Introduction
Hypertension is one of the major risk factors for
cardiovascular diseases and an important healthcare concern
worldwide (1). A growing body of
evidence indicates that oxidative stress plays an important role in
the pathophysiology of high-salt-induced hypertension (2–4).
High-salt intake is a significant environmental factor, strongly
associated with blood pressure (BP) regulation and hypertensive
responses and may increase oxidative stress, thus affecting the
pathophysiological procedure of adverse events in the sympathetic
nervous system (5,6). This breaks the balance between
reactive oxygen species (ROS) generation and the antioxidant
defenses, and triggers oxidative stress in central and peripheral
tissues (7). ROS, such as
super-oxide anion, hydrogen peroxide and hydroxyl radical, not only
participate in numerous cellular signaling pathways, but also
modulate systemic vascular resistance and balance in salt and water
homeostasis (8,9). Moreover, a previous study
demonstrated that the overproduction of ROS in the central nervous
system is extremely critical for arterial pressure regulation by
enhancing renal sympathetic nerve activity (RSNA) (10). The rostral ventrolateral medulla
(RVLM) is one of the main active regions for the central regulation
of resting BP and sympathetic outflow (11–13). Therefore, the overproduction of
ROS in the RVLM plays a key role in high BP and sympathetic
overactivity in salt-induced hypertension.
Alpha-lipoic acid (ALA), chemically known as
1,2-dithiolane-3-pentanoic acid
(C8H14O2S2), is widely
recognized for its potent superoxide inhibitory properties both as
natural diet constituent and a synthetic isolate. It is soluble in
aqueous and lipid portions of the cell (14,15). The antioxidant capacity of ALA is
more potent than that of vitamins C and E, and glutathione
(16). ALA and its reduced form,
dihydrolipoic acid (DHLA), have been shown to be potent naturally
occurring antioxidants by scavenging a variety of ROS (14). Furthermore, ALA appears to
regenerate other endogenous antioxidants, including vitamins C and
E and glutathione, and has the salubrious property of promoting the
body's antioxidant capacity. In addition, it is also a key
regulator of energy metabolism in the mitochondria, which is a
naturally occurring dithiol compound synthesized enzymatically in
the mitochondrion from octanoic acid (16). Thus, ALA is closely related to the
body's antioxidant activity. This suggests that ALA may be a
possible candidate as a protective agent against the risk factors
of hypertension. It is also possible that ALA may decrease BP in
hypertension by resisting the superoxide damage and protecting the
body's biological systems from cardiovascular diseases. Therefore,
in the present study, we aimed to explore whether ALA
supplementation attenuates oxidative stress in the RVLM, thus
decreasing BP and sympathetic nerve activity in salt-induced
hypertension.
Materials and methods
Animals
Adult male Wistar rats (n=56; aged 7 weeks; weighing
180–220 g) were obtained from the Experimental Animal Center of
Wuhan University of Science and Technology. All rats were housed in
a room with a temperature-controlled (23±2°C) environment with a
normal 12-h light-dark cycle and allowed access to normal rat chow
ad libitum.
All procedures were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (the US National Institutes of Health
Publication no. 85–23, revised 1996) and approved by the Committee
on the Ethics of Animal Experiments of Wuhan University of Science
and Technology, Wuhan, China.
General experimental protocol
The male Wistar rats were randomly divided into 2
groups (n=28) as follows: the normal salt diet group administered
0.3% NaCl (NS group) as a control, the high-salt diet group
administered 8% NaCl (HS group) in their food for 8 weeks to induce
hypertension, as previously described (5,6).
After 8 weeks, the rats in the NS and HS group were respectively
administered ALA (60 mg/kg) dissolved in the vehicle (0.9% saline)
or an equal volume of the vehicle daily by gastric perfusion for 8
weeks, as previously described (17). Thus, there were now 4 groups of
rats (HS + vehicle, HS + ALA, NS + vehicle and NS + ALA) with 14
rats in each group.
BP measurements
In the rats from all the chronic feeding groups,
arterial pressure was measured non-invasively using a tail-cuff
instrument and a recording system (Kent Scientific Corp.,
Torrington, CT, USA), as previously described (18). Briefly, unanesthetized rats from
each group were warmed to an ambient temperature of 30°C by placing
them in a holding device mounted on a thermostatically controlled
warming plate. Tail cuffs were placed on the animals, and each rat
was allowed to become accustomed to the cuff for 10 min prior to
performing the BP measurements. All measurements were taken within
the same 2-h time window each day. Each session consisted of 30
cycles. BP was measured on 5 consecutive days each week, and values
were averaged from ≥6 consecutive cycles. BP was measured at
baseline (7 weeks of age) and then weekly until the end of either
chronic study period.
Collection of blood and tissue
samples
Each group of rats (n=14) was anesthetized with a
ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture via
intraperitoneal (i.p.) injection. From each group, 7 rats were
perfused with 4% paraformaldehyde for immunofluorescence and
immunohistochemistry. The remaining rats were decapitated and trunk
blood was collected for high performance liquid chromatography
(HPLC) fresh tissue was obtained for western blot analysis, ELISA
and other experiments. The RVLM tissue was isolated following
Palkovits' microdissection procedure as previously described
(19). Plasma and tissue samples
were stored at −80°C until analysis.
Immunofluorescence and
immunohistochemistry
The rats were anesthetized and perfused through the
heart with 4% paraformaldehyde in phosphate-buffered saline (PBS,
pH 7.4). The brains were dehydrated in graded sucrose, and
OCT-embedded. The RVLM was identified as the region extending
caudally 500–700 µm from the caudal pole of the facial
nucleus. Serial coronal sections (14-µm-thick) were cut and
mounted on glass slides, which were stored at −80°C until use for
measurements, as previously described (20).
Immunohistochemical and immunofluorescence staining
was carried out on brain sections as described previously to
identify NAD(P)H oxidase (NOX2 and NOX4) expression in the RVLM
using respective antibodies [NOX2, sc-20782, 1:200; NOX4, sc-5827,
1:200; and copper/zinc (Cu/Zn)-superoxide dismutase (SOD),
sc-11407, 1:200; all from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA] (19). The brain
sections were washed in PBS, permeabilized in 0.5% Triton, blocked
using 5% normal goat serum and incubated with the primary
antibodies in blocking buffer at 4°C overnight. Following
incubation with the primary antibodies (anti-NOX4 and
anti-Cu/Zn-SOD antibodies), the sections were incubated with
secondary antibodies for immunofluorescence [Alexa 488-labeled
anti-rabbit (1:200, green fluorescence) or Alexa 594-labeled
anti-rabbit (1:200, red fluorescence); Invitrogen Life
Technologies, Carlsbad, CA, USA] for 60 min at 37°C.
For immunohistochemistry, the brain sections were
incubated with anti-NOX2 primary antibody and then with anti-rabbit
secondary antibody from a Histostain™-Plus kit (SP-9001; ZSGB-Bio,
Beijing, China) for 60 min. Antibody binding was visualized using a
3,3′-diaminobenzidine (DAB) kit (AR-1002; Boster Bio-Engineering,
Wuhan, China) according to the manufacturer's instructions.
Follwing a 10-min wash in tap water, the slices were stained in
Harris' hematoxylin solution for 8 min and then differentiated in
1% acid alcohol for 30 sec. Processing was terminated with
H2O and the sections were imaged using a Nikon camera
(Tokyo, Japan), as previously described (21).
Superoxide anion levels in the RVLM were determined
by fluorescent-labeled dihydroethidium (DHE; Molecular Probes,
Eugene, OR, USA) staining. The coronal sections
(14-µm-thick) were incubated with 1 µmol/l DHE at
37°C for 10 min as previously described (20).
Western blot analysis
Protein extracted from the RVLM tissues was prepared
as previously described (21).
For NOX2, NOX4 and Cu/Zn-SOD detection, protein extracts (5
µl) from the RVLM were resolved by 10–15% SDS-polyacrylamide
gels, and electroblotted onto nitrocellulose membranes
(Immobilon-P; EMD Millipore, Billerica, MA, USA) that were blocked
in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5%
bovine serum albumin for 1 h at room temperature (20). The blots were incubated overnight
at 4°C with the primary antibodies to NOX2 (sc-20782, 1:400), NOX4
(sc-5827, 1:400) and Cu/Zn-SOD (sc-11407, 1:200) (all form Santa
Cruz Biotechnology, Inc.) to determine the relative expression
levels in the RVLM. After washing with wash buffer (1X TBS, 0.1%
Tween-20), the blots were then incubated for 1 h with the secondary
antibody (1:10,000 dilution; Santa Cruz Biotechnology) labeled with
horseradish peroxidase. Protein loading was controlled by probing
all blots with β-actin antibody (Thermo Fisher Scientific, Waltham,
MA, USA) and normalizing their protein intensities to those of
β-actin. The immuno-complexes were visualized using an
ECL-immunoblotting detection kit (PerkinElmer, Inc., Waltham, MA,
USA). Band densities were analyzed using NIH ImageJ software, as
previously described (21).
Preparation of mitochondria matrix
fraction in RVLM
Mitochondrial matrix (stroma) was prepared by
applying the method described as follows: brain tissues were
rapidly removed and washed with 0.86% cold normal saline, then
chopped into small sections, and placed into ice-cold isolation
buffer for mitochondria (10 mM Tris-HCl, pH 7.4, 250 mM sucrose,
0.5 M methylene diamine tetra-acetic acid (EDTA), and 0.5 % bovine
serum albumin). After being homogenized, the homogenate was
centrifuged at 750 × g for 10 min. The supernatant was then
centrifuged at 10,000 rpm for 10 min at 4°C. Mitochondrial pellets
were washed twice with isolation buffer and then resuspended in the
same buffer solution. The mitochondrial matrix was extracted from
freshly prepared mitochondria by freezing and defrosting with
repeated homogenization in order to burst mitochondria. Following
centrifugation at 10,000 rpm for 10 min, the supernatant was the
source of SOD, glutathione (GSH) and malondialdehyde (MDA), as
previously described (22).
Biochemical evaluation of MDA, GSH, and
SOD in RVLM mitochondria
Lipid peroxidation product in the RVLM was
determined by measuring the MDA content in tissue homogenates
according to the method of Begue and Aust spec-trophotometrically
at 532 nm (23). Values were
expressed as mm/g protein. SOD activity was determined by following
the method of Kono at 550 nm (24). Values were expressed as U/mg
protein. The level of reduced GSH was measured as protein-free
sulfhydryl content by the method of Sedlak and Lindsay at 412 nm
and values were expressed as µm/g protein (25).
According to the manufacturer's instructions, the
standards or sample diluents were added to the appropriate well of
a microtiter plate pre-coated with specific antibodies and
incubated. Conjugate was added followed by incubation at 37°C for 1
h and then washing. The reactions were terminated with stop
solution and read at 450 nm for MDA, GSH and SOD measurements using
a microtiter plate reader (MK3; Thermo Fisher Scientific).
Measurement of plasma levels of
norepinephrine (NE)
Plasma NE levels were measured by HPLC as described
previously with minor modifications in plasma sample preparation
Plasma samples were prepared by adding activated alumina, Tris
buffer, EDTA, and internal standard 3,4-dihydrobenzylamine, along
with 0.5 ml of rat plasma. The samples were centrifuged, and
supernatant was separated, rinsed 2 times in ultrapure water, and
filtered through a Millipore filter (Ultrafree MC UFC30GV00;
Millipore). The composition of the mobile phase was as follows:
monochloroacetic acid (14.14 g/l), sodium hydroxide (4.675 g/l),
octanesulfonic acid disodium salt (0.3 g/l),
ethylenedi-aminetetraacetic acid (0.25 g/l), acetonitrile (3.5%)
and tetrahydrofuran (1.4%). The mobile phase was carried out in
pyrogen-free water and then filtered and degassed through the
Millipore filter and pumped at a flow rate of 1.8 ml/min. The
sensitivity of the detector was 1 nA full scale, and the potential
of the working electrode was 0.65 V. The column oven maintained the
temperature of the column at 37°C. At the time of HPLC analysis,
tissue samples were homogenized in 150 µl of 0.1 M
HClO4 using a micro-ultrasonic cell disruptor (Kontes,
Vineland, NJ, USA) and centrifuged at 10,000 × g for 10 min. 50
µl of the supernatant along with 25 µl of the
internal standard (0.05 M dihydroxybenzylamine) were injected into
the HPLC system (26,27).
Statistical analysis
All data are expressed as the means ± standard error
of the mean (SEM). The significance of differences between mean
values was analyzed by ANOVA followed by Tukey's test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
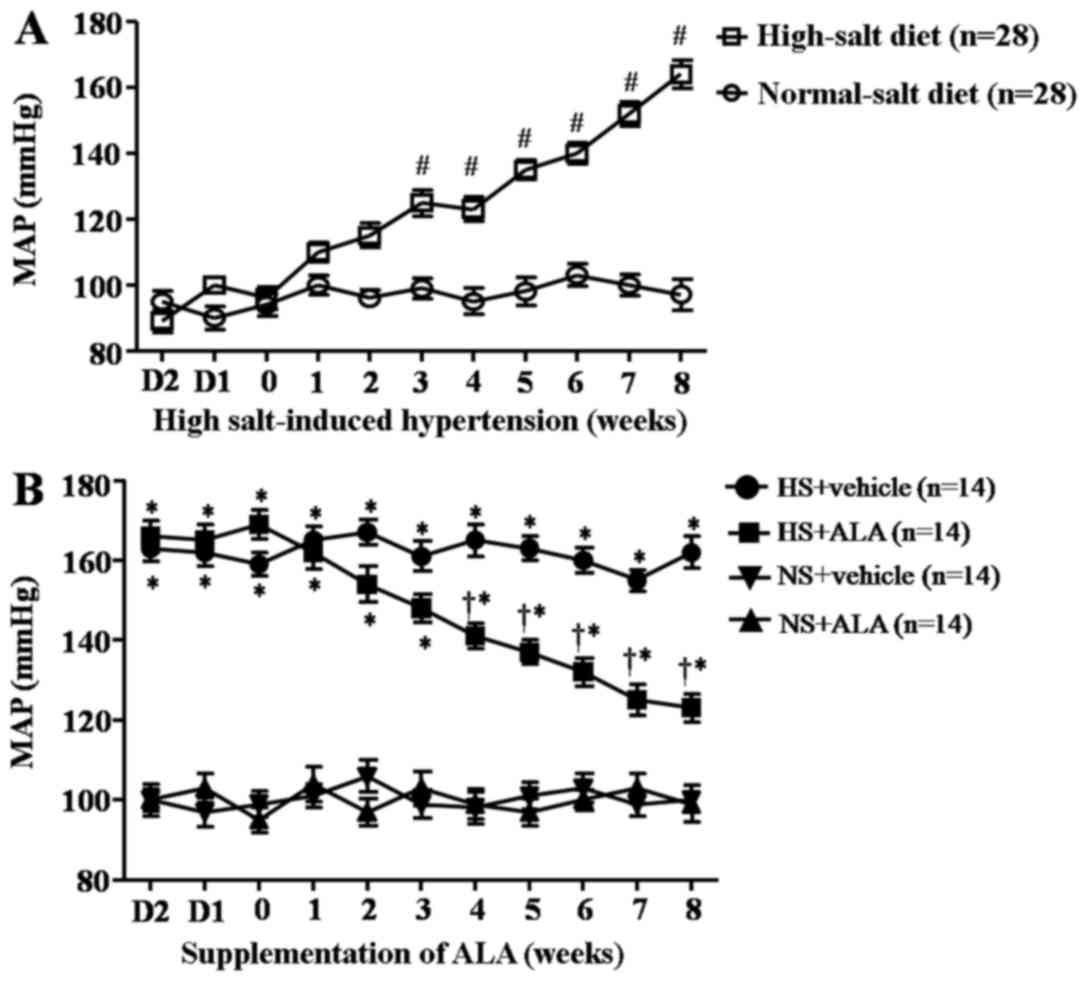
Mean arterial pressure (MAP)
A high-salt diet induced a significant increase in
MAP compared with the control rats after 8 weeks prior to the
supplementation of ALA (Fig. 1A).
Fig. 1B presents the MAP trends
for each group of rats treated with ALA for 8 weeks. The MAP of
rats fed a high-salt diet was significantly higher compared to that
of the control animals (NS group). The supplementation of ALA
decreased the MAP in the rats with high salt-induced
hypertension.
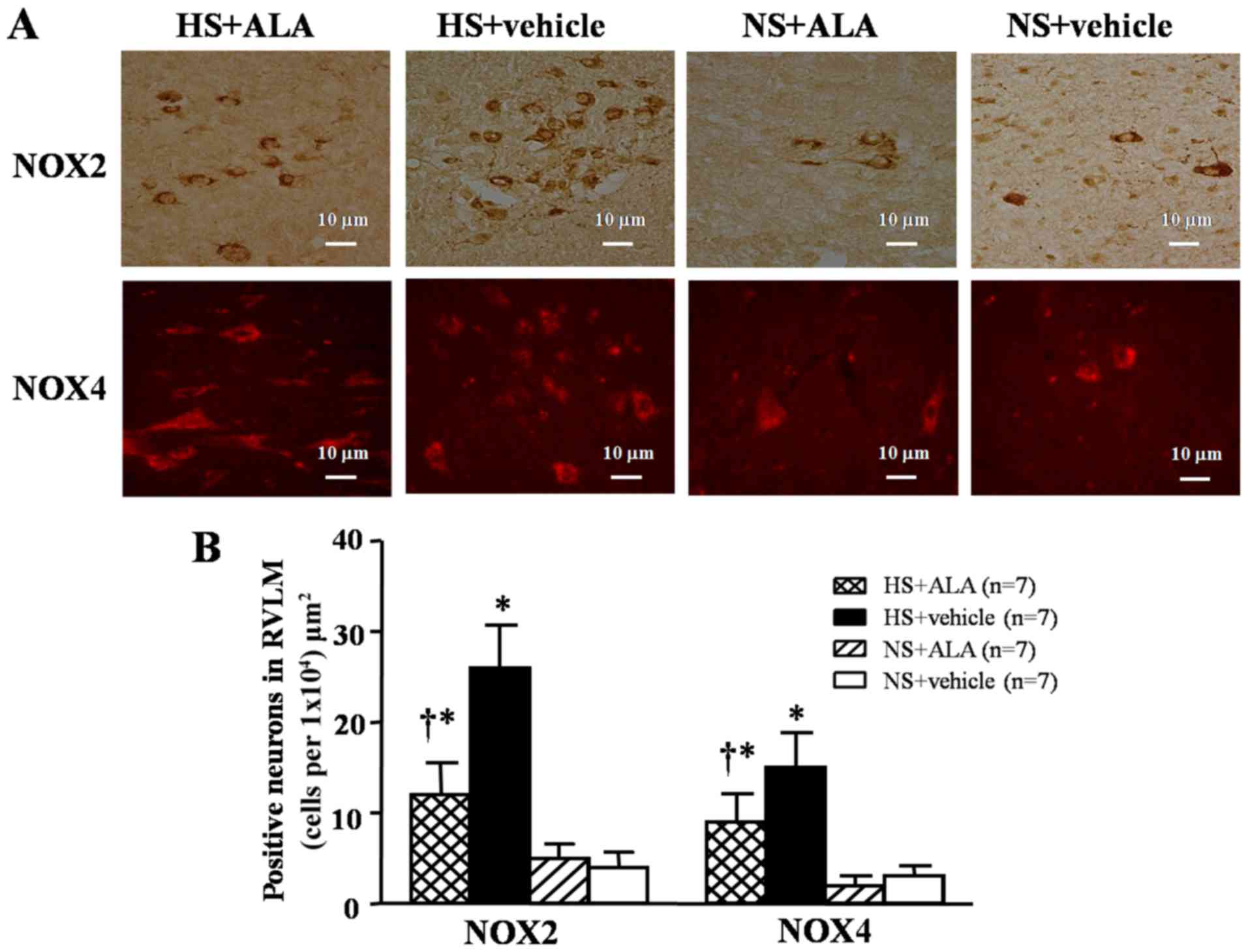
NOX2- or NOX4-positive neurons in the
RVLM
Imunohistochemisty and immunofluorescence staining
revealed that the high-salt diet induced a significant increase in
the expression of NOX2 and NOX4 in the RVLM compare to the control
rats. The supplementation of ALA decreased the number of NOX2- and
NOX4-positive neurons in the hypertensive rats (Fig. 2).
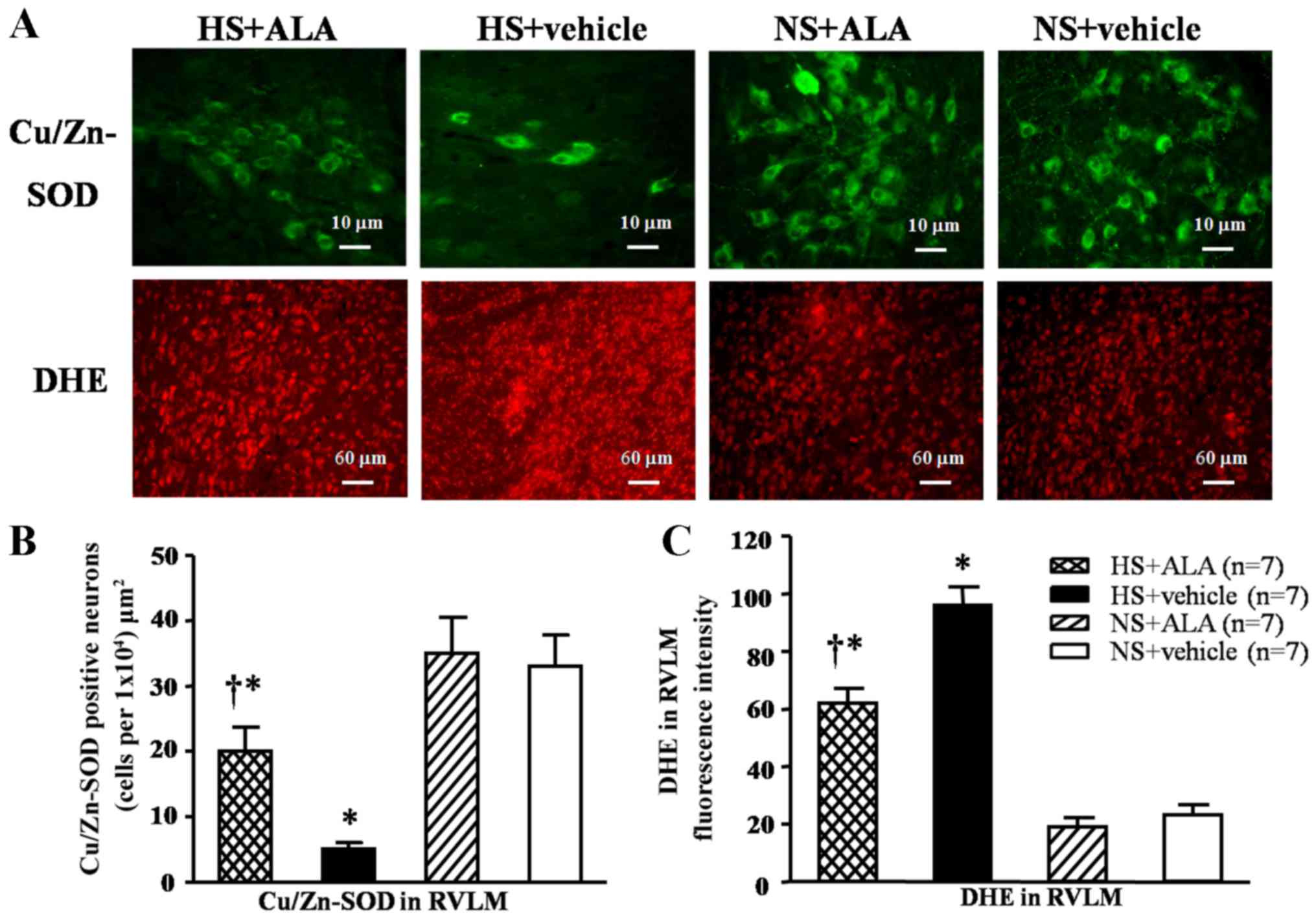
Superoxide- and Cu/Zn-SOD-positive
neurons in the RVLM
Immunofluorescence staining revealed that the
high-salt diet induced a significant decrease in Cu/Zn-SOD levels,
and an increase in fluorescence-labeled DHE compared with the
control rats. The supplementation of ALA decreased the DHE
fluorescence intensity and increased the number of
Cu/Zn-SOD-positive neurons in the hypertensive rats (Fig. 3).
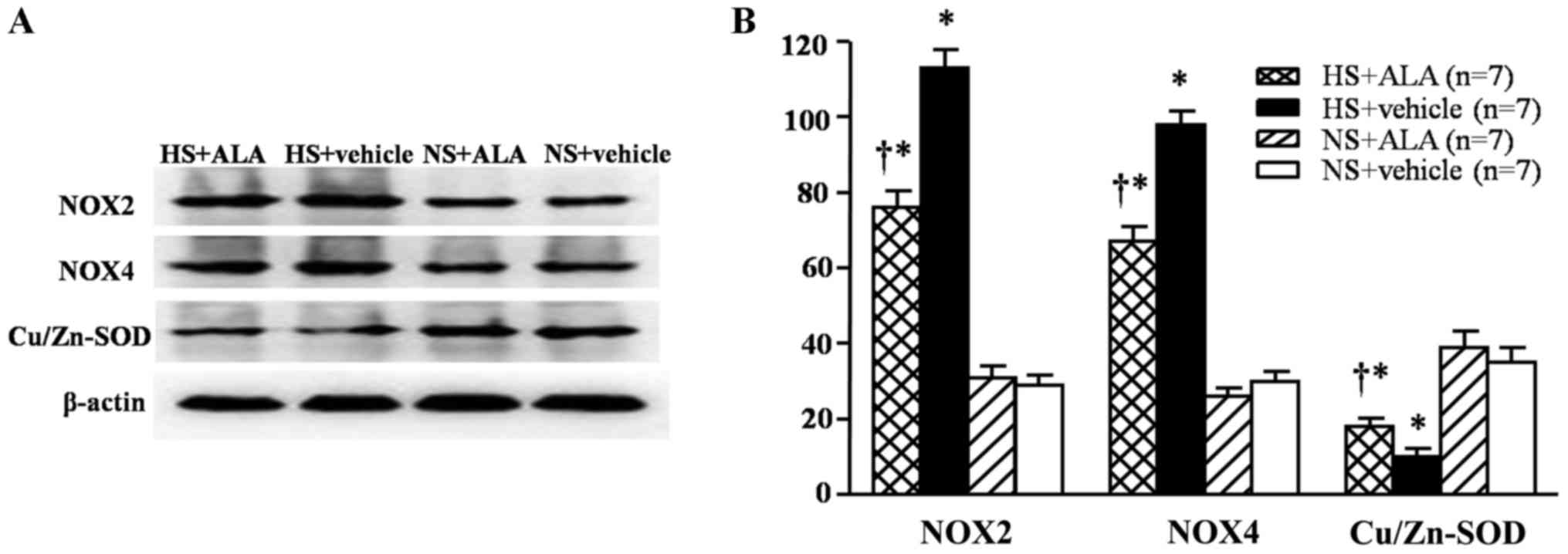
Protein expression levels of NOX2, NOX4
and Cu/Zn-SOD in the RVLM
The results of western blot analysis indicated that
the rats fed a high-salt diet exhibited significantly increased
levels of NOX2 and NOX4, and decreased expression levels of
Cu/Zn-SOD in the RVLM compared with the control rats. The
supplementation of ALA decreased the levels of NOX2 and NOX4, and
increased the Cu/Zn-SOD expression levels in the hypertensive rats
(Fig. 4).
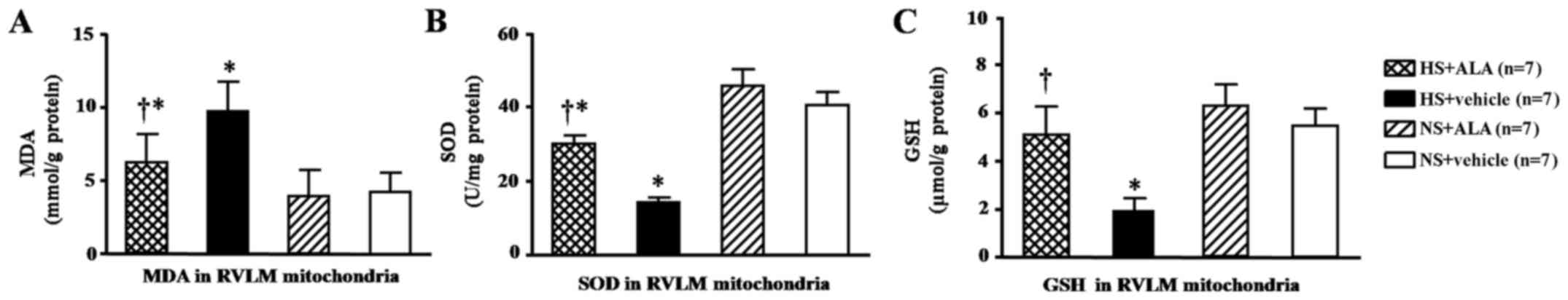
Levels of MAD, SOD and GSH in the RVLM
mitochondria
The MDA levels in the RVLM mitochondria were
significantly higher in the rats fed a high-salt diet than in those
in the normal control group. The supplementation of ALA decreased
the levels of MDA as compared with the respective control group (HS
+ vehicle; Fig. 5A). On the other
hand, the results revealed that the levels of SOD and GSH in the
RVLM mitochondria were decreased in the rats fed the high-salt
diet. The supplementation of ALA increased the levels of SOD and
GSH (Fig. 5B and C). The GSH
level in the ALA-treated rats was similar to that in the control
groups (Fig. 5C).
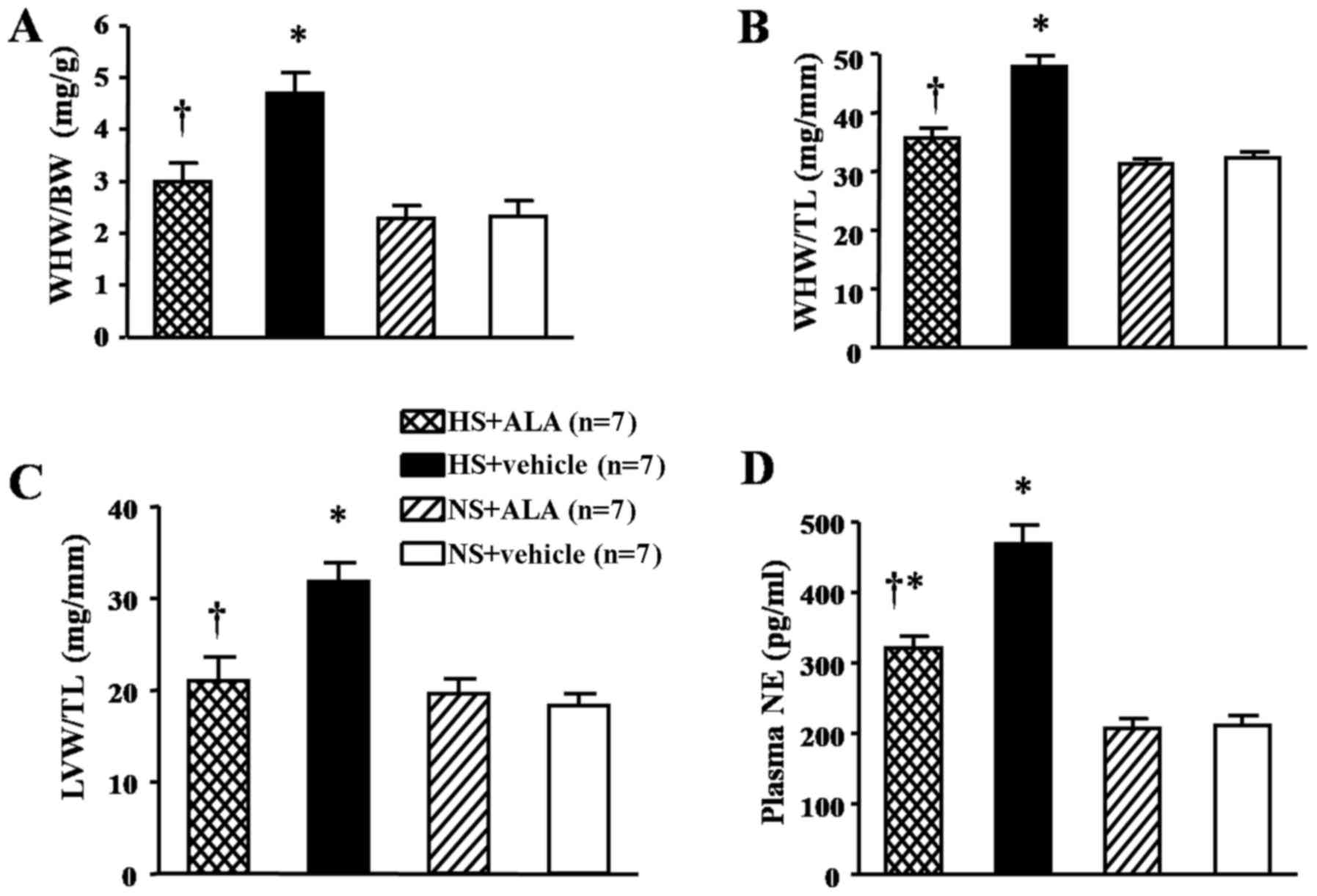
Effect of ALA supplementation on cardiac
hypertrophy and plasma NE levels
Whole heart weight/body weight (WHW/BW) ratio,
WHW/tibia length (TL) ratio and left-ventricular weight (LVW)/TL
ratio were measured as indicators of cardiac hypertrophy. Plasma NE
presents the activity of the sympathetic nervous system. The rats
fed a high-salt diet exhibited increased cardiac hypertrophy as
indicated by the increased WHW/BW ratio, WHW/TL ratio, and LVW/TL
ratio, which were decreased by ALA supplementation (Fig. 6A–C). In addition, the plasma NE
levels in the rats fed a high-salt diet were higher than those in
the control group. The supplemenation of ALA decreased the levels
of plasma NE in the hypertensive rats (Fig. 6D).
Discussion
The results of our study demonstrated that ALA
supplementation for 8 weeks markedly alleviated high salt-induced
hypertensive responses, as evidenced by the reduction in MAP and
plasma NE levels, that represent the activity of the sympathetic
nervous system. Moreover, ALA supplementation not only decreased
the expression of NAD(P)H subunits (NOX2 and NOX4) in the RVLM and
attenuated the overproduction of ROS in the RVLM mitochondria, but
it also enhanced the antioxidant capacity and attenuated cardiac
hypertrophy, as indicated by the decreased WHW/BW ratio, WHW/TL
ratio and LVW/TL ratio in the hypertensive rats administered ALA.
Therefore, the novel findings of this study are that the long-term
administration of ALA attenuates MAP, decreases sympathetic nervous
system activity and body oxidative damage in rats with high
salt-induced hypertension by decreasing the expression of NAD(P)H
subunits (NOX2 and NOX4), increased the levels of mitochondrial
bioenergetic enzymes, and enhancing the intracellular antioxidant
capacity in the RVLM during the development of hypertension.
It is well known that a high-salt intake is
responsible for the development of high BP in human communities
(28,29). Moreover, studies over the past
decade have demonstrated that a high-salt diet increases oxidative
stress in brain regions, such as the hippocampus and cerebral
cortex, which contributes to the pathological mechanisms of
hypertension (30–32). The RVLM is considered to be a
cardiovascular center that determines basal sympathetic tone, and
to be responsible for activating the sympathetic nervous system
(5). In the present study, we
found that a high-salt diet not only increased sympathetic nervous
system activity, but also elevated arterial BP. We also observed
that the production of superoxide was significantly increased,
whereas the antioxidant capacities (SOD and GSH in RVLM
mitochondria) were significantly decreased in the RVLM in the rats
with high salt-induced hypertension. Consistent with previous
studies (33–35), the findings of our study
demonstrated that a high-salt diet enhanced superoxide generation
in the RVLM, and activated the sympathetic nervous system during
the development of hypertension.
ALA has been described as a potent biological
antioxidant and an essential co-factor for mitochondrial
bioenergetic enzymes, which has extensively been applied as a
therapy for preventing diabetic polyneuropathies, and restoring
intracellular glutathione levels (36–38). It is also unique among
antioxidants that could be soluble in both lipid and aqueous
environments (36,38). Therefore, ALA can safely penetrate
deep into the brain, helping to scavenge free radicals and
reversing the damaging effects of ROS overproduction. Our present
study demonstrated that the long-term supplementation of ALA
decreased MAP, delayed the progress of cardiac hypertrophy, and
reduced the levels of NAD(P)H subunits (NOX2 and NOX4) and
mitochondrial superoxide in RVLM in rats with high salt-induced
hypertension. These results provide sufficient evidence that ALA
can cross the blood-brain barrier, reach the RVLM, and scavenge
free radicals derived from NAD(P)H in the mitochondria. Thus, in
this study, we hypothesized that ALA supplementation may decrease
oxidative stress in the RVLM by decreasing NOX2 and NOX4
expression, increasing the levels of mitochondrial bioenergetic
enzymes, and enhancing the intracellular antioxidant capacity in
the RVLM, finally leading to reduced BP and cardiac hypertrophy in
rats with high salt-induced hypertension.
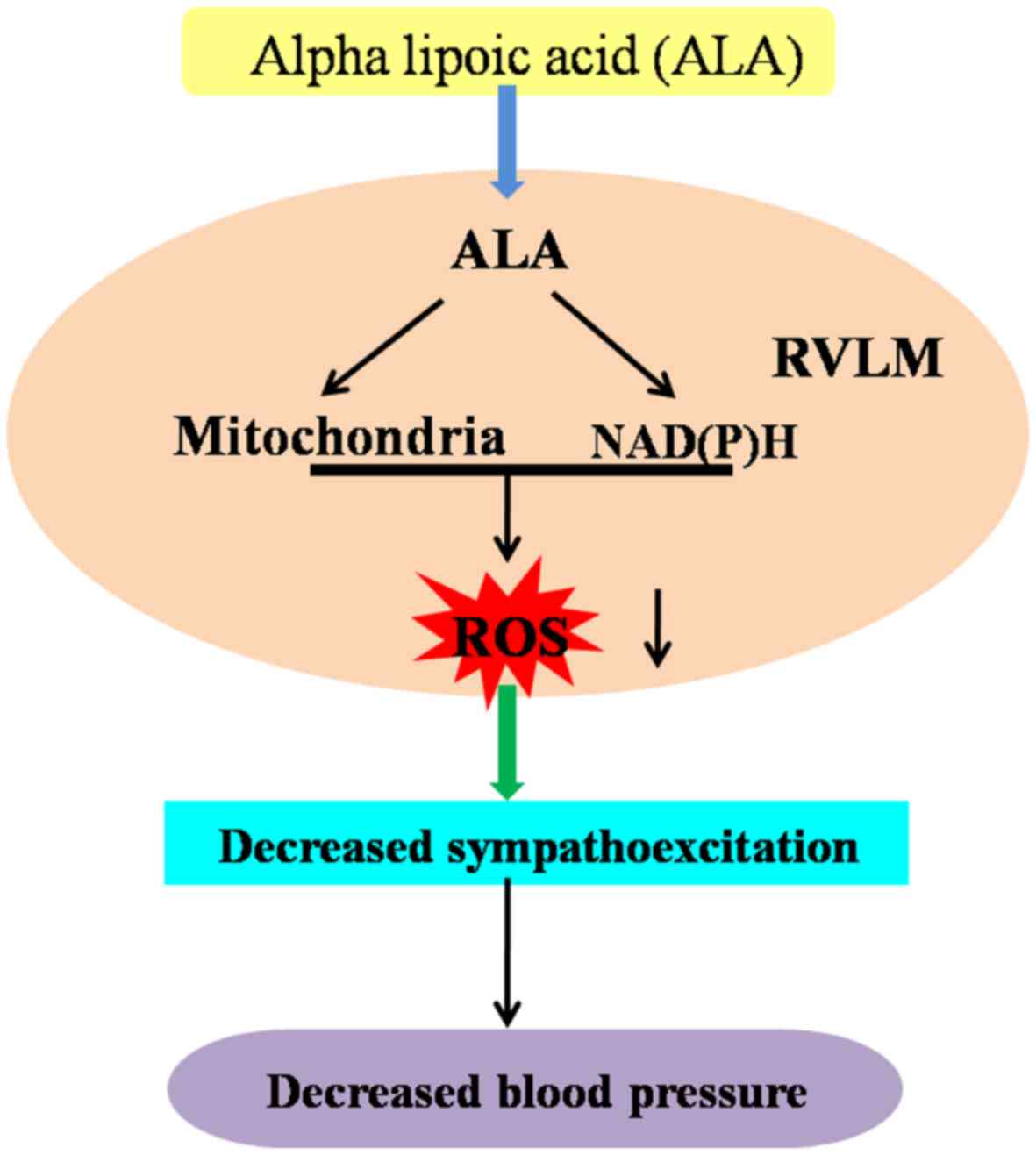
In conclusion, the present findings suggest that the
long-term consumption of a high-salt diet augments BP and induces
the overproduction of ROS derived from NAD(P)H in the mitochondria
in the RVLM, which plays an important pathophysiological role in
the development of hypertension. More importantly, our results
indicate that the long-term supplementation of ALA attenuates
hypertensive responses and attenuates cardiac hypertrophy by
decreasing the expression of NAD(P)H subunits (NOX2 and NOX4),
increasing the levels of mitochondrial bioenergetic enzymes, and
enhancing intracellular antioxidant capacity in the RVLM during the
development of hypertension. The mechanisms responsible for the
effects of ALA on hypertension are presented in Fig. 7.
References
|
1
|
Hall JE, Granger JP, do Carmo JM, da Silva
AA, Dubinion J, George E, Hamza S, Speed J and Hall ME:
Hypertension: Physiology and pathophysiology. Compr Physiol.
2:2393–2442. 2012.
|
|
2
|
Montezano AC, Dulak-Lis M, Tsiropoulou S,
Harvey A, Briones AM and Touyz RM: Oxidative stress and human
hypertension: Vascular mechanisms, biomarkers, and novel therapies.
Can J Cardiol. 31:631–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Virdis A, Bacca A, Colucci R, Duranti E,
Fornai M, Materazzi G, Ippolito C, Bernardini N, Blandizzi C,
Bernini G, et al: Endothelial dysfunction in small arteries of
essential hypertensive patients: Role of cyclooxygenase-2 in
oxidative stress generation. Hypertension. 62:337–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanhoutte PM, Shimokawa H, Tang EH and
Feletou M: Endothelial dysfunction and vascular disease. Acta
Physiol (Oxf). 196:193–222. 2009. View Article : Google Scholar
|
|
5
|
Koga Y, Hirooka Y, Araki S, Nozoe M, Kishi
T and Sunagawa K: High salt intake enhances blood pressure increase
during development of hypertension via oxidative stress in rostral
ventrolateral medulla of spontaneously hypertensive rats. Hypertens
Res. 31:2075–2083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YZ, Chen JK, Li ZP, Zhao T, Ni M, Li
DJ, Jiang CL and Shen FM: High-salt diet enhances hippocampal
oxidative stress and cognitive impairment in mice. Neurobiol Learn
Mem. 114:10–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higashi Y, Maruhashi T, Noma K and Kihara
Y: Oxidative stress and endothelial dysfunction: Clinical evidence
and therapeutic implications. Trends Cardiovasc Med. 24:165–169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrades ME, Ritter C and Dal-Pizzol F:
The role of free radicals in sepsis development. Front Biosci
(Elite Ed). 1:277–287. 2009.
|
|
9
|
Barichello T, Fortunato JJ, Vitali AM,
Feier G, Reinke A, Moreira JC, Quevedo J and Dal-Pizzol F:
Oxidative variables in the rat brain after sepsis induced by cecal
ligation and perforation. Crit Care Med. 34:886–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang ZH, Wei SG, Francis J and Felder RB:
Cardiovascular and renal sympathetic activation by blood-borne
TNF-alpha in rat: The role of central prostaglandins. Am J Physiol
Regul Integr Comp Physiol. 284:R916–R927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumagai H, Oshima N, Matsuura T, Iigaya K,
Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, et al:
Importance of rostral ventrolateral medulla neurons in determining
efferent sympathetic nerve activity and blood pressure. Hypertens
Res. 35:132–141. 2012. View Article : Google Scholar :
|
|
12
|
Brooks VL, Haywood JR and Johnson AK:
Translation of salt retention to central activation of the
sympathetic nervous system in hypertension. Clin Exp Pharmacol
Physiol. 32:426–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carlson SH, Roysomutti S, Peng N and Wyss
JM: The role of the central nervous system in NaCl-sensitive
hypertension in spontaneously hypertensive rats. Am J Hypertens.
14:155S–162S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith AR, Shenvi SV, Widlansky M, Suh JH
and Hagen TM: Lipoic acid as a potential therapy for chronic
diseases associated with oxidative stress. Curr Med Chem.
11:1135–1146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McNeilly AM, Davison GW, Murphy MH, Nadeem
N, Trinick T, Duly E, Novials A and McEneny J: Effect of α-lipoic
acid and exercise training on cardiovascular disease risk in
obesity with impaired glucose tolerance. Lipids Health Dis.
10:2172011. View Article : Google Scholar
|
|
16
|
Wollin SD and Jones PJ: Alpha-lipoic acid
and cardiovascular disease. J Nutr. 133:3327–3330. 2003.PubMed/NCBI
|
|
17
|
Petronilho F, Florentino D, Danielski LG,
Vieira LC, Martins M, Vieira A, Bonfante S, Goldim MP and Vuolo F:
Alpha-Lipoic Acid Attenuates Oxidative Damage in Organs After
Sepsis. Inflammation. 39:357–365. 2016. View Article : Google Scholar
|
|
18
|
Elks CM, Reed SD, Mariappan N,
Shukitt-Hale B, Joseph JA, Ingram DK and Francis J: A
blueberry-enriched diet attenuates nephropathy in a rat model of
hypertension via reduction in oxidative stress. PLoS One.
6:e240282011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao L, Wang W, Li YL, Schultz HD, Liu D,
Cornish KG and Zucker IH: Superoxide mediates sympathoexcitation in
heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ
Res. 95:937–944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su Q, Liu JJ, Cui W, Shi XL, Guo J, Li HB,
Huo CJ, Miao YW, Zhang M, Yang Q, et al: Alpha lipoic acid
supplementation attenuates reactive oxygen species in hypothalamic
paraventricular nucleus and sympathoexcitation in high salt-induced
hypertension. Toxicol Lett. 241:152–158. 2016. View Article : Google Scholar
|
|
21
|
Agarwal D, Welsch MA, Keller JN and
Francis J: Chronic exercise modulates RAS components and improves
balance between pro- and anti-inflammatory cytokines in the brain
of SHR. Basic Res Cardiol. 106:1069–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lakroun Z, Kebieche M, Lahouel A, Zama D,
Desor F and Soulimani R: Oxidative stress and brain mitochondria
swelling induced by endosulfan and protective role of quercetin in
rat. Environ. Sci Pollut Res Int. 22:7776–7781. 2015. View Article : Google Scholar
|
|
23
|
Begue JA and Aust SD: Microsomal lipid
peroxidation. Methods Enzymol. 52:302–310. 1978. View Article : Google Scholar
|
|
24
|
Kono Y: Generation of superoxide radical
during autoxidation of hydroxylamine and an assay for superoxide
dismutase. Arch Biochem Biophys. 186:189–195. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sedlak J and Lindsay RH: Estimation of
total protein bound and nonprotein sulfhydryl groups in tissue with
Ellman's reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guggilam A, Cardinale JP, Mariappan N,
Sriramula S, Haque M and Francis J: Central TNF inhibition results
in attenuated neurohumoral excitation in heart failure: A role for
superoxide and nitric oxide. Basic Res Cardiol. 106:273–286. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guggilam A, Haque M, Kerut EK, McIlwain E,
Lucchesi P, Seghal I and Francis J: TNF-alpha blockade decreases
oxidative stress in the paraventricular nucleus and attenuates
sympathoex-citation in heart failure rats. Am J Physiol Heart Circ
Physiol. 93:H599–H609. 2007. View Article : Google Scholar
|
|
28
|
Karppanen H and Mervaala E: Sodium intake
and hypertension. Prog Cardiovasc Dis. 49:59–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frohlich ED: The salt conundrum: A
hypothesis. Hypertension. 50:161–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang BS, Van Vliet BN and Leenen FH:
Increases in CSF [Na+] precede the increases in blood
pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol
Heart Circ Physiol. 287:H1160–H1166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang BS, Cheung WJ, Wang H, Tan J, White
RA and Leenen FH: Activation of brain renin-angiotensin-aldosterone
system by central sodium in Wistar rats. Am J Physiol Heart Circ
Physiol. 291:H1109–H1117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang BS, Amin MS and Leenen FH: The
central role of the brain in salt-sensitive hypertension. Curr Opin
Cardiol. 21:295–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Huang BS and Leenen FH: Brain
sodium channels and ouabainlike compounds mediate central
aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol.
285:H2516–H2523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nozoe M, Hirooka Y, Koga Y, Araki S, Konno
S, Kishi T, Ide T and Sunagawa K: Mitochondria-derived reactive
oxygen species mediate sympathoexcitation induced by angiotensin II
in the rostral ventrolateral medulla. J Hypertens. 26:2176–2184.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zimmerman MC and Davisson RL: Redox
signaling in central neural regulation of cardiovascular function.
Prog Biophys Mol Biol. 84:125–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goraca A, Huk-Kolega H, Piechota A,
Kleniewska P, Ciejka E and Skibska B: Lipoic acid - biological
activity and therapeutic potential. Pharmacol Rep. 63:849–858.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM
and Farrag MM: The potential protective role of alpha-lipoic acid
against acetaminophen-induced hepatic and renal damage. Toxicology.
243:261–270. 2008. View Article : Google Scholar
|
|
38
|
Anto SK, Koyada N, Khan S and Jena G:
α-Lipoic acid attenuates transplacental nicotine-induced germ cell
and oxidative DNA damage in adult mice. J Basic Clin Physiol
Pharmacol. 27:585–593. 2016. View Article : Google Scholar : PubMed/NCBI
|