1. Introduction
The physiological and pathophysiological roles of
the cannabinoid and sex hormone systems have been studied
separately. In the present review, we suggest some common molecular
pathways and possible interactions between cannabinoids and sex
hormones in physiological and selected pathophysiological
conditions. We hypothesized that the endocannabinoid system may
have a body-wide protective role against the harmful effects of sex
hormones.
2. Cannabinoids
Cannabinoid receptors are membrane receptors of the
G protein-coupled receptor (GPR) superfamily. There are two
subtypes of cannabinoid receptors, termed cannabinoid type 1 (CB1)
and CB2 receptors. CB1 receptors are mostly present in the central
nervous system, but are also expressed in peripheral tissues, such
as endothelial cells, adipocytes and peripheral nerves. They are
linked via Gi to the inhibition of adenylyl cyclase and
voltage-operated calcium channels, influencing many secondary
messengers (1). CB2 receptors are
linked via Gi to adenylyl cyclase and mitogen-activated protein
kinase (MAPK), but not to voltage-operated calcium channels. They
are expressed in the immune system, gastrointestinal tract,
peripheral nervous system and microglia of the brain (1). Recent findings suggest that
cannabinoids can also activate other receptors, including vanilloid
receptor 1, GPR18, GPR19, GPR55 receptor, the latter being
suggested as the CB3 receptor (2–5).
There are many exogenous [i.e., tetrahydrocannabinol (THC),
cannabidiol and cannabinol] and endogenous [anandamide,
2-arachidonoyl glycerol (2-AG), virhodamine, 2-arachidonoyl
glycerol ether (noladin) and N-arachidonoyl dopamine] substances
that effect cannabinoid receptors (1).
3. Estrogens
Estrogens bind to estrogen receptors (ERs)α and β.
ER complexes bind with high affinity and specificity to estrogen
response elements (EREs) to regulate the transcription of target
genes involved in the regulation of many complex physiological
processes. ERs can sometimes regulate the expression of genes that
lack EREs by modulating the transcriptional activity of other
transcription factors (6). Some
non-genomic effects of estrogens are known and are caused by the
direct activation of ERs in the plasma membrane (7,8).
A third estrogen receptor, G protein coupled
estrogen receptor (GPER, which is also known as GPR30), has also
been discovered, although its functional role is still unclear
(9). Modulation of the estrogen
receptors is currently being considered for the prevention and
treatment of a wide variety of pathological conditions, including
osteoporosis, metabolic and cardiovascular diseases, inflammation,
neurodegenerive disorders and cancer (10). Three estrogens are present in
significant quantities in the plasma of human females:
17β-estradiol, estrone and estriol. The estrogenic potency of
17β-estradiol is kown to be 12-fold greater than that of estrone
and 80-fold greater than that of estriol, making the total
estrogenic efficiency of 17β-estradiol much greater than that of
the other two combined. 17β-estradiol is the principal estrogen
secreted by the ovaries; small amounts of estrone are also
secreted, but most of the circulating estrone is formed in
peripheral tissues from androgens (11).
4. Cannabinoids and estrogens
On the level of the hypothalamic-pituitary-gonadal
axis, interactions between cannabinoids and estrogens have been
well documented. Studies have indicated that the acute
administration of THC, a non-selective CB1 and CB2 receptor
agonist, decreases serum luteinizing hormone (LH) and
gonadotropin-releasing hormone (GnRH) secretion in ovariectomized
female and intact male rats (12–14). Lower concentrations of GnRH [and
consequently a decrease in LH and follicle-stimulating hormone
(FSH) concentrations] result in lower circulating estrogen levels.
Anandamide, the main endogenous cannabinoid, produces similar
results in both, female and male rats (15). Cannabinoids appear to modulate the
release of GnRH through their effect on hypothalamic GnRH-releasing
neurons with a high density of CB1 receptors and a relatively low
density of CB2 receptors (16).
Fatty acid amide hydrolase (FAAH) is responsible for
anandamide degradation (17).
Estrogens decrease FAAH activity in the mouse uterus (18) and this leads to higher cannabinoid
concentrations. In association with these findings, a previous
study found that there was a positive correlation between peak
plasma anandamide with peak plasma 17β-estradiol and gonadotrophin
levels at ovulation (19). A
possible underlying mechanism responsible for this phenomenon is
that increased levels of estrogens at ovulation inhibit FAAH
activity and consequently increase endocannabinoid plasma levels.
Gorzalka and Dang published a detailed review describing the
behavioral and reproductive aspects of cannabinoid and sex hormone
interactions (20).
In addition, studies have demonstrated that
17β-estradiol increases the expression of CB2 receptors in
osteoclasts in vitro, as well as the expression of CB1
receptors in human colon cancer (21,22). In the brain, 17β-estradiol
regulates CB1 expression in a region-dependent manner, providing a
possible explanation for gender-related differences in sensitivity
for the central effects of cannabinoids (23). Recently, selective estrogen
receptor modulators (raloxifene, bazedoxifene and lasofoxifene)
were discovered to act as inverse CB2 agonists (24). Furthermore, tamoxifen has been
demonstrated to act as an inverse CB1 and CB2 agonist in breast
cancer cells (25). This finding
indicates that estrogens may also have a direct influence on CB1
and CB2 receptors.
5. Overlapping molecular pathways of
cannabinoids and estrogens
Adenylate cyclase (AC) and protein kinase
A (PKA)
Cannabinoid receptor agonists signal through the
inhibition of the AC and PKA pathways (26). This is also one of the main
signaling pathways of estrogens (27–29), activated by the binding of
17β-estradiol to ERs (Fig. 1) and
partly by non-genomic mechanisms of estrogen action (30).
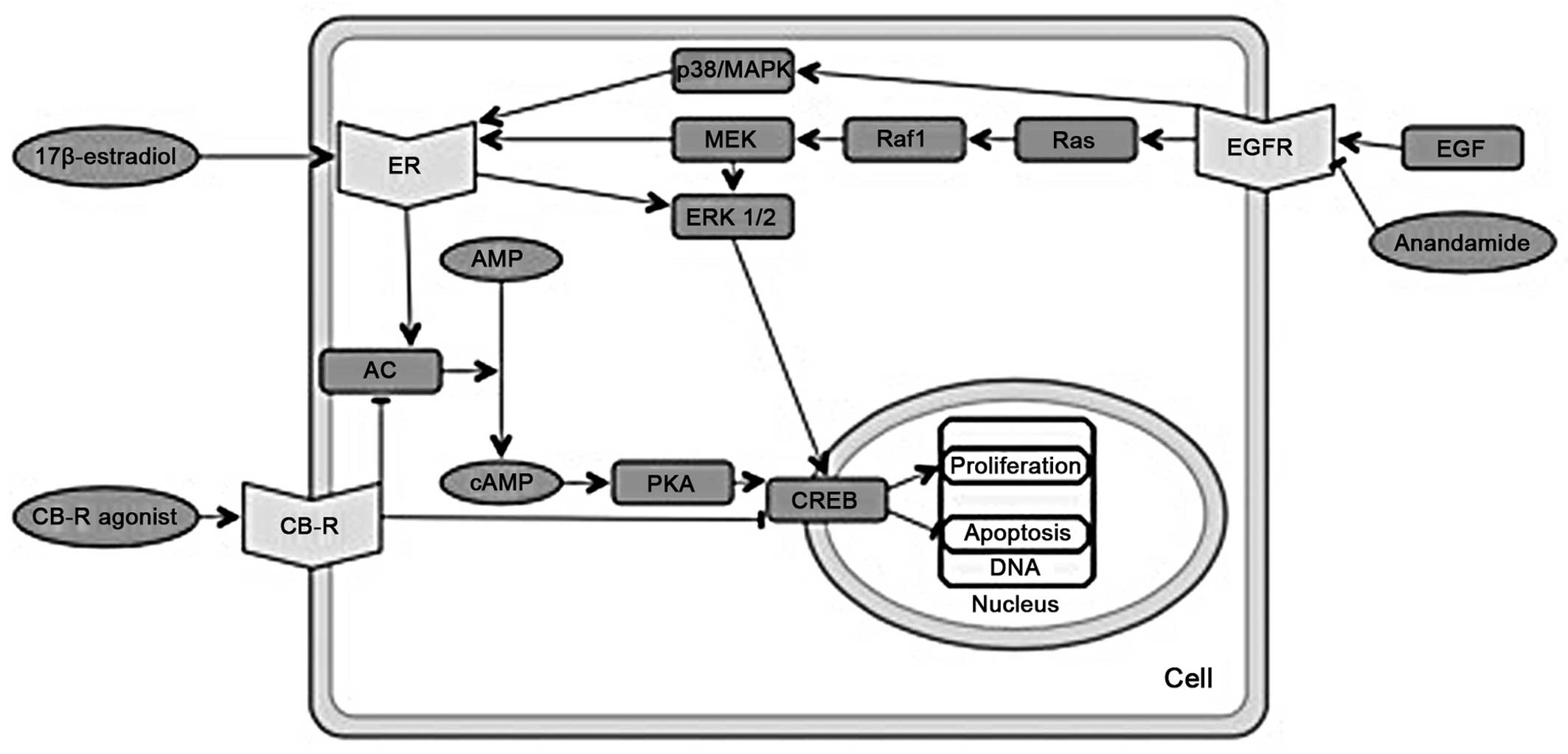 | Figure 1Overlapping molecular pathways of
cannabinoids and estrogens. Part I. ER, estrogen receptor; CB-R,
cannabinoid receptor; AC, adenylate cyclase; (c)AMP, (cyclic)
adenosine monophosphate; PKA, protein kinase A; CREB, cAMP response
element binding protein; CRE, cAMP response elements; EFG,
epidermal growth factor; EGFR, epidermal growth factor receptor;
Ras, rats sarcoma protein; Raf1, proto-oncogene
serine/threonine-protein kinase; MEK, mitogen-activated protein
kinase kinase; ERK1/2, extracellular-signal-regulated kinases 1/2;
p38/MAPK, p38 mitogen-activated protein kinase. |
Epidermal growth factor (EGF)
receptor
Endocannabinoids decrease the expression of EGF
receptors (31) and significantly
inhibit the EGF-induced proliferation, migration and invasion of
non-small cell lung cancer cell lines (32). The EGF cytoplasmic signaling
pathways influence ER activity. The activation of EGF receptors
leads to the MAPK-mediated phosphorylation of ERα; in addition, EGF
receptors activate p38/MAPKs that activate ERα/β (33) (Fig.
1).
Cyclic adenosine monophosphate (cAMP)
response element binding protein (CREB)
After binding to ERs, estrogens can promote the
activation of activating transcription factor (ATF)-2/CREB to
induce the expression of cyclin D1 and can promote the activation
of ATF-1/CREB to induce the expression of B-cell lymphoma 2 protein
(Bcl-2). Cyclin D1 and Bcl-2 are important for their proliferative
and anti-apoptotic effects (34).
The β-estradiol through the MAPK pathway, independently from the
PKA pathway (35). Yet again,
cannabinoids appear to have an opposite effect. It has been shown
that cannabinoid agonists inhibit cAMP response elements (CRE)
(36), the binding
deoxyribonucleic acid (DNA) sequences for CREB (Fig. 1).
Prolactin
Endocannabinoids inhibit the mitogenic action of
prolactin (37), which is among
its others functions also an important inducer of carcinogenesis in
breast cancer. Both estrogens and endocannabinoids regulate the
expression of prolactin receptors (37,38).
Vascular endothelial growth factor
(VEGF)
Cannabinoids cause a reduction in VEGF expression
and inhibit angiogenesis through CB1 receptors. In mouse thyroid
carcinoma, the reported anticancer effects of the CB1 receptor
agonist, Met-F-anandamide, may be due to the inhibition of
angiogenesis, as a consequence of VEGF signal blocking, the
overexpression of cyclin-dependent kinase inhibitor 1 (p21)
(39) and interference with VEGF
receptor type 2 activation (40).
By binding to EREs, 17β-estradiol directly regulates VEGF
gene transcription in endometrial cells and in Ishikawa
adenocarcinoma cells. This mechanism may also be important in the
estrogenic regulation of VEGF production and angiogenesis in
estrogen target tissues, i.e., breast, bone, heart and skin
(41,42).
Proto-oncogene serine/threonine-protein
kinase (Raf)
Cannabinoids signal apoptosis via a pathway
involving CB receptors. This pathway is sustained by ceramide
accumulation and extracellular signal-regulated Raf kinase
activation (43). The Raf kinase
is activated by ER through non-genomic mechanisms. It has been
demonstrated that in Chinese hamster ovary cells, serine 522 in the
ligand binding domain of ERα interacts with caveolin-1. Caveolin-1
is a structural protein in caveolae that binds Raf, proto-oncogene
tyrosine-protein kinase (Src), growth factor receptor-bound protein
7, rat sarcoma protein (Ras), mitogen-activated protein kinase
kinase (MEK), EGF receptor and ERα at the plasma membrane, forming
a 'signalsome' for the rapid activation of intracellular signaling
(44, and refs therein). The protein Raf is important in the
activation of MAPK and other kinases that are activated by
estrogens (35).
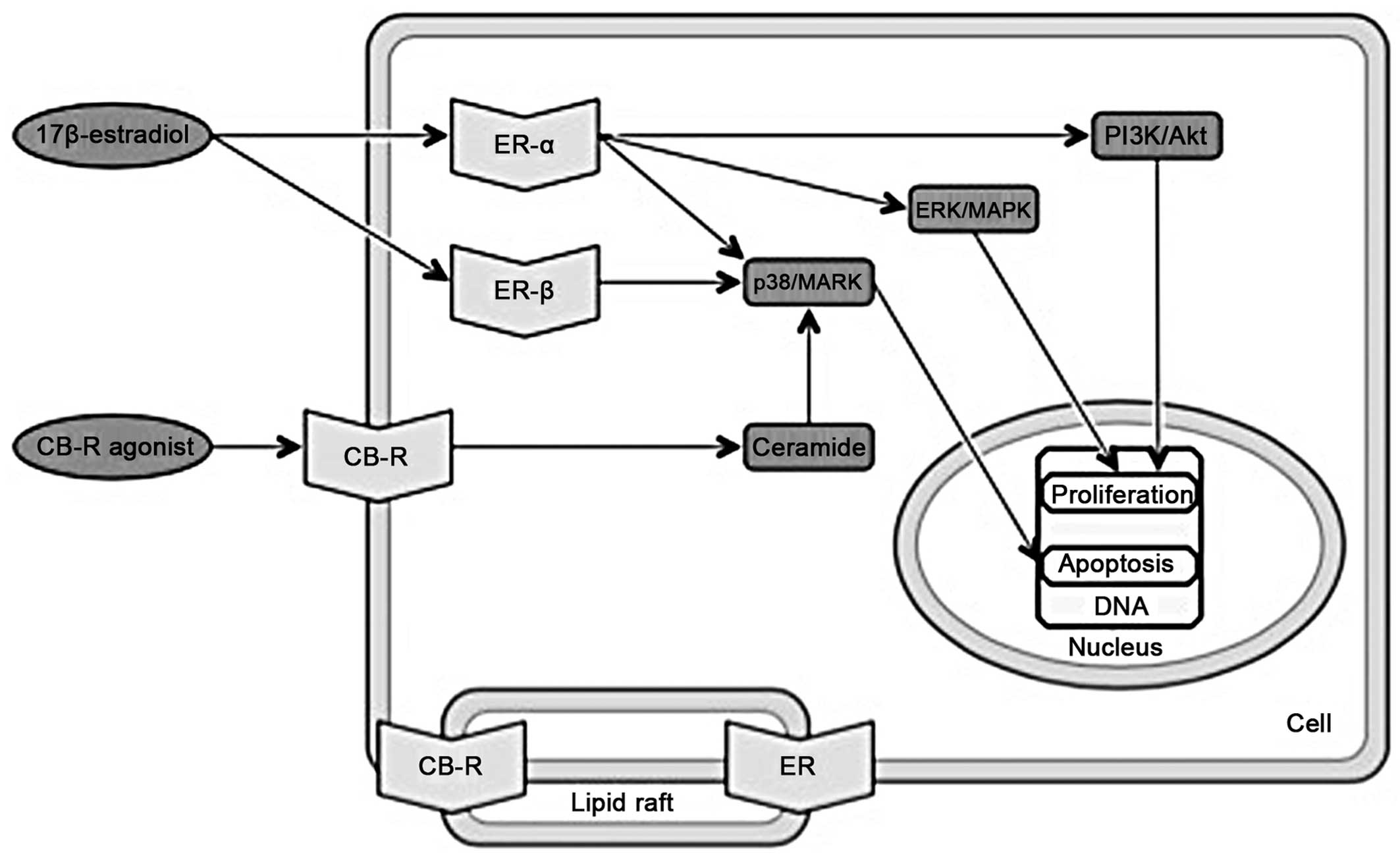
MAPK
The MAPK pathway is generally important in gene
expression, cell proliferation and apoptosis (45). Moreover, the activation of ERα or
ERβ differentially affects proliferation and apoptosis. The
17β-estradiol-ERα complex activates multiple signaling pathways,
including p38/MAPK, extracellular-signal-regulated kinase
(ERK)/MAPK and phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/protein kinase B (Akt) which are involved in cell cycle
progression and apoptotic cascade prevention. The 17β-estradiol-ERβ
complex activates only the p38/MAPK pathway, which in turn leads to
cell apoptosis (46). The
ER-17β-estradiol complex combined with the insulin-like growth
factor-1 (IGF-1) receptor is also a MAPK signaling pathway
activator (47). Many apoptotic
effects are also linked to CB receptors and the activation of MAPK
pathways (48); e.g., ceramide
synthesis is induced by cannabinoids and leads to the activation of
the p38/MAPK pathway (49)
(Fig. 2).
PI3K
Cannabinoids cause the downregulation of PI3K-Akt
and ERK1/2 kinase signaling, which in turn inhibits proliferation
and induces apoptosis (51). By
contrast, 17β-estradiol activates PI3K-mediated signaling, which
causes rapid endothelial nitric-oxide synthesis (52) and promotes cell cycle progression
(Fig. 3), also involving
increased cyclin D1 expression (53).
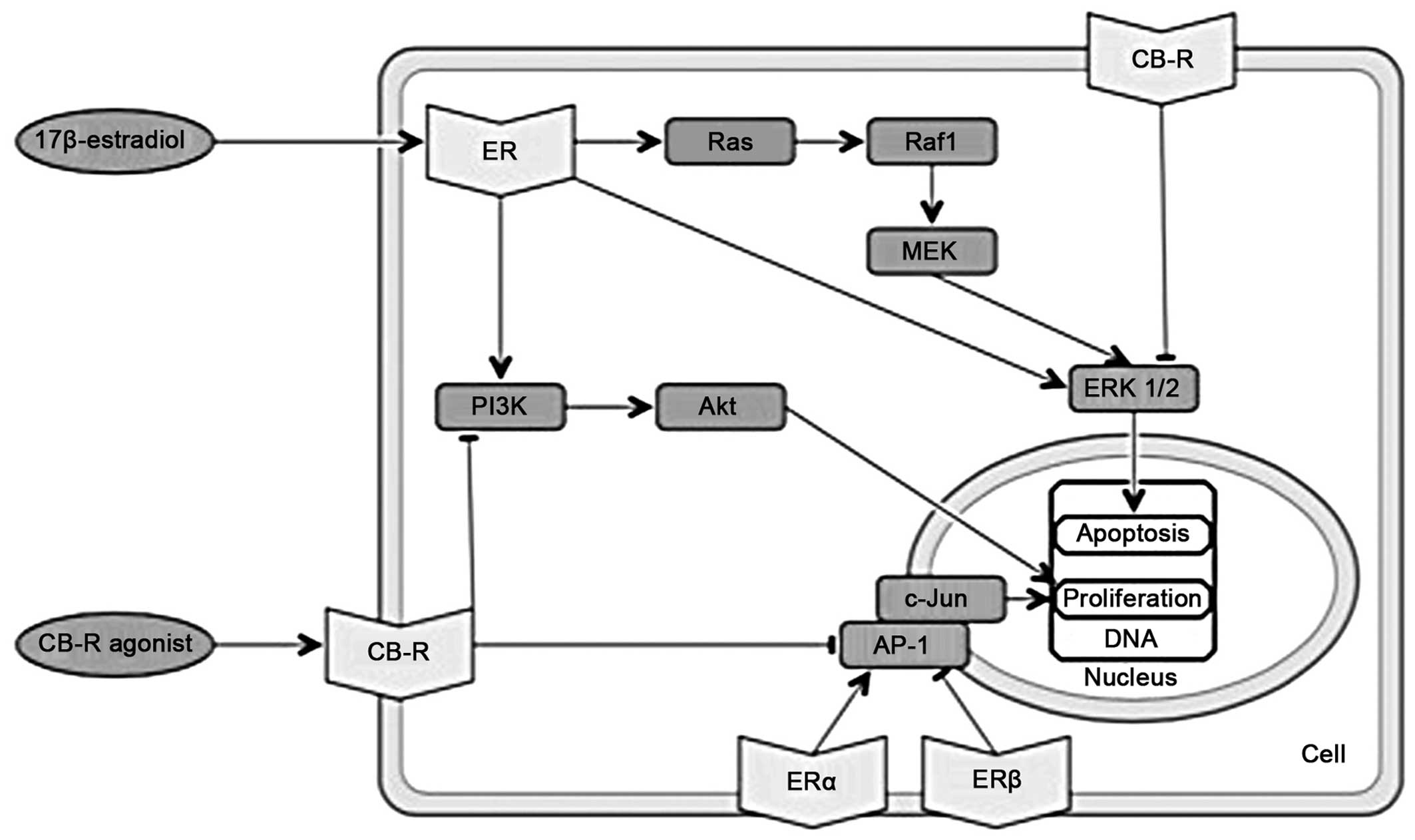 | Figure 3Overlapping molecular pathways of
cannabinoids and estrogens. Part III. ER: estrogen receptor; CB-R,
cannabinoid receptor; PI3K, phosphatidylinositol-4,5-bisphosphate
3-kinase; Akt, serine-threonine specific protein kinase; AP-1,
activator protein 1; c-Jun, protein encoded by the JUN gene;
Ras, rats sarcoma protein; Raf1, proto-oncogene
serine/threonine-protein kinase; MEK, mitogen-activated protein
kinase kinase; ERK1/2, extracellular-signal-regulated kinases
1/2. |
C-Jun N-terminal kinase
C-Jun N-terminal kinase may be a potential target of
ceramide action in the induction of apoptosis in a number of cell
types (43). Estrogens also
influence c-Jun N-terminal kinase. Estrogen receptors can associate
with promoters/enhancers of the transcription factors ATF-2 and
c-Jun. The activator protein-1 (AP-1) complex, consisting also of
c-Jun, plays an important role in cell proliferation (34). Estrogen receptors enhance the
transcription of genes that contain AP-1 (54). The activation of ERα activates
AP-1-dependent transcription, whereas the activation of ERβ
inhibits AP-1-dependent transcription (55). Cannabinoid agonists inhibit
AP-1-mediated transcriptional activities, the latter being induced
in several types of tumors (34)
(Fig. 3).
ERK1/2
Current evidence indicates that a small population
of ERα and ERβ localized at the plasma membrane exists within
caveolar lipid rafts. It is at the plasma membrane that
17β-estradiol-ER associates with the scaffolding protein,
caveolin-1, and a variety of signal transduction cascades
activations occur, including ERK and other enzymes [phospholipase C
(PLC), protein kinase C (PKC), PI3K, nitric oxide synthase]
(35). In bones, ERα is present
in the caveolae of bone-forming osteoblasts. The ERα transmits
survival signals through the activation of the Src/adapter protein
Shc/ERK pathway and prolongs the lifespan of osteoblasts (56). Cannabinoids activate ERK1/2
kinases, leading to G1 cell cycle arrest (57) (Fig.
3). The activation of either CB1 or CB2 receptors in colon
cancer cells induces the Raf-MEK-ERK pathway to promote apoptosis
and triggers the synthesis of ceramide (58).
Other molecular interactions
There is evidence to indicate that certain enzymes
involved in the synthesis or degradation of endocannabinoids are
also regulated by estrogens. Estrogens and progesterone
downregulate uterine enzyme N-acyl phosphatidylethanolamine
phospholipase D (NAPE-PLD) expression via their nuclear receptors
(59). Protein NAPE-PLD is an
important enzyme in the synthesis of anandamide (60). Two endocannabinoids, anandamide
and 2-AG, are oxidized by cyclooxygenase-2 (COX-2) (61). The 17β-estradiol exhibits a
tendency to increase COX-2 expression and prostaglandin E2
synthesis in primary human uterine microvascular endothelial cells
(62). As mentioned above, FAAH
activity is inhibited by 17β-estradiol, which probably leads to
higher concentrations of endocannabinoids after rising plasma
estrogen levels (19).
Accordingly, a homeostatic association between cannabinoids and
estrogens can be proposed; increased levels of estrogens result in
higher concentrations of endocannabinoids, which in turn inhibits
the hypothalamic-pituitary-gonadal axis and leads to a decrease in
estrogen levels (21). It also
appears that endocannabinoids are released as a response to
estrogen action and that they act via several molecular pathways,
generally contradicting the effects of estrogens.
6. Cannabinoids and estrogens and in common
diseases
Cannabinoids and estrogens in breast
cancer
There is strong epidemiological, biological and
clinical data that connects sex hormones, particularly estrogens,
to breast cancer. The ERα is most directly implicated in the
pathophysiology of the disease and its presence in tumor tissue is
one of the most important disease prognostic factors. The role of
other sex hormone receptors in the pathophysiology of breast
cancer, i.e., ERβ and androgen receptors (ARs), has been less
clearly investigated (63).
A recent study suggested that tamoxifen can act as a
CB1 and CB2 inverse agonist, thus producing cytotoxicity via an
ER-independent mechanism of action (25).
It has been shown that the activation of CB2
receptor by THC reduces the progression of the cell cycle and
promotes the apoptosis of human breast cancer cells (64). Cannabidiol and most potently its
analogue, 01663, induce breast cancer cell death through apoptosis
and autophagy in vitro (65), and they inhibit breast cancer cell
proliferation and invasion in vivo (66); other synthetic cannabinoid
receptor agonists also inhibit tumor growth and breast cancer
metastasis (67). On the other
hand, a previous study found that THC stimulates breast cancer
growth and metastasis in vivo by the suppression of the
body's antitumor immune response (68).
Cannabinoids and estrogens in endometrial
cancer
Endometrial cancer is the most common gynecological
malignancy. Prolonged unopposed estrogen exposure is associated
with the majority of type I endometrial cancers. Estrogen
replacement therapy, prescribed to control menopausal symptoms,
increases the risk of developing endometrial cancer by 2–20-fold
(69). Endometrial cancers are
thought to arise from estrogen exposure, not balanced by the
differentiating effects of progesterone (70). Currently, estrogen antagonists and
progesterone analogues are used in endometrial cancer treatment
(70).
A previous study demonstrated that anandamide may be
a possible risk factor in endometrial cancer. Anandamide was shown
to decrease both CB1 and CB2 receptor transcript levels in
endometrial cancer tissues. In addition, plasma anandamide
concentrations were significantly higher in patients with
endometrial cancer than in the control group. This suggests that
increased tissue and plasma anandamide concentrations may be in
some way be linked to the pathophysiology of endome-trial cancer
(71).
In another study, the immunohistochemical analysis
of endometrial biopsies revealed that CB2 receptors were
selectively expressed in cancer cells, with a very weak expression
in healthy cells in the same biopsies. Mass spectrometry analysis
of endometrial carcinoma lipid extracts also revealed a significant
increase in 2-AG levels in comparison with samples obtained from
healthy subjects. No significant increase in the levels of
anandamide was detected. The elevation of 2-AG is possibly due to
the decrease in the expression of monoacylglycerol (MAGL), an
important enzyme necessary for 2-AG breakdown (72).
Cannabinoids and estrogens in
osteoporosis
The bone remodeling process is influenced by many
factors, including estrogens and endocannabinoids. Imbalances in
bone remodeling mechanisms cause one of the most common
degenerative diseases in developed countries, osteoporosis
(73).
Osteoblasts and osteoclasts are influenced by
estrogens at both the cellular and molecular level. Estrogens
increase collagen I and osteoprotegerin expression (74,75), there is evidence to suggests that
estrogens have inhibitory effects on osteoblast apoptosis (76).
Cannabinoids also appear to modulate bone structure.
Compared to CB1 receptors, CB2 receptors have been reported to have
a significantly higher expression in osteoblasts, osteoclasts and
osteocytes (77). Selective CB2
receptor agonists/antagonists may therefore successfully regulate
bone remodeling. Importantly, selective CB2 receptor ligands are
not generally psychoactive, making them more suitable for potential
clinical use (78).
There is evidence of estrogen and cannabinoid
interactions in bone cells. Exposure to 17β-estradiol has been
shown to lead to the increased expression of CB2 receptors in
osteoclasts (21). In our recent
study on primary human osteoblasts, we reported a possible
synergistic interaction between 17β-estradiol and a selective CB2
antagonist/inverse agonist (79).
Cannabinoids and estrogens in
atherosclerosis
The pathophysiology underlying atherosclerosis is a
combination of endothelial cell dysfunction and vascular
inflammation, accompanied by a build-up of lipids, cholesterol and
calcium within the tunica intima. In combination, these can result
in plaque formation, thrombosis and cardiovascular insufficiency
(80).
An increase in cardiovascular incidents in
post-menopausal women suggests that estrogens play an essential
protective role against cardiovascular disease. Menopause creates
unhealthy changes in plasma lipoprotein levels that can be reversed
by post-menopausal estrogen replacement therapy (81). Studies have demonstrated that
estrogens are important for normal cell proliferation in blood
vessels. When physiological angiogenesis is lacking or
insufficient, a setting is created for various cardiovascular
diseases (82). Estrogens
regulate lipid and cholesterol levels and may provide protection by
increasing plasma high-density lipoprotein levels (83). Furthermore, estrogens may modulate
inflammatory responses within vascular cells, may cause stem cell
death and may also be involved in the development of hypertrophy
(84).
The high expression of CB1 and CB2 receptors in
atherosclerotic plaques indicates an important role of the
endocannabinoid system in atherosclerosis (80). A higher expression of CB1
receptors is also associated with cardiovascular risk factors, such
as obesity and dyslipidemia and CB1 agonists have been shown to
increase the amount of reactive oxygen species, and thus to induce
the apoptosis of endothelial cells in coronary arteries (85,86). In an animal model of
atherosclerosis, CB1 antagonists have proven useful; they reduce
the accumulation of oxygenated low-density lipoproteins in
macrophages, reduce inflammatory reactions in small blood vessels,
decrease the proliferation of smooth muscle cells in vessel walls
and, consequently, delay disease progression (80). The CB2 receptors have also been
proven to play a significant role in the pathogenesis of
atherosclerosis. In a previous study, the progression of
atherosclerotic plaques in a mouse model was shown to be attenuated
by the administration of THC. This effect was nullified by the
subsequent administration of a selective CB2 antagonist (87). On the other hand, CB2 agonists
reduce the accumulation of lipids in human foam cells (88). Cannabinoids also lower the
expression of CD36 receptor, which promotes the release of
pro-inflammatory cytokines and increases its own expression
(89).
7. Androgens
The principle steroidal androgen testosterone and
its more potent metabolite, 5-dihydrotestosterone (DHT),
synthetized by enzyme 5α-reductase, mediate their biological
effects by binding to the AR. AR functions as a ligand-inducible
transcription factor (90). In
addition, evidence suggests that androgens can exert non-genomic
effects. Non-genomic activity typically involves the rapid
induction of conventional second messenger signal transduction
cascades. The non-genomic effects of androgens can be mediated by
at least three androgen-binding proteins, the classical
intracellular AR, the transmembrane AR and the transmembrane sex
hormone-binding globulin (SHBG) receptor (91). The non-genomic effects for
transmembrane receptors are converted via a G-protein coupled
processes, whereas binding to intracellular ARs may lead to an
activation of several cytosolic pathways. Other androgen hormones
that effect ARs are androstanediol, androstenedione,
dehydroepiandrosterone and androsterone (91).
8. Cannabinoids and androgens
Studies have provided conflicting results as to
whether chronic or acute marijuana use reduces the levels of
circulating testosterone in humans. The influence of cannabinoids
on androgens appears to be more consistent in animal models
(92,93). The exposure to THC in vitro
has been shown to cause a decrease in testosterone production by
mouse testes (94), and the
chronic administration of THC to male mice has been shown to cause
a reversible regression in Leyding cell tissues and the elimination
of spermatogenesis (95). THC has
been shown to exert anti-androgenic effects in adult castrated rats
(96). The chronic administration
of high doses of THC to male dogs has been shown to cause
testicular degeneration (97).
The acute administration is effective in reducing serum
testosterone levels (14).
9. Overlapping molecular pathways of
cannabinoids and androgens
Androgens are involved in molecular pathways that
are also affected by endocannabinoids (Fig. 4). Non-genomic androgen activity
involves the rapid induction of conventional second messenger
signal transduction cascades, including increases in free
intracellular calcium and the activation of PKA, PKC and MAPK,
leading to diverse cellular effects, including smooth muscle
relaxation, neuromuscular signal transmission and neuronal
plasticity (90). In prostate
cancer cells, MAPK activation by AR/Src/protein MNAR pathway has
been shown to be both androgen-dependent and -independent (98,99).
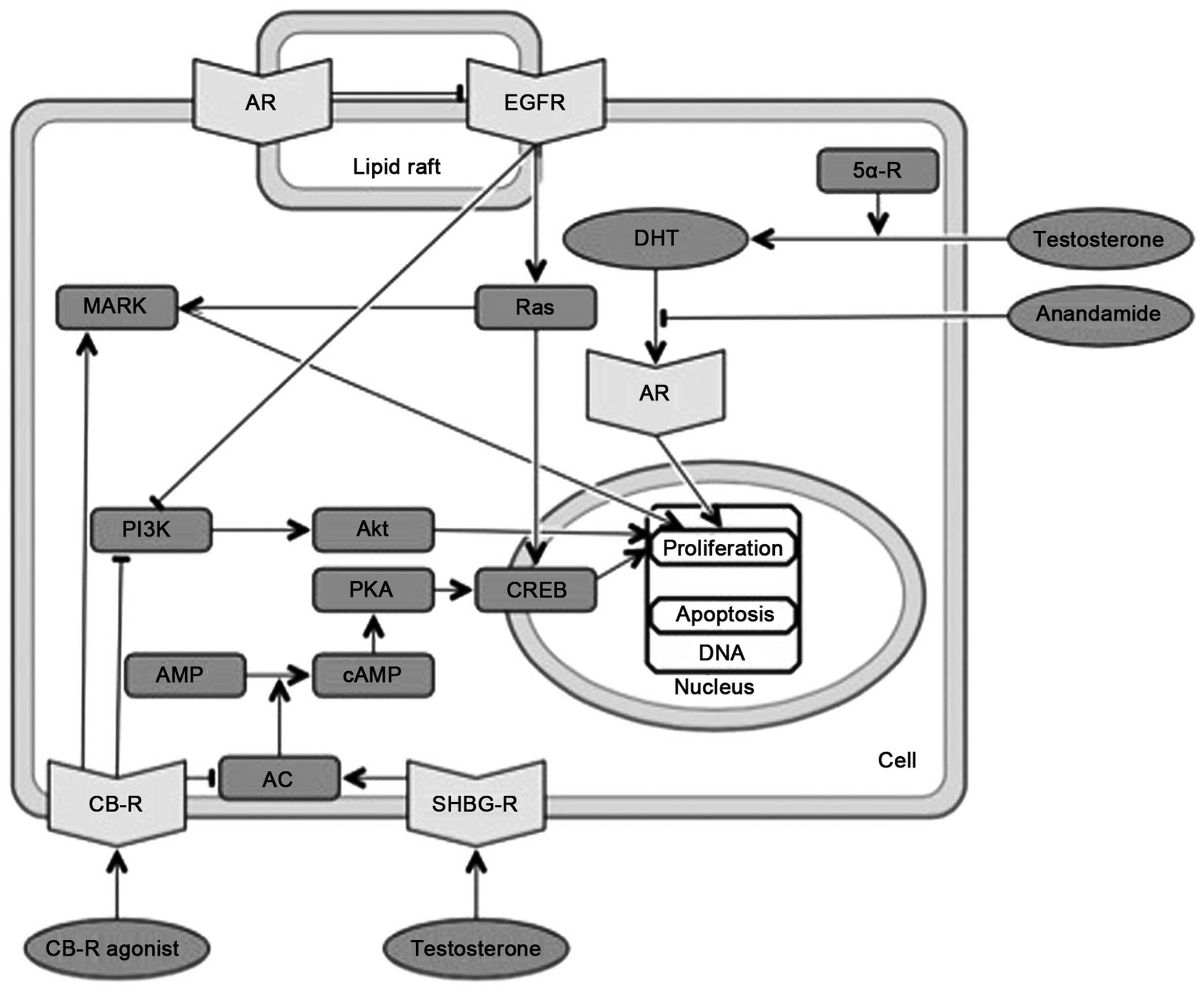 | Figure 4Overlapping molecular pathways of
cannabinoids and androgens. CB-R, cannabinoid receptor; AR,
androgen receptor; AC, adenylat cyclase; DHT, dihydrotestosterone;
SHBG-R, sex hormone-binding globulin receptor; (c)AMP, (cyclic)
adenosine monophosphate; PKA, protein kinase A; CREB, cAMP response
element-biding protein; PI3K, phosphatidylinositol-4,5-bisphosphate
3-kinase; EGFR, epidermal growth factor receptor; Ras, rats sarcoma
protein; MAPK, mitogen-activated protein kinase. |
Testosterone may exert its effects through the SHBG
receptor complex. The SHBG binds gonadal steroids and acts as a
docking station for free testosterone or other steroids to act on
cells without entering them. The activation of the SHBG receptor
results in the activation of AC, the rapid generation of cAMP and
the activation of PKA. These secondary messenger actions affect the
transcriptional activity of classic, intracellular receptors for
steroid hormones (100).
Androgens can interact with certain growth factors
and ARs are activated by growth factors, such as IGF, EGF,
interleukin-6 and others (101).
In PC3-AR cells it was observed that a pool of classical ARs is
located within lipid rafts and a population of EGF receptors is
located within cholesterolrich membrane microdomains (102). The interaction between ARs and
EGF receptors on the plasma membrane decreases the EGF-mediated
phosphorylation and PI3K/Akt downstream signaling of EGF receptor
(103).
There is little direct evidence of interactions
between cannabinoids and the androgen system. However, it has been
demonstrated that marihuana, THC and cannabinol inhibit
dihydrotestosterone binding to ARs (104).
10. Cannabinoids and androgens in common
diseases
Cannabinoids and androgens in prostate
cancer
The induction of prostatic cancer by androgens
(specifically by testosterone replacement therapy) is still a
controversial issue (105), but
there is little doubt that the activation of AR by testosterone and
dihydroxytestosterone contributes significantly to the progression
of metastatic prostate cancer (106). Cannabinoids, on the other hand,
have some protective functions. Synthetic cannabinoid quinoines
have anti-proliferative effects in vitro and prostate
antitumor activity in vivo (107). Another study found that
cannabidiol inhibited prostate carcinoma growth in vitro and
in vivo via the stimulation of intrinsic pathways of
apoptosis. The pro-apoptotic effects of cannabidiol were due to
transient receptor potential cation channel subfamily M member 8
(TRPM8) antagonism, the downregulation of AR, p53 activation and
the elevation of reactive oxygen species (108).
11. Conclusions
Sex hormones play a very important evolutionary role
in reassuring the propagation of species. They may be protective in
some instances (i.e., the protective properties of estrogens
against cardiovascular diseases); however, they often have toxic
effects due to their cancer-inducing properties and their
involvement in autoimmune diseases. The cancer-inducing properties
of estrogens have been particularly well documented in breast and
endometrial cancer. It has been shown that there is a positive
correlation of peak plasma anandamide with 17β-estradiol and
gonadotropin levels at ovulation (19). This may indicate the body's
protective reaction against rising levels of sex hormones. The same
principles may apply to cannabinoids and prostate cancer. We
propose that the endocannabinoid system may be a body-wide
protective system against the harmful effects of sex hormones. The
two systems are probably antagonistic, thus maintaining
homeostasis. Common molecular pathways, in which both cannabinoids
and sex hormones affect cell proliferation and apoptosis, require
further investigation in order to clarify their molecular
interactions.
Abbreviations:
|
AC
|
adenylate cyclase
|
|
2-AG
|
2-arachidonoyl glycerol
|
|
Akt
|
serine-threonine specific protein
kinase
|
|
cAMP
|
cyclic adenosine monophosphate
|
|
AP-1
|
activator protein-1
|
|
AR
|
androgen receptor
|
|
ATF-1
|
activating transcription factor-1
|
|
ATF-2
|
activating transcription factor 2
|
|
Bcl-2
|
B-cell lymphoma 2 protein
|
|
CB1
|
cannabinoid type 1
|
|
CB2
|
cannabinoid type 2
|
|
CB3
|
cannabinoid type 3
|
|
c-Jun
|
protein encoded by the JUN
gene
|
|
COX-2
|
cyclo-oxygenase-2
|
|
CRE
|
cAMP response elements
|
|
CREB
|
cAMP response element binding
protein
|
|
DHT
|
dihydrotestosterone
|
|
DNA
|
deoxyribonucleic acid
|
|
EGF
|
epidermal growth factor
|
|
ER
|
estrogen receptor
|
|
EREs
|
estrogen response elements
|
|
ERK1/2
|
extracellular-signal-regulated kinases
1/2
|
|
FAAH
|
fatty acid amide hydrolase
|
|
FSH
|
follicle-stimulating hormone
|
|
GnRH
|
gonadotropin-releasing hormone
|
|
GPER
|
G protein coupled estrogen
receptor
|
|
GPR18
|
G protein coupled receptor 18
|
|
GPR19
|
G protein coupled receptor 19
|
|
GPR55
|
G protein coupled receptor 55
|
|
IGF
|
insulin-like growth factor
|
|
LH
|
luteinizing hormone
|
|
MAGL
|
monoacylglycerol lipase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
NAPE-PLD
|
N-acyl phosphatidylethanolamine
phospholipase D
|
|
p21
|
cyclin-dependent kinase inhibitor
1
|
|
PI3K
|
phosphatidylinositol-4,5-bisphosphate
3-kinase
|
|
PKA
|
protein kinase A
|
|
PKC
|
protein kinase C
|
|
PLC
|
phospholipase C
|
|
Raf
|
proto-oncogene
serine/threonine-protein kinase
|
|
Ras
|
rat sarcoma protein
|
|
SHBG
|
sex hormone-binding globulin
|
|
Src
|
proto-oncogene tyrosine-protein
kinase
|
|
THC
|
tetrahydrocannabinol
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Han S, Thatte J, Buzard DJ and Jones RM:
Therapeutic utility of cannabinoid receptor type 2 (CB(2))
selective agonists. J Med Chem. 56:8224–8256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryberg E, Larsson N, Sjögren S, Hjorth S,
Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T and
Greasley PJ: The orphan receptor GPR55 is a novel cannabinoid
receptor. Br J Pharmacol. 152:1092–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McHugh D, Hu SS, Rimmerman N, Juknat A,
Vogel Z, Walker JM and Bradshaw HB: N-arachidonoyl glycine, an
abundant endogenous lipid, potently drives directed cellular
migration through GPR18, the putative abnormal cannabidiol
receptor. BMC Neurosci. 11:442010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown AJ: Novel cannabinoid receptors. Br
J Pharmacol. 152:567–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Starowicz K, Nigam S and Di Marzo V:
Biochemistry and pharmacology of endovanilloids. Pharmacol Ther.
114:13–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kushner PJ, Agard DA, Greene GL, Scanlan
TS, Shiau AK, Uht RM and Webb P: Estrogen receptor pathways to
AP-1. J Steroid Biochem Mol Biol. 74:311–317. 2000. View Article : Google Scholar
|
|
7
|
Hammes SR and Levin ER: Minireview: Recent
advances in extranuclear steroid receptor actions. Endocrinology.
152:4489–4495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coleman KM and Smith CL: Intracellular
signaling pathways: Nongenomic actions of estrogens and
ligand-independent activation of estrogen receptors. Front Biosci.
6:D1379–D1391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prossnitz ER, Arterburn JB and Sklar LA:
GPR30: A G protein-coupled receptor for estrogen. Mol Cell
Endocrinol. 265–266:138–142. 2007. View Article : Google Scholar
|
|
10
|
Paterni I, Granchi C, Katzenellenbogen JA
and Minutolo F: Estrogen receptors alpha (ERα) and beta (ERβ):
Subtype-selective ligands and clinical potential. Steroids.
90:13–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John E: Hall: Guyton and Hall Textbook of
Medical Physiology. 12th edition. Saunders; pp. 991–992. 2010
|
|
12
|
Tyrey L: delta-9-Tetrahydrocannabinol
suppression of episodic luteinizing hormone secretion in the
ovariectomized rat. Endocrinology. 102:1808–1814. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tyrey L: delta 9-Tetrahydrocannabinol: A
potent inhibitor of episodic luteinizing hormone secretion. J
Pharmacol Exp Ther. 213:306–308. 1980.PubMed/NCBI
|
|
14
|
Kumar MS and Chen CL: Effect of an acute
dose of delta 9-THC on hypothalamic luteinizing hormone releasing
hormone and met-enkephalin content and serum levels of testosterone
and corticosterone in rats. Subst Alcohol Actions Misuse. 4:37–43.
1983.PubMed/NCBI
|
|
15
|
Scorticati C, Fernández-Solari J, De
Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A,
Billi S, Franchi A, McCann SM, et al: The inhibitory effect of
anandamide on luteinizing hormone-releasing hormone secretion is
reversed by estrogen. Proc Natl Acad Sci USA. 101:11891–11896.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gammon CM, Freeman GM Jr, Xie W, Petersen
SL and Wetsel WC: Regulation of gonadotropin-releasing hormone
secretion by cannabinoids. Endocrinology. 146:4491–4499. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cravatt BF, Giang DK, Mayfield SP, Boger
DL, Lerner RA and Gilula NB: Molecular characterization of an
enzyme that degrades neuromodulatory fatty-acid amides. Nature.
384:83–87. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacCarrone M, De Felici M, Bari M, Klinger
F, Siracusa G and Finazzi-Agrò A: Downregulation of anandamide
hydrolase in mouse uterus by sex hormones. Eur J Biochem.
267:2991–2997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Talatini MR, Taylor AH and Konje JC:
The relationship between plasma levels of the endocannabinoid,
anandamide, sex steroids, and gonadotrophins during the menstrual
cycle. Fertil Steril. 93:1989–1996. 2010. View Article : Google Scholar
|
|
20
|
Gorzalka BB and Dang SS: Minireview:
Endocannabinoids and gonadal hormones: bidirectional interactions
in physiology and behavior. Endocrinology. 153:1016–1024. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossi F, Bellini G, Luongo L, Mancusi S,
Torella M, Tortora C, Manzo I, Guida F, Nobili B, de Novellis V and
Maione S: The 17-β-oestradiol inhibits osteoclast activity by
increasing the cannabinoid CB2 receptor expression. Pharmacol Res.
68:7–15. 2013. View Article : Google Scholar
|
|
22
|
Notarnicola M, Messa C, Orlando A, Bifulco
M, Laezza C, Gazzerro P and Caruso MG: Estrogenic induction of
cannabinoid CB1 receptor in human colon cancer cell lines. Scand J
Gastroenterol. 43:66–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riebe CJ, Hill MN, Lee TT, Hillard CJ and
Gorzalka BB: Estrogenic regulation of limbic cannabinoid receptor
binding. Psychoneuroendocrinology. 35:1265–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar P and Song ZH: CB2 cannabinoid
receptor is a novel target for third-generation selective estrogen
receptor modulators bazedoxifene and lasofoxifene. Biochem Biophys
Res Commun. 443:144–149. 2014. View Article : Google Scholar
|
|
25
|
Prather PL, FrancisDevaraj F, Dates CR,
Greer AK, Bratton SM, Ford BM, Franks LN and Radominska-Pandya A:
CB1 and CB2 receptors are novel molecular targets for Tamoxifen and
4OH-Tamoxifen. Biochem Biophys Res Commun. 441:339–343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melck D, Rueda D, Galve-Roperh I, De
Petrocellis L, Guzmán M and Di Marzo V: Involvement of the
cAMP/protein kinase A pathway and of mitogen-activated protein
kinase in the anti-proliferative effects of anandamide in human
breast cancer cells. FEBS Lett. 463:235–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu Q and Moss RL: 17 beta-Estradiol
potentiates kainate-induced currents via activation of the cAMP
cascade. J Neurosci. 16:3620–3629. 1996.PubMed/NCBI
|
|
28
|
Picotto G, Massheimer V and Boland R:
Acute stimulation of intestinal cell calcium influx induced by 17
beta-estradiol via the cAMP messenger system. Mol Cell Endocrinol.
119:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watters JJ and Dorsa DM: Transcriptional
effects of estrogen on neuronal neurotensin gene expression involve
cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci.
18:6672–6680. 1998.PubMed/NCBI
|
|
30
|
Szego CM and Davis JS: Adenosine
3′,5′-monophosphate in rat uterus: Acute elevation by estrogen.
Proc Natl Acad Sci USA. 58:1711–1718. 1967. View Article : Google Scholar
|
|
31
|
Mimeault M, Pommery N, Wattez N, Bailly C
and Hénichart JP: Anti-fn of epidermal growth factor receptor
downregulation and ceramide production. Prostate. 56:1–12. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Preet A, Qamri Z, Nasser MW, Prasad A,
Shilo K, Zou X, Groopman JE and Ganju RK: Cannabinoid receptors,
CB1 and CB2, as novel targets for inhibition of non-small cell lung
cancer growth and metastasis. Cancer Prev Res (Phila). 4:65–75.
2011. View Article : Google Scholar
|
|
33
|
Driggers PH and Segars JH: Estrogen action
and cytoplasmic signaling pathways. Part II: The role of growth
factors and phosphorylation in estrogen signaling. Trends
Endocrinol Metab. 13:422–427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Lone R, Frith MC, Karlsson EK and Hansen
U: Genomic targets of nuclear estrogen receptors. Mol Endocrinol.
18:1859–1875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marino M, Galluzzo P and Ascenzi P:
Estrogen signaling multiple pathways to impact gene transcription.
Curr Genomics. 7:497–508. 2006. View Article : Google Scholar
|
|
36
|
Bosier B, Hermans E and Lambert D:
Differential modulation of AP-1- and CRE-driven transcription by
cannabinoid agonists emphasizes functional selectivity at the CB1
receptor. Br J Pharmacol. 155:24–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Melck D, De Petrocellis L, Orlando P,
Bisogno T, Laezza C, Bifulco M and Di Marzo V: Suppression of nerve
growth factor Trk receptors and prolactin receptors by
endocannabinoids leads to inhibition of human breast and prostate
cancer cell proliferation. Endocrinology. 141:118–126. 2000.
|
|
38
|
Watters JJ, Chun TY, Kim YN, Bertics PJ
and Gorski J: Estrogen modulation of prolactin gene expression
requires an intact mitogen-activated protein kinase signal
transduction pathway in cultured rat pituitary cells. Mol
Endocrinol. 14:1872–1881. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Portella G, Laezza C, Laccetti P, De
Petrocellis L, Di Marzo V and Bifulco M: Inhibitory effects of
cannabinoid CB1 receptor stimulation on tumor growth and metastatic
spreading: Actions on signals involved in angiogenesis and
metastasis. FASEB J. 17:1771–1773. 2003.PubMed/NCBI
|
|
40
|
Blázquez C, González-Feria L, Alvarez L,
Haro A, Casanova ML and Guzmán M: Cannabinoids inhibit the vascular
endothelial growth factor pathway in gliomas. Cancer Res.
64:5617–5623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mueller MD, Vigne JL, Minchenko A, Lebovic
DI, Leitman DC and Taylor RN: Regulation of vascular endothelial
growth factor (VEGF) gene transcription by estrogen receptors alpha
and beta. Proc Natl Acad Sci USA. 97:10972–10977. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stoner M, Wang F, Wormke M, Nguyen T,
Samudio I, Vyhlidal C, Marme D, Finkenzeller G and Safe S:
Inhibition of vascular endothelial growth factor expression in
HEC1A endometrial cancer cells through interactions of estrogen
receptor alpha and Sp3 proteins. J Biol Chem. 275:22769–22779.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Galve-Roperh I, Sánchez C, Cortés ML,
Gómez del Pulgar T, Izquierdo M and Guzmán M: Anti-tumoral action
of cannabinoids: Involvement of sustained ceramide accumulation and
extracellular signal-regulated kinase activation. Nat Med.
6:313–319. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klinge CM, Blankenship KA, Risinger KE,
Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM and
Keynton RS: Resveratrol and estradiol rapidly activate MAPK
signaling through estrogen receptors alpha and beta in endothelial
cells. J Biol Chem. 280:7460–7468. 2005. View Article : Google Scholar
|
|
45
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
46
|
Acconcia F, Totta P, Ogawa S, Cardillo I,
Inoue S, Leone S, Trentalance A, Muramatsu M and Marino M: Survival
versus apoptotic 17beta-estradiol effect: Role of ER alpha and ER
beta activated non-genomic signaling. J Cell Physiol. 203:193–201.
2005. View Article : Google Scholar
|
|
47
|
Kahlert S, Nuedling S, van Eickels M,
Vetter H, Meyer R and Grohe C: Estrogen receptor alpha rapidly
activates the IGF-1 receptor pathway. J Biol Chem. 275:18447–18453.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pertwee RG, Howlett AC, Abood ME,
Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos
G, Mackie K, et al: International Union of Basic and Clinical
Pharmacology. LXXIX Cannabinoid receptors and their ligands: Beyond
CB1 and CB2. Pharmacol Rev. 62:588–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ramer R, Weinzierl U, Schwind B, Brune K
and Hinz B: Ceramide is involved in r(+)-methanandamide-induced
cyclooxygenase-2 expression in human neuroglioma cells. Mol
Pharmacol. 64:1189–1198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pisanti S, Picardi P, D'Alessandro A,
Laezza C and Bifulco M: The endocannabinoid signaling system in
cancer. Trends Pharmacol Sci. 34:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ellert-Miklaszewska A, Kaminska B and
Konarska L: Cannabinoids downregulate PI3K/Akt and Erk signalling
pathways and activate proapoptotic function of Bad protein. Cell
Signal. 17:25–37. 2005. View Article : Google Scholar
|
|
52
|
Haynes MP, Li L, Sinha D, Russell KS,
Hisamoto K, Baron R, Collinge M, Sessa WC and Bender JR: Src kinase
mediates phosphatidylinositol 3-kinase/Akt-dependent rapid
endothelial nitric-oxide synthase activation by estrogen. J Biol
Chem. 278:2118–2123. 2003. View Article : Google Scholar
|
|
53
|
Marino M, Acconcia F and Trentalance A:
Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN
via MAP kinase in HepG2 cells. Mol Biol Cell. 14:2583–2591. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gaub MP, Bellard M, Scheuer I, Chambon P
and Sassone-Corsi P: Activation of the ovalbumin gene by the
estrogen receptor involves the fos-jun complex. Cell. 63:1267–1276.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Paech K, Webb P, Kuiper GG, Nilsson S,
Gustafsson J, Kushner PJ and Scanlan TS: Differential ligand
activation of estrogen receptors ERalpha and ERbeta at AP1 sites.
Science. 277:1508–1510. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kousteni S, Han L, Chen JR, Almeida M,
Plotkin LI, Bellido T and Manolagas SC: Kinase-mediated regulation
of common transcription factors accounts for the bone-protective
effects of sex steroids. J Clin Invest. 111:1651–1664. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sarfaraz S, Afaq F, Adhami VM, Malik A and
Mukhtar H: Cannabinoid receptor agonist-induced apoptosis of human
prostate cancer cells LNCaP proceeds through sustained activation
of ERK1/2 leading to G1 cell cycle arrest. J Biol Chem.
281:39480–39491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cianchi F, Papucci L, Schiavone N, Lulli
M, Magnelli L, Vinci MC, Messerini L, Manera C, Ronconi E,
Romagnani P, et al: Cannabinoid receptor activation induces
apoptosis through tumor necrosis factor alpha-mediated ceramide de
novo synthesis in colon cancer cells. Clin Cancer Res.
14:7691–7700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo Y, Wang H, Okamoto Y, Ueda N, Kingsley
PJ, Marnett LJ, Schmid HH, Das SK and Dey SK:
N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an
important determinant of uterine anandamide levels during
implantation. J Biol Chem. 280:23429–23432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsuboi K, Okamoto Y, Ikematsu N, Inoue M,
Shimizu Y, Uyama T, Wang J, Deutsch DG, Burns MP, Ulloa NM, et al:
Enzymatic formation of N-acylethanolamines from N-acylethanolamine
plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing
phospholipase D-dependent and -independent pathways. Biochim
Biophys Acta. 1811:565–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Urquhart P, Nicolaou A and Woodward DF:
Endocannabinoids and their oxygenation by cyclo-oxygenases,
lipoxygenases and other oxygenases. Biochim Biophys Acta.
1851:366–376. 2015. View Article : Google Scholar
|
|
62
|
Tamura M, Deb S, Sebastian S, Okamura K
and Bulun SE: Estrogen up-regulates cyclooxygenase-2 via estrogen
receptor in human uterine microvascular endothelial cells. Fertil
Steril. 81:1351–1356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Higa GM and Fell RG: Sex hormone receptor
repertoire in breast cancer. Int J Breast Cancer. 2013:2840362013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Caffarel MM, Sarrió D, Palacios J, Guzmán
M and Sánchez C: Delta9-tetrahydrocannabinol inhibits cell cycle
progression in human breast cancer cells through Cdc2 regulation.
Cancer Res. 66:6615–6621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shrivastava A, Kuzontkoski PM, Groopman JE
and Prasad A: Cannabidiol induces programmed cell death in breast
cancer cells by coordinating the cross-talk between apoptosis and
autophagy. Mol Cancer Ther. 10:1161–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Murase R, Kawamura R, Singer E, Pakdel A,
Sarma P, Judkins J, Elwakeel E, Dayal S, Martinez-Martinez E, Amere
M, et al: Targeting multiple cannabinoid anti-tumour pathways with
a resorcinol derivative leads to inhibition of advanced stages of
breast cancer. Br J Pharmacol. 171:4464–4477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qamri Z, Preet A, Nasser MW, Bass CE,
Leone G, Barsky SH and Ganju RK: Synthetic cannabinoid receptor
agonists inhibit tumor growth and metastasis of breast cancer. Mol
Cancer Ther. 8:3117–3129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
McKallip RJ, Nagarkatti M and Nagarkatti
PS: Delta-9-tetrahydrocannabinol enhances breast cancer growth and
metastasis by suppression of the antitumor immune response. J
Immunol. 174:3281–3289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
SGO Clinical Practice Endometrial Cancer
Working Group; Burke WM, Orr J, Leitao M, Salom E, Gehrig P,
Olawaiye AB, Brewer M, Boruta D, Villella J, Herzog T and Abu
Shahin F; Society of Gynecologic Oncology Clinical Practice
Committee: Endometrial cancer: A review and current management
strategies: part I. Gynecol Oncol. 134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tangen IL, Werner HM, Berg A, Halle MK,
Kusonmano K, Trovik J, Hoivik EA, Mills GB, Krakstad C and Salvesen
HB: Loss of progesterone receptor links to high proliferation and
increases from primary to metastatic endometrial cancer lesions.
Eur J Cancer. 50:3003–3010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ayakannu A, Taylor AH, Marczylo TH,
Willets JM, Brown L, Davies Q, Moss E and Konje JC: Association of
cannabinoid receptor expression with anandamide concentrations in
endometrial cancer. Lancet Volume. 383:S232014. View Article : Google Scholar
|
|
72
|
Guida M, Ligresti A, De Filippis D,
D'Amico A, Petrosino S, Cipriano M, Bifulco G, Simonetti S, Orlando
P, Insabato L, et al: The levels of the endocannabinoid receptor
CB2 and its ligand 2-arachidonoylglycerol are elevated in
endometrial carcinoma. Endocrinology. 151:921–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Centrella M and McCarthy TL: Estrogen
receptor dependent gene expression by osteoblasts - direct,
indirect, circumspect, and speculative effects. Steroids.
77:174–184. 2012. View Article : Google Scholar
|
|
75
|
Bilezikian JP, Raisz LG and Martin TJ:
Principles of Bone Biology. 3rd edition. Elsevier; Amsterdam: pp.
855–885. 2008
|
|
76
|
Bradford PG, Gerace KV, Roland RL and
Chrzan BG: Estrogen regulation of apoptosis in osteoblasts. Physiol
Behav. 99:181–185. 2010. View Article : Google Scholar :
|
|
77
|
Ofek O, Karsak M, Leclerc N, Fogel M,
Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, et
al: Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc
Natl Acad Sci USA. 103:696–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hanus L, Breuer A, Tchilibon S, Shiloah S,
Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R and
Fride E: HU-308: A specific agonist for CB(2), a peripheral
cannabinoid receptor. Proc Natl Acad Sci USA. 96:14228–14233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hojnik M, Dobovišek L, Knez Ž and Ferk P:
A synergistic interaction of 17-β-estradiol with specific
cannabinoid receptor type 2 antagonist/inverse agonist on
proliferation activity in primary human osteoblasts. Biomed Rep.
3:554–558. 2015.PubMed/NCBI
|
|
80
|
Steffens S, Veillard NR, Arnaud C, Pelli
G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL and Mach F:
Low dose oral cannabinoid therapy reduces progression of
atherosclerosis in mice. Nature. 434:782–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Paganini-Hill A, Dworsky R and Krauss RM:
Hormone replacement therapy, hormone levels, and lipoprotein
cholesterol concentrations in elderly women. Am J Obstet Gynecol.
174:897–902. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Boosani CS and Sudhakar YA:
Proteolytically derived endogenous angioinhibitors originating from
the extracellular matrix. Pharmaceuticals (Basel). 4:1551–1577.
2011. View Article : Google Scholar
|
|
83
|
Deroo BJ and Korach KS: Estrogen receptors
and human disease. J Clin Invest. 116:561–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Murphy E: Estrogen signaling and
cardiovascular disease. Circ Res. 109:687–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dol-Gleizes F, Paumelle R, Visentin V,
Marés AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P
and Bono F: Rimonabant, a selective cannabinoid CB1 receptor
antagonist, inhibits atherosclerosis in LDL receptor-deficient
mice. Arterioscler Thromb Vasc Biol. 29:12–18. 2009. View Article : Google Scholar
|
|
86
|
Pacher P: Cannabinoid CB1 receptor
antagonists for atherosclerosis and cardiometabolic disorders: New
hopes, old concerns? Arterioscler Thromb Vasc Biol. 29:7–9. 2009.
View Article : Google Scholar :
|
|
87
|
Mach F, Montecucco F and Steffens S:
Cannabinoid receptors in acute and chronic complications of
atherosclerosis. Br J Pharmacol. 153:290–298. 2008. View Article : Google Scholar
|
|
88
|
Chiurchiù V, Lanuti M, Catanzaro G, Fezza
F, Rapino C and Maccarrone M: Detailed characterization of the
endocannabinoid system in human macrophages and foam cells, and
anti-inflammatory role of type-2 cannabinoid receptor.
Atherosclerosis. 233:55–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Collot-Teixeira S, Martin J, McDermott-Roe
C, Poston R and McGregor JL: CD36 and macrophages in
atherosclerosis. Cardiovasc Res. 75:468–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Heinlein CA and Chang C: The roles of
androgen receptors and androgen-binding proteins in nongenomic
androgen actions. Mol Endocrinol. 16:2181–2187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Michels G and Hoppe UC: Rapid actions of
androgens. Front Neuroendocrinol. 29:182–198. 2008. View Article : Google Scholar
|
|
92
|
Cohen S: The 94-day cannabis study. Ann NY
Acad Sci. 282:211–220. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Block RI, Farinpour R and Schlechte JA:
Effects of chronic marijuana use on testosterone, luteinizing
hormone, follicle stimulating hormone, prolactin and cortisol in
men and women. Drug Alcohol Depend. 28:121–128. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dalterio S, Bartke A and Burstein S:
Cannabinoids inhibit testosterone secretion by mouse testes in
vitro. Science. 196:1472–1473. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dixit VP, Sharma VN and Lohiya NK: The
effect of chronically administered cannabis extract on the
testicular function of mice. Eur J Pharmacol. 26:111–114. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ghosh SP, Chatterjee TK and Ghosh JJ:
Antiandrogenic effect of delta-9-tetrahydrocannabinol in adult
castrated rats. J Reprod Fertil. 62:513–517. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dixit VP, Gupta CL and Agrawal M:
Testicular degeneration and necrosis induced by chronic
administration of cannabis extract in dogs. Endokrinologie.
69:299–305. 1977.PubMed/NCBI
|
|
98
|
Migliaccio A, Castoria G, Di Domenico M,
de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini
MS, Abbondanza C and Auricchio F: Steroid-induced androgen
receptor-oestradiol receptor beta-Src complex triggers prostate
cancer cell proliferation. EMBO J. 19:5406–5417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Unni E, Sun S, Nan B, McPhaul MJ, Cheskis
B, Mancini MA and Marcelli M: Changes in androgen receptor
nongenotropic signaling correlate with transition of LNCaP cells to
androgen independence. Cancer Res. 64:7156–7168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li J and Al-Azzawi F: Mechanism of
androgen receptor action. Maturitas. 63:142–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Taichman RS, Loberg RD, Mehra R and Pienta
KJ: The evolving biology and treatment of prostate cancer. J Clin
Invest. 117:2351–2361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bonaccorsi L, Nosi D, Quercioli F,
Formigli L, Zecchi S, Maggi M, Forti G and Baldi E: Prostate
cancer: A model of integration of genomic and non-genomic effects
of the androgen receptor in cell lines model. Steroids.
73:1030–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bonaccorsi L, Nosi D, Muratori M, Formigli
L, Forti G and Baldi E: Altered endocytosis of epidermal growth
factor receptor in androgen receptor positive prostate cancer cell
lines. J Mol Endocrinol. 38:51–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Purohit V, Ahluwahlia BS and Vigersky RA:
Marihuana inhibits dihydrotestosterone binding to the androgen
receptor. Endocrinology. 107:848–850. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Grech A, Breck J and Heidelbaugh J:
Adverse effects of testosterone replacement therapy: An update on
the evidence and controversy. Ther Adv Drug Saf. 5:190–200. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ahmed A, Ali S and Sarkar FH: Advances in
androgen receptor targeted therapy for prostate cancer. J Cell
Physiol. 229:271–276. 2014. View Article : Google Scholar
|
|
107
|
Morales P, Vara D, Goméz-Cañas M, Zúñiga
MC, Olea-Azar C, Goya P, Fernández-Ruiz J, Díaz-Laviada I and
Jagerovic N: Synthetic cannabinoid quinones: Preparation, in vitro
antiproliferative effects and in vivo prostate antitumor activity.
Eur J Med Chem. 70:111–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
De Petrocellis L, Ligresti A, Schiano
Moriello A, Iappelli M, Verde R, Stott CG, Cristino L, Orlando P
and Di Marzo V: Non-THC cannabinoids inhibit prostate carcinoma
growth in vitro and in vivo: Pro-apoptotic effects and underlying
mechanisms. Br J Pharmacol. 168:79–102. 2013. View Article : Google Scholar :
|