Introduction
The anterior cruciate ligament (ACL) plays a pivotal
role in maintaining the stability of the knee (1). The normal structural composition of
the native ACL-to-bone interface consists of four distinct tissue
regions, including the ligament, the non-mineralized
fibrocartilage, mineralized fibrocartilage and bone. Due to the
relative avascularity of the fibrocartilage zone and bone loss at
the site of injury (2), the
anatomic insertion site is difficult to regenerate, which has led
orthopedic surgeons to perform ACL reconstructions in the majority
of cases (3). Functional
rehabilitation post-ACL reconstruction largely depends on the
successful healing of the tendon-to-bone interface (4).
In recent years, a number of strategies, including
cell therapy and various growth factors in tissue engineering have
been investigated in an aim to biologically accelerate and improve
the healing of tendon-to-bone interface (5,6).
Bone marrow-derived mesenchymal stem cells (BMSCs) have been
utilized to investigate the regeneration of the tendon-to-bone
interface due to their self-renewal potential and pluripotency for
possible clinical use (7–11). However, there is still
unsatisfactory rehabilitation resulting from the lack of adequate
growth factors which have been shown to be powerful regulators of
biological function (12). Bone
morphogenetic protein 2 (BMP2) belongs to the transforming growth
factor (TGF)-β superfamily, known for its osteoinductive capacity,
which has been well-investigated in the study of tendon-to-bone
healing (13,14). Dong et al reported that the
topical application of BMSCs infected with recombinant BMP2
lentivirus promoted the formation of fibrocartilage-like tissue and
further improved the mechanical properties of the reconstructed ACL
(15), indicating that the
interactions between cells derived from BMSCs and tendon cells
(fibroblasts) play a role in fibrocartilage formation by initiating
the differentiation of BMSCs and phenotypic alteratoins in
fibroblasts through suitable growth factors.
Basic fibroblast growth factor (bFGF) has been found
to be involved in numerous cellular functions, including
angiogenesis, cell proliferation, wound healing, limb formation and
tissue remodeling (16–19). However, to date, there are only a
few studies available on the effects of bFGF (by gene therapy) on
tendon-to-bone regeneration following ACL reconstruction. Kohno
et al demonstrated the abundance of bFGF and BMP2 at the
tendon-bone interface through an immunohistochemical investigation
during the early post-operative stage (12). Their results implied that the
implanted BMSCs genetically modified with both bFGF and BMP2 may
potentially promote tendon-to-bone regeneration.
To examine this hypothesis, we designed a
three-dimensional BMSC-ligament fibroblast co-culture model using
an alginate hydrogel microsphere, which mimics the cellular
organization at the interface in vivo, as well as
facilitating paracrine interactions. To the best of our knowledge,
this is the first report of the interaction of gene-transfected
BMSCs with ACL-derived fibroblasts. We also evaluated the early
effects of co-culture on the differentiation of BMSCs and the
phenotypic maintenance of ligament fibroblasts. The findings of our
study provide a theoretical foundation for the regeneration of the
tendon-to-bone interface.
Materials and methods
Construction of adenoviral vectors
Replication-defective human adenovirus type 5
(SinoGenoMax Co., Ltd., Beijing, China) was used to generate the
recombinant adenoviral vectors as previously described (20). To obtain adenoviral vectors
carrying bFGF and BMP2, the human entire coding sequence of bFGF
(480 bp) and BMP2 (1.2 kb) was inserted into an adenoviral plasmid
containing an enhanced green fluorescent protein (EGFP) under the
control of the cytomegalovirus (CMV) promoter (SinoGenoMax Co.,
Ltd.). The produced vectors were designated as AdbFGF and AdBMP2.
Subsequently, 293 cells purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA) were used to reproduce
recombinant viruses, as previously described (21). Viral titers were measured using a
fluorescence spectrophotometer (F-4500; Hitachi, Tokyo, Japan), as
previously described (22). The
50% tissue culture infective dose (TCID50/ml) was
utilized to detect the quantification of virus infectious titers,
as previously described (23).
Isolation, expansion and characterization
of BMSCs
Two specific pathogen-free Wistar rats weighing
80–100 g (male) were purchased from the Laboratory Animal Center of
Wuhan University (Wuhan, China). The protocol for the use of rats
was approved by the Committee on the Ethics of Animal Experiments
of Wuhan University. The animals were sacrificed by anesthesia with
5% isoflurane. The femurs and tibias were harvested and the
metaphysis on both sides was removed using a rongeur under sterile
conditions. Bone marrow was collected by flushing the femur and
tibia with medium. Following centrifugation at 150 × g for 8 min at
25°C, the cell pellets were mixed thoroughly with Dulbecco's
modified Eagle's medium (DMEM)/F12 (Invitrogen, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY,
USA) and 100 U/ml penicillin-streptomycin in 5% CO2 at
37°C and the cells were subcultured to passage 3 for the following
experiments. The pluripotency of the BMSCs was confirmed by
culturing for osteo-genic and chondrogenic differentiation in
controlled medium as previously described (24,25). Generally, for osteogenic
differentiation, the BMSCs were seeded at a density of
4×104 cells/cm2 in a 6-well plate for 2 weeks
in the osteogenic medium and von Kossa staining (Baso Biotech Co.,
Ltd., Wuhan, China) was then utilized to detect the calcium
deposits. For chondrogenic differentiation, the BMSCs were
suspended at a concentration of 5×106 cells/ml in 1.25%
alginate (Sigma-Aldrich, St. Louis, MO, USA) in 0.15 M NaCl and
slowly dropped into 102 mM CaCl2 solution and then
encapsulated in alginate beads. After 4 weeks of culture, the
alginate bead sections were stained with Alcian blue (ALB; Baso
Biotech Co., Ltd.) and Safranin O (Saf-O; Baso Biotech Co., Ltd.)
for the evaluation of chondrogenesis. Furthermore, BMSC markers
were also analyzed by flow cytometry. Approximately
5×105 cells were incubated with specific phycoerythrin-
or fluorescein isothiocyanate-conjugated monoclonal antibodies for
rat CD29 (25-0291), CD90 (15-0900), CD45 (11-0461) and CD11
(12-0110) (Biolegend, San Diego, CA, USA) and subjected to flow
cytometric analysis using a BD FACSAria™ III flow cytometer (BD
Biosciences, San Jose, CA, USA).
Isolation and expansion of ligament
fibroblasts
The tissue of ACLs were removed from both knee
joints of the rats under aseptic conditions and sliced into
sections (approximately 1 mm3) and then digested with
0.2% collagenase type I (Sigma, Santa Clara, CA, USA) for 3 h at
37°C in an incubator. Following centrifugation at 150 × g for 8 min
at 25°C, the supernatant was discarded, and the recovered cells
were cultured with the same growth medium described above and
subcultured to passage 3 for use in the following experiments.
Gene transfer and establishment of
three-dimensional co-culture model
Adenoviral vectors were transfected into the BMSCs
at a multiplicity of infection (MOI) of 0, 25, 50, 100, 150 and
200. After 48 h, the cells were harvested and used to detect EGFP
expression by flow cytometry, and the cells were also observed
under an inverted fluorescence microscope (4J41302; Olympus,
Tokoyo, Japan). The percentage of live infected cells expressing
EGFP was counted to determine the optimal MOI value. Each
experiment was repeated at least 3 times. Additionally, the
expression of the transfected genes was examined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
The BMSCs were transfected with AdEGFP, AdbFGF, or
AdBMP2, or with AdbFGF plus AdBMP2. At 24 h post-transfection, the
BMSCs were trypsinized and encapsulated in the beads following the
method described above. Twenty beads were placed on one side of the
well each containing a sterile satinless metal cell strainer. The
third passage ligament fibroblasts cultured in alginate beads at a
concentration of 5×106 cells/ml were placed on the other
side of the well. Based on the different treatment of the BMSCs,
the co-culture model was divided into 4 groups as follows: i) the
AdEGFP group, ii) the AdbFGF group, iii) the AdBMP2 group, iv) the
AdbFGF plus AdBMP2 group. The co-cultured cells were incubated for
6 days and the medium was changed every 3 days in all groups.
Cell proliferation assay
The proliferation of the transfected BMSCs and the
ligament fibroblasts in the co-culture model was determined on days
0, 3 and 6. Briefly, on days 0, 3 and 6, the tests were performed
on 96-well plates with one bead in each well. Culture medium
containing MTS solution (Promega, Shanghai, China) was added
followed by incubation for 4 h at 37°C, as previously described
(26). The absorbance at 490 nm
was then measured using a spectrophotometer (Shimadzu, Kyoto,
Japan).
Concentration of bFGF and BMP2 in the
cell supernatant
The concentrations of bFGF and BMP2 in the cell
supernatant following co-culture for 3 days were determined using
enzyme linked immunosorbent assay (ELISA) kits (R&D Systems,
Minneapolis, MN, USA) according to the manufacturer's
instructions.
RNA extraction and RT-qPCR
mRNA expression of related genes in the BMSCs and
ligament fibroblasts was detected by RT-qPCR. Following co-culture
for 6 days, the BMSCs and ligament fibroblasts were recovered from
the alginate beads. Total RNA was isolated using TRIzol reagent
(Invitrogen) following manufacturer's instructions, and was
converted to cDNA using the PrimScript® RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). cDNA was assayed
for mRNA expression, including that of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), scleraxis (SCX), collagen (COL) type 1
(COL1), alkaline phosphatase (ALP), osteocalcin (OCN) and COL3. The
tests were performed on an ABI Step One RT-PCR thermal cycler (ABI
Stepone, Applied Biosystems, Foster City, CA, USA) using the
SYBR® Premix Ex Taq™ kit (Takara Biotechnology Co.,
Ltd.). The housekeeping gene, GAPDH, was used as a quantitative
control. The sequences of the primers used for PCR and the
annealing temperature for the genes used in this experiment are
shown in Table I. The PCR cycling
conditions were as follows: pre-denaturation at 95°C for 30 sec,
denaturation at 95°C for 5 sec, and the annealing conditions for
each gene, and final elongation at 72°C for 30 sec. The Ct value of
fluorescent product was detected at the extension period, and gene
expression in all samples was analyzed by applying the
2−ΔΔCt relative quantification method.
 | Table ISequences of primers for used for
RT-qPCR. |
Table I
Sequences of primers for used for
RT-qPCR.
| Genes | Primer
sequences | Annealing temp
(°C) |
|---|
| GAPDH | F:
GCAAGTTCAACGGCACAG | 60 |
| R:
GCCAGTAGACTCCACGACA | |
| bFGF | F:
GTGTTACGGATGAGTGTTTCT | 60 |
| R:
CAGCTCTTAGCAGACATTGG | |
| BMP2 | F:
AGTGGGTGCTGCTCTTCCTA | 60 |
| R:
ATGGGACACTCCTCTGTTGG | |
| SCX | F:
TGGGTGAAGCCTGCGGTGAC | 60 |
| R:
CGTCTTTCTGTCACGGTCTTTGCT | |
| OCN | F:
CAGACCTAGCAGACACCATG | 60 |
| R:
GCTTGGACATGAAGGCTTTG | |
| ALP | F:
GCCTTACCAACTCATTTGTGC | 60 |
| R:
CATACCATCTCCCAGGAACATG | |
| COL1 | F:
CATGTCTGGTTTGGAGAGAG | 60 |
| R:
CGCTGTTCTTGCAGTGATA | |
| COL3 | F:
CTGGAGTCGGAGGAATGG | 60 |
| R:
GCCAGATGGACCAATAGCA | |
Western blot analysis
Proteins were extracted from the harvested BMSCs and
ligament fibroblasts on day 6 following co-culture. The protein
concentrations were determined by BCA assay (Sigma). Samples of 50
μl protein were separated on 12% sodium dodecyl
sulphate-polyacrylamide gels before being transferred onto
polyvinylidene difluoride membranes (Millipore, Boston, MA, USA).
The membranes were blocked with 5% non-fat milk powder and
incubated with anti-SCX (1:250; sc-87425), anti-ALP (1:200;
sc-79839), anti-OCN (1:1,000; sc-18319), anti-COL1 (1:100; sc-8784)
and anti-COL3 (1:150; sc-8781) antibodies (all from Santa Cruz
Biotechnology Co., Ltd., Santa Cruz, CA, USA), and then incubated
with peroxidase-conjugated secondary antibodies (A0001H; Bluegene
Biotech Co., Ltd., Shanghai, China). The proteins were visualized
by ECL detection (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
following the manufacturer's instructions. GAPDH (sc-48166; Santa
Cruz Biotechnology Co., Ltd.) was used as an internal control.
Statistical analysis
For the quantification of data, each assay was
repeated at least 3 times independently. The results of the
quantitative analyses are expressed as the means ± standard error
of mean (SEM). The means were compared using one-way analysis of
variance (one-way ANOVA) followed by Tukey's post-hoc assuming
equal variances with the SPSS 17 (SPSS, Inc., Chicago, IL, USA). A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of BMSCs
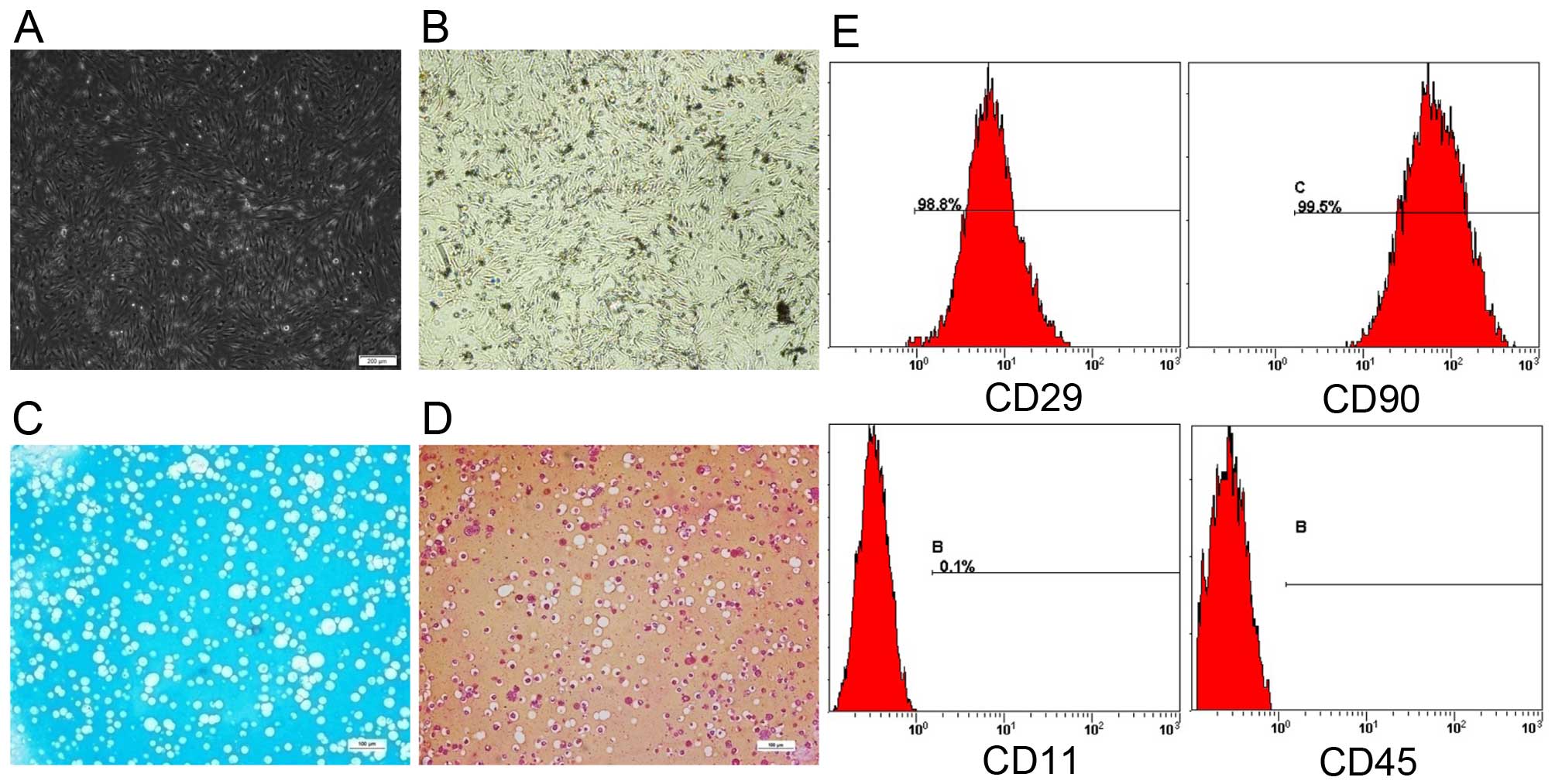
Firstly, BMSCs exhibited a long spindle-like shape
and grew well under the microscope (Fig. 1A). Subsequently, to identify
whether the isolated cells were rat BMSCs, the pluripotency of the
BMSCs was assessed by differentiation toward the osteogenic lineage
by von Kossa staining (Fig. 1B)
and the chondrogenic lineage by ALB staining (Fig. 1C) and Saf-O staining (Fig. 1D). Black calcium deposits were
spotted and the alginate beads sections were positively stained for
glycosaminoglycan with ALB and Saf-O staining, which indicated the
pluripotency of the BMSCs. Lastly, we performed flow cytometric
analysis to demonstrate that the rat BMSCs expressed the specific
mesenchymal stem cell markers, CD29 and CD90, but not the
hematopoietic lineage markers, CD11 and CD45 (Fig. 1E).
Efficiency of adenoviral infection of
BMSCs and successful establishment of the co-culture model
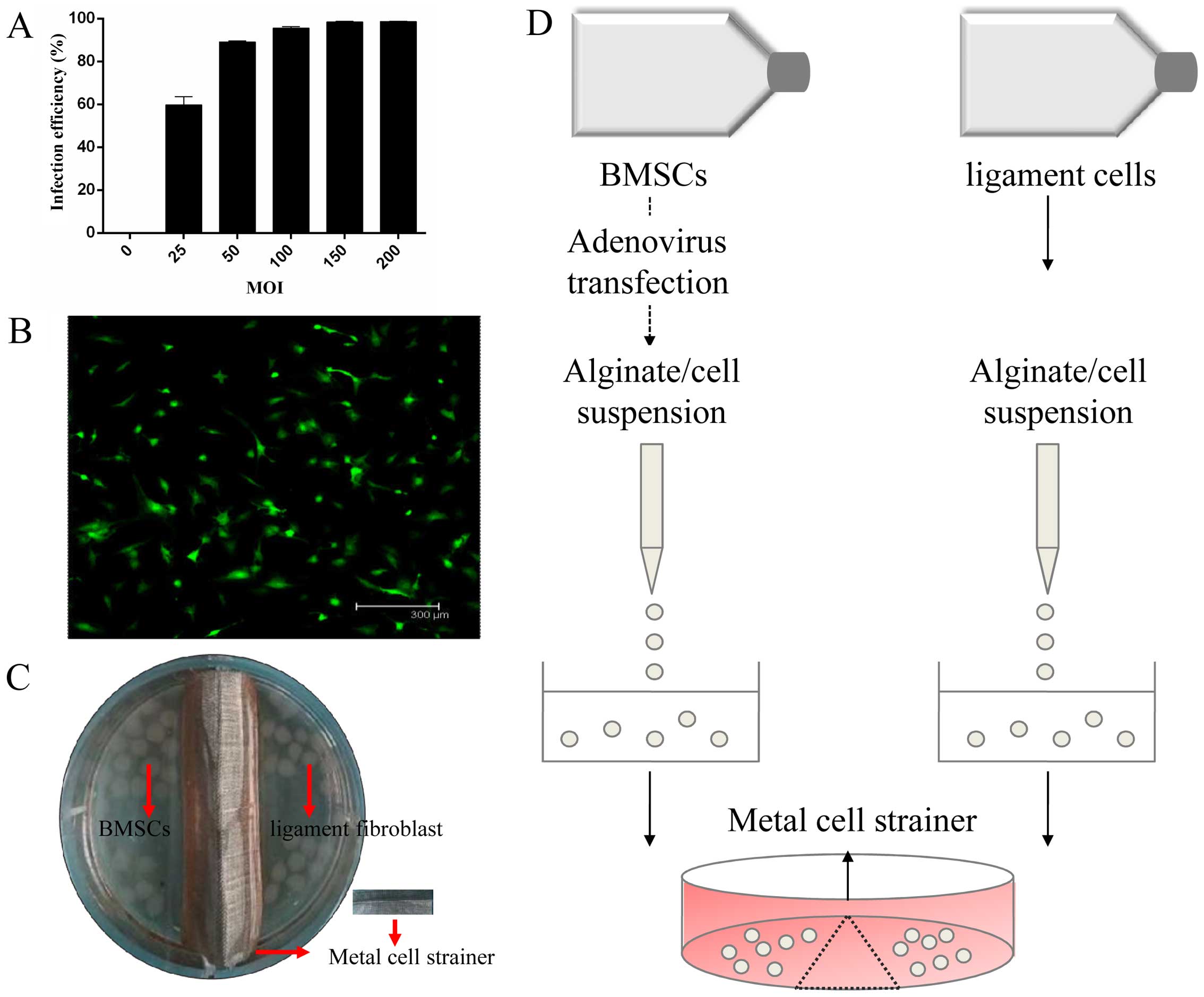
The infection efficiency firmly correlated with the
dosage of adenoviral vectors. The efficiency was 89.1% at an MOI of
50 and 95.5% of the BMSCs were infected at an MOI of 100 (Fig. 2A). The basic principle to
determine the optimal MOI is selecting the most cost-effective
recombinant virus with the least cytotoxicity and higher infection
efficiency; we selected the MOI of 50 as the optimal MOI.
Fluorescence microscopic visualization revealed a high efficiency
of adenovirus at an MOI 50 following transfection (Fig. 2B). The co-culture model developed
by our own laboratory was established using a stainless metal cell
strainer in a 6-well plate (Fig. 2C
and D).
Gene and protein expression of bFGF and
BMP2
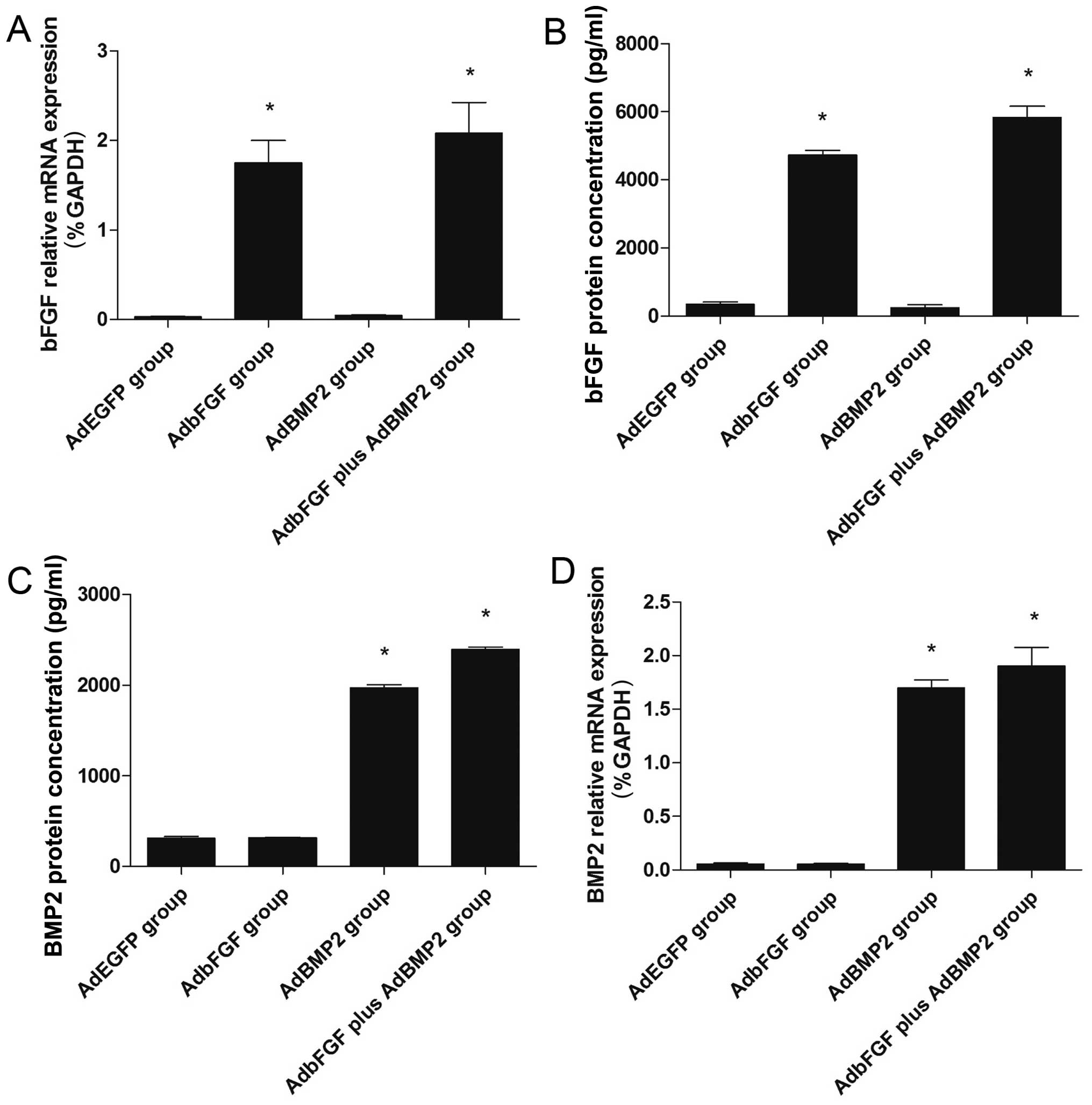
The gene expression of bFGF and BMP2 within 72 h
following transfection was verified by RT-qPCR. The mRNA expression
of bFGF was markedly increased in the AdbFGF group and in the
AdbFGF plus AdBMP2 group, compared with the AdEGFP group (Fig. 3A). The mRNA expression of BMP2 was
significantly enhanced in the AdBMP2 group and in the AdbFGF plus
AdBMP2 group, compared with the AdEGFP group (Fig. 3D).
Similar to the increase observed in mRNA expression,
the protein concentrations in the supernatant were also increased.
The protein concentration of bFGF was higher in the AdbFGF group
(4731.57±224.82 pg/ml) and in the AdbFGF plus AdBMP2 group
(5835.27±568.27 pg/ml), than in the AdEGFP group (343.17±134.18
pg/ml) following co-culture for 3 days (Fig. 3B). The protein concentration of
BMP2 was higher in the Ad-BMP2 group (1948.47±326.86 pg/ml) and in
the AdbFGF plus AdBMP2 group (2135.33±228.09 pg/ml), than in the
AdEGFP group (312.16±31.85 pg/ml) following co-culture for 3 days
(Fig. 3C).
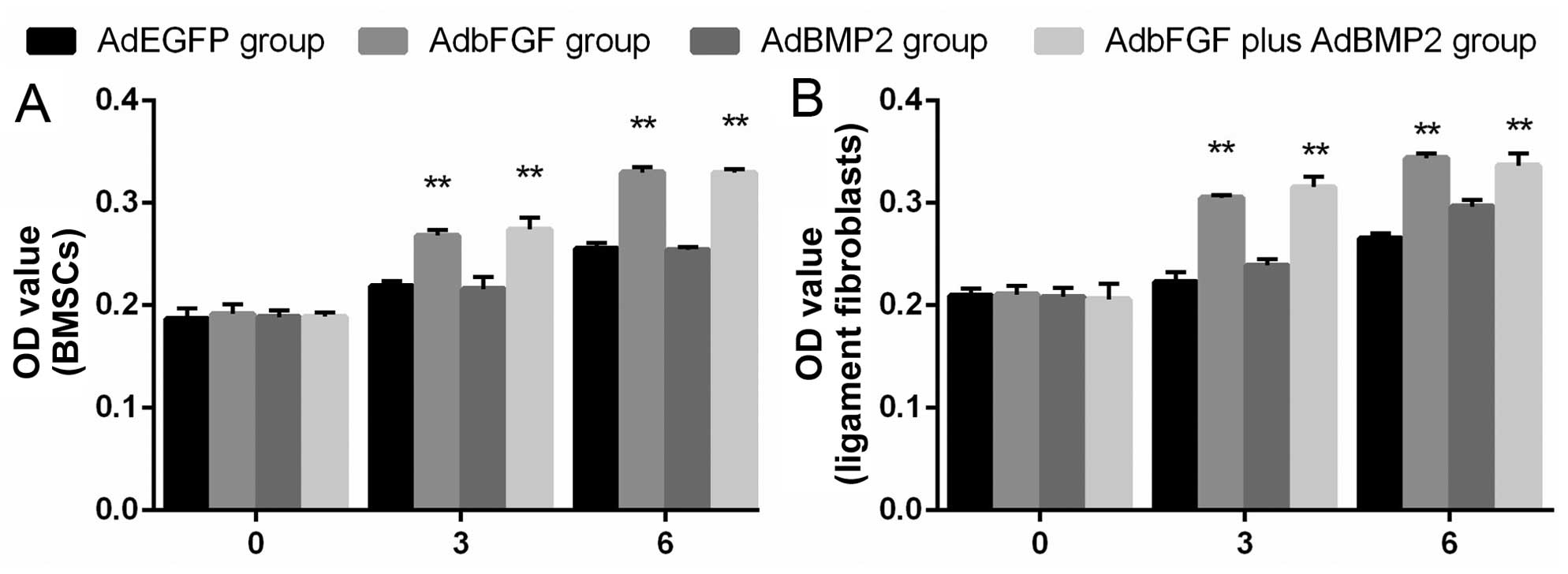
Cell proliferation assay
BMSC proliferation exhibited no obvious changes
among the groups on day 0, which suggested the non-toxicity of the
adenovirus on BMSC viability following transfection at the
indicated MOI. Cell proliferation was markedly enhanced in the
AdbFGF group and in the AdbFGF plus AdBMP2 group on days 3 and 6.
However, there was no significant difference between the AdBMP2
group and AdEGFP group (Fig.
4A).
Moreover, ligament fibroblast proliferation
exhibited similar changes to those of the BMSCs. Cell proliferation
was distinctly increased in the AdbFGF group and in the AdbFGF plus
AdBMP2 group on days 3 and 6; however, no significant difference
was observed between the AdBMP2 group and AdEGFP group (Fig. 4B).
mRNA and protein expression in cells by
RT-qPCR and western blot analysis
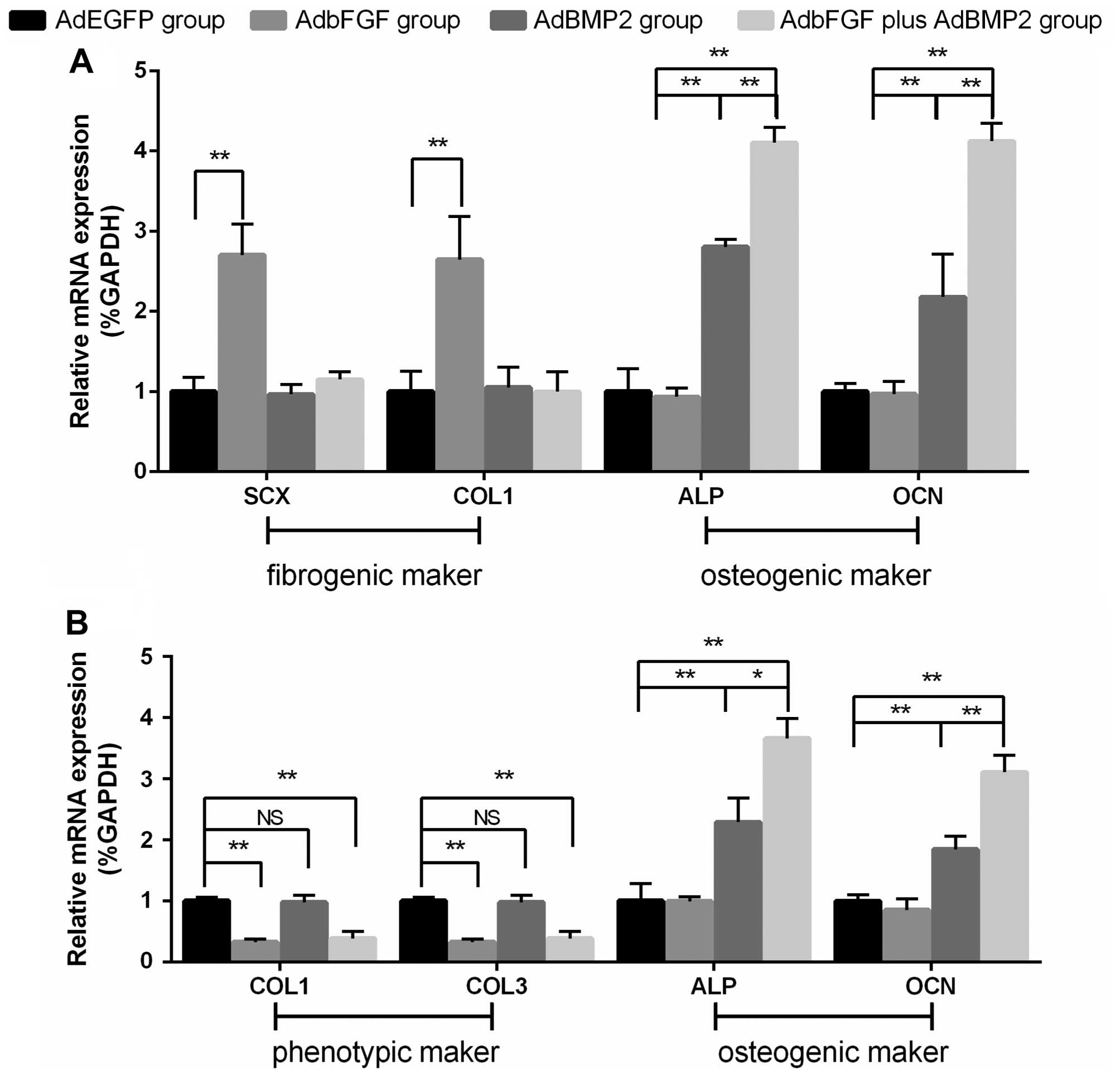
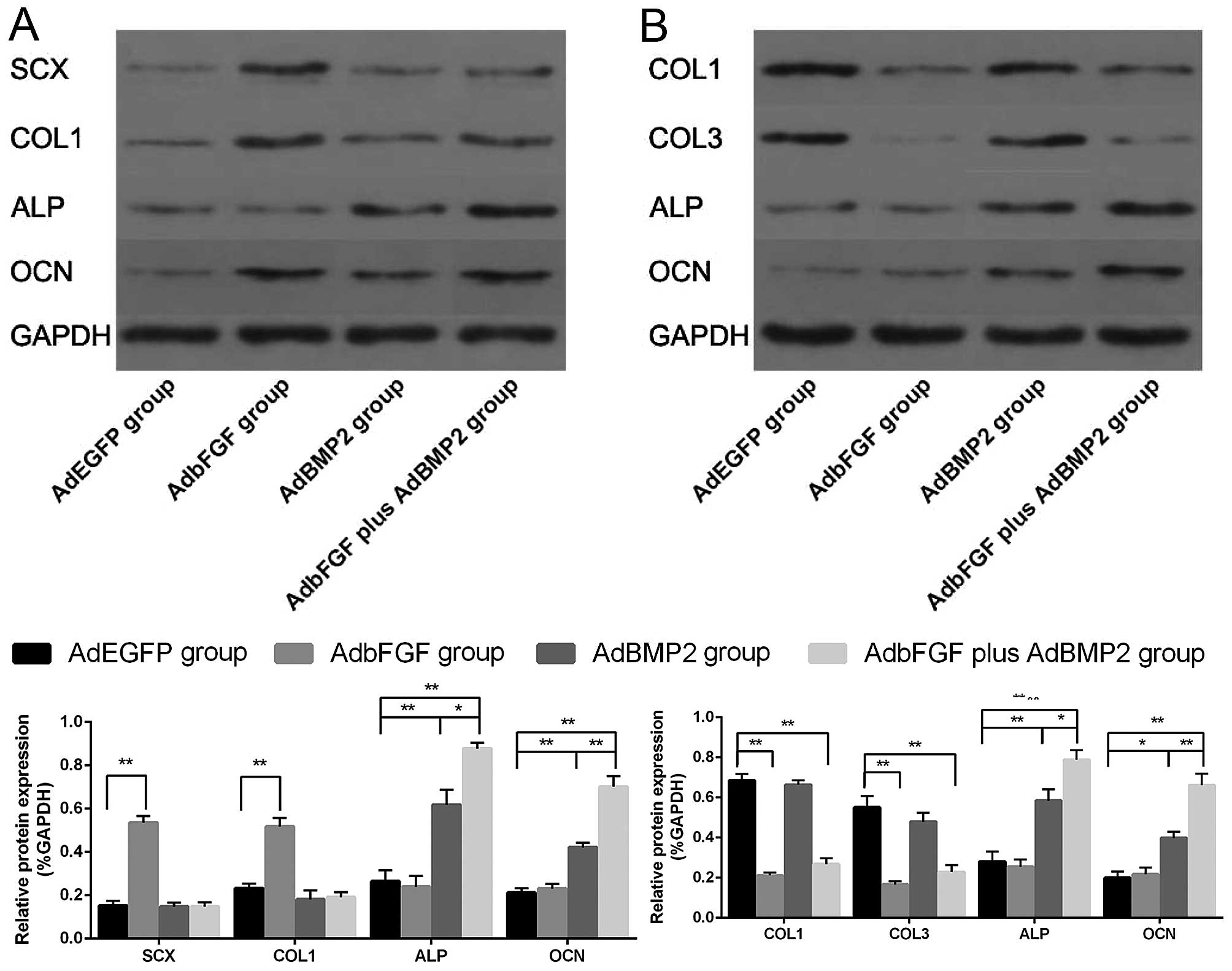
As regards the BMSCs (Figs. 5A and 6A), compared with the AdEGFP group, the
mRNA and protein expression of SCX and COL1 was significantly
elevated in the AdbFGF group. In addition, the mRNA and protein
expression of ALP and OCN was distinctly increased in the AdBMP2
group and in the AdbFGF plus AdBMP2 group. As regards the ligament
fibroblasts (Figs. 5B and
6B), compared with the AdEGFP
group, the mRNA and protein expression of COL1 and COL3 was
significantly decreased in the AdbFGF group and in the AdbFGF plus
AdBMP2 group, although there was no obvious difference between the
AdBMP2 group and the AdEGFP group. Moreover, the mRNA and protein
expression of ALP and OCN was markedly enhanced in the AdBMP2 group
and AdbFGF plus AdBMP2 group. In addition, a higher expression of
ALP and OCN in both the BMSCs and ligament fibroblasts was observed
in the AdbFGF plus AdBMP2 group compared with the AdBMP2 group.
Discussion
Our goal was to elucidate the possible mechanisms
responsible for the regeneration of the tendon-to-bone interface,
and this study focused on the early cellular interactions between
BMSCs expressing bFGF/BMP2 and ligament fibroblasts. A biomimetic
three-dimensional co-culture model was developed to evaluate the
interactions between transfected BMSCs and ligament fibroblasts and
to analyze the potential effects of these communications on the
interface. Our results revealed that the combination of bFGF and
BMP2 promoted the proliferation and osteogenic differentiation of
the BMSCs. On the other hand, the two cytokines not only promoted
the proliferation and differentiation of the ligament fibroblasts,
but also decreased the expression of COL1 and COL3, which are the
main components of the ligament matrix. These effects in the
co-culture system suggest that the implantation of BMSCs expressing
bFGF/BMP2 at the interface may achieve a solid osseointegration
between the grafts and the bone tunnel.
Scaffolds are important components of the tissue
engineering strategy as they define the ultimate shape of the
construct while providing the required mechanical strength during
regeneration and proper cell attachment sites (27). The alginate hydrogel microsphere
as a unique three-dimensional cell delivery scaffold, has been
widely used in tissue engineering, drug delivery and wound healing,
due to retainment of the structural similarity with the
extracellular tissues (28). Due
to these features, we adopted this strategy to encapsulate both
genetically modified BMSCs that served as a source of growth
factors and ligament fibroblasts for maintaining the phenotype
during culture (29). It has been
confirmed that macromolecules with molecular weight of <49 kDa
can penetrate the pores of the alginate hydrogel microspheres to
influence cell behavior (30),
which suggessts that bFGF and BMP2 can enter the microsphere and
regulate the biological behavior of encapsulated BMSCs and ligament
fibroblasts. Furthermore, our previous study also reported the
proliferation and differentiation of BMSCs in alginate hydrogel
microspheres for cartilage tissue engineering (25). Thus, it is possible for alginate
hydrogel microspheres to be used as scaffolds in tissue engineering
techniques.
SCX, detected in the ligament progenitor cells, is
important for the development of the musculoskeletal system and
COL1 and COL3 is the major constituent of ligament (31,32). In our study, for the AdbFGF group,
our results demonstrated that cell proliferation and the expression
of SCX and COL1 in the BMSCs was markedly enhanced, indicating a
shift of BMSCs towards the more mature state of fibroblast-like
cells following the gene transfer of bFGF. Our findings collaborate
with the findings of the in vitro model by Cai et al,
which suggested that bFGF may be an important regulator of the
proliferation and differentiation of BMSCs (33). As regards the ligament
fibroblasts, cell proliferation was markedly enhanced, and this was
accompanied by the decreased expression of COL1 and COL3. Qiu et
al reported similar results, showing that the expansion of
tenocytes treated by bFGF was supported, while collagen synthesis
was significantly decreased (34). Caliari and Harley also confirmed
that bFGF increased the proliferation of equine tenocytes, but
reduced the expression of phenotype-related genes (COL1 and COL3)
within an anisotropic collagen-GAG scaffold, for which they
considered that a single factor led to a dose-dependent trade-off
between driving tenocyte proliferation versus the maintenance of a
tenocyte phenotype (31). In this
study, the possible reason may be that for the expansion phase of
fibroblasts in vitro, early cell differentiation and target
structure formation has to be minimized to enhance nutrient
diffusion. Fully differentiated fibroblasts tend to form thick
layers of collagen around the scaffold, which could prevent the
cells within the scaffold from gaining sufficient nutrients from
the culture medium and consequently would be less likely to
proliferate.
In this study, with respect to the AdBMP2 group, the
early osteogenic differentiation of the BMSCs was noted, evidenced
by the increased expression of OCN and ALP, which was consistent
with other findings reported by other studies (35,36). However, the proliferation and the
expression of collagen (COL1 and COL3) concerning the exposure of
ligament fibroblasts to BMP2 exhibited no obvious changes. Other
in vitro studies have reported consistent results that
neither the proliferation of tenocyte-like cells nor collagen
production was influenced by BMP2 (13,37,38). In our study, the increased
expression of OCN and ALP in ligament fibroblasts was also
observed. This result supports those of the study by
Salingcarnboriboon et al, who showed that the mRNA
expression of ALP and osterix in tendon cell lines (TT-E4, TT-G11
and TT-D6) was extensively increased following culture in the
presence of BMP2 for 3 days (38). Additionally, Steinert et al
demonstrated that ACL-derived cells express stem cell markers and
are able to undergo osteogenic differentiation (39). Our present results indicated that
BMP2 promoted the osteogenic differentiation of ligament
fibroblasts. Hashimoto et al reported that an engineered
bone-to-bone graft, generated by injecting BMP2 into the
semitendinosus tendon to achieve ectopic ossicles, resulted in the
restoration of morphology and function equivalent to those of the
normal ACL (40). Martinek et
al reported that adenoviral BMP-2 transfection of ACL grafts
led to improved bone tunnel integration in rabbits (41). The effects of osteogenic
differentiation of BMP2 on the ligament fibroblasts in our findings
may be a reason for the improved tendon-to-bone healing as reported
above and further suggest that a beneficial effect of implanted
BMSCs expressing BMP2 may be partially caused by enhancing the
osteogenic differentiation of both BMSCs and ligament
fibroblasts.
A cocktail of various growth factors has been
developed to manipulate the biological healing of the
tendon-to-bone interface. Hou et al revealed that the
healing of experimentally injured Achilles tendons in rabbits would
be enhanced by the cell-based gene transfer of vascular endothelial
growth factor (VEGF) and TGF-β1 (42). In an in vitro study, Pauly
et al investigated BMP-2 in combination with BMP-7 and
showed that it could positively affect human rotator cuff ligament
fibroblasts in terms of stimulating cell activity and COL1
production and the expression of several markers (13). In this study, the combination of
bFGF and BMP2 yielded better results, since the combination of both
factors more potently promoted the proliferation and osteogenic
differentiation of BMSCs and ligament fibroblasts along with the
decreased expression of collagen in the ligament fibroblasts,
suggesting the synergistic effects of bFGF and BMP2 in the
co-culture system. Wang et al demonstrated similar results,
showing that the combination of BMP-2 and bFGF was more effective
than either one alone in promoting the formation of new bone
(43). Based on the findings of
this study, it may be presumed that the synergistic effects firstly
began with the continuous fission and proliferation of BMSCs and
ligament fibroblasts, as well as the decreased expression of
phenotype makers in ligament fibroblasts induced by bFGF. During
this process, BMP2 was provided to induce cell differentiation.
However, the detailed mechanisms responsible for these synergistic
effects and whether more benefits could be achieved if more genes
are transfected warrants further investigation.
The duration (6 days) was simply provided for our
preliminary knowledge about the early cellular responses of BMSCs
transfected with bFGF/BMP2 and ligament fibroblast in
three-dimensional co-culture. However, as a limitation to our
study, the terminal effects of long term co-culture between the two
types of cells in vitro remain unknown. In addition, further
studies are required to confirm whether the co-application of bFGF
and BMP2 will achieve a solid osseointegration between the grafts
and the bone tunnel in vivo.
In conclusion, we developed a biomimetic
three-dimensional co-culture model to evaluate the interactions
between transfected BMSCs and ligament fibroblasts. Co-culture of
two types of cells gave rise to cell differentiation and phenotypic
changes. The findings of this study demonstrated the superiority of
combinational growth factors in inducing osteogenic differentiation
and provided a theoretical foundation for the improvement of the
tendon-to-bone interface in vivo.
Acknowledgments
This study was supported by grants from the Natural
Science Foundation of China (no. 81201401 and 81371940).
References
|
1
|
Laurencin CT and Freeman JW: Ligament
tissue engineering: An evolutionary materials science approach.
Biomaterials. 26:7530–7536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong MW, Qin L, Tai JK, Lee SK, Leung KS
and Chan KM: Engineered allogeneic chondrocyte pellet for
reconstruction of fibrocartilage zone at bone-tendon junction - a
preliminary histological observation. J Biomed Mater Res B Appl
Biomater. 70:362–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zantop T, Petersen W, Sekiya JK, Musahl V
and Fu FH: Anterior cruciate ligament anatomy and function relating
to anatomical reconstruction. Knee Surg Sports Traumatol Arthrosc.
14:982–992. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurosaka M, Yoshiya S and Andrish JT: A
biomechanical comparison of different surgical techniques of graft
fixation in anterior cruciate ligament reconstruction. Am J Sports
Med. 15:225–229. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothrauff BB and Tuan RS: Cellular therapy
in bone-tendon interface regeneration. Organogenesis. 10:13–28.
2014. View Article : Google Scholar :
|
|
6
|
Atesok K, Fu FH, Wolf MR, Ochi M, Jazrawi
LM, Doral MN, Lubowitz JH and Rodeo SA: Augmentation of
tendon-to-bone healing. J Bone Joint Surg Am. 96:513–521. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YG, Wei JN, Lu J, Wu XT and Teng GJ:
Labeling and tracing of bone marrow mesenchymal stem cells for
tendon-to-bone tunnel healing. Knee Surg Sports Traumatol Arthrosc.
19:2153–2158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ouyang HW, Goh JC and Lee EH: Use of bone
marrow stromal cells for tendon graft-to-bone healing: Histological
and immunohistochemical studies in a rabbit model. Am J Sports Med.
32:321–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gulotta LV, Kovacevic D, Ehteshami JR,
Dagher E, Packer JD and Rodeo SA: Application of bone
marrow-derived mesenchymal stem cells in a rotator cuff repair
model. Am J Sports Med. 37:2126–2133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gulotta LV, Kovacevic D, Montgomery S,
Ehteshami JR, Packer JD and Rodeo SA: Stem cells genetically
modified with the developmental gene MT1-MMP improve regeneration
of the supraspinatus tendon-to-bone insertion site. Am J Sports
Med. 38:1429–1437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim JK, Hui J, Li L, Thambyah A, Goh J and
Lee EH: Enhancement of tendon graft osteointegration using
mesenchymal stem cells in a rabbit model of anterior cruciate
ligament reconstruction. Arthroscopy. 20:899–910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kohno T, Ishibashi Y, Tsuda E, Kusumi T,
Tanaka M and Toh S: Immunohistochemical demonstration of growth
factors at the tendon-bone interface in anterior cruciate ligament
reconstruction using a rabbit model. J Orthop Sci. 12:67–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pauly S, Klatte F, Strobel C, Schmidmaier
G, Greiner S, Scheibel M and Wildemann B: BMP-2 and BMP-7 affect
human rotator cuff tendon cells in vitro. J Shoulder Elbow Surg.
21:464–473. 2012. View Article : Google Scholar
|
|
14
|
Wang CJ, Weng LH, Hsu SL, Sun YC, Yang YJ,
Chan YS and Yang YL: pCMV-BMP-2-transfected cell-mediated gene
therapy in anterior cruciate ligament reconstruction in rabbits.
Arthroscopy. 26:968–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Y, Zhang Q, Li Y, Jiang J and Chen S:
Enhancement of tendon-bone healing for anterior cruciate ligament
(ACL) reconstruction using bone marrow-derived mesenchymal stem
cells infected with BMP-2. Int J Mol Sci. 13:13605–13620. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woad KJ, Hunter MG, Mann GE, Laird M,
Hammond AJ and Robinson RS: Fibroblast growth factor 2 is a key
determinant of vascular sprouting during bovine luteal
angiogenesis. Reproduction. 143:35–43. 2012. View Article : Google Scholar
|
|
17
|
Fei Y, Xiao L, Doetschman T, Coffin DJ and
Hurley MM: Fibroblast growth factor 2 stimulation of osteoblast
differentiation and bone formation is mediated by modulation of the
Wnt signaling pathway. J Biol Chem. 286:40575–40583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsurushima H, Marushima A, Suzuki K, Oyane
A, Sogo Y, Nakamura K, Matsumura A and Ito A: Enhanced bone
formation using hydroxyapatite ceramic coated with fibroblast
growth factor-2. Acta Biomater. 6:2751–2759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Song K, Rao N, Huang M, Huang Z
and Cao Y: Roles of exogenously regulated bFGF expression in
angiogenesis and bone regeneration in rat calvarial defects. Int J
Mol Med. 27:545–553. 2011.PubMed/NCBI
|
|
20
|
Chen B, Qin J, Wang H, Magdalou J and Chen
L: Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene
transfer on human osteoarthritic chondrocytes and osteoarthritis in
rabbits. Exp Mol Med. 42:684–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hillgenberg M, Hofmann C, Stadler H and
Löser P: High-efficiency system for the construction of adenovirus
vectors and its application to the generation of representative
adenovirus-based cDNA expression libraries. J Virol. 80:5435–5450.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mi Z, Ghivizzani SC, Lechman ER, Jaffurs
D, Glorioso JC, Evans CH and Robbins PD: Adenovirus-mediated gene
transfer of insulin-like growth factor 1 stimulates proteoglycan
synthesis in rabbit joints. Arthritis Rheum. 43:2563–2570. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin HT, Tsai HY, Liu CP and Yuan TT:
Comparability of bovine virus titers obtained by
TCID50/ml and FAID50/ml. J Virol Methods. 165:121–124.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae SE, Bhang SH, Kim BS and Park K:
Self-assembled extracellular macromolecular matrices and their
different osteogenic potential with preosteoblasts and rat bone
marrow mesenchymal stromal cells. Biomacromolecules. 13:2811–2820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng Y, Li TQ, Yan YE, Magdalou J, Wang H
and Chen LB: Effect of nicotine on chondrogenic differentiation of
rat bone marrow mesenchymal stem cells in alginate bead culture.
Biomed Mater Eng. 22:81–87. 2012.PubMed/NCBI
|
|
26
|
Deng Y, Zhou H, Yan C, Wang Y, Xiao C, Gu
P and Fan X: In vitro osteogenic induction of bone marrow stromal
cells with encapsulated gene-modified bone marrow stromal cells and
in vivo implantation for orbital bone repair. Tissue Eng Part A.
20:2019–2029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Venkatesan J, Bhatnagar I, Manivasagan P,
Kang KH and Kim SK: Alginate composites for bone tissue
engineering: A review. Int J Biol Macromol. 72:269–281. 2015.
View Article : Google Scholar
|
|
28
|
Lee KY and Mooney DJ: Alginate: Properties
and biomedical applications. Prog Polym Sci. 37:106–126. 2012.
View Article : Google Scholar
|
|
29
|
Qiu Y, Wang X, Zhang Y, Carr AJ, Zhu L,
Xia Z and Sabokbar A: In vitro two-dimensional and
three-dimensional tenocyte culture for tendon tissue engineering. J
Tissue Eng Regen Med. 10:E216–E226. 2016. View Article : Google Scholar
|
|
30
|
Moshaverinia A, Xu X, Chen C, Akiyama K,
Snead ML and Shi S: Dental mesenchymal stem cells encapsulated in
an alginate hydrogel co-delivery microencapsulation system for
cartilage regeneration. Acta Biomater. 9:9343–9350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caliari SR and Harley BA: Composite growth
factor supplementation strategies to enhance tenocyte bioactivity
in aligned collagen-GAG scaffolds. Tissue Eng Part A. 19:1100–1112.
2013. View Article : Google Scholar :
|
|
32
|
Caliari SR, Weisgerber DW, Ramirez MA,
Kelkhoff DO and Harley BA: The influence of
collagen-glycosaminoglycan scaffold relative density and
microstructural anisotropy on tenocyte bioactivity and
transcriptomic stability. J Mech Behav Biomed Mater. 11:27–40.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai TY, Zhu W, Chen XS, Zhou SY, Jia LS
and Sun YQ: Fibroblast growth factor 2 induces mesenchymal stem
cells to differentiate into tenocytes through the MAPK pathway. Mol
Med Rep. 8:1323–1328. 2013.PubMed/NCBI
|
|
34
|
Qiu Y, Wang X, Zhang Y, Carr AJ, Zhu L,
Xia Z and Sabokbar A: Development of a refined tenocyte expansion
culture technique for tendon tissue engineering. J Tissue Eng Regen
Med. 8:955–962. 2014. View Article : Google Scholar
|
|
35
|
Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T
and Wei F: BMP2 and VEGF promote angiogenesis but retard terminal
differentiation of osteoblasts in bone regeneration by
up-regulating Id1. Acta Biochim Biophys Sin (Shanghai). 43:796–804.
2011. View Article : Google Scholar
|
|
36
|
Park SY, Kim KH, Shin SY, Koo KT, Lee YM
and Seol YJ: Dual delivery of rhPDGF-BB and bone marrow mesenchymal
stromal cells expressing the BMP2 gene enhance bone formation in a
critical-sized defect model. Tissue Eng Part A. 19:2495–2505. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murray SJ, Santangelo KS and Bertone AL:
Evaluation of early cellular influences of bone morphogenetic
proteins 12 and 2 on equine superficial digital flexor tenocytes
and bone marrow-derived mesenchymal stem cells in vitro. Am J Vet
Res. 71:103–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salingcarnboriboon R, Yoshitake H, Tsuji
K, Obinata M, Amagasa T, Nifuji A and Noda M: Establishment of
tendon-derived cell lines exhibiting pluripotent mesenchymal stem
cell-like property. Exp Cell Res. 287:289–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steinert AF, Kunz M, Prager P, Barthel T,
Jakob F, Nöth U, Murray MM, Evans CH and Porter RM: Mesenchymal
stem cell characteristics of human anterior cruciate ligament
outgrowth cells. Tissue Eng Part A. 17:1375–1388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashimoto Y, Naka Y, Fukunaga K, Nakamura
H and Takaoka K: ACL reconstruction using bone-tendon-bone graft
engineered from the semitendinosus tendon by injection of
recombinant BMP-2 in a rabbit model. J Orthop Res. 29:1923–1930.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinek V, Latterman C, Usas A,
Abramowitch S, Woo SL, Fu FH and Huard J: Enhancement of
tendon-bone integration of anterior cruciate ligament grafts with
bone morphogenetic protein-2 gene transfer: A histological and
biomechanical study. J Bone Joint Surg Am. 84-A:1123–1131.
2002.PubMed/NCBI
|
|
42
|
Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang
H, Fu X, Zhang J and Yu C: Effects of transforming growth
factor-beta1 and vascular endothelial growth factor 165 gene
transfer on Achilles tendon healing. Matrix Biol. 28:324–335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang L, Zou D, Zhang S, Zhao J, Pan K and
Huang Y: Repair of bone defects around dental implants with bone
morphogenetic protein/fibroblast growth factor-loaded porous
calcium phosphate cement: A pilot study in a canine model. Clin
Oral Implants Res. 22:173–181. 2011. View Article : Google Scholar
|