Introduction
Pain is defined as an unpleasant sensory and
emotional experience associated with actual or potential tissue
damage (1). Our understanding of
pain has developed and evolved over recent years owing to the
identification and investigation of various molecules and pathways
including bradykinin, cyclooxygenases (COXs), nerve growth factor
(NGF), interleukins (ILs), and other channels associated with pain
(2–7). Owing to the improved knowledge of
pain signaling mechanisms, novel therapeutic targets for the
treatment of chronic pain have emerged. Nevertheless, effective
therapies designed to prevent or treat both acute and chronic pain
are limited in number.
Inflammation has been reported as a common symptom
of acute and chronic pain as it is believed that pain is initiated
by local inflammatory events (8,9).
Inflammatory pain is typically treated with opioids or nonsteroidal
anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen,
which act as COX inhibitors (10,11). However, side effects limit the use
of these medications in both short- and long-term settings
(12). Thus, the treatment of
inflammatory pain is challenging, and effective, novel medications
with fewer side effects are needed.
Many animal models and methods have been developed
to measure pain intensity for different types of pain, including
the hot plate test, the formalin test, acetic acid-induced
writhing, the tail flick test and the von Frey hair test. These are
reflex response-based tests which involve the application of
physical or chemical stimuli to the abdomen, paw, or tail. Cho
et al (13) devised a
voluntary movement test to measure inflammatory- and neuropathic
pain-related behavior in animals without the use of stimuli. In the
present study, we administered lipopolysaccharide (LPS) to mice to
induce systemic inflammatory pain and thereby established a mouse
model of LPS-induced pain; we then evaluated their voluntary
movements and performed biochemical analysis.
LPS is a major, bacterial Toll-like receptor 4
(TLR4) ligand that activates the innate immune response to
infection. The administration of LPS not only induces
pro-inflammatory cytokine production but also inhibits neurotrophic
factor production (14). The
response to systemic inflammatory pain induced by LPS
administration involves the release of numerous pro-inflammatory
cytokines such as IL-1β and IL-6.
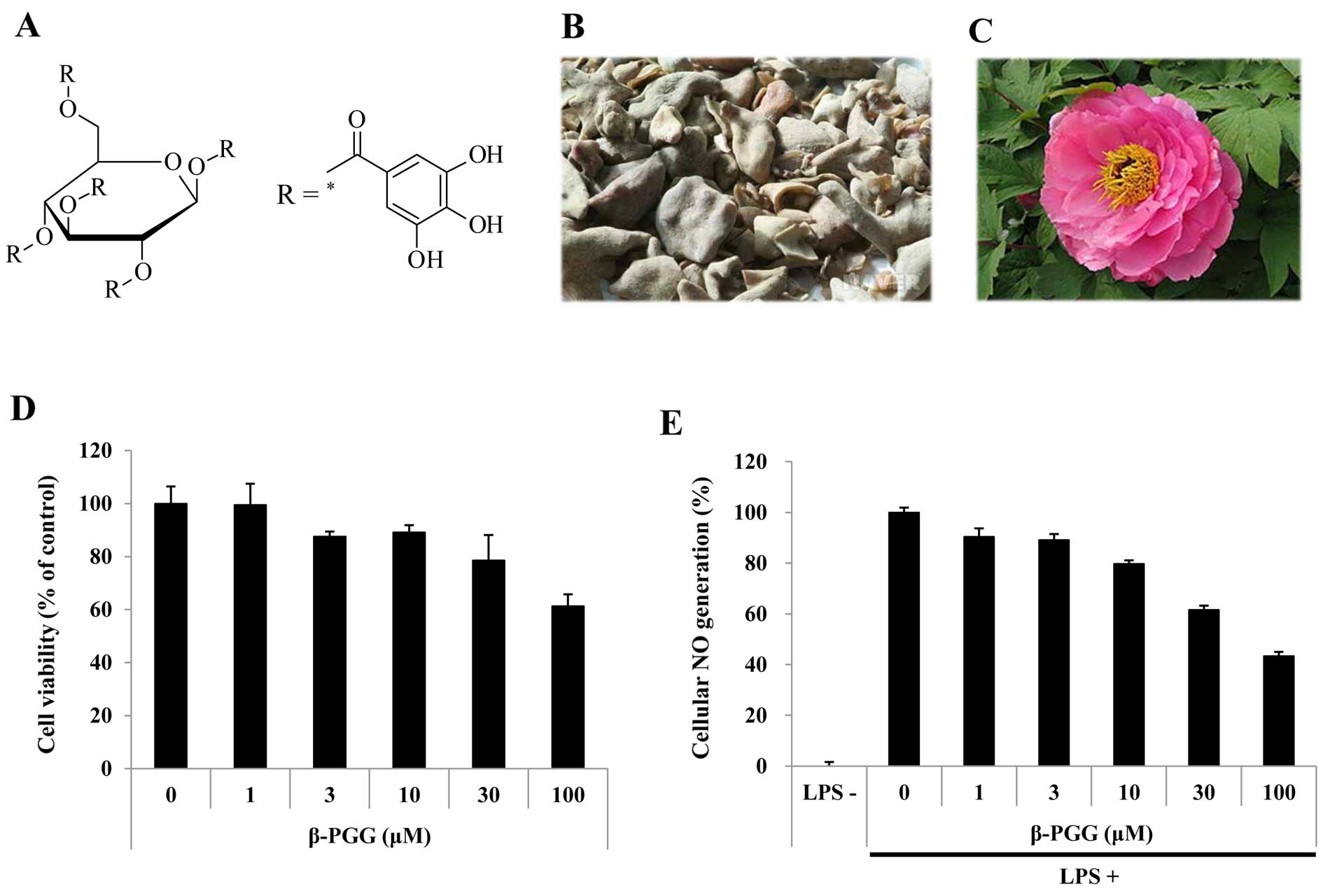
1,2,3,4,6-Penta-O-galloyl-β-D-glucose (β-PGG), a prototypical
gallotannin (Fig. 1A), is found
in medicinal herbs such as Rhus chinensis Mill. (Fig. 1B) and Paeonia suffruticosa
(Fig. 1C) (15). β-PGG has been demonstrated to
exhibit antioxidant, anti-diabetic and anticancer effects (16–18). However, to the best of our
knowledge, the analgesic effects of β-PGG in an animal model of
LPS-induced pain have not been reported to date.
In this study, we evaluated the analgesic effects of
β-PGG on LPS-exposed RAW 264.7 cells and in an animal model of
LPS-induced pain in order to determine whether β-PGG affects
cellular nitric oxide (NO) generation, the voluntary movements of
animals experiencing inflammatory pain and the mRNA expression of
inflammation-related cytokines, each of which is associated with
pain symptoms.
Materials and methods
Chemicals, reagents and cells
All chemicals, solvents, reagents and standards used
in the experiments were purchased from Sigma Chemical Co. (St.
Louis, MO, USA). All solutions were freshly prepared with distilled
water. RAW 264.7 cells were purchased from the American Type
Culture Collection (no. TIB-71™; ATCC, Manassas, VA, USA).
Cell viability
Cell viability was assessed using the MTT assay as
previously described (19).
Briefly, RAW 264.7 cells were seeded in a 96-well flat-bottom
microplate at a density of 2×104 cells/well and
incubated at 37°C for 1 h. The cells were then treated with various
concentrations of β-PGG (1–100 µM). After an additional 24 h
incubation, 20 µl MTT [5 mg/ml in phosphate-buffered saline
(PBS)] solution was added to each well, and the plate was incubated
for a further 2 h. The absorbance was measured at 450 nm using a
microplate reader (Victor3; PerkinElmer, Inc., Waltham, MA,
USA).
Measurement of cellular NO
generation
The concentration of NO in the medium was measured
by Griess reagent as an indicator of NO production. Briefly, RAW
264.7 cells were seeded in a 96-well flat-bottom microplate at a
density of 2×104/well and incubated at 37°C for 1 h. The
cells were then exposed to LPS at 1 µg/ml and treated with
various concentrations of β-PGG (1–100 µM). After an
additional 24 h incubation, NO concentrations in the supernatants
were measured by adding Griess reagent (20). The absorbance of the mixtures was
determined using a microplate reader at a wavelength of 540 nm.
Animals and care
Male BALB/c mice aged 6 weeks and weighing 18–23 g
(Samtako, Osan, Korea) were used in this study. The animals were
housed in an air-conditioned room at a temperature of 22±1°C and a
humidity of 55±1% on a 12 h light/dark cycle. They were fed a
standard commercial rodent pellet diet (Samtako) and water was
provided ad libitum. The animal experiments complied with
the in-house guidelines of Kyungpook National University Animal
Care and Use Committee, and protocols (approval no. #KNU 2014-148)
were in accordance with the guidelines of the Committee on Research
and Ethical Issues of the International Association for the Study
of Pain (21). All animals were
permitted to adapt to the laboratory environment for at least 1
week prior to performing the experiments.
Behavioral test to examine the effect of
pain on voluntary movements
Behavior was examined using a slightly modified
method of Cobos et al (22). Briefly, a total of 35 animals were
acclimated to the laboratory environment for at least 1 week prior
to initiating the experiments and were randomized into 7 groups,
each containing 5 animals: two saline-treated control groups
(normal and negative); four positive control groups (ibuprofen,
gabapentin, capsaicin, and ascorbic acid); and one treatment group
(β-PGG). Animals received intraperitoneal injections of the samples
daily for 3 days and the LPS (1 mg/kg) challenge was performed on
the last day. Thirty minutes after the administration of LPS, the
mice were subjected to behavioral tests for 60 min by placing each
animal in a plastic cage (26×15 cm). We recorded the voluntary
movement distances and cumulative voluntary movement distances
covered by the animals at 10 min intervals over a 60 min period
using Kinovea (http://www.kinovea.org/), an open-source motion
tracking software program. At 120 min after LPS administration, the
animals were euthanized by carbon dioxide inhalation. Brain tissues
were removed and frozen for subsequent analyses.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the brain tissues using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's instructions (23). The RNA (1–10 µg) was
reverse transcribed into first-strand cDNA using an RT-&GO
Master Mix (MP Biomedicals, Santa Ana, CA, USA), and the product
was used as the PCR template. RT-PCR was performed using a Takara
PCR Thermal Cycler (Takara Bio, Otsu, Japan) and the following
oligonucleotides primers were used: mouse IL-1β forward,
5′-AGAGCCCATCCTCTGTGACT-3′ and reverse, 5′-CTCTGCTTGTGAGGTGCTGA-3′;
mouse IL-6 forward, 5′-CACTTCACAAGTCGGAGGCT-3′ and reverse,
5′-GCCACTCCTTCTGTGACTCC-3′. Mouse glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as aninternal control (for ward,
5′-GGCAAATTCAACGGCACAGT-3′ and reverse,
5′-CTCGTGGTTCACACCCATCA-3′). Genes for IL-1β, IL-6 and GAPDH were
amplified with a denaturation step at 94°C for 20 sec, an annealing
step at 58°C for 40 sec, and an extension step at 72°C for 30 sec.
The PCR products were electrophoresed at 100 V for 40 min on a 2%
agarose gel in TBE buffer. The mRNA levels were normalized to the
housekeeping gene, GAPDH.
Statistical analysis
The experiments were performed in triplicate and the
results are expressed as the means ± standard deviation (SD).
Statistical significance was determined by one-way analysis of
variance (ANOVA), using the program IBM SPSS statistics (23). When the data from the ANOVA were
significant, the differences were analyzed by Tukey's HSD post hoc
test or Duncan's test. The critical level for statistical
significance was defined as P<0.05 or P<0.01.
Results
Cell viability and antioxidant potential
of β-PGG
The effects of various doses (1–100 µM) of
β-PGG on the viability of RAW 264.7 cells were evaluated using an
MTT assay in the present study. The results showed that the cell
viability at various doses was 99.55±7.93%, 87.55±1.87%,
89.11±2.76%, 78.61±9.48%, and 61.31±4.51%, respectively. These
findings indicated that there were no cytotoxic effects at doses up
to 30 µM (Fig. 1D).
Inhibitory effect of β-PGG on cellular NO
generation
We then examined whether β-PGG had the potential to
suppress NO generation in stressed RAW 264.7 cells. The amount of
NO accumulated was used as an indicator of NO generation in the
medium. Treatment with LPS (1 µg/ml) increased NO
accumulation (114.80%) compared with that observed in the untreated
control cells. Treatment with β-PGG at concentrations of 1, 3, 10,
and 30 µM, decreased NO release by 9.62±3.29%, 10.86±2.36%,
20.25±1.38%, and 38.38±1.65%, respectively, compared with the
stressed cells (Fig. 1E). As
β-PGG did not induce cytotoxicity at these concentrations, the
inhibition of NO generation may not be attributed to cytotoxicity.
NO is a member of the reactive nitrogen species (RNS) family and
the interaction of NO with reactive oxygen species (ROS) produces
several types of RNS including NO, nitrogen dioxide, and
peroxynitrite, which cause nitrosative stress and tissue damage at
the cellular level (24). Thus,
β-PGG induced a decrease in NO generation which indicates that
β-PGG possesses anti-inflammatory potential.
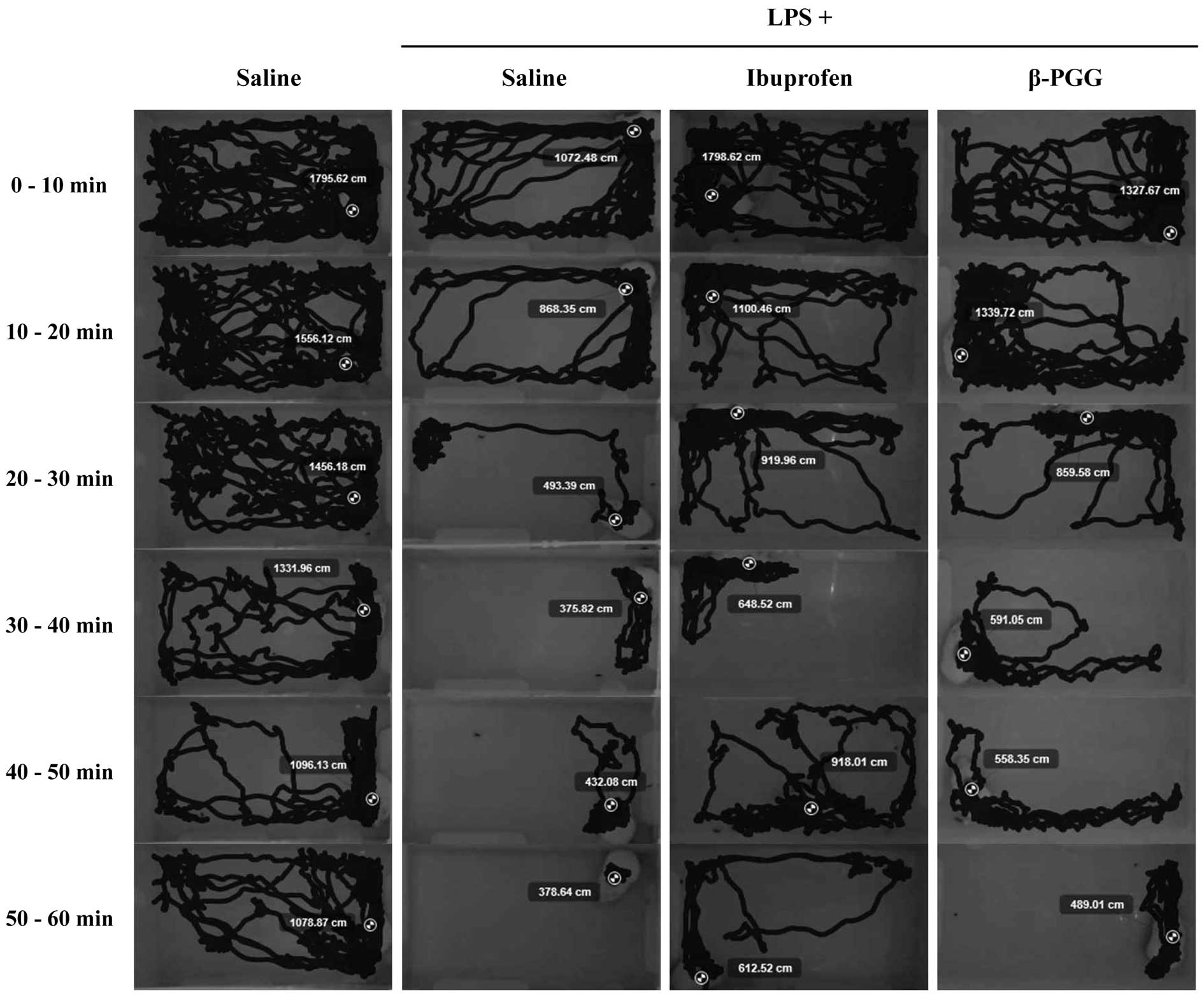
β-PGG recovers voluntary movements in an
animal model of LPS-induced pain
To determine the effect of pain on voluntary
movements, each animal was placed in a plastic cage (26×15 cm) and
the voluntary movements of the mice were subsequently recorded.
When the saline-treated animals were placed in the plastic cage,
they moved in a normal manner. The total distance traveled for 1 h
was analyzed at 10 min intervals using Kinovea (http://www.kinovea.org/), an open-source motion
tracking software program.
Fig. 2 shows
representative motion tracking results of the saline-, LPS-,
ibuprofen- and β-PGG-treated animals free to travel the plastic
cage. We observed a marked difference in total voluntary movements
between LPS- and ibuprofen-treated animals. We then evaluated
whether ibuprofen (an NSAID), gabapentin (an NSAID), capsaicin [a
ligand of transient receptor potential cation channel subfamily V
member 1 (TRPV1)] and ascorbic acid (an antioxidant) could recover
the voluntary movements. Ibuprofen (30 mg/kg), gabapentin (100
mg/kg), capsaicin (10 mg/kg), ascorbic acid (100 mg/kg) and β-PGG
(10 mg/kg) were injected intraperitoneally to LPS-exposed animals
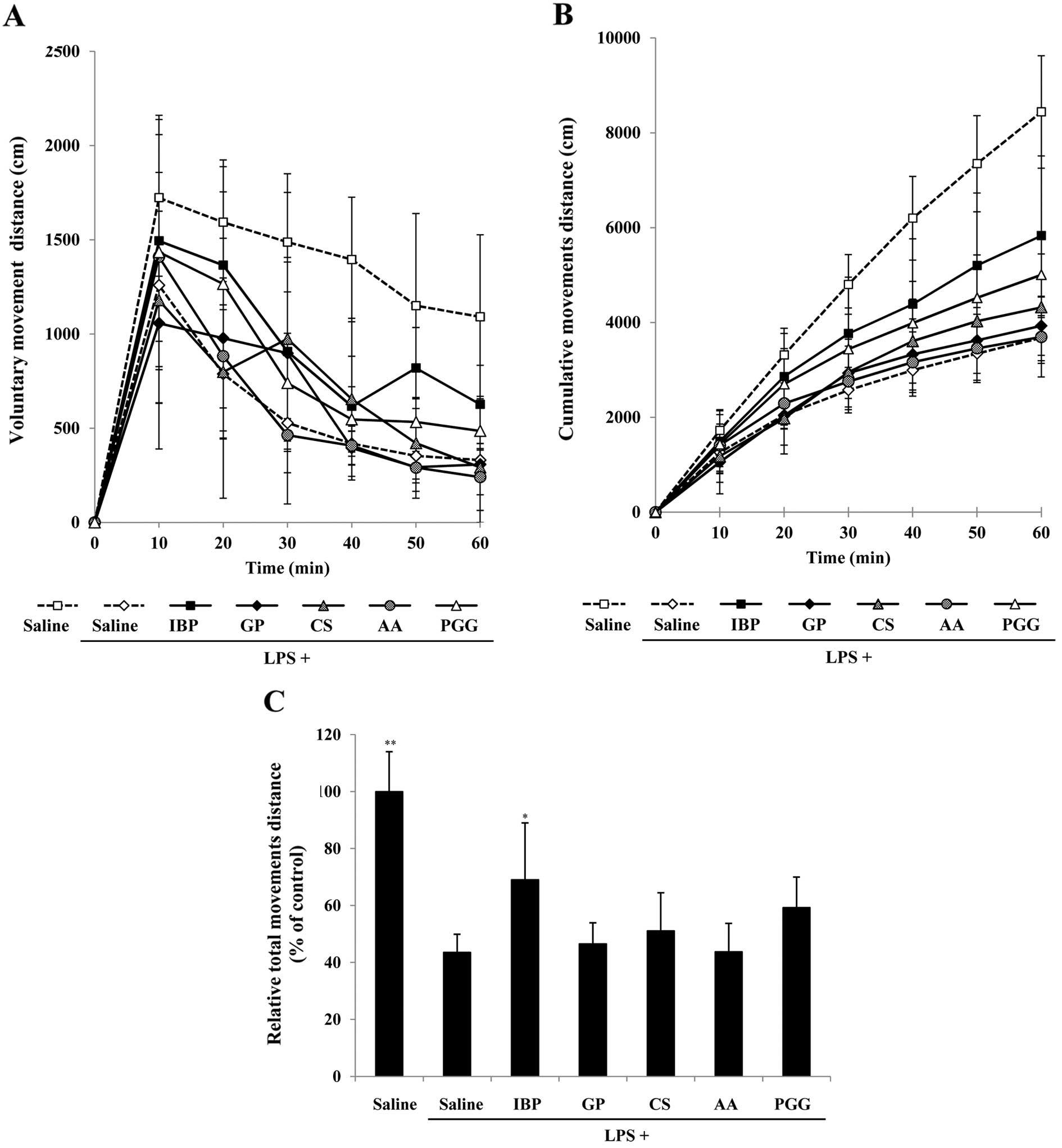
30 min before measuring the travelled distance. As shown in
Fig. 3A, the distances travelled
by LPS-exposed animals measured at 10-min intervals (0–10 min,
1259.20±392.77 cm; 10–20 min, 787.75±341.62 cm; 20–30 min,
528.04±202.52 cm; 30–40 min, 418.44±67.55 cm; 40–50 min,
353.12±62.43 cm; 50–60 min, 330.84±61.09 cm) significantly
decreased after 20 min, compared with those of the animals treated
with saline (0–10 min, 1722.91±415.83 cm; 10–20 min, 1592.70±295.27
cm; 20–30 min, 1487.65±263.81 cm; 30–40 min, 1395.34±330.80 cm;
40–50 min, 1150.06±489.36 cm; 50–60 min, 1091.26±434.85 cm),
ibuprofen (0–10 min, 1494.47±666.93 cm; 10–20 min, 1365.26±388.63
cm; 20–30 min, 907.40±475.31 cm; 30–40 min, 616.64±265.66 cm; 40–50
min, 819.51±215.12 cm; 50–60 min, 626.84±207.32 cm) and β-PGG (0–10
min, 1434.79±623.69 cm; 10–20 min, 1265.63±658.47 cm; 20–30 min,
739.82±263.73 cm; 30–40 min, 546.70±173.29 cm; 40–50 min,
533.26±122.90 cm; 50–60 min, 486.00±183.54 cm), indicating that
LPS-induced pain effectively slowed motion, whereas β-PGG recovered
37.87% of voluntary movement (ibuprofen recovered 50.7%).
Analysis of total distance travelled in
an animal model of LPS-induced pain
Measuring the cumulative distance travelled by
animals for 60 min (Fig. 3B)
showed that distance travelled by the animals treated with saline
only (10 min, 1722.91±415.83 cm; 20 min, 3315.60±452.01 cm; 30 min,
4803.25±630.14 cm; 40 min, 6198.60±881.01 cm; 50 min,
7348.66±1014.98 cm; 60 min, 8439.91±1184.55 cm), as well as
LPS-exposed animals treated with ibuprofen (10 min, 1494.47±666.93
cm; 20 min, 2859.73±1020.23 cm; 30 min, 3767.13±1189.96 cm; 40 min,
4383.77±1382.03 cm; 50 min, 5203.29±1525.56 cm; 60 min,
5830.13±1683.06 cm) and β-PGG (10 min, 1434.79±623.69 cm; 20 min,
2700.42±748.07 cm; 30 min, 3440.24±867.53 cm; 40 min,
3986.93±879.61 cm; 50 min, 4520.19±905.16 cm; 60 min,
5006.19±902.04 cm) increased steadily compared with the cumulative
distances covered by LPS-exposed animals treated with saline (10
min, 1259.20±392.77 cm; 20 min, 2046.95±291.00 cm; 30 min,
2574.99±479.84 cm; 40 min, 2993.43±543.33 cm; 50 min,
3346.55±560.98 cm; 60 min, 3677.39±537.28 cm), gabapentin (10 min,
1057.10±667.47 cm; 20 min, 2034.88±619.42 cm; 30 min,
2933.02±714.53 cm; 40 min, 3328.61±749.11 cm; 50 min,
3622.07±692.24 cm; 60 min, 3930.24±622.75 cm), capsaicin (10 min,
1179.05±547.39 cm; 20 min, 1978.11±750.12 cm; 30 min,
2952.47±551.15 cm; 40 min, 3606.89±885.12 cm; 50 min,
4027.77±1097.88 cm; 60 min, 4319.74±1124.37 cm), and ascorbic acid
(10 min, 1409.75±448.57 cm; 20 min, 2292.04±524.36 cm; 30 min,
2755.69±593.79 cm; 40 min, 3163.50±639.97 cm; 50 min,
3454.54±720.15 cm; 60 min, 3695.48±842.12 cm). As shown in Fig. 3C, the treatment of LPS-exposed
animals with ibuprofen (69.08±19.94%) or β-PGG (59.32±10.69%)
recovered the initial decrease in total voluntary movements
compared with the saline plus LPS treatment group (43.57±6.37%) and
saline alone group (100.00±14.04%). By contrast, voluntary movement
in the gabapentin (46.57±7.38%), capsaicin (51.18±13.32%) and
ascorbic acid (43.79±9.98%)-treated groups decreased in a similar
manner to the saline plus LPS treatment group (43.57±6.37%). Thus,
only β-PGG and ibuprofen recovered the reduction in total distance
travelled due to LPS-induced pain.
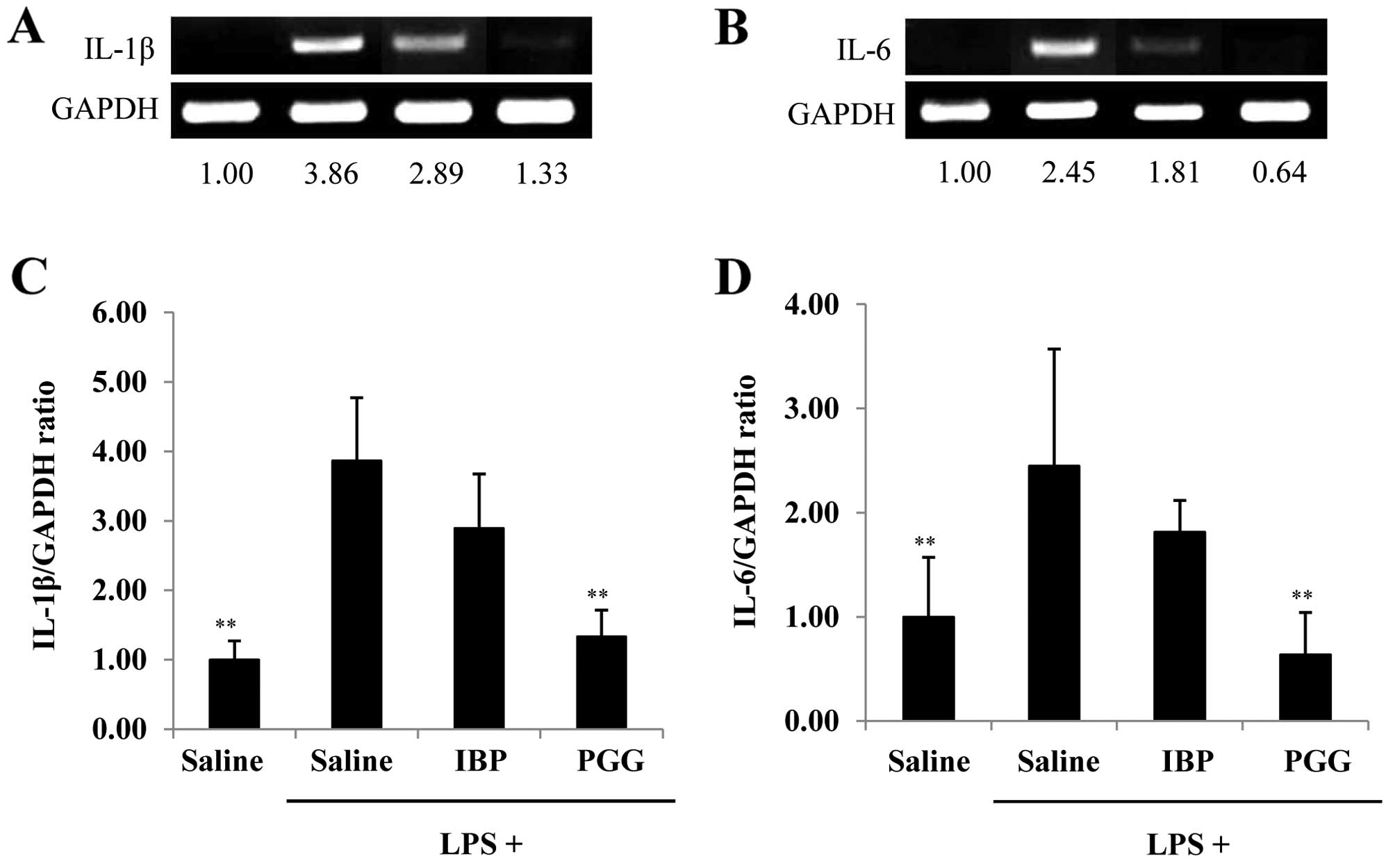
Gene expression of IL-1β and IL-6 by
β-PGG
We also examined whether the mRNA expression of
IL-1β and IL-6 in brain tissues from LPS-exposed mice was altered
by treatment with β-PGG or ibuprofen. We isolated brain tissues
from mice treated with β-PGG or ibuprofen, and then compared the
gene expression profiles to those of the control using RT-PCR. As
shown in Fig. 4A and B, LPS
exposure (1 mg/kg) increased the mRNA expression of IL-1 and IL-6.
β-PGG treatment significantly decreased the mRNA levels of IL-1β
(1.33±0.38-fold) and IL-6 (0.64±0.40-fold) compared with those in
the the LPS plus saline group (3.86±0.91 and 2.45±1.12-fold) and
the ibuprofen (2.89±0.78 and 1.81±0.30-fold) group,
respectively.
Discussion
Previous studies have investigated the antioxidant,
anti-diabetic and anti-inflammatory effects as well as the
anticancer potential of β-PGG (25–29). However, to date, no studies have
evaluated the effects of β-PGG in a mouse model LPS-induced pain,
to the best of our knowledge. The results of the present study
suggest that β-PGG possesses analgesic potential comparable to
other known analgesic compounds, namely ibuprofen, gabapentin,
capsaicin and ascorbic acid.
Antioxidants, among other nutritional substances,
aid in the eliminiation of potentially harmful free radicals from
the body. We examined whether β-PGG was capable of eliminating free
radicals in immune cells. We treated RAW 264.7 cells with β-PGG and
using the ORAC assay measured how much β-PGG scavenges free
radicals (data not shown). The ORAC assay utilizes an AAPH-derived
peroxyl radical that mimics the lipid peroxyl radicals involved in
the lipid peroxidation chain reaction in vivo. The
inhibition of peroxyl radical-induced oxidation of a fluorescent
probe, fluorescein, by antioxidants was serially monitored. β-PGG
exhibited potent antioxidant activity, which is possibly conferred
by the hydroxyl groups in its structure. This suggests that β-PGG
has the potential to eliminate such radicals during numerous
antioxidative events and in several antioxidant pathways. Chronic
pain is associated with oxidative stress processes, thus the
presence of free radicals may play a role in the development and
persistence of pain. It follows that antioxidants such as vitamins
C and E, selenium and β-carotene, those present in fruits,
vegetables, green tea, and red wine, may be used as therapy for
acute pain, fibromyalgia, dysmenorrhea, diabetic neuropathy,
osteoarthritis, and recurrent pancreatitis. We therefore
hypothesized that various food ingredient(s) exhibit analgesic
activity. This hypothesis can be proven using an animal model of
LPS-induced pain.
Pain has long been defined as an unpleasant sensory
and emotional experience which is associated with actual or
potential tissue damage as well as with major stresses on both the
mind and the body. Generally, pain intensity has been measured with
one of several different tests: the hot plate test, the formalin
test, acetic acid-induced writhing, the tail flick test, or the von
Frey hair test, among others. We selected a model of LPS-induced
pain for use in this study as the model is easy and simple to
establish and it generates reproducible data.
Fig. 3 shows the
voluntary movement distances covered by mice subjected to
LPS-induced pain. Previous research has shown that voluntary
movement distances provide measurements of some types of chronic
pain including inflammatory and neuropathic pain (13). We examined voluntary movement in
an animal model of LPS-induced pain, and the results showed that
β-PGG reduced the effect of inflammation on voluntary movement and
recovered the reduction in movement distances lost owing to
LPS-induced pain. The findings of the present study suggest that
β-PGG possesses analgesic potential and is superior in terms of
movement distance recovery compared with other compounds including
gabapentin, capsaicin and ascorbic acid. Although all compounds
used in this study are used as analgesic drugs, they have different
pharmacokinetic activities in the mammalian body. For example,
gabapentin increases GABA biosynthesis, glutamate decarboxylase and
branched chain aminotransferase, and is used for the treatment of
neuropathic pain (30). Capsaicin
binds to a TRPV1, and produces similar sensations to excessive heat
(31). Ibuprofen is an NSAID used
for relieving pain by reducing inflammation, and inhibits COX-2
which is involved in mediating fever, inflammation, and pain
(32). The cytokines IL-1β and
IL-6 are associated with the molecular inflammation of specified
tissues, marking them as potential biomarkers of local inflammatory
events. We evaluated changes in the mRNA expression of both IL-1β
and IL-6 using RT-PCR and found that β-PGG decreased the mRNA
expression of both IL-1β and IL-6, suggesting that β-PGG exhibits
anti-inflammatory effects. We also found that β-PGG was more
effective at reducing IL-1β and IL-6 levels than ibuprofen, but
that it was not more effective at reducing NGF (data not shown). We
anticipate that NGF levels will markedly decrease during pain
exposure; however, we are unable to prove this currently.
Furthermore, the molecular targets which are involved with the pain
signaling pathways remain unknown, and further studies are
warranted since chronic pain can cause severe depression and
emotional distress, potentially leading to suicide.
Notably, as β-PGG possesses anti-diabetic activity,
we can investigate ways to alleviate the acute or chronic pain
associated with diabetes if we use dual effects (analgesic and
anti-diabetic) of this excellent food ingredient/biomaterial. Our
study has several limitations, including the fact that we only
investigated the analgesic effects of β-PGG in inflammatory pain
and not in other various types of pain such as nociceptive and
neuropathic pain. Future studies of the pain-related biomarkers
expressed by β-PGG, if any, in an animal model of pain and other
analgesic models are warranted, and should seek to determine the
clinical viability of β-PGG as a curative or preventive therapy in
the management of acute and chronic pain.
References
|
1
|
Tick H: Nutrition and pain. Phys Med
Rehabil Clin N Am. 26:309–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okuse K: Pain signalling pathways: from
cytokines to ion channels. Int J Biochem Cell Biol. 39:490–496.
2007. View Article : Google Scholar
|
|
3
|
Pesquero JB, Araujo RC, Heppenstall PA,
Stucky CL, Silva JA Jr, Walther T, Oliveira SM, Pesquero JL, Paiva
AC, Calixto JB, et al: Hypoalgesia and altered inflammatory
responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci
USA. 97:8140–8145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khasar SG, Lin YH, Martin A, Dadgar J,
McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, et
al: A novel nociceptor signaling pathway revealed in protein kinase
C epsilon mutant mice. Neuron. 24:253–260. 1999. View Article : Google Scholar
|
|
5
|
An S, Yang J, Xia M and Goetzl EJ: Cloning
and expression of the EP2 subtype of human receptors for
prostaglandin E2. Biochem Biophys Res Commun. 197:263–270. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Numazaki M, Tominaga T, Toyooka H and
Tominaga M: Direct phosphorylation of capsaicin receptor VR1 by
protein kinase Cepsilon and identification of two target serine
residues. J Biol Chem. 277:13375–13378. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMahon SB: NGF as a mediator of
inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 351:431–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kidd BL and Urban LA: Mechanisms of
inflammatory pain. Br J Anaesth. 87:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basbaum AI, Bautista DM, Scherrer G and
Julius D: Cellular and molecular mechanisms of pain. Cell.
139:267–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scholz J and Woolf CJ: Can we conquer
pain? Nat Neurosci. 5(Suppl): 1062–1067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schnitzer TJ: Update on guidelines for the
treatment of chronic musculoskeletal pain. Clin Rheumatol. 25(Suppl
1): S22–S29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carter GT, Duong V, Ho S, Ngo KC, Greer CL
and Weeks DL: Side effects of commonly prescribed analgesic
medications. Phys Med Rehabil Clin N Am. 25:457–470. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho H, Jang Y, Lee B, Chun H, Jung J, Kim
SM, Hwang SW and Oh U: Voluntary movements as a possible
non-reflexive pain assay. Mol Pain. 9:252013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hofmann AS and Gross GG: Biosynthesis of
gallotannins: formation of polygalloylglucoses by enzymatic
acylation of 1,2,3,4,6-penta-O-galloylglucose. Arch Biochem
Biophys. 283:530–532. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhimani RS, Troll W, Grunberger D and
Frenkel K: Inhibition of oxidative stress in HeLa cells by
chemopreventive agents. Cancer Res. 53:4528–4533. 1993.PubMed/NCBI
|
|
17
|
Li Y, Kim J, Li J, Liu F, Liu X,
Himmeldirk K, Ren Y, Wagner TE and Chen X: Natural anti-diabetic
compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin
receptor and activates insulin-mediated glucose transport signaling
pathway. Biochem Biophys Res Commun. 336:430–437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh GS, Pae HO, Oh H, Hong SG, Kim IK, Chai
KY, Yun YG, Kwon TO and Chung HT: In vitro anti-proliferative
effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on human
hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett.
174:17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heo JC, Woo SU, Kweon MA, Park JY, Lee HK,
Son M, Rho JR and Lee SH: Aqueous extract of the Helianthus annuus
seed alleviates asthmatic symptoms in vivo. Int J Mol Med.
21:57–61. 2008.
|
|
20
|
Giustarini D, Rossi R, Milzani A and
Dalle-Donne I: Nitrite and nitrate measurement by Griess reagent in
human plasma: evaluation of interferences and standardization.
Methods Enzymol. 440:361–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Charlton E: Ethical guidelines for pain
research in humans. Committee on Ethical Issues of the
International Association for the Study of Pain. Pain. 63:277–278.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cobos EJ, Ghasemlou N, Araldi D, Segal D,
Duong K and Woolf CJ: Inflammation-induced decrease in voluntary
wheel running in mice: a nonreflexive test for evaluating
inflammatory pain and analgesia. Pain. 153:876–884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heo JC and Lee SH: Alleviation of
asthma-related symptoms by a derivative of L-allo threonine. Int J
Mol Med. 31:881–887. 2013.PubMed/NCBI
|
|
24
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
25
|
Park KY, Lee HJ, Jeong SJ, Lee HJ, Kim HS
and Kim SH, Lim S, Kim HC, Lü J and Kim SH:
1,2,3,4,6-Penta-O-galloly-beta-D-glucose suppresses hypoxia-induced
accumulation of hypoxia-inducible factor-1α and signaling in LNCaP
prostate cancer cells. Biol Pharm Bull. 33:1835–1840. 2010.
View Article : Google Scholar
|
|
26
|
Lee HJ, Jeong SJ, Lee HJ, Lee EO, Bae H,
Lieske JC and Kim SH: 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose
reduces renal crystallization and oxidative stress in a
hyperoxaluric rat model. Kidney Int. 79:538–545. 2011. View Article : Google Scholar
|
|
27
|
Lee JH, Yehl M, Ahn KS, Kim SH and Lieske
JC: 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose attenuates renal cell
migration, hyaluronan expression, and crystal adhesion. Eur J
Pharmacol. 606:32–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pae HO, Oh GS, Jeong SO, Jeong GS, Lee BS,
Choi BM, Lee HS and Chung HT:
1,2,3,4,6-Penta-O-galloyl-beta-D-glucose up-regulates heme
oxygenase-1 expression by stimulating Nrf2 nuclear translocation in
an extracellular signal-regulated kinase-dependent manner in HepG2
cells. World J Gastroenterol. 12:214–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Li L, Kim SH, Hagerman AE and Lü
J: Anti-cancer, anti-diabetic and other pharmacologic and
biological activities of penta-galloyl-glucose. Pharm Res.
26:2066–2080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taylor CP: Mechanisms of action of
gabapentin. Rev Neurol (Paris). 153(Suppl 1): S39–S45. 1997.
|
|
31
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: a
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao P and Knaus EE: Evolution of
nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX)
inhibition and beyond. J Pharm Pharm Sci. 11:81s–110s. 2008.
View Article : Google Scholar
|