Introduction
An increasing number of patients requiring surgical
implantation are expected to attend orthopedic clinics. As a
consequence of age or systemic diseases, the risk of implant
loosening is also increasing, becoming an intractable problem.
Thus, increasing efforts are focusing on the development of methods
or approaches to prevent implant loosening with particular focus on
enhancing bone-implant osseointegration. Various approaches have
been investigated to promote implant osseointegration, including
modification of the implant surface and implant material (1,2),
as well as the administration of medicines (3,4)
and local growth factors (5).
Unfortunately, these approaches are associated with several
drawbacks including unstable implants, high costs and an adverse
side effect profile (6,7). Thus, clinically, it is imperative to
develop approaches that are effective, without side effects and
that promote osseointegration between the host bone and the
implant.
It has long been recognized that bone is capable of
adapting its mass and microstructure in response to mechanical
stimuli (8,9). Some researchers have focused on
biophysical stimulation methods to treat osteoporosis (10). The non-invasive and
non-pharmacological intervention of low-magnitude (LM, <1 × g,
g=9.81 m/sec2), high-frequency (HF, 20–90 Hz) (LMHF)
vibrations has gained interest as studies have shown that such a
mechanical signal may promote bone formation, prevent bone loss and
stimulate bone healing (11–15). Additionally, the effect of LMHF
vibration on bone-implant osseointegration has been confirmed
(16–18). Particularly, our previous study
demonstrated that LMHF enhanced bone-implant osseointegration in
ovariectomized rats, which was confirmed by histomorphometrical and
biomechanical analysis (19).
However, the underlying mechanism through which LMHF vibration
induces bone-implant osseointegration at the cellular and molecular
level remains largely unknown.
Previously, several studies have determined the
effect of the chemical composition of the implants and their
surface properties on osteoblast behavior (20–22). However, these studies were
performed under static conditions and did not take into account the
presence of mechanical stimuli at the cell-implant interface and
their effects on the osteoblastic phenotype. It is well known that
the efficacy of orthopedic implants requires the creation of a
direct structural and functional connection between the material
and the bone. Adhesion is a factor involved in the first phase of
cell-material interactions and the quality of adhesion determines
the ability of cells to proliferate and differentiate in contact
with the implant. Several in vitro studies have determined
the effects of mechanical strain on the adhesion of osteoblastic
cells (23,24). On the other hand, several studies
have reported that the exposure of osteoblastic cells or bone
marrow-derived mesenchymal stem cells (BMSCs) to LMHF vibration may
stimulate osteoblast differentiation (25–27). However, whether LMHF vibration
exerts an effect on the adhesion and the differentiation of BMSCs
cultured on the surface of an implant currently remains unclear.
Thus, we hypothesized that LMHF vibration enhances the adhesion and
the osteogenic differentiation of BMSCs cultured on an implant
surface in vitro, leading to the increased expression of
adhesion molecules and osteoblastic markers, as well as enhancing
extracellular matrix (ECM) synthesis.
Thus far, the molecular mechanism responsible for
the effects of mechanical stimuli on bone formation has yet to be
addressed. Several studies have demonstrated that Wnt/β-catenin
signaling is a normal physiological response to mechanical stimuli
(28–30). Canonical Wnt signaling has been
found to encourage mesenchymal progenitor cells to differentiate
into osteoblasts (31). Hou et
al (30) investigated the
mechanobiological mechanisms of vibration-enhanced osteogenic
responses in osteoblasts, and found that the protein expression of
Wnt10B and οsteoprotegerin (OPG) increased, whereas the levels of
sclerostin and receptor activator of nuclear factor κB ligand
(RANKL) were reduced. These findings suggest that Wnt signaling is
an essential pathway in the mechanotransduction process. Thus, we
hypothesized that Wnt/β-catenin signaling may also play an
important role in the LMHF vibration-mediated osteogenic
differentiation of BMSCs.
In this study, we established an in vitro
model of hydroxyapatite (HA)-coated substrates. We aimed to explore
the cellular and molecular mechanisms responsible for the effects
of LMHF vibration on the adhesion and the osteogenic
differentiation of BMSCs cultured on HA-coated surfaces, and to
examine whether Wnt/β-catenin signaling is involved in osteogenesis
following exposure to LMHF vibration.
Materials and methods
In vitro material preparation
Titanium substrates (circular substrates 34 or 14.75
mm in diameter and 1 mm in thickness) were made by the Engineering
Research Center in Biomaterials of Sichuan University (Chengdu,
China) and plasma sprayed with HA (100 µm in thickness)
using a Metco MN Plasma System and an AR-2000 Thermal Spray Robot
(Metco, Westbury, NY, USA).
Isolation and culture of BMSCs
Twenty male Sprague-Dawley (SD) rats (4–6 weeks old)
were obtained from the Experimental Animal Center of the First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China).
The current study was approved by the Animal Care Committee of Sun
Yat-sen University (approval no. [2014]52). The BMSCs were isolated
from SD rats as previously described (26). Briefly, femora and tibiae were
harvested from euthanized rats (excess 10% chloral hydrate by
intraperitoneal injection), cut at each bone end, and then flushed
with 5 ml Dulbecco's modified Eagle's medium (DMEM; Gibco, Beijing,
China) culture medium twice using a syringe. The cells were
resuspended following centrifugation (1,000 × g, 5 min) and placed
in the culture medium, and cultured in an 5% CO2
incubator at 37°C. The culture medium was replaced every 2 days.
The cells were subcultured to 80–90% confluence. Passage 3 (P3)
BMSCs were used for all experiments (26).
LMHF vibration in vitro
HA-coated titanium substrates (Φ34 or 14.75 mm) were
placed into 6-well plates, 24-well plates and 35 mm cultured dishes
after high temperature sterilization. The BMSCs were seeded on the
HA-coated surface in 6-well plates/35 mm cultured dishes
(5×104 cells/cm2) and 24-well plates
(5×103 cells/cm2) and randomly divided into
two groups: i) osteogenic medium (control group) and ii) osteogenic
medium and LMHF vibration (LMHF group). In the control group, the
medium was replaced with osteogenic medium (medium containing 10 nM
dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml
ascorbic acid) (Sigma, St. Louis, MO, USA) after 24 h. The
osteogenic medium was replaced every 2 days for 14 days.
For the LMHF group, the plates cultured with BMSCs
were mounted on the platform of a GJX-5 vibration sensor (Beijing
Sendig Technology, Beijing, China) and subjected to mechanical
stimuli (magnitude, 0.3 g; frequency, 40 Hz) for 30 min every 24 h
starting from day 0. The vibration parameter (40 Hz, 0.3 g) was
similar to that of previously published methods (26,32,33). After 30 min of vibration, the
cells in the LMHF group received fresh osteogenic medium.
The culture medium in all groups was replaced every
48 h. All groups were cultured for 14 days (34).
Immunofluorescence staining
After 1, 3 and 5 days of administration,
immunostaining was performed. The cells were then washed with
phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for
20 min at room temperature, and washed again with PBS. They were
then immersed in 0.1% Triton X-100 for 4 min and washed in PBS. The
cells were blocked with 1% bovine serum albumin (BSA) and incubated
with anti-fibronectin (FN; mouse; 1:100; F7387) and anti-F-actin
(1:100; 94072) primary antibodies (both from Sigma-Aldrich, St
Louis, MO, USA) at 4°C overnight followed by incubation with
FITC-conjugated secondary antibody (anti-mouse; 1:2,000; A-21202;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h at room
temperature. The cells were rinsed with PBS, and the nuclei were
stained with 4′,6-diamino-2-phenylindole hydrochloride (DAPI) and
incubated for 30 min at room temperature. Imaging experiments were
conducted using a fluorescence microscope (Leica DMI3000 B) and a
laser scanning confocal microscope (ZEISS LSM710) (both from Leica
Microsystems, Wetzlar, Germany).
Scanning electron microscope (SEM)
observations
The cells in all groups were evaluated using an SEM
to examine cell morphology and attachment. The BMSCs of all groups
were seeded in 35-mm culture dishes (containing HA-coated titanium
substrates) at a density of 5×104 cells/cm2
in osteogenic medium for 14 days. The medium was removed and fixed
with 2.5% glutaraldehyde in 0.1 M cacodylate buffer overnight at
4°C. After fixation, the samples were dehydrated at room
temperature in increasing concentrations of ethanol (30, 50, 70, 95
and 100%), and then point-dried with CO2 (35). The specimens were mounted on
aluminum stubs and sputter-coated with gold. Finally, the samples
were observed under an SEM.
Assay of alkaline phosphatase (ALP)
activity
To evaluate ALP activity, the BMSCs were seeded on
the HA-coated surface in 6-well plates at a density of
5×104 cells/cm2 and osteogenesis was induced
in different groups. ALP activity was then measured using a
SensoLyte p-nitrophenyl phosphate (pNPP) ALP assay kit (AnaSpec,
Fremont, CA, USA) according to the manufacturer's instructions on
days 3, 7, 10 and 14. Briefly, the cells were lysed with lysis
buffer, which was provided in the kit. After three rapid
freeze-thaw cycles, the proteins were extracted. The supernatant
was collected after the cell lysate was centrifuged for 15 min
(10,000 × g, 4°C) and subsequently combined with pNPP. ALP activity
was detected at 405 nm using a microplate reader (Sunrise; Tecan,
Männedorf, Switzerland). Values were normalized to total protein
content, which was measured using a spectrophotometer (NanoDrop
2000; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells in
different groups using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). RNA was then used for cDNA synthesis with a SYBR-PrimeScript
RT-PCR kit (Takara, Dalian, China). Each cDNA samples was analysed
in triplicate in a 10 µl reaction volume containing 1
µl cDNA, 0.3 µl forward primer, 0.3 µl reverse
primer, 5 µl SYBR® Premix Ex Taq™, and 3.4
µl diethylpyrocarbonate (DEPC) water. Fluorescence data was
analyzed using a CFX96™ Real-Time PCR Detection System (Bio-Rad,
Hercules, CA, USA). The PCR conditions were as follows: initial
denaturation, 95℃ for 5min (1 cycle); denaturation, 94℃ for 30 sec;
annealing, 60℃ for 10 sec; elongation, 72℃ for 20 sec; and final
extension step, 72℃ for 10 min (for 40 cycles). Gene expression
levels were calculated in relation to the β-actin CT value by the
2−ΔΔCT method. Data are normalized to the control group.
The primer sequences of FN, β1 integrin, vinculin, paxillin,
Runt-related transcription factor 2 (Runx2), osterix (Osx),
collagen I (Col-I), osteocalcin (OCN) and β-actin are presented in
Table I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene | Primer
sequences | Product size
(bp) |
|---|
| Fibronectin | F:
5′-CGGTGGCTGTCAGTCAAAG-3′ | 130 |
| R:
5′-AAACCTCGGCTTCCTCCATAA-3′ | |
| β1 integrin | F:
5′-TGAATGTGAATGCCAAAGCGA-3′ | 128 |
| R:
5′-CAATGTCTACCAACACGCCC-3′ | |
| Vinculin | F:
5′-TCTGACCTCCTGCTTACCTTT-3′ | 188 |
| R:
5′-CAACTCCTGCTGTCTCTCATC-3′ | |
| Paxillin | F: 5′-
GCCAGCAGCAGACCAGAA-3′ | 171 |
| R: 5′-
TAGTGGGAGGAGGAGGAAGG-3′ | |
| Runx2 | F:
5′-CCACCTCTGACTTCTGCCTC-3′ | 111 |
| R:
5′-GGGATGAAATGCCTGGGAAC-3′ | |
| Osterix | F:
5′-CAGTAATCTTCGTGCCAGACC-3′ | 180 |
| R:
5′-GGCTTCTTTGTGCCTCCTTT-3′ | |
| Collagen I | F:
5′-GTGTTCAAGGTGGCAAAGGTG-3′ | 141 |
| R:
5′-CAGGACCAGGGAGACCGAA-3′ | |
| Osteocalcin | F:
5′-GCGGAACCTATGCCTGCT-3′ | 118 |
| R:
5′-GTGGTGTTGGTGATTTGGATGG-3′ | |
| β-actin | F:
5′-GGAAATCGTGCGTGACATTA-3′ | 183 |
| R:
5′-AGGAAGGAAGGCTGGAAGAG-3′ | |
LMHF vibration and Dkk-1
administration
In order to examine whether Wnt/β-catenin signaling
is involved in osteogenesis after LMHF vibration treatment, the
BMSCs were seeded in 6-well plates at a density of 5×104
cells/cm2 and then randomly divided into three groups:
i) osteogenic medium (control group), ii) osteogenic medium and
LMHF vibration (LMHF group) and iii) osteogenic medium and LMHF
vibration with 100ng/ml dickkopf-1 (Dkk-1; Sigma-Aldrich), a WNT
signaling pathway inhibitor (Dkk-1 group). The culture medium in
all groups was replaced every 48 h. All groups were cultured for 14
days (34).
Western blot analysis
The samples for western blot analysis were collected
on day 4. To obtain whole-cell protein extracts, the cells were
washed twice with ice-cold PBS and lysed in 80 µl Mammalian
Protein Extraction reagent (Thermo Fisher Scientific, Inc.). The
supernatant protein samples were harvested after centrifugation for
15 min (12,000 × g, 4°C) and boiled for 5 min. Equal volumes of the
samples were run on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and subsequently transferred to a
PVDF membrane. The membrane was blocked for 1 h with 5% nonfat dry
milk and then incubated overnight at 4°C with the following primary
antibodies: Wnt10b (ab106522; Abcam, Cambridge, CA, USA); β-catenin
(2968) and Runx2 (12556) (both from Cell Signaling Technology,
Danvers, MA, USA); Osx (ab22552) and GAPDH (ab8245) (both from
Abcam). Following incubation with secondary antibody (Cell
Signaling Technology), the immunoreactive proteins were visualized
using a chemiluminescence kit (Millipore, Billerica, MA, USA). Gray
analysis was performed using Photoshop CS5 (Adobe Systems Inc., San
Jose, CA, USA).
Statistical analysis
All data are presented as the means ± standard
deviation. For the control and the LMHF cultures, comparisons were
performed using the two-tailed Mann-Whitney test, and statistical
analysis to compare the results among groups was conducted using
one-way analysis of variance (ANOVA), and the least significant
difference (LSD) post hoc test was applied for multiple comparisons
using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Immunofluorescence staining of matrix
organization and cytoskeleton
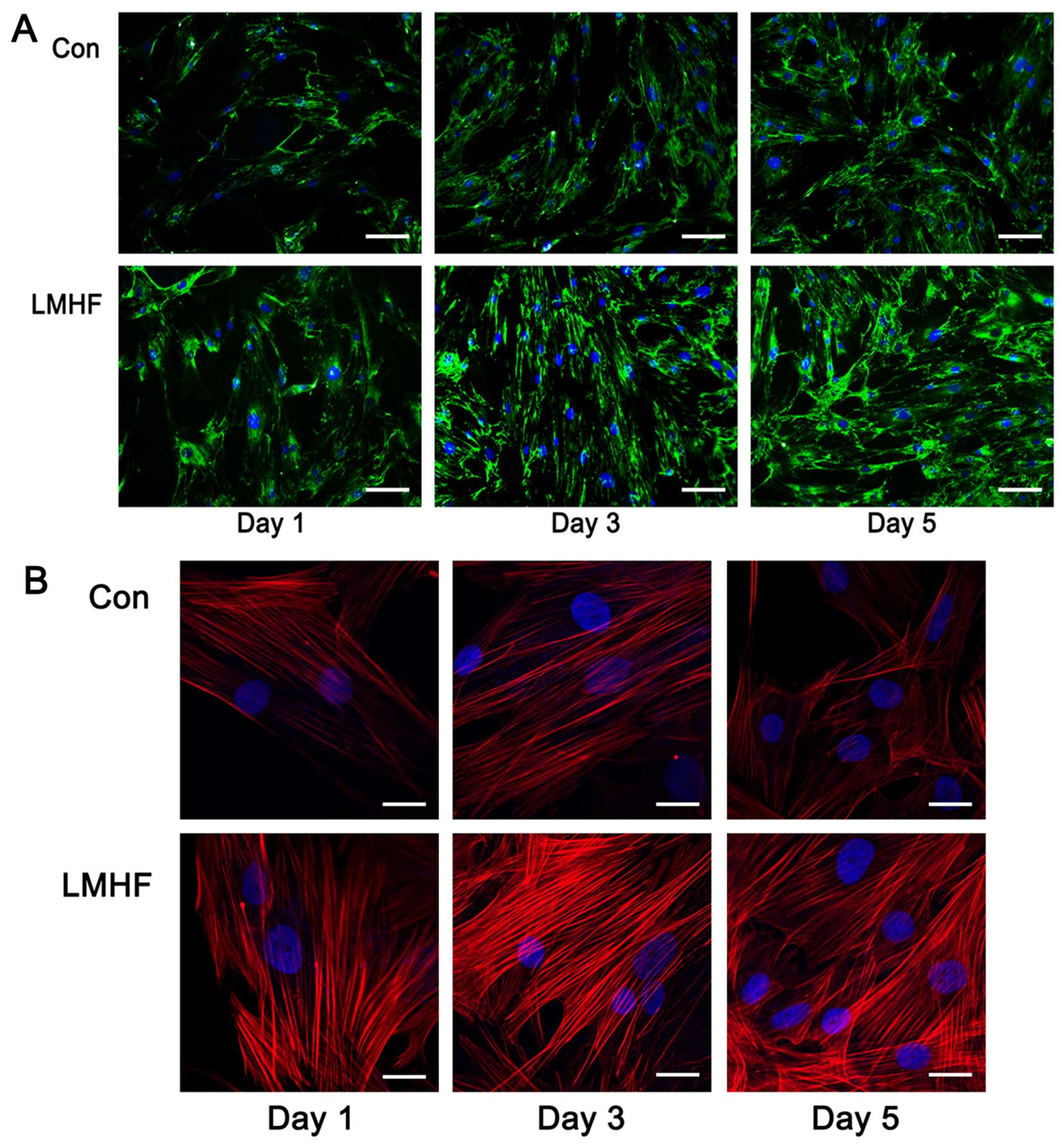
The effect of LMHF vibration on FN matrix
organisation was investigated on days 1, 3 and 5. As shown in
Fig. 1, increasing fluorescence
intensity and increasing numbers of FN fibres were observed after
1, 3 and 5 days of LMHF vibration (Fig. 1A), confirming the increased FN
production quantified by RT-qPCR. Actin rearrangement is an
important event in cell attachment. Cytoskeletal organization was
assessed by F-actin labeling following LMHF stimulation (1, 3 and 5
days). Actin filaments were clearly visible after staining with the
F-actin antibody (Fig. 1B). The
stimulation of BMSCs with LMHF vibration resulted in the
rearrangement of the actin cytoskeleton with more prominent
F-actin. The majority of the actin filaments were situated parallel
to the main direction of the cell, and the fluorescence intensity
was significantly stronger than that in the control group.
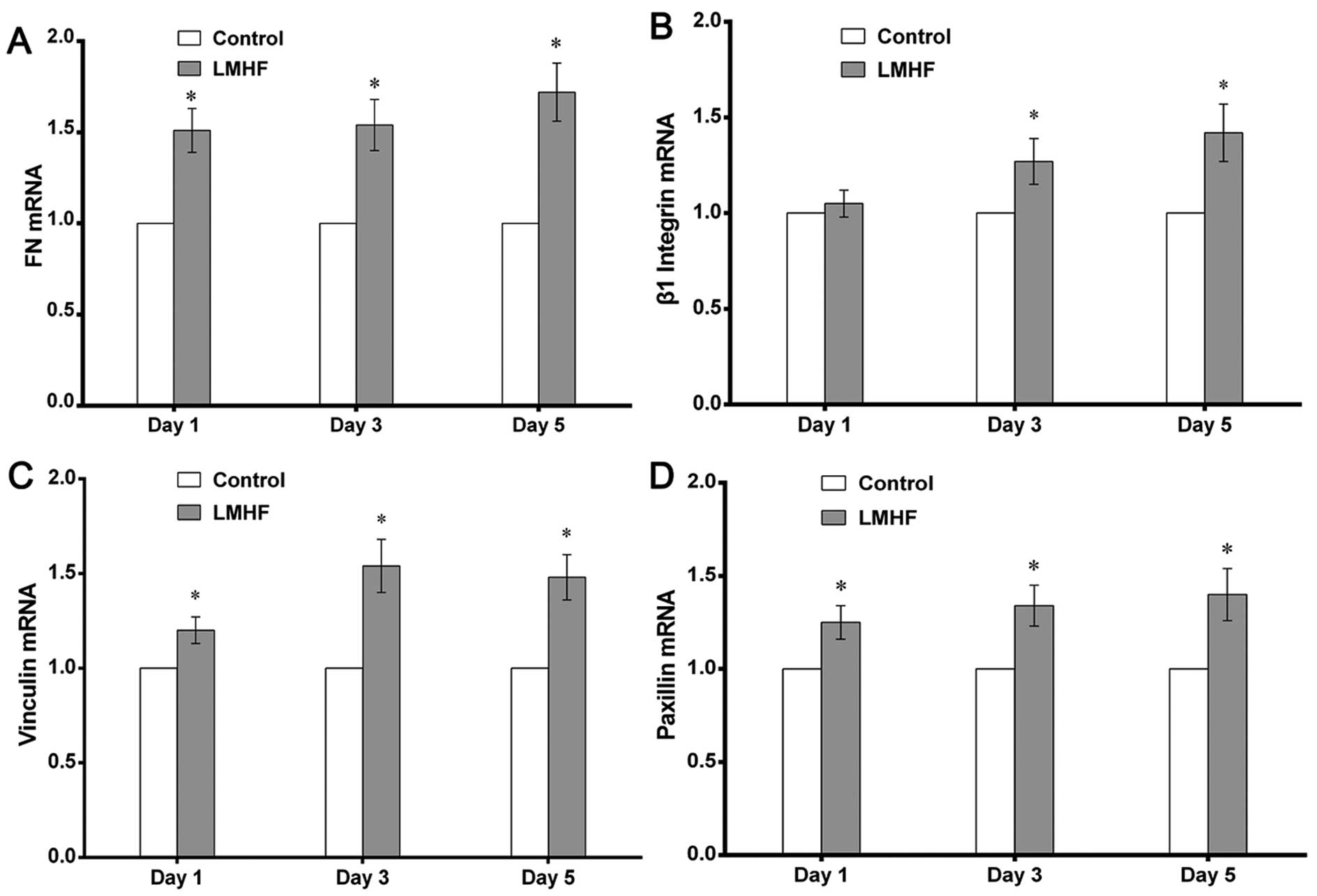
Analysis of the gene expression of cell
adhesion molecules by RT-qPCR
To determine the effect of LMHF vibration on
cell-biomaterial interactions, the mRNA expression of FN, β1
integrin, vinculin and paxillin, which are involved in the adhesion
of cells to the substrates, was measured by RT-qPCR on days 1, 3
and 5. The mRNA expression of FN was greatly enhanced in
LMHF-vibrated cultures (P<0.05) (Fig. 2A); β1 integrin mRNA expression was
also significantly increased after LMHF vibration on days 3 and 5
(P<0.05) (Fig. 2B). Similarly,
LMHF vibration also resulted in an increase in the mRNA expression
of vinculin and paxillin on days 1, 3 and 5 (P<0.05) (Fig. 2C and D).
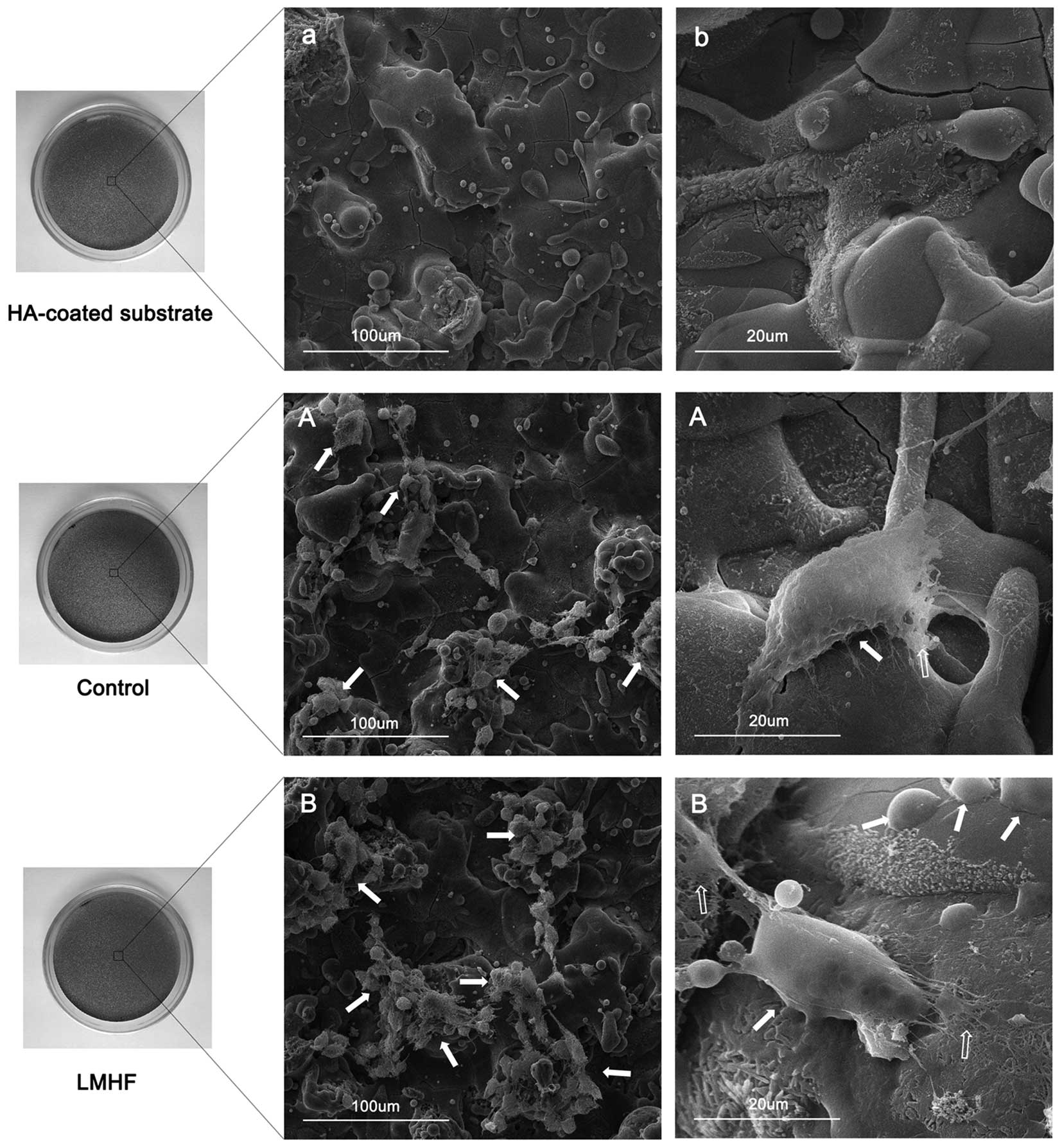
SEM observation of cell morphology
SEM images of the HA-coated surface without any
cells are shown in Fig. 3a and b.
Fourteen days after culture, the cells in the two groups adhered
tightly to the HA-coated surface and SEM images of the cells are
presented in Fig. 3A and B. The
cells were connected with each other by filopodia and established
their ECM on the HA surface, which suggests that the cells are
capable of commendably integrating with the HA-coated substrate.
Most strikingly, an increased number of cells and more plentiful
ECM are present on the HA-coated surface in the LMHF group compared
with that in the control group (Fig.
3A and B).
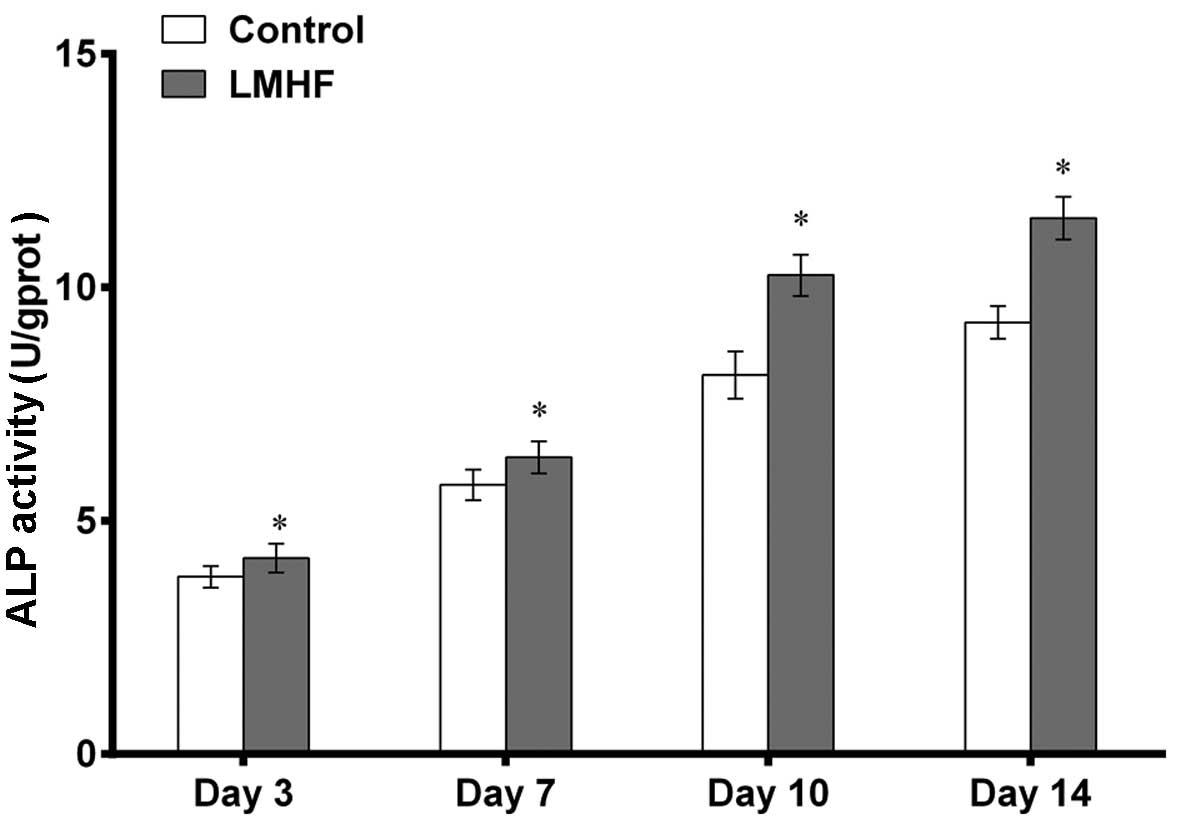
Assessment of ALP activity
The BMSCs were cultured in osteogenic medium in
different treatment groups for measuring ALP activity on days 3, 7,
10 and 14 (Fig. 4). ALP activity
gradually increased in a time-dependent manner in all groups. In
comparison with the control group, the level of ALP activity of the
LMHF group was significantly increased at different time-points
(P<0.05).
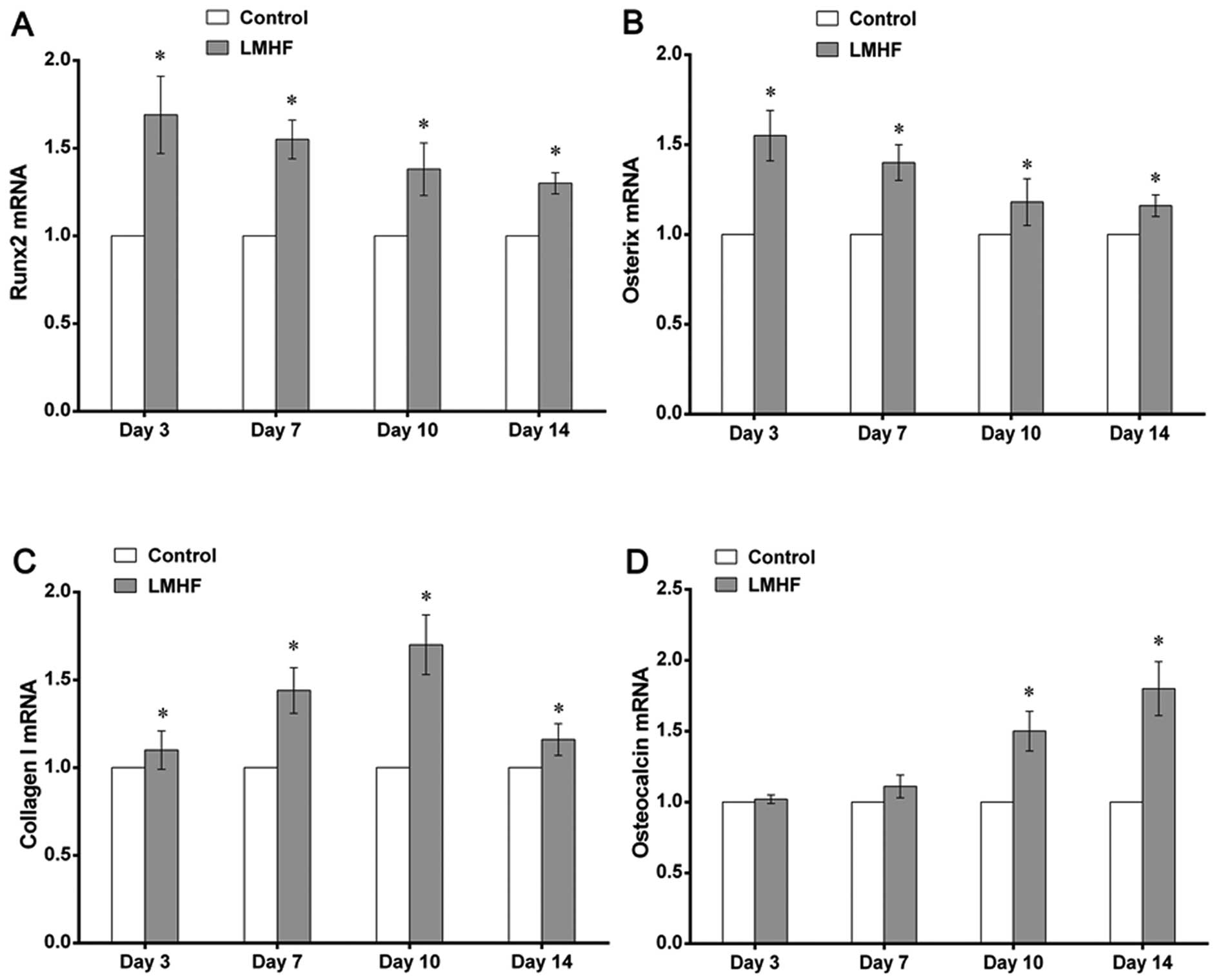
Analysis of osteogenic-specific gene
expression by RT-qPCR
To examine whether LMHF vibration affected the
osteogenic differentiation of BMSCs, the expression of genes
associated with osteogenesis, including Runx2, Osx, Col-I and OCN,
was evaluated using RT-qPCR on days 3, 7, 10 and 14. LMHF vibration
upregulated the mRNA expression of Runx2 and Osx on days 3, 7, 10
and 14 (P<0.05), and the expression of Runx2 and Osx was
apparent on day 3 (P<0.05) (Fig.
5A and B). Similarly, with LMHF vibration treatment, the mRNA
expression of Col-I showed a marked increase on days 3, 7, 10 and
14 (P<0.05) (Fig. 5C). The
mRNA expression of OCN was also significantly increased following
exposure to LMHF vibration on days 10 and 14 (P<0.05) (Fig. 5D), although no visible difference
in the mRNA expression of OCN was observed on days 3 or 7
(P>0.05) (Fig. 5D).
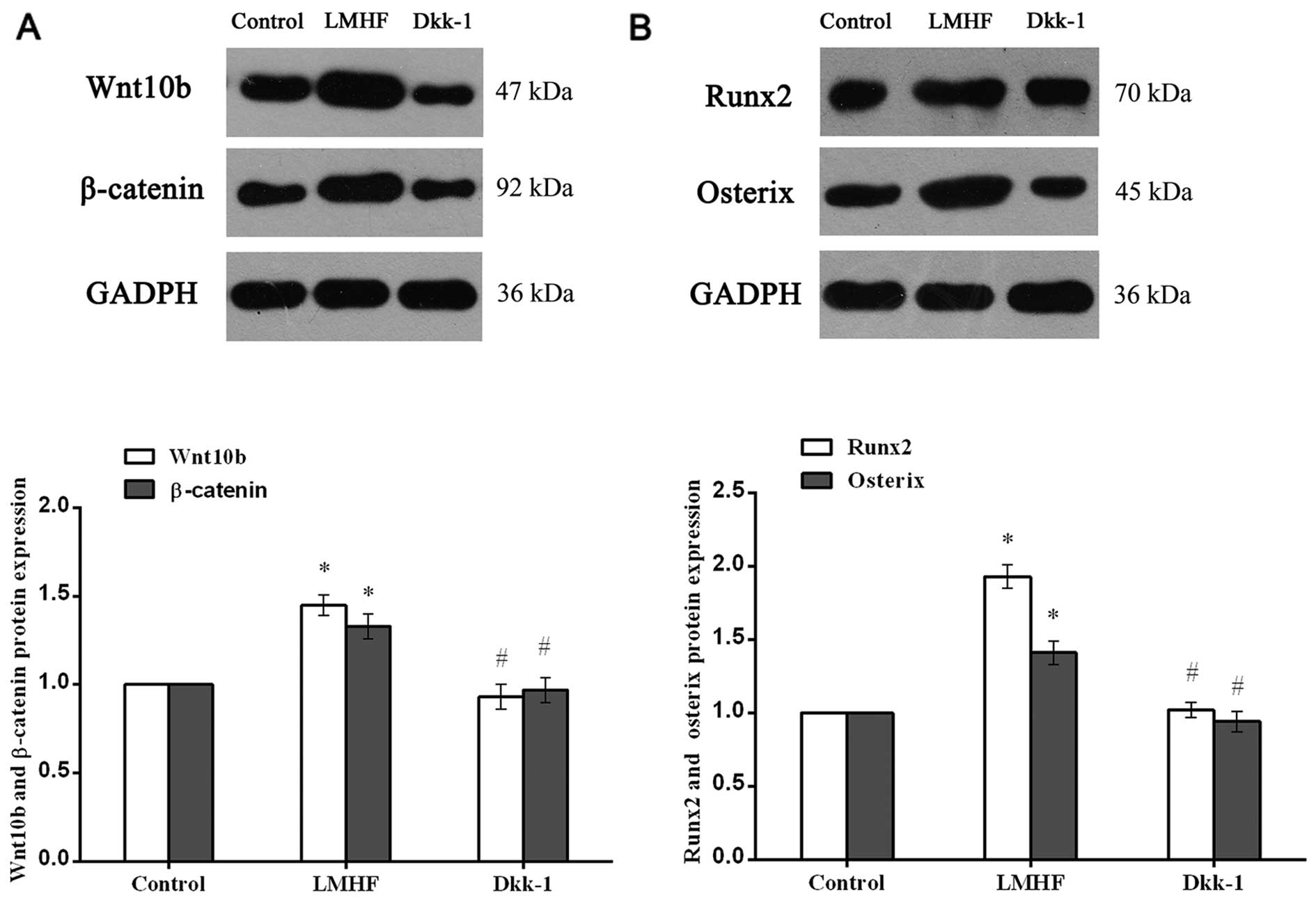
Involvement of Wnt/β-catenin signaling in
LMHF vibration-induced osteogenesis
Western blot analysis and gray analysis showed that
Wnt10b and β-catenin expression in the LMHF group was significantly
increased compared with the control group (P<0.05) (Fig. 6A). Additionally, following the
inhibition of Wnt/β-catenin signaling by Dkk-1, the protein
expression of Wnt10b and β-catenin was reduced when compared with
that in the LMHF group (P<0.05), although there was no
statistically significant difference compared with the control
group (P>0.05) (Fig. 6A).
Similar results were seen with regard to the protein expression of
Runx2 and Osx (Fig. 6B). Runx2
and Osx protein levels were significantly increased in the LMHF
group, when compared with those in the control group (P<0.05).
Similarly, after the administration of Dkk-1, a significant
decrease in Runx2 and Osx protein expression was observed when
compared with that in the LMHF group (P<0.05), and there was no
significant difference between the control and Dkk-1 groups
(P>0.05).
Discussion
A number of in vivo studies have proved that
LMHF vibration is beneficial for osseointegration at the
bone-implant interface (17–19). Previously, we established a rat
model of osteoporosis and inserted implants into the proximal
tibiae, and then treated the rats with LMHF vibration (0.3 g, 30–35
Hz). Our results indicated that LMHF vibration significantly
increased bone-to-implant contact (%) and the peri-implant bone
fraction, as well as biomechanical parameters, such as maximum
push-out force and interfacial shear strength (19). As a continuation of our previous
in vivo study, the current in vitro study aimed to
examine the cellular and molecular mechanisms responsible for the
effects of LMHF vibration on bone-implant osseointegration.
Consistent with our hypothesis, the findings demonstrated that LMHF
vibration enhanced the adhesion and the osteogenic differentiation
of the BMSCs cultured on the HA-coated surface, and Wnt/β-catenin
was responsible for the LMHF vibration-induced osteogenesis.
In the present experiment, we used HA-coated
titanium substrates. HA is the major mineral component of human
bone tissue and has been widely used as a coating of load-bearing
metallic implants due to the osteoconductivity and chemical
similarity of HA to bone (35,36). The HA coating has been found to
increase bone-implant contact and new bone formation around implant
surfaces (37). Previous studies
have demonstrated that the HA coating exhibits good in vitro
cell adhesion and cytocompatibility (20,35–37). Thus, HA-coated substrates were
used in this in vitro study to provide insights into the
adhesion and the osteogenic differentiation of BMSCs in response to
LMHF vibration, and this is a novel contribution of the present
study.
In the present study, the LMHF vibration regime (0.3
g, 40 Hz) used has been previously demonstrated to exert an
osteogenic effect (26,32,33). Zhou et al (26) demonstrated that microvibration
(magnitude, 0.3 g; frequency, 40 Hz) has a stimulatory effect on
the osteodifferentiation of BMSCs. Zhang et al (32) cultured periodontal ligament stem
cells (PDLSCs) and treated them with vibration (magnitude, 0.3 g;
frequency, 10–180 Hz), and the results suggested that the
osteogenic markers were significantly increased by LMHF vibration
at frequencies of 40 and 50 Hz. Additionally, in a study by Kim
et al (33), human
mesenchymal stromal cells (hMSCs) were subjected to vibration
stimuli (magnitude, 0.1–0.6 g; frequency, 10–40 Hz), and they found
that daily exposure to vibration increased the proliferation and
the osteogenic differentiation of hMSCs, with the highest
efficiency occurring at a peak magnitude of 0.3 g and a frequency
of 30 to 40 Hz. Thus, based on the findings of these studies, a
magnitude of 0.3 g and a frequency of 40 Hz was selected for use in
this study.
The ability of BMSCs to adhere to the implant
surface and their differentiation on the implant surface are
important components of successful osseointegration (38,39). In order to analyze cell adhesion
to the implant surface, we assessed the matrix organization (FN)
and the cytoskeleton rearrangement (F-actin), as well as the gene
expression of FN, β1 integrin, vinculin and paxillin, which are
involved in the adhesion of cells to substrates. FN is a major
protein of the ECM and plays a central role in regulating cell
adhesion (23). This study
indicated that LMHF vibration significantly increased the
expression of FN. This upregulation of FN by mechanical stress has
also been reported in other studies (23,40). Mechanical strain is known to
induce changes in cytoskeletal organization, and actin filaments
are crucial for cell adhesion (41). The stimulation of BMSCs by LMHF
vibration resulted in rearrangement of the actin cytoskeleton with
more prominent F-actin, and the fluorescence intensity was
significantly stronger. A similar actin fiber pattern has been
observed in myoblasts following exposure to cyclic strain (42). To the best of our knowledge,
various adhesion molecules are involved in the adhesion of cells to
implants, such as β1 integrin, vinculin and paxillin (43–45). The results showed that LMHF
stimulation notably increased the mRNA expression of β1 integrin,
vinculin and paxillin. Dumas et al (40) suggested that LMHF stimulation
increased the expression of vinculin in mesenchymal stem cells
(MSCs). Carvalho et al (46) showed that human osteosarcoma cells
treated with mechanical stimulation exhibited significantly
increased mRNA expression of β1 integrin. Furthermore, SEM
observation was performed to evaluate cell morphology on the
HA-coated surface. SEM images revealed that the cells were
connected to each other by filopodia and were tightly adhered to
the HA-coated surface, whereas there were higher cell numbers and
more ECM attached to the HA-coated surface in the LMHF group. The
SEM images illustrated that LMHF vibration significantly promoted
the integration of cells with the HA-coated surface. Taken
together, these results demonstrate that LMHF vibration may promote
cell adhesion to the HA-coated surface. This finding was unique in
that it is the first to describe the effects of LMHF vibration on
the cell-implant interaction in vitro, to the best of our
knowledge.
The osteogenic differentiation of cells is usually
divided into three discrete stages: commitment to osteogenic
lineage, matrix synthesis, and matrix mineralization. Runx2 and Osx
are usually highly expressed at the early stage (commitment to
osteogenic lineage) (47,48), Col-I and ALP at the middle stage
(matrix synthesis) (32,48), and OCN at the late stage (matrix
mineralization) of osteogenesis (49). Based on the results of the ALP
activity assay and RT-qPCR, we observed that ALP activity and the
expression of Runx2, Osx, Col-I and OCN were increased by LMHF
vibration. Taken together, these results regarding the
osteogenic-specific markers indicated that LMHF vibration promotes
the osteogenic differentiation of BMSCs cultured on HA-coated
surfaces in vitro. Zhou et al (26) investigated the effect of LMHF
vibration on the osteogenic differentiation of BMSCs seeded on
human bone-derived scaffolds. They found that LMHF vibration
promoted BMSC differentiation by upregulating the mRNA and protein
expression of osteogenic markers including Runx2, ALP, Col-I and
OCN. Zhang et al (32)
cultured PDLSCs under conditions of LMHF vibration. The results
showed that LMHF vibration increased the levels of ALP, Col-I,
Runx2, Osx and OCN, which demonstrated that LMHF mechanical
vibration promotes the osteogenic differentiation of PDLSCs.
Additionally, Prè et al (27) treated BMSCs with mechanical
vibration, and the results showed that the expression of ALP and
Runx2 was significantly increased after mechanical vibration
treatment. Even though a marked enhancement of osteogenic
differentiation in different cell types was observed in these
studies, none of them used HA-coated material to observe the effect
of LMHF vibration on the osteogenic differentiation of BMSCs. Our
experiment covered the limitations of the studies mentioned above.
In addition, the results suggested that LMHF vibration is
beneficial for the osteogenic differentiation of BMSCs cultured on
HA-coated surfaces in vitro.
Although LMHF vibration has been demonstrated to
promote the osteogenic differentiation of BMSCs cultured on implant
surfaces, the molecular mechanism responsible for the effects of
LMHF vibration on the osteogenic differentiation of BMSCs remains
elusive. Several studies have suggested that cells convert the
mechanical stimulus into a biochemical signal, thus playing a role
in regulating osteogenic differentiation (29,30,50). Canonical Wnt signaling promotes
MSCs to differentiate into osteoblasts. In osteoblasts, the Wnt
pathway also promotes proliferation and mineralization whereas it
blocks apoptosis and osteoclastogenesis by increasing the OPG/RANKL
ratio (31). In the present
study, we analyzed the protein expression of Wnt10b, β-catenin,
Runx2 and Osx to probe the direct effect of LMHF vibration on the
osteogenic differentiation of BMSCs through the activation of the
Wnt/β-catenin signaling pathway. We found that LMHF vibration
significantly increased the expression of Wnt10b, β-catenin, Runx2
and Osx; however, after the administration of Dkk-1, these proteins
were downregulated markedly. These results suggest that LMHF
vibration may directly promote the osteogenic differentiation of
BMSCs through the Wnt/β-catenin signaling pathway.
Previously, Robinson et al (28) demonstrated that Wnt/β-catenin
signaling is a normal physiological response to mechanical loading
and that the activation of the Wnt/β-catenin pathway enhances the
sensitivity of osteoblasts to mechanical loading. Hou et al
(30) investigated the
mechanobiological mechanisms of vibration-enhanced osteogenic
responses in MC3T3-E1 cells and they demonstrated that Wnt
signaling was involved in mechanotransduction at LM vibrations and
the RANKL/OPG ratio and levels of sclerostin were also
significantly decreased. It has been found that RANKL/OPG is the
major signaling axis that controls osteoclast formation, activation
and survival, and furthermore, sclerostin was shown to modulate the
activity of osteoblastic cells by reducing ALP activity, type I
collagen synthesis, and mineralization (51). Thus, all of these studies have
suggested that the Wnt/β-catenin signaling pathway is activated by
mechanical vibration in osteoblastic cells, and that mechanical
vibration played a role in promoting osteogenesis indirectly by
activating the Wnt/β-catenin pathway. However, in the present
study, we have demonstrated that LMHF vibration may directly
promote the osteogenic differentiation of BMSCs by activating the
Wnt/β-catenin signaling pathway.
Our in vitro results demonstrated the
positive effects of LMHF vibration on the adhesion and on the
osteogenic differentiation of BMSCs cultured on HA-coated surfaces.
Moreover, the effect of LMHF vibration on the osteogenic
differentiation of BMSCs by activating the Wnt/β-catenin signaling
pathway was also proved. However, it remains unknown whether the
activation of Wnt/β-catenin signaling by LMHF vibration is involved
in the regulation of bone-implant osseointegration. Further
investigations may elucidate the in-depth role of LMHF
vibration-induced activation of Wnt/β-catenin on the osteogenic
differentiation of BMSCs.
In conclusion, LMHF vibration promotes the adhesion
and the osteogenic differentiation of BMSCs on HA-coated surfaces
in vitro. LMHF vibration may directly induce osteogenesis by
activating the Wnt/β-catenin signaling pathway. These data provide
a scientific foundation for improving bone-implant osseointegration
through the application of LMHF vibration.
Acknowledgments
This study was supported by the Guangdong Nature
Science Foundation (no. S2013010015784) and the Guangdong
Provincial Science and Technology Foundation of International
Cooperation Projects (no. 2012B050600026). The authors would like
to thank Ms. Wenhui Zheng for her help with SEM observations and
Mr. Richard Tran for improving the English of this paper.
References
|
1
|
Meirelles L, Arvidsson A, Andersson M,
Kjellin P, Albrektsson T and Wennerberg A: Nano hydroxyapatite
structures influence early bone formation. J Biomed Mater Res A.
87:299–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kujala S, Ryhänen J, Danilov A and
Tuukkanen J: Effect of porosity on the osteointegration and bone
ingrowth of a weight-bearing nickel-titanium bone graft substitute.
Biomaterials. 24:4691–4697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen BL, Xie DH, Zheng ZM, Lu W, Ning CY,
Li YQ, Li FB and Liao WM: Comparison of the effects of alendronate
sodium and calcitonin on bone-prosthesis osseointegration in
osteoporotic rats. Osteoporos Int. 22:265–270. 2011. View Article : Google Scholar
|
|
4
|
Ohkawa Y, Tokunaga K and Endo N:
Intermittent administration of human parathyroid hormone (1–34)
increases new bone formation on the interface of
hydroxyapatitecoated titanium rods implanted into ovariectomized
rat femora. J Orthop Sci. 13:533–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boden SD, Kang J, Sandhu H and Heller JG:
Use of recombinant human bone morphogenetic protein-2 to achieve
posterolateral lumbar spine fusion in humans: a prospective,
randomized clinical pilot trial: 2002 Volvo Award in clinical
studies. Spine. 27:2662–2673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hokugo A, Saito T, Li A, Sato K, Tabata Y
and Jarrahy R: Stimulation of bone regeneration following the
controlled release of water-insoluble oxysterol from biodegradable
hydrogel. Biomaterials. 35:5565–5571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizzoli R, Reginster JY, Boonen S, Bréart
G, Diez-Perez A, Felsenberg D, Kaufman JM, Kanis JA and Cooper C:
Adverse reactions and drug-drug interactions in the management of
women with postmenopausal osteoporosis. Calcif Tissue Int.
89:91–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frost HMA: A 2003 update of bone
physiology and Wolff's Law for clinicians. Angle Orthod. 74:3–15.
2004.PubMed/NCBI
|
|
9
|
Burr DB, Robling AG and Turner CH: Effects
of biomechanical stress on bones in animals. Bone. 30:781–786.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubin C, Recker R, Cullen D, Ryaby J,
McCabe J and McLeod K: Prevention of postmenopausal bone loss by a
low-magnitude, high-frequency mechanical stimuli: a clinical trial
assessing compliance, efficacy, and safety. J Bone Miner Res.
19:343–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubin C, Turner AS, Bain S, Mallinckrodt C
and McLeod K: Anabolism. Low mechanical signals strengthen long
bones. Nature. 412:603–604. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie L, Jacobson JM, Choi ES, Busa B,
Donahue LR, Miller LM, Rubin CT and Judex S: Low-level mechanical
vibrations can influence bone resorption and bone formation in the
growing skeleton. Bone. 39:1059–1066. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubin C, Turner AS, Müller R, Mittra E,
McLeod K, Lin W and Qin YX: Quantity and quality of trabecular bone
in the femur are enhanced by a strongly anabolic, noninvasive
mechanical intervention. J Bone Miner Res. 17:349–357. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garman R, Gaudette G, Donahue LR, Rubin C
and Judex S: Low-level accelerations applied in the absence of
weight bearing can enhance trabecular bone formation. J Orthop Res.
25:732–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Judex S, Lei X, Han D and Rubin C:
Low-magnitude mechanical signals that stimulate bone formation in
the ovariectomized rat are dependent on the applied frequency but
not on the strain magnitude. J Biomech. 40:1333–1339. 2007.
View Article : Google Scholar
|
|
16
|
De Smet E, Jaecques SV, Wevers M, Jansen
JA, Jacobs R, Sloten JV and Naert IE: Effect of controlled early
implant loading on bone healing and bone mass in guinea pigs, as
assessed by micro-CT and histology. Eur J Oral Sci. 114:232–242.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa T, Possemiers T, Zhang X, Naert I,
Chaudhari A, Sasaki K and Duyck J: Influence of whole-body
vibration time on peri-implant bone healing: a histomorphometrical
animal study. J Clin Periodontol. 38:180–185. 2011. View Article : Google Scholar
|
|
18
|
Ogawa T, Zhang X, Naert I, Vermaelen P,
Deroose CM, Sasaki K and Duyck J: The effect of whole-body
vibration on peri-implant bone healing in rats. Clin Oral Implants
Res. 22:302–307. 2011. View Article : Google Scholar
|
|
19
|
Chen B, Li Y, Xie D and Yang X:
Low-magnitude high-frequency loading via whole body vibration
enhances bone-implant osseointegration in ovariectomized rats. J
Orthop Res. 30:733–739. 2012. View Article : Google Scholar
|
|
20
|
Liu X, Bao C, Hu J, Yin G and Luo E:
Effects of clodronate combined with hydroxyapatite on
multi-directional differentiation of mesenchymal stromal cells.
Arch Med Sci. 6:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fassina L, Saino E, Sbarra MS, Visai L, De
Angelis MG, Magenes G and Benazzo F: In vitro electromagnetically
stimulated SAOS-2 osteoblasts inside porous hydroxyapatite. J
Biomed Mater Res A. 93:1272–1279. 2010.
|
|
22
|
Gong SH, Lee H, Pae A, Noh K, Shin YM, Lee
JH and Woo YH: Gene expression of MC3T3-E1 osteoblastic cells on
titanium and zirconia surface. J Adv Prosthodont. 5:416–422. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Palma F, Chamson A, Lafage-Proust MH,
Jouffray P, Sabido O, Peyroche S, Vico L and Rattner A:
Physiological strains remodel extracellular matrix and cell-cell
adhesion in osteoblastic cells cultured on alumina-coated titanium
alloy. Biomaterials. 25:2565–2575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lacouture ME, Schaffer JL and Klickstein
LB: A comparison of type I collagen, fibronectin, and vitronectin
in supporting adhesion of mechanically strained osteoblasts. J Bone
Miner Res. 17:481–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel MJ, Chang KH, Sykes MC, Talish R,
Rubin C and Jo H: Low magnitude and high frequency mechanical
loading prevents decreased bone formation responses of 2T3
preosteoblasts. J Cell Biochem. 106:306–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Guan X, Zhu Z, Gao S, Zhang C, Li
C, Zhou K, Hou W and Yu H: Osteogenic differentiation of bone
marrow-derived mesenchymal stromal cells on bone-derived scaffolds:
effect of microvibration and role of ERK1/2 activation. Eur Cell
Mater. 22:12–25. 2011.PubMed/NCBI
|
|
27
|
Prè D, Ceccarelli G, Visai L, Benedetti L,
Imbriani M, Cusella De Angelis MG and Magenes G: High-frequency
vibration treatment of human bone marrow stromal cells increases
differentiation toward bone tissue. Bone Marrow Res.
2013:8034502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P,
Brown EL, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Premaraj S, Souza I and Premaraj T:
Mechanical loading activates β-catenin signaling in periodontal
ligament cells. Angle Orthod. 81:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou WW, Zhu ZL, Zhou Y, Zhang CX and Yu
HY: Involvement of Wnt activation in the micromechanical
vibration-enhanced osteogenic response of osteoblasts. J Orthop
Sci. 16:598–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubota T, Michigami T and Ozono K: Wnt
signaling in bone metabolism. J Bone Miner Metab. 27:265–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Li J, Zhang L, Zhou Y, Hou W,
Quan H, Li X, Chen Y and Yu H: Effects of mechanical vibration on
proliferation and osteogenic differentiation of human periodontal
ligament stem cells. Arch Oral Biol. 57:1395–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim IS, Song YM, Lee B and Hwang SJ: Human
mesenchymal stromal cells are mechanosensitive to vibration
stimuli. J Dent Res. 91:1135–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau E, Lee WD, Li J, Xiao A, Davies JE, Wu
Q, Wang L and You L: Effect of low-magnitude, high-frequency
vibration on osteogenic differentiation of rat mesenchymal stromal
cells. J Orthop Res. 29:1075–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roy M, Fielding GA, Beyenal H,
Bandyopadhyay A and Bose S: Mechanical, in vitro antimicrobial, and
biological properties of plasma-sprayed silver-doped hydroxyapatite
coating. ACS Appl Mater Interfaces. 4:1341–1349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su B, Peng X, Jiang D, Wu J, Qiao B, Li W
and Qi X: In vitro and in vivo evaluations of
nano-hydroxyapatite/polyamide 66/glass fibre (n-HA/PA66/GF) as a
novel bioactive bone screw. PLoS One. 8:e683422013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duan Y, Zhu S, Guo F, Zhu J, Li M, Ma J
and Zhu Q: The effect of adhesive strength of hydroxyapatite
coating on the stability of hydroxyapatite-coated prostheses in
vivo at the early stage of implantation. Arch Med Sci. 8:199–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olivares-Navarrete R, Hyzy SL, Hutton DL,
Erdman CP, Wieland M, Boyan BD and Schwartz Z: Direct and indirect
effects of microstructured titanium substrates on the induction of
mesenchymal stem cell differentiation towards the osteoblast
lineage. Biomaterials. 31:2728–2735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Puleo DA and Nanci A: Understanding and
controlling the bone-implant interface. Biomaterials. 20:2311–2321.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dumas V, Ducharne B, Perrier A, Fournier
C, Guignandon A, Thomas M, Peyroche S, Guyomar D, Vico L and
Rattner A: Extracellular matrix produced by osteoblasts cultured
under low-magnitude, high-frequency stimulation is favourable to
osteogenic differentiation of mesenchymal stem cells. Calcif Tissue
Int. 87:351–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sato K, Adachi T, Matsuo M and Tomita Y:
Quantitative evaluation of threshold fiber strain that induces
reorganization of cytoskeletal actin fiber structure in
osteoblastic cells. J Biomech. 38:1895–1901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng L, Song J, Li Z, Fan Y, Zhao Z, Chen
Y, Deng F and Hu Y: The mechanism of myoblast deformation in
response to cyclic strain - A cytomechanical study. Cell Biol Int.
32:754–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wixler V, Geerts D, Laplantine E, Westhoff
D, Smyth N, Aumailley M, Sonnenberg A and Paulsson M: The LIM-only
protein DRAL/FHL2 binds to the cytoplasmic domain of several alpha
and beta integrin chains and is recruited to adhesion complexes. J
Biol Chem. 275:33669–33678. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mierke CT: The role of vinculin in the
regulation of the mechanical properties of cells. Cell Biochem
Biophys. 53:115–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mierke CT: The role of focal adhesion
kinase in the regulation of cellular mechanical properties. Phys
Biol. 10:0650052013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carvalho RS, Scott JE and Yen EH: The
effects of mechanical stimulation on the distribution of beta 1
integrin and expression of beta 1-integrin mRNA in TE-85 human
osteosarcoma cells. Arch Oral Biol. 40:257–264. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marie PJ: Transcription factors
controlling osteoblastogenesis. Arch Biochem Biophys. 473:98–105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakashima K and de Crombrugghe B:
Transcriptional mechanisms in osteoblast differentiation and bone
formation. Trends Genet. 19:458–466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mantila Roosa SM, Liu Y and Turner CH:
Gene expression patterns in bone following mechanical loading. J
Bone Miner Res. 26:100–112. 2011. View Article : Google Scholar
|
|
51
|
Winkler DG, Sutherland MK, Geoghegan JC,
Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR,
Staehling-Hampton K, et al: Osteocyte control of bone formation via
sclerostin, a novel BMP antagonist. EMBO J. 22:6267–6276. 2003.
View Article : Google Scholar : PubMed/NCBI
|