
|
30
|
Katsumata Y, Shinmura K, Sugiura Y,
Tohyama S, Matsuhashi T, Ito H, Yan X, Ito K, Yuasa S, Ieda M, et
al: Endogenous prostaglandin D2 and its metabolites protect the
heart against ischemia-reperfusion injury by activating Nrf2.
Hypertension. 63:80–87. 2014. View Article : Google Scholar
|
|
31
|
Chen XQ, Wu SH, Zhou Y and Tang YR:
Lipoxin A4-induced heme oxygenase-1 protects cardiomyocytes against
hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE
complex. PLoS One. 8:e671202013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wells G: Peptide and small molecule
inhibitors of the Keap1-Nrf2 protein-protein interaction. Biochem
Soc Trans. 43:674–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abed DA, Goldstein M, Albanyan H, Jin H
and Hu L: Discovery of direct inhibitors of Keap1-Nrf2
protein-protein interaction as potential therapeutic and preventive
agents. Acta Pharm Sin B. 5:285–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song
D, Dai Y, Wang T, Qiu L, Wen L, et al: Aldose reductase regulates
miR-200a-3p/141-3p to coordinate Keap1-Nrf2, Tgfβ1/2, and Zeb1/2
signaling in renal mesangial cells and the renal cortex of diabetic
mice. Free Radic Biol Med. 67:91–102. 2014. View Article : Google Scholar
|
|
35
|
Shi L, Wu L, Chen Z, Yang J, Chen X, Yu F,
Zheng F and Lin X: MiR-141 activates Nrf2-dependent antioxidant
pathway via down-regulating the expression of Keap1 conferring the
resistance of hepatocellular carcinoma cells to 5-fluorouracil.
Cell Physiol Biochem. 35:2333–2348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin
X and Wang MJ: High glucose induces renal tubular epithelial injury
via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med.
13:3522015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eades G, Yang M, Yao Y, Zhang Y and Zhou
Q: miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in
breast cancer cells. J Biol Chem. 286:40725–40733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng
ZY and Li J: MicroRNA-200a controls Nrf2 activation by target Keap1
in hepatic stellate cell proliferation and fibrosis. Cell Signal.
26:2381–2389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sangokoya C, Telen MJ and Chi JT: microRNA
miR-144 modulates oxidative stress tolerance and associates with
anemia severity in sickle cell disease. Blood. 116:4338–4348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang M, Yao Y, Eades G, Zhang Y and Zhou
Q: MiR-28 regulates Nrf2 expression through a Keap1-independent
mechanism. Breast Cancer Res Treat. 129:983–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh B, Ronghe AM, Chatterjee A, Bhat NK
and Bhat HK: MicroRNA-93 regulates NRF2 expression and is
associated with breast carcinogenesis. Carcinogenesis.
34:1165–1172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma Q and Zhang L: Epigenetic programming
of hypoxic-ischemic encephalopathy in response to fetal hypoxia.
Prog Neurobiol. 124:28–48. 2015. View Article : Google Scholar
|
|
43
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar
|
|
44
|
Santra M, Chopp M, Santra S, Nallani A,
Vyas S, Zhang ZG and Morris DC: Thymosin beta 4 up-regulates
miR-200a expression and induces differentiation and survival of rat
brain progenitor cells. J Neurochem. 136:118–132. 2016. View Article : Google Scholar
|
|
45
|
Li R, He JL, Chen XM, Long CL, Yang DH,
Ding YB, Qi HB and Liu XQ: MiR-200a is involved in proliferation
and apoptosis in the human endometrial adenocarcinoma cell line
HEC-1B by targeting the tumor suppressor PTEN. Mol Biol Rep.
41:1977–1984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ahmed RP, Haider HK, Buccini S, Li L,
Jiang S and Ashraf M: Reprogramming of skeletal myoblasts for
induction of pluripotency for tumor-free cardiomyogenesis in the
infarcted heart. Circ Res. 109:60–70. 2011. View Article : Google Scholar : PubMed/NCBI
|