Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
joint disease and is characterized by the proliferation of
synoviocytes that produce inflammatory cytokines and chemokines
(1). Despite significant
therapeutic advances, there is still a need for effective
treatments for RA (2). RA not
only decreases the quality of life and life expectancy of patients,
but also and most commonly, accelerates atherosclerosis (3). The hallmarks of RA are leukocytic
infiltration of the synovium and the expansion of fibroblast-like
synoviocytes (FLS) (4). FLS are
the resident mesenchymal cells of synovial joints and are located
in the lining of the joints. They play an increasingly important
role in the pathogenesis of RA, and participate in all the
pathological events of RA (5).
Apoptosis is a key mechanism that regulates tissue
composition and homeostasis (6).
It can be considered a therapeutic tool in RA, and alterations in
the apoptosis of synovial cells have been described in residential
synoviocytes, as well as in inflammatory cells (7,8).
Long non-coding RNAs (lncRNAs) are a recently discovered class of
non-protein coding RNAs (9,10),
and metastasis associated lung adenocarcinoma transcript 1
(MALAT1), a well-described lncRNA of of >8,000 nt in length
expressed on chromosome 11q13, is widely expressed in normal
tissues (11–13). MALAT1 has been recently observed
to be upregulated in various human cancers and has been shown to be
associated with cancer metastasis and recurrence of hepatocellular
carcinoma following liver transplantation (14). In bladder cancer, the upregulation
of MALAT1 has been reported to contribute to enhanced cell
migration by inducing epithelial-to-mesenchymal transition
(15). MALAT1 has also been
reported to control cell cycle progression by regulating the
expression of the oncogenic transcription factor, B-MYB (16). In osteosarcoma, MALAT1 has been
shown to promote the proliferation and metastasis of osteosarcoma
cells by activating the phosphoinositide 3-kinase (PI3K)/AKT
pathway (17). The P13K/AKT
signaling pathway plays a critical role in regulating basic
cellular functions, such as the control of transcription and
translation (18). The P13K/AKT
pathway is also involved in apoptosis; when this pathway is
activated, apoptosis is then decreased and cell proliferation
increases (19).
Quercetin is a flavonoid molecule ubiquitous in
nature (20). It is a dietary
antioxidant that prevents the oxidation of low-density lipoprotein
in vitro by the scavenging of free oxygen radicals and the
inhibition of the growth of certain types of cancer cell (21,22). It has been shown to prevent and
protect against streptozotocin-induced oxidative stress and β-cell
damage in the rat pancreas (23).
Quercetin has been shown to be effective in the management of
arthritis (24). It has also been
shown to inhibit the release of macrophage-derived cytokines and
nitric oxide (25).
In this study, we aimed to elucidate the mechanisms
through which quercetin affects FLS from patients with RA (termed
RAFLS) and the involvement of possible signaling pathways. First,
we determined the relative expression levels of differentially
expressed lncRNAs and the proliferation of RAFLS, and screened the
most significantly differentially expressed lncRNA. The screened
lncRNA was then knocked down and the apoptosis of RAFLS was
analyzed. At the same time, the protein expression levels of
caspase-3 and caspase-9, Bax, Bcl-2, phosphorylated (p-)P13K, P13K,
p-AKT, AKT, p-mammalian target of rapamycin (mTOR) and mTOR were
determined by western blot analysis.
Materials and methods
Cell culture and treatment
Rheumatoid arthritis fibroblast-like synoviocytes
(RAFLS) used in this study were obtained from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and
100 µg/ml streptomycin in a humidified cell incubator at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
The medium was changed every 24 h and cells at passages 3 to 8 were
used in the following experiments. Each experiment performed
thrice. All the samples were stored at −80°C for further use.
Cell tranfection
MALAT1 and HOTAIR small interfering RNAs (siRNAs)
and scrambled RNA were purchased from Dharmacon Research, Inc.
(Lafayette, CO, USA). Transfection was conducted with cationic
lipopolyamines (Invitrogen, Carlsbad, CA, USA). RAFLS at
approximately 70–80% confluence were used for transfection. siRNA
targeting MALAT1 (siRNA-MALAT1-1, -2, -3 or si-MALAT1-1, -2, -3),
siRNA targeting HOTAIR or scrambled RNA control (siRNA-Scramble or
si-Scramble) in Opti-MEM (Invitrogen) was added at a final
concentration of 5 µg/ml DNA. The control cells represent
normal RAFLS without any transfection. Following incubation for 4
h, fresh growth medium was added, and the cells were cultured as
described above.
RNA extraction and PCR array
Total RNA was extracted and isolated from the cells
using the Agilent Technologies Total RNA isolation mini kit
(Agilent Technologies, Palo Alto, CA, USA) according to the
manufacturer's instructions. Spectrophotometric methods were used
to assess the quality and quantity of the RNA samples. PCR array
was used to screen the differentially expressed lncRNAs following
treatment with quercetin.
A PCR array is a highthroughput RT-PCR system which
is based on ATAC-PCR. A 96-well PCR plate was prepared with a
different primer-probe in each well as the PCR array. In brief, the
experimental procedure was as follows: RNA was converted into cDNA,
digested by a restriction enzyme, and then ligated to an adaptor
with the cohesive ends created by the enzyme. After mixing the
ligated samples, PCR amplification was conducted with an adaptor
primer and a gene-specific primer. The PCR specific procedure was:
denaturing at 95°C for 15 sec, annealing for 30 sec at 60°C, and
extension for 30 sec at 72°C. A total of 40 PCR cycles were
performed.
Quantitative PCR (qPCR)
To quantitatively determine the messenger RNA (mRNA)
expression levels of MALAT1, HOX transcript antisense RNA (HOTAIR),
prostate cancer associated rranscript 1 (PCAT1), WD repeat
containing, antisense to TP53 (WRAP53), long stress-induced
non-coding transcript 5 (LSINCT5), 7SK, C1QTNF9B antisense RNA 1
(C1QTNF9B-AS1), breast cancer anti-estrogen resistance 4 (BCAR4),
maternally expressed 3 (MEG3), carbonyl reductase 3 antisense RNA
(CBR3-AS), growth arrest-specific 5 (GAS5), ZNFX1 antisense RNA 1
(ZNFX1-AS1), HIF1A anti-sense RNA 1 (HIF1A-AS1), DNM3 opposite
strand/antisense RNA (DNM3OS), brain cytoplasmic RNA 1 (BCYRN1) and
Yiya in the RAFLS treated with quercetin (RAFLS + quercetin) or in
the untreated RAFLS (RAFLS − quercetin), as well as those of MALAT1
in normal cells (controls) or in the cells tranfected with
siRNA-MALAT1 (si-MALAT1-1, -2, -3) or siRNA-Scramble (si-Scramble),
qPCR was used. Genes were amplified by specific oligonucleotide
primers, and the human glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) gene was used as an endogenous control. The primers were
the following: PCAT-1 forward, 5′-AATGGCATGAACCTGGGAGGCG-3′ and
reverse, 5′-GGCTTTGGGAAGTGCTTTGGAG-3′; WRAP53 forward,
5′-TGGCACAAAGCTGGACAGT-3′ and reverse 5′-GCTGGGTCCTGGTCTGAAG-3′;
CBR3-AS1 forward, 5′-CTTCTGGTTACAATGATTCTC-3′ and reverse,
5′-CACTTACTGCCTACATTAGA-3′; HIF1A-AS1 forward,
5′-AATGTGTTCCTTGCTCTT-3′ and reverse, 5′-GTATGTCTCAGTTATCTTCCT-3′;
LSINCT5 forward, 5′-TTCGGCAAGCTCCTTTTCTA-3′ and reverse,
5′-GCCCAAGTCCCAAAAAGTTCT-3′; HOTAIR forward,
5′-TGCTACTTGTGTAGACCCAG-3′ and reverse,
5′-AGCAAAGGCTGGACCTTTGCT-3′; MALAT1 forward,
5′-TGATAGCCAAATTGAGACAA-3′ and reverse 5′-TTCAGGGTGAGGAAGTAAAA-3′;
7SK forward, 5′-AAACAAGCTCTCAAGGTC-3′ and reverse,
5′-CCTCATTTGGATGTGTCT-3′; BCAR4 forward, 5′-GGACTCATTGTTGTTCTAC-3′
and reverse, 5′-ACCTATGGCTATCATTGTT-3′; GAS5 forward,
5′-CCCAAGGAAGGATGAG-3′ and reverse, 5′-ACCAGGAGCAGAACCA-3′; MEG3
forward, 5′-CTGCCCATCTACACCTCACG-3′ and reverse,
5′-CTCTCCGCCGTCTGCGCTAGGGGCT-3′; CIQTNF9B -AS1 forward,
5′-CGGCGTGGTGTAGCGT-TC-3′ and reverse, 5′-GTGCAGCCTGCGACGGT-3′;
Yiya forward, 5′-TATCCTATTCTTAGCAACTG-3′ and reverse,
5′-ACATACCTGGCATATAGT-3′; ZNFX1-AS1 forward,
5′-CCAGTTCCACAAGGTTAC-3′ and reverse, 5′-GCAGGTAGGCAGTTAGAA-3′;
DNM3OS forward, 5′-ATAGAGCAAGTCTGGATT-3′ and reverse,
5′-GGATGAGGCAATAACATT-3′; BCYRN1 forward, 5′-CTGGGCAATATAGCGAGAC-3′
and reverse, 5′-TGCTTTGAGGGAAGTTACG-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTGATTTTGGAGGGATCTC-3′.
The detection and quantification of mRNA expression involved the
following steps: first, reverse transcription was performed at 55°C
for 30 min, initial activation for 15 min at 95°C, next 40 cycles
of denaturation were conducted at 94°C for 15 sec, then annealing
for 30 sec at 55°C, extension for 30 sec at 72°C. The expression
level was normalized using U6 small nuclear RNA by the
2−ΔCt method. The ΔCt values were normalized to GAPDH
level.
Western blot analysis
To determine the protein expression of signaling
pathways which may be part of the molecular mechanisms involved in
the effects of quercetin on RAFLS, western blot analysis was used.
The cultured cells were lysed in RIPA buffer consisting of 1%
sodium dodecyl sulfate (SDS), 0.1 mM phenylmethylsulfonyl fluoride
(PMSF) and complete protease inhibitors (Roche, Mannheim, Germany).
The lysates were centrifuged at 13,000 × g for 15 min, and the
supernatants were frozen at −80°C until use. The BCA protein assay
kit (Pierce Chemical Co., Rockford, IL, USA) was used to measure
the protein concentration of the lysates. Protein samples (20
µg) prepared as indicated above were resolved by
SDS-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes (Millipore Corp., Bedford, MA, USA). After
blocking in 5% non-fat milk in Tris-buffered saline for 1 h at room
temperature, the membranes were incubated in primary antibodies
[including rabbit polyclonal to caspase-3 (1:500, Cat. no. ab13847;
Abcam, Cambridge, MA, USA), mouse monoclonal to caspase-9 (1:300,
Cat. no. sc-56076; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit polyclonal to Bax (1:500, Cat. no. ab10813; Abcam),
rabbit polyclonal to Bcl2 (1:1,000, Cat. no. ab59348; Abcam), mouse
monoclonal to GAPDH (1:400, Cat. no. sc-365062; Santa Cruz
Biotechnology, Inc.), rabbit polyclonal to p-PI3K (1:300, Cat. no.
BS4811; Biogot Technology Co., Ltd., Nanjing, China), rabbit
polyclonal to PI3K (1:1,000, Cat. no. ab10813; Abcam), mouse
monoclonal to Akt (1:200, Cat. no. sc-5298; Santa Cruz
Biotechnology, Inc.), mouse monoclonal to p-Akt (1:800, Cat. no.
ab38449; Abcam), rabbit polyclonal to mTOR (1:2,000, Cat. no.
ab2732; Abcam) and rabbit polyclonal to p-mTOR (1:800, Cat. no.
ab1093; Abcam)], followed by horseradish peroxidase-conjugated
secondary antibody (both from Santa Cruz Biotechnology, Inc.). The
blots were scanned on the Fluor-S MAX MultiImager, and signal
intensities were determined using Quantity One image software (both
from Bio-Rad Laboratories, Inc., Hercules, USA).
Cell viability assay
To analyze RAFLS viability, the Cell Counting kit-8
(CCK-8) (Dojindo Laboratories, Kumamoto, Japan) was used in
accordance with the manufacturer's instructions. An RAFLS
suspension of 100 ml following treatment with quercetin was plated
in a 96-well plate supplemented with DMEM supplemented with 10% FBS
with various concentrations (0, 10, 50, 100, 150, 200 and 300
µM) of quercetin (Sigma-Aldrich, St. Louis, MO, USA).
Following culture for 24, 48 and 72 h at 37°C with 5%
CO2, CCK-8 (10 ml) was added to each well. Following
incubation for 1–4 h at 37°C with 5% CO2, the optical
density (OD) values were measured using an enzyme-labeled
instrument (Varian Medical Systems, Inc., Palo Alto, CA, USA) at
450 nm.
Analysis of apoptosis
RAFLS apoptosis in the control, si-MALAT1 and
si-Scramble group was analyzed using an Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit I (BD Pharmingen,
Heidelberg, Germany) according to the manufacturer's instructions.
Following transfection with si-MALAT1 or si-Scramble, the cells
were collected by trypsinization and washed with phosphate-buffered
saline (PBS), and then plated on 6-well plates at the concentration
of 1×106 cells/ml. Following incubation for 72 h at 37°C
with 5% CO2, the cells were fixed by 70% pre-cooled
ethanol and 100 µl RNase (10 mg/ml) was then added. The
cells were then stained with 5 µl Annexin V-FITC and
propidium iodide (PI). The samples were then incubated for 15 min
in the dark. Apoptosis was analyzed using a FACSCalibur flow
cytometry with CellQuest software (both from Becton-Dickinson,
Mountain View, CA, USA). The cells undergoing apoptosis were
Annexin V-FITC-positive and PI-negative.
Statistical analysis
The differences between the quercetin-treated and
control cells were analyzed using the Student's t-test and a
probability value of p<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using the SAS 6.12 software package.
Results
Cell viability following treatment with
quercetin
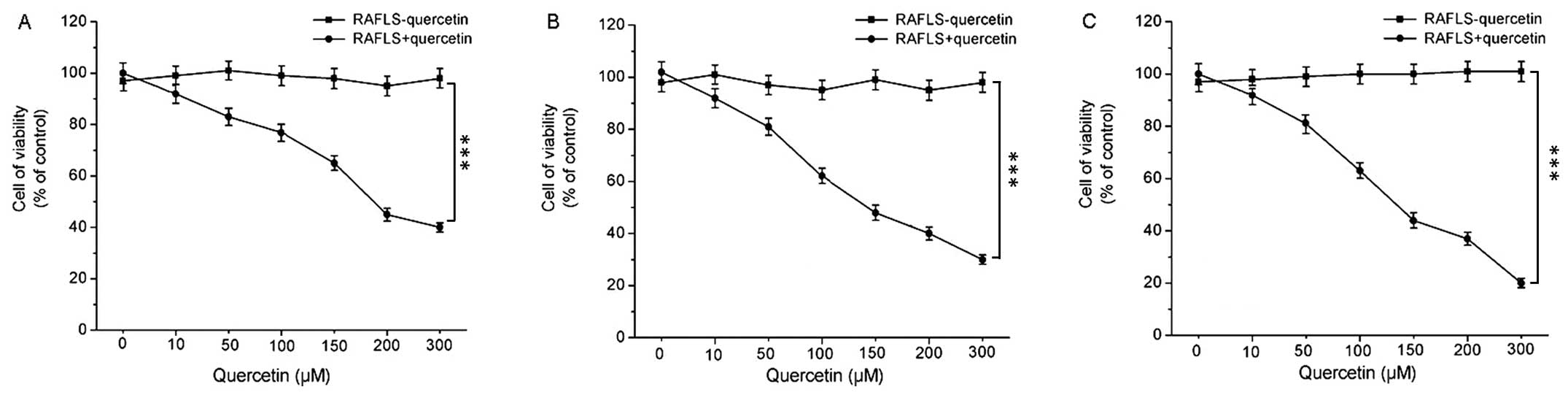
In order to examine the effects of quercetin on
RAFLS viability, the cells were treated with various concentrations
of quercetin for 24, 48 and 72 h and the RAFLS were then examined
by flow cytometry. The results of cell viability determined at 24,
48 and 72 h are shown in Fig.
1A–C, respectively. RAFLS viability markedly decreased
following treatment with increasing concentrations of quercetin,
and the cell viability also markedly decreased in a time-dependent
manner even at the same quercetin concentration. The cell viability
remained stable at all time points examined when no quercetin was
used.
Cell apoptosis following treatment with
quercetin
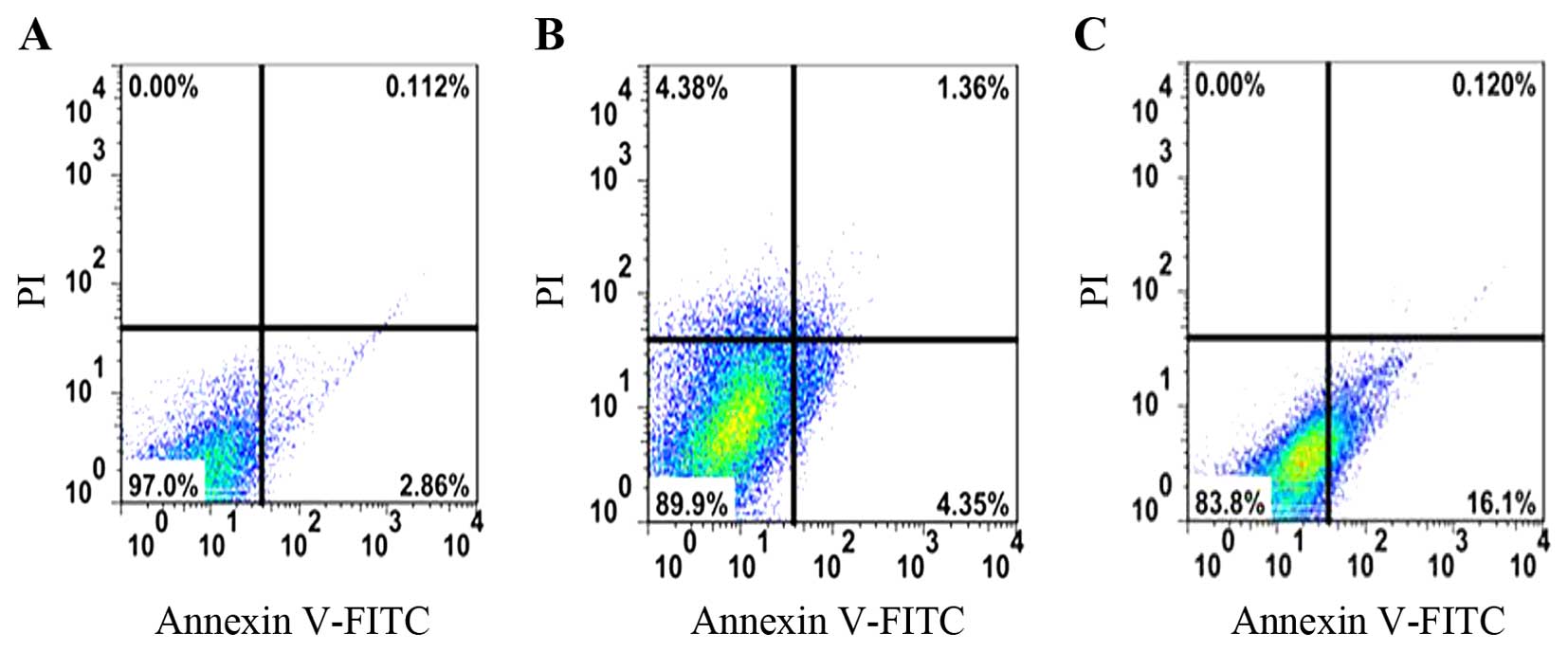
Apoptosis plays a key role in tissue homoeostasis
both under physiological and pathological conditions (26). It has been demonstrated that
certain characteristic changes occur in the composition and
structure of the inflamed synovial membrane in RA (27). Therefore, in this study, the RAFLS
apoptotic rate in the controls, or in the cells treated with 200
µM quercetin (effective concentration from Fig. 1) for 24, 48 and 72 h was analyzed
and the results are shown in Fig.
2. The apoptotic rate of the cells treated with quercetin for
24, 48 and 72 h was 0.112, 1.36 and 0.120%, respectively. These
rates were much higher than those of the controls.
Treatment with quercetin results in the
upregulation of lncRNA MALAT1
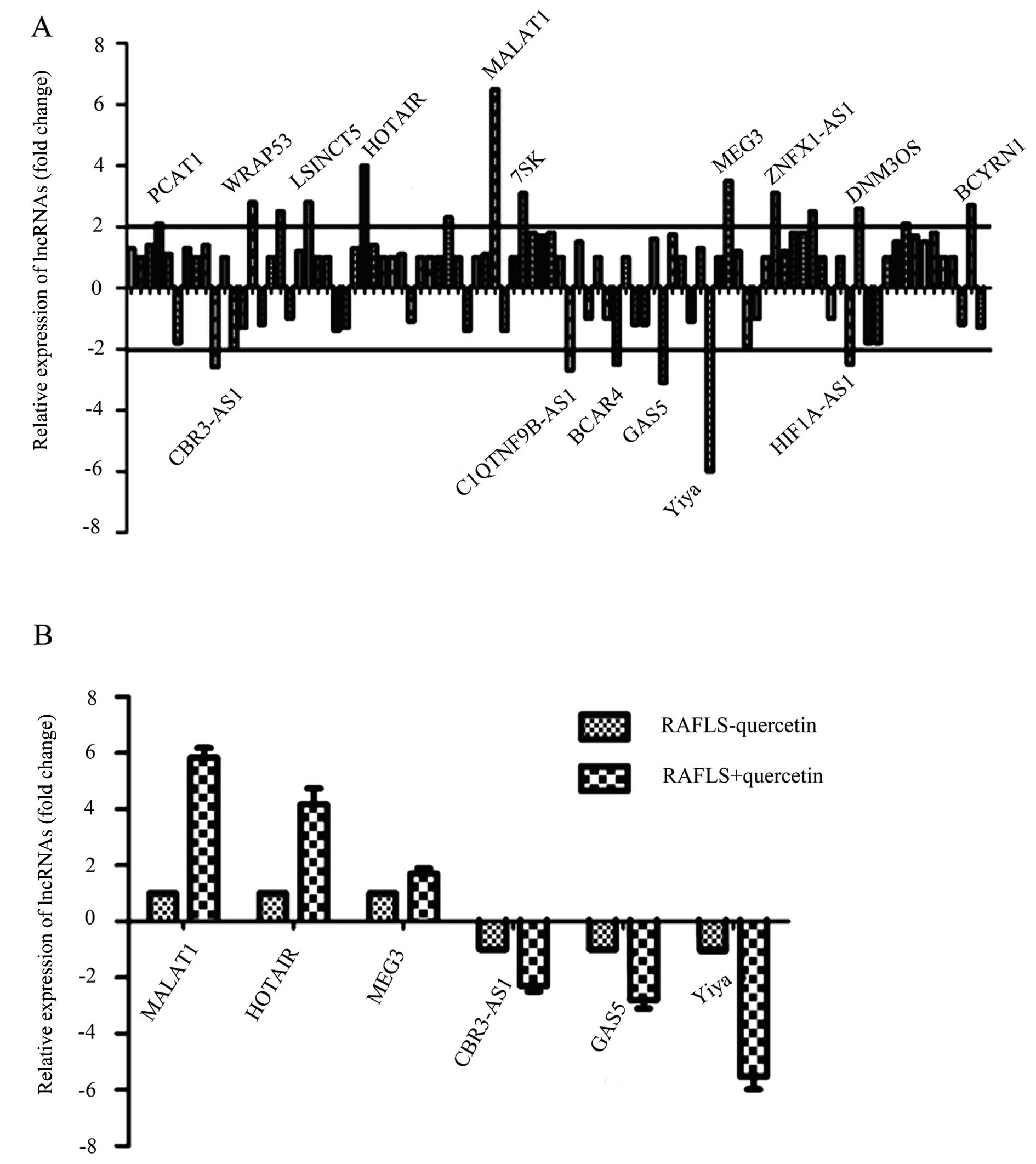
To explore the molecular mechanisms responsible for
the effects of quercetin on RAFLS, a PCR array was used to analyze
the changes in lncRNA expression. The relative expression levels of
lncRNAs which were differentially expressed in the control and the
group treated with quercetin are shown in Fig. 3A. The lncRNAs examined included
PCAT1, CBR3-AS1, WRAP53, LSINCT5, HOTAIR, MALAT1, 7SK,
C1QTNF9B-AS1, BCAR4, GAS5, MEG3, Yiya, ZNFX1-AS1, HIF1A-AS1, DNM3OS
and BCYRN1; these lncRNAs all exhibited a difference in expression
of ≥2-fold, and those with the greatest differences in expression
were marked. From these lncRNAs listed, the most significantly
differentially expressed ones were selected: MALAT1, HOTAIR, MEG3,
CBR3-AS1, GAS5 and Yiya. To further analyze the differences in the
expression of these lncRNAs, their relative expression levels in
the control cells and in the cells treated with quercetin were
determined by qPCR and comparisons were made between the 2 groups
of cells. As shown in Fig. 3B,
MALAT1, HOTAIR and MEG3 were upregulated, whereas CBR3-AS1, GAS5
and Yiya were downregulated in cells treated with quercetin.
Moreover, MALAT1 was upregulated by approximately 4-fold;
therefore, MALAT1 was selected as the key lncRNA for analysis in
the following experiments.
MALAT1 is necessary for the
quercetin-induced apoptosis of RAFLS
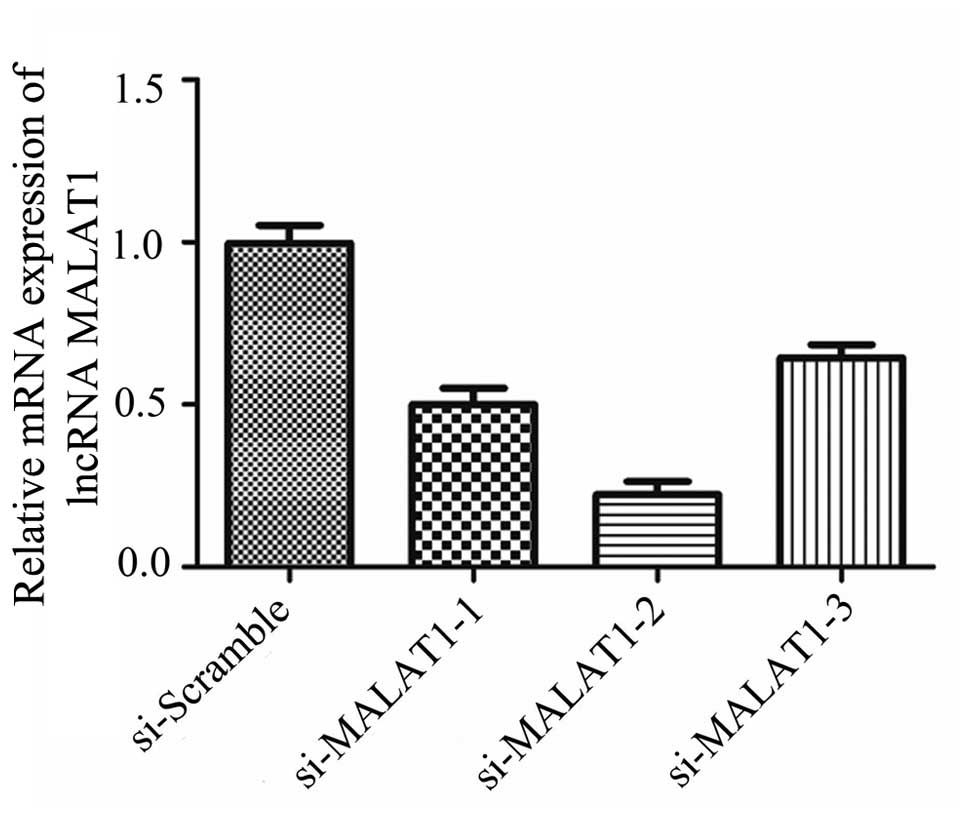
To further determine whether the upregulation of
MALAT1 is necessary for the quercetin-induced apoptosis of RAFLS,
siRNAs were used to knock down the expression of MALAT1 and the
results are shown in Fig. 4. The
relative expression of MALAT1 in the RAFLS following transfection
with si-MALAT1-1, -2, -3 was significantly decreased compared with
the cells transfected with si-Scramble. Following transfection,
western blot analysis was used to detect the expression level of
MALAT1 in different groups. Transfection with si-MALAT1-2 induced
the most significant decrease in MALAT1 expression (Fig. 4). Thus, si-MALAT1-2 was selected
for use in the subsequent experiments.
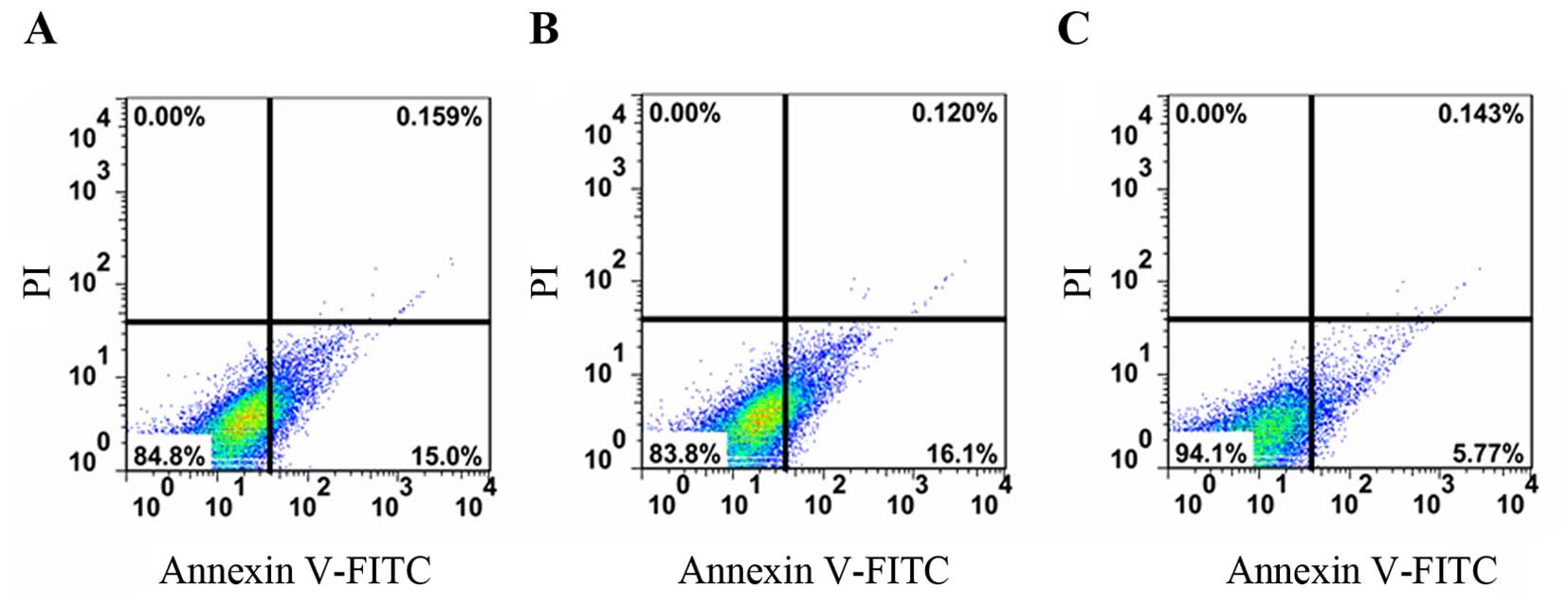
We also examined the apoptosis of the control cells,
and those transfected with si-MALAT1 or si-Scramble and the results
are shown in Fig. 5. The apototic
rate of the control cells, and those transfected with si-MALAT1 or
si-Scramble was 0.159, 0.120 and 0.143%, respectively. This
indicated the that the knockdown of MALAT1 inhibited the apoptosis
of the RAFLS.
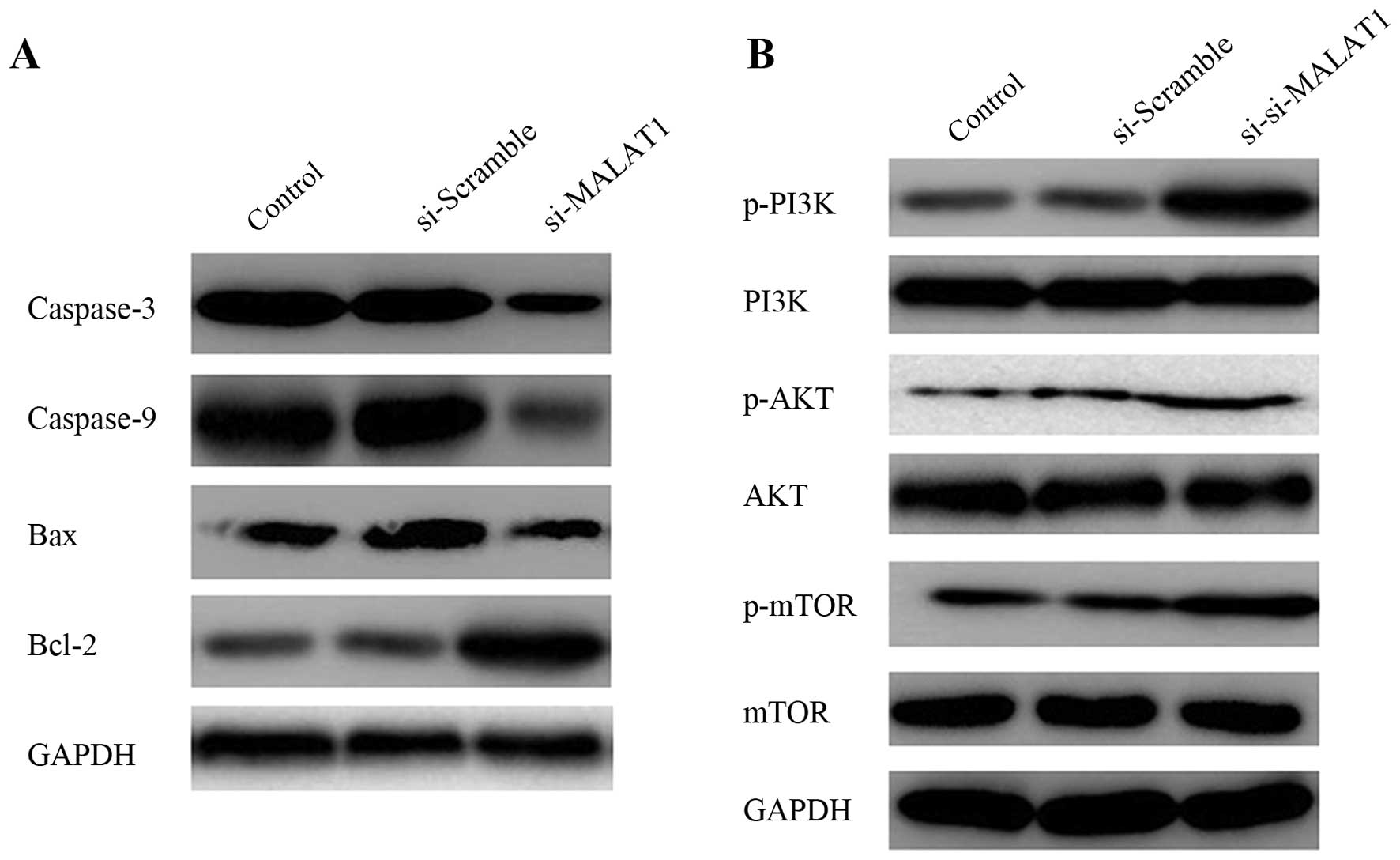
Knockdown of MALAT1 influences protein
expression
To explore the mechanisms responsible for the
apoptosis induced by quercetin, we examined the involvement of
caspase-3 and caspase-9 by measuring their expression levels in the
cells in which MALAT1 was knocked down by western blot analysis.
Bcl-2 family members have either pro-(i.e., Bax) or anti-apoptotic
(i.e., Bcl-2) activities. The proteins of the Bcl-2 family regulate
the mitochondrial pathway by controlling the permeabilization of
the outer mitochondrial membrane in response to many types of
stress or damage (28).
Therefore, in this study, the expression of Bcl-2 and Bax was also
determined. As shown in Fig. 6A,
the protein expression of caspase-3 and caspase-9, as well as that
of Bax in the cells transfected with si-MALAT1 was significantly
lower than that of the cells transfected with si-Scramble. The
protein expression of Bcl-2 increased in the cells transfected with
si-MALAT1 compared with the cells transfected with the blank or
si-Scramble.
Activation of the PI3K/AKT pathway by
MALAT1 knockdown
The PI3K/AKT pathway is often aberrantly activated
in human cancers and contributes to enhanced cell proliferation and
metastasis (29,30). In this study, to whether MALAT1
regulates the proliferation of RAFLS, western blot analysis was
used to examine the effects of MALAT1 knockdown on two relative
signaling pathways. The expression levels of proteins associated
with the PI3K/AKT pathway were determined. As shown in Fig. 6B, the downregulation of MALAT1
significantly increased the levels of p-P13K, p-AKT and p-mTOR, and
decreased those of AKT; no detectable changes were observed in the
levels of P13K and mTOR. These results indicated that the knockdown
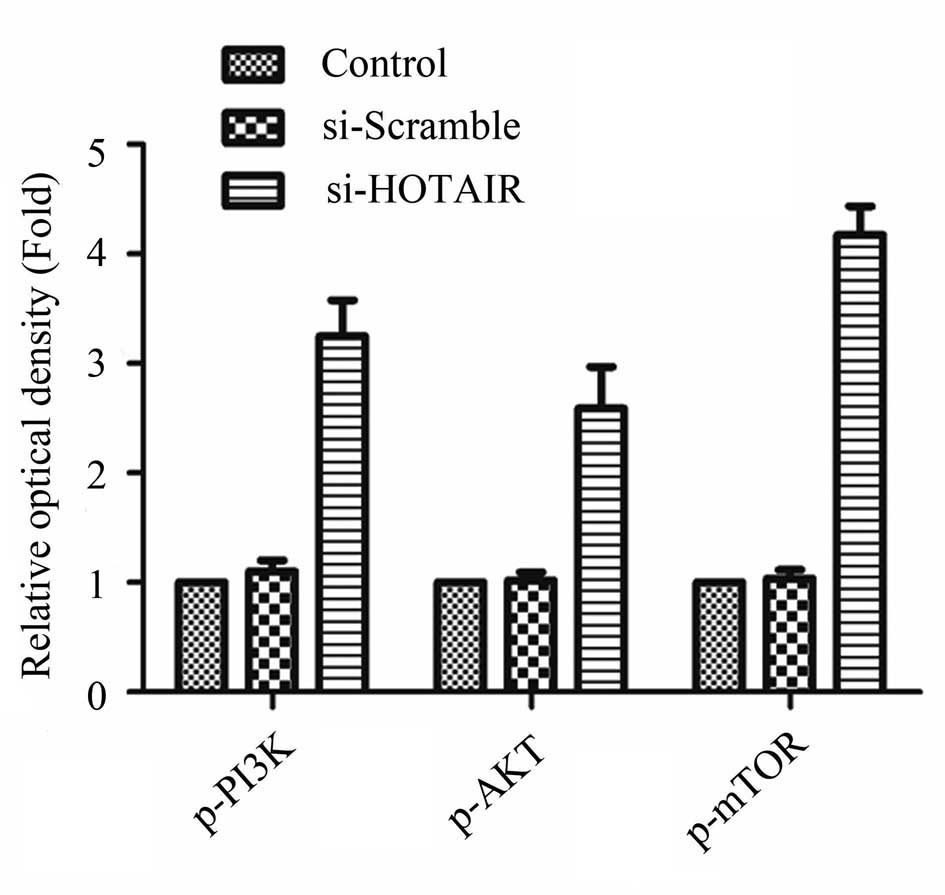
of MALAT1 led to the activation of the PI3K/AKT pathway. The
relative optical density of p-P13K, p-AKT and p-mTOR in the control
cells, as well as in the cells transfected with si-Scramble and or
si-HOTAIR had been determined previously and the results are shown
in Fig. 7. The relative optical
density of p-P13K, p-AKT and p-mTOR in the cells transfected with
si-HOTAIR was higher than that of the controls or the cells
transfected with si-Scramble.
Discussion
RA is the most common type of inflammatory arthritis
and is characterized by the presence of activated T lymphocytes,
macrophages and synoviocytes. It is a major cause of disability
(31,32). RA is also associated with
degeneration of cartilage and the erosion of juxta-articular bone,
as well as with an increased risk of cardiovascular disease
(33,34). FLS are resident mesenchymal cells
of the synovial joints which are recognized to play a key role in
the pathogenesis of RA (35). It
can invade normal human cartilage and bone during the course of RA
(36,37). Quercetin, a member of the
flavonoid family, is an antioxidant that prevents the oxidation of
low-density lipoprotein in vitro by scavenging free oxygen
radicals (21,38). It has been shown to induce cell
cycle arrest and apoptosis in human breast cancer (22). In human hepatoma cell lines,
quercetin has been shown to induce apoptosis by activating
caspases, regulating Bcl-2, and inhibiting the activation of the
PI3K/AKT and ERK pathways (39).
Moreover, it has also been reported to have therapeutic potential
for the treatment of RA (24,40). In this study, we aimed to
elucidate the molecular mechanisms responsible for the effects of
quercetin on RAFLS.
First, the viability of RAFLS following treatment
with various concentrations of quercetin for 24, 48 and 72 h was
determined. We found that RAFLS viability markedly decreased
following treatment with quercetin in a concentration- and
time-dependent manner. The determination of the apoptosis of RAFLS
following treatment with 200 µM quercetin for 24, 48 and 72
h indicated that the apoptotic rate increased significantly, which
was consistent with the results obtained in the study by Sung et
al (41). These results
indicate that quercetin decreases cell viability and promotes the
apoptosis of RAFLS. To elucidate the molecular mechanisms involved,
the differentially expressed lncRNAs were screened in the RAFLS
treated with quercetin by PCR array and qPCR, and lncRNA MALAT1 was
selected for further analysis, as it was the most significantly
differentially expressed lncRNA. Transcription generates ncRNAs or
lncRNAs, which influence diverse cellular processes, such as cell
proliferation, cell cycle progression, apoptosis, or cell growth
(42). lncRNAs have diverse modes
of action and also regulate gene expression (43,44). MALAT1 is an lncRNA that is highly
expressed in several types of tumor and its elevated expression is
associated with hyper-proliferation (16,45). It plays a pivotal role in
colorectal cancer (CRC) metastasis, has been shown to play a key
role in the biological processes of cell proliferation, migration
and invasion (13). It has been
discovered as a marker for lung cancer metastasis, and has been
shown to be associated with many RNA binding proteins and is highly
conserved throughout evolution (46). Therefore, we inferred that MALAT1
may be a key molecule involved in the mechanisms of action of
quercetin. In this study, RAFLS were treated with quercetin after
MALAT1 was knocked down and apoptosis was determined. The results
revealed that the apoptotic rate of the cells transfected with
si-MALAT1 was almost the same as that of the control cells; this
indicated that the induction of apoptosis can be prevented
following treatjment with quercetin when MALAT1 is knocked down. We
thus inferred that MALAT1 is essential for the apoptotic effects of
quercetin in RA.
The protein expression of caspase-3 and -9, Bcl-2
and Bax was determined by western blot analysis. The result
revealed that the expression of caspase-3 and caspase-9 and Bax
decreased, and that of Bcl-2 increased in the cells transfected
with si-MALAT1. Caspases are crucial mediators of programmed cell
death (apoptosis) (47).
Caspase-3 is a member of the cysteine protease family, which plays
a key role in apoptotic pathways by cleaving a variety of key
cellular proteins (48). It can
be activated by various death-inducing signals, including
chemotherapeutic agents (49).
Caspase-9 is a member of the caspase family of cysteine proteases
that have been implicated in apoptosis and cytokine processing
(50). It is one caspase upstream
of caspase-3 and its activation is stimulated by Apaf-1/cytochrome
c and inhibited by AKT signals (51). It is critical for cytochrome
c-dependent apoptosis and normal brain development (52). Bcl-2 family members have been
proposed to play a key role in regulating apoptosis (53). The Bcl-2 family can be divided
into three classes: the anti-apoptotic Bcl-2, the pro-apoptotic Bax
and the BH3-only subfamilies (54). Bax is essential for the death
receptor-mediated apoptosis of cancer cells (55). Bcl-2 is an integral membrane
protein located mainly on the outer membrane of mitochondria
(56). It inhibits most types of
apoptotic cell death, implying a common mechanism of lethality
(57). The Bcl-2/Bax ratio has
been identified to be of clinical relevance in B-cell chronic
lymphocytic leukemia (CLL) (58).
In this study, the activities of caspase-3 and caspase-9 were
reduced, and the Bcl-2/Bax ratio was increased following the
knockdown of MALAT1. We thus concluded that the apoptosis of RAFLS
was inhibited by MALAT1 knockdown.
The relative protein expression of p-P13K, p-AKT,
p-mTOR, AKT, P13K and mTOR in the quercetin-treated RAFLS was
determined. MALAT1 knockdown significantly enhanced the expression
of p-P13K, p-AKT and p-mTOR and reduced the expression of AKT.
However, no detectable changes in P13K and mTOR protein expression
were observed. The PI3K/AKT signal transduction cascade has been
investigated extensively for its roles in oncogenic transformation
(59). PI3K/AKT can regulate the
signaling of multiple biological processes such as apoptosis,
metabolism, cell proliferation and cell growth (60). Both PI3K and AKT play a role in
the prevention of apoptosis. Therefore, we concluded that the
knockdown of MALAT1 inhibits cell growth and metastasis by
activating the PI3K/AKT signaling pathway. Moreover, the relative
optical density of p-P13K, p-AKT and p-mTOR also increased when
HOTAIR was knocked down.
In conclusion, in the present study, we found that
MALAT1 expression was significantly upregulated in the
quercetin-treated RAFLS. The knockdown of MALAT1 inhibited the
apoptosis of RAFLS and led to the activation of the PI3K/AKT
pathway. Therefore, we concluded that quercetin promotes RAFLS
apoptosis by upregulating lncRNA MALAT1.
References
|
1
|
Nanki T, Nagasaka K, Hayashida K, Saita Y
and Miyasaka N: Chemokines regulate IL-6 and IL-8 production by
fibroblast-like synoviocytes from patients with rheumatoid
arthritis. J Immunol. 167:5381–5385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabriel SE and Michaud K: Epidemiological
studies in incidence, prevalence, mortality, and comorbidity of the
rheumatic diseases. Arthritis Res Ther. 11:2292009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurowska M, Rudnicka W, Kontny E, Janicka
I, Chorazy M, Kowalczewski J, Ziółkowska M, Ferrari-Lacraz S, Strom
TB and Maśliński W: Fibroblast-like synoviocytes from rheumatoid
arthritis patients express functional IL-15 receptor complex:
endogenous IL-15 in autocrine fashion enhances cell proliferation
and expression of Bcl-x(L) and Bcl-2. J Immunol. 169:1760–1767.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen S, Yang Y, Feng H, Wang H, Zhao R and
Liu H: Baicalein inhibits interleukin-1β-induced proliferation of
human rheumatoid arthritis fibroblast-like synoviocytes.
Inflammation. 37:163–169. 2014. View Article : Google Scholar
|
|
6
|
Liu H and Pope RM: The role of apoptosis
in rheumatoid arthritis. Curr Opin Pharmacol. 3:317–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pope RM: Apoptosis as a therapeutic tool
in rheumatoid arthritis. Nat Rev Immunol. 2:527–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baier A, Meineckel I, Gay S and Pap T:
Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol. 15:274–279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gloss BS and Dinger ME: The specificity of
long noncoding RNA expression. Biochim Biophys Acta. 1859:16–22.
2016. View Article : Google Scholar
|
|
10
|
Pedram Fatemi R, Salah-Uddin S, Modarresi
F, Khoury N, Wahlestedt C and Faghihi MA: Screening for
small-molecule modulators of long noncoding RNA-protein
interactions using AlphaScreen. J Biomol Screen. 20:1132–1141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber DG, Johnen G, Casjens S, Bryk O,
Pesch B, Jöckel KH, Kollmeier J and Brüning T: Evaluation of long
noncoding RNA MALAT1 as a candidate blood-based biomarker for the
diagnosis of non-small cell lung cancer. BMC Res Notes. 6:5182013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jalali S, Bhartiya D, Lalwani MK,
Sivasubbu S and Scaria V: Systematic transcriptome wide analysis of
lncRNA-miRNA interactions. PLoS One. 8:e538232013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Yang M, Tian J, Wang X and Li Z:
MALAT-1: A long non-coding RNA and its important 3′ end functional
motif in colorectal cancer metastasis. Int J Oncol. 39:169–175.
2011.PubMed/NCBI
|
|
14
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
over-expression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar
|
|
15
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tripathi V, Shen Z, Chakraborty A, Giri S,
Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A and
Prasanth KV: Long noncoding RNA MALAT1 controls cell cycle
progression by regulating the expression of oncogenic transcription
factor B-MYB. PLoS Genet. 9:e10033682013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar
|
|
18
|
Bjelogrlić S, Srdić T and Radulović S:
Mammalian target of rapamycin is a promising target for novel
therapeutic strategy against cancer. J BUON. 11:267–276. 2006.
|
|
19
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: an update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar
|
|
20
|
Lamson DW and Brignall MS: Antioxidants
and cancer, part 3: quercetin. Altern Med Rev. 5:196–208.
2000.PubMed/NCBI
|
|
21
|
Hollman PC, vd Gaag M, Mengelers MJ, van
Trijp JM, de Vries JH and Katan MB: Absorption and disposition
kinetics of the dietary antioxidant quercetin in man. Free Radic
Biol Med. 21:703–707. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ,
Yoo YD, Kim TW, Lee YS and Lee SJ: Induction of cell cycle arrest
and apoptosis in human breast cancer cells by quercetin. Int J
Oncol. 19:837–844. 2001.PubMed/NCBI
|
|
23
|
Coskun O, Kanter M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and β-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Natarajan V, Krithica N, Madhan B and
Sehgal PK: Formulation and evaluation of quercetin polycaprolactone
microspheres for the treatment of rheumatoid arthritis. J Pharm
Sci. 100:195–205. 2011. View Article : Google Scholar
|
|
25
|
Mamani-Matsuda M, Kauss T, Al-Kharrat A,
Rambert J, Fawaz F, Thiolat D, Moynet D, Coves S, Malvy D and
Mossalayi MD: Therapeutic and preventive properties of quercetin in
experimental arthritis correlate with decreased macrophage
inflammatory mediators. Biochem Pharmacol. 72:1304–1310. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Loof A, Vandersmissen T, Marchal E and
Schoofs L: Initiation of metamorphosis and control of ecdysteroid
biosynthesis in insects: The interplay of absence of Juvenile
hormone, PTTH, and Ca(2+)-homeostasis. Peptides. 68:120–129. 2015.
View Article : Google Scholar
|
|
27
|
Korb A, Pavenstädt H and Pap T: Cell death
in rheumatoid arthritis. Apoptosis. 14:447–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Sun S, Chen J, Ren P, Hu Y, Cao
Z, Sun H and Ding Y: Oxymatrine induces mitochondria dependent
apoptosis in human osteosarcoma MNNG/HOS cells through inhibition
of PI3K/Akt pathway. Tumour Biol. 35:1619–1625. 2014. View Article : Google Scholar
|
|
30
|
Hong SK, Yoon S, Moelling C, Arthan D and
Park JI: Noncatalytic function of ERK1/2 can promote
Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem.
284:33006–33018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McInnes IB, al-Mughales J, Field M, Leung
BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC and Liew FY: The
role of interleukin-15 in T-cell migration and activation in
rheumatoid arthritis. Nat Med. 2:175–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solomon DH, Karlson EW, Rimm EB, Cannuscio
CC, Mandl LA, Manson JE, Stampfer MJ and Curhan GC: Cardiovascular
morbidity and mortality in women diagnosed with rheumatoid
arthritis. Circulation. 107:1303–1307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feldmann M and Maini RN: Anti-TNF α
therapy of rheumatoid arthritis: what have we learned? Annu Rev
Immunol. 19:163–196. 2001. View Article : Google Scholar
|
|
35
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tolboom TC, van der Helm-Van Mil AH,
Nelissen RG, Breedveld FC, Toes RE and Huizinga TW: Invasiveness of
fibroblast-like synoviocytes is an individual patient
characteristic associated with the rate of joint destruction in
patients with rheumatoid arthritis. Arthritis Rheum. 52:1999–2002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Wu J, Cao Q, Xiao L, Wang L, He
D, Ouyang G, Lin J, Shen B, Shi Y, et al: A critical role of Cyr61
in interleukin-17-dependent proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
60:3602–3612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: from antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
40
|
Sato M, Miyazaki T, Kambe F, Maeda K and
Seo H: Quercetin, a bioflavonoid, inhibits the induction of
interleukin 8 and monocyte chemoattractant protein-1 expression by
tumor necrosis factor-alpha in cultured human synovial cells. J
Rheumatol. 24:1680–1684. 1997.PubMed/NCBI
|
|
41
|
Sung MS, Lee EG, Jeon HS, Chae HJ, Park
SJ, Lee YC and Yoo WH: Quercetin inhibits IL-1β-induced
proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid
synovial fibroblast. Inflammation. 35:1585–1594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schmidt LH, Spieker T, Koschmieder S,
Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D,
Marra A, et al: The long noncoding MALAT-1 RNA indicates a poor
prognosis in non-small cell lung cancer and induces migration and
tumor growth. J Thorac Oncol. 6:1984–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eißmann M, Gutschner T, Hämmerle M,
Günther S, Caudron-Herger M, Groß M, Schirmacher P, Rippe K, Braun
T, Zörnig M and Diederichs S: Loss of the abundant nuclear
non-coding RNA MALAT1 is compatible with life and development. RNA
Biol. 9:1076–1087. 2012. View Article : Google Scholar
|
|
47
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Devarajan E, Sahin AA, Chen JS,
Krishnamurthy RR, Aggarwal N, Brun AM, Sapino A, Zhang F, Sharma D,
Yang XH, et al: Down-regulation of caspase 3 in breast cancer: a
possible mechanism for chemoresistance. Oncogene. 21:8843–8851.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Du J, Wang X, Miereles C, Bailey JL,
Debigare R, Zheng B, Price SR and Mitch WE: Activation of caspase-3
is an initial step triggering accelerated muscle proteolysis in
catabolic conditions. J Clin Invest. 113:115–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuida K: Caspase-9. Int J Biochem Cell
Biol. 32:121–124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujita E, Jinbo A, Matuzaki H, Konishi H,
Kikkawa U and Momoi T: Akt phosphorylation site found in human
caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun.
264:550–555. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Krajewski S, Krajewska M, Ellerby LM,
Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal
RE, Fiskum G and Reed JC: Release of caspase-9 from mitochondria
during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci
USA. 96:5752–5757. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lindsten T, Ross AJ, King A, Zong WX,
Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K,
et al: The combined functions of proapoptotic Bcl-2 family members
bak and bax are essential for normal development of multiple
tissues. Mol Cell. 6:1389–1399. 2000. View Article : Google Scholar
|
|
54
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor-induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fulda S, Meyer E and Debatin K-M:
Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression.
Oncogene. 21:2283–2294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saxena A, Viswanathan S, Moshynska O,
Tandon P, Sankaran K and Sheridan DP: Mcl-1 and Bcl-2/Bax ratio are
associated with treatment response but not with Rai stage in B-cell
chronic lymphocytic leukemia. Am J Hematol. 75:22–33. 2004.
View Article : Google Scholar
|
|
59
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|