Introduction
Inhaled β2-adrenoceptor agonists are among the most
effective and safest bronchodilators currently available.
Epinephrine (EPI), a β2-adrenergic receptor agonist, relaxes
bronchial smooth muscle and is used widely to treat asthma.
Endogenous EPI levels are low in patients with asthma, which may
explain why EPI delivery is such an effective treatment (1,2).
In our previous studies involving the induction of adrenal
medullary chromaffin cell (AMCC) transformation into neurons, we
observed decreased EPI levels and found that this phenomenon was
inhibited by administration of the anti-nerve growth factor
(anti-NGF) antibody (3,4). Thus, transformation of AMCCs into
neurons reduces EPI synthesis and induces bronchoconstriction in
asthma, with reduced phenylethanolamine N-methyltransferase (PNMT)
expression in the neurons. Of course, both EPI synthesis and
release determine serum EPI concentrations, and little is currently
known concerning EPI release in asthma patients.
The adrenomedullary hormonal system plays an
important role in the regulation of bronchial dilatation via
secretion of EPI (5). At the
adrenomedullary synapses, neuron terminals release the classical
transmitter acetylcholine. Acetylcholine interacts with nicotinic
acetylcholine receptors (nAChRs) on the adrenal medulla to induce
elevation of the intracellular calcium concentration
([Ca2+]i) (6) and then secretion of EPI (7). In this process, the
[Ca2+]i directly influences EPI secretion
from the adrenal medulla, and the [Ca2+]i is
dependent on the regulation of nAChRs. Pentameric combinations of
homologous nAChRs are formed by the α2–10 and β2–4 subunits, and
differential associations of these subunits confer distinct
functional and structural properties (6,8).
Heteromeric nAChRs consisting of α- and β-subunits have a
fractional Ca2+ current (7), whereas nAChRs containing the α7
subunit have the highest fractional Ca2+ current among
all nAChRs (9).
Redistribution of nAChR subunits in PC12 cells in
response to exposure to nicotine, NGF and hypoxia has been
demonstrated within only a couple of hours following treatment
(9–11). The redistribution did not change
the total number of receptors, but instead changed the proportions
of different receptor subunits (12). Based on these previous
demonstrations of nAChR subunit redistribution and increased
expression of the α7 nAChR subunit in neuronal cells exposed to
various stress (9,11), we hypothesized that redistribution
of adrenomedullary nAChRs occurs in asthma, with increased
expression of the α7 subunit, and that this phenomenon influences
EPI secretion. To test this hypothesis, we employed a mouse model
of ovalbumin (OVA)-induced asthma to determine whether the adrenal
medulla of these mice exhibits redistribution of nAChRs. We then
investigated the effects of an agonist and an antagonist of the α7
nAChR subunit on EPI secretion and asthma symptoms in mice with
asthma.
Materials and methods
Animals and treatments
Conventionally bred 6- to 8-week-old female C57BL/6
mice (Experimental Animal Center of Central South University,
Changsha, China) were used in all animal experiments. This study
was carried out in strict accordance with the recommendations from
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (Bethesda, MD, USA). The study
protocol was approved by the Ethics Committee of the Asthma
Research Institute (Hunan, China) (20100606).
For the first set of experiments, 32 mice were
divided among the following 4 groups (n=8/group) using a random
number table: a control group injected with saline (control group);
a control group treated with anti-NGF only (anti-NGF group); a
group sensitized by chicken OVA (asthma group); and a group
sensitized by OVA and treated with anti-NGF (asthma + anti-NGF
group). The mice in the asthma group and asthma + anti-NGF group
were sensitized intraperitoneally (i.p.) with 0.2 ml of a mixture
containing 50 µg OVA (grade V) and 2 mg aluminum hydroxide
(Sigma-Aldrich, St. Louis, MO, USA) in sterile saline on days 1 and
14. Beginning on day 21, the asthma group and asthma + anti-NGF
group were exposed to 1% OVA (w/v) aerosol for 30 min every day
from day 21 to 23 and treated with 1.0 mg/kg anti-NGF (i.p.) for 60
min before each aerosol treatment. The control group and anti-NGF
group received aerosol treatment with sterile saline only.
For the second set of experiments, 240 mice were
divided into two groups (n=120/group) using a random number table:
an original control group injected with saline (control O group);
and a group sensitized with OVA (OVA group). The mice in the OVA
group were sensitized i.p. with 0.2 ml of a mixture of 50 µg
OVA (grade V) and 2 mg aluminum hydroxide in sterile saline on days
1 and 14. Beginning on day 21, the OVA group was exposed to 1% OVA
(w/v) aerosol for 30 min every day from day 21 to 23. The control O
group received aerosol treatment with sterile saline only. Then the
OVA group and the control O group were divided into three subgroups
(n=40/group) each using a random number table. Mice in the OVA
group were divided into: i) a group sensitized with OVA and treated
with methyllycaconitine (MLA; Sigma-Aldrich) (asthma + MLA group);
ii) a group sensitized with OVA and given saline (asthma + saline
group); and iii) a group sensitized with OVA and trea ted with
PNU-282987 (Sigma-Aldrich) (asthma + PNU group). Mice in the
control O group were divided into: iv) a control group given saline
only (control 2 group); v) a group treated with MLA but not
sensitized with OVA (MLA group); and vi) a group treated with
PNU-282987 but not sensitized with OVA (PNU group). Mice were
treated with MLA or PNU-282987 at 30 min after the last aerosol
treatment, and then we measured bronchial responsiveness of the
mice chosen using a random number table. At the same time, we
sacrificed five mice in each group at 30, 35, 40, 45, 50, 55 and 60
min after MLA or PNU-282987 treatment (5 mice per group per time
selected using a random number table), because excessive blood
draws would interfere with the experiment.
Measurement of bronchial
responsiveness
The airway responsiveness to methacholine was
measured at 1 h after the final OVA challenge using a whole-body
plethysmography device (model PLY 3211; Buxco Electronics, Inc.,
Wilmington, NC, USA). The airway resistance (RL) of individual mice
was calculated by dividing the driving pressure by the rate of air
flow (P/V). We injected 10 mg/kg MLA i.p. into mice in the asthma +
MLA and MLA groups, 0.4 mg/kg PNU-282987 i.p. into mice in the
asthma + PNU and PNU groups, and saline into mice in the asthma +
saline group and the control 2 group 30 min before measurement of
bronchial responsiveness. After measuring airway responsiveness,
blood samples were collected by cardiac puncture, and the mice were
sacrificed.
Histological analysis
Lung tissues were collected, fixed in 4%
paraformaldehyde, and then embedded in paraffin. Tissue sections (4
µm) were stained with hematoxylin and eosin (H&E), and
morphological changes were observed via light microscopy.
Inflammatory parameters in lung tissue (peribronchial, perivascular
and parenchymal infiltration of inflammatory cells) were evaluated
in a blinded manner by a senior lung pathologist.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the adrenal medulla of mice was
extracted using TRIzol reagent and the SuperScript™ total RNA
isolation kit (both from Invitrogen, Carlsbad, CA, USA), according
to the manufacturer's instructions. The mRNA levels of target gene
transcripts and β-actin, as an internal control, were determined by
quantitative PCR using the SYBR-Green PCR kit in a 7900HR Fast
Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The primer sequences used are listed in Table I. The PCR amplification was
performed at 95°C for 5 min then 42 cycles of 95°C for 30 sec plus
59°C for 30 sec, followed by 72°C for 20 sec and 95°C for 10 sec.
The relative mRNA expression levels of each target gene transcript
compared to those of the control β-actin were analyzed by the
2−ΔΔCt method.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primers | Sequences
(5′→3′) | Amplicon length
(bp) |
|---|
| nAChR-α3-F |
GCTAGCTTAGCTGTGCTTCG | 71 |
| nAChR-α3-R |
GTCTGAAGACCGCATGGACA | |
| nAChR-α4-F |
AGTCGAGACCCAGCCTACAT | 81 |
| nAChR-α4-R |
GGACTGGCCTTCTCAACCTC | |
| nAChR-α5-F |
TTACAATGCCAAGTGATGAC | 124 |
| nAChR-α5-R |
GAGGACTCTGAAGGACAAC | |
| nAChR-α6-F |
CTGCGTCACATCTGGAAG | 185 |
| nAChR-α6-R |
GTTATCACACCGTCATACTTG | |
| nAChR-α7-F |
GTCACCTACACAGTAACCAT | 109 |
| nAChR-α7-R |
CAGGCAGCAAGAATACCA | |
| nAChR-α9-F |
ATTGTCAACCTCCTCATCC | 146 |
| nAChR-α9-R |
ATCTCTGCCACCATTAGC | |
| nAChR-β2-F |
ACTTGTGTTCCCTAGAAGAGCAG | 284 |
| nAChR-β2-R |
TGTCAGTACCCAAAACCCCTG | |
| nAChR-β4-F |
CACCTCCCTTGACATTCCCC | 135 |
| nAChR-β4-R |
CAGGATGCCATGGTGTGAGT | |
| GAL-F |
GCCCACATGCCATTGACAAC | 140 |
| GAL-R |
GCGGACAATGTTGCTCTCAG | |
| PNMT-F |
GTGTCGGGACGGGTTCTCAT | 120 |
| PNMT-R |
GTGTCGGGACGGGTTCTCAT | |
| TH-F |
GTCTACTGTCTGCCCGTGAT | 180 |
| TH-R |
CAATGTCCTGGGAGAACTGG | |
| GAPDH-F |
AGGAGCGAGACCCCACTAAC | 87 |
| GAPDH-R |
CGGAGATGATGACCCTTTTG | |
Western blot analysis
Following homogenization and quantification, the
protein lysates from mouse adrenal medulla (30 µg/lane) were
loaded on 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and then transferred onto
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). The membranes were blocked with 0.05 g/ml skim milk at room
temperature for 2 h before incubation with the primary anti-α7
nAChR antibody (1:1,000; ab10096; Abcam, Cambridge, MA, USA) and
primary anti-β-actin antibody (1:1,000; AM1021B; Abgent, Inc., San
Diego, CA, USA) overnight at 4°C. Protein levels were detected via
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibodies (goat anti-rabbit IgG/HRP; dilution 1:5,000; 074–1506;
KPL, Gaithersburg, MD, USA) and visualized using enhanced
chemiluminescence detection. The relative protein expression levels
and/or phosphorylation levels of target proteins were determined by
intensity analysis using Image Pro-Plus 6.0 software (IPP 6.0;
Media Cybernetics, Inc., Rockville, MD, USA).
Enzyme-linked immunosorbent assay
(ELISA)
EPI and corticosterone protein levels in serum of
the mouse models were quantified using commercial ELISA kits
(Abnova, Taipei, Taiwan), following the manufacturer's
instructions. The intensities were measured at 450 nm using a
spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
and the concentrations of target proteins were calculated and
analyzed. We injected 10 mg/kg MLA i.p. into mice in the asthma +
MLA group and MLA group, 0.4 mg/kg PNU-282987 i.p. into mice in the
asthma + PNU group and PNU group, and saline into mice in the
asthma + saline group and the control 2 group. We then sacrificed
five mice at 30, 35, 40, 45, 50, 55 and 60 min (5 mice per group
per time selected using a random number table).
Statistical analysis
Data are presented as the means ± standard deviation
(SD) values. One-way analysis of variance (ANOVA) was used for
multiple comparisons, followed by the Fisher's protected least
significant difference test. A P-value <0.05 was considered
statistically significant.
Results
α7 nAChR expression is enhanced in the
asthma group
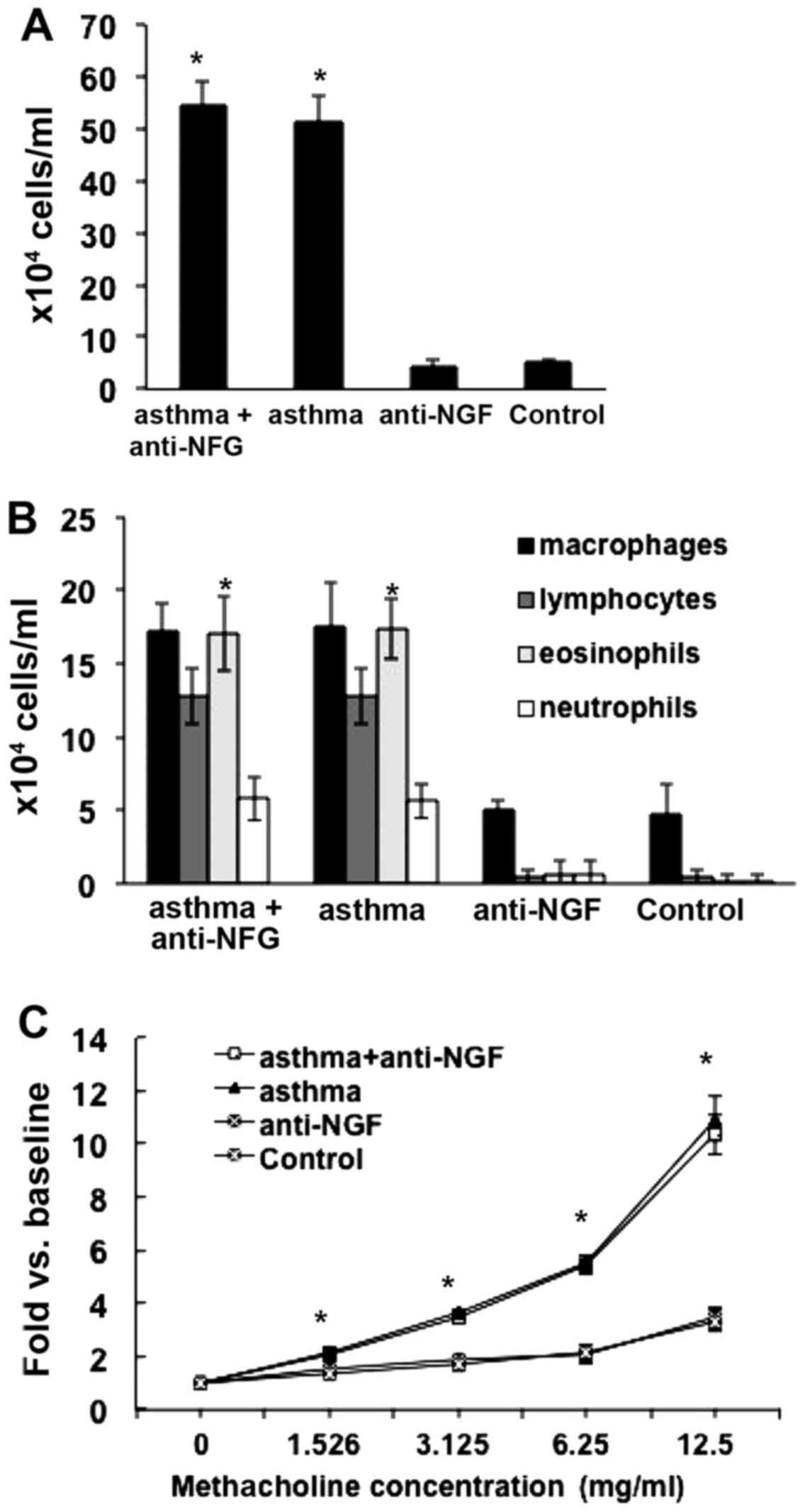
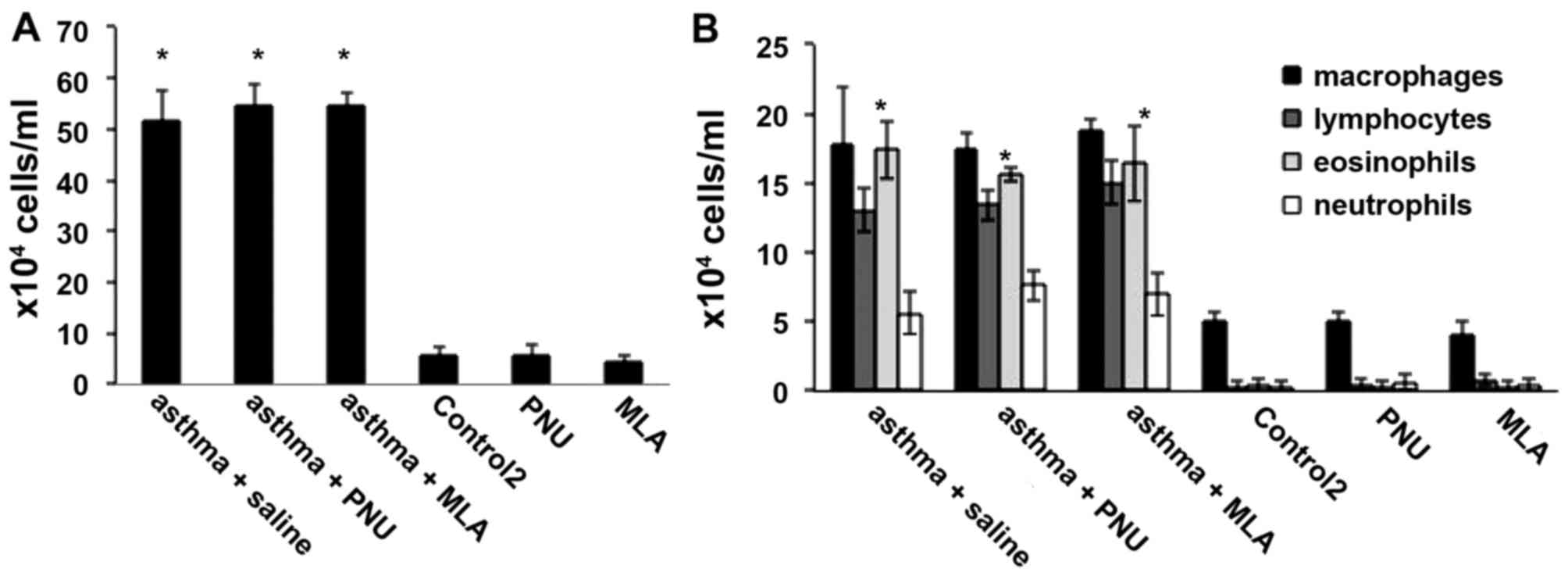
Compared to that in the control group, the total
white cell counts in the bronchoalveolar lavage fluid from mice in
the asthma and asthma + anti-NGF groups were significantly
increased (P<0.05), and eosinophil counts were also
significantly increased (P<0.05; Fig. 1A and B). As shown in Fig. 1C, RL measurements in the asthma
and asthma + anti-NGF groups were significantly higher than those
in the control group (P<0.05) in response to methacholine at a
concentration of 1.526 mg/ml or higher. In addition, as the
concentration of methacholine increased, the increase in RL became
greater. RL measurements in the asthma and asthma + anti-NGF groups
did not differ significantly (P>0.05). However, no significant
differences in cell counts were observed between the asthma and
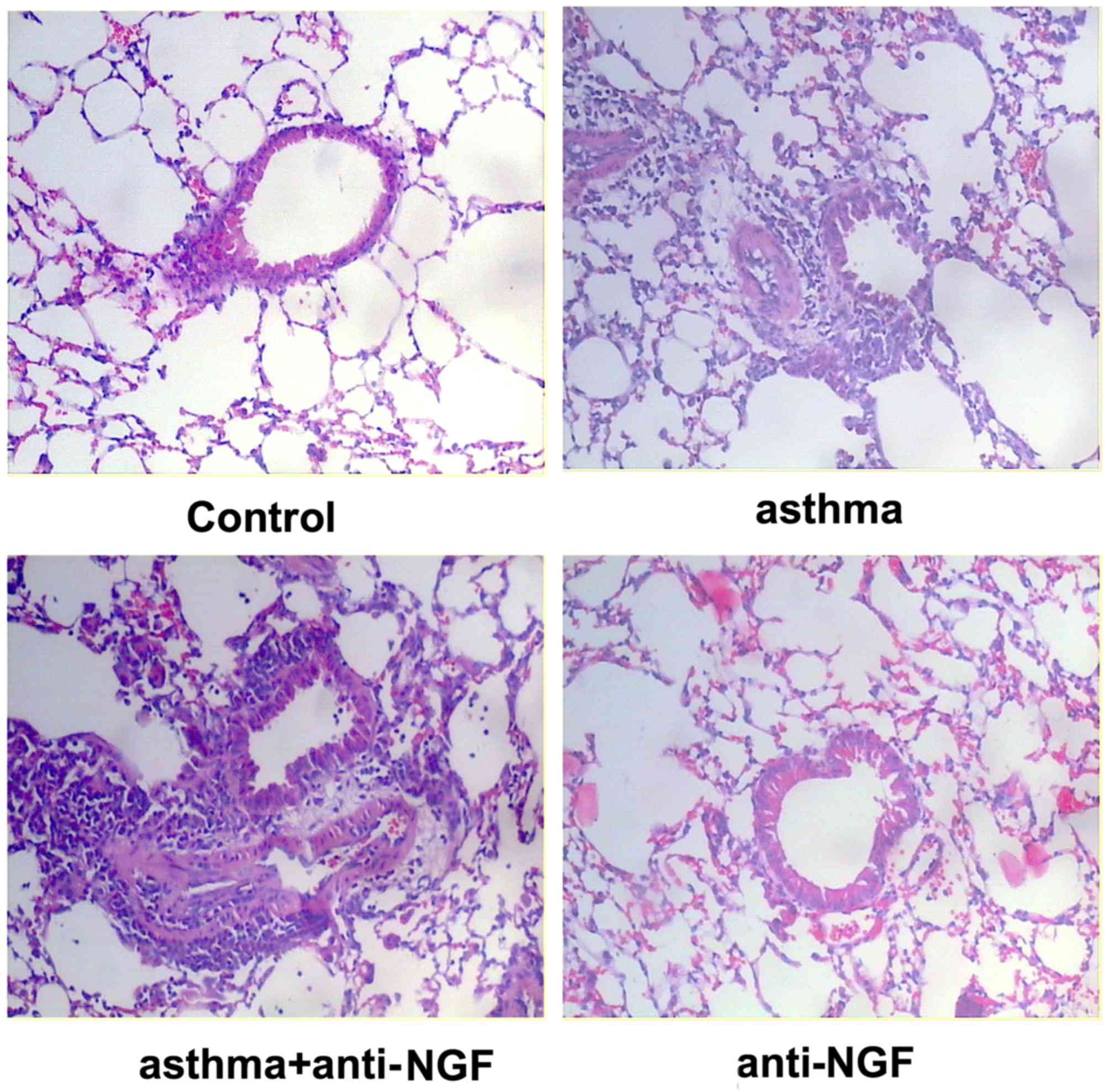
asthma + anti-NGF groups (P>0.05). As shown in Fig. 2, no obvious inflammation,
bronchial smooth muscle thickening, or airway mucus were observed
in lung tissue of the control group. In contrast, the asthma group
and asthma + anti-NGF group displayed considerable muscle
thickening, epithelial edema, and inflammatory cell infiltration.
There were no obvious difference in epithelial edema and
inflammatory cell infiltration between the asthma group and the
asthma + anti-NGF group. Together, these results demonstrate that
we successfully generated a mouse model of asthma.
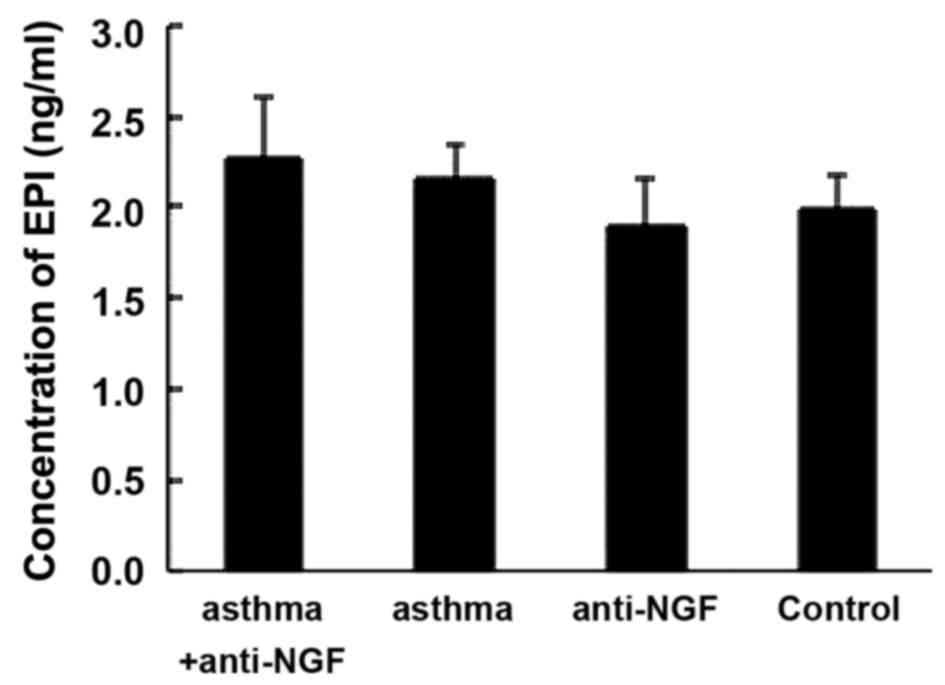
Next we measured serum EPI concentrations by ELISA.
As shown in Fig. 3, EPI
concentrations did not differ between the asthma group and the
control group (P>0.05). Since the body needs much higher than
normal levels of EPI to cope with asthma symptoms (2), the similar EPI levels in these
groups indicate an obvious insufficiency in the OVA group.
Using the established asthma model, we first
evaluated the redistribution of adrenomedullary nAChR subunits in
mice of the asthma group by assessing the expression of all
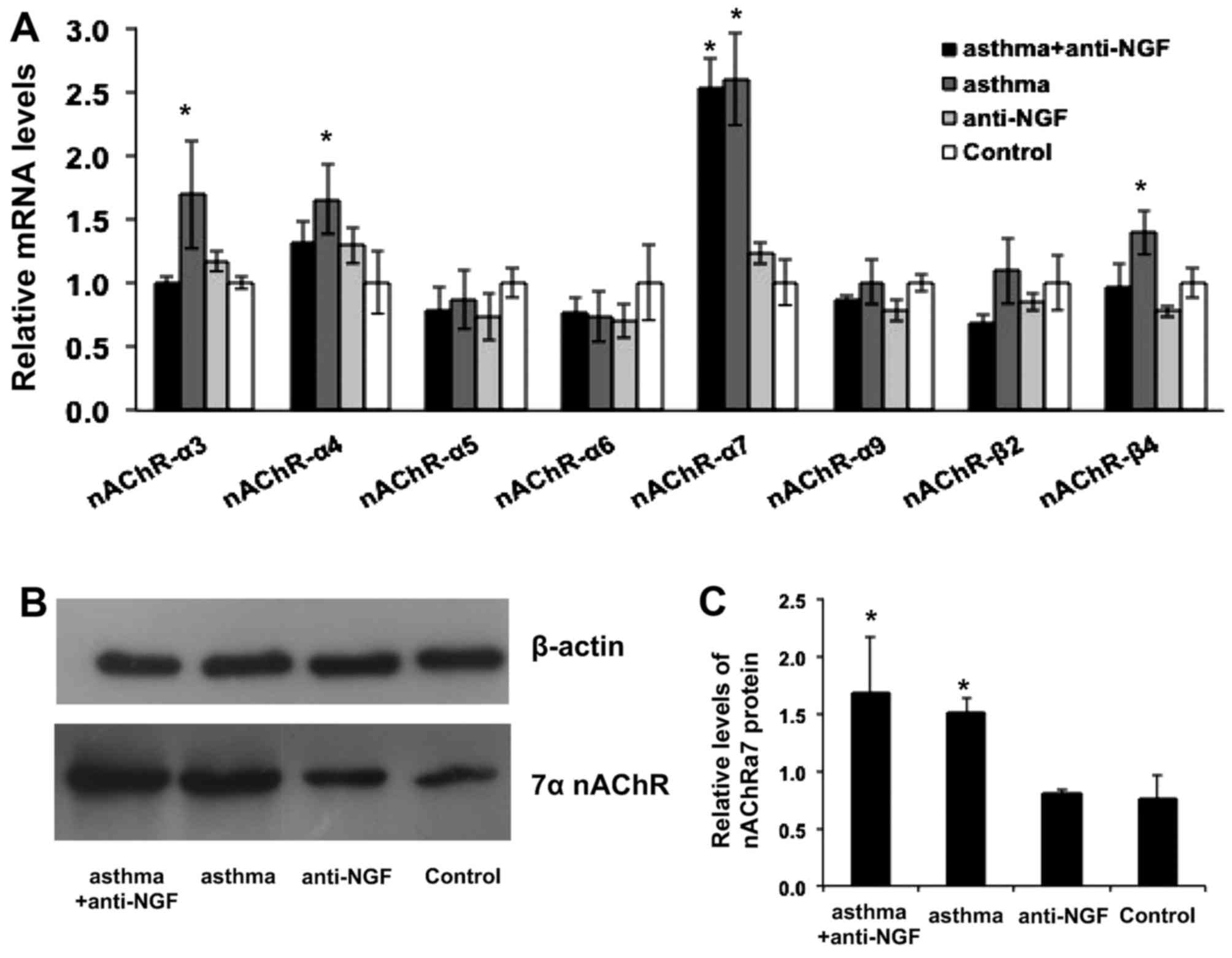
possible subunits including α3–9 and β3–5 (13). As shown in Fig. 4A, the relative expression level of
the α7 nAChR subunit in the asthma group was significantly higher
than that in the control group (P<0.05), which demonstrates that
nAChR subunit redistribution occurred in the asthmatic mice. The
results also showed that the α7 nAChR subunit was associated with
major changes in distribution. The relative level of α7 nAChR
expression was ~2.6-fold higher after the induction of asthma in
these mice. Interestingly, the levels of α3, α4 and β4 subunits in
the asthma group were also relatively enhanced by 69, 66 and 39%,
respectively. We next confirmed our findings by western blot
analysis. We measured the concentration of α7 nAChR protein in each
group separately, as shown in Fig. 4B
and C, and the expression of α7 nAChRs in the asthma group was
significantly higher than that in the control group, consistent
with the results of our PCR analysis. We further measured enzymes
involved in the synthesis of catechol-amine (CA) such as tyrosine
hydroxylase (TH), PNMT, and a neuropeptide referred to as galanin
(GAL) to show that CA synthesis was not related to the nAChR
distribution (14,15).
Effects of agonists and antagonists of α7
nAChR on mice with asthma
To investigate the effect of α7 nAChR overexpression
on circulating EPI levels in mice with asthma, our mouse asthmatic
model was treated with the α7 nAChR agonist PNU or the α7 nAChR
antagonist MLA, individually 30 min before evaluation. First, as
shown in Fig. 5A and B, compared
to that in the control 2 group, the total white cell counts in
bronchoalveolar lavage fluid from mice in the asthma + saline,
asthma + MLA, and asthma + PNU groups were significantly increased
(P<0.05), and eosinophil counts showed significant increases in
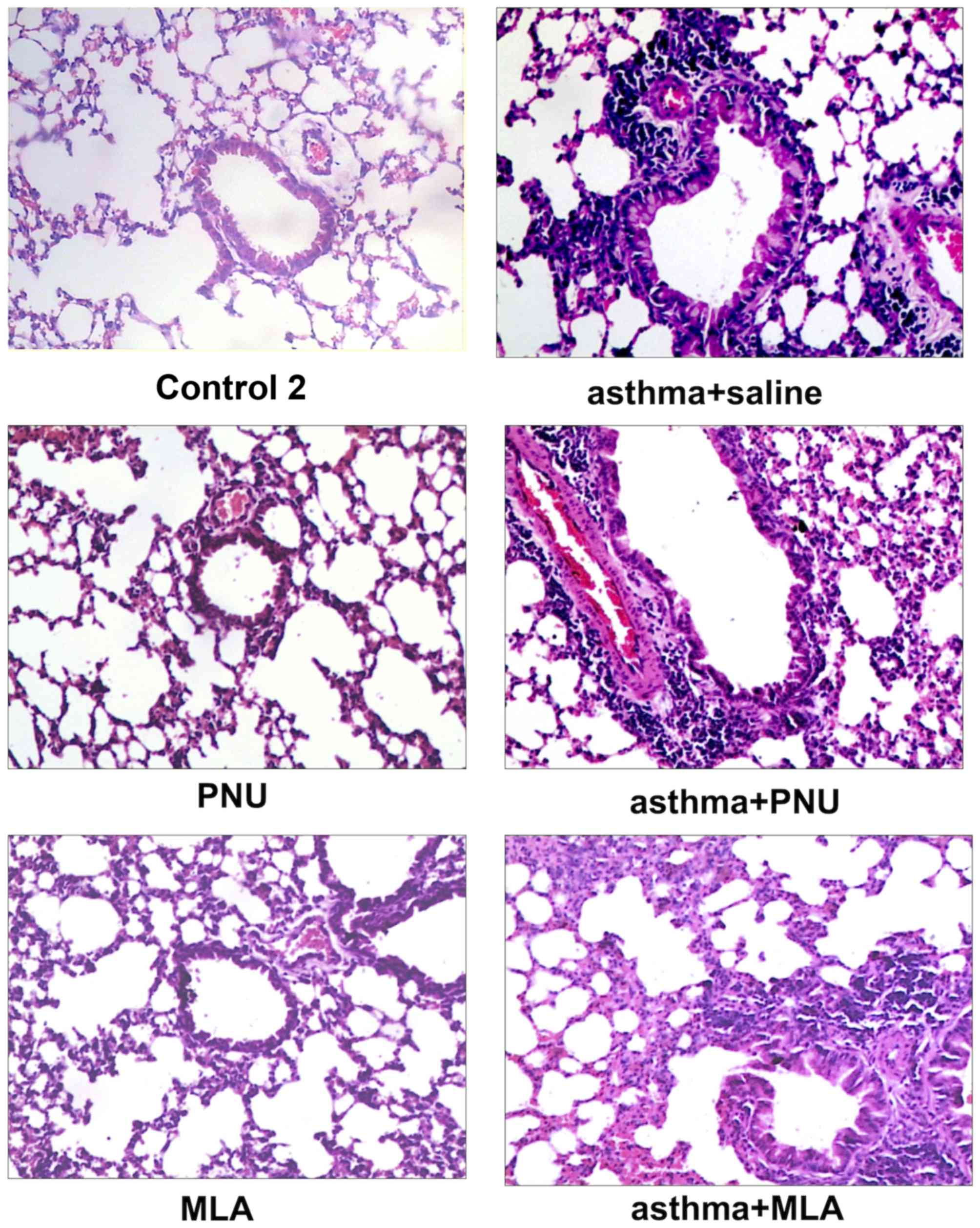
these groups (P<0.05). The results in Fig. 6 show that, similar to the asthma
group and control group in the previous experiments, the control 2,
PNU, and MLA groups showed no obvious inflammation, bronchial
smooth muscle thickening, or airway mucus in the lung tissues. In
contrast, all groups sensitized with OVA (asthma + saline, asthma +
MLA, and asthma + PNU groups) displayed considerable muscle
thickening, epithelial edema, and inflammatory cell infiltration.
Compared to the asthma + saline group, the asthma + PNU group that
was treated with the α7 nAChR agonist exhibited less leukocyte
infiltration and no significant bronchial epithelial swelling.
Compared to those for the asthma + saline group, the histology
results for the asthma + MLA group (treated with the α7 nAChR
antagonist) were similar. However, compared to the asthma + PNU
group, there was obvious leukocyte infiltration around the bronchus
and pulmonary vessel as well as epithelial swelling in the asthma +
MLA group.
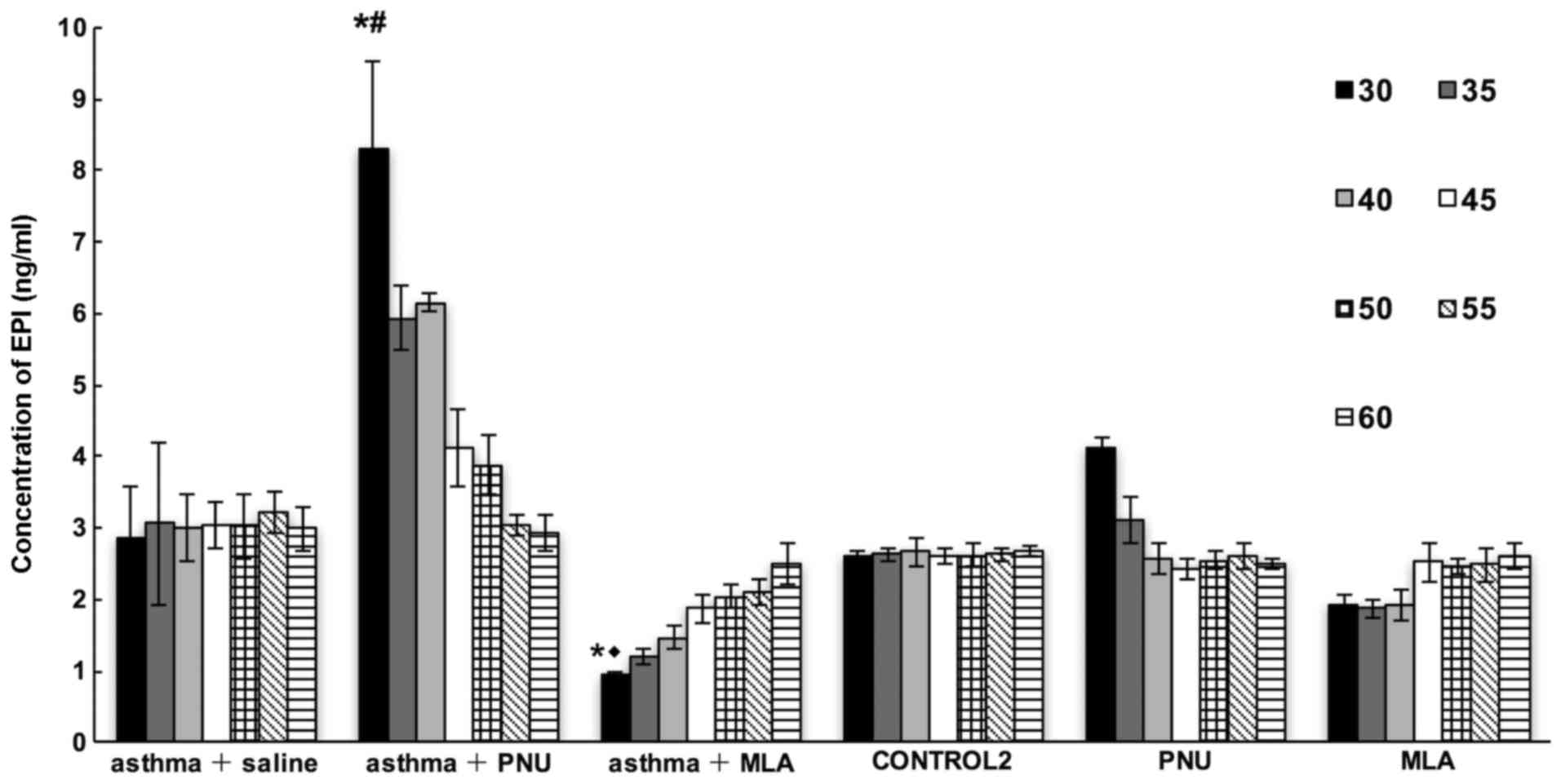
We then measured EPI levels in mice in the asthma +
saline, asthma + MLA, asthma + PNU, PNU, MLA, and control 2 groups
sacrificed at different time-points within 1 h after treatment
according to the different group assignments. As shown in Fig. 7, the concentration of circulating
EPI progressively increased in the asthma + PNU group and decreased
in the asthma + MLA group relative to levels in the asthma + saline
group in a time-dependent manner (P<0.05). Compared to that in
the PNU group, the serum EPI level in the asthma + PNU group was
increased by nearly 2-fold. Similarly, the serum EPI level in the
asthma + MLA group was significantly lower than that in the MLA
group (P<0.05). Finally, the serum EPI concentrations in the
control 2 and asthma + saline groups were relatively stable.
The RL measurements observed in response to
methacholine in each group are shown in Fig. 8. Compared to that in the asthma +
saline group, the RL in the asthma + PNU group was significantly
reduced upon exposure to the lowest methacholine concentration of
1.526 mg/ml and for all higher concentrations tested (P<0.05).
As the concentration of methacholine was increased, RL measurements
in mice in the asthma + PNU group were reduced by 19.3, 84.1, 92.8
and 218.7% with exposure to 1.526, 3.125, 6.25 and 12.5 mg/ml
methacholine, respectively, compared to the average measurements in
the asthma + saline group. Similarly, RL was significantly
increased with exposure to increasing concentrations of
methacholine in the asthma + MLA group. With exposure to 1.526,
3.125, 6.25 and 12.5 mg/ml methacholine, RL was increased by 53.3,
162.8, 118.3 and 130.8%, respectively, in the asthma + MLA group
compared to measurements in the asthma + saline group. RL
measurements in the PNU group and MLA group in response to
increasing methacholine concentrations did not differ significantly
from those in the control 2 group (P>0.05).
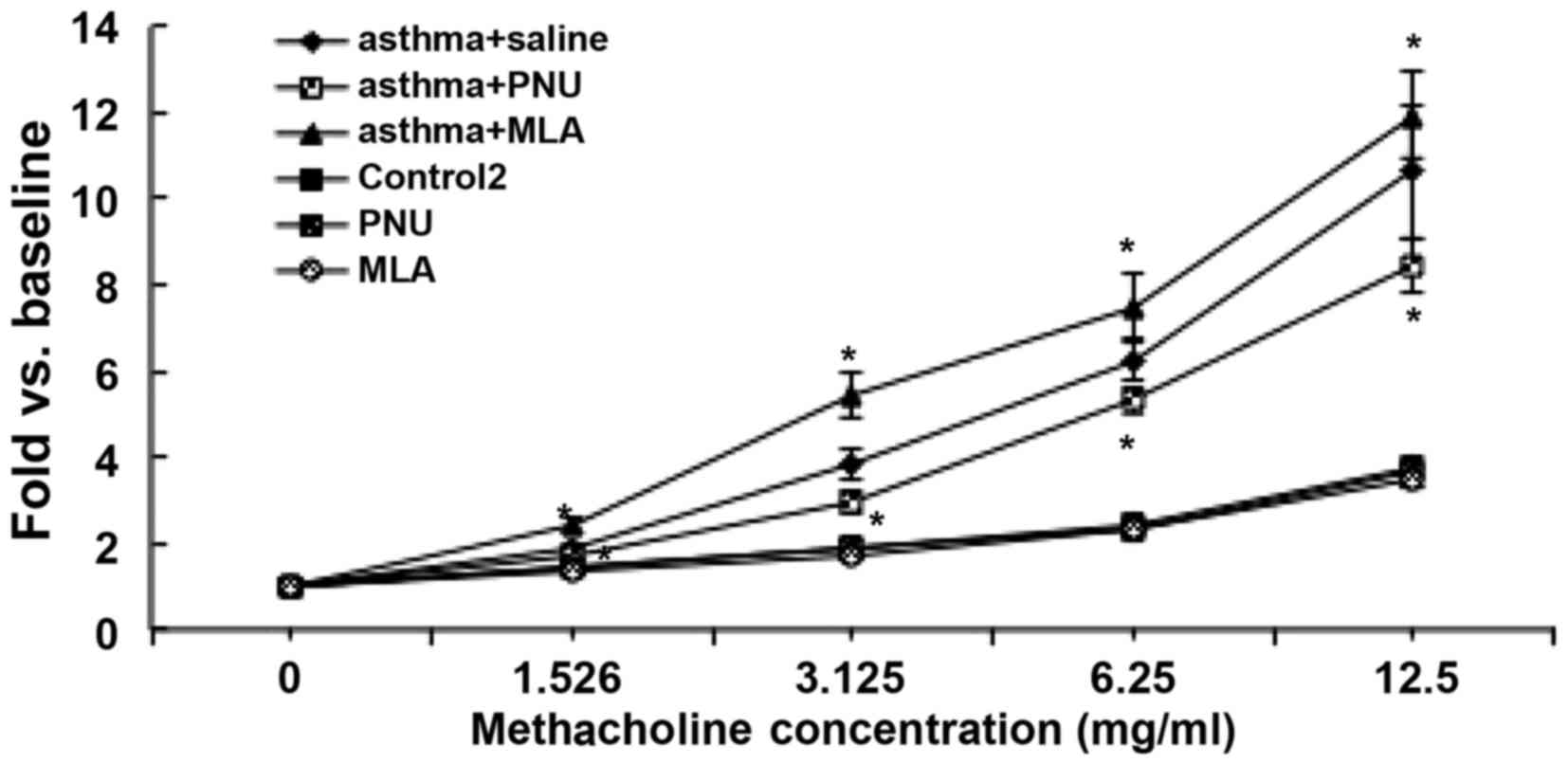 | Figure 8Changes in airway resistance after α7
nAChR agonist (PNU) or antagonist (MLA) treatment. Changes in mice
airway resistance (RL) in response to increasing concentrations of
methacholine 1 h after last OVA challenge. The airway resistance
measurements in mice in the asthma + PNU group were reduced by
19.3, 84.1, 92.8 and 218.7% following exposure to 1.526, 3.125,
6.25 and 12.5 mg/ml methacholine compared to the average
measurements in the asthma + saline group. Following exposure to
1.526, 3.125, 6.25 and 12.5 mg/ml methacholine, airway resistance
was increased by 53.3, 162.8, 118.3 and 130.8%, respectively, in
the asthma + MLA group compared to measurements in the asthma +
saline group. Data are expressed as fold vs. baseline
(saline-induced RL) and are shown as the means ± SD (n=5).
*P<0.05 vs. the asthma + saline group. |
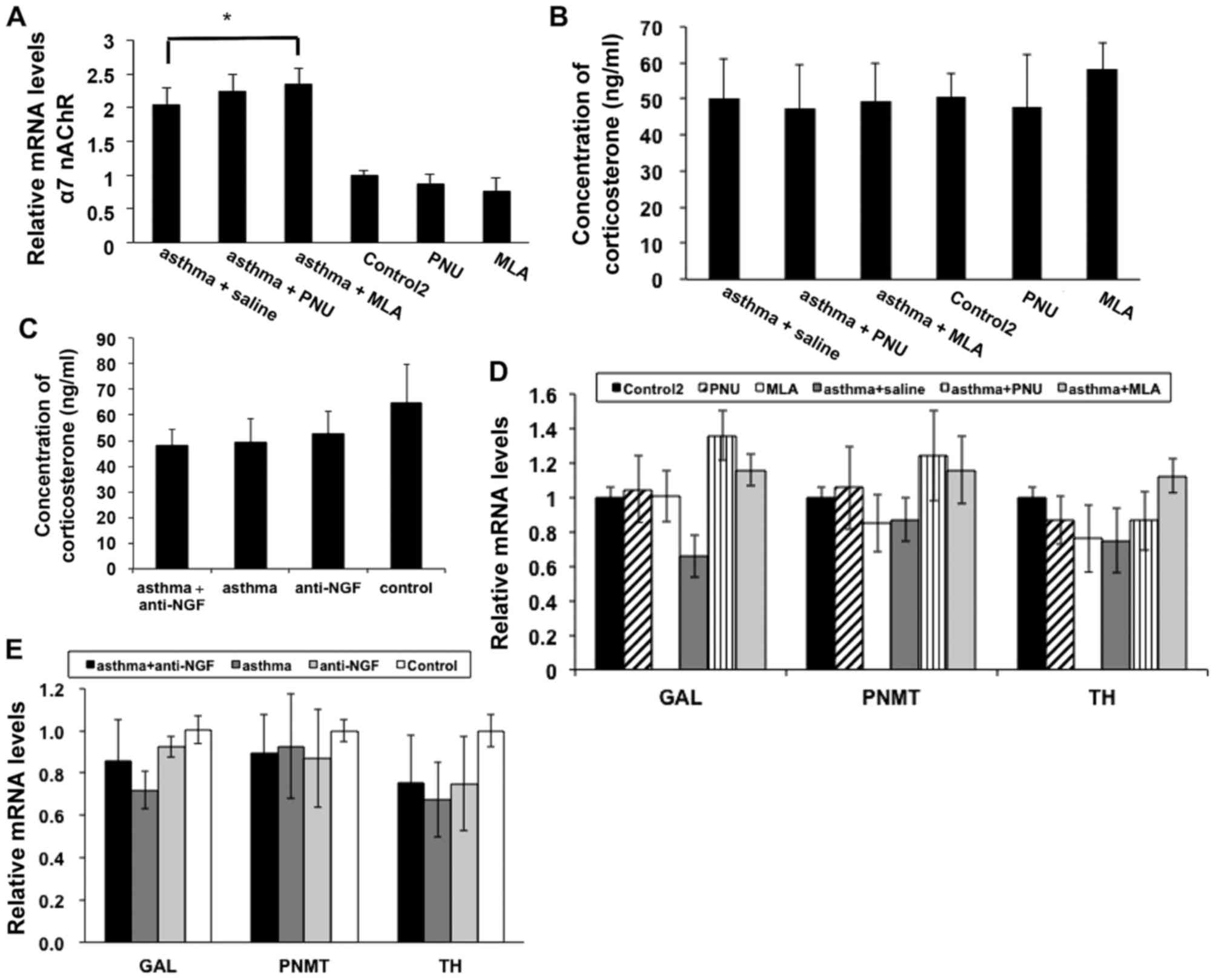
Finally, we evaluated α7 nAChR expression in each
group, and as shown in Fig. 9A,
α7 nAChR expression in the asthma + saline, asthma + MLA and asthma
+ PNU groups was significantly higher than that in the control 2
group. As shown in Fig. 9B and C,
we also examined corticosterone levels in each group and did not
observe any significant differences (P>0.05), which indicates
that the effects of α7 nAChR expression were not mediated by
glucocorticoid. In fact, recent studies also confirmed that α7
nAChR expression during mouse development is primarily limited to
the adrenal medulla (16).
Therefore, we next detected the expression of two rate-limiting
enzymes involved in EPI synthesis, PNMT and TH, as well as a
mediating neuropeptide GAL in each group of mice to rule out the
possibility of interference with differences in the levels of EPI
synthesis, and the observed expression levels showed no significant
differences (P>0.05; Fig. 9D and
E).
Discussion
The bronchus relies on circulating EPI for dilation
due to the lack of adrenergic innervation. Interestingly, EPI
levels in asthma patients do not increase as rapidly as needed to
alleviate bronchoconstriction (17,18). For this reason, researchers have
proposed that this weak increase in circulating EPI is an important
factor in the pathogenesis of asthma (19). nAChRs, which are ligand-gated ion
channels, have many different subtypes with different calcium
permeability, and the flux of Ca2+ is controlled by
these receptors on AMCCs, which regulates EPI release. A change in
α7 nAChR expression has been found in most relevant EPI release
experiments.
In the present study, we explored adrenal medulla
nAChR subtype redistribution and the impact on circulating levels
of EPI in a mouse model of asthma. However, our previous studies
have shown that long-term OVA challenge results in increased NGF
expression and causes ultrastructural changes, causing EPI
synthesis disorders (4,26–28). Therefore, we needed to exclude the
impact of reduced synthesis of EPI. We used two methods to
eliminate interference of synthesis. i) To reduce AMCC
transformation, we used an acute asthmatic model, in which mice
received aerosol treatment for a shorter time period; and ii) we
established the anti-NGF group to eliminate the transformation
effect of NGF using an NGF antibody. Currently, mouse models of
asthma can be divided into three types: acute asthma, chronic
asthma and severe asthma models. Acute asthma models show
significant infiltration of eosinophils and airway
hyperresponsiveness as the main feature (20). This model type is used primarily
in allergic disease studies (21,22). Models of chronic asthma, which are
mainly used for airway remodeling research, exhibit smooth muscle
cell proliferation, epithelial thickening, goblet cell metaplasia,
and mild fibrosis (23,24). Severe asthma models are
characterized by infiltration of a large number of eosinophils and
extensive neutrophils. Significant airway wall thickening, airway
smooth muscle layer thickening, and reticular basement membrane
thickening also are observed (25). This model type is usually employed
in severe asthma research. In the present study, there were no
obvious differences in epithelial edema and inflammatory cell
infiltration between the asthma group and the asthma + anti-NGF
group. Therefore, there was no significant difference in pulmonary
inflammation between the asthma group and the asthma + anti-NGF
group. Thus, we eliminated the interference of EPI synthesis
disorders.
EPI release is controlled by nAChRs that consist of
a series of different subtypes with different ion permeability in
the adrenal medulla. The distributions of these subtypes regulate
the ion permeability and the capability for EPI release. Studies
have shown that many factors can increase the expression of α7
nAChR in PC12 cells, including nicotine, hypoxia, and NGF in a
time- and dose-dependent manner (9–11).
α7 nAChR has received increasing attention in recent years, because
α7 nAChR generally forms a single homologous subunit pentamer, and
α7 nAChR has the highest Ca2+ permeability among all
nAChRs (13). In this study, we
found that obvious nAChR subtype redistribution occured in the
acute asthma mouse model, and α7 nAChR subtype expression was
significantly increased by up to 260%. In addition, the expression
of α3, α4, and β4 subunits was relatively increased by 69, 66 and
39%, respectively. However, subunits containing the α3–6 and β
subunits tend to constitute heteromeric receptors, which have a
fractional Ca2+ current (13). Therefore, based on previous
studies and our findings, we believe that the changes in the α7
nAChR subtype play a primary role in promoting EPI release in
response to asthma symptoms. α7 nAChR has become a hot topic in
recent years, but primarily with a focus on the brain α7 nAChR. For
example, Morioka et al found that α7 nAChR can be activated
to increase the expression of excitatory amino acid transporter 1,
which may be useful for the treatment of neurological disorders
associated with disturbance of the glutamatergic system (29). O'Neill et al found that α7
nAChR can improve cognitive function in animal models of
Alzheimer's disease (30,31). In addition, α7 nAChR activation
also has been shown to reduce stroke damage by reducing oxidative
stress as well as via a neuron protective effect in Parkinson's
disease (32). However, little is
known about the function of α7 nAChR in asthma, and for the first
time, our study showed that α7 nAChR expression was significantly
increased in asthmatic mice. This increase in α7 nAChR expression
in asthmatic mice significantly promotes flow of calcium ions and
release of EPI.
To further test the effect of the α7 nAChR subtype
in EPI release function, we treated mice with PNU (α7 nAChR
agonist) or MLA (α7 nAChR antagonist) at 30 min after the last
aerosol treatment and detected EPI levels 30 min later. We tested
lung function in each mouse at 1 h after the last treatment. PNU
and MLA cannot influence the adrenal gland through the central
nervous system as these molecules cannot pass the blood-brain
barrier (33). Therefore, adrenal
α7 nAChR expression directly affects the level of serum EPI. The
levels of EPI were significantly increased after application of
PNU, an α7 nAChR agonist, in the asthmatic mice (asthma PNU
interference group). On the one hand, EPI levels were increased by
~2-fold compared to levels in the asthma saline treatment group at
30 min after treatment with PNU and then gradually decreased until
reaching the same level as in the asthma saline treatment group at
55 min after PNU treatment. On the other hand, pulmonary function
tests showed that RL in the asthma PNU interference group was
significantly reduced by up to 218.7%. The EPI level in the MLA
asthma intervention group markedly declined by ~50% compared to the
level in the asthma saline treatment group and then gradually
decreased until reaching the same level in the asthma saline
treatment group at 55 min after MLA treatment. The RL in the asthma
MLA interference group was significantly increased by up to 162.8%.
Because α7 nAChR subtype expression increased by more than 200%,
the α7 nAChR subtype counteracted the increased EPI level after PNU
treatment and caused a more than 200% decrease in RL in the mice.
Similarly, due to increased expression of α7 nAChR, the α7 nAChR
antagonist MLA resulted in a significant decrease in the EPI level
and ~150% increase in the RL. In conclusion, we found that an
increase in α7 nAChR may facilitate the release of more EPI by
AMCCs and an increase in the EPI level could relieve RL.
Glucocorticoid can increase circulating EPI
concentrations, and glucocorticoid has a potent anti-inflammatory
effect. Thus, it can produce a therapeutic effect in asthma.
Therefore, we examined the serum levels of glucocorticoid, and
similar results were observed between each group. These findings
indicate that overexpression of α7 nAChR is not caused by
glucocorticoid. Recent studies have also indicated that during
mouse development, α7 nAChR is basically expressed in the adrenal
medulla, whereas the adrenal cortex shows slight expression of α7
nAChR (16). Therefore, this
study showed that the nicotinic acetylcholine subtype
redistribution in a mouse model of acute asthma indeed occurs, and
the main subtype increased is α7 nAChR. EPI release in the
asthmatic mice was significantly increased after α7 nAChR agonist
treatment and the ability to deal with methacholine stimulation was
significantly enhanced. Moreover, we measured PNMT and TH, which
are two rate-limiting enzymes of EPI synthesis, and an important
neuromedin GAL in mice of all groups (14,15). The results showed that the level
of EPI synthesis was not affected, indicating that the mice did not
have an EPI synthesis disorder.
In conclusion, the present study demonstrates that
the redistribution of nAChR subtypes, primarily α7 nAChR occurs in
the adrenal medulla in asthmatic mice. Increased α7 nAChR
expression can rapidly increase serum EPI levels and decrease
airway responsiveness.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (no. 81370127) and the Fundamental
Research Funds for the Central Universities of Central South
University (no. 2013zzts315).
References
|
1
|
Barnes PJ, Brown MJ, Silverman M and
Dollery CT: Circulating catecholamines in exercise and
hyperventilation induced asthma. Thorax. 36:435–440. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ind PW, Causon RC, Brown MJ and Barnes PJ:
Circulating catecholamines in acute asthma. Br Med J (Clin Res Ed).
290:267–269. 1985. View Article : Google Scholar
|
|
3
|
Wang J, Hu CP and Feng JT: Dysfunction of
releasing adrenaline in asthmatic adrenaline medullary chromaffin
cells due to functional redundancy primed by nerve growth factor.
Zhonghua Jie He He Hu Xi Za Zhi. 29:812–815. 2006.In Chinese.
|
|
4
|
Feng JT and Hu CP: Dysfunction of
releasing adrenaline in asthma by nerve growth factor. Med
Hypotheses. 65:1043–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi-Shima N, Okada S, Shimizu T,
Usui D, Nakamura K, Lu L and Yokotani K: Adrenal adrenaline- and
noradrena- line-containing cells and celiac sympathetic ganglia are
differentially controlled by centrally administered
corticotropin-releasing factor and arginine-vasopressin in rats.
Eur J Pharmacol. 564:94–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fenster CP, Rains MF, Noerager B, Quick MW
and Lester RA: Influence of subunit composition on desensitization
of neuronal acetylcholine receptors at low concentrations of
nicotine. J Neurosci. 17:5747–5759. 1997.PubMed/NCBI
|
|
7
|
Fucile S: Ca2+ permeability of
nicotinic acetylcholine receptors. Cell Calcium. 35:1–8. 2004.
View Article : Google Scholar
|
|
8
|
Nai Q, McIntosh JM and Margiotta JF:
Relating neuronal nicotinic acetylcholine receptor subtypes defined
by subunit composition and channel function. Mol Pharmacol.
63:311–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi T, Yamashita H, Nakamura S,
Ishiguro H, Nagatsu T and Kawakami H: Effects of nerve growth
factor and nicotine on the expression of nicotinic acetylcholine
receptor subunits in C12 cells. Neurosci Res. 35:175–181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin MK, Han W, Bevans-Fonti S, Jun JC,
Punjabi NM and Polotsky VY: The effect of adrenal medullectomy on
metabolic responses to chronic intermittent hypoxia. Respir Physiol
Neurobiol. 203:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Utsugisawa K, Nagane Y, Obara D and Tohgi
H: Increased expression of alpha7 nAChR after transient hypoxia in
C12 cells. Neuroreport. 11:2209–2212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rogers SW, Mandelzys A, Deneris ES, Cooper
E and Heinemann S: The expression of nicotinic acetylcholine
receptors by C12 cells treated with NGF. J Neurosci. 12:4611–4623.
1992.PubMed/NCBI
|
|
13
|
Dajas-Bailador F and Wonnacott S:
Nicotinic acetylcholine receptors and the regulation of neuronal
signalling. Trends Pharmacol Sci. 25:317–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer-Colbrie R, Eskay RL, Eiden LE and
Maas D: Transsynaptic regulation of galanin, neurotensin, and
substance P in the adrenal medulla: combinatorial control by
second-messenger signaling pathways. J Neurochem. 59:780–783. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fischer-Colbrie R, Iacangelo A and Eiden
LE: Neural and humoral factors separately regulate neuropeptide Y,
enkephalin, and chromogranin A and B mRNA levels in rat adrenal
medulla. Proc Natl Acad Sci USA. 85:3240–3244. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gahring LC, Myers E, Palumbos S and Rogers
SW: Nicotinic receptor alpha7 expression during mouse adrenal gland
development. PLoS One. 9:e1038612014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barnes P, FitzGerald G, Brown M and
Dollery C: Nocturnal asthma and changes in circulating epinephrine,
histamine, and cortisol. N Engl J Med. 303:263–267. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Aalderen WM, Postma DS, Koëter GH and
Knol K: Nocturnal airflow obstruction, histamine, and the autonomic
central nervous system in children with allergic asthma. Thorax.
46:366–371. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bates ME, Clayton M, Calhoun W, Jarjour N,
Schrader L, Geiger K, Schultz T, Sedgwick J, Swenson C and Busse W:
Relationship of plasma epinephrine and circulating eosinophils to
nocturnal asthma. Am J Respir Crit Care Med. 149:667–672. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bates JH, Rincon M and Irvin CG: Animal
models of asthma. Am J Physiol Lung Cell Mol Physiol.
297:L401–L410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy AT, Lakshmi SP and Reddy RC: Murine
model of allergen induced asthma. J Vis Exp. 63:e37712012.
|
|
22
|
Secor ER, Carson WF, Singh A, Pensa M,
Guernsey LA, Schramm CM and Thrall RS: Oral bromelain attenuates
inflammation in an ovalbumin-induced murine model of asthma. Evid
Based Complement Alternat Med. 5:61–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahn JH, Kim CH, Kim YH, Kim SJ, Lee SY,
Kim YK, Kim KH, Moon HS, Song JS, Park SH, et al: Inflammatory and
remodeling events in asthma with chronic exposure to house dust
mites: a murine model. J Korean Med Sci. 22:1026–1033. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson JR Jr, Wiley RE, Fattouh R,
Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R,
Inman MD and Jordana M: Continuous exposure to house dust mite
elicits chronic airway inflammation and structural remodeling. Am J
Respir Crit Care Med. 169:378–385. 2004. View Article : Google Scholar
|
|
25
|
Jiang XB, Zhu Y and Yin KS: Reproduction
of severe asthma model in mice. Zhongguo Wei Zhong Bing Ji Jiu Yi
Xue. 18:733–736. 2006.In Chinese. PubMed/NCBI
|
|
26
|
Hu CP, Zou YQ, Feng JT and Li XZ: The
effect of unilateral adrenalectomy on transformation of adrenal
medullary chromaffin cells in vivo: a potential mechanism of asthma
pathogenesis. PLoS One. 7:e445862012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu CP, Zou JT, Zou YQ, Li XZ and Feng JT:
Kidney-tonifying recipe can repair alterations in adrenal medullary
chromaffin cells in asthmatic rats. Evid Based Complement Alternat
Med. 2012:5426212012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng JT, Wu XM, Li XZ, Zou YQ, Qin L and
Hu CP: Transformation of adrenal medullary chromaffin cells
increases asthmatic susceptibility in pups from allergen-sensitized
rats. Respir Res. 13:992012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morioka N, Tokuhara M, Nakamura Y,
Idenoshita Y, Harano S, Zhang FF, Hisaoka-Nakashima K and Nakata Y:
Primary cultures of rat cortical microglia treated with nicotine
increases in the expression of excitatory amino acid transporter 1
(GLAST) via the activation of the α7 nicotinic acetylcholine
receptor. Neuroscience. 258:374–384. 2014. View Article : Google Scholar
|
|
30
|
O'Neill MJ, Murray TK, Lakics V, Visanji
NP and Duty S: The role of neuronal nicotinic acetylcholine
receptors in acute and chronic neurodegeneration. Curr Drug Targets
CNS Neurol Disord. 1:399–411. 2002. View Article : Google Scholar
|
|
31
|
Vicens P, Ribes D, Heredia L, Torrente M
and Domingo JL: Effects of an alpha7 nicotinic receptor agonist and
stress on spatial memory in an animal model of Alzheimer's disease.
Biomed Res Int. 2013:9527192013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Z, Shen F, He Y, Degos V, Camus M,
Maze M, Young WL and Su H: Correction: activation of α-7 nicotinic
acetylcholine receptor reduces ischemic stroke injury through
reduction of pro-inflammatory macrophages and oxidative stress.
PLoS One. 11:e01522182016. View Article : Google Scholar
|
|
33
|
Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh
DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK,
et al: Discovery and structure-activity relationship of
quinuclidine benzamides as agonists of alpha7 nicotinic
acetylcholine receptors. J Med Chem. 48:905–908. 2005. View Article : Google Scholar : PubMed/NCBI
|