Introduction
Pulmonary fibrosis (PF) is a c\hronic and
progressive disease characterized by diffuse inflammation,
interstitial fibrosis and the distortion of the lung architecture,
which contributes to extensive damage and dysfunction of the lungs
(1). However, the explicit
pathogenesis of PF remains inadequately understood, and there is an
unmet need for effective treatments. Novel strategies aimed at
enhancing the therapeutic effects have gained significant interest.
Previous studies have demonstrated that the imbalance of oxidants
and antioxidants caused by oxidative stress plays an important role
in the development of PF (2–4),
including bleomycin (BLM)-induced PF (5,6).
Thus, the key components involved in oxidative stress have been
receiving increased attention and are considered as promising
therapeutic targets for the treatment of PF.
The transcription factor Bach1, which is a member of
the cap 'n' collar family of basic leucine zipper, is known to be
involved in the regulation of oxidative stress response (7). Bach1 executes transcriptional
inhibition via competitive binding to the Maf-recognition element
closely related to antioxidant response element (ARE), thus
antagonizing the activation of nuclear factor-erythroid 2-related
factor 2 (Nrf2) (8,9). Nrf2 functions as one of the most
important molecules involved in oxidative stress that promotes the
expression of Nrf2-dependent antioxidant genes and proteins
(10,11). It has been reported that Nrf2
deficiency is associated with the pathogenesis of lung fibrosis in
mice and humans (12,13). Nrf2 agonists protect against PF by
regulating the lung oxidant levels in mice with BLM-induced lung
fibrosis (14). Furthermore,
ARE/Nrf2-dependent antioxidants, including glutathione peroxidase 1
(GPx1), heme oxygenase-1 (HO-1) and NAD(P) H:quinone
oxidoreductase-1 (NQO1) may be critical in pulmonary protection
(15–17). Additionally, the expression of
Nrf2-dependent antioxidants can be suppressed by the
transcriptional induction of Bach1 (8). Although the evidence suggests that
Bach1 is a transcriptional repressor of Nrf2, the inhibitory
effects of Bachl on Nrf2-dependent antioxidants and fibrotic
processes in pulmonary tissue remain still poorly understood. To
date, there are no available published studies investigating
whether targeting Bachl attenuates BLM-induced lung fibrosis, at
least to the best of our knowledge.
As BLM causes cell damage, and the emergence of free
radicals and the subsequent induction of oxidative stress
ultimately results in inflammation and fibrosis (18,19), it is currently the most commonly
used animal model to investigate PF (20). It has been demonstrated that
multiple factors, including tumor necrosis factor (TNF)-α,
transforming growth factor (TGF)-β1, interleukin (IL)-1 and -6, and
connective tissue growth factor (CTGF) are implicated in the
development and progression of PF (21,22). A previous study found markedly
increased levels of TGF-β1 and IL-6 in both bronchoalveolar lavage
fluid (BALF) and in the serum of mice following exposure to BLM
(23). TGF-β1 has been identified
as a key mediator of lung fibrosis, and it induces the
proliferation and migration of lung fibroblasts, as well as the
remodeling of the extracellular matrix (ECM) (24,25). In this context, TGF-β1 and IL-6
are considered to be useful parameters for observing the
progression of PF.
Small interfering RNA (siRNA), which can attain
target-specific gene silencing, may be a potent tool for gene
therapy (26,27). In this study, we focused on Bach1,
since it is an important mediator of oxidative stress and may be a
critical target for PF therapy. Based on these reasons, we
hypothesized that adenovirus-mediated Bach1 siRNA may be effective
in silencing Bach1 transcripts in vitro and in vivo,
representing a potential means with which to promote the expression
of Nrf2-dependent antioxidants and ameliorate fibrotic process in
BLM-induced lung fibrosis. For our experiments, Bach1 siRNAs were
administered to mouse lung fibroblasts (MLFs) and to mice with
BLM-induced PF in order to monitor the antioxidant and
anti-fibrotic effects via the measurement of antioxidant factors,
fibrosis-related cytokines and histological changes. Taken
together, the findings of our study may provide novel insight into
the role of Bach1 in the regulation of oxidative stress involved in
the pathogenesis of PF, thus leading to the development of
promising strategies for the treatment of PF.
Materials and methods
Construction of Bach1 siRNA expression
vectors and recombinant adenovirus
Two siRNAs targeting mouse Bach1 mRNA were designed
using a software available at www.ambion.com via a cDNA sequence (SEQ ID,
XM_006522879), and an empty adenoviral vector was used as a
negative control. The specific siRNA coding sequences were designed
to contain the target sequences and their reverse complement
19-nucleotide sequences which are separated by 9-nucleotide
sequences of stem-loop structure (Table I).
 | Table ISequence of forward and reverse
strands of the Bach1 shDNA oligonucleotides. |
Table I
Sequence of forward and reverse
strands of the Bach1 shDNA oligonucleotides.
| Target | Target siRNA
sequences |
|---|
| Bach1 siRNA#1 | F: 5′-GCG TAC ACA
ATA TCG AGGA TTCAAGACG TCC TCG ATA TTG TGT ACGC TTTTTT-3′ |
| R: 5′-AAAAAA GCG
TAC ACA ATA TCG AGGA CGTCTTGAA TCC TCG ATA TTG TGT ACGC-3′ |
| Bach1 siRNA#2 | F: 5′-GAA TCT CAC
CTT GTA GACC TTCAAGACG GGT CTA CAA GGT GAG ATTC TTTTTT-3′ |
| R: 5′-AAAAAA GAA
TAT CTC CTT GTA GACC CGTCTTGAA GGT CTA CAA GGT GAG ATTC-3′ |
The forward and reverse oligonucleotides were
chemically synthesized and annealed to form a duplex. Two siRNA
sequences were cloned into the plasmid vector, pGenesil-1. The
siRNA expression cassette was subsequently excised from pGenesil-1
using EcoRI and HindIII, and also ligated into the
linearized adenoviral shuttle vector, pShuttle-CMV. The ligation
products were transformed into E. coli DH5α, and positive
clones were selected. The recombinant adenovirus vectors were
obtained by homologous recombination with pShuttle-CMV-Bach1-siRNA
and the skeleton plasmid of pAdeasy-1 in bacteria BJ5183. The
recombinant cosmids titled Ad-siBach1 carrying either Bach1 siRNA
or adenoviral vector with green fluorescent protein (GFP) were
linearized by PacI digestion and then transfected 293A cells
(Wuhan Cell Marker Biotechnology, Wuhan, China) using Lipofectamine
2000 reagent (Invitrogen, Carlsbad, CA, USA). The success of Bach1
siRNA insertion into adenoviral plasmid was confirmed by DNA
sequencing and the titer of each virus stock was determined by
plaque assay on 293A cells. Finally, the siRNA adenoviral vector
was calculated as 1.1×109 plaque-forming units (PFU)/ml
and the empty adenoviral vector was 1.4×109 PFU/ml. The
virus was purified by double CsCl gradient ultracentrifugation used
for in vivo experiments. The transfection efficiency was
monitored by fluorescence microscopy and flow cytometry. The
silencing efficiency of target gene and protein were determined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis. The selected siRNA sequence
with the highest inhibitory effect was used in the animal
experiments.
Animal experiments and sample
preparation
Seven-week-old female C57BL/6 mice (body weight,
18–20 g) were purchased from Vital River Laboratories (Beijing,
China). The animals were maintained under specific pathogen-free
conditions and kept for 1 week prior to use. The room temperature
was maintained at 22±3°C, with a 12-h/12-h day/night cycle and
relative humidity of 50±10%. All animals had free access to rodent
chow and water. The mice were randomly divided into 4 groups as
follows: a control with saline (n=5), BLM (n=5), BLM + control
siRNA (n=5) and BLM + Bach1 siRNA groups (n=5). For the model of
PF, 5 mg/kg BLM (Nippon Kayaku Co., Ltd., Tokyo, Japan) in 50
μl phosphate-buffered saline (PBS) were administered
intratracheally to the mice; the control animals were injected
intratracheally with the same volume of sterile saline. BLM and
saline were administered only once. The mice in the control siRNA
and Bach1 siRNA group were injected with control siRNA or Bach1
siRNA, respectively, via the tail vein (one injection every other
day), with a total dose of 1×109 PFU viruses per mouse
after 2 weeks of BLM administration. At the designated time points
(28 days post-BLM administration), the mice were humanely
sacrificed by an overdose of the anesthesia (inhalation of ether)
for serum, BALF and histological measurements. We lavaged the lungs
using 0.9% saline using a tracheal cannula for 3 times and
collected 1.5 ml BALF in each mouse. The blood of the experimental
mice was collected using a capillary through the conjunctiva and
into the orbital sinus. Following centrifugation (2000 × g for 5
min at 4°C), the serum and supernatants were stored at −80°C until
use. In addition, the lungs were removed and immediately frozen in
liquid nitrogen, and then stored at −80°C until further processing.
All procedures involving animals were approved by the Ethics
Committee of Capital Medical University, Beijing, China and
complied with guidelines on the Use of Experimental Animals.
Lung histopathology
The left lung tissues from the mice were dissected
and fixed in 4% paraformaldehyde. After 24 h, the tissues were
dehydrated and embedded in paraffin. Paraffin blocks of 3–4
μm thickness were cut and stained with hematoxylin and eosin
(H&E) and Masson's reagent (Solarbio, Beijing, China) for the
assessment of PF histopathology. The histological severity of
pulmonary alveolitis was scored as previously described (28), and PF was scored as described in
the study by Ashcroft et al (29).
Cell culture and treatment
The MLF cell line (MIC-CELL-0040) was purchased from
PriCells Biomedical Technology Co., Ltd. (Hubei, China) and
maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen)
supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA),
100 U/ml penicillin and streptomycin. The cells were plated at
1×105 cells/100 mm and cultured at 37°C in a 5%
CO2 humidified atmosphere. We used 0.25% trypsin for
digestion and the cells were filled in 2 culture flasks for passage
(1:2). All experiments proceeded using cells between 3 and 4 cell
passages. For the induction of fibrosis using the recombinant
protein, TGF-β1, the cells were seeded in 12-well plates at a
density of 1×105 cells/well and starved for 24 h and
then incubated with TGF-β1 (5 ng/ml) in complete medium for 24 h.
To knockdown Bach1 expression, the MLFs were infected with the
Bach1 siRNA at a multiplicity of infection (MOI) of 50 for 2 h and
were then washed to remove the virus. The cells in in the control
group (control siRNA) were transfected with empty vector. The cells
were then cultured for a further 48 h and then analyzed for their
GFP intensity using a FACScan flow cytometer (Becton-Dickinson,
Mountain View, CA, USA) and directly observed under a fluorescence
microscope (Olympus BX51; Olympus, Tokyo, Japan).
RT-qPCR
Total RNA was isolated from the mouse pulmonary
tissue samples and the MLFs using the simple Total RNA kit (Tiangen
Biotech Co., Ltd., Beijing, China) according to the manufacturer's
instructions. RNA was reverse transcribed using the Revert Aid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.,
Shanghai, China) and quantitative PCR (qPCR) was performed using
the SYBR PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China). The initial denaturation step was at 95°C for 10
min, and then PCR amplification was achieved by 40 cycles at 95°C
for 15 sec and 60°C for 1 min. The process of RT-qPCR was
implemented by ABI Prism 7500 (Applied Biosystems, Foster City, CA,
USA). The sequences of the primers used are listed in Table II.
 | Table IISequences of primers used for PCR in
this study. |
Table II
Sequences of primers used for PCR in
this study.
| Target | Primer
sequence |
|---|
| Bach1 | F: 5′-GAACAG GGCTAC
TCGCAA AG-3′ |
| R: 5′-AAAGGG CAGTTG
ACGGAA C-3′ |
| HO-1 | F:
5′-GACAGAAGAGGCTAAGACCGC-3′ |
| R:
5′-TGACGAAGTGACGCCATCT-3′ |
| GPx1 | F:
5′-GCACATCTACCACGCAGTCA-3′ |
| R:
5′-AGAGTCTCAAGAACATCGCCT-3′ |
| NQO1 | F:
5′-GCTTTAGGGTCGTCTTGGC-3′ |
| R:
5′-TGGCGTAGTTGAATGATGTCT-3′ |
| GAPDH | F:
5′-AAGACCCAGAAATGAAC-3′ |
| R:
5′-TCTACACGATAACAACCA-3′ |
The level of gene expression was quantified using a
standard curve and the comparative CT method normalized to GADPH
mRNA expression. We used the formula 2−ΔΔCT to calculate
the relative expression levels of genes. Both samples were examined
in triplicate, and the experiment was repeated at least 3
times.
Western blot analysis
Cell pellets or lung tissues were lysed in RIPA
lysis buffer containing 1 mM phenylmethanesulfonyl fluoride
(Applygen Technologies, Inc., Beijing, China) on ice, and the
concentration of the sample proteins was tested using the BCA
method before being mixed in 5X SDS loading buffer. Following heat
denaturation at 100°C for 5 min, equal amounts of lysate (60 mg)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto nitrocellulose
membranes (Applygen Technologies, Inc.). The membranes were blocked
with 5% fat-free milk for 1 h at room temperature, followed by
incubation with primary antibodies against β-actin (1:500;
sc-130301; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
Bach1 (1:300; ab49657), HO-1 (1:500; ab13248), GPx1 (1:1,000;
ab140883) and NQO1 (1:500; ab28947) (all from Abcam, Cambridge, UK)
at 4°C overnight. The following day, the membranes were incubated
with fluorescent-labeled secondary antibodies (1:10,000;
600-101-096; Rockland, Inc., Gilbertsville, PE, USA) for 1 h at
room temperature, and the bands were visualized using a
double-infrared laser scanning imaging system (LI-COR Biosciences,
Lincoln, NE, USA). Protein expression was analyzed and normalized
to that of β-actin.
Measurements of cytokine levels using
enzyme-linked immunosorbent assay (ELISA)
BALF and blood from the mice were sampled at the end
of 4 weeks after the initiation of the animal experiment. The
supernatants of MLFs were also collected after 48 h of the
transfection of siRNA for measurements. We then used the BALF,
serum and cell supernatants to analyze the levels of cytokines in
lung fibrosis. ELISA was used to detect TGF-β1 and IL-6 using
respective ELISA kits (R&D Systems, Minneapolis, MN, USA). The
supernatants of MLFs, serum and BALF of mice were diluted according
to different proportions along with reagents and standard dilutions
prepared before the experiment. Standard, control and samples (100
μl/well) were incubated for 2 h at room temperature. They
were then washed with wash buffer 5 times and 100 μl of
conjugate was then added for 2 h followed by substrate solution for
30 min away from light. After the addition of 100 μl of stop
solution, the sample concentrations were determined to measure the
absorbance at a 450 nm using a microplate reader (Bio Rad,
Hercules, CA, USA). Both assays were performed in duplicate.
Statistical analysis
Data were analyzed using a statistical software
package (SPSS 13.0; SPSS, Inc., Chicago, IL, USA), and expressed as
mean ± SD. One-way analysis of variance (ANOVA) was performed for
multiple group comparisons. A value of P<0.05 was considered to
indicate a statistically significant difference and a value of
P<0.01 was considered to indicate a marked statistically
significant difference.
Results
Transfection efficiency and targeting of
Bach1 siRNA
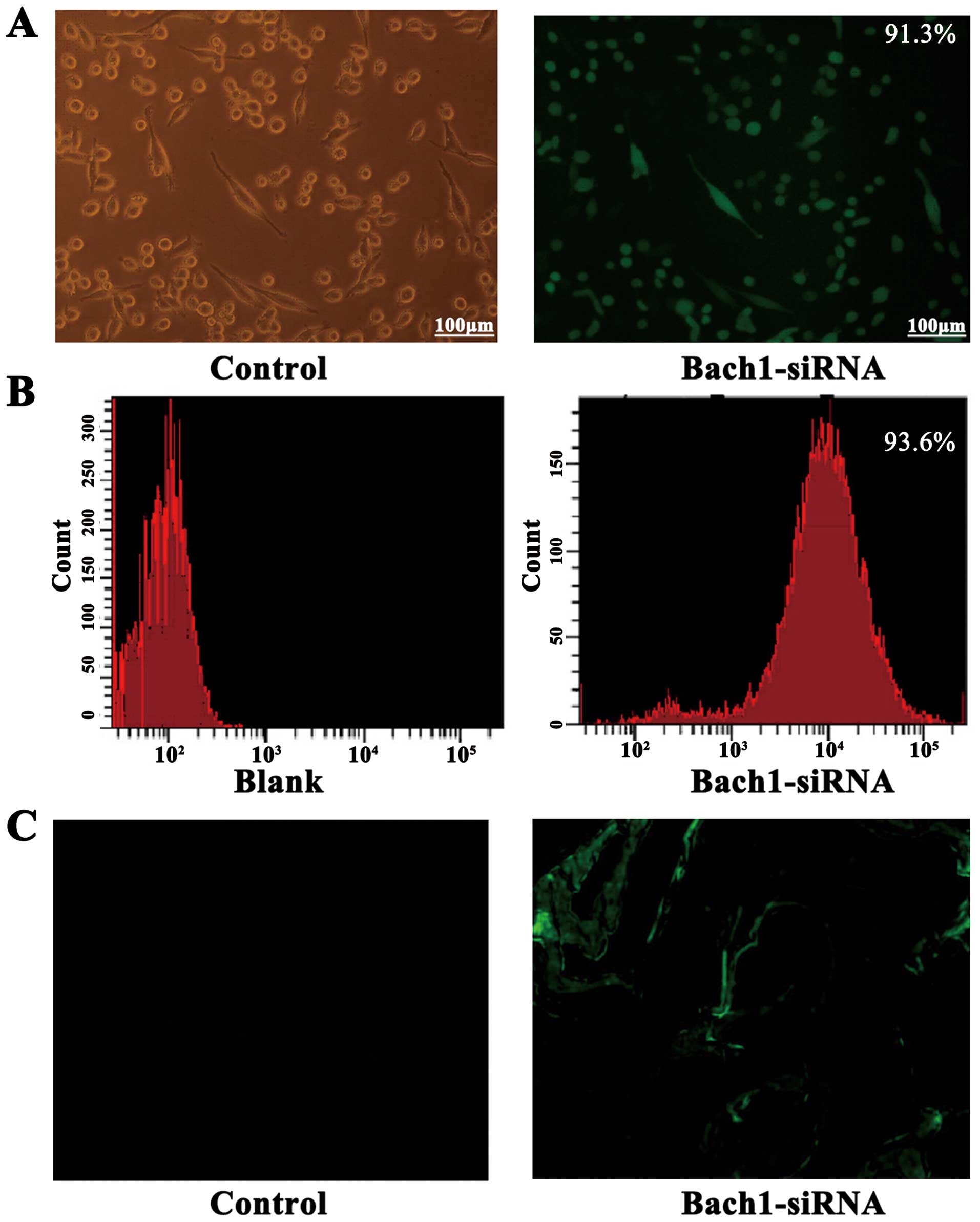
The successful construction of Bach1 siRNA
adenoviral vectors was determined by gene sequencing. To
demonstrate the transfection efficiency of Bach1 siRNA on MLFs and
its targeting in vivo, we used a fluorescent microscope and
flow cytometric analysis. After the Bach1 siRNA was transfected
into the MLFs, >90% GFP was expressed in the fluorescence states
(Fig. 1A), and the results of
flow cytometry also suggested that the transfection efficiency of
Bach1 siRNA was 93.6% (Fig. 1B).
After 2 weeks of siRNA administration, GFPs were observed in the
tissue sections of the lungs of mice, suggesting that Bach1 siRNA
targeted the lungs (Fig. 1C).
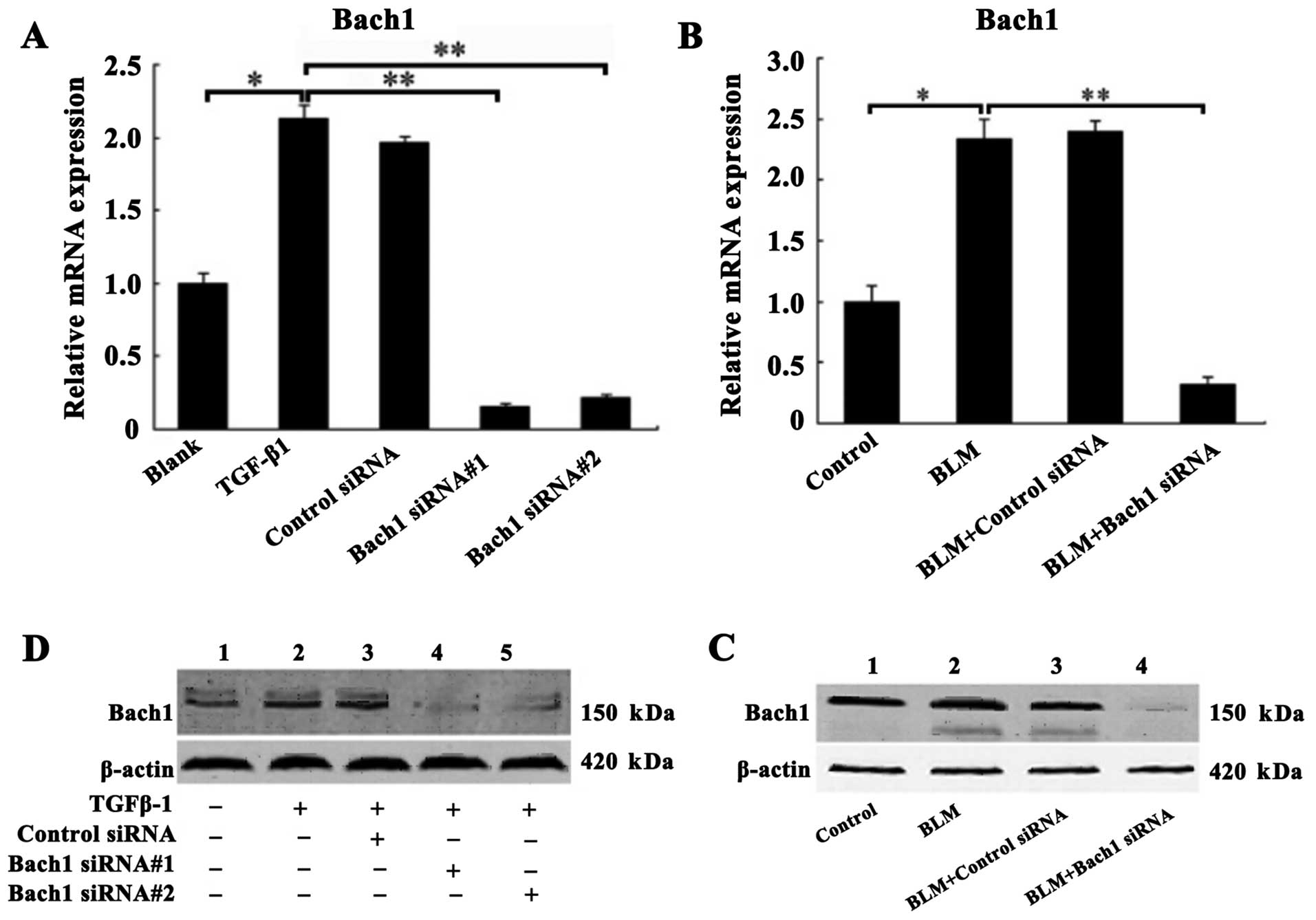
Confirmation of Bach1 gene knockdown in
vivo and in vitro
The MLFs were incubated with recombinant protein
TGF-β1 (5 ng/ml) for the induction of fibrosis prior to
transfection with control siRNA, or Bach1 siRNA#1 and #2 in
vitro. Similarly, superior Bach1 siRNA (Bach#1) was injected
into the mice with BLM-induced PF in vivo. The inhibitory
effects of Bach1 siRNA on Bach1 mRNA and protein expression were
analyzed by RT-qPCR and western blot analysis. As shown in Fig. 2A, the mRNA level of Bach1 in the
TGF-β1 group was significantly increased compared with that of the
blank group (P<0.05), suggesting that TGF-β1 promoted Bach1
generation. The mRNA expression of Bach1 in the MLFs following
transfection with Bach1 siRNA#1 or #2 was significantly decreased
compared with that of the TGF-β1 group or the control siRNA group
(P<0.01), and was also significantly decreased by 15.3 and 22%,
respectively (P<0.01) compared with the blank group (P<0.01)
(Fig. 2A). Similarly, the results
of western blot analysis also revealed that the protein expression
of Bach1 in the MLFs following transfection with Bach1 siRNA#1 or
#2 was markedly decreased (Fig.
2C). To further characterize Bach1 expression in vivo,
we detected Bach1 expression in the lungs of mice and found that
Bach1 mRNA and protein expression in the mice was decreased
significantly following the administration of BLM and Bach1 siRNA,
compared with the control group and the control siRNA group
(Fig. 2B and D).
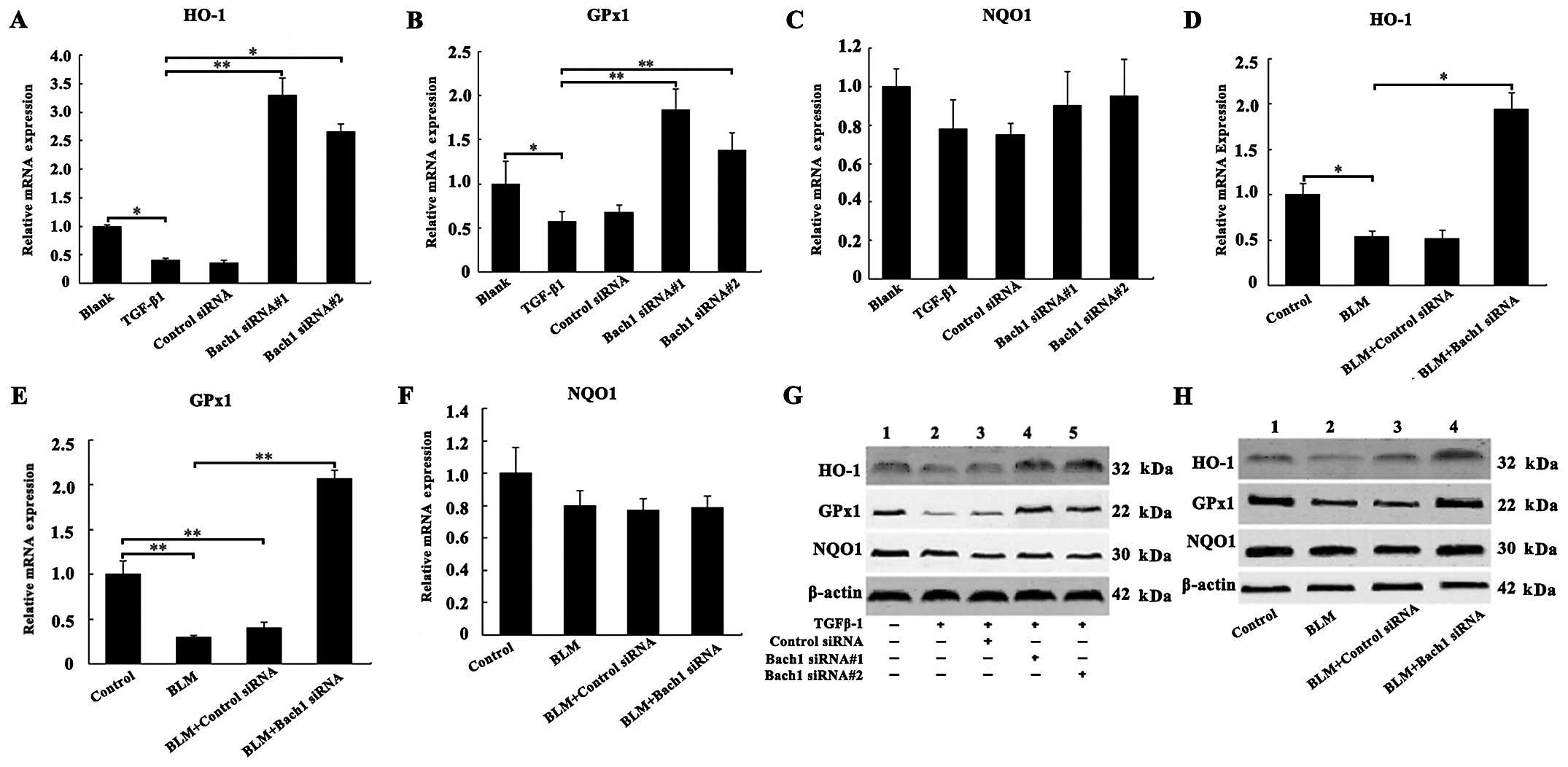
Effects of Bach1 knockdown on the
expression of antioxidant genes and proteins
To examine the mechanisms through which Bach1 siRNA
affects the expression of antioxidant factors, such as HO-1, GPx1
and NQO1, we examined their mRNA and protein expression levels in
the MLFs and lungs of mice. The results of RT-qPCR revealed that
the mRNA levels of HO-1 and GPx1 were decreased following exposure
of the MLFs to TGF-β1 compared with the blank group (both
P<0.05; Fig. 3A and B), and
the knockdown of Bach1 significantly increased the HO-1 and GPx1
mRNA levels compared with the TGF-β1 group (P<0.01; Fig. 3A and B). The effect of Bach1
siRNA#1 was more prominent than that of Bach1 siRNA#2 in the MLFs.
Furthermore, as shown in Fig. 3D and
E, we observed a significant increase in the mRNA levels of
HO-1 and GPx1 in the lung tissues of mice following treatment with
Bach1 siRNA compared with the BLM group (P<0.05 and P<0.01).
Concomitantly, the knockdown of Bach1 significantly increased the
protein levels of HO-1 and GPx1 in the MLFs in the blank group or
TGF-β1 group, and in the lung tissues from mice in the control
group or BLM group (Fig. 3G and
H). However, Bach1 siRNA did not alter the mRNA and protein
levels of NQO1 neither in the MLFs nor in the lung tissues
(Fig. 3C and F). The results of
protein expression were similar to those of mRNA expression. All
these results suggest that Bach1 siRNA promotes the generation of
oxidation resistance factors, and it may thus inhibit the oxidative
stress induced by TGF-β1 and BLM.
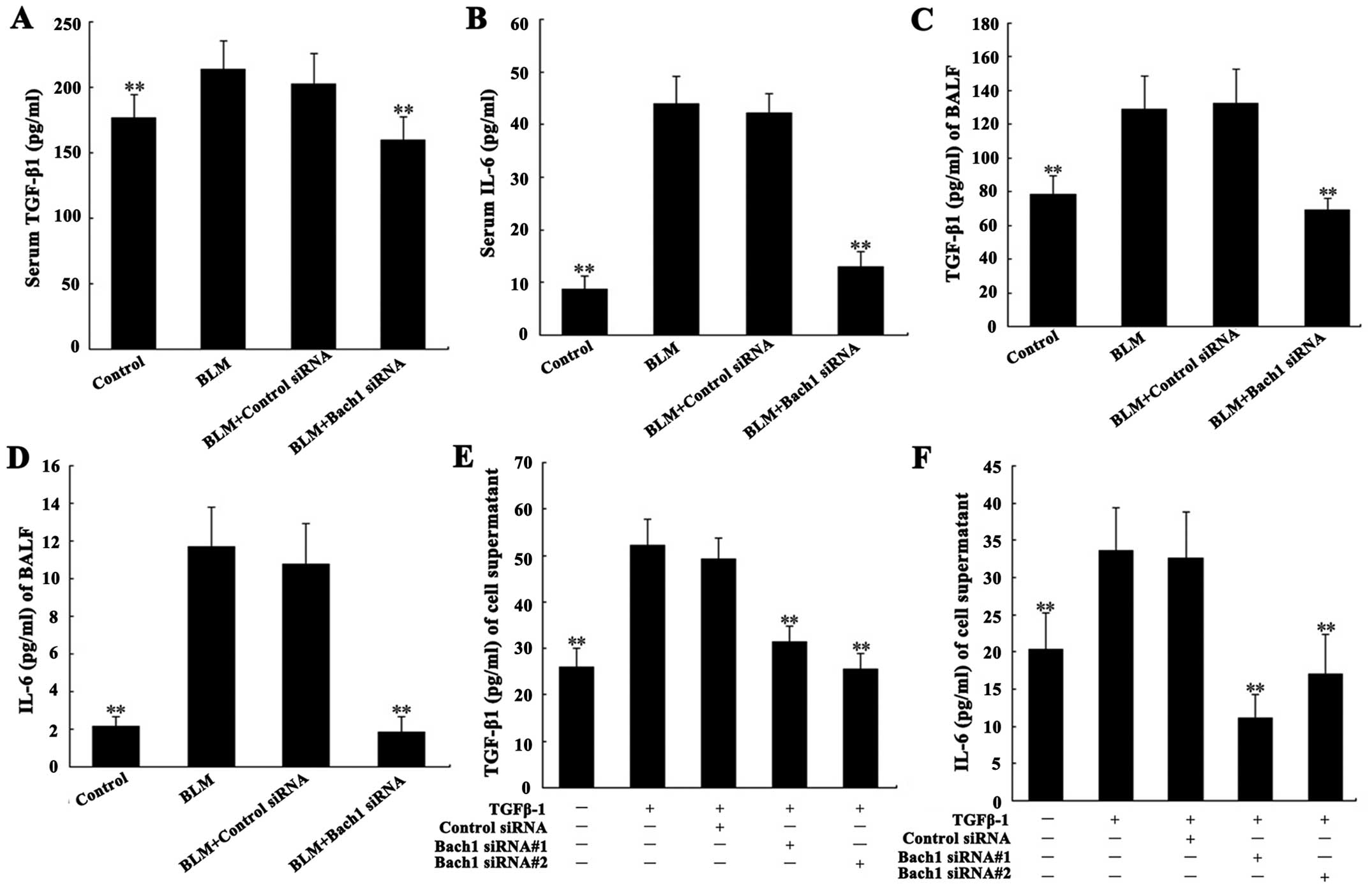
Effects of Bach1 siRNA on the expression
of fibrosis-related cytokines
Due to the important role of TGF-β1 and IL-6 in the
pathogenesis of PF, we analyzed their concentrations in the
supernatant of MLFs, and in serum and BALF from mice. The levels of
TGF-β1 and IL-6 in serum and BALF from mice with BLM-induced lung
fibrosis were significantly increased compared with the control
group (both P<0.01; Fig.
4A–D). Their concentrations in the supernatant of the MLFs
following stimulation with TGF-β1 were significantly increased
compared with the blank group (both P<0.01; Fig. 4E and F). Additionally, the levels
of IL-6 and TGF-β1 in serum and BALF from the mice in the Bach1
siRNA group were significantly decreased compared with those of the
mice BLM treated with control siRNA or in the control group (both
P<0.01; Fig. 4A–D). The
results of the analysis of the expression of IL-6 and TGF-β1 in the
supernatant of MLFs were similar to those obtained from the serum
and BALF of mice. These results suggest that the use of Bach1 siRNA
suppresses the expression of TGF-β1 and that of related cytokines
in BLM-induced fibrosis.
Effects of Bach1 siRNA on
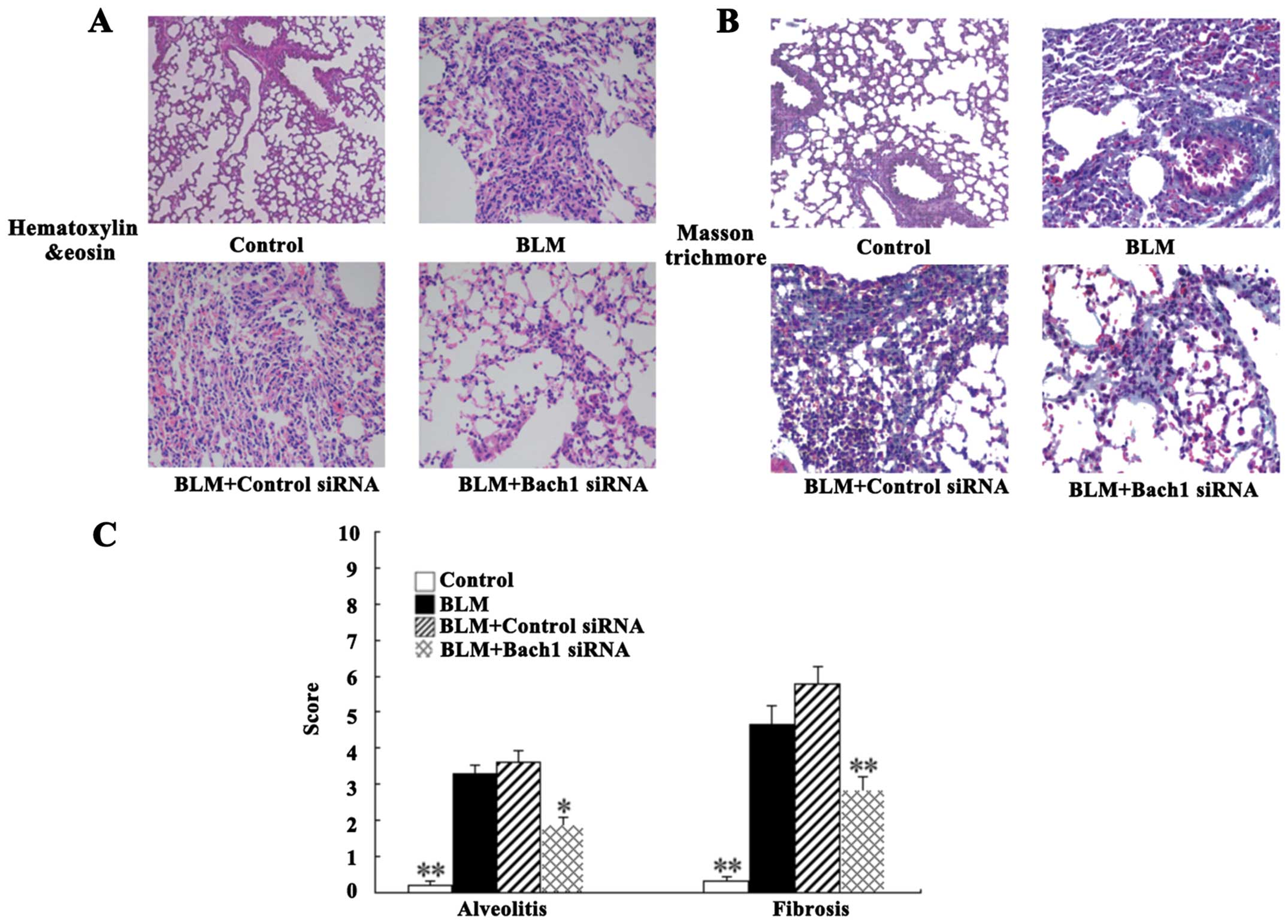
histopathological changes in mice with BLM-induced fibrosis
For histological analysis, the lung tissues of mice
were stained with H&E and Masson's staining. The degree of lung
fibrosis was accessed by alveolitis, fibering and the integrity of
the structure. Intact alveoli, a normal interstitium and a few
inflammatory cells in the lung tissues were observed in the control
group (Fig. 5A). Nevertheless,
H&E staining of the BLM-adminstered mice (at 28 days) revealed
the extensive destruction of alveoli (collapsed and disappeared),
the extensive thickening of the lung interstitium, peribronchial
and interstitial infiltrations of inflammatory cells, predominant
lymphocytes, and multiple focal fibrotic lesions. There were milder
inflammatory infiltrations and the destruction of alveoli following
treatment with Bach1 siRNA compared with the BLM group and control
siRNA group (Fig. 5A). As shown
by Masson's trichrome staining, we observed massive fibrosis (blue
dye), the accumulation of inflammatory cells and the extensive
destruction of alveoli in the BLM group (Fig. 5B). The same change was detected in
the control siRNA group. However, treatment with Bach1 siRNA
significantly attenuated these fibrosis-related changes in the mice
with BLM-induced fibrosis (Fig.
5B).
The alveolitis and fibrosis scores indicated that
the mice in the Bach1 siRNA group had significantly lower
alveolitis and fibrosis scores than those in the BLM group and
control siRNA group (P<0.05 and P<0.01; Fig. 5C). Therefore, treatment with Bach1
siRNA potentially inhibits the histopathological progress of
BLM-induced lung fibrosis in mice.
Discussion
Oxidative stress is considered a prominent mechanism
associated with the pathogenesis of PF (30,31). The decline in antioxidant capacity
and the elevated oxidant burden contribute to the progression of
lung fibrosis via the activation of inflammation and growth
regulatory cytokines, the regulation of related enzymes essential
to the induction of antioxidants, and the stimulation of the
production of myofibroblasts and ECM (32). Bach1 is involved in the induction
of oxidative stress by competing with Nrf2 and negatively
regulating ARE-mediated gene expression (33,34). Previous studies have demonstrated
the importance of Nrf2 and its antioxidant pathway in PF (12–14), suggesting that Bach1 may play a
potential role in the pathogenesis of the disease. In the present
study, we demonstrated that the expression of Bach1 was markedly
increased in both MLF following TGF-β1 stimulation and in the lung
tissues of mice with BLM-induced fibrosis. It has been established
that fibroblasts are associated with PF due to the conversion from
fibroblasts into myofibroblasts, which is one of the principal
characteristics of the pathogenesis of PF (35). TGF-β1 stimulation results in the
proliferation and differentiation of fibroblasts, and in the
increase of matrix synthesis in the lungs related to the subsequent
development of PF (36). The use
of BLM results in the elevation of TGF-β1 in fibroblasts, advanced
inflammation and epithelial-mesenchymal transition (EMT),
subsequent progressive fibrosis, and the exacerbated destruction of
pulmonary structure in animal models (37). Furthermore, the model of
BLM-induced lung fibrosis is rather an acute lung injury with major
oxidative and inflammatory responses (18). The results of the present study
supported our hypothesis that the overexpression of Bach1 may be
associated with the pathogenesis of PF by affecting the
antioxidant/oxidant balance.
To suppress a target gene, siRNA is considered a
powerful tool due to the same effects as a knockout gene (38). Our data demonstrated that two
adenovirus-mediated Bach1 siRNAs were successfully transfected into
MLFs and specifically targeted the lungs of mice by custom
designing. More importantly, adenovirus-mediated siRNAs effectively
suppressed Bach1 expression in MLFs following TGF-β1 stimulation.
For in vivo experiments, the superior Bach1 siRNA was
systemically administered to mice with lung fibrosis to silence
Bach1, and the evidence suggested that the mRNA and protein
expression levels of Bach1 were markedly inhibited in the lung
tissues of mcie with BLM-induced fibrosis.
A key mechanism in the cellular defense against
oxidative stress is mediated by the transcriptional induction of
ARE-driven genes that include stress-response genes (e.g., HO-1),
direct antioxidants (e.g., GPx) and phase 2 detoxifying enzymes
(e.g., NQO1) (13). Nrf2 forms a
heterodimer with basic-region leucine zipper (bZIP) transcription
factors for ARE binding and ARE-driven gene transcription that may
be critical in pulmonary protection (13). Bach1 binds to ARE-like sequences,
functioning as a competitive antagonist of Nrf2, thus inhibiting
the transcriptional activation of ARE-driven genes (8). The inhibition of Nrf2 and ARE-driven
antioxidants enhances the oxidative burden and has been implicated
in the pathogenesis of PF (39).
The altered expression of ARE-driven antioxidants in PF also
suggests that oxidative stress may contribute to pathogenesis of
PF. Among these ARE-driven antioxidants, GPx has been regarded as
the direct antioxidant whose functional roles in oxidative tissue
stress have been widely defined (40,41). The phase 2 detoxifying enzyme,
NQO1, contributes to facilitate the excretion of oxidized, reactive
secondary metabolites via xenobiotic detoxification (42). In addition, the stress-response
protein, HO-1, protects cells from various oxidant insults
(39). A previous study
demonstrated that the deficiency of ARE antioxidative signaling in
mice exacerbates BLM-induced lung injury and fibrosis (15). It has been found that GPx activity
and GPx1 expression are decreased in C57BL/6 mice with BLM-induced
fibrosis (17). The
downregulation of NQO1 and HO-1 have been observed in the lung
tissues of BLM-treated animals (43). Furthermore, Nrf2 siRNA has been
shown to decrease NQO1 and HO-1 mRNA expression in fibroblasts, and
induces the conversion from fibroblasts to myofibroblasts (44). As previously described, our
results demonstrated that the downregulation of antioxidants, such
as HO-1 and GPx1 was observed in MLFs following TGF-β1 stimulation
and in the lungs of C57BL/6 mice following exposure to BLM. We also
investigated whether Bach1 siRNA regulates ARE-driven antioxidants
involved in lung fibrosis. Whether Bach1 affects the expression of
other ARE-dependent genes remains controversial. Previous studies
have reported that Bach1 represses the expression of ARE-dependent
genes, such as HO-1 and NQO1 (33,45). However, in studies in which the
effects of Bach1 siRNA on ARE-dependent gene expression were
examined by microarray analysis or qPCR, little or no effect on the
expression of genes other than HO-1 was observed (46,47). In this study, our data
demonstrated that the suppression of HO-1 and GPx1 in TGF-β1 and
BLM-induced lung fibrosis was alleviated by Bach1 siRNA; however,
no effect was observed on the expression of NQO1, thereby
indicating that Bach1 siRNA participates in the mechanism of
protection in TGF-β1 and BLM-mediated oxidative stress in PF. The
complete mechanisms involved are not known, although it has been
postulated that Bach1 is involved in regulating the expression of
antioxidant genes. The present study further supports the notion
that Bach1 siRNA alleviates fibrotic processes in pulmonary
tissues.
There are several growth factors and fibrogenic
cytokines contributing to lung fibrosis, including TGF-β1 and IL-6
(21). These molecules result in
the proliferation and differentiation of lung fibroblasts that are
responsible for remodeling of the ECM (23). Choe et al (23) demonstrated that TGF-β1 and IL-6
were upregulated in BALF and serum following the exposure of mice
to BLM. In this study, we provide compelling evidence that BLM
induced a marked increase in the levels of TGF-β1 and IL-6 in both
BALF and serum of mice. By contrast, Bach1 siRNA inhibited the
expression of fibrosis-related cytokines, such as TGF-β1 and IL-6
in the serum and BALF of mice with BLM-induced fibrosis. Moreover,
the elevation of TGF-β1 and IL-6 expression was also found in MLFs
following TGF-β1 stimulation, and Bach1 siRNA significantly
decreased the expression of these two cytokines. To further explore
whether Bach1 siRNA exerted anti-fibrotic effects on lung fibrosis,
we determined the pathological changes in lung tissues. The results
revealed that the administration of BLM led to the thickness of
alveolar septa, narrowing of the alveolar space, the extensive
infiltration of inflammatory cells, and the accumulation of
collagen deposition in the lungs, which is consistent with results
of previous studies (48,49). In addition, Bach1 siRNA markedly
attenuated inflammatory cell infiltration, the destruction of
alveoli and the accumulation of collagen deposition. On the whole,
our findings demonstrated that the silencing of Bach1 attenuated
the histopathological changes in the lung tissues from mice with
BLM-induced fibrosis and suppressed the expression of
fibrosis-related cytokines. On the basis of these findings, we
believe that Bach1 represents a crucial molecule in the
pathogenesis of PF, thereby highlighting Bach1 as a potential
therapeutic target in PF.
In conclusion, the present study demonstrated that
the silencing Bach1 inhibited TGF-β1 and BLM-induced PF. This
anti-fibrotic effect was associated with the expression of
antioxidants and the regulation of antioxidant capabilities. Bach1
siRNA may play an important role in the pathogenesis of PF and may
have potential for use in the treatment of lung fibrosis. Further
studies on the role of Bach1 may provide further insight into the
mechanisms responsible for the development of PF.
Acknowledgments
This study was supported by the Project of the
National Natural Science Foundation of China in 2014 [project name:
The role and mechanism of the LOXL2 in the pulmonary fibrosis of
the connective tissue disease (no. 81471616)].
References
|
1
|
Gross TJ and Hunninghake GW: Idiopathic
pulmonary fibrosis. N Engl J Med. 345:517–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kliment CR, Englert JM, Gochuico BR, Yu G,
Kaminski N, Rosas I and Oury TD: Oxidative stress alters syndecan-1
distribution in lungs with pulmonary fibrosis. J Biol Chem.
284:3537–3545. 2009. View Article : Google Scholar :
|
|
3
|
Kaya V, Yazkan R, Yıldırım M, Doğuç DK,
Süren D, Bozkurt KK, Yüksel Ö, Demırpence Ö, Şen CA and Yalçın AY:
The relation of radiation-induced pulmonary fibrosis with stress
and the efficiency of antioxidant treatment: an experimental study.
Med Sci Monit. 20:290–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuzawa Y, Kawashima T, Kuwabara R,
Hayakawa S, Irie T, Yoshida T, Rikitake H, Wakabayashi T, Okada N,
Kawashima K, et al: Change in serum marker of oxidative stress in
the progression of idiopathic pulmonary fibrosis. Pulm Pharmacol
Ther. 32:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teixeira KC, Soares FS, Rocha LG, Silveira
PC, Silva LA, Valença SS, Dal Pizzol F, Streck EL and Pinho RA:
Attenuation of bleomycin-induced lung injury and oxidative stress
by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther.
21:309–316. 2008. View Article : Google Scholar
|
|
6
|
Serrano-Mollar A, Closa D, Prats N, Blesa
S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ and Bulbena
O: In vivo antioxidant treatment protects against bleomycin-induced
lung damage in rats. Br J Pharmacol. 138:1037–1048. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa M, Numazawa S and Yoshida T:
Redox regulation of the transcriptional repressor Bach1. Free
Radical Biol Med. 38:1344–1352. 2005. View Article : Google Scholar
|
|
8
|
Jyrkkänen HK, Kuosmanen S, Heinäniemi M,
Laitinen H, Kansanen E, Mella-Aho E, Leinonen H, Ylä-Herttuala S
and Levonen AL: Novel insights into the regulation of
antioxidant-response-element-mediated gene expression by
electrophiles: induction of the transcriptional repressor BACH1 by
Nrf2. Biochem J. 440:167–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Igarashi K and Sun J: The heme-Bach1
pathway in the regulation of oxidative stress response and
erythroid differentiation. Antioxid Redox Signal. 8:107–118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itoh K, Tong KI and Yamamoto M: Molecular
mechanism activating Nrf2-Keap1 pathway in regulation of adaptive
response to electrophiles. Free Radic Biol Med. 36:1208–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giudice A, Arra C and Turco MC: Review of
molecular mechanisms involved in the activation of the Nrf2-ARE
signaling pathway by chemopreventive agents. Methods Mol Biol.
647:37–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zucker SN, Fink EE, Bagati A, Mannava S,
Bianchi-Smiraglia A, Bogner PN, Wawrzyniak JA, Foley C, Leonova KI,
Grimm MJ, et al: Nrf2 amplifies oxidative stress via induction of
Klf9. Mol Cell. 53:916–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho HY, Reddy SP and Kleeberger SR: Nrf2
defends the lung from oxidative stress. Antioxid Redox Signal.
8:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kikuchi N, Ishii Y, Morishima Y, Yageta Y,
Haraguchi N, Itoh K, Yamamoto M and Hizawa N: Nrf2 protects against
pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2
balance. Respir Res. 11:312010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho HY, Reddy SP, Yamamoto M and
Kleeberger SR: The transcription factor NRF2 protects against
pulmonary fibrosis. FASEB J. 18:1258–1260. 2004.PubMed/NCBI
|
|
16
|
Tsuburai T, Suzuki M, Nagashima Y, Suzuki
S, Inoue S, Hasiba T, Ueda A, Ikehara K, Matsuse T and Ishigatsubo
Y: Adenovirus-mediated transfer and overexpression of heme
oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary
fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther.
13:1945–1960. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santos-Silva MA, Pires KM, Trajano ET,
Martins V, Nesi RT, Benjamin CF, Caetano MS, Sternberg C, Machado
MN, Zin WA, et al: Redox imbalance and pulmonary function in
bleomycin-induced fibrosis in C57BL/6, DBA/2, and BALB/c mice.
Toxicol Pathol. 40:731–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moeller A, Ask K, Warburton D, Gauldie J
and Kolb M: The bleomycin animal model: a useful tool to
investigate treatment options for idiopathic pulmonary fibrosis?
Int J Biochem Cell Biol. 40:362–382. 2008. View Article : Google Scholar
|
|
19
|
Moore BB and Hogaboam CM: Murine models of
pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
294:L152–L160. 2008. View Article : Google Scholar
|
|
20
|
B Moore B, Lawson WE, Oury TD, Sisson TH,
Raghavendran K and Hogaboam CM: Animal models of fibrotic lung
disease. Am J Respir Cell Mol Biol. 49:167–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida M, Sakuma J, Hayashi S, Abe K,
Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N and Kaneda
Y: A histologically distinctive interstitial pneumonia induced by
overexpression of the interleukin 6, transforming growth factor
beta 1, or platelet-derived growth factor B gene. Proc Natl Acad
Sci USA. 92:9570–9574. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Selman M and Pardo A: Role of epithelial
cells in idiopathic pulmonary fibrosis: from innocent targets to
serial killers. Proc Am Thorac Soc. 3:364–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choe JY, Jung HJ, Park KY, Kum YS, Song
GG, Hyun DS, Park SH and Kim SK: Anti-fibrotic effect of
thalidomide through inhibiting TGF-beta-induced ERK1/2 pathways in
bleomycin-induced lung fibrosis in mice. Inflamm Res. 59:177–188.
2010. View Article : Google Scholar
|
|
24
|
Xu YD, Hua J, Mui A, O'Connor R,
Grotendorst G and Khalil N: Release of biologically active
TGF-beta1 by alveolar epithelial cells results in pulmonary
fibrosis. Am J Physiol Lung Cell Mol Physiol. 285:L527–L539. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CM, Park JW, Cho WK, Zhou Y, Han B,
Yoon PO, Chae J, Elias JA and Lee CG: Modifiers of TGF-β1 effector
function as novel therapeutic targets of pulmonary fibrosis. Korean
J Intern Med. 29:281–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pecot CV, Calin GA, Coleman RL,
Lopez-Berestein G and Sood AK: RNA interference in the clinic:
challenges and future directions. Nat Rev Cancer. 11:59–67. 2011.
View Article : Google Scholar :
|
|
27
|
Zhao Y, Li H, Wu R, Li S, Wang P, Wang H,
Wang J and Zhou J: Antitumor effects of oncolytic
adenovirus-carrying siRNA targeting potential oncogene EphA3. PLoS
One. 10:e01267262015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
29
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kinnula VL and Myllärniemi M:
Oxidant-antioxidant imbalance as a potential contributor to the
progression of human pulmonary fibrosis. Antioxid Redox Signal.
10:727–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andersson-Sjöland A, Karlsson JC and
Rydell-Törmänen K: ROS-induced endothelial stress contributes to
pulmonary fibrosis through pericytes and Wnt signaling. Lab Invest.
96:206–217. 2016. View Article : Google Scholar
|
|
32
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: a possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhakshinamoorthy S, Jain AK, Bloom DA and
Jaiswal AK: Bach1 competes with Nrf2 leading to negative regulation
of the antioxidant response element (ARE)-mediated NAD(P)H:quinone
oxidoreductase 1 gene expression and induction in response to
antioxidants. J Biol Chem. 280:16891–16900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Warnatz HJ, Schmidt D, Manke T, Piccini I,
Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M,
et al: The BTB and CNC homology 1 (BACH1) target genes are involved
in the oxidative stress response and in control of the cell cycle.
J Biol Chem. 286:23521–23532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: one
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim S, Lim JH and Woo CH: ERK5
inhibition ameliorates pulmonary fibrosis via regulating
Smad5 acetylation. Am J Pathol. 183:1758–1768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Li Y, Wang Y, Xu D, Li C, Liu M, Sun
X and Li Z: Tanshinone IIA attenuates bleomycin-induced pulmonary
fibrosis via modulating angiotensin-converting enzyme
2/angiotensin-(1–7) axis in rats. Int J Med Sci. 11:578–586. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walters DM, Cho HY and Kleeberger SR:
Oxidative stress and antioxidants in the pathogenesis of pulmonary
fibrosis: a potential role for Nrf2. Antioxid Redox Signal.
10:321–332. 2008. View Article : Google Scholar
|
|
40
|
Koo HC, Davis JM, Li Y, Hatzis D, Opsimos
H, Pollack S, Strayer MS, Ballard PL and Kazzaz JA: Effects of
transgene expression of superoxide dismutase and glutathione
peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J
Physiol Lung Cell Mol Physiol. 288:L718–L726. 2005. View Article : Google Scholar
|
|
41
|
Reddy NM, Kleeberger SR, Cho HY, Yamamoto
M, Kensler TW, Biswal S and Reddy SP: Deficiency in Nrf2-GSH
signaling impairs type II cell growth and enhances sensitivity to
oxidants. Am J Respir Cell Mol Biol. 37:3–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holtzclaw WD, Dinkova-Kostova AT and
Talalay P: Protection against electrophile and oxidative stress by
induction of phase 2 genes: the quest for the elusive sensor that
responds to inducers. Adv Enzyme Regul. 44:335–367. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ni S, Wang D, Qiu X, Pang L, Song Z and
Guo K: Bone marrow mesenchymal stem cells protect against
bleomycin-induced pulmonary fibrosis in rat by activating Nrf2
signaling. Int J Clin Exp Pathol. 8:7752–7761. 2015.PubMed/NCBI
|
|
44
|
Artaud-Macari E, Goven D, Brayer S, Hamimi
A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Römer S,
Boutten A and Bonay M: Nuclear factor erythroid 2-related factor 2
nuclear translocation induces myofibroblastic dedifferentiation in
idiopathic pulmonary fibrosis. Antioxid Redox Signal. 18:66–79.
2013. View Article : Google Scholar
|
|
45
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: from basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reichard JF, Sartor MA and Puga A: BACH1
is a specific repressor of HMOX1 that is inactivated by arsenite. J
Biol Chem. 283:22363–22370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
MacLeod AK, McMahon M, Plummer SM, Higgins
LG, Penning TM, Igarashi K and Hayes JD: Characterization of the
cancer chemopreventive NRF2-dependent gene battery in human
keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not
the BACH1-NRF2 pathway, controls cytoprotection against
electrophiles as well as redox-cycling compounds. Carcinogenesis.
30:1571–1580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cui K, Kou JQ, Gu JH, Han R, Wang G, Zhen
X and Qin ZH: Naja naja atra venom ameliorates pulmonary fibrosis
by inhibiting inflammatory response and oxidative stress. BMC
Complement Altern Med. 14:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao R, Cao Y, He YR, Lau WB, Zeng Z and
Liang ZA: Adiponectin attenuates lung fibroblasts activation and
pulmonary fibrosis induced by paraquat. PLoS One. 10:e01251692015.
View Article : Google Scholar : PubMed/NCBI
|