1. Introduction
Over the past decade, various diagnostic criteria
for metabolic syndrome (MetS) have been proposed by different
organizations (1–6). However, generally speaking, MetS is
defined by a cluster of factors including obesity/central obesity,
insulin resistance (IR), dyslipidemia and hypertension. As a
worldwide problem, the prevalence of age-adjusted MetS estimated by
the National Health and Nutrition Examination Survey (NHANES) is
22.9% in the US adult population (age ≥20) in the last decade
(7). According to a
representative sample of elderly people aged 60–95 in Beijing in
two cross-sectional surveys, the prevalence of MetS in elderly
Chinese individuals was 50.4% in 2001 and increased to 58.1% in
2010 (8). Besides high morbidity,
MetS is associated with the development of cardiovascular disease
(9), type 2 diabetes and cancer
(10). Mottillo et al
(11) found that MetS is
associated with a 2-fold increase in cardiovascular outcomes. In
addition, MetS is also involved in the development of cancer, as
colorectal cancer is one of the most common types of cancer in
patients with MetS (12).
Thus, it is important to identify a biomarker
significantly associated with MetS. As a biological molecule
involved in carbohydrate and lipid metabolism, sphingosine
1-phosphate (S1P) is a potent sphingolipid mediator that regulates
an array of cellular responses, including cell migration,
differentiation, apoptosis, lymphocyte trafficking and
inflammation, by acting as both an extracellular signal and an
intracellular second messenger (13). Herein, we reviewed the association
between S1P and MetS, with emphasis upon the ways in which
researchers have gained knowledge of this correlation.
2. S1P metabolism and signaling
Sphingosine, the substrate for S1P synthesis which
is produced by the degradation of ceramide, may be phosphorylated
by sphingosine kinases (SphKs) to form S1P. In subcellular
organelles, S1P synthesis is initiated in the endoplasmic reticulum
(ER), whereas later steps of the complex metabolism mainly occur in
the Golgi apparatus, together with the involvement of lysosomes,
nuclei and mitochondria (14).
SphK1 is mainly localized in the cytoplasm and translocates to the
cell membrane for activation, whereas SphK2 is primarily but not
exclusively localized in the nucleus (15). S1P is either dephosphorylated by
two S1P-specific phosphatases (SPP1 and SPP2), or irreversibly
degraded by S1P lyase (SPL) to phosphoethanolamine (PE) and
hexadecenal which are incorporated into glycerolipids.
Extracellular S1P may also be degraded by lysophospholipid
phosphatase (LPP)1 and LPP3 into sphingosine, which is taken up by
cells for further metabolism (16).
In healthy subjects, the concentration of plasma S1P
is 100–300 nM and approximately 100 nM in lymph (17–21). Plasma S1P is principally derived
from erythrocytes (22) and
vascular endothelial cells (23),
whereas the majority of lymph S1P originates from lymphatic
endothelial cells (24). S1P in
vascular and lymphatic endothelial cells is exported by the
specific transporter Spns2 (25,26). However, in erythrocytes, S1P is
exported by an ATP-dependent, vanadate- and glyburide-sensitive
transporter. Requiring an extracellular stimulus such as thrombin,
S1P is exported from platelets through two independent
transporters, a Ca2+-dependent transporter and an
ATP-dependent glyburide-sensitive transporter (27). The majority of plasma S1P is
combined with apolipoprotein M (apoM) which preferentially
associates with high-density lipoprotein (HDL) (28,29). The remaining part binds to
albumin, low density lipoproteins (LDLs) and very low density
lipoproteins (VLDLs) (29).
S1P signals through 5 specific G-coupled S1P
receptors designated as S1P1R-5 (30,31) and each subtype exhibits
differential coupling efficacy to G protein α subunits. Windh et
al (32) revealed that S1P1R
coupled exclusively to Gi, whereas both S1P2R and S1P4R
coupled to Gi and to G12/13 as well as to the
Gq family using the heterologous expression system of
insect Sf9 cells. S1P4R (33) and
S1P5R (34) were shown to couple
to Gi and G12, by determining
35S-GTPγS binding to G proteins in CHO cells. This
coupling resulted in the activation of small GTPases such as Rho,
Rac and Ras (35–37). Further downstream effectors of S1P
receptors include adenylate cyclase, phosphatidylinositol 3-kinase
(PI3K), phospholipase C, protein kinase C and intracellular calcium
(38). Although widely expressed,
S1P1R, S1P2R and S1P3R were principally expressed in vascular
tissues, whereas S1P5R and S1P4R were largely expressed in the
hematopoietic and nervous systems, respectively (39).
On the other hand, S1P has been suggested to
function as an intracellular messenger. Parham et al found
that S1P activated a luciferase-tagged peroxisome
proliferator-activated receptor γ (PPARγ)-specific gene reporter by
~12-fold in human endothelial cells, independently of its known
effects on canonical signaling through S1P receptors (40). S1P in the nucleus, produced by
SphK2 bound to the histone deacetylases HDAC1 and HDAC2
specifically, inhibited their enzymatic activity, which then
affects the dynamic balance of histone acetylation and thus, the
epigenetic regulation of specific target genes (41). Similar to nuclear S1P, the
majority of mitochondrial S1P produced by mitochondrial SphK2
specifically binds to homomeric PHB2, which regulates mitochondrial
assembly and function. Furthermore, mitochondrial respiration
through cytochrome oxidase was reduced by depleting SphK2 or PHB2,
suggesting that the interaction between S1P and PHB2 is important
for mitochondrial assembly and respiration (42). On the other hand, independently of
S1P extracellular signals, SphK1 as well as the production of
intracellular S1P are necessary for nuclear factor-κB (NF-κB)
activation by tumor necrosis factor-α (TNF-α) (43) (Fig.
1).
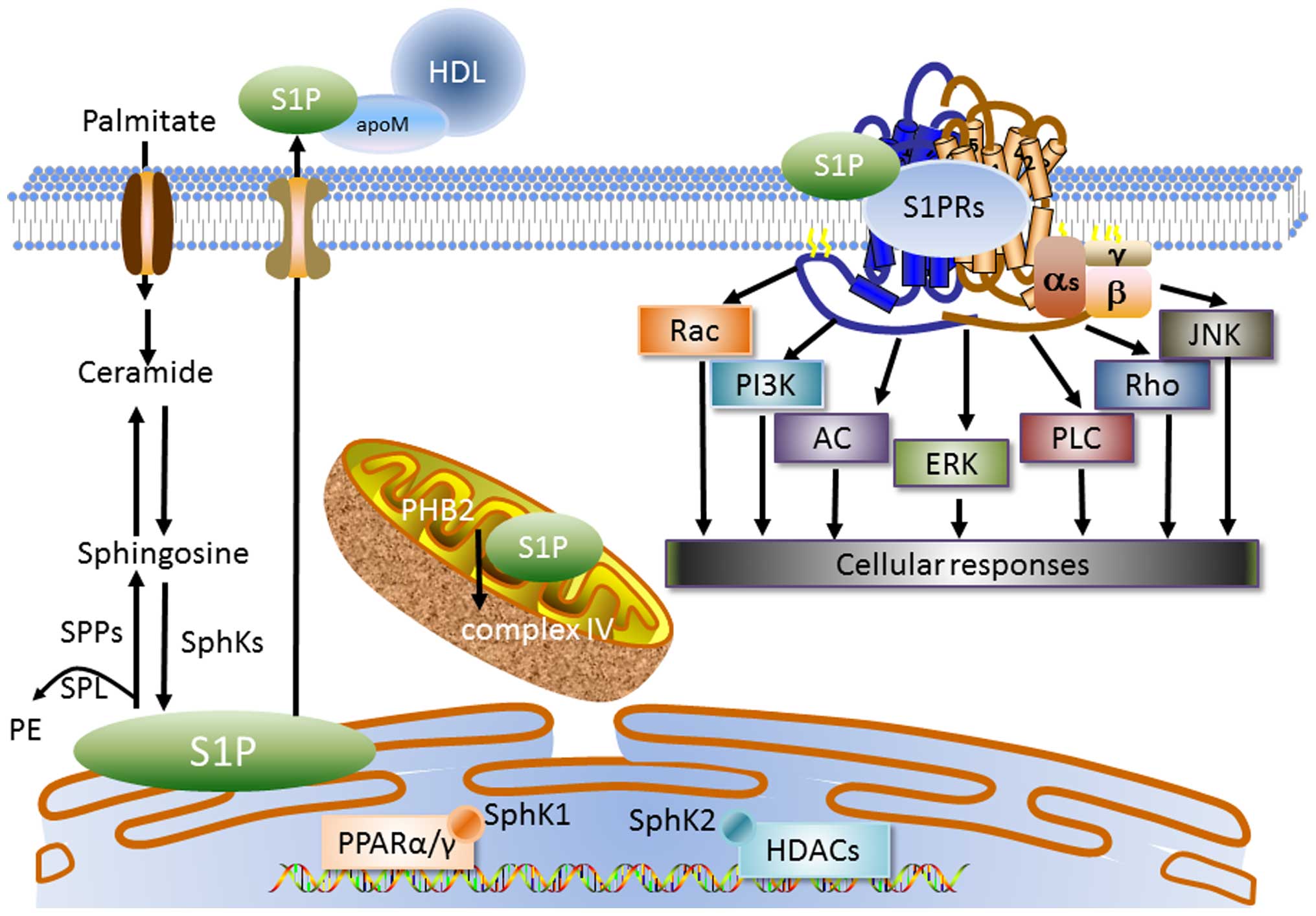 | Figure 1Sphingosine 1-phosphate (S1P)
metabolism and signaling. Sphingosine, the substrate for S1P
synthesis, which is produced by the degradation of ceramide, may be
phosphorylated by sphingosine kinases (SphK1 and SphK2) to form
S1P. SphK1 is generally localized in the cytoplasm, whereas SphK2
is primarily but not exclusively localized in nucleus. In
subcellular organelles, S1P synthesis initiates in the endoplasmic
reticulum (ER), where S1P is irreversibly degraded by S1P lyase
(SPL) to phosphoethanolamine (PE) or dephosphorylated by S1P
phosphatases (SPPs) to sphingosine. In vascular and lymphatic
endothelial cells, S1P is transported out by specific Spns2
transporters. S1P is exported out of vascular and lymphatic
endothelial cells by the specific transporter Spns2. However, in
erythrocytes, S1P is exported by an ATP-dependent, vanadate- and
glyburide-sensitive transporter. The majority of plasma S1P is
bound to apolipoprotein M (apoM) which preferentially associates
with high densiy lipoprotein (HDL). The extracellular S1P signals
through specific G-coupled S1P receptors designated as S1P1R-5,
activating Rac, phosphatidylinositol 3-kinase (PI3K), adenylate
cyclase (AC), extracellular-regulated protein kinase (ERK),
phospholipase C (PLC), Rho and Jun N-terminal kinase (JNK) to
contribute to cellular responses. Intracellular S1P also directly
activates prohibitin 2 (PHB2) in the mitochondria, which stabilizes
cytochrome c oxidase. In the nucleus, S1P is also involved
in the activation of nuclear transcription factors PPARα and PPARγ,
and histone deacetylases (HDACs). |
3. S1P in obesity
S1P is positively associated with obesity. Plasma
levels of S1P were higher in obese patients than those in non-obese
and lean individuals (44). In
addition, it was demonstrated that plasma S1P positively correlates
with body mass index (BMI), total body fat percentage and waist
circumference (45). The
pathophysiology of obesity is complex; it is known to involve
abnormal energy metabolism, deviant adipogenesis and inflammation.
Recent studies have suggested that S1P is involved in these
pathophysiological processes and in fact, reduces obesity.
S1P ameliorates obesity through the regulation of
energy homeostasis. Silva et al (46) found that S1P1R protein is highly
expressed in the hypothalamic pro-opiomelanocortin neurons of rats.
Additionally, food consumption was decreased and energy expenditure
was increased by intra-cerebroventricular injections of S1P through
the activation of signal transducer and activator of transcription
3 (STAT3) and the melanocortin system, and vice versa. Moreover,
the expression of S1P1R in neurons was controlled by STAT3 in
neurons through a positive feedback mechanism. As several models of
obesity display an imbalance of the hypothalamic S1P/S1P1R/STAT3
axis, whereas pharmacological intervention improves these
phenotypes, this indicates that the S1P/S1P1R/STAT3 signaling
pathway in neurons plays a vital role in controlling energy
homeostasis in rats.
S1P reduces fat mass by affecting adipogenesis and
lipolysis through several pathways. In maturing 3T3-L1
preadipocytes, S1P significantly decreased lipid accumulation in a
dose-dependent manner with the downregulation of the
transcriptional levels of the CCAAT/enhancer binding proteins α
(C/EBPα), PPARγ and adiponectin, which are markers of adipogenic
differentiation. Moreover, the activation of Jun N-terminal kinase
(JNK) and p38 mitogen-activated protein kinase (p38 MAPK) were also
downregulated by S1P treatment in human preadipocytes. These
results suggest that S1P mediates the downregulation of adipogenic
transcription factors and by inactivating the JNK and p38 MAPK
signaling pathways subsequently affects adipogenesis (47). FTY720, an analogue of S1P,
phosphorylated by SphK2 to form FTY720(S)-phosphate (FTY720-P),
which binds to S1P1R, S1P3R, S1P4R and S1P5R, significantly
down-regulated the markers of adipogenic differentiation (PPARγ,
C/EBPα and adiponectin) and upregulated the regulators of
lipolysis, [hormone-sensitive lipase (HSL), adipose triglyceride
lipase (ATGL) and perilipin], indicating that FTY720 prevented
obesity by modulating adipogenesis and lipolysis (48). This evidence suggests that S1P
affects several pathways to adjust the balance between adipogenesis
and lipolysis, subsequently altering the fat mass which reduces
obesity, indicating that S1P may be used in the treatment of
obesity. However, the internal mechanisms responsible for these
effects remain to be elucidated. Further investigation is warranted
into whether extracellular and intracellular S1P signals are
involved in this process as well as into the mechanisms responsible
for achieving these effects.
As chronic low-grade inflammation and activation of
the immune system participate in the pathogenesis of MetS (49) inflammation is also involved in the
interaction between S1P and obesity. A cross-sectional study
recruited 30 healthy overweight and 15 lean adolescents, and found
that there was a significant association between S1P and TNF-α
(50). In animal studies, Samad
et al found that S1P was elevated in obese (ob/ob)
mice, and S1P induced the gene expression of interleukin (IL)-6,
TNF-α, monocyte chemoattractant protein-1 and keratinocyte-derived
chemokine in cultured adipocytes (51). On the other hand, Wang et
al found that genetic disruption of the SphK1/S1P signaling
pathway in mice with diet-induced obesity (DIO) increased adipose
gene expression of the anti-inflammatory molecules IL-10 and
adiponectin whereas it reduced adipose tissue macrophage
recruitment and the expression of the proinflammatory molecules
TNF-α and IL-6 (52). These
findings suggest that the SK1/S1P signaling pathway contributed to
the proinflammatory phenotype of the obese adipose tissue, and
played a critical role in the pathogenesis of obesity-mediated
metabolic disease.
As S1P ameliorates abnormal energy metabolism and
deviant adipogenesis, while mediating inflammation, it plays an
alternative role in the development of obesity. There is a
possibility that, in the early stages of obesity, S1P increases the
expression of proinflammatory factors, which activate the
protective mechanisms of the body, thereby attenuating further
development of obesity. Thus, S1P effectively adjusted the balance
between adipogenesis and lipolysis in order to decrease the fat
mass, which is the most prominent feature of obesity. On the other
hand, S1P is a lipid with multiple biological activities mediated
through intracellular and extracelluar pathways, and the activation
of different pathways leads to different results. Thus, it is
necessary to explore more specific mechanisms, and additional
animal models and clinical trials are required in order to confirm
the effects of S1P.
4. S1P in IR
S1P is synthesized by SphKs. SphK1 and SphK2 mediate
opposite effects in insulin sensitivity. Qi et (53) found that all high fat diet
(HFD)-fed SphK1(−/−) mice manifested evident diabetes, accompanied
by a nearly 3-fold reduction in insulin levels compared with the WT
mice which developed glucose intolerance and compensatory
hyperinsulinemia. Pancreatic β-cell mass was increased by 140% in
HFD-fed WT mice and decreased to 50% in HFD-fed SphK1(−/−) mice, in
comparison with the chow diet control groups, respectively. The
SphK1 level was also elevated in obese, type 2 diabetic humans.
Enhanced insulin signaling in adipose and muscle as well as
improved systemic insulin sensitivity and glucose tolerance were
demonstrated in SphK1(−/−) mice, suggesting that the SphK1-S1P axis
improves IR associated with obesity and type 2 diabetes (52). In addition, primary islets
isolated from SphK1(−/−) mice exhibited higher susceptibility to
lipotoxicity than WT controls. Of note, S1P profoundly abrogated
lipotoxicity in β cells or the cells lacking SphK1 activity and
SphK1(−/−) islets, highlighting a pivotal role of S1P in β-cell
survival under lipotoxic conditions (53). Recognizing that S1P is metabolized
by SphK2, the change of SphK2 is also involved in the process of
IR. Cantrell Stanford et al (54) demonstrated that glucose elevates
intracellular S1P by activating SphK2 in MIN6 cells and mouse
pancreatic islets. Notably, the elevation of S1P correlates with
the increase in glucose-stimulated insulin secretion (GSIS). On the
other hand, downregulated levels of S1P and the knockdown of SphK2
in MIN6 cells or primary islets results in decreased GSIS, whereas
the knockdown of the S1P phosphatase, SPP1, leads to a rise in
GSIS. These data suggest that glucose-activated SphK2/S1P is
important for GSIS in pancreatic β cells.
Apart from pancreatic islets, SphK1/S1P/S1P
receptors also acted as an insulin-mimetic cue in insulin sensitive
tissues. SphK1 transgenic mice fed an HFD showed increased SphK1
activity in skeletal muscle, accompanied by ameliorated muscle
insulin resistance, which may be associated with a concomitant
reduction in the phosphorylation of c-JNK, a serine threonine
kinase associated with IR, thus indicting that skeletal muscle and
whole-body insulin sensitivity were improved in SphK1 transgenic
mice on an HFD (55). In
addition, the inhibition of SphK1 decreased the effect of palmitate
on insulin-stimulated glucose uptake by attenuating the activity of
the AKT/glycogen synthase kinase 3β (GSK3β) signaling pathway and
insulin signaling proteins in L6 myotubes (56) Furthermore, SphK1 gene delivery
significantly enhanced the phosphorylation of insulin-signaling
kinases such as Akt and GSK3β in the livers of diabetic animals
(57). S1P, through engagement
with S1P2R, has been found to produce a transient burst of reactive
oxygen species (ROS) through calcium-dependent activation of the
small GTPase Rac1 in skeletal muscle cells, accompanied by a redox
modulation of protein tyrosine phosphatase-1B (TPT-1B) activity,
the main negative regulator of insulin receptor phosphorylation. It
is indicative of critical crosstalk occurring between S1P, insulin
pathways and ROS (58). The
extra- and intracellular S1P from the liver, another important
organ involved in IR, was markedly increased by palmitate. Once
generated, S1P bound to S1P2R via reduced insulin-mediated glycogen
synthesis and PI3K/Akt-GSK3β activation to impair insulin signaling
(59).
The malignant crosstalk of pancreatic islet β-cell
dysfunction and insulin sensitivity leads to insulin resistance. In
spite of the fact that SphK2/S1P increases the GSIS of β-cells,
more evidence showed that SphK1/S1P/S1P2R pathway activation
inhibits the feedback loop of insulin secretion and
sensitivity.
5. S1P in hyperglycemia
Plasma levels of S1P in patients with type 2
diabetes were significantly higher (~50%) than those in healthy
individuals (60). Additionally,
plasma S1P positively correlated with HbA1c (%) levels in humans
(45). It is well known that
β-cell dysfunction and insulin resistance are the most important
pathophysiological mechanisms associated with the development of
diabetes. Zhao et al found that oral administration of
FTY720 to diabetic (db/db) mice increased β-cell mass and
blood insulin levels to normalize fasting blood glucose levels.
Further investigation confirmed that S1P controls β-cell
regeneration in the islets isolated from the treated mice by
decreasing cyclin-dependent kinase inhibitor p57 (KIP2) levels and
increasing cyclin D3 expression via the PI3K pathway (61).
More importantly, S1P ameliorates the complications
of diabetes. Diabetic nephropathy (DN) is the leading cause of
end-stage renal failure, which may be attenuated by S1P. FTY720 or
SEW2871, a selective S1P1R agonist, significantly reduced urinary
albumin excretion which led to tubule injury and increased urinary
TNF-α in rats with streptozotocin (STZ)-induced diabeties (62). A dorsal skin wound was made in the
healthy and diabetic mice; S1P injection alone promoted wound
healing in the diabetic mice compared with the control, and the
combination of S1P and JTE-013 (S1P2R antagonist) administration
induced maximal wound healing in diabetic mice (63). These findings indicate that S1P
possesses unique potential in the therapy of DN and diabetic
wounds.
However, controversy still exists. As the connective
tissue growth factor (CTGF) is a marker in mesangial cells for the
progression of DN, El-Shewy et al found that SphK1/S1P1/3R
activation is upstream of extracellular regulated protein kinases
(ERK)1/2 and JNK, which are essential for LDL-regulated expression
of CTGF in renal mesangial cells (64). The SphK1/S1P pathway also plays a
critical role in fibronectin accumulation, which is a glomerular
extracellular matrix protein associated with DN, in both kidneys
from STZ-induced diabetic rats and high glucose-treated glomerular
mesangial cells (65). Lan et
al (66) identified that
fibronectin expression was upregulated via S1P-S1P2R-MAPK (ERK1/2
and p38 MAPK) axis activation in mesangial cells under high glucose
conditions, suggesting that SphK1-S1P-S1P2R-MAPK may be a potential
therapeutic target for the treatment of DN.
In diabetes, elevated S1P levels are evident. While
the downstream receptors have the opposite effect under
hyper-glycemic conditions, S1P1R activation ameliorates diabetes
whereas S1P2R activation worsens the condition (Table I). Finding an equilibrium point
between S1P1R and S1P2R, and then balancing the activation of S1P1R
and S1P2R, may provide a novel target for the treatment of
diabetes.
 | Table IRole of S1P and downstream signaling
in insulin resistance and hyperglycemia. |
Table I
Role of S1P and downstream signaling
in insulin resistance and hyperglycemia.
| Subjects | Intervening
substance/target molecules | Downstream
signal | Biological
outcome | Refs. |
|---|
| SphK1 transgenic
mice | Fed an HFD | A concomitant
reduction in the phosphorylation of c-JNK | Ameliorate muscle
insulin resistance | (55) |
| MIN6 cells,
pancreatic islets | Glucose | Activate
SphK2/S1P | Increased GSIS | (54) |
| db/db mice | FTY720 (10 mg/kg,
intraperitoneal injection daily for 6 weeks) | Increase cyclin D3
and decline KIP2 via PI3K | Increase β-cell
mass and blood insulin levels | (61) |
| STZ-exposed
diabetic rats | FTY720 (0.3 mg/kg,
oral gavage, 9 weeks) | Upregulate
S1P1R | Reduce the urinary
albumin excretion | (62) |
| db/db mice | S1P (10 or 100 mM
injected into the wound bed daily) and JTE-013 | Downregulate
S1P2R | Promote the healing
of wound | (63) |
| DIO mice | SphK1
deficiency | Increase IL-10 and
adiponectin levels; reduce ATM recruitment, TNF and IL-6 | Enhanced insulin
signaling in adipose and muscle and improved systemic insulin
sensitivity and glucose tolerance | (52) |
| C2C12 cells | S1P (0.01–5
µM, 15–90 min) | Calcium-dependent
activation of the small GTPase Rac1 | Produce a transient
burst of ROS | (58) |
| Primary rat
hepatocytes | S1P (1 Mm, 5
h) | S1PR2, reduces
PI3K/Akt-GSK-3β activation | Reduce insulin
mediated glycogen synthesis and impair insulin signaling | (59) |
| Renal mesangial
cells | SphKs inhibitor,
VPC23019 | Inhibit activation
of ERK1/2 and JNK via S1PR1 and S1P3R | Essential for
expression of CTGF induced by LDL | (64) |
| SphK1, S1P (1
µm, 12 h) | S1P2R-MAPK (ERK1/2
and p38 MAPK) | Mediate high
glucose-induced fibronectin expression | (66) |
6. S1P in hyperlipidemia
S1P contributes to the protective role of HDL. Tong
et al (67) found that HDL
in diabetic patients with elevated S1P levels exhibited more
powerful protective effects, inducing COX-2 expression and PGI2
release from human umbilical vein endothelial cells, than control
HDL through the activation of S1P1R/3R. Additionally, S1P
compensated for the reduction in COX-2 expression through glycated
HDL by phosphorylating the ERK/MAPK-CREB pathway (68). These findings confirmed the
protective role of HDL-binding S1P in patients with type 2 diabetes
mellitus, suggesting a possible novel therapeutic target. There is
no S1P in HDL in apoM(−/−) mice, whereas HDL in
transgenic mice overexpressing human apoM has an increased S1P
content, indicating that the bridge between HDL and S1P in plasma
is apoM. The 1.7-Å structure of the S1P-apoM complex suggests that
S1P specifically interacts with an amphiphilic pocket in the
lipocalin fold of apoM. Human apoM(+) HDL induced S1P1R
internalization, downstream MAPK and Akt activation, endothelial
cell migration, and formation of endothelial adherence junctions,
whereas apoM(−) HDL did not. Moreover, the HDL fraction of
apoM(−/−) mice which lacked S1P showed dampened basal endothelial
barrier function in lung tissue. This demonstrated that apoM is an
essential vascular protective constituent of HDL by delivering S1P
to the S1P1R on endothelial cells (28).
Plasma S1P not only positively correlated with LDL
cholesterol in a human study (45), but also in experimental animal
studies, showing the important role of S1P in the context of
hyperlipidemia. High fructose-fed rats induced hyperlipidemia and
activated the SphK1/S1P signaling pathway, which in turn led to the
activation of NF-κB signaling and inflammation in rat livers.
Importantly, lipid metabolic disorder as well as hepatic insulin
and leptin signaling impairment were observed in this animal model,
which resulted in lipid accumulation in the liver (69). Additionally, SphK1 gene delivery
significantly attenuated elevated levels of plasma non-esterified
fatty acid (NEFA), triacylglycerol, LDL and cholesterol, which
markedly ameliorated liver, heart and kidney injuries induced by
hyperglycaemia in KK/Ay diabetic mice (57). As in other animal models of
hyperlipidemia, the capability to generate and release S1P were
significantly higher in the hypersensitized platelets and blood
plasma obtained from rabbits with hypercholesterolemia. Moreover,
co-treatment with S1P (0.125–0.5 mM) potentiated the ox-LDL-induced
proliferation of vascular smooth muscle cells in a dose-dependent
manner through the S1P1R dependent pathway (70). Thus, the SphK1/S1P/S1P receptor
signaling pathway may be a novel mechanism which is important in
hyperlipidemia.
It is well known that hyperlipidemia is always
accompanied by a low HDL level, and the majority of plasma S1P is
bound to HDL. It has been hypothesized that amount of S1P which
binds to HDL is less, whereas more S1P may bind to LDL and VLDL
under conditions of hyperlipidemia. From the above studies, we can
deduce that S1P exhibits a protective function by binding HDL,
wheras the combination with LDL shows the opposite effects.
7. S1P in hypertension
Plasma S1P levels are significantly higher in
patients with hypertension in comparison with normotensive
patients. In fact, the S1P1R gene is a candidate for the control of
salt sensitivity and hypertension in the stroke-prone spontaneously
hypertensive rat (SHRSP) (71).
In SHR, application of dimethylsphingosine, a sphingosine kinase
inhibitor, led to a marked upregulation in mean arterial pressure
(MAP) with a further rise in carotid artery resistance. However, in
Wistar-Kyoto (WKY) rats, dimethylsphingosine had little effect on
MAP (72). S1P induced transient
relaxation of isolated pressurized mesenteric arterioles with low
concentrations (10–100 nM) in a dose dependent manner. Maximal
vasodilation (55±8%) was demonstrated at 2 min after S1P addition
and returned to baseline levels by 5 min via G protein-dependent,
calcium-sensitive, and PI3K signaling pathways (73).
However, controversy continues to exist. FTY720
produced modest hypertension (2–3 mmHg) in patients in a 1 year
trial. Furthermore, FTY720 elicited dose-dependent hypertension
after multiple days of oral administration through the activation
of S1P3R in rats (74).
Furthermore, S1P induced vasocontraction through the activation of
the Rho/Rho-kinase signaling pathway in the canine basilar artery
at high concentrations ranging between 100 and 10 mM (75). The S1P extracellular signals
induced hypertension. S1P/S1P3R selectively constricted isolated
cerebral arteries mainly by Rho and partly through
G(i/o) protein (76).
On the other hand, Yogi et al (77) identified that S1P/S1P1R is
associated with specific pathways through epidermal growth factor
receptor and platelet-derived growth factor transactivation, a
process upregulated in the vascular smooth muscle cells of SHRSP
rats which contributes to vascular inflammation in
hypertension.
The complex cardiovascular effects of S1P are likely
to be due to differential concentrations of S1P. Low concentrations
(10–100 nM) lead to vasodilation whereas high concentrations
(100–10,000 nM) result in the vasocontraction of isolated
arterioles. The activation of different S1P receptor subtypes may
also contribute to different results. Thus, the investigation of
more mechanisms is necessary in order to identify the multiple
roles of S1P in hypertension.
8. Conclusion
Due to the high prevalence of MetS worldwide, a
condition which is closely assicated with the development and
prognosis of diabetes and cardiovascular disease, studies were
taken to find a biomarker or effective way to diminish the
development and progress of MetS. S1P has been demonstrated to play
multiple roles in the occurrence and development of this disease,
mainly through S1P1R-S1P3R. There is controversy regarding the role
of S1P in MetS, such as anti-inflammation versus pro-inflammation,
and anti-hypertension versus pro-hypertension. Different SphKs, S1P
concentration or S1P receptor subtypes result in diverse results
(Fig. 2). Thus, the possible
implications of S1P-directed mechanisms on the occurrence and
development of MetS remains to be elucidated. Further
investigations may provide novel candidates for the treatment of
MetS.
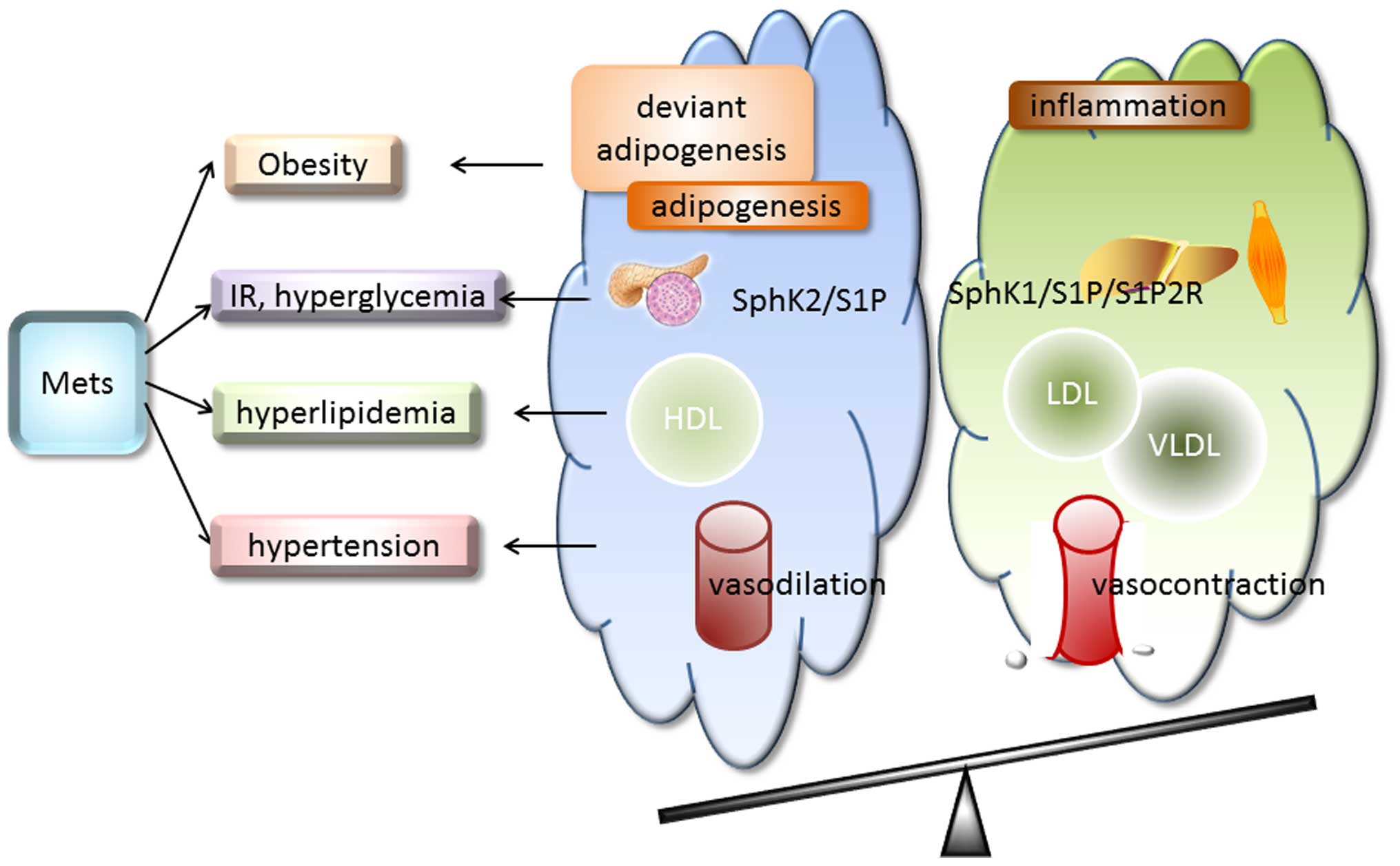 | Figure 2Sphingosine 1-phosphate (S1P) in
metabolic syndrome (MetS). MetS is defined by a cluster of factors
including obesity/central obesity, hyperglycemia, dyslipidemia,
hypertension and insulin resistance (IR). S1P plays multiple roles
in the occurrence and development of this disease. However, the
specific role played by S1P remains unclear. It ameliorates
abnormal energy metabolism and deviant adipogenesis and mediates
inflammation in obesity. The majority of S1P1R activation improves
diabetes, whereas S1P2R activation worsens the condition. In
hyperlipidemia, S1P binds to high-density lipoprotein (HDL),
low-density lipoprotein (LDL) and very low-denity lipoprotein
(VLDL) to exert direct different effects. Moreover, low
concentrations of S1P (10–100 nM) lead to vasodilation whereas high
concentrations (100–10,000 nM) result in the vasocontraction of
isolated arterioles. SphK2/S1P increases the glucose-stimulated
insulin secretion (GSIS) of β-cells. More evidence showed that
activation of the sphingosine kinase 1 (SphK1)/S1P/S1P2R pathway
deteriorates the feedback loop of insulin secretion and
sensitivity. Different SphKs, S1P concentrations or S1P receptor
subtypes result in diverse results; alterations to the balance
between these factors may provide novel treatments for MetS. |
Acknowledgments
The present study was supported by funding from the
National Natural Science Foundation of China (81470593), the
National Basic Research Program of China (2014CB542400) and the
National Natural Science Foundation of China (81300053), the
Fundamental Research Funds for the Central Universities of Central
South University (2015zzts123).
References
|
1
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balkau B and Charles MA: Comment on the
provisional report from the WHO consultation. European Group for
the Study of Insulin Resistance (EGIR). Diabet Med. 16:442–443.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults: Executive Summary of
The Third Report of The National Cholesterol Education Program
(NCEP) Expert Panel on Detection, Evaluation, And Treatment of High
Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA.
285:2486–2497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bloomgarden ZT: American Association of
Clinical Endocrinologists (AACE) consensus conference on the
insulin resistance syndrome: 25–26 August 2002, Washington, DC.
Diabetes Care. 26:933–939. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alberti KG and Zimmet P: The metabolic
syndrome - a new worldwide definition. Lancet. 366:1059–1062. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM and
Smith SC Jr; International Diabetes Federation Task Force on
Epidemiology and Prevention; Hational Heart, Lung, and Blood
Institute; American Heart Association; World Heart Federation;
International Atherosclerosis Society; International Association
for the Study of Obesity: Harmonizing the metabolic syndrome: a
joint interim statement of the International Diabetes Federation
Task Force on Epidemiology and Prevention; National Heart, Lung,
and Blood Institute; American Heart Association; World Heart
Federation; International Atherosclerosis Society; and
International Association for the Study of Obesity. Circulation.
120:1640–1645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beltrán-Sánchez H, Harhay MO, Harhay MM
and McElligott S: Prevalence and trends of metabolic syndrome in
the adult U.S. population, 1999–2010. J Am Coll Cardiol.
62:697–703. 2013. View Article : Google Scholar
|
|
8
|
Liu M, Wang J, Jiang B, Sun D, Wu L, Yang
S, Wang Y, Li X and He Y: Increasing prevalence of metabolic
syndrome in a Chinese elderly population: 2001–2010. PLoS One.
8:e662332013. View Article : Google Scholar
|
|
9
|
Wilson PW, D'Agostino RB, Parise H,
Sullivan L and Meigs JB: Metabolic syndrome as a precursor of
cardiovascular disease and type 2 diabetes mellitus. Circulation.
112:3066–3072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mendonça FM, de Sousa FR, Barbosa AL,
Martins SC, Araújo RL, Soares R and Abreu C: Metabolic syndrome and
risk of cancer: Which link? Metabolism. 64:182–189. 2015.
View Article : Google Scholar
|
|
11
|
Mottillo S, Filion KB, Genest J, Joseph L,
Pilote L, Poirier P, Rinfret S, Schiffrin EL and Eisenberg MJ: The
metabolic syndrome and cardiovascular risk a systematic review and
meta-analysis. J Am Coll Cardiol. 56:1113–1132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esposito K, Chiodini P, Capuano A,
Bellastella G, Maiorino MI, Rafaniello C, Panagiotakos DB and
Giugliano D: Colorectal cancer association with metabolic syndrome
and its components: a systematic review with meta-analysis.
Endocrine. 44:634–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaho VA and Hla T: Regulation of
mammalian physiology, development, and disease by the sphingosine
1-phosphate and lysophosphatidic acid receptors. Chem Rev.
111:6299–6320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maceyka M and Spiegel S: Sphingolipid
metabolites in inflammatory disease. Nature. 510:58–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan H and Pitson SM: Post-translational
regulation of sphingosine kinases. Biochim Biophys Acta.
1831:147–156. 2013. View Article : Google Scholar
|
|
16
|
Escalante-Alcalde D, Hernandez L, Le
Stunff H, Maeda R, Lee HS, Gang-Cheng Jr, Sciorra VA, Daar I,
Spiegel S, Morris AJ and Stewart CL: The lipid phosphatase LPP3
regulates extra-embryonic vasculogenesis and axis patterning.
Development. 130:4623–4637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knapp M, Lisowska A, Zabielski P, Musiał W
and Baranowski M: Sustained decrease in plasma
sphingosine-1-phosphate concentration and its accumulation in blood
cells in acute myocardial infarction. Prostaglandins Other Lipid
Mediat. 106:53–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baranowski M, Charmas M, Długołęcka B and
Górski J: Exercise increases plasma levels of sphingoid base-1
phosphates in humans. Acta Physiol (Oxf). 203:373–380. 2011.
View Article : Google Scholar
|
|
19
|
Knapp M, Baranowski M, Lisowska A and
Musiał W: Decreased free sphingoid base concentration in the plasma
of patients with chronic systolic heart failure. Adv Med Sci.
57:100–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knapp M, Lisowska A, Knapp P and
Baranowski M: Dose-dependent effect of aspirin on the level of
sphingolipids in human blood. Adv Med Sci. 58:274–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baranowski M, Górski J, Klapcinska B,
Waskiewicz Z and Sadowska-Krepa E: Ultramarathon run markedly
reduces plasma sphingosine-1-phosphate concentration. Int J Sport
Nutr Exerc Metab. 24:148–156. 2014. View Article : Google Scholar
|
|
22
|
Pappu R, Schwab SR, Cornelissen I, Pereira
JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG and
Coughlin SR: Promotion of lymphocyte egress into blood and lymph by
distinct sources of sphingosine-1-phosphate. Science. 316:295–298.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venkataraman K, Lee YM, Michaud J,
Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C and Hla T:
Vascular endothelium as a contributor of plasma sphingosine
1-phosphate. Circ Res. 102:669–676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pham TH, Baluk P, Xu Y, Grigorova I,
Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR and
Cyster JG: Lymphatic endothelial cell sphingosine kinase activity
is required for lymphocyte egress and lymphatic patterning. J Exp
Med. 207:17–27. 2010. View Article : Google Scholar :
|
|
25
|
Mendoza A, Bréart B, Ramos-Perez WD, Pitt
LA, Gobert M, Sunkara M, Lafaille JJ, Morris AJ and Schwab SR: The
transporter Spns2 is required for secretion of lymph but not plasma
sphingosine-1-phosphate. Cell Rep. 2:1104–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukuhara S, Simmons S, Kawamura S, Inoue
A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, et
al: The sphingosine-1-phosphate transporter Spns2 expressed on
endothelial cells regulates lymphocyte trafficking in mice. J Clin
Invest. 122:1416–1426. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi N, Kobayashi N, Yamaguchi A and
Nishi T: Characterization of the ATP-dependent sphingosine
1-phosphate transporter in rat erythrocytes. J Biol Chem.
284:21192–21200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christoffersen C, Obinata H, Kumaraswamy
SB, Galvani S, Ahnström J, Sevvana M, Egerer-Sieber C, Muller YA,
Hla T, Nielsen LB and Dahlbäck B: Endothelium-protective
sphingosine-1-phosphate provided by HDL-associated apolipoprotein
M. Proc Natl Acad Sci USA. 108:9613–9618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okajima F: Plasma lipoproteins behave as
carriers of extracellular sphingosine 1-phosphate: is this an
atherogenic mediator or an antiatherogenic mediator? Biochim
Biophys Acta. 1582:132–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MJ, Van Brocklyn JR, Thangada S, Liu
CH, Hand AR, Menzeleev R, Spiegel S and Hla T:
Sphingosine-1-phosphate as a ligand for the G protein-coupled
receptor EDG-1. Science. 279:1552–1555. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosen H, Stevens RC, Hanson M, Roberts E
and Oldstone MB: Sphingosine-1-phosphate and its receptors:
structure, signaling, and influence. Annu Rev Biochem. 82:637–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Windh RT, Lee MJ, Hla T, An S, Barr AJ and
Manning DR: Differential coupling of the sphingosine 1-phosphate
receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12)
families of heterotrimeric G proteins. J Biol Chem.
274:27351–27358. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamazaki Y, Kon J, Sato K, Tomura H, Sato
M, Yoneya T, Okazaki H, Okajima F and Ohta H: Edg-6 as a putative
sphingosine 1-phosphate receptor coupling to Ca(2+) signaling
pathway. Biochem Biophys Res Commun. 268:583–589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Im DS, Heise CE, Ancellin N, O'Dowd BF,
Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, et
al: Characterization of a novel sphingosine 1-phosphate receptor,
Edg-8. J Biol Chem. 275:14281–14286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye D and Lin F: S1pr2/Gα13 signaling
controls myocardial migration by regulating endoderm convergence.
Development. 140:789–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singleton PA, Dudek SM, Chiang ET and
Garcia JG: Regulation of sphingosine 1-phosphate-induced
endothelial cytoskeletal rearrangement and barrier enhancement by
S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J.
19:1646–1656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishimaru N, Yamada A, Nitta T, Arakaki R,
Lipp M, Takahama Y and Hayashi Y: CCR7 with S1P1 signaling through
AP-1 for migration of Foxp3+ regulatory T-cells controls
autoimmune exocrinopathy. Am J Pathol. 180:199–208. 2012.
View Article : Google Scholar
|
|
38
|
Mendelson K, Evans T and Hla T:
Sphingosine 1-phosphate signalling. Development. 141:5–9. 2014.
View Article : Google Scholar :
|
|
39
|
Waeber C; Sphingosine 1-phosphate (S1P)
signaling and the vasculature: Lysophospholipid Receptors:
Signaling and Biochemistry. Chun J, Hla T, Spiegel S and Moolenaar
W: John Wiley and Sons, Inc; Hoboken, NJ: pp. 313–347. 2013,
View Article : Google Scholar
|
|
40
|
Parham KA, Zebol JR, Tooley KL, Sun WY,
Moldenhauer LM, Cockshell MP, Gliddon BL, Moretti PA, Tigyi G,
Pitson SM and Bonder CS: Sphingosine 1-phosphate is a ligand for
peroxisome proliferator-activated receptor-γ that regulates
neoangiogenesis. FASEB J. 29:3638–3653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hait NC, Allegood J, Maceyka M, Strub GM,
Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S
and Spiegel S: Regulation of histone acetylation in the nucleus by
sphingosine-1-phosphate. Science. 325:1254–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Strub GM, Paillard M, Liang J, Gomez L,
Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, et
al: Sphingosine-1-phosphate produced by sphingosine kinase 2 in
mitochondria interacts with prohibitin 2 to regulate complex IV
assembly and respiration. FASEB J. 25:600–612. 2011. View Article : Google Scholar :
|
|
43
|
Alvarez SE, Harikumar KB, Hait NC,
Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T,
et al: Sphingosine-1-phosphate is a missing cofactor for the E3
ubiquitin ligase TRAF2. Nature. 465:1084–1088. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ito S, Iwaki S, Koike K, Yuda Y, Nagasaki
A, Ohkawa R, Yatomi Y, Furumoto T, Tsutsui H, Sobel BE and Fujii S:
Increased plasma sphingosine-1-phosphate in obese individuals and
its capacity to increase the expression of plasminogen activator
inhibitor-1 in adipocytes. Coron Artery Dis. 24:642–650.
2013.PubMed/NCBI
|
|
45
|
Kowalski GM, Carey AL, Selathurai A,
Kingwell BA and Bruce CR: Plasma sphingosine-1-phosphate is
elevated in obesity. PLoS One. 8:e724492013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Silva VR, Micheletti TO, Pimentel GD,
Katashima CK, Lenhare L, Morari J, Mendes MC, Razolli DS, Rocha GZ,
de Souza CT, et al: Hypothalamic S1P/S1PR1 axis controls energy
homeostasis. Nat Commun. 5:48592014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moon MH, Jeong JK, Lee YJ, Seol JW and
Park SY: Sphingosine-1-phosphate inhibits the adipogenic
differentiation of 3T3-L1 preadipocytes. Int J Mol Med.
34:1153–1158. 2014.PubMed/NCBI
|
|
48
|
Moon MH, Jeong JK, Lee JH, Park YG, Lee
YJ, Seol JW and Park SY: Antiobesity activity of a sphingosine
1-phosphate analogue FTY720 observed in adipocytes and obese mouse
model. Exp Mol Med. 44:603–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Majumdar I and Mastrandrea LD: Serum
sphingolipids and inflammatory mediators in adolescents at risk for
metabolic syndrome. Endocrine. 41:442–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Samad F, Hester KD, Yang G, Hannun YA and
Bielawski J: Altered adipose and plasma sphingolipid metabolism in
obesity: a potential mechanism for cardiovascular and metabolic
risk. Diabetes. 55:2579–2587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang J, Badeanlou L, Bielawski J, Ciaraldi
TP and Samad F: Sphingosine kinase 1 regulates adipose
proinflammatory responses and insulin resistance. Am J Physiol
Endocrinol Metab. 306:E756–E768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qi Y, Chen J, Lay A, Don A, Vadas M and
Xia P: Loss of sphingosine kinase 1 predisposes to the onset of
diabetes via promoting pancreatic β-cell death in diet-induced
obese mice. FASEB J. 27:4294–4304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cantrell Stanford J, Morris AJ, Sunkara M,
Popa GJ, Larson KL and Özcan S: Sphingosine 1-phosphate (S1P)
regulates glucose-stimulated insulin secretion in pancreatic beta
cells. J Biol Chem. 287:13457–13464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bruce CR, Risis S, Babb JR, Yang C,
Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K,
Takuwa Y, et al: Overexpression of sphingosine kinase 1 prevents
ceramide accumulation and ameliorates muscle insulin resistance in
high-fat diet-fed mice. Diabetes. 61:3148–3155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mikłosz A, Łukaszuk B, Baranowski M,
Górski J and Chabowski A: Effects of inhibition of serine
palmitoyltransferase (SPT) and sphingosine kinase 1 (SphK1) on
palmitate induced insulin resistance in L6 myotubes. PLoS One.
8:e855472013. View Article : Google Scholar
|
|
57
|
Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li
JF, Yi J, Yuan YJ, Zhang QW, Mi J, et al: Sphingosine kinase 1
participates in insulin signalling and regulates glucose metabolism
and homeostasis in KK/Ay diabetic mice. Diabetologia. 50:891–900.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rapizzi E, Taddei ML, Fiaschi T, Donati C,
Bruni P and Chiarugi P: Sphingosine 1-phosphate increases glucose
uptake through trans-activation of insulin receptor. Cell Mol Life
Sci. 66:3207–3218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fayyaz S, Henkel J, Japtok L, Krämer S,
Damm G, Seehofer D, Püschel GP and Kleuser B: Involvement of
sphingosine 1-phosphate in palmitate-induced insulin resistance of
hepatocytes via the S1P2 receptor subtype. Diabetologia.
57:373–382. 2014. View Article : Google Scholar
|
|
60
|
Randriamboavonjy V, Badenhoop K, Schmidt
H, Geisslinger G, Fisslthaler B and Fleming I: The S1P(2) receptor
expressed in human platelets is linked to the RhoA-Rho kinase
pathway and is down regulated in type 2 diabetes. Basic Res
Cardiol. 104:333–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao Z, Choi J, Zhao C and Ma ZA: FTY720
normalizes hyperglycemia by stimulating β-cell in vivo regeneration
in db/db mice through regulation of cyclin D3 and p57(KIP2). J Biol
Chem. 287:5562–5573. 2012. View Article : Google Scholar
|
|
62
|
Awad AS, Rouse MD, Khutsishvili K, Huang
L, Bolton WK, Lynch KR and Okusa MD: Chronic sphingosine
1-phosphate 1 receptor activation attenuates early-stage diabetic
nephropathy independent of lymphocytes. Kidney Int. 79:1090–1098.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kawanabe T, Kawakami T, Yatomi Y, Shimada
S and Soma Y: Sphingosine 1-phosphate accelerates wound healing in
diabetic mice. J Dermatol Sci. 48:53–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Shewy HM, Sohn M, Wilson P, Lee MH,
Hammad SM, Luttrell LM and Jaffa AA: Low-density lipoprotein
induced expression of connective tissue growth factor via
transactivation of sphingosine 1-phosphate receptors in mesangial
cells. Mol Endocrinol. 26:833–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu W, Lan T, Xie X, Huang K, Peng J,
Huang J, Shen X, Liu P and Huang H: S1P2 receptor mediates
sphingosine-1-phosphate-induced fibronectin expression via MAPK
signaling pathway in mesangial cells under high glucose condition.
Exp Cell Res. 318:936–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lan T, Liu W, Xie X, Xu S, Huang K, Peng
J, Shen X, Liu P, Wang L, Xia P and Huang H: Sphingosine kinase-1
pathway mediates high glucose-induced fibronectin expression in
glomerular mesangial cells. Mol Endocrinol. 25:2094–2105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tong X, Peng H, Liu D, Ji L, Niu C, Ren J,
Pan B, Hu J, Zheng L and Huang Y: High-density lipoprotein of
patients with type 2 diabetes mellitus upregulates cyclooxgenase-2
expression and prostacyclin I-2 release in endothelial cells:
relationship with HDL-associated sphingosine-1-phosphate.
Cardiovasc Diabetol. 12:272013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tong X, Lv P, Mathew AV, Liu D, Niu C,
Wang Y, Ji L, Li J, Fu Z, Pan B, et al: The compensatory enrichment
of sphingosine-1-phosphate harbored on glycated high-density
lipoprotein restores endothelial protective function in type 2
diabetes mellitus. Cardiovasc Diabetol. 13:822014. View Article : Google Scholar
|
|
69
|
Wang X, Zhang DM, Gu TT, Ding XQ, Fan CY,
Zhu Q, Shi YW, Hong Y and Kong LD: Morin reduces hepatic
inflammation-associated lipid accumulation in high fructose-fed
rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate
signaling pathway. Biochem Pharmacol. 86:1791–1804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Son DJ, Lee HW, Shin HW, Lee JJ, Yoo HS,
Kim TJ, Yun YP and Hong JT: Enhanced release of
sphingosine-1-phosphate from hypercholesterolemic platelets: role
in development of hypercholesterolemic atherosclerosis.
Prostaglandins Leukot Essent Fatty Acids. 78:383–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Graham D, McBride MW, Gaasenbeek M, Gilday
K, Beattie E, Miller WH, McClure JD, Polke JM, Montezano A, Touyz
RM and Dominiczak AF: Candidate genes that determine response to
salt in the stroke-prone spontaneously hypertensive rat: congenic
analysis. Hypertension. 50:1134–1141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Spijkers LJ, van den Akker RF, Janssen BJ,
Debets JJ, De Mey JG, Stroes ES, van den Born BJ, Wijesinghe DS,
Chalfant CE, MacAleese L, et al: Hypertension is associated with
marked alterations in sphingolipid biology: a potential role for
ceramide. PLoS One. 6:e218172011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dantas AP, Igarashi J and Michel T:
Sphingosine 1-phosphate and control of vascular tone. Am J Physiol
Heart Circ Physiol. 284:H2045–H2052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fryer RM, Muthukumarana A, Harrison PC,
Nodop Mazurek S, Chen RR, Harrington KE, Dinallo RM, Horan JC,
Patnaude L, Modis LK and Reinhart GA: The clinically-tested S1P
receptor agonists, FTY720 and BAF312, demonstrate subtype-specific
bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS One.
7:e529852012. View Article : Google Scholar
|
|
75
|
Tosaka M, Okajima F, Hashiba Y, Saito N,
Nagano T, Watanabe T, Kimura T and Sasaki T: Sphingosine
1-phosphate contracts canine basilar arteries in vitro and in vivo:
possible role in pathogenesis of cerebral vasospasm. Stroke.
32:2913–2919. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Salomone S, Yoshimura S, Reuter U, Foley
M, Thomas SS, Moskowitz MA and Waeber C: S1P3 receptors mediate the
potent constriction of cerebral arteries by
sphingosine-1-phosphate. Eur J Pharmacol. 469:125–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yogi A, Callera GE, Aranha AB, Antunes TT,
Graham D, McBride M, Dominiczak A and Touyz RM:
Sphingosine-1-phosphate-induced inflammation involves receptor
tyrosine kinase transactivation in vascular cells: Upregulation in
hypertension. Hypertension. 57:809–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
















