1. Introduction
Magnetic resonance imaging (MRI) contrast agents are
widely used to increase the contrast difference between normal and
abnormal tissues. Shortly after the introduction of clinical MRI,
the first contrast-enhanced human MRI study was reported in 1981
using ferric chloride as the contrast agent in the gastrointestinal
(GI) tract (1). In 1984, Carr
et al first proved the use of a gadolinium compound as a
diagnostic intravascular MRI contrast agent (2). Almost half of the MRI studies
performed nowadays are contrast-enhanced studies, and this is a
growing trend (3). Newer contrast
agents are constantly being discovered and investigated. The safety
of contrast agents for clinical use is under strict scrutiny. This
review therefore, aims to classify the MRI contrast agents
discovered to date into relevant groups and to also discuss their
applications, structures, mechanisms of action, pharmacokinetics
and pharmacodynamics.
MRI contrast agents may be categorised according to
the following features (4):
magnetic properties, chemical composition, the presence or absence
of metal atoms, route of administration, effect on the magnetic
resonance image, biodistribution and application.
2. Magnetic properties
The majority of MRI contrast agents are either
paramagnetic gadolinium ion complexes or superparamagnetic (iron
oxide) magnetite particles. The paramagnetic contrast agents are
usually made from dysprosium (Dy3+), the lanthanide
metal gadolinium (Gd3+), or the transition metal
manganese (Mn2+) and possess water soluble properties.
The most commonly selected metal atom used in MRI contrast agents
is the lanthanide ion gadolinium (III) as it possesses a high
magnetic moment and it is the most stable ion with unpaired
electrons. Due to the presence of unpaired electrons, these
contrast agents possess paramagnetic properties; gadolinium has
seven, dysprosium has four and manganese has five unpaired
electrons. Contrast agents containing gadolinium shorten the T1 (or
longitudinal) and T2 (or transverse) relaxation time of
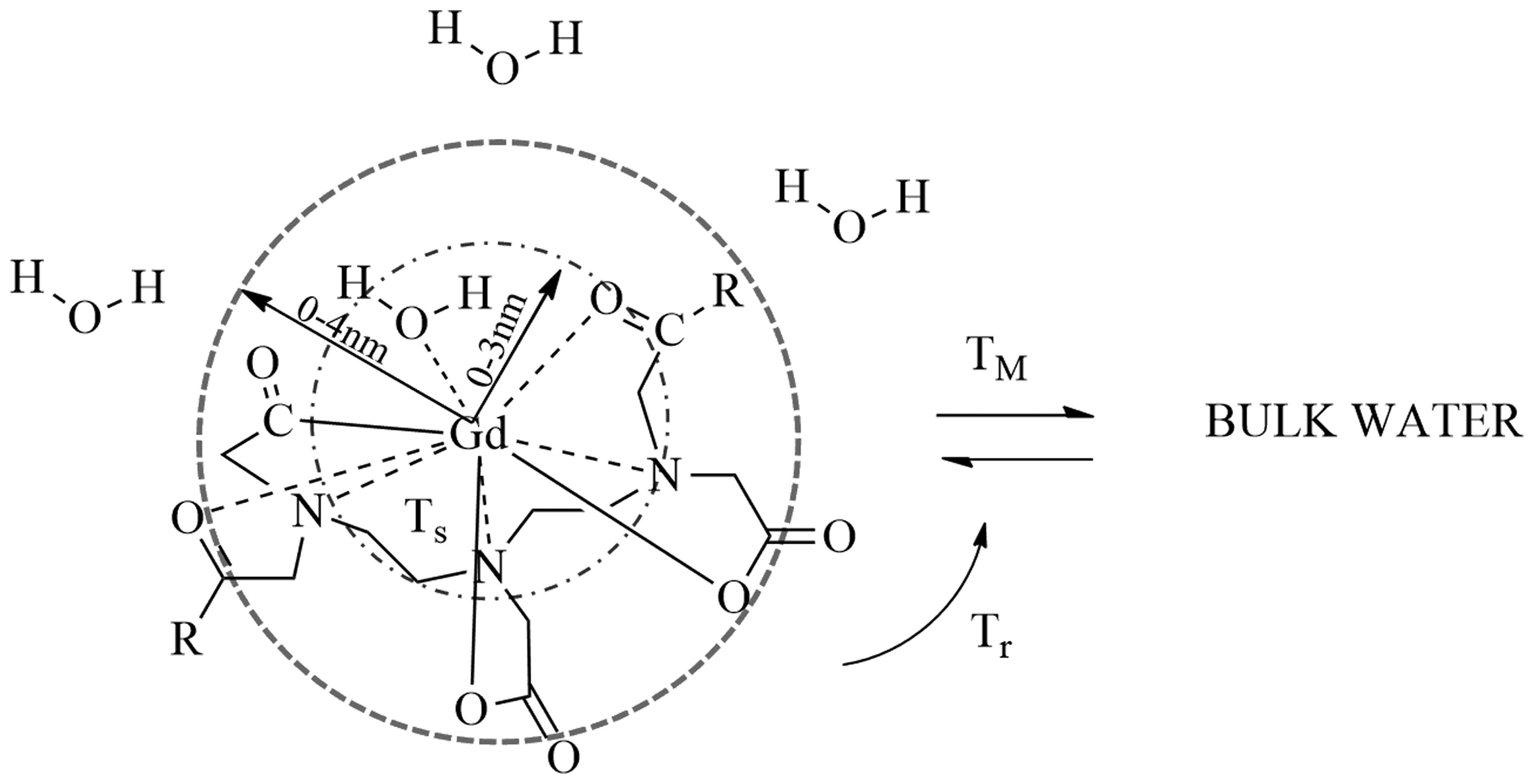
neighbouring water protons (Fig.
1). These effects increase the signal intensity of T1-weighted
images, and reduce the signal intensity of T2-weighted images
(5,6). T1 shortening occurs at lower
gadolinium concentrations, whereas T2 shortening occurs at higher
gadolinium concentrations, which is of limited clinical use due to
the increased risk of toxicity. Therefore, in conventional clinical
practice T1 is evaluated after the administration of extracellular
agents (7). Contrast agents
containing transition metal ions, such as high spin manganese (II)
and superparamagnetic iron oxide such as iron (III) oxides, affect
the T2 relaxation strongly (8,9).
Gadolinium-based contrast agents:
paramagnetic
Gadolinium (III)-based contrast agents are
categorised into three groups: extracellular fluid (ECF) agents,
blood pool contrast agents (BPCAs) and organ-specific agents.
Manganese-based contrast agents:
paramagnetic
Manganese, in the form of manganese chelates or
manganese-based nanoparticles, is used as a contrast agent.
Manganese chelates, including manganese dipyridoxyl diphosphate
(Mn-DPDP), markedly enhance the T1 signal intensity, and has been
used to detect hepatic lesions. In the human body, the chelate
dissociates into manganese and DPDP. Manganese is taken up by the
liver cells and excreted into the bile, whereas the DPDP component
is excreted by the kidneys (10).
Research on Mn-based nanoparticles is not as detailed in comparison
with other well-studied nanoparticles based on iron oxide (11).
Manganese-enhanced MRI (MEMRI) uses manganese ions
(Mn2+) and this contrast agent has applications in
animal experiments (12).
Mn2+ enters cells through calcium (Ca2+)
channels and thus, this group of contrast agents may be used for
functional brain imaging (13). A
previous MRI study has suggested that Mn2+ carbon
nanostructure complexes of graphene oxide nanoplatelets and
graphene oxide nanoribbons are highly effective MRI contrast agents
(14).
Iron oxide contrast agents:
superparamagnetic
There are two types of iron oxide contrast agents:
superparamagnetic iron oxide (SPIO) and ultrasmall
superparamagnetic iron oxide (USPIO). Superparamagnetic contrast
agents consist of suspended colloids of iron oxide nanoparticles.
When applied during imaging, they reduce the intensity of the T2
signals in the tissues which absorb the contrast agent. SPIO and
USPIO have achieved successful outcomes in the diagnosis of liver
tumors in some cases (15). Two
decades ago, SPIO was the first nanoparticulate MRI contrast agent
to be introduced as a liver contrast agent, and it is still used
for clinical imaging (16). SPIOs
and USPIOs such as Feridex I.V., Resovist, Sinerem and Clariscan
have been approved for use in the past. However, these agents are
currently unavailable apart from the oral iron oxide contrast
agent, Lumirem/GastroMARK.
The nano-sized dimensions and the particle shapes of
this group of contrast materials allow for different
biodistribution and applications that are not observed with other
contrast agents. At present, nanoparticulate iron oxide is a
popular and unique nanoparticulate agent used in clinical practice.
However, owing to the sophisticated modern technology of molecular
and cellular imaging, which makes disease-specific biomarkers
visible at microscopic and molecular levels, other nanoparticles
have also obtained greater attention as potential MRI contrast
agents. Due to the enormous improvement in nanotechnology, novel
nanoparticulate MRI contrast agents have been developed with
further improved contrast abilities as well as other functions
(16).
Iron platinum contrast agents:
superparamagnetic
Compared with iron oxide nanoparticles,
superparamagnetic iron platinum particles (SIPPs) are thought to
possess significantly improved T2 relaxation properties. SIPPs have
been encapsulated with phospholipids to create multifunctional SIPP
stealth immunomicelles in order to specifically target human
prostate cancer cells (17).
These contrast agents are still under investigation and have not
yet been studied in humans, to the best of our knowledge. This
study revealed that multifunctional SIPP micelles have been
synthesized and conjugated to a monoclonal antibody against
prostate-specific membrane antigen. In addition, the complex
specifically targeted human prostate cancer cells in vitro,
suggesting that SIPPs may have the potential to be tumor-specific
in the future (17).
3. Chemical composition and the presence or
absence of metal atoms
MRI contrast agents may be divided into two
classifications as mentioned previously. The first group is
comprised of paramagnetic compounds, which include lanthanides such
as gadolinium. The second group is comprised of transition elements
such as manganese and iron.
In order to reduce the toxicity of metal ions, the
concept of chelation has been introduced. To prepare contrast
agents based on metallic ions, the technique of chelated complex
formation is widely used. The acute and the chronic toxic
side-effects induced by the metal ion as well as the chelating
agent are markedly reduced due to complexation (18).
As mentioned previously, gadolinium is used as a
gadolinium (III) ion. Gadolinium (III) is weakly bound to serum
proteins and may be displaced by ligands. Lanthanide salts
generally hydrolyse into hydroxides, which are taken up by the
reticuloendothelial system (RES) and accumulate in the body,
particularly in the liver, spleen and bone, thereby causing
potential toxicity. Lanthanide ions are excreted into both urine
and faeces, unlike manganese ions which are almost exclusively
excreted by GI elimination, via the biliary route. To overcome the
aforementioned problems, these elements are administered in
chelated forms.
Gadolinium-based chelation complexes
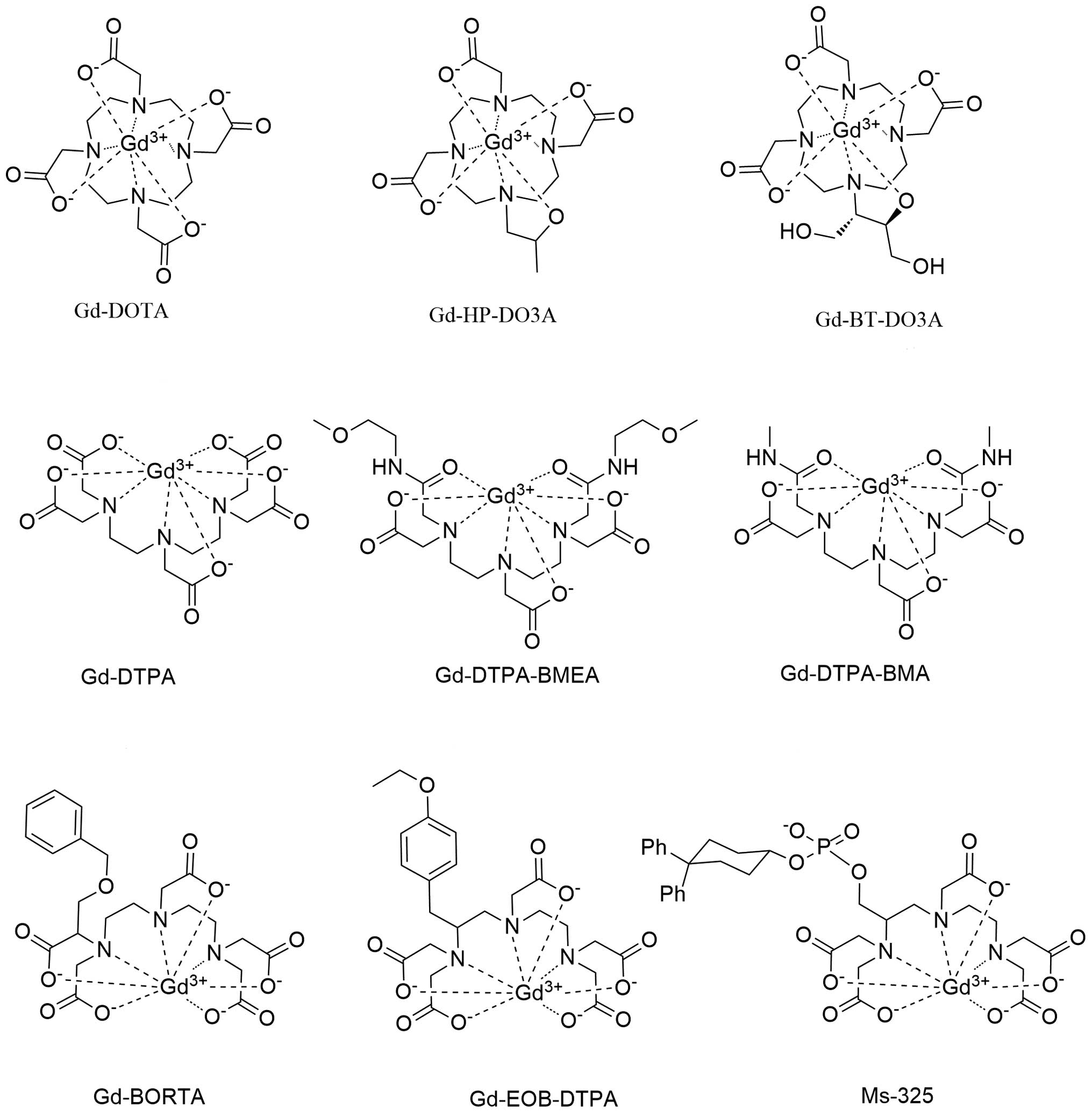
Fig. 2 illustrates
the gadolinium-based chelation complexes used in clinical practice.
There are three types of gadolinium (III)-based chelates (18).
Ionic and hydrophilic complexes
Ionic and hydrophilic complexes include gadolinium
(III) diethylenetriamine pentaacetate (Gd-DTPA, also known as
gadopentate dimeglumine), Gd(III) 1,4,7,10-tetrazacyclododecane
NN′N″N‴-tetra-acetate (Gd-DOTA, gadoterate) (19) and Gd(III) polyaspartate.
Nonionic and hydrophilic complexes
Nonionic hydrophilic chelates of gadolinium (III)
include Gd3-diethylenetriamine pentaacetate-bis(methylamide)
(Gd-DTPA-BMA, also known as gadodiamide) and a macrocyclic chelate
analog of Gd-DOTA, where an acetic acid function is replaced by a
2-propanol radical (Gd-HP-DO3A, also known as gadoteridol)
(20).
Ionic and lipophilic complexes
The other group of gadolinium complexes includes the
Gd benzyl-oxy-methyl derivative of diethyltriamine pentaacetate
dimethylglucamine salt (Gd-BOPTA, also known as gadobenate
dimeglumine) and Gd ethoxybenzyl diethylentriamine pentaacetate
(Gd-EOB-DTPA, also known as gadoxetate) (18).
4. Route of administration
MRI contrast agents may be administered
intravenously or orally. The route of administration is dependent
on the subject of interest. A list of contrast agents is presented
in Table I.
 | Table IAgents administered orally. |
Table I
Agents administered orally.
| Short name | Generic name | Trade name | Enhancement |
|---|
| Gd-DTPAa | Gadopentate
dimeglumine | Magnevist
Enteral | Positive |
| –a | Ferric amonium
citrate | Ferriseltz | Positive |
| –a | Manganese
chloride | LumenHance | Positive |
| –a | Gadolinium-loaded
zeolite | Gadolite | Positive |
| OMPa | Ferristene
(MPIO) | Abdoscan | Negative |
| AMI-121b | Ferumoxsil
(MPIO) |
Lumirem/GastroMARK | Negative |
| PFOBb |
Perfluoro-octylbromide | Imagent GI | Negative |
Intravenous contrast agents
Intravenous MRI contrast agents are comprised of
chelates of paramagnetic ions, both ionic and nonionic. The
particulates are isolated in the liver, spleen and lymph nodes. The
intravascular agents are confined to the blood pool and to specific
tumors.
Ionic intravenous contrast agents
The first intravenous contrast agents to be used
were the chelates of paramagnetic ions Cr and Gd in combination
with ethylenediaminetetraacetic acid (EDTA). However, EDTA was
relatively unstable, and was found to cause toxic effects in an
animal study (21). Gd-DTPA has
been successfully used due to its stability and reliability, and it
is the first intravenous MRI contrast agent to be approved for use
in humans (Magnevist; Berlex Laboratories). Gd has a large magnetic
moment, exceeded only by dysprosium (III) and holmium (III). Even
at low concentrations, it possesses strong paramagnetic properties
and low toxicity. The paramagnetic properties are due to the fact
that it has seven unpaired electrons, as stated previously.
Following intravenous administration, it is distributed in the
intravascular and extracellular fluid spaces, and then rapidly
excreted into urine (22).
Nonionic intravenous contrast agents
Nonionic contrast agents have been developed in
parallel with iodinated contrast materials. Some side effects are
due to the fact that ionic chelates are hyperosmolar. In contrast
to ionic agents, nonionic agents are relatively hypoosmolar.
Gadodiamide (Omniscan; Winthrop Pharmaceuticals) is a nonionic
complex, which has only two-fifths of the osmolality of Gd-DTPA.
Owing to a median lethal dose of 34 mmol/kg, gadodiamide has a
safety ratio 2–3 times that of Gd-DOTA, and 3–4 times that of
Gd-DTPA. The administration of gadodiamide does not cause
abnormalities in serum bilirubin levels. However, one study
conducted in 73 individuals demonstrated that elevated serum iron
levels are a potential concern with an incidence of 8.2%, and a
similar efficacy to that of Gd-DTPA (23). Gadoteridol (ProHance; Squibb) is
the third kind of intravenous contrast agent sold on the market. It
is a nonionic contrast agent with osmolarity similar to that of
gadodiamide (24).
MRI oral contrast agents (OCAs)
The oral administration of contrast agents is
appropriate for GI tract scans. Naturally prepared fruit juices
such as Medlar fruit juice, blueberry juice and green tea, have
been studied as MRI contrast agents for several years. Artificial
OCAs are based on the heavy metal ions such as gadolinium,
manganese (III), manganese (II), copper (II) and iron (III). Air
and clay are used to reduce the T2 signal intensity (25,26). Gadolinium-based agents, SPIO,
manganese-containing agents and barium sulfate suspensions have
been studied as oral MRI contrast agents (27,28). The oral administration of MRI
contrast agents containing manganese is a novel, noninvasive method
for imaging (27). Barium sulfate
suspensions are useful as negative oral MRI contrast agents
(28). Divalent manganese ions
are paramagnetic and greatly reduce the T1 relaxation time, thereby
increasing T1 signal intensity. Manganese enters excitable cells
such as neurons and myocardiocytes through various calcium
channels. Thus, manganese acts as an indicator of calcium channel
activity (27). However, the
intravascular route of administration of MRI contrast agents is
more useful and is the more commonly used route for MRI scans.
5. Effect on the image
Paramagnetic contrast agents, apart from
dysprosium-based compounds, are positive agents and they exert
similar effects on T1 imaging and T2 imaging. However, as the T1 of
tissues is much higher than the T2, the predominant effect at low
does is that of T1 shortening (29). The tissues absorbing such agents
become bright on T1-weighted images.
Negative contrast agents reduce T2 signals by
shortening the T2 relaxation time. Superparamagnetic and
ferromagnetic agents belong to this group. However, reducing the
particle size of ferromagnetic particles size results in the
permanent loss of magnetic properties, and a change to become
superparamagnetic particles (30). These compounds may also become T1
agents, depending on particle size and coating.
6. Biodistribution and applications
Extracellular fluid (ECF) agents
ECF agents (so called intravenous contrast agents)
are distributed within the extracellular space. These agents have
been used for the longest period of time in liver imaging, and they
remain the most commonly used and well-documented. ECF agents are
comprised of gadolinium chelated to an organic compound such as
DTPA (31–33). A list of the ECF agents is
presented in Table II.
 | Table IIECF space agents. |
Table II
ECF space agents.
| Short name | Generic name | Trade name | Enhancement and
physiochemical effects |
|---|
| Gd-DTPAa | Gadopentate
dimeglumine | Magnevist |
Positive-ionic-linear |
| Gd-DOTAa | Gadoterate
meglumine | Dotarem,
Artirem |
Positive-ionic-macrocyclic |
| Gd-DTPA-BMAa | Gadodiamide
injection | Omniscan |
Positive-nonionic-linear |
| Gd-HP-DO3Aa | Gadoteridol
injection | ProHance |
Positive-nonionic-macrocylic |
|
Gd-DTPA-BMEAa |
Gadoversetamide | OptiMARK |
Positive-nonionic-linear |
|
Gd-DO3A-butrola | Gadobutrol | Gadovist |
Positive-nonionic-macrocyclic |
| Gd-BOPTAa | Gadobenate
dimeglumine | MultiHance |
Positive-ionic-linear |
The pharmacokinetics of gadolinium chelates mimic
that of iodinated contrast agents for computed tomography (CT). The
contrast agents circulate and then freely distribute in the
extracellular space. ECF agents are mainly eliminated by renal
excretion. Gadolinium enters the liver through the hepatic artery
and portal vein, and is freely redistributed into the interstitial
space. In contrast to iodine molecules which are imaged by CT, the
effect of gadolinium is assessed by MRI rather than the molecule
itself. Gadolinium exhibits an amplification effect as a number of
adjacent water protons are relaxed by a single gadolinium atom. As
a result, MRI is more sensitive to the effects of gadolinium than
CT is to the effects of iodine (29,33).
BPCAs
BPCAs, also known as intravascular contrast agents,
remain in the intravascular space much longer and are excreted more
slowly than their ECF counterparts, thus providing a longer time
window for the imaging of blood vessels. These agents are currently
under investigation for use in angiography, which may be performed
in the equilibrium phase (34). A
list of BPCAs is presented in Table
III.
 | Table IIIBPCAs. |
Table III
BPCAs.
| Short name | Generic name | Trade name | Enhancement |
|---|
| NC-100150b | PEG-feron
(USPIO) | Clariscan | Positive |
| SH U 555 Cb | Ferucarbotran
(USPIO) | Supravist | Positive |
| MS-325a | Gadofosveset | AngioMARK,
Vasovist, Ablavar | Positive |
| Gadomer-17b | – | – | Positive |
|
Gabofluorine-Mb | – | – | Positive |
| P792b | Gadomelitol | Vistarem | Positive |
| AMI-227c | Ferumoxtran-10
(USPIO) |
Sinerem/Combidex | Positive or
negative |
| Gd-BOPTAa | Gadobenate
dimeglumine | MultiHance | Positive |
The BPCAs may be classified into the following three
categories based on their mechanism of action: i) systems based on
the noncovalent binding of low-molecular Gd to human serum albumin
(HSA) to prevent immediate leakage into the interstitial space
(35,36); ii) systems incorporating polymers
or liposomes based on an increase in the size of the contrast
agent, which slows down leakage through endothelial pores (37,38); and iii) systems based on
nanoparticles, involving a change in the route of elimination
(39–41). These agents may be classified
broadly into three categories: USPIO particles, agents that
reversibly bind to plasma proteins, and macromolecules (42,43).
Targeted and organ-specific contrast
agents
The primary aim of MRI contrast agent development is
to identify agents which are capable of targeting specific tissues.
A list of such compounds is presented in Table IV. It is important to consider
the following three parameters in order to optimize the development
of targeted and organ-specific contrast agents: i) improvement of
the enhancing effect as high and ultrahigh fields call for a
different contrast agent compared with low and medium high fields;
ii) selective distribution in the body as it is necessary for these
contrast agents to accumulate (organ-or pathology-specific tracers)
at the required site in order to reach high local concentrations
and iii) improvement of tolerance: although tolerance of existing
compounds is already very good, this means, the compound has to be
inert chemically and biologically, and also has to be completely
eliminated from the body.
 | Table IVTargeted/organ-specific agents. |
Table IV
Targeted/organ-specific agents.
| Short name | Generic name | Trade name | Enhancement and
physiochemical effects |
|---|
| Mn-DPDPc | Mangafodipir
trisodium | Treslascan | Positive/liver |
| Gd-EOB-DTPAa | Gadoxetate | Primovist,
Eovist |
Positive-ionic-linear/liver |
| Gd-BOPTAa | Gadobenate
dimeglumine | MultiHance |
Positive-ionic-linear/liver |
| AMI-25a | Ferumoxides
(SPIO) | Endorem,
Feridex | Negative/liver |
| SH U 555 Ac | Ferucarbotran
(SPIO) | Resovist,
Cliavist | Negative/liver |
| AMI-227c | Ferumoxtran-10
(USPIO) | Sinerem,
Combidex | Positive or
negative/lymph nodes |
|
Gadofluorine-Mb | – | – | Positive/(lymph
nodes, CNS) |
| Mn-DPDPb | Mangafodipir
trisodium | – |
Positive/myocardium |
| Dy-DTPA-BMAb | Sprodiamide
injection | – | Negative/myocardial
and brain perfusion |
|
Gd-DTPA-mesoporphyrinb | Gadophrin | – |
Positive/myocardium, necrosis |
Iron oxides and liposomes have attracted particular
interest as potential organ-specific agents. Iron oxide particles
are imported into the cells of the RES through phagocytosis, which
provides selective access to the liver, spleen, lymph nodes, and
bone marrow. These agents can either be positive (T1) or negative
(T2/T2*) enhancers, depending on particle size,
composition, concentration and saturation magnetization of the
material as well as the equipment hardware and pulse sequences
used. The biodistribution of iron oxides is determined by size,
shape, charge, hydrophilicity, chemical composition and surface
coating (44). The majority of
compounds are polydisperse and polycrystalline. However, actively
targeted iron oxides, which tend to contain smaller
superparamagnetic labels, are monodisperse and monocrystalline. For
intravenous use, iron oxide particles should be <50 nm in order
to avoid entrapment in the lungs.
Another group of particulate contrast agents are
liposomes. Paramagnetic ions may either be encapsulated in the
aqueous compartment of the liposomes or be linked to their lipid
bilayers. More sophisticated liposome compounds have been developed
including phospholipid spin-labeled and amphipathic chelate
complexes.
The primary organ selected for developing passive
targeting compounds (vascular, hepatobiliary, and
reticuloendothelial) is the liver. In addition to vascular
structures, both hepatocytes and the RES may be targeted. By
dynamic examinations, vascular structures as well as highly
vascularized lesions are commonly highlighted with the conventional
low molecular weight contrast agents. Both Gd-EOB-DTPA and Gd-BOPTA
are positive gadolinium-based agents with lipophilic side groups.
Gd-EOB-DTPA is a liver-specific agent whereas Gd-BOPTA is a
multipurpose contrast agent, well suited for liver imaging
(45). Mn-DPDP is a positive
multipurpose agent, which taken up by hepatocytes (46). Contrast enhancement appears to be
due to the limited release of the manganese ion and this effect is
long lasting and may be achieved with doses as low as 10 mmol/kg
body weight.
Further applications
Some contrast agents may also be capable of
targeting other organs such as the spleen, pancreas, bone marrow,
lymph nodes, adrenals, muscles and particularly the heart as well
as inflammation and specific tumors. However, they are not yet
ready for use in clinical practice.
7. Future prospects and conclusions
The first MRI contrast agent to be used was ferric
chloride in 1981. Over the past 3 decades, many contrast agents
have been developed for use in clinical practice and some of them
were withdrawn as result of safety concerns. The MRI contrast
agents discovered to date may be classified into various groups
according to a number of criteria: chemical composition, the
presence of metal atoms, route of administration, magnetic
properties, effect on the image, biodistribution and further
applications. As a result there are variations in the clinical
implications, mechanisms of action, safety, pharmacokinetics and
pharmacodynamics of these contrast agents. Currently, newer and
safer MRI agents capable of targeting organs, sites of inflammation
and specific tumors are under investigation in order to develop
contrast agents with higher disease specificity.
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
Gd
|
gadolinium
|
|
Mn
|
manganese
|
|
Dy
|
dysprosium
|
|
SPIO
|
superparamagnetic iron oxide
|
|
USPIO
|
ultrasmall superparamagnetic iron
oxide
|
|
SIPP
|
superparamagnetic iron platinum
particle
|
|
Mn-DPDP
|
manganese dipyridoxyl diphosphate or
mangafodipir trisodium
|
|
MEMRI
|
manganese-enhanced MRI
|
|
Gd-DTPA
|
gadolinium (III) diethylenetriamine
pentaacetate
|
|
Gd-DOTA
|
gadoterate dotarem
|
|
Gd-EOB-DTPA
|
gadolinium ethoxybenzyl
diethylenetriamine pentaacetate or gadoxetate
|
|
Cr
|
chromium
|
|
Gd-DTPA-BMA
|
gadolinium 3-diethylenetriamine
pentaacetate-bis(methylamide)
|
|
Gd-HP-DO3A
|
gadoteridol
|
|
Gd-BOPTA
|
gadobenate dimeglumine
|
|
OCA
|
oral contrast agent
|
|
GI
|
gastrointestinal
|
|
CT
|
computed tomography
|
|
ECF
|
extracellular fluid
|
|
BPCA
|
blood pool contrast agent
|
|
HSA
|
human serum albumin
|
|
RES
|
reticuloendothelial system
|
References
|
1
|
Young IR, Clarke GJ, Bailes DR, Pennock
JM, Doyle FH and Bydder GM: Enhancement of relaxation rate with
paramagnetic contrast agents in NMR imaging. J Comput Tomogr.
5:543–547. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carr DH, Brown J, Bydder GM, Weinmann HJ,
Speck U, Thomas DJ and Young IR: Intravenous chelated gadolinium as
a contrast agent in NMR imaging of cerebral tumours. Lancet.
1:484–486. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang JB, Sheng YQ, Hu HJ and Shen YQ:
Macromolecular MRI contrast agents: structures, properties and
applications. Prog Polym Sci. 38:462–502. 2013. View Article : Google Scholar
|
|
4
|
Geraldes CFGC and Laurent S:
Classification and basic properties of contrast agents for magnetic
resonance imaging. Contrast Media Mol Imaging. 4:1–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell DG: Liver I: Currently available
gadolinium chelates. Magn Reson Imaging Clin N Am. 4:37–51.
1996.PubMed/NCBI
|
|
6
|
Wood ML and Hardy PA: Proton relaxation
enhancement. J Magn Reson Imaging. 3:149–156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gandhi SN, Brown MA, Wong JG, Aguirre DA
and Sirlin CB: MR contrast agents for liver imaging: what, when,
how. Radiographics. 26:1621–1636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shokrollahi H: Contrast agents for MRI.
Mater Sci Eng C. 33:4485–4497. 2013. View Article : Google Scholar
|
|
9
|
Yurt A and Kazanci N: Investigation of
magnetic properties of various complexes prepared as contrast
agents for MRI. J Mol Struct. 892:392–397. 2008. View Article : Google Scholar
|
|
10
|
Harisinghani MG, Jhaveri KS, Weissleder R,
Schima W, Saini S, Hahn PF and Mueller PR: MRI contrast agents for
evaluating focal hepatic lesions. Clin Radiol. 56:714–725. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhen ZP and Xie J: Development of
manganese-based nanoparticles as contrast probes for magnetic
resonance imaging. Theranostics. 2:45–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silva AC, Lee JH, Aoki L and Koretsky AR:
Manganese-enhanced magnetic resonance imaging (MEMRI):
methodological and practical considerations. NMR Biomed.
17:532–543. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YJ and Koretsky AP: Manganese ion
enhances T-1-weighted MRI during brain activation: An approach to
direct imaging of brain function. Magn Reson Med. 38:378–388. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paratala BS, Jacobson BD, Kanakia S,
Francis LD and Sitharaman B: Physicochemical characterization, and
relaxometry studies of micro-graphite oxide, graphene
nanoplatelets, and nanoribbons. PloS One. 7:e381852012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura H, Ito N, Kotake F, Mizokami Y
and Matsuoka T: Tumor-detecting capacity and clinical usefulness of
SPIO-MRI in patients with hepatocellular carcinoma. J
Gastroenterol. 35:849–855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Na HB, Song IC and Hyeon T: Inorganic
nanoparticles for MRI contrast agents. Adv Mater. 21:2133–2148.
2009. View Article : Google Scholar
|
|
17
|
Taylor RM, Huber DL, Monson TC, Ali AMS,
Bisoffi M and Sillerud LO: Multifunctional iron platinum stealth
immunomicelles: targeted detection of human prostate cancer cells
using both fluorescence and magnetic resonance imaging. J Nanopart
Res. 13:4717–4729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thunus L and Lejeune R: Overview of
transition metal and lanthanide complexes as diagnostic tools.
Coordin Chem Rev. 184:125–155. 1999. View Article : Google Scholar
|
|
19
|
Sijens PE, van den Bent MJ, Nowak PJ, van
Dijk P and Oudkerk M: 1H chemical shift imaging reveals loss of
brain tumor choline signal after administration of Gd-contrast.
Magn Reson Med. 37:222–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang CA: Magnetic resonance imaging
contrast agents. Design and physicochemical properties of
gadodiamide. Invest Radiol. 28(Suppl 1): S21–S27. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Runge VM, Clanton JA, Herzer WA, Gibbs SJ,
Price AC, Partain CL and James AE Jr: Intravascular contrast agents
suitable for magnetic resonance imaging. Radiology. 153:171–176.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Runge VM, Schoerner W, Niendorf HP,
Laniado M, Koehler D, Claussen C, Felix R and James AE Jr: Initial
clinical evaluation of gadolinium DTPA for contrast-enhanced
magnetic resonance imaging. Magn Reson Imaging. 3:27–35. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaplan GD, Aisen AM and Aravapalli SR:
Preliminary clinical trial of gadodiamide injection: a new nonionic
gadolinium contrast agent for MR imaging. J Magn Reson Imaging.
1:57–62. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Runge VM, Dean B, Lee C, Carolan F and
Heard G: Phase III clinical evaluation of Gd-HP-DO3A in head and
spine disease. J Magn Reson Imaging. 1:47–56. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cordova-Fraga T, Sosa M,
Hernandez-Gonzalez MA, Reyes-Aguilera JA, Solorio S, Ramirez C,
Bautista-Flores E, Reynaga G, Avila-Rodriguez M and De la
Roca-Chiapas JM: Medlar (Achras sapota L.) as oral contrast agent
for MRI of the gastrointestinal tract. Appl Magn Reson. 42:161–167.
2012. View Article : Google Scholar
|
|
26
|
Mayo-Smith WW: Computed body tomography
with MRI correlation. AJR Am J Roentgenol. 173:2661999.
|
|
27
|
Jacobs KE, Behera D, Rosenberg J, Gold G,
Moseley M, Yeomans D and Biswal S: Oral manganese as an MRI
contrast agent for the detection of nociceptive activity. NMR
Biomed. 25:563–569. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li KC, Tart RP, Fitzsimmons JR, Storm BL,
Mao J and Rolfes RJ: Barium sulfate suspension as a negative oral
MRI contrast agent: in vitro and human optimization studies. Magn
Reson Imaging. 9:141–150. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verloh N, Utpatel K, Haimerl M, Zeman F,
Fellner C, Fichtner-Feigl S, Teufel A, Stroszczynski C, Evert M and
Wiggermann P: Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A
histopathologic correlation. Sci Rep. 5:154082015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weissleder R, Bogdanov A and Papisov M:
Drug targeting in magnetic resonance imaging. Magn Reson Q.
8:55–63. 1992.PubMed/NCBI
|
|
31
|
Ahmad MW, Xu W, Kim SJ, Baeck JS, Chang Y,
Bae JE, Chae KS, Park JA, Kim TJ and Lee GH: Potential dual imaging
nanoparticle: Gd2O3 nanoparticle. Sci Rep. 5:85492015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Edelman RR, Siegel JB, Singer A, Dupuis K
and Longmaid HE: Dynamic MR imaging of the liver with Gd-DTPA:
initial clinical results. AJR Am J Roentgenol. 153:1213–1219. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balci NC and Semelka RC: Contrast agents
for MR imaging of the liver. Radiol Clin North Am. 43:887–898.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nolte-Ernsting C, Adam G, Bücker A, Berges
S, Bjørnerud A and Günther RW: Abdominal MR angiography performed
using blood pool contrast agents: comparison of a new
superparamagnetic iron oxide nanoparticle and a linear gadolinium
polymer. AJR Am J Roentgenol. 171:107–113. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lauffer RB, Parmelee DJ, Dunham SU,
Ouellet HS, Dolan RP, Witte S, McMurry TJ and Walovitch RC: MS-325:
albumin-targeted contrast agent for MR angiography. Radiology.
207:529–538. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cavagna FM, Lorusso V, Anelli PL, Maggioni
F and de Haën C: Preclinical profile and clinical potential of
gadocoletic acid trisodium salt (B22956/1), a new intravascular
contrast medium for MRI. Acad Radiol. 9(Suppl 2): S491–S494. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Curtet C, Maton F, Havet T, Slinkin M,
Mishra A, Chatal JF and Muller RN: Polylysine-Gd-DTPAn and
polylysine-Gd-DOTAn coupled to anti-CEA F(ab′)2 fragments as
potential immunocontrast agents. Relaxometry, biodistribution, and
magnetic resonance imaging in nude mice grafted with human
colorectal carcinoma. Invest Radiol. 33:752–761. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Løkling KE, Fossheim SL, Skurtveit R,
Bjørnerud A and Klaveness J: pH-sensitive paramagnetic liposomes as
MRI contrast agents: in vitro feasibility studies. Magn Reson
Imaging. 19:731–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung CW and Jacobs P: Physical and
chemical properties of superparamagnetic iron oxide MR contrast
agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging.
13:661–674. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taupitz M, Schnorr J, Abramjuk C, Wagner
S, Pilgrimm H, Hünigen H and Hamm B: New generation of
monomer-stabilized very small superparamagnetic iron oxide
particles (VSOP) as contrast medium for MR angiography: preclinical
results in rats and rabbits. J Magn Reson Imaging. 12:905–911.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung KH, Kim HK, Park JA, Nam KS, Lee GH,
Chang Y and Kim TJ: Gd Complexes of DO3A-(Biphenyl-2,2′-bisamides)
Conjugates as MRI Blood-Pool Contrast Agents. ACS Med Chem Lett.
3:1003–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bourrinet P, Bengele HH, Bonnemain B,
Dencausse A, Idee JM, Jacobs PM and Lewis JM: Preclinical safety
and pharmacokinetic profile of ferumoxtran-10, an ultrasmall
superparamagnetic iron oxide magnetic resonance contrast agent.
Invest Radiol. 41:313–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weinmann HJ, Ebert W, Misselwitz B and
Schmitt-Willich H: Tissue-specific MR contrast agents. Eur J
Radiol. 46:33–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Corot C and Warlin D: Superparamagnetic
iron oxide nanoparticles for MRI: contrast media pharmaceutical
company R&D perspective. Wiley Interdiscip Rev Nanomed
Nanobiotechnol. 5:411–422. 2013.PubMed/NCBI
|
|
45
|
Runge VM: A comparison of two MR
hepatobiliary gadolinium chelates: Gd-BOPTA and Gd-EOB-DTPA. J
Comput Assist Tomogr. 22:643–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torres CG, Lundby B, Sterud AT, McGill S,
Gordon PB and Bjerknes HS: MnDPDP for MR imaging of the liver -
Results from the European phase III studies. Acta Radiol.
38:631–637. 1997.PubMed/NCBI
|