Introduction
The majority of neurons in the adult mammalian
central nervous system (CNS) fail to spontaneously regenerate
following injury, predominantly due to the presence of
myelin-associated inhibitors and the development of glial scars
that form an inhibitory environment for regeneration (1–5).
Thus, elimination of inhibitory factors, blocking of inhibitory
signaling pathways and increasing the intrinsic growth state of
neurons are the strategies currently used to promote regeneration
of CNS neurons.
Growth associated protein-43 (GAP-43) is a
well-known specific marker of axonal regeneration predominantly
localized at the growth cone and presynaptic terminals of
developing axons. GAP-43 is involved in neuronal pathfinding and
branching during development and regeneration, and aids the
formation and regulation of synapses, which is crucial for synaptic
plasticity. It is highly expressed following CNS injury to promote
neural regeneration (6,7). However, axonal regeneration is
severely restricted by the inhibitory microenvironment formed by
myelin-associated neurite outgrowth inhibitor (Nogo-A) and other
components of the glial scar. Currently, Nogo-A and its receptor
(NgR) are regarded as crucial inhibitory factors for axonal
regeneration following CNS injury (8,9).
The cytokine interleukin-6 (IL)-6, is a major
mediator of inflammation and IL-6 levels are elevated at varying
degrees within the brain, blood and serum following CNS damage. It
has been previously reported that the accumulation of IL-6 is
involved in causing secondary damage following CNS injury and
connective tissue scar formation (10-12). However, several previous studies
have demonstrated that IL-6 is a highly versatile cytokine and
exerts a beneficial effect on neural regeneration and functional
recovery (5–7,13).
The aim of our present study was to provide evidence
validating the neuroprotective and regeneration-enhancing effects
of IL-6. The study initially demonstrated that IL-6 promoted
regeneration of neurons and axons in cultured dissociated dorsal
root ganglion (DRG) neurons and an establish rat spinal cord injury
(SCI) model. Furthermore, the data indicated that the
pro-regenerative effects of IL-6 are associated with the
upregulation of GAP-43 and the downregulation Nogo-A and NgR.
Materials and methods
Materials
The following products were utilized in the present
study: rabbit anti-β-III-tubulin polyclonal antibody (cat. no.
1967-1; Abcam, Cambridge, MA, USA); fluorescein isothiocyanate
(FITC)-labeled goat anti-rabbit IgG (Beijing Zhongshan Jinqiao
Biotechology Co., Ltd., Beijing, China); recombinant rat IL-6
(R&D Systems, Inc., Minneapolis, MN, USA) biotinylated dextran
amine (BDA)-10000 (cat. no. D1956; Molecular Probes, Thermo Fisher
Scientific, Inc., Waltham, MA, USA); 10% BDA (pH 7.3) was prepared
with 0.01 mol/l phosphate-buffered saline (PBS); diaminobenzidine
(DAB) powder (Sigma-Aldrich, St. Louis, MO, USA); 2.5% nickel
ammonium sulfate-DAB was prepared with 0.82 g natrium aceticum, 2.5
g nickel ammonium sulfate and 100 ml H2O2;
CNS myelin proteins (Sigma-Aldrich); Takara RNA PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China); rabbit anti-GAP-43
polyclonal antibody (cat. no. 1751-1; Abcam); rabbit anti-Nogo-A
polyclonal antibody (cat. no. bs-0134R) and rabbit anti-NgR
polyclonal antibody (cat. no. bs-0129R) (both from Bioss, Beijing,
China) and mouse anti-β-actin monoclonal antibody (Boster
Biotechnology Co., Ltd., Wuhan, China).
DRG culture
Adult male Wister rats were deeply anaesthetized
with an intraperitoneal injection of 3.5% chloral hydrate (10
ml/kg). DRG neurons were harvested under a stereomicro-scope and
placed in a 10-ml tube on ice containing Ham's-F12 culture medium.
Following removal of the attached roots and connective tissue
capsules using forceps, DRG neurons were washed with F12 medium 3
times and centrifuged at 500 rpm for 4 min, then subsequently
digested with 0.125% collagenase IV in an incubator for 45 min and
with 0.25% collagenase IV for 15 min. The neurons were washed with
F12 medium following digestion, following which digestion was
terminated with F12 solution containing 20% fetal calf serum (FCS).
The cell suspension was centrifuged at 400 rpm for 5 min following
mechanical isolation of the cells in N2 medium. The supernatant was
discarded and the cell pellet was resuspended in 2 ml N2 medium
added with 15% bovine serum albumin (BSA) then centrifuged again at
900 rpm for 10 min. Cell debris was collected at the BSA/N2
interface and discarded with the supernatant. DRG neurons were
again washed with N2 medium and centrifuged at 500 rpm for 5 min
and plated onto poly-L-lysine pre-coated culture dishes. In the
myelin protein and IL-6 groups, culture wells were also pre-coated
with myelin protein in addition to poly-L-lysine. After
approximately 1 h, cell adherence was detected and then sufficient
N2 medium was added to the culture dish. Cultured DRG neurons were
divided into: sham control, myelin protein and IL-6 intervention
groups. The IL-6 intervention group was further subdivided into 3
groups treated with either 50, 100 or 200 ng/ml IL-6. All
experiments in each group were performed 3 times. After 48 h in
culture, DRG neurons were fixed for immunostaining and mRNA
extraction.
Identification of DRG neurons and
evaluation of purity
The cultured DRG neurons were evaluated for purity
by counting positive neurons demonstrated by the formation of
neuronal processes under a microscope. Cell counting was performed
in 5 random ×400 microscopic fields/well, and the proportion of
positive neurons to the total cell number in 3 wells was calculated
to indicate the purity of the DRG neurons.
Immunocytochemistry
DRG neuronal suspensions were transferred onto
poly-L-lysine pre-coated coverslips in 24-well plates
(1×105 DRG neurons/well). Following incubation, the
cells were washed with 0.01 M PBS, 3×5 min and fixed with 4%
formaldehyde for 30 min. The cells were then incubated with 0.4%
Triton X-100 for 20 min and goat serum for 30 min to block
non-specific protein binding. Subsequently, the cells were
incubated overnight with the primary antibody (rabbit
anti-β-III-tubulin polyclonal antibody; 0.01 M PBS was used in the
sham control group) at 4°C.
Following overnight incubation, the coverslips were
washed with PBS (3×5 min) and incubated with FITC goat anti-rabbit
IgG diluted 1:100 for 2 h at room temperature, and then the
coverslips were washed again (3×5 min) with 0.01 M PBS. Finally,
the coverslips were mounted onto slides with 50% glycerinum. The
lengths of the neural processes in the 50 longest neurons were
measured in 10 randomly selected areas from each coverslip using
confocal laser scanning microscopy. The values are represented as
the mean ± standard error.
Treatment of animals
Pathogen-free Sprague-Dawley (SD) rats (6-week-old;
200–240 g body weight) were purchased from the Laboratory Animal
Center of Chongqing Medical University (Chongqing, China)
[certificate, SCXK (YU) 2007-0001]. The SD rats were maintained
under optimal conditions for hygiene, temperature (20±2°C) and
photo-periods (12-h light:12-h dark), and were provided food and
water ad libitum according to the Institutional Guidelines
for the Care and Use of Laboratory Animals. All animal procedures
were approved by the Ethics Committee of Chongqing Medical
University.
Acute SCI model and subarachnoid
injection
The rat SCI model was produced by a modification of
the classic Allen's weight drop method. A 5 g metal bar with a
2.5-mm diameter was dropped through a guidance glass tube from a
height of 5 cm onto the exposed surface of the spinal cord to
inflict acute SCI centering on the T9 vertebra. With the aid of the
operating microscope, an 8-cm polyethylene catheter was inserted at
a 30° angle through the foramen magnum, advanced 3 cm caudally to
the spinal subarachnoid space according to a procedure described
previously (14). IL-6 (10, 50
and 100 pmol/kg/day) and 5 ml saline were administered daily
through the implanted catheter separately using a microsyringe for
7 consecutive days in the IL-6 group and saline group, whereas the
sham control group did not receive IL-6 or saline
administration.
Behavioral testing
The lower limb function of rats was tested via
Basso, Beattie and Bresnahan (BBB) scoring (15) prior to the operation and at day 1,
3, 5, 7, 10 and 14 post-operation.
BDA injection
Rats were randomly divided into 2 groups and
received (n=5 each) saline or treatment with IL-6. At day 14
following the establishment of the SCI model, the rats were
anesthetized with 3.5% chloral hydrate (10 ml/kg, i.p.), and then
placed on a stereotaxic apparatus, according to previously
described methods with appropriate modifications (16). An incision was made along the
midline of the scalp, the periosteum was cleared and the bregma was
exposed. Points (A=-1.0 mm, R=+1.0 mm; A=+4.0 mm, R=+1.0 mm; A=-1.0
mm, R=+5.0 mm; A=+4.0 mm, R=+5.0 mm; A, indicates anterior to the
bregma; R, indicates right of the bregma) were marked centering on
the bregma to locate the right sensorimotor cortex and a
rectangular-shaped flap between the 4 points was removed and bone
windows measuring 5×4 mm were prepared. Multiple injections of BDA
(molecular weight, 10,000; 10% 0.1 M phosphate buffer; pH 7.3;
Molecular Probes, Thermo Fisher Scientific, Inc.) through a 10
μl microsyringe were performed at the right sensorimotor
cortex at 5 points with depths of 2 mm and 1 mm separately per
site. For each injection, 1 μl of BDA solution was gradually
delivered.
Antegrade tracing with BDA
At 3 weeks after the injection of BDA, the rats were
anaesthetized with 3.5% chloral hydrate, the L8-L10 vertebrae (with
measured intervals from 5 mm above to 5 mm below the lesion site)
were removed and fixed in 4% formaldehyde at 4°C overnight, then
transferred to a 30% sucrose solution and incubated at 4°C until
the tissue was deposited. Transverse sections (50-μm
thickness) were cut using a cryostat (Leica-1850; Leica Biosystems,
Wetzlar, Germany) and incubated in Tris-buffered saline (TBS)
solution (0.05 M, pH 7.6) with 0.3% Triton X-100 at room
temperature for 4 h. The sections were washed with 0.05 M TBS (3×5
min) and then incubated in horseradish peroxidase (HRP)-labeled
streptavidin (1:200) at 4°C overnight. The HRP-labeled streptavidin
was removed, and the sections were washed with 0.05 M TBS (3×5
min). After 5-10 min preincubation in 2.5% ammonium nickel sulfate
and 0.035% DAB, 0.05 mol/l TBS was used to terminate the reaction.
The sections were then transferred to glass slides pre-coated with
3-aminopropyltriethoxysilane and dehydrated in an ascending ethanol
gradient, deparaffinized with xylene and embedded in epoxy resin.
Finally, images were captured using a fluorescence microscope.
Immunofluorescence for the detection of
GAP-43
The procedures performed to detect GAP-43 by
immunofluorescence were identical to the immunohistochemistry
experiments. Rabbit anti-GAP-43 polyclonal antibody (1:300
dilution) was used as the primary antibody, and fluorescence
intensity was quantified based on the mean value of 4-5 cells/field
measured using Quant Report software and laser confocal
microscopy.
Western blotting for GAP-43, Nogo-A and
NgR
Briefly, total protein was extracted from DRG
neurons at 48 h after culture and removal from injured spinal cord
tissue. The protein concentration was determined by Bradford assay.
Equivalent amounts of protein (30 μg) from each sample were
separated on 10% SDS-PAGE gels and transferred onto a
polyvinylidene fluoride membrane (Sigma-Aldrich). The membrane was
blocked with 5% non-fat milk in TBS, incubated with a rabbit
anti-GAP-43 polyclonal antibody (1:200), rabbit anti-Nogo-A
polyclonal antibody (1:200), rabbit anti-NgR polyclonal antibody
(1:200) and a mouse anti-β-actin monoclonal antibody (1:1,000)
overnight at 4°C, followed by incubation with corresponding
HRP-conjugated secondary antibodies (1:2,000) overnight at 4°C.
Specific blots were developed using ECL-Plus chemiluminescence. The
densitometric quantification of the bands was performed using
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The results are expressed as the ratio of the expression of
GAP-43, Nogo-A and NgR to β-actin.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was purified from the SCI DRG neurons
after 48 h in culture. The primers for GAP-43, Nogo-A, NgR and
glycer-aldehyde 3-phosphate dehydrogenase (GAPDH) were designed
according to the published cDNA sequences (Table I). PCR was performed under the
following conditions: denaturation at 94°C for 2 min; 29 cycles of
denaturation at 94°C for 30 sec, annealing at 53°C for 30 sec and
extension at 72°C for 1 min; followed by a final extension at 72°C
for 10 min.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Genes | Sense (5′→3′) | Antisense
(5′→3′) | Product
size
(bp) | GenBank
accession no. |
|---|
| GAP-43 |
aggccaaggagaaggatgatg |
tagctttagcagcactttctg | 220 | NM_017195 |
| NgR |
ttctgcatggcaaccgtatcc |
ttggcaaacaggtagagggtc | 157 | NM_053613 |
| Nogo-A |
cttccttctctatctcctctc |
atggatttgttgccctctctg | 148 | NM_031831 |
| GAPDH |
gtctacatgttccagtatgac |
ccaaagttgtcatggatgacc | 376 | NM_017008 |
Statistical analysis
Data are presented as the means ± 6 standard
deviations. Data analyses were performed with SPSS 10.0 software
(SPSS, Inc, Chicago, IL, USA). Differences were examined for
statistical significance using one-way analysis of variance (ANOVA)
for comparisons involving more than 2 groups and the Student's
t-test for comparisons between 2 groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification and purity determination
of DRG neurons
DRG neuronal identification was performed following
48 h in culture. Neurons exhibiting axonal sprouting under
microscopic examination were considered as positive cells. The
number of positive cells and the number of cells were counted in 5
random ×400 microscopic fields of 3 wells. The proportion of
positive cells represented the purity of the DRG neurons. The
purity of DRG was >95% in the present study.
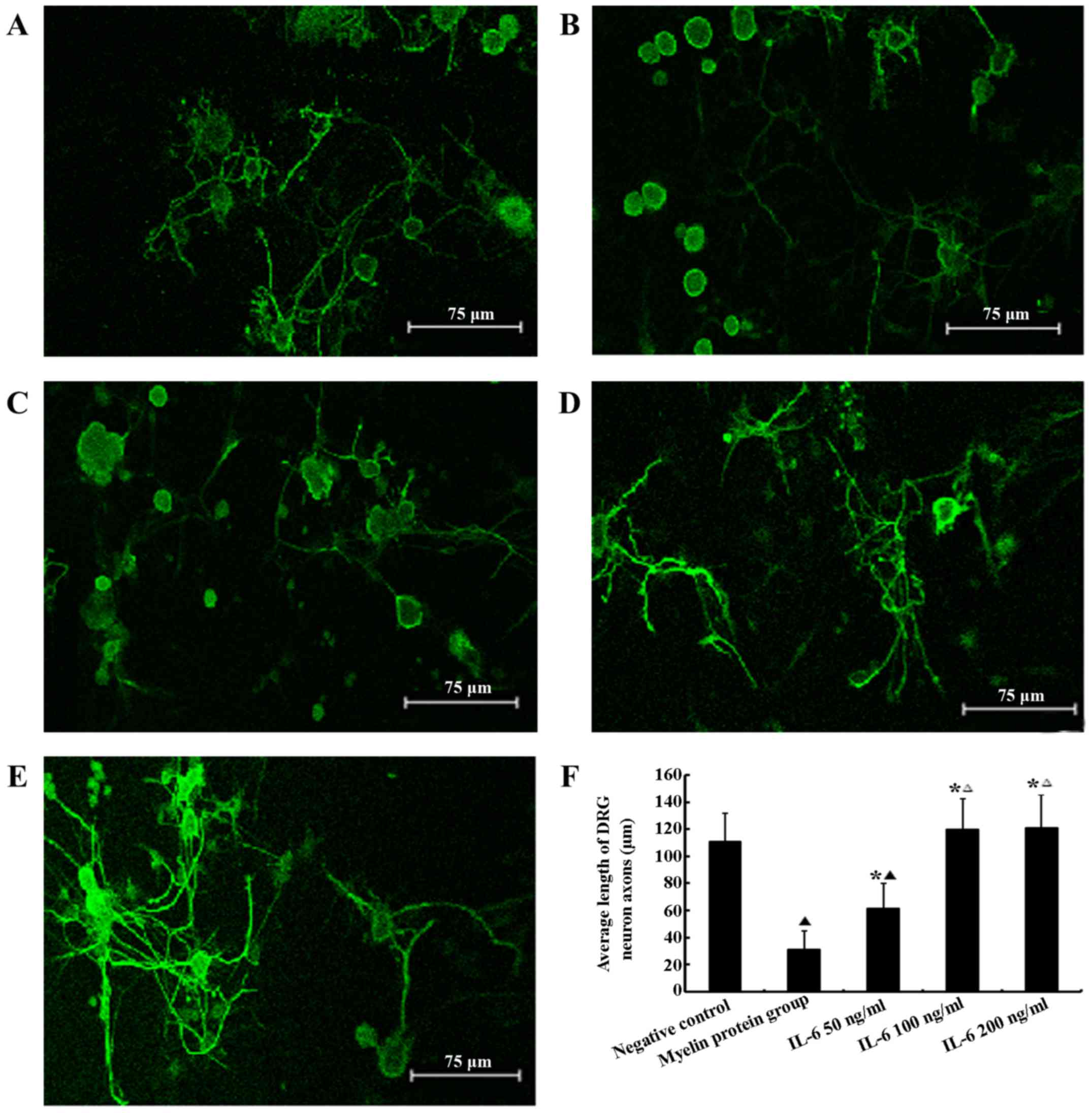
Effect of myelin proteins and IL-6 on the
survival of DRG neurons in vitro
MTT assays indicated no significant difference in
DRG neuronal survival among groups treated with the various
concentrations of IL-6 and the sham control (P>0.05), whereas
myelin proteins significantly decreases DRG neuronal survival
(P<0.05). Following 48 h in culture, DRG neurons in the myelin
protein group exhibited significantly shorter neurites compared
with the sham control and IL-6 groups (P<0.05). All of the IL-6
concentrations used promoted neurite outgrowth resulting in marked
neurite extension compared with the myelin protein group. Thus,
IL-6 reduced the inhibitory effect of myelin proteins (P<0.05).
Furthermore, IL-6 treatment enhanced axonal regrowth in a
dose-dependent manner demonstrating partial resistance to the
effects of myelin proteins with 50 ng/ml IL-6 and complete
resistance in the 100 and 200 ng/ml groups. Neurites in the 50
ng/ml IL-6 group were significantly shorter compared with the 100
and 200 ng/ml groups (P<0.05). However, at a concentration of
100 ng/ml IL-6, the neurite length did not increase further as the
concentration increased. There were no significant differences in
neurite length among the 100, 200 ng/ml IL-6 and the sham control
group (P>0.05; Fig. 1).
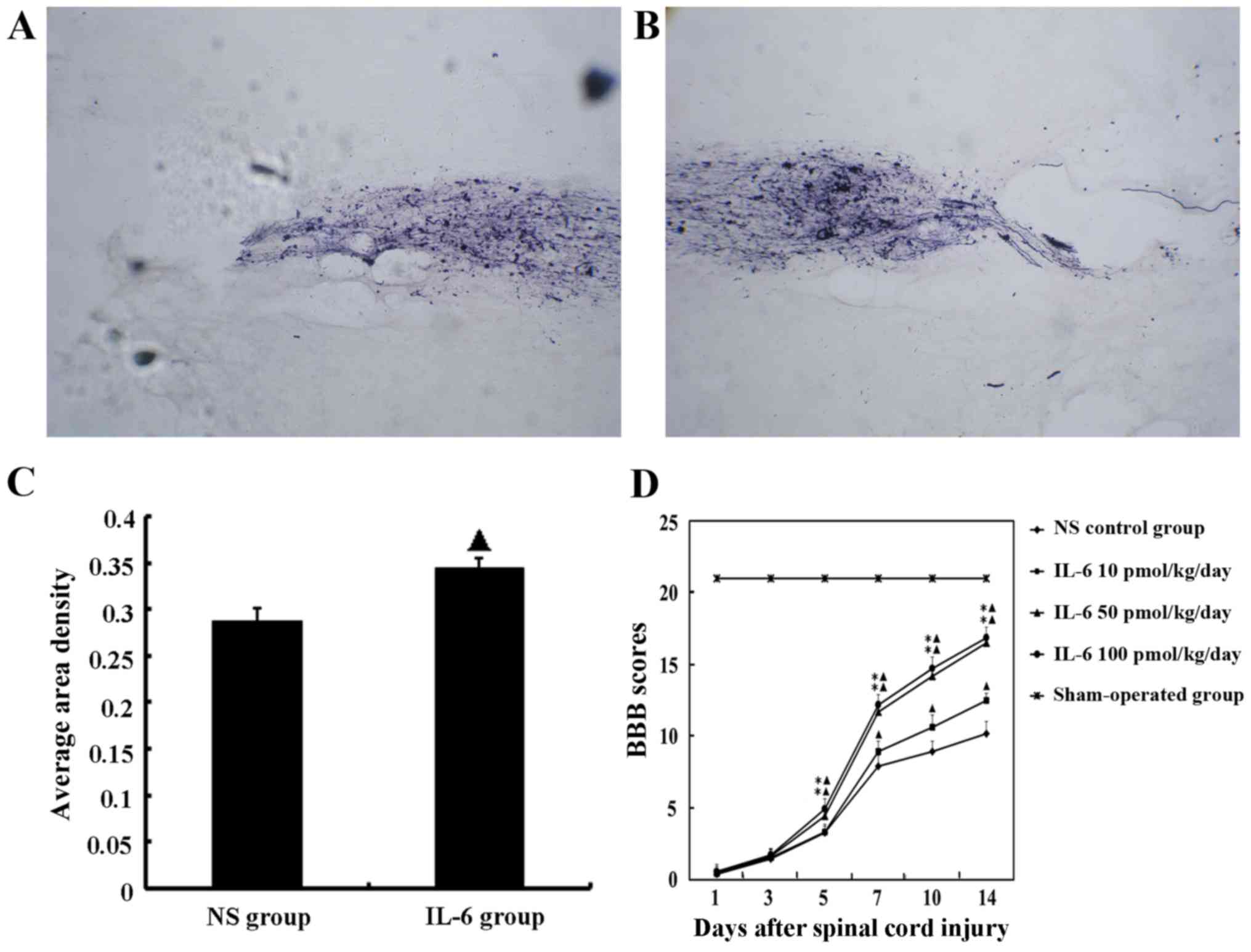
IL-6 promotes functional recovery
following SCI in vivo
The functional deficit in rats was measured at day 1
following SCI operation using the BBB scoring. Animals with a score
>1 were excluded from further analyses. According to this
criterion, 73 rats with experimentally induced SCI were randomly
divided into 5 groups: sham operation, saline and IL-6 treatment
with low-, moderate- and high-doses. Routine manually assisted
urination was provided 2-3 times/day following the operation until
the recovery of voluntary urination 7–10 days later. Due to
difficulty in urination assistance, 3 rats died from bladder
rupture; thus, 72 rats were finally included in the BBB scoring
analysis with 14 rats in each group.
BBB scores of the sham operation group were
significantly different compared with SCI rats at all time-points
investigated (P<0.05). Rats treated with the low-dose of IL-6
exhibited gradually increasing BBB scores from day 7 post-surgery
and were consistently increased compared with the saline group
during observation (P<0.05). In the groups that received
moderate- and high-dose IL-6, BBB scores began to increase from day
5 post-surgery and were significantly higher compared with the
saline and low-dose IL-6 groups (P<0.05; Fig. 2D).
Anterograde tracing of corticospinal
tract (CST) axons by BDA indicates that IL-6 enhances axonal
regeneration
Hematuria was resolved post-operatively in 8 out of
10 rats and disappeared within 4 days. Routine manually assisted
urination was performed 2-3 times/day post-surgery until the
recovery of voluntary urination after 7–10 days.
Rats were suspended by lifting of the tails at 2 h
after BDA administration. When flexion and arm holding were
observed in the left upper limbs and disappeared 3-5 h later,
tracing to the right motor cortex was considered to have been
successful.
In the saline group, the damaged segment of the
spinal cord exhibited CTS interruption, axon retraction and an
absence of growth cones at the rostral end, and few BDA-labeled
fibers were observed to have assembled and grown through the lesion
to reach the segment distal to the lesion center. Whereas in the
IL-6 treatment group, very robust growth cone formation and
aggregation of BDA-labeled fibers were observed, and several
BDA-labeled axons were observed at the caudal end of the lesion
(Fig. 2A and B).
Furthermore, pathological image analysis (CM-2000B;
Beijing, China) demonstrated that the density of BDA-labeled axons
was significantly increased in the IL-6 treatment group compared
with the saline group. The mean area density values were
0.344±0.011 and 0.288±0.013, respectively (P<0.05; Fig. 2C).
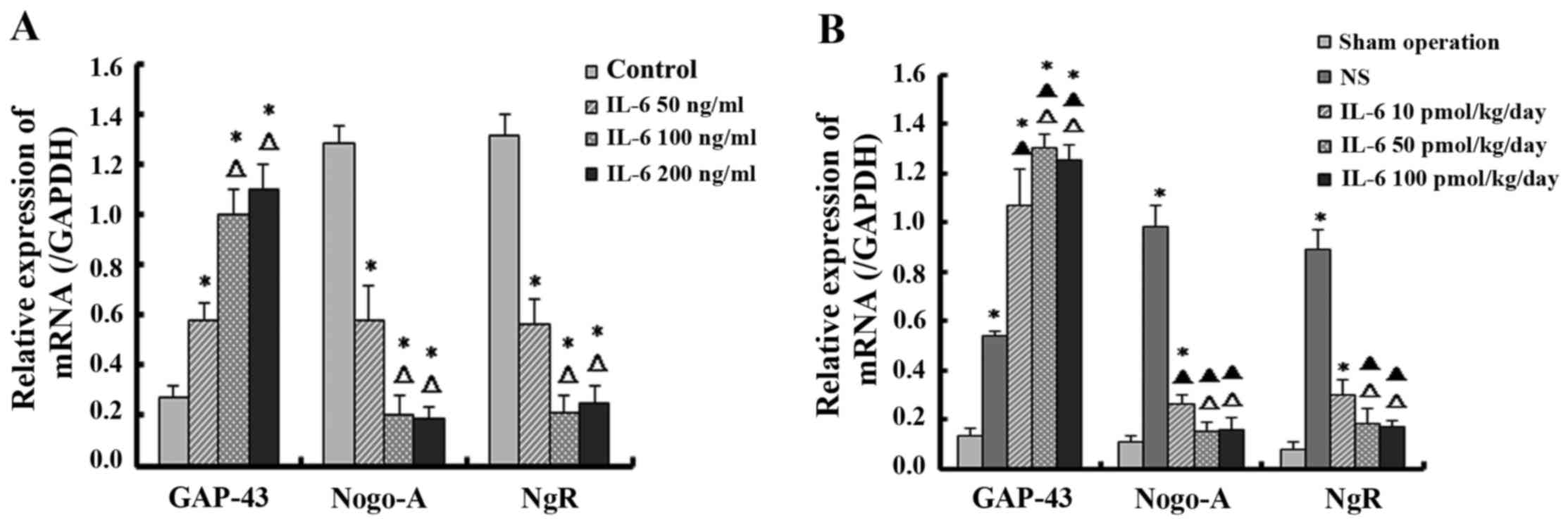
IL-6 upregulates the expression of GAP-43
and downregulates the expression of Nogo-A and NgR in dissociated
DRG neurons and SCI tissue
Semi-quantitative analyses of PCR bands were
performed using the Quantity One software analysis system and the
results were normalized to the optical density of the reference
gene bands. The isolated DRG neurons treated with low-dose IL-6
demonstrated significantly higher expression of GAP-43 mRNA and
lower expression of Nogo-A and NgR mRNA compared with the control
group (P<0.05). Furthermore, compared with the control and
low-dose IL-6 groups, the moderate- and high-dose IL-6 groups
exhibited a significantly higher level of GAP-43 mRNA and lower
levels of Nogo-A and NgR mRNA (P<0.05). However, there was no
significant difference between mRNA levels in the moderate- and
high-dose IL-6 groups (P>0.05; Fig. 3A).
The mRNA expression of GAP-43 was increased in
groups administrated with various doses of IL-6 and saline compared
with the sham control group. Furthermore, the GAP-43 level was
significantly increased in the IL-6 treatment group compared with
the saline group (P<0.05). Additionally, among groups treated
with IL-6, the level of GAP-43 mRNA in the moderate- and high-dose
groups were significantly higher compared with the lose-dose group
(P<0.05). However, the difference in GAP-43 levels between the
moderate- and high-dose groups was not significant (P>0.05).
Regarding Nogo-A and NgR, compared with the sham group, the mRNA
level was highest in the saline group, and was decreased in the
groups treated with IL-6 (P<0.05) compared with the saline
group. The moderate- and high-dose IL-6 significantly downregulated
the expression of Nogo-A and NgR mRNA compared with the saline
group to the level detected in the sham operation group
(P>0.05). Additionally, there was no significant difference
between the moderate- and high-dose group (P>0.05; Fig. 3B).
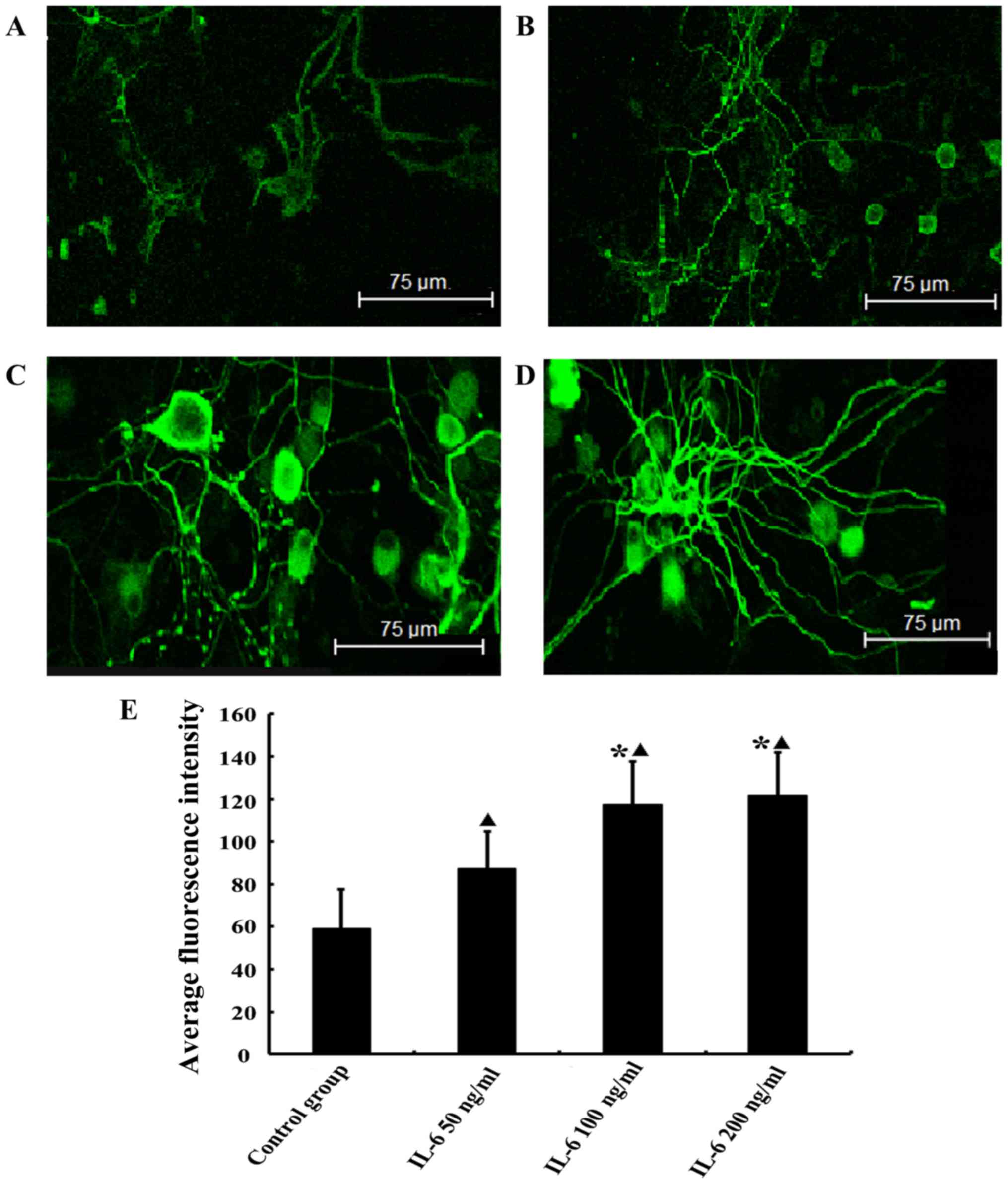
IL-6 upregulates the expression of GAP-43
in isolated DRG neurons
Green fluorescence represents the FITC-labeled
target protein. The GAP-43 staining was more intense in the
low-dose IL-6 group compared with the control group (P<0.05) and
the intensity of staining was increased further in the moderate-
and high-dose IL-6 groups. There was no observable difference
between the GAP-43 staining in the moderate- and high-dose groups
(P>0.05; Fig. 4).
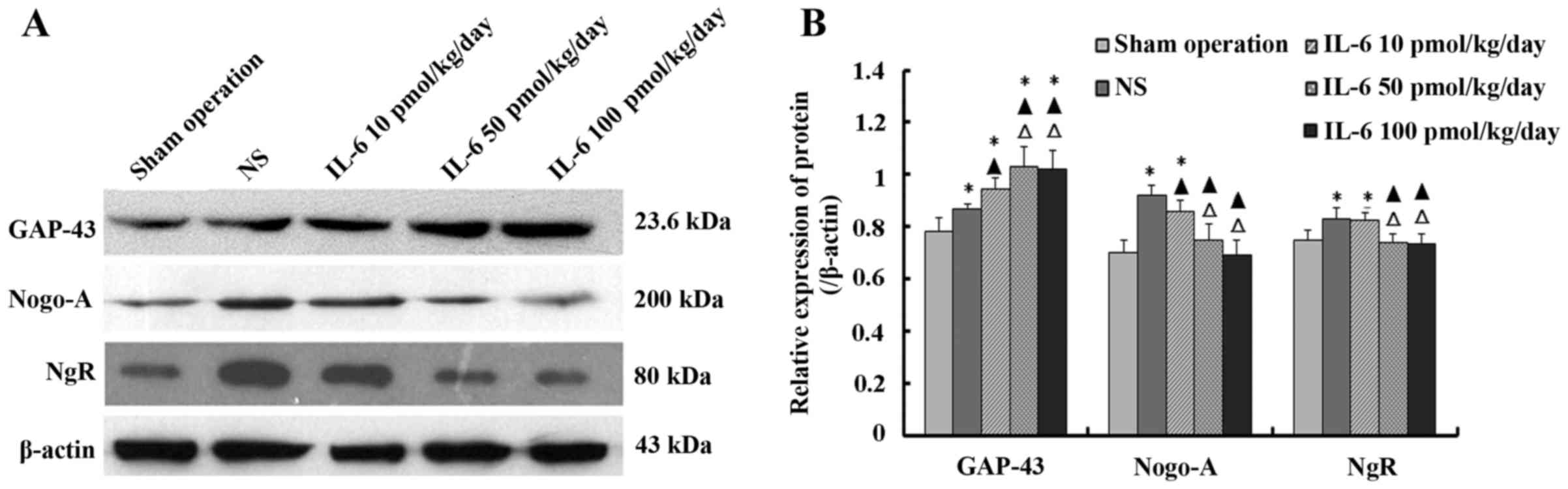
Effect of IL-6 on the expression of
GAP-43, Nogo-A and NgR protein in injured spinal cord tissue
The levels of GAP-43, Nogo-A and NgR protein in
injured spinal cord tissue were determined by western blotting. The
results indicated that the expression level of GAP-43 protein was
highest in the moderate- and high-dose IL-6 groups, compared to the
low-dose IL-6 and saline groups, and with the lowest level noted in
the sham operation group. The difference between the moderate- and
high-dose IL-6 group was not significant (P>0.05).
Compared with the sham operation group, the levels
of Nogo-A and NgR protein were significantly higher in the saline
and low-dose IL-6 groups (P<0.05), whereas the difference
between the moderate- and high-dose IL-6 group was not significant
(P>0.05). Furthermore, the levels of Nogo-A and NgR protein were
highest in the saline group, than these levels in the low-dose IL-6
group, and lowest in the moderate- and high-dose IL-6 groups. The
difference between the moderate- and high-dose IL-6 group was not
significant (P>0.05; Fig.
5).
Discussion
Damage to the adult spinal cord often leads to
persistent deficits due to the inability of mature axons to
regenerate following injury. Mounting evidence suggests that the
inhibitory local extracellular environment, formation of the glial
scar and a decrease in the intrinsic regeneration capacity of
mature neurons are the primary obstacles for axon regeneration.
Various oligo-dendrocyte-derived myelin-associated neurogrowth
inhibitory factors, including myelin-associated protein (MAP), Nogo
and oligodendrocyte myelin glycoprotein (OMgp) are the main
components of the extracellular inhibitory environment (1–4,17).
Recently, an increasing number of studies have demon
strated that regeneration of a damaged adult axon is possible by
either increasing the permissive cues or decreasing the
non-permissive cues of the existing environment. This observation
was followed by a series of significant results within animal
spinal cord injury research. As a highly versatile cytokine, IL-6
has been previously reported to have both detrimental and
beneficial effects in the nervous system. The detrimental effects
are usually attributed to its pro-inflammatory actions contributing
to inflammation following SCI. However, various studies have
demonstrated that IL-6 is also involved in neuroprotection
following SCI injury (5–7,13,18). IL-6 is downstream of cyclic AMP,
which has proven to be one of the most effective means of
overcoming inhibition of axonal regeneration (18–22).
To mimic the inhibitory environment following SCI,
myelin proteins were used as a culture substrate in the present
study to observe the effect of IL-6 on the survival and neurite
outgrowth of isolated DRG neurons. The in vitro results
indicated that the adminstration of exogenous IL-6 to the culture
medium promoted neurite outgrowth in dissociated DRG neurons
cultured on myelin proteins in a dose-dependent manner, exerting a
partial effect at the concentration of 50 ng/ml, and a complete
effect at 100 ng/ml. Enhancement of neuron regeneration ability was
not observed as the concentration of IL-6 increased from 100 to 200
ng/ml. Different doses of IL-6 exhibited varying effects on
functional recovery following SCI. Faster functional recovery and
higher BBB scores were observed in rats treated with moderate- and
high-dose IL-6. This demonstrated that the myelin proteins
inhibited neurite outgrowth of dissociated DRG neurons and that
exogenous IL-6 is beneficial for axonal regeneration by abrogating
myelin protein-mediated inhibition of regeneration in vitro.
The promoting effect of IL-6 on axonal regeneration and spinal
functional recovery was dose-dependent, which was in accordance
with previous findings by Hakkoum et al (6). The mechanism of action of IL-6 in
overcoming myelin inhibitors is via activation of the classic IL-6
trimeric receptor, resulting in activation of the Janus
kinase/signal transducer and activator of transcription 3 (STAT3)
and mitogen-activated protein kinase (MAPK) signaling cascade
(5,13,18,23,24). These different signaling pathways
have been proposed to be important for the intracellular signaling
mechanisms triggered by injury or associated with synaptic
plasticity. For example, activation of STAT3 in retinal ganglion
cells is essential for inflammatory stimulation, including
IL-6-induced neuroprotection and axonal regeneration (5). Furthermore, the MAPK pathway is
crucial for the stimulatory effects of neurotrophic factors,
including nerve growth factor and glial-derived neuro-trophic
factor, on neurite outgrowth (25–27). Thus, IL-6 may activate these
signaling cascades to promote regeneration in lesioned neurons
(28).
BDAs are highly sensitive tools for anterograde and
retrograde pathway tracing studies of the nervous system (16,29). In the present study,
microinjection of BDA was performed at multiple sites in the rat
sensorimotor cortex to evaluate axonal sprouting following SCI, as
impaired axon plasmic transport is followed by distal and partial
proximal axon degeneration and fracture. The BDA tracing study
demonstrated retraction and disruption of axons from the initial
site the CST. In the saline-treated rats, no axons passed through
the lesion site, whereas IL-6-treated rats exhibited a high-density
of BDA-positive fibers in the sagittal section, with the
compensatory spouts either directly growing through the lesion or
circumventing the injury site to the distal segment of the spinal
cord. These morphological results suggested that intrathecal
administration of IL-6 promoted compensatory sprouting of the CST
following SCI.
To maintain the stable and complex state in the
mature nervous system, the regeneration and plasticity of adult
neurons are restricted. There is, however, evidence from animal
studies demonstrating that axonal elongation and partial neural
reconstruction is triggered by axonal injury, and that this process
is largely dependent on the synthesis of certain proteins, to which
GAP-43 is closely associated. As a marker of axonal regeneration,
GAP-43 is a nervous tissue-specific cytoplasmic protein highly
expressed in neuronal growth cones, regenerated Schwann and glial
cells during development, and during axonal regeneration. It is
thought to be involved in neural development, neurite elongation
and synapse formation (30,31). The first step of axonal
regeneration is the formation of the growth cone. Normally, the
combination of G proteins and receptors within growth cones induces
growth cone collapse and growth inhibition. GAP-43 is expressed on
the surface of growth cones, and its binding to G proteins induces
release of G proteins from its combination and termination of
subsequent inhibitory signaling pathways, which ultimately leads to
elongation and regeneration of axons (32). The activated growth cones extend
the presynaptic membrane and form synaptic connections resulting in
enhanced release of vesicular transmitters and stimulation of
various biological effects (33,34). However, the presence of an
inhibitory environment in the CNS extremely limits axonal
regeneration following SCI. To date, myelin-associated protein
Nogo-A has been identified as the most potent inhibitor of neurite
growth. The lack of CNS regeneration in adult mammals is largely
attributed to the presence of Nogo-A, and its receptor NgR
(9,35–37). Nogo-A has been demonstrated to
bind to its specific receptor, NgR, to initiate a signaling cascade
resulting in inhibition of neurite growth. Nogo-A is involved in
regulating actin cyto-skeleton dynamics in local growth cones,
inducing retraction of filopodia and lamellipodia, and ultimately
stimulating the collapse of growth cones (38). In addition to Nogo-A, two other
myelin-associated neurite growth inhibitors, myelin-associated
glycoprotein and OMgp, also bind to NgR. NgR knockdown or
downregulation may be an effective disinhibitory strategy to
promote CNS axon regeneration (39,40).
Axonal regeneration is the outcome of a
counterbalance between stimulatory and inhibitory factors. Thus,
there are two approaches to encourage regeneration: altering the
environment by blocking/neutralizing inhibitors of regeneration
and/or altering the intrinsic growth state of the neuron. The
results presented in the present study demonstrated that the levels
of GAP-43 mRNA and protein were significantly increased in damaged
spinal cord tissue. Administration of IL-6 significantly increased
the levels of GAP-43 mRNA and protein in damaged spinal cord tissue
and upregulation of the GAP-43 mRNA and protein levels in isolated
DRG neurons. The present study demonstrated that addition of
exogenous IL-6 to the culture medium or by subarachnoid injection
resulted in a dose-dependent increase in GAP-43 expression, which
is in accordance with a previous investigation performed using an
organotypic hippocampal slice culture model (6). Additionally, in the present study,
the protein expression levels of Nogo-A and NgR were significantly
increased in the damaged spinal cord tissue, and this increase was
downregulated by IL-6 in the damaged tissue and isolated
neurons.
Taken together, the results of the present study
suggest that IL-6 promotes axonal regeneration via stimulating the
intrinsic growth state of neurons and resisting the extrinsic
inhibitory environment. The cellular and molecular mechanisms by
which IL-6 exerts its beneficial effect may be attributed to its
upregulation of GAP-43 expression and simultaneous downregulation
of Nogo-A and NgR levels. This provides new insights into the
mechanisms of the Nogo-A system in axonal regeneration and will aid
the development of novel treatment strategies for SCI.
Acknowledgments
This study was supported by grants from the
Chongqing Nature Science Foundation (grant no. CSTC2013jcyjA10079).
The authors would also like to thank the editors of the Spandidos
Publications - English Language Editing Service, for professional
English language editing of this article.
References
|
1
|
Wang KC, Koprivica V, Kim JA, Sivasankaran
R, Guo Y, Neve RL and He Z: Oligodendrocyte-myelin glycoprotein is
a Nogo receptor ligand that inhibits neurite outgrowth. Nature.
417:941–944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Filbin MT: Myelin-associated inhibitors of
axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci.
4:703–713. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geoffroy CG and Zheng B: Myelin-associated
inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol.
27:31–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JK and Zheng B: Role of
myelin-associated inhibitors in axonal repair after spinal cord
injury. Exp Neurol. 235:33–42. 2012. View Article : Google Scholar
|
|
5
|
Leibinger M, Andreadaki A, Diekmann H and
Fischer D: Neuronal STAT3 activation is essential for CNTF- and
inflammatory stimulation-induced CNS axon regeneration. Cell Death
Dis. 4:e8052013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hakkoum D, Stoppini L and Muller D:
Interleukin-6 promotes sprouting and functional recovery in
lesioned organotypic hippocampal slice cultures. J Neurochem.
100:747–757. 2007. View Article : Google Scholar
|
|
7
|
Chidlow G, Wood JP, Ebneter A and Casson
RJ: Interleukin-6 is an efficacious marker of axonal transport
disruption during experimental glaucoma and stimulates
neuritogenesis in cultured retinal ganglion cells. Neurobiol Dis.
48:568–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang T, Xiong JQ, Ren XB and Sun W: The
role of Nogo-A in neuroregeneration: a review. Brain Res Bull.
87:499–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pernet V and Schwab ME: The role of Nogo-A
in axonal plasticity, regrowth and repair. Cell Tissue Res.
349:97–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu CH, Yhee JY, Kim JH, Im KS, Kim NH,
Jung DI, Lee HC, Chon SK and Sur JH: Pro- and anti-inflammatory
cytokine expression and histopathological characteristics in canine
brain with traumatic brain injury. J Vet Sci. 12:299–301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada S, Nakamura M, Mikami Y, Shimazaki
T, Mihara M, Ohsugi Y, Iwamoto Y, Yoshizaki K, Kishimoto T, Toyama
Y, et al: Blockade of interleukin-6 receptor suppresses reactive
astrogliosis and ameliorates functional recovery in experimental
spinal cord injury. J Neurosci Res. 76:265–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerrero AR, Uchida K, Nakajima H,
Watanabe S, Nakamura M, Johnson WE and Baba H: Blockade of
interleukin-6 signaling inhibits the classic pathway and promotes
an alternative pathway of macrophage activation after spinal cord
injury in mice. J Neuroinflammation. 9:402012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XQ, Peng YP, Lu JH, Cao BB and Qiu
YH: Neuroprotection of interleukin-6 against NMDA attack and its
signal transduction by JAK and MAPK. Neurosci Lett. 450:122–126.
2009. View Article : Google Scholar
|
|
14
|
Ou S, Zhao YD, Xiao Z, Wen HZ, Cui J and
Ruan HZ: Effect of lappaconitine on neuropathic pain mediated by
P2X3 receptor in rat dorsal root ganglion. Neurochem
Int. 58:564–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bareyre FM, Haudenschild B and Schwab ME:
Long-lasting sprouting and gene expression changes induced by the
monoclonal antibody IN-1 in the adult spinal cord. J Neurosci.
22:7097–7110. 2002.PubMed/NCBI
|
|
17
|
Matsushita H, Endo S, Kobayashi E,
Sakamoto Y, Kobayashi K, Kitaguchi K, Kuroki K, Söderhäll A,
Maenaka K, Nakamura A, et al: Differential but competitive binding
of Nogo protein and class i major histocompatibility complex (MHCI)
to the PIR-B ectodomain provides an inhibition of cells. J Biol
Chem. 286:25739–25747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry
N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, et al: The
cytokine interleukin-6 is sufficient but not necessary to mimic the
peripheral conditioning lesion effect on axonal growth. J Neurosci.
26:5565–5573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiq MM and Hannila SS: Looking
downstream: the role of cyclic AMP-regulated genes in axonal
regeneration. Front Mol Neurosci. 8:262015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hannila SS and Filbin MT: The role of
cyclic AMP signaling in promoting axonal regeneration after spinal
cord injury. Exp Neurol. 209:321–332. 2008. View Article : Google Scholar
|
|
21
|
Lau BY, Fogerson SM, Walsh RB and Morgan
JR: Cyclic AMP promotes axon regeneration, lesion repair and
neuronal survival in lampreys after spinal cord injury. Exp Neurol.
250:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peace AG and Shewan DA: New perspectives
in cyclic AMP-mediated axon growth and guidance: the emerging epoch
of Epac. Brain Res Bull. 84:280–288. 2011. View Article : Google Scholar
|
|
23
|
Schumann G, Huell M, Machein U, Hocke G
and Fiebich BL: Interleukin-6 activates signal transducer and
activator of transcription and mitogen-activated protein kinase
signal transduction pathways and induces de novo protein synthesis
in human neuronal cells. J Neurochem. 73:2009–2017. 1999.PubMed/NCBI
|
|
24
|
Pizzi M, Sarnico I, Boroni F, Benarese M,
Dreano M, Garotta G, Valerio A and Spano P: Prevention of neuron
and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6
receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol
Cell Neurosci. 25:301–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agthong S, Koonam J, Kaewsema A and
Chentanez V: Inhibition of MAPK ERK impairs axonal regeneration
without an effect on neuronal loss after nerve injury. Neurol Res.
31:1068–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu RY and Snider WD: Different signaling
pathways mediate regenerative versus developmental sensory axon
growth. J Neurosci. 21:RC1642001.PubMed/NCBI
|
|
27
|
Wiklund P, Ekström PA and Edström A:
Mitogen-activated protein kinase inhibition reveals differences in
signalling pathways activated by neurotrophin-3 and other
growth-stimulating conditions of adult mouse dorsal root ganglia
neurons. J Neurosci Res. 67:62–68. 2002. View Article : Google Scholar
|
|
28
|
Teng FY and Tang BL: Axonal regeneration
in adult CNS neurons - signaling molecules and pathways. J
Neurochem. 96:1501–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hellenbrand DJ, Kaeppler KE, Hwang E,
Ehlers ME, Toigo RD, Giesler JD, Vassar-Olsen ER and Hanna A: Basic
techniques for long distance axon tracing in the spinal cord.
Microsc Res Tech. 76:1240–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grasselli G, Mandolesi G, Strata P and
Cesare P: Impaired sprouting and axonal atrophy in cerebellar
climbing fibres following in vivo silencing of the
growth-associated protein GAP-43. PLoS One. 6:e207912011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan Q, Hu B, Su H, So KF, Lin Z and Wu W:
GAP-43 expression correlates with spinal motoneuron regeneration
following root avulsion. J Brachial Plex Peripher Nerve Inj.
4:182009.PubMed/NCBI
|
|
32
|
Strittmatter SM: GAP-43 as a modulator of
G protein transduction in the growth cone. Perspect Dev Neurobiol.
1:13–19. 1992.PubMed/NCBI
|
|
33
|
Fenrich KK, Skelton N, MacDermid VE,
Meehan CF, Armstrong S, Neuber-Hess MS and Rose PK: Axonal
regeneration and development of de novo axons from distal dendrites
of adult feline commissural interneurons after a proximal axotomy.
J Comp Neurol. 502:1079–1097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Denny JB: Molecular mechanisms, biological
actions, and neuropharmacology of the growth-associated protein
GAP-43. Curr Neuropharmacol. 4:293–304. 2006. View Article : Google Scholar
|
|
35
|
Huo Y, Yin XL, Ji SX, Zou H, Lang M, Zheng
Z, Cai XF, Liu W, Chen CL, Zhou YG, et al: Amino-Nogo inhibits
optic nerve regeneration and functional recovery via the integrin
αv signaling pathway in rats. Cell Physiol Biochem. 35:616–626.
2015. View Article : Google Scholar
|
|
36
|
Schwab ME and Strittmatter SM: Nogo limits
neural plasticity and recovery from injury. Curr Opin Neurobiol.
27:53–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kempf A and Schwab ME: Nogo-A represses
anatomical and synaptic plasticity in the central nervous system.
Physiology (Bethesda). 28:151–163. 2013. View Article : Google Scholar
|
|
38
|
Wälchli T, Pernet V, Weinmann O, Shiu JY,
Guzik-Kornacka A, Decrey G, Yüksel D, Schneider H, Vogel J, Ingber
DE, et al: Nogo-A is a negative regulator of CNS angiogenesis. Proc
Natl Acad Sci USA. 110:E1943–E1952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmed Z, Dent RG, Suggate EL, Barrett LB,
Seabright RJ, Berry M and Logan A: Disinhibition of
neurotrophin-induced dorsal root ganglion cell neurite outgrowth on
CNS myelin by siRNA-mediated knockdown of NgR, p75NTR
and Rho-A. Mol Cell Neurosci. 28:509–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang T, Wang J, Yin C, Liu R, Zhang JH and
Qin X: Down-regulation of Nogo receptor promotes functional
recovery by enhancing axonal connectivity after experimental stroke
in rats. Brain Res. 1360:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|