Introduction
Type 2 diabetes and metabolic syndrome are prevalent
worldwide (1). These metabolic
disorders are characterized by the disruption of glucose and lipid
metabolism. Obesity is major risk for the development of type 2
diabetes and related metabolic disorders (2). The prevalence of obesity has
increased worldwide, with more than a billion adults being
overweight and over 300 million adults being classified as obese
(3). Obesity is defined not as an
excess of body weight, but as an excessive accumulation of fat mass
in white adipose tissue to the extent that health may be adversely
affected.
The accumulation of body fat greatly depends on
adipocyte differentiation, also known as adipogenic differentiation
or adipogenesis (4). Adipocyte
differentiation is a complex process which is divided into four
steps, including initial growth arrest, mitotic clonal expansion
(MCE), early differentiation and terminal differentiation, namely
the development of the mature adipocyte phenotype (5,6).
Adipocyte differentiation is regulated by a series of signaling
pathways triggered by an adipogenic stimulus. Among these factors,
peroxisome proliferator-activated receptor γ (PPARγ) and
CCAAT/enhancer-binding protein (C/EBP)α family members are the key
transcription factors responsible for adipogenesis (7,8).
In response to adipogenic stimuli, C/EBPβ is rapidly activated and
functions at the early stage of differentiation by initiating the
MCE of pre-adipocytes and activating the PPARγ and C/EBPα
regulatory network (9–12). C/EBPα and PPARγ mutually interact
and form a positive feedback loop that plays pivotal roles during
the later stage of adipocyte differentiation by inducing and
maintaining the expression of multiple adipocyte-specific genes,
i.e. fatty-acid binding protein (aP2), cluster of differentiation
36 (CD36, a receptor for lipoproteins) and fatty acid transport
protein-1 (FATP-1) (13–16). Abnormal adipocyte differentiation
is involved in the development of multiple metabolic disorders.
Over the past years, many researchers have focused
on the investigation of dietary agents and natural products that
can be used for the intervention of metabolic disorders.
Amentoflavone (AMF) is a polyphenolic compound derived from the
extracts of Selaginella tamariscina which has been found to
possess potent anti-inflammatory effects (17–20). A previous study demonstrated that
AMF inhibits protein tyrosine phosphatase 1B activity, which has
been proposed as a strategy for the treatment of type 2 diabetes
and obesity (21). The present
study was designed to examine the effects of AMF on high fat (HF)
diet-induced metabolic dysfunction, focusing on the regulation of
adipocyte differentiation.
Materials and methods
Materials
Anti-β-actin (sc-130656), anti-PPARγ (sc-7273),
anti-C/EBPα (sc-61) and anti-C/EBPβ (sc-746) antibodies and
3-isobutyl-1-methylxanthine (IBMX) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). AMF, Oil Red O, insulin,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA),
N-acetylcysteine (NAC) and most of the chemicals and reagents used
in this study were procured from Sigma (St. Louis, MO, USA).
Animal experiments
All animal experiments were performed according to
the procedures approved by the Affiliated Hospital of the Chinese
Academy of Military Medical Sciences, Beijing, China (approval no.
CAMS-2014-12-115). A total of 32 male Wistar rats (6–8 weeks old;
weighing 180–220 g) were purchased from the Animal Centre of
Capital Medical University, Beijing, China. The rats were housed
under controlled temperature (23±2°C) and humidity (55±5%)
conditions with a standard light and dark (12 h light/dark) cycle.
The rats were randomly divided into 4 groups as follows: the
control group, the HF diet group, the HF diet + 10 mg/kg AMF group
and the HF diet + 50 mg/kg AMF group. The rats in the control group
were fed a standard diet (22% protein, 63% carbohydrate and 5% fat;
Animal Center of Chinese Academy of Medical Sciences). The rats in
the HF diet group were given a HF diet. The HF diet consisted of
10% protein, 72% carbohydrate and 8% fat; (Animal Center of Chinese
Academy of Medical Sciences). Rats in HF diet + AMF group were
administrated with 10 or 50 mg/kg/day AMF via intraperitoneal
injection (AMF was dissolved in DMSO and diluted in saline) for 4
months. The experimental period lasted for 4 months. After being
fed the different diets, fasting blood glucose (FBG) levels (12 h)
were measured. At the end of the experiment, the rats were weighed
using a scale used for animals prior to anesthesia. The mice were
anesthetized with sodium pentobarbital, perirenal adipose tissues
were weighed (perirenal adipose tissues were cut using surgical
scissors and weighed using a precision balance) and tissue and
blood samples (obtained from the vena cava) were then harvested for
the assays.
Intraperitoneal glucose tolerance test
(IPGTT) and intraperitoneal insulin tolerance test (IPITT)
After the animals were fed the different diets, the
IPGTT and the IPITT were performed as previously described
(22). The rats were fasted for
12 and 4 h, respectively, and the blood samples were obtained from
the tail vein for the detection of basal glucose levels.
Subsequently, each rat was intraperitoneally injected with glucose
(1 g/kg body weight), or insulin (0.75 U/kg body weight). Blood
samples were obtained from the tail vein at 30, 60 and 120 min
after the injection and were analyzed immediately for the glucose
concentration. The area under curve (AUC) was calculated to
determine the relative efficiency of AMF. In the IPGTT and IPITT
tests, AUC was used to evaluate response to glucose and insulin
load. AUC was calculated as: (glucose level at 0 min + glucose
level at 30 min) ×0.5/2 + (glucose level at 30 min + glucose level
at 60 min) ×0.5/2 + (glucose level at 60 min + glucose level at 120
min) ×1/2.
Biochemical analysis
After being fed the different diets, the blood
samples were harvested and serum was separated for the estimation
of triglyceride (TG) levels (Nanjing Jiancheng Co., Nanjing, China)
and insulin levels (Crystal Chem, Downers Grove, IL, USA) using
commercial kits. The assays were conducted according to the
manufacture's instructions. The TG content was determined according
to the formation of quinonyl compounds and the absorbance at 510 nm
was measured. Insulin was determined with the ultra-sensitive rat
insulin ELISA kit. At the end of detection, the A450 and subtract
A630 values were measured within 30 min and insulin concentrations
were calculated using the standard curve. Homeostatic model
assessment-insulin resistance index (HOMA-IR) is an index
reflecting the insulin sensitivity. HOMA-IR was calculated as:
(glucose level × insulin level)/22.5.
Cell culture and treatment
The 3T3-L1 pre-adipocytes were cultured and
differentiation was induced as previously described (23). The 3T3-L1 pre-adipocytes were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% bovine calf serum, penicillin (100 U/ml), streptomycin
(100 µg/ml) and glutamine (292 µg/l). The cells were
maintained at 37°C in a humidified 5% CO2 atmosphere. AT
2 days post-confluence (day 0), the 3T3-L1 pre-adipocytes were
stimulated with 0.5 mM IBMX, 1 µM dexamethasone and 167 nM
insulin in DMEM containing 10% fetal bovine serum (FBS) (also known
as DMI differentiation induction medium) for 2 days (day 2). The
cells were then incubated with 10% FBS/DMEM medium with 167 nM
insulin for a further 2 days (day 4) and were then cultured in 10%
FBS/DMEM medium for an additional 4 days (day 8). Following 8 days
of differentiation, >90% of the cells became mature adipocytes
with lipid-filled droplets. For AMF treatment, indicated
concentrations (1–10 µg/ml) of AMF were added to the DMI
induction medium for the indicated incubation time periods (2 days)
maintained when the medium was changed.
Cell transfection
The scramble plasmids, and the C/EBPα and PPARγ
plasmids were synthesized (GeneChem, Shanghai, China). Transient
transfection was performed using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
C/EBPα (500 ng), or PPARγ (500 ng) or their empty vectors were
mixed with 4 µl Lipofectamine 2000 (Invitrogen) in 400
µl serum-free medium and placed at temperature for 20 min.
The mixture was then added to the wells and incubated for 4–6 h.
The medium was then changed to complete culture medium. The cells
were transfected with the plasmids 2 days before confluence.
Determination of lipid accumulation
After the treatments, the adipocytes were fixed with
4% paraformaldehyde for 30 min and rinsed with water. Subsequently,
the fixed cells were stained with Oil Red O staining solution (6
parts of saturated Oil Red O dye in isopropanol + 4 parts water)
for 30 min. The cells were washed with water to remove the excess
dye. Lipid accumulation in the cells was observed under a
microscope (Olympus, Tokyo, Japan). The cells were then incubated
with 4% Nonidet P-40 in isopropanol for 5 min to dissolve the
stained oil droplets. The absorbance of the dye lipid complex was
measured at 510 nm using a plate reader (Tecan, Männedorf,
Switzerland).
Cell viability and proliferation
Cell viability was examined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. A total of 0.5 mg/ml MTT was added to each well and
incubated for 4 h. After aspiration of the supernatants, formazan
crystals were dissolved using DMSO. The absorbance at 550 nm was
measured using a plate reader (Tecan). Cell proliferation was
determined using a CCK8 assay kit (Beyotime, Shanghai, China)
according to the manufacturer's instructions. Cells were plated in
96-well culture plates. After the treatment, 10 µl of the
CCK-8 solution was added to each well and cells were cultured at
37°C for an additional hour. The absorbance at 450 nm was measured
to evaluate cell proliferation (Tecan). The absorbance was detected
at 570 and 450 nm, respectively, using a microplate reader (Bio-Rad
Biosystems, Hercules, CA, USA).
Determination of reactive oxygen species
(ROS) generation
ROS were detected usng the specific probe, DCFH-DA,
as previously described (24).
After the treatments, the cells were incubated with 10 µM
DCFH-DA in the dark at 37°C for 30 min. After washing twice with
PBS, the ROS levels were analyzed by flow cytometry (C6; BD
Biosciences, San Jose, CA, USA).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the cells using an RNA
isolation kit (Tiangen, Beijing, China) according to the
manufacturer's instructions. RNA (500 ng) was reverse transcribed
into cDNA using the First Strand cDNA synthesis kit (Takara, Tokyo,
Japan). Quantitative PCR (qPCR) was performed using SYBR-Green
(Takara) in a real-time PCR system from Bio-Rad Biosystems. The
primers used were as follows: PPARγ forward, 5′-ACA AGA GCT GAC CCA
ATG-3′and reverse, 5′-CAT GAG GGA GTT AGA AGG-3′; aP2 forward,
5′-CGT TCT CTT TCT CCC TGT-3′ and reverse, 5′-TTG AAG GAA ATC TCG
GTG-3′; CD36 forward, 5′-TTT GGA TCT TTG ATG TGC-3′ and reverse,
5′-TTT CCT TGG CTA GAT AAC-3′; C/EBPα forward,
5′-AGGTGCTGGAGTTGACCAGT-3′ and reverse, 5′-CAGCCTAGAGATCCAGCGAC-3′;
β-actin forward, 5′-AGG CCA ACC GTG AAA AGA TG-3′ and reverse,
5′-TGG CGT GAG GGA GAG CAT AG-3′. The 2−ΔΔCT method was
used to measure gene expression compared with the endogenous
control (β-actin). Amplification was performed with an initial step
at 94°C for 5 min, followed by 40 cycles of denaturation at 94°C
for 30 sec, annealing at 63°C for 30 sec, and then extension at
72°C for 10 sec.
Protein extraction and western blot
analysis
Total protein was extracted from the cells using
RIPA lysis buffer according to the manufacturer's instructions. The
cell lysates were washed with cold phosphate-buffered saline (PBS)
and incubated on ice in lysis buffer for 30 min. The lysates were
centrifuged at 20,000 × g for 30 min at 4°C and the protein
concentrations in the supernatants were measured using a Bradford
protein assay kit (Pierce, Rockford, IL, USA). Approximately 20
µg of protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins
were then transferred onto PVDF membranes (Millipore, Billerica,
MA, USA), and the membranes were then blocked with 8% non-fat milk.
The membranes were incubated overnight at 4°C with the primary
antibodies (anti-β-actin, anti-PPARγ, anti-C/EBPα and anti-C/EBPβ
antibodies). After washing with TBST, the membranes was incubated
with a horseradish peroxidase-conjugated secondary antibody (Cat.
no. 32260; Pierce) at 37°C for 30 min. Protein bands were
visualized by ECL and captured using a Bio-Rad Imaging System
(Bio-Rad Biosystems).
Statistical analysis
Results were expressed as the means ± SEM.
Statistical analysis was carried out by one-way analysis of
variance (ANOVA) followed by the Newmane Keuls multiple-comparison
post hoc test using GraphPad Prism software. Data were considered
statistically significant for p<0.05.
Results
AMF attenuates metabolic dysfunction in
rats fed a HF diet
In the present study, we first evaluated the effects
of AMF on metabolic dysfunction in rats fed a HF diet. As shown in
Table I, the FBG levels in the HF
diet group were 118.5±12.8 mg/dl, which were significantly higher
than those of the control group rats (65.9±6.3 mg/dl). The
administration of AMF at 10 and 50 mg/kg significantly decreased
the FBG levels to 101.1±9.7 and 82.9±8.1 mg/dl, respectively in the
HF diet group rats. The HF diet markedly increased the fasting
insulin levels from 1.5±0.4 (control group) to 4.3±0.6 ng/dl. The
administration of 10 and 50 mg/kg AMF significantly decreased the
fasting insulin levels to 3.2±0.5 and 2.8±0.4 ng/dl, respectively
in the HF diet-fed rats. As also shown in Table I, the HOMA-IR value was increased
from 0.5±0.2 to 8.6±2.7 in the rats fed the HF diet. The
administration of 10 and 50 mg/kg AMF significantly inhibited the
increase in the HOMA-IR value (5.7±1.1 and 3.2±2.3, respectively)
in the HF diet-fed rats.
 | Table IEffect of AMF on high fat
diet-induced metabolic dysfunction. |
Table I
Effect of AMF on high fat
diet-induced metabolic dysfunction.
| Group | FBG (mg/dl) | FI (ng/dl) | HOMA-IR |
|---|
| Control | 65.9±6.3 | 1.5±0.4 | 0.5±0.2 |
| High-fat diet | 118.5±12.8a | 4.3±0.6a | 8.6±2.7a |
| 10 mg/kg AMF | 101.1±9.7b | 3.2±0.5b | 5.7±1.1b |
| 50 mg/kg AMF | 82.9±8.1b | 2.8±0.4b | 3.2±2.3b |
We also examined the effects of AMF on glucose and
insulin tolerance in the HF diet-fed rats (Table II). In the HF diet group,
following the injection of glucose, the glucose level increased
from 128.1±5.1 to 183.3±5.4 mg/dl at 30 min and then decreased to
145.8±6.7 mg/dl at 120 min, which was significantly higher than
that of the control group. In the 10 mg/kg AMF group, following the
injection of glucose, the glucose level increased from 105.2±4.9 to
163.8±4.8 mg/dl at 30 min at 30 min and then decreased to 129.9±6.4
mg/dl at 120 min. In the 50 mg/kg AMF group, following the
injection of glucose, the glucose level increased from 87.8±5.1 to
131.9±5.2 mg/dl at 30 min and then decreased to 116.5±6.6 mg/dl at
120 min. The glucose levels in the AMF groups were significantly
lower than those of the HF-diet group, as indicated by the IPGTT.
The AUC was calculated and the results revealed that AMF
significantly inhibited the increase in the AUC value in the HF
diet-fed rats, as indicated by the IPGTT.
 | Table IIEffect of AMF on high-fat
diet-induced glucose and insulin intolerance. |
Table II
Effect of AMF on high-fat
diet-induced glucose and insulin intolerance.
| Group | IPGTT (mg/dl)
|
|---|
| 0 min | 30 min | 60 min | 120 min | AUC |
|---|
| Control | 64.8±4.6 | 118.2±5.8 | 97.1±6.3 | 77.4±5.5 | 191±16 |
| High-fat diet | 128.1±5.1a | 183.3±5.4a | 165.2±6.6a | 145.8±6.7a | 319±23a |
| 10 mg/kg AMF | 105.2±4.9b | 163.8±4.8b | 145.6±5.3b | 129.9±6.4b | 285±18b |
| 50 mg/kg AMF | 87.8±5.1b | 131.9±5.2b | 127.5±5.7b | 116.5±6.6b | 247±22b |
|
| IPITT (mg/dl)
|
| Control | 77.18±5.2 | 51.3±5.3 | 43.1±4.9 | 44.7±5.4 | 121±8 |
| High-fat diet | 154.8±5.1a | 97.9±6.6a | 85.6±5.8a | 92.4±5.7a | 209±9a |
| 10 mg/kg AMF | 125.6±5.4b | 79.5±4.7b | 68.9±5.6b | 75.8±5.1b | 184±7b |
| 50 mg/kg AMF | 91.7±4.8b | 61.6±5.2b | 53.9±5.7b | 57.8±5.5b | 149±8b |
As regards insulin tolerance, in the HF-diet group,
following the injection of insulin, the glucose level decreased
from 154.8±5.1 to 85.6±5.8 mg/dl at 60 min and then increased to
92.4±5.7 mg/dl at 120 min, which was significantly higher than that
of the control group. In the 10 mg/kg AMF group, following the
injection of insulin, the glucose level decreased from 125.6±5.4 to
68.9±5.6 mg/dl at 60 min and then increased to 75.8±5.1 mg/dl at
120 min. In the 50 mg/kg AMF group, following the injection of
insulin, the glucose level decreased from 91.7±4.8 to 53.9±5.7
mg/dl at 60 min and then increased to 57.8±5.5 mg/dl at 120 min.
The glucose levels in the AMF groups were significantly lower than
those of the HF diet group, as indicated by IPITT. As shown by the
AUC values, AMF significantly inhibited the increase in the AUC
values in the HF diet-fed rats, as indicated by the IPITT. These
results indicated that the administration of AMF significantly
attenuated HF diet-induced metabolic dysfunction in rats.
AMF attenuates lipid accumulation in rats
fed a HF diet
A shown in Table
III, the HF diet significantly increased the body weights of
the rats from 362±38 to 484±37 g. The administration of 10 and 50
mg/kg AMF significantly inhibited the HF diet-induced increase in
body weight (434±29 and 402±25, respectively). We also determined
the weights of perirenal adipose tissues and the results revealed
that the weights of the perirenal adipose tissues were
significantly increased in the HF diet group (13.8±3.9 g). The
administration of 10 and 50 mg/kg AMF markedly decreased the
weights of the perirenal adipose tissues to 10.2±1.6 and 8.3±2.5 g,
respectively, in the HF diet-fed rats (Table III). The serum TG content was
also determined. As shown in Table
III, the HF diet induced a significant increase in the TG
content (249±25 mg/dl). The administration of 10 and 50 mg/kg AMF
significantly inhibited the increase in the serum TG content
(211±15 and 176±14, respectively) in the rats fed the HF diet.
These results indicated that the administration of AMF
significantly attenuated HF diet-induced lipid accumulation in
rats.
 | Table IIIEffect of AMF on high fat
diet-induced lipid accumulation. |
Table III
Effect of AMF on high fat
diet-induced lipid accumulation.
| Group | BW (g) | PATW (g) | Serum TG
(mg/dl) |
|---|
| Control | 362±38 | 3.5±1.4 | 146±21 |
| High fat | 484±37a | 13.8±3.9a | 249±25a |
| 10 mg/kg AMF | 434±29b | 10.2±1.6b | 211±15b |
| 50 mg/kg AMF | 402±25b | 8.3±2.5b | 176±14b |
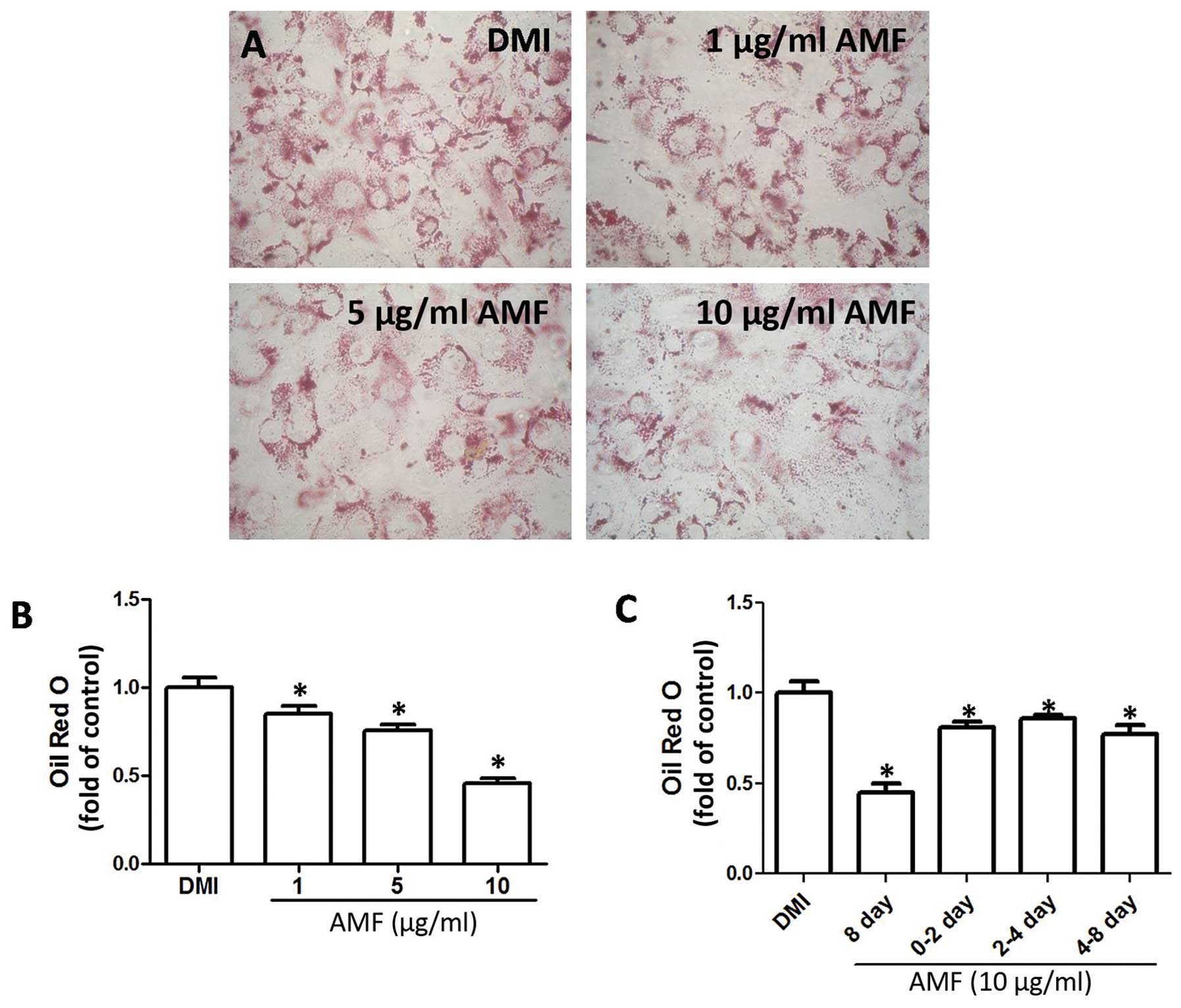
AMF inhibits 3T3-L1 adipocyte
differentiation
We examined the effects of AMF on 3T3-L1 adipocyte
differentiation in vitro. AMF inhibited the accumulation of
oil droplets in the differentiated adipocytes in a
concentration-dependent manner, indicating that AMF suppressed
3T3-L1 adipocyte differentiation (Fig. 1A and B). Moreover, we evaluated
the effect of AMF on 3T3-L1 adipocyte differentiation during
different stages of differentiation. 3T3-L1 pre-adipocytes were
subjected to adipogenic differentiation in the presence of 10
µg/ml AMF for 0–8, 0–2, 2–4 and 4–8 days. As shown in
Fig. 1C, 0–2, 2–4 and 4–8 days of
incubation of the cells with AMF significantly decreased Oil Red O
staining in adipocytes, compared with the control, indicating that
AMF exerted inhibitory effects on 3T3-L1 adipocyte differentiation
at different stages of differentiation.
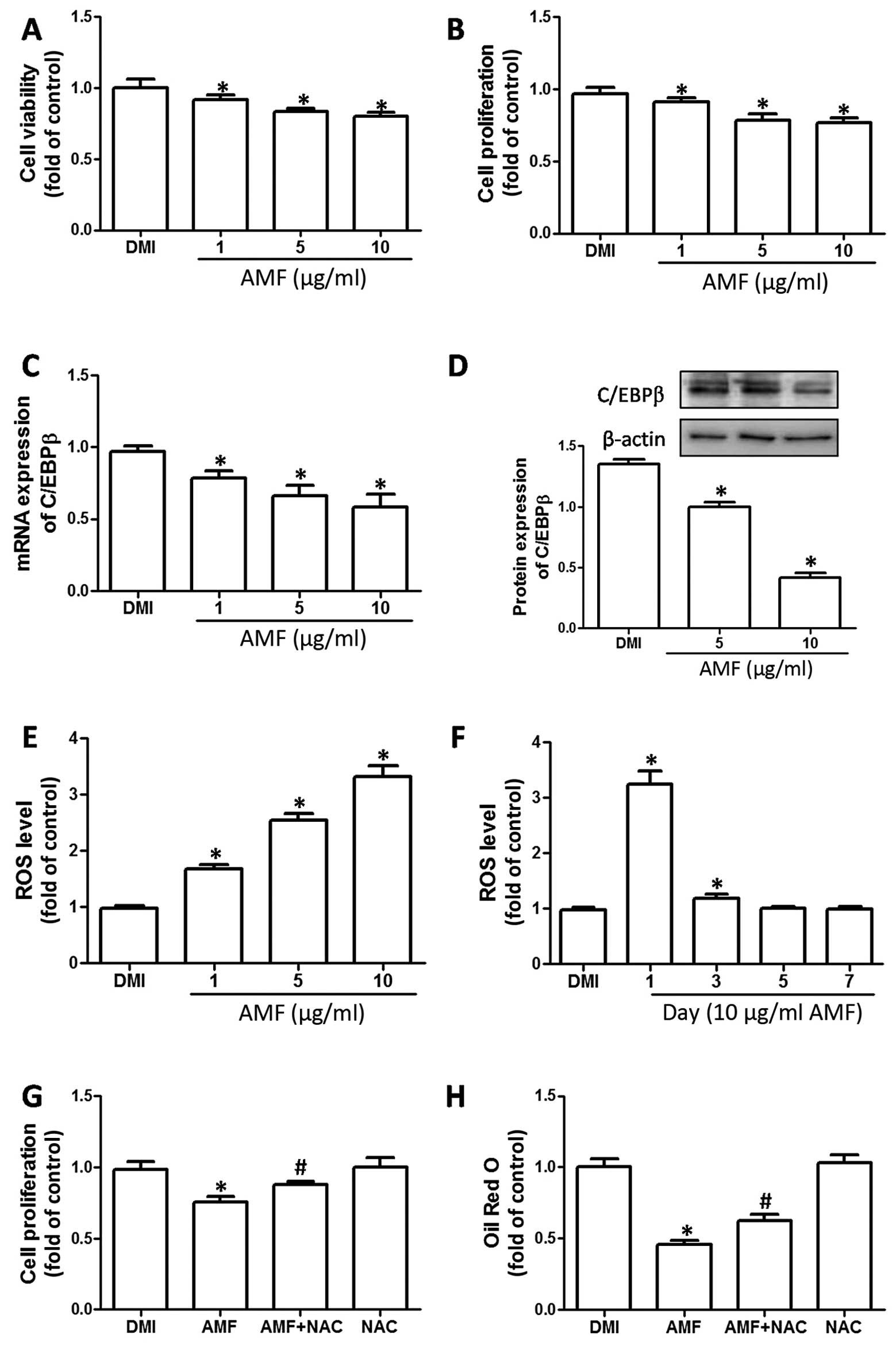
AMF inhibits MCE during 3T3-L1 adipocyte
differentiation by promoting ROS generation
To examine the effects of AMF on MCE, 3T3-L1
pre-adipocytes were subjected to adipogenic differentiation in the
presence of indicated concentrations of AMF for 2 days and cell
viability and proliferation were detected. AMF inhibited cell
viability and proliferation in a concentration-dependent manner at
day 2 of differentiation, indicating the suppression of MCE
(Fig. 2A and B). To examine the
mechanisms resposible for the AMF-induced inhibition of MCE, the
effect of AMF on C/EBPβ expression was determined. AMF inhibited
the mRNA and protein expression of C/EBPβ in a
concentration-dependent manner (Fig.
2C and D). Moreover, we examined the effect of AMF on ROS
generation during MCE. As shown in Fig. 2E, at day 1 of differentiation, AMF
markedly increased the ROS level in a concentration-dependent
manner. Furthermore, AMF only increased the ROS level at the early
stages of adipocyte differentiation, indicating that ROS generated
by AMF mainly influence MCE (Fig.
2F). A shown in Fig. 2G and
H, we found that the antioxidant, NAC, significantly suppressed
the inhibitory effect of AMF on cell proliferation and oil droplet
accumulation, confirming that ROS generation was involved in the
AMF-induced inhibition of adipocyte differentiation through the
suppression of MCE.
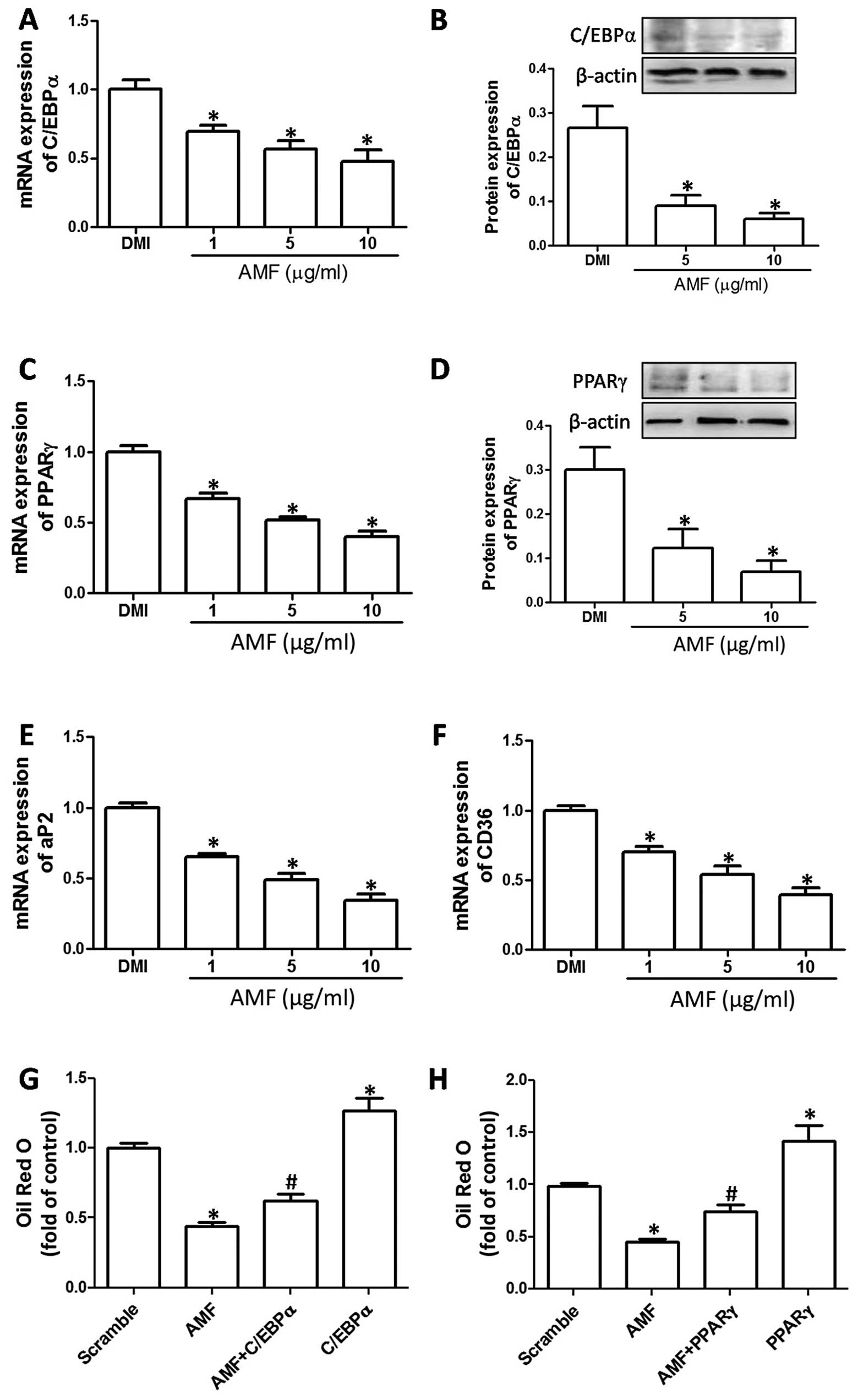
AMF inhibits 3T3-L1 adipocyte
differentiation through the inhibition of C/EBPα/PPARγ
signaling
To evaluate the mechanisms responsible for the
inhibitory effect of AMF on the early and terminal differentiation
of 3T3-L1 adipocytes, the effects of AMF on C/EBPα and PPARγ
expression were determined. AMF inhibited the mRNA and protein
expression of C/EBPα and PPARγ in a concentration-dependent manner
(Fig. 3A–D). Moreover, we
detected the expression of aP2 and CD36, which are direct targets
of PPARγ in the regulation of adipogenic differentiation. The mRNA
expression of aP2 and CD36 was significantly inhibited by AMF in a
concentration-dependent manner (Fig.
3E and F). To further examine the role of the downregulation of
C/EBPα and PPARγ in the AMF-induced inhibition of adipocyte
differentiation, 3T3-L1 pre-adipocytes were transfected with C/EBPα
and PPARγ plasmids. As shown in Fig.
2G and H, the overexpression of C/EBPα and PPARγ significantly
prohibited the AMF-induced inhibition of adipocyte differentiation.
These results indicated that AMF inhibited 3T3-L1 adipocyte
differentiation through the inhibition of C/EBPα/PPARγ
signaling.
Discussion
AMF is a polyphenolic compound derived from the
extracts of Selaginella tamariscina. A number of studies
have demonstrawted that AMF exerts anti-inflammatory (17–20), antioxidant (25–27), anti-viral (28,29) and antitumor effects (30,31), as well as protective effects
against radiation-induced damaqge (32,33) and neuroprotective effects
(32,33). Na et al found that AMF
inhibited protein tyrosine phosphatase 1B activity, implicating a
potential use of AMF in the treatment of type 2 diabetes and
obesity (21). In the current
study, we evaluated the protective effects of AMF against metabolic
dysfunction and focused on the influence of AMF on adipocyte
differentiation.
We found that AMF protected against HF diet-induced
metabolic dysfunction in a dose-dependent manner, as evidenced by a
decrease in fasting blood glucose levels, fasting insulin levels,
HOMA-IR and a decrease in the level of glucose (as shown by IPGTT
and IPITT). Lipid accumulation and thus, obesity is the major
contributor to metabolic disroders induced by a HF diet (34,35). In the present study, we focused on
whether the improvement of metabolic function induced by AMF was
attributed to the regulation of lipid accumulation. We found that
AMF significantly inhibited the increase in body weights, the
weights of perirenal adipose tissues and the serum TG content in a
dose-dependent manner in rats fed a HF diet. Our data demonstrated
that the inhibition of lipid accumulation may be involved in the
inhibitory effects of AMF on HF diet-induced metabolic
dysfunction.
We then examined the possible mechanism responsible
for the AMF-induced inhibition of lipid accumulation. The
accumulation of body fat largely depends on adipocyte
differentiation. The course of adipocyte differentiation includes
four steps, initial growth arrest, MCE, early differentiation and
terminal differentiation, namely the development of the mature
adipocyte phenotype (5,6). Our results revealed that AMF
inhibited the different stages of adipocyte differentiation. MCE is
a synchronous process required for adipogenesis (36). C/EBPβ is required for MCE during
adipogenesis (12). We found that
AMF decreased C/EBPβ expression in a concentration-dependent
manner, leading to the inhibition of MCE. Moreover, our results
indicated that ROS generation was involved in the AMF-induced
inhibition of MCE. Hoever, it has been demonstrated that ROS
enhance MCE and facilitate adipogenesis by promoting C/EBPβ
expression (37). This
discrepancy may be attributed to different levels or sources of ROS
production. It has been demonstrated that AMF exhibits potential
antioxidant activities (25–27). For example, Xu et al found
the free radical scavenging and antielastase activities of AMF
(26). Yamaguchi et al
indicated that AMF from Brazilian pine Araucaria
angustifolia exerted protective effects against DNA damage and
lipoperoxidation (27). Combined
with our results, it is suggested that AMF exerts differential
effects on the redox state in different cell lines or under
different conditions.
After MCE, early and terminal differentiation is
mainly regulated by the PPARγ and C/EBPα interactive network.
Without C/EBPα, the formation of white adipose tissue is disrupted
(38). The ectopic expression of
C/EBPα promotes adipogenesis in various fibroblastic cell lines
(39). However, C/EBPα is
incapable of inducing adipogenesis without PPARγ (39). PPARγ regulates adipocyte
differentiation by transcriptionally modulating a battery of target
genes responsible for the terminal maturation of fat cells,
including aP2 and CD36. Our results indicated that AMF inhibited
PPARγ and C/EBPα expression and the expression of downstream
targets in a concentration-dependent manner. The overexpression of
PPARγ and C/EBPα suppressed the AMF-induced inhibition of the
formation of oil droplets, indicating that the downregulation of
PPARγ and C/EBPα was involved in the AMF-induced inhibitory effect
on adipocyte differentiation.
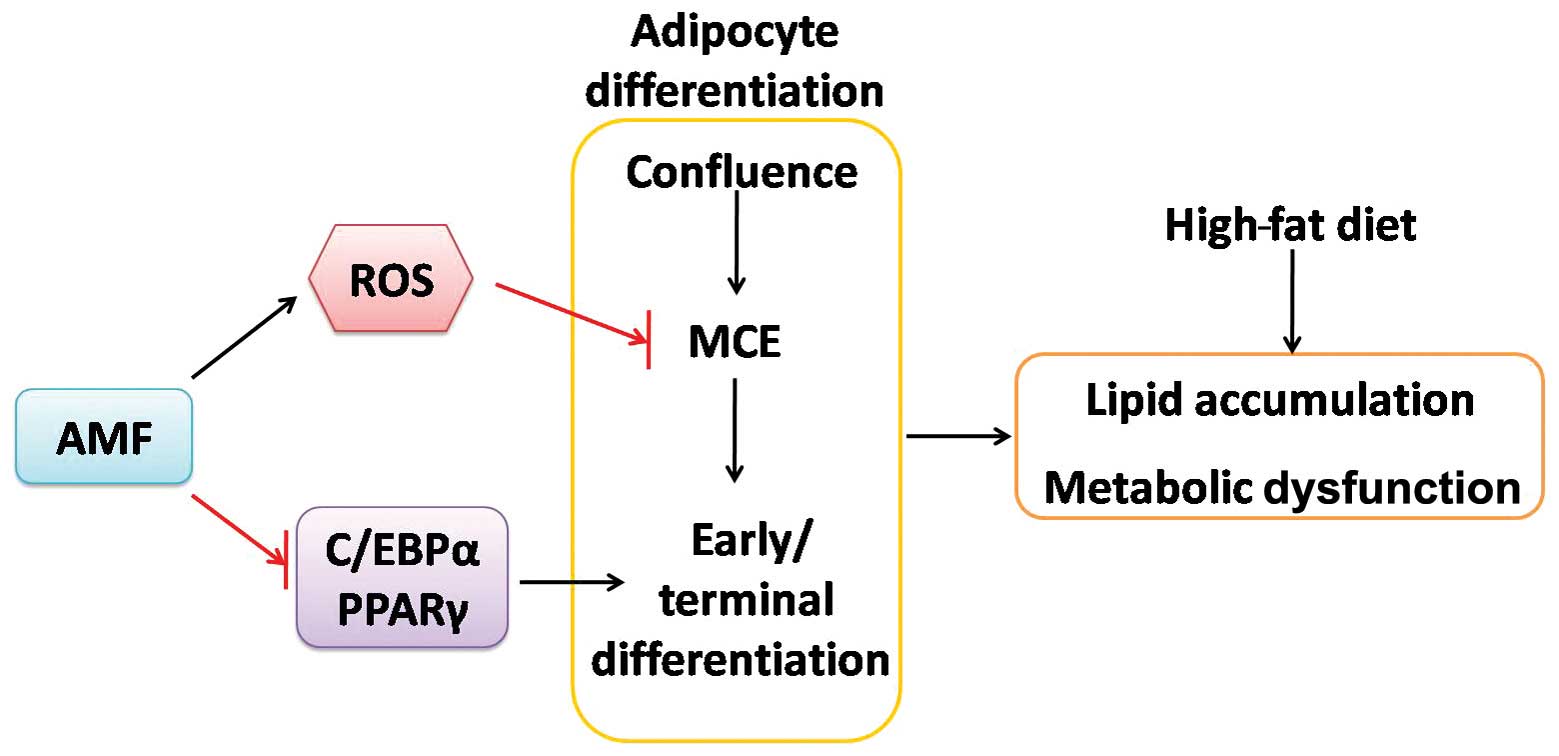
In conclusion, in the present study, we found that
AMF attenuated metabolic dysfunction in HF diet-fed rats and
exerted inhibitory effects on 3T3-L1 adipocyte differentiation
(Fig. 4). Furthermore, AMF
promoted ROS generation, decreased C/EBPβ, resulting in inhibition
of MCE; on the other way, AMF inhibited PPARγ and C/EBPα
expression, suppressed molecular pathways that responsible for the
formation of lipid droplets, leading to inhibition of early and
terminal differentiation (Fig.
4). The inhibitory effects on adipocyte differentiation may
contribute to the protective effects of AMF against HF diet-induced
metabolic dysfunction (Fig. 4).
Overall, the data from the present study provide novel insight into
the mechanisms underlying the protective effects of AMF against
metabolic dysfunction and the inhibitory effects of AMF on
adipocyte differentiation.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Diabetes Association: 6. Obesity
management for the treatment of type 2 diabetes. Diabetes Care.
39(Suppl 1): S47–S51. 2016. View Article : Google Scholar
|
|
3
|
Tseng YH, Cypess AM and Kahn CR: Cellular
bioenergetics as a target for obesity therapy. Nat Rev Drug Discov.
9:465–482. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X and Hai C: Redox modulation of
adipocyte differentiation: Hypothesis of 'Redox Chain' and novel
insights into intervention of adipogenesis and obesity. Free Radic
Biol Med. 89:99–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
6
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
7
|
Lefterova MI and Lazar MA: New
developments in adipogenesis. Trends Endocrinol Metab. 20:107–114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang QQ and Lane MD: Activation and
centromeric localization of CCAAT/enhancer-binding proteins during
the mitotic clonal expansion of adipocyte differentiation. Genes
Dev. 13:2231–2241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeh WC, Cao Z, Classon M and McKnight SL:
Cascade regulation of terminal adipocyte differentiation by three
members of the C/EBP family of leucine zipper proteins. Genes Dev.
9:168–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka T, Yoshida N, Kishimoto T and Akira
S: Defective adipocyte differentiation in mice lacking the
C/EBPbeta and/or C/EBPdelta gene. EMBO J. 16:7432–7443. 1997.
View Article : Google Scholar
|
|
12
|
Tang QQ, Otto TC and Lane MD:
CCAAT/enhancer-binding protein beta is required for mitotic clonal
expansion during adipogenesis. Proc Natl Acad Sci USA. 100:850–855.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clarke SL, Robinson CE and Gimble JM:
CAAT/enhancer binding proteins directly modulate transcription from
the peroxisome proliferator-activated receptor gamma 2 promoter.
Biochem Biophys Res Commun. 240:99–103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Z, Rosen ED, Brun R, Hauser S, Adelmant
G, Troy AE, McKeon C, Darlington GJ and Spiegelman BM:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao D and Lazar MA: Peroxisome
proliferator activated receptor gamma, CCAAT/enhancer-binding
protein alpha, and cell cycle status regulate the commitment to
adipocyte differentiation. J Biol Chem. 272:21473–21478. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans RM, Barish GD and Wang YX: PPARs and
the complex journey to obesity. Nat Med. 10:355–361. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang N, Rizshsky L, Hauck CC, Nikolau BJ,
Murphy PA and Birt DF: The inhibition of lipopolysaccharide-induced
macrophage inflammation by 4 compounds in Hypericum perforatum
extract is partially dependent on the activation of SOCS3.
Phytochemistry. 76:106–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai SC, Liang YH, Chiang JH, Liu FC, Lin
WH, Chang SJ, Lin WY, Wu CH and Weng JR: Anti-inflammatory effects
of Calophyllum inophyllum L. in RAW264.7 cells. Oncol Rep.
28:1096–1102. 2012.PubMed/NCBI
|
|
19
|
Woo ER, Lee JY, Cho IJ, Kim SG and Kang
KW: Amentoflavone inhibits the induction of nitric oxide synthase
by inhibiting NF-kappaB activation in macrophages. Pharmacol Res.
51:539–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banerjee T, Valacchi G, Ziboh VA and van
der Vliet A: Inhibition of TNFalpha-induced cyclooxygenase-2
expression by amentoflavone through suppression of NF-kappaB
activation in A549 cells. Mol Cell Biochem. 238:105–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Na M, Kim KA, Oh H, Kim BY, Oh WK and Ahn
JS: Protein tyrosine phosphatase 1B inhibitory activity of
amentoflavone and its cellular effect on tyrosine phosphorylation
of insulin receptors. Biol Pharm Bull. 30:379–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Gu C, He W, Ye X, Chen H, Zhang X
and Hai C: Glucose oxidase induces insulin resistance via
influencing multiple targets in vitro and in vivo: The central role
of oxidative stress. Biochimie. 94:1705–1717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Peng H, Fan H, Zou X, Liu Q, Zhang
Y, Xu H, Chu Y, Wang C, Ayyanathan K, et al: The LIM protein Ajuba
promotes adipogenesis by enhancing PPARgamma and p300/CBP
interaction. Cell Death Differ. 23:158–168. 2016. View Article : Google Scholar
|
|
24
|
Hou X, Tong Q, Wang W, Xiong W, Shi C and
Fang J: Dihydromyricetin protects endothelial cells from hydrogen
peroxide-induced oxidative stress damage by regulating
mitochondrial pathways. Life Sci. 130:38–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erdogan-Orhan I, Altun ML, Sever-Yilmaz B
and Saltan G: Anti-acetylcholinesterase and antioxidant assets of
the major components (salicin, amentoflavone, and chlorogenic acid)
and the extracts of Viburnum opulus and Viburnum lantana and their
total phenol and flavonoid contents. J Med Food. 14:434–440. 2011.
View Article : Google Scholar
|
|
26
|
Xu GH, Ryoo IJ, Kim YH, Choo SJ and Yoo
ID: Free radical scavenging and antielastase activities of
flavonoids from the fruits of Thuja orientalis. Arch Pharm Res.
32:275–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi LF, Vassão DG, Kato MJ and Di
Mascio P: Biflavonoids from Brazilian pine Araucaria angustifolia
as potentials protective agents against DNA damage and
lipoperoxidation. Phytochemistry. 66:2238–2247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coulerie P, Nour M, Maciuk A, Eydoux C,
Guillemot JC, Lebouvier N, Hnawia E, Leblanc K, Lewin G, Canard B,
et al: Structure-activity relationship study of biflavonoids on the
Dengue virus polymerase DENV-NS5 RdRp. Planta Med. 79:1313–1318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma SC, But PP, Ooi VE, He YH, Lee SH, Lee
SF and Lin RC: Antiviral amentoflavone from Selaginella sinensis.
Biol Pharm Bull. 24:311–312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S, Kim H, Kang JW, Kim JH, Lee DH, Kim
MS, Yang Y, Woo ER, Kim YM, Hong J and Yoon DY: The biflavonoid
amentoflavone induces apoptosis via suppressing E7 expression, cell
cycle arrest at sub-G1 phase, and mitochondria-emanated
intrinsic pathways in human cervical cancer cells. J Med Food.
14:808–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siveen KS and Kuttan G: Effect of
amentoflavone, a phenolic component from Biophytum sensitivum, on
cell cycling and apoptosis of B16F-10 melanoma cells. J Environ
Pathol Toxicol Oncol. 30:301–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee CW, Na Y, Park NH, Kim HS, Ahn SM, Kim
JW, Kim HK and Jang YP: Amentoflavone inhibits UVB-induced matrix
metalloproteinase-1 expression through the modulation of AP-1
components in normal human fibroblasts. Appl Biochem Biotechnol.
166:1137–1147. 2012. View Article : Google Scholar
|
|
33
|
Park NH, Lee CW, Bae JH and Na YJ:
Protective effects of amentoflavone on Lamin A-dependent
UVB-induced nuclear aberration in normal human fibroblasts. Bioorg
Med Chem Lett. 21:6482–6484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jo YH, Choi KM, Liu Q, Kim SB, Ji HJ, Kim
M, Shin SK, Do SG, Shin E, Jung G, et al: Anti-obesity effect of
6,8-diprenylgenistein, an isoflavonoid of Cudrania tricuspidata
fruits in high-fat diet-induced obese mice. Nutrients.
7:10480–10490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Oliveira PR, da Costa CA, de Bem GF,
Cordeiro VS, Santos IB, de Carvalho LC, da Conceição EP, Lisboa PC,
Ognibene DT, Sousa PJ, et al: Euterpe oleracea Mart.-derived
polyphenols protect mice from diet-induced obesity and fatty liver
by regulating hepatic lipogenesis and cholesterol excretion. PLoS
One. 10:e01437212015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang QQ, Otto TC and Lane MD: Mitotic
clonal expansion: A synchronous process required for adipogenesis.
Proc Natl Acad Sci USA. 100:44–49. 2003. View Article : Google Scholar :
|
|
37
|
Lee H, Lee YJ, Choi H, Ko EH and Kim JW:
Reactive oxygen species facilitate adipocyte differentiation by
accelerating mitotic clonal expansion. J Biol Chem.
284:10601–10609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Linhart HG, Ishimura-Oka K, DeMayo F, Kibe
T, Repka D, Poindexter B, Bick RJ and Darlington GJ: C/EBPalpha is
required for differentiation of white, but not brown, adipose
tissue. Proc Natl Acad Sci USA. 98:12532–12537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Freytag SO, Paielli DL and Gilbert JD:
Ectopic expression of the CCAAT/enhancer-binding protein alpha
promotes the adipogenic program in a variety of mouse fibroblastic
cells. Genes Dev. 8:1654–1663. 1994. View Article : Google Scholar : PubMed/NCBI
|