Gap junctions are intercellular channels that
mediate both electrical and biochemical coupling through the
exchange of ions, second messengers and small metabolites (1,2).
Gap junction intercellular communication (GJIC) is essential for
regulating cellular differentiation and apoptosis, movement of
cells within tissues, and intracellular signalling (3). In excitable tissues, GJIC also
governs conduction of electrical signals between successive cells
(4–8). Each gap junction is formed by two
connexons (hexamers of connexins, Cx) that align in the
extracellular space (9).
Currently 21 members of the human connexin gene family have been
identified (10). Some connexin
isoforms are cell-type specific, and their expression varies during
different metabolic states, such as pluripotent stem cell induction
(11), epidermal wound healing
(12), epithelial-to-mesenchymal
transition (EMT) (3) and
pathological states such as hepatitis (13).
Connexin can be found in both excitable and
non-excitable tissues. An example of excitable tissue, the cardiac
myocardium, has abundant expression of the isoforms Cx30.2, Cx40,
Cx43 and Cx45 (14). Their
expression levels vary with the region concerned. Thus, Cx40 is
only expressed in the atria, whereas the ventricles show extensive
expression of Cx43 and Cx45 but not Cx40. Other connexin isoforms
have been detected in many non-excitable tissues (15). Cx43 can be found in breasts,
kidneys, skin and lungs. Cx26 is expressed in liver, kidneys and
oesophageal epithelium, and Cx32 is found in liver and kidneys
(16).
Gap junctions function through two distinct gating
mechanisms: membrane voltage-dependent and transjunctional
voltage-dependent gating (also known as fast and slow gating)
(17). Besides voltage
sensitivity, both mechanosensitivity and chemosensitivity have been
reported (17,18). For example, connexin activity is
influenced by intracellular Ca2+, pH, chemical
uncouplers (19), phosphorylation
events (20,21), and lipid availability in the
immediate environment, including LDL, apo-B (22) and cholesterol (23).
In recent years, there has been a growing interest
in the role of connexins in different physiological and
pathological states, and the use of gap junction modulators in
different clinical conditions (24). Apart from modifying gap junction
function, interventions can be applied through modulating
synthesis, transport, assembly, phosphorylation, and degradation of
gap junction proteins (25). It
has been shown that gene therapy restores or increases GJIC in
transfected cells or 'knock-in' animals (25,26). This review focuses on reviewing
the therapeutic applications of gap junction modulators in
inflammatory and neoplastic disorders. Potential directions for
further investigation and treatment development are also
discussed.
Several autosomal dominant hereditary epidermal
diseases are attributed to mutations in genes encoding for
connexins. These diseases include Vohwinkel syndrome, Bart-Pumphrey
syndrome, hystrix-like ichthyosis with deafness syndrome,
keratitis-ichthyosis-deafness (KID) syndrome, erythrokeratoderma
variabilis, hidrotic ectodermal dysplasia and oculodentodigital
dysplasia (27,28).
Cx26 is known to be a significantly upregulated gene
in psoriatic patients. In contrast to normal skin, it is detected
intensely in keratinocytes in psoriatic plaques (29,30). It has been proposed that Cx26
regulates epidermal differentiation, more specifically epidermal
barrier acquisition. There is therapeutic potential in the
reestablishment of skin barrier and inflammatory response
regulation, particularly in hyperproliferative skin conditions
(31).
Currently, 10 missense substitution mutations in the
Cx26 gene are known to cause KID syndrome (32). It has been hypothesized that the
abnormally high activity of defective Cx26 hemi-channels allows
leakage of cytoplasmic contents, and is therefore detrimental to
cell survival and tissue integrity (33). Due to repeated skin fissuring and
micro-wounding, bacterial and fungal infections are common, thus
requiring a combination of drugs such as emollients, barrier
creams, topical keratolytics and anti-microbial agents (33). Retinoic acid is a prospect for
novel treatment in hyperkeratotic skin. It unexpectedly causes: i)
significant Cx26 upregulation; ii) Cx43 upregulation; and iii)
increased epidermal thickness (34). Yet, the mechanisms by which
elevated Cx26 expression results in beneficial therapeutic effects
in KID syndrome without exacerbating this condition remain unknown.
The precise underlying mechanism of action will need to be
understood before further testing.
Lymphatic vessels collect lymph from excess tissue
fluid, return it to the blood circulation and mediate the uptake of
lipids, including lipid-soluble vitamins. Previous studies have
demonstrated the variable expression of Cx37, Cx43 and Cx47 during
development of the lymphatic system, with the first two segregated
at the downstream and upstream sides of valves respectively, while
Cx47 was found in a subset of endothelial cells on the upstream of
adult valves (35). It is known
that differential expression is involved in initiating the
formation and determining the cell polarity of the valve (36); whereas Cx37 and Cx43-knockout
mouse models developed defective valves and abnormal thoracic duct
formation (35). Several connexin
gene mutations have been identified to cause both primary (37) and secondary lymphedema (38,39). Underlying mechanisms and the
importance in physiological functioning of the lymphatic system
remain unclear; however, future studies may provide answers to
developing potential regimens for lymphatic diseases.
In the respiratory tract, connexins are found in the
epithelium, from the airways to alveoli, with regional specific
expression patterns (40). At the
upper respiratory tract Cx26, Cx30, Cx31, Cx32, Cx37, Cx43 and Cx46
are found, and Cx26, Cx32, Cx37, Cx40, Cx43 and Cx46 are present at
lower levels (41). Cx43 is also
found extensively throughout the rest of the lung tissue, including
smooth muscles, both alveolar epithelial cell types and even
alveolar macrophages (41). Cx32
and Cx43 are both found in cultured human pulmonary artery
endothelial cells (42). Gap
junctions contribute to mucociliary clearance, surfactant secretion
and synchronization of pulmonary vascular smooth muscle contraction
(41).
Carbenoxolone, a gap junction uncoupler, was tested
in a mouse model of asthma, where it was found to reduce
infiltration of inflammatory cells and interleukin production,
thereby decreasing lung inflammation (43). It acted by preventing the increase
in interleukins 4 and 5 and eosinophils (43,44). These findings suggest that use of
gap junction uncouplers can be used in nebulized form for the
treatment of asthma.
In a mouse model of allergen-induced airway
inflammation, Cx37 expression levels were found to be negatively
correlated with airway inflammation, airway responsiveness, and
levels of Th2 cytokines (45).
Cx37, Cx40 and Cx43 are thought to play a role in regulating
vascular resistance and right ventricular function (46). Decreased expression of these
connexins are implicated in the pathogenesis of pulmonary arterial
hypertension (PAH) by increasing airway inflammation and
sensitivity (41,47).
The role of Cx40 in pulmonary vascular function was
explored in an animal model of acute lung injury (48). During the course of lung injury,
Cx40 expression was decreased in a time-dependent manner with
increased vascular permeability. The latter was aggravated by the
gap junction uncoupler heptanol, which produced abnormal
Ca2+ handling in smooth muscle cells. In Cx40-knockout
mice, increased inflammation with induced leukocyte infiltration
was observed (49). Cx40 was
found to mediate anti-inflammatory effects by activating CD73,
which reduced adhesion by adenosine production. Another study
tested the hypothesis that a reduction in Cx40 expression may limit
acute lung inflammation (50).
However, these authors found that the development of acute lung
inflammation did not differ between wild-type and Cx40-knockout
mice.
Various pharmacologically active substances have
been reported to enhance connexin expression in the lungs.
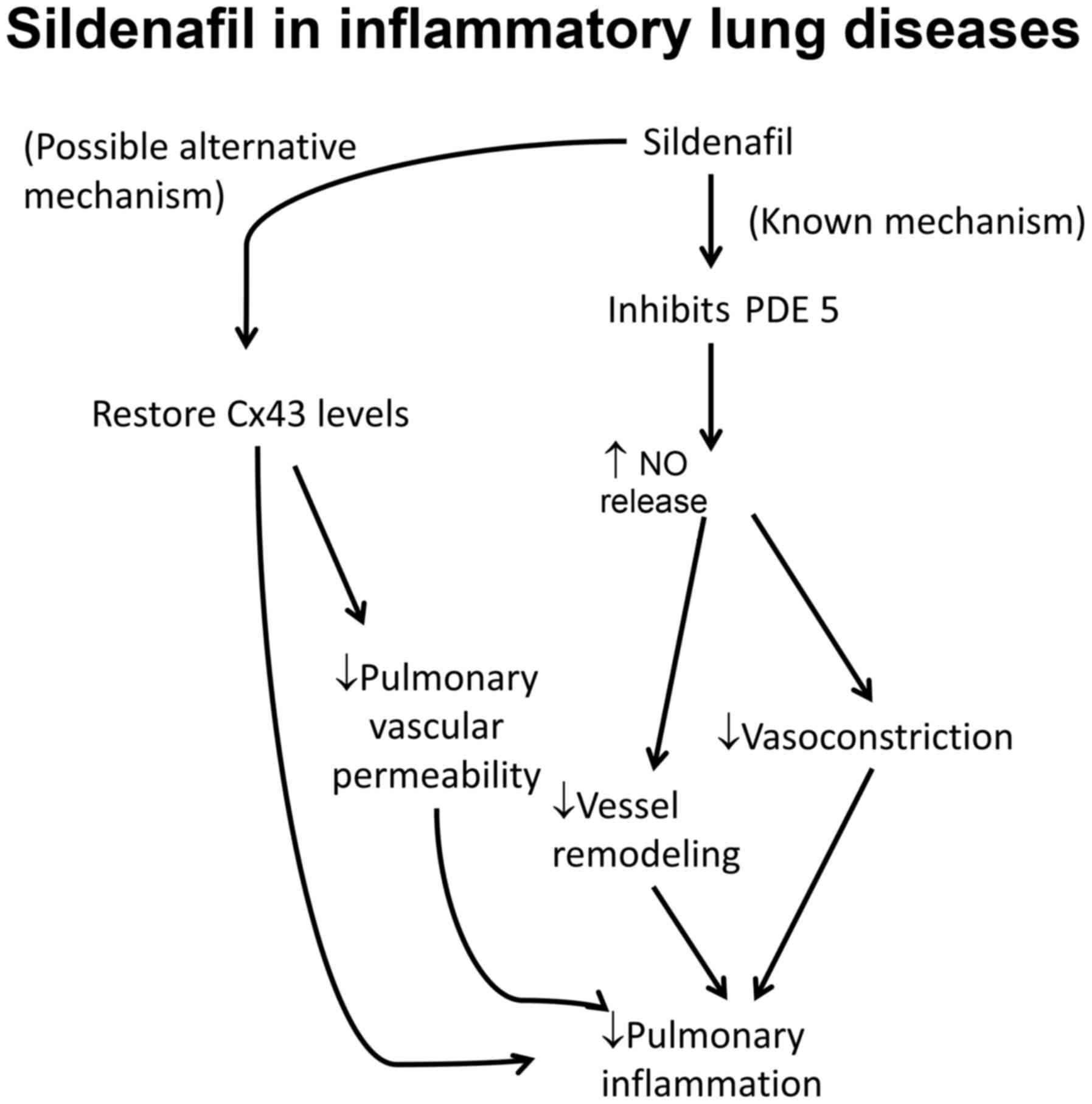
Sildenafil, a phosphodiesterase 5 inhibitor, is a common agent used
to treat PAH owing to its ability to vasodilate and suppress
adverse vessel remodeling (57).
Experiments in a mouse model of PAH suggest that it also acts to
restore Cx43 to normal levels (Fig.
1) (58). Rotigaptide, a
synthetic peptide which acts to enhance gap junction function, is
currently under clinical trial for preventing cardiac arrhythmias.
It is also being investigated for its potential protective effects
in pulmonary inflammatory diseases (41). Expression of various connexins in
pulmonary endothelial and smooth muscle cells can be interfered
with by using siRNA (54,59), which can potentially be exploited
to treat pulmonary inflammatory diseases (41).
Hepatic gap junctions are known to play a crucial
role in inter-cellular communication (60) and local propagation of antiviral
immune response signaling (61).
In chronic liver disease, Cx32 is lost from the hepatocyte membrane
by apoptosis as the condition progresses (62). Cx32-knockout mice exhibited
resistance to liver cell death induced by D-galactosamine and
carbon tetrachloride (63), but
increased predisposition to liver cancer (64). In contrast, Cx43 was induced in
the cytoplasm of damaged liver cells, and a Cx43 inhibitor
downregulated the activity of caspase-3, a major contributor in the
apoptotic cascade (62). The
underlying mechanism is therefore suggested to be Cx43-induced
hepatocyte apoptosis regulated by GJIC. Upon Cx32 removal, injured
hepatocytes may escape apoptosis and their persistence may pose as
a risk factor in carcinogenesis (62). The exact mechanism remains to be
elucidated; however, there is counter evidence against the notion
that Cx43 directly induces apoptosis (65,66). In addition to acute liver injury,
altered levels and localisation of certain connexins such as Cx26,
Cx32, and Cx43 are associated with cholestasis and liver fibrosis
(13).
The liver is responsible for the metabolism of
drugs, which can often induce liver injury in a dose-dependent
manner and produce fulminant hepatic failure (67). Cx32 and Cx40 have been implicated
in paracetamol-induced liver injury (65,68,69). Gap junction inhibition was shown
to protect against this injury by inhibiting cytochrome P450
enzymes and c-jun N-terminal kinase activation (70) as well as apoptotic signalling
(62), thereby preventing
fulminant liver failure (71).
Hepatocellular carcinoma (HCC) is associated with
the presence of Cx43 expression, while reduced Cx43 levels have
been associated with reduced invasion, migration and metastasis
(Fig. 2) (72). However, several studies have
demonstrated differing results. In one study Cx43 overexpression
was noted in HCC and in especially rapidly growing cells with
limited differentiation (73). In
another, induced Cx43 expression in rat HCC cells reduced the
growth rate and even led to cytoskeletal reorganization similar to
the effects noted following treatment with all-trans
retinoic acid, which induces differentiation (74). It is unclear whether Cx43 serves
as a definitive oncogene or tumour-suppressor gene, or that its
activity depends on its expression level. Cx32 displays
characteristics of a tumour-suppressor gene, as its removal in
rodents led to a significant increase in hepatocarcinogenesis
(75).
Lindane (hexachlorocyclohexane) is an insecticide
that is also used to induce carcinogenesis in pre-clinical
research. It induces Cx43 endocytosis through activation of
extracellular signal-regulated kinases and Ser368 phosphorylation,
leading to GJIC uncoupling in liver and myometrial cells (76,77). Oxidation of glutathione was also
observed (78). This is thought
to contribute to the promotion of neoplastic growth (77,78). Lindane was also found to inhibit
GJIC and induce changes in Cx43 and ZO-1 localisation from the
membrane to the cytoplasmic perinuclear region (79). Lindane can be used in further
investigations to investigate the mechanisms of carcinogenesis and
the involvement of connexins to identify therapeutic targets.
Metastatic breast cancer can be aggressive,
metastasizing to distant organs such as the lungs, liver, bones or
brain (80). Cx43-mutant mice
have reduced Cx43 levels, extensive mammary gland hyperplasia but
delayed onset of palpable tumours (81). Increased metastasis to the lungs
was observed when compared to control mice with normal Cx43 levels.
Cx43 therefore appears to exert protective effects. Regarding colon
cancer, Cx43 downregulation was found in colon cancer cell lines
and in colorectal carcinomas, and was found to be associated with
shorter relapse-free and overall survival (82). Normally, Cx43 co-localizes with
β-catenin and negatively regulates the Wnt pathway, mediating
apoptosis. When Cx43 levels are reduced, apoptosis of cancer cells
is lost.
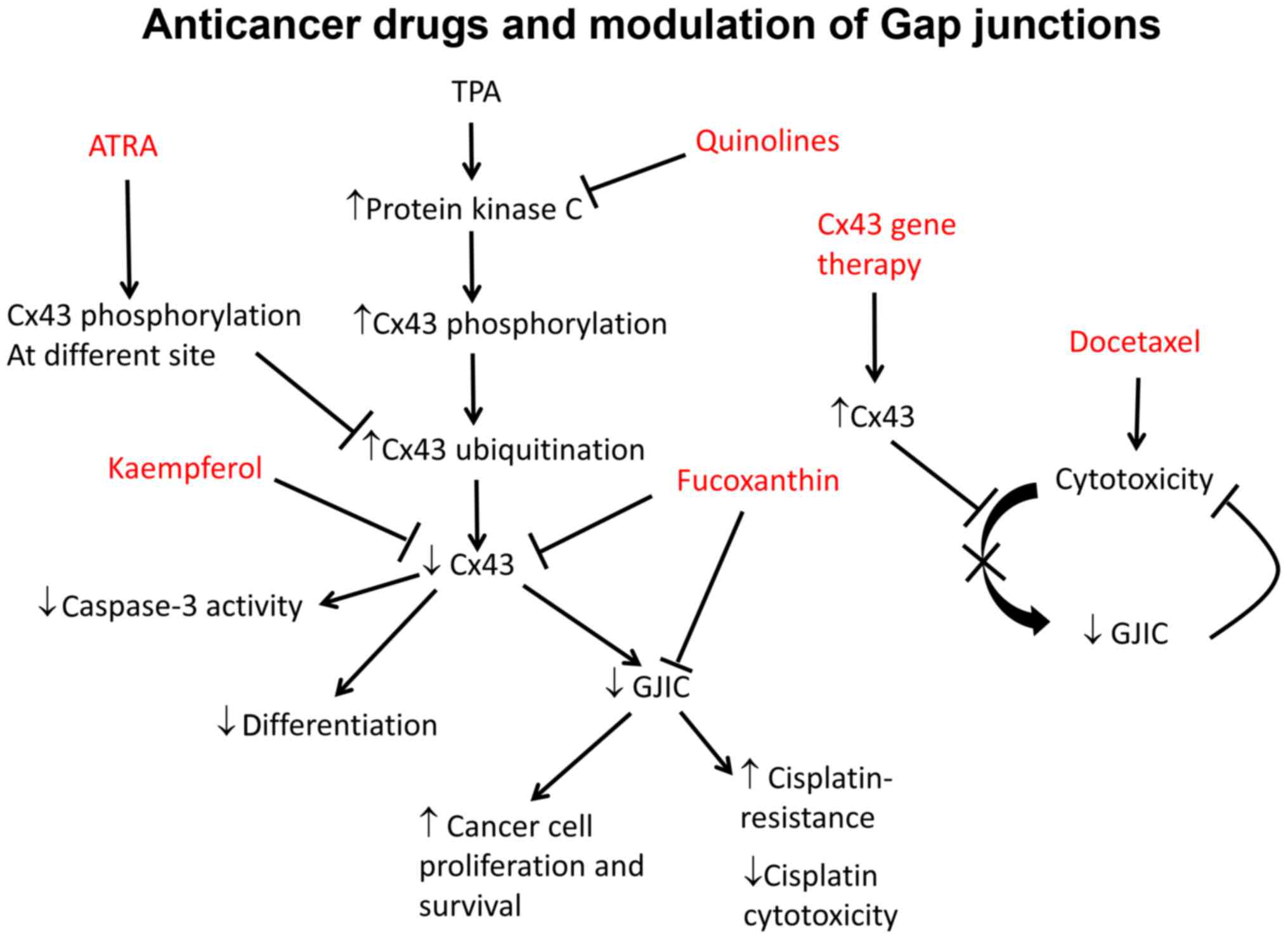
Several chemotherapeutic agents have been studied
for their anti-neoplastic effects, in which connexin proteins have
been implicated (83–85). Fucoxanthin, a carotenoid, was
found to inhibit tumorigenesis in human cancer cells from colon,
prostate, leukemia and cervical epithelium (86). At high doses it inhibits the
tumour suppressor p53, thereby promoting apoptosis (87) and inducing cell cycle arrest
(88). In hepatic cancer SK-Hep-1
cells, fucoxanthin increased Cx32 and Cx43 expression and enhanced
GJIC (84). Kaempferol, an
antiflavonoid anticancer agent, promoted the differentiation of
partially differentiated colon cancer cells with low Cx43
expression. This was associated with higher levels of Cx43 and
phosphorylation status (89).
Docetaxel is the first cytotoxic drug reported to
demonstrate benefits in the treatment for advanced hormone
refractory prostate cancer (105). However, resistance against
docetaxel has always been a challenge, and is found in more than
50% of patients receiving this drug as first-line therapy (106). Extensive efforts have focused on
improving the responsiveness and overcoming resistance in
metastatic prostate cancer (107). Cx43 expression has shown
promising potential in its application as an adjunct agent to
docetaxel. In PC-3 cells, Cx43 expression downregulated Bcl-2
expression, and apoptosis is associated with significantly
increased sensitivity to docetaxel both in vitro and in
vivo, and addition of non-viral Cx43 gene therapy to
conventional docetaxel treatment caused a significant increment in
the tumour xenograft suppression effect (108). Taxels have differential
cytotoxicities that are dependent upon the presence of functional
gap junctions (109). The
distribution and combination of gap junctions may therefore need to
be taken into consideration when using taxols in different types of
cancers. In another study, forced Cx43 expression enhanced prostate
cancer cell sensitivity to TNFα (110). The presence of Cx26, another
commonly investigated connexin, has been associated with tumour
prognosis, oncogene expression, recurrence and higher tumour grade
(111,112). Cx26 may therefore be a good
candidate for prediction of prognosis and recurrence (111). Cx26 may also be involved in
tumour suppression. It was demonstrated that Cx26 expression
suppressed the growth of HeLa cells in vivo and in
vitro, with insignificant changes in GJIC (113). Organic selenium compounds are
Cx26 transcriptional upregulators, and have been evaluated in
clinical trials for adenomatous polyp recurrence (114).
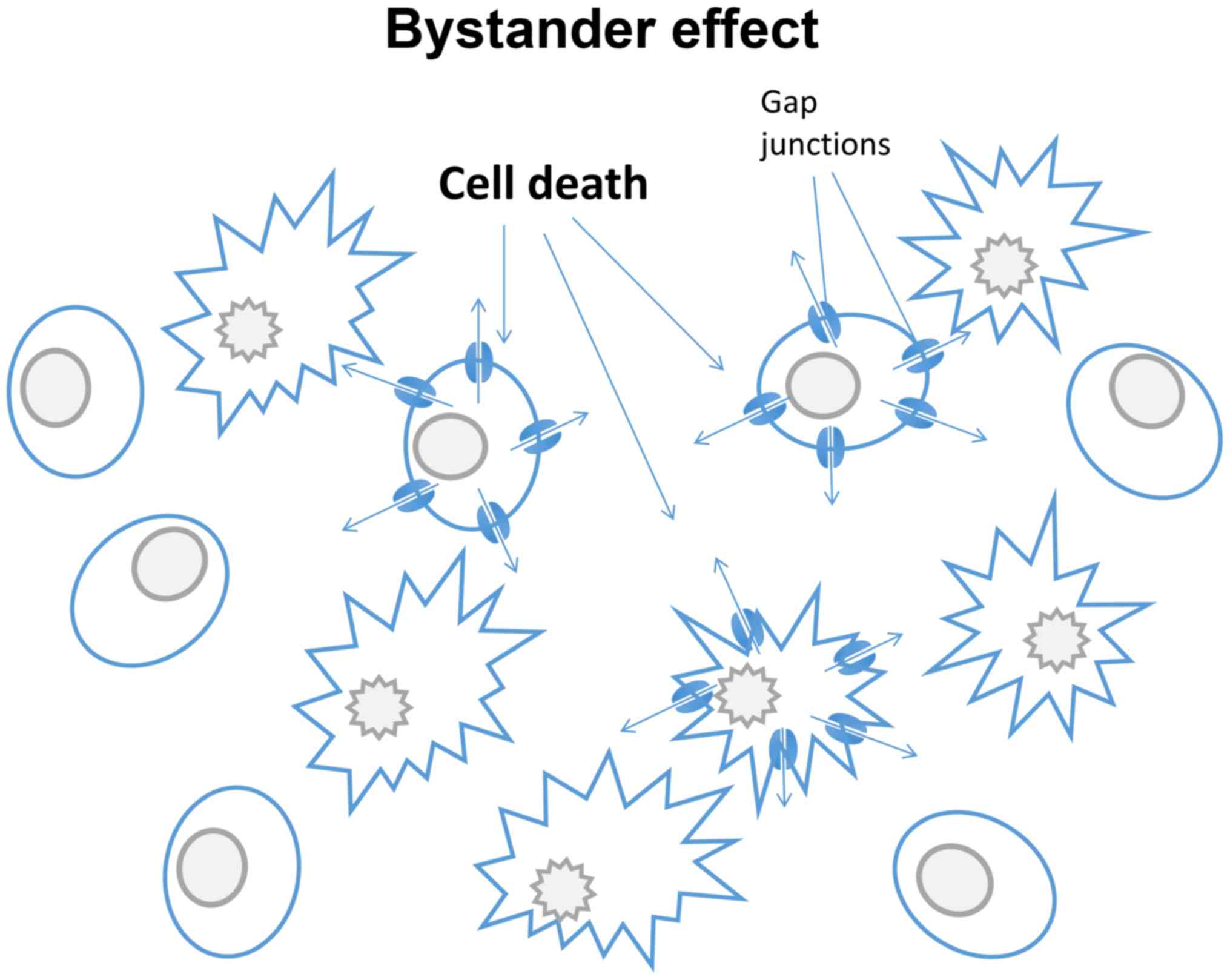
Suicide gene therapy has become an area of intense
investigation in the treatment of different cancers (115). Suicide genes are defined as
those with protein products, when expressed, are non-toxic to
cells, but are converted into toxic metabolites upon exposure to a
pro-drug. However, various suicide gene products may induce a
bystander effect (Fig. 3). This
describes a situation where a toxic effect, such as cell death,
propagates from nongene-modified tumour cells to neighbouring
cells. This is dependent on the function of gap junctions, and can
be exploited for therapeutic use. For example, the bystander effect
can be enhanced by treating cancer cells with both Cx43 and human
herpes simplex virus thymidine kinase type (HSV-TK) transfection,
leading to cell death (116,117). Similar effects were noted
following the replacement of Cx32 with Cx43 (118). By utilizing the bystander effect
it may be possible to amplify the cytotoxicity of certain cell
type-specific drugs (119). The
effect was however limited to cancer cells that were able to
utilize and assemble the induced connexins into functional gap
junctions (120). When treating
prostate cancer, tumour cell responsiveness was significantly
enhanced when Cx26 was applied. This bystander effect can also be
utilized in therapies using ionizing radiation. Radiation traversed
the cell nucleus to induce response or damage in neighboring cells,
and nearby non-irradiated cells showed characteristics of damages
and responses induced by irradiation. It was then confirmed that
Cx43 mediated GJIC that transmited radiation stress from the
irradiated cells to the bystander cells (121). This opens up an opportunity for
improving therapy to enhance the efficacy of not only chemotherapy,
but also radiotherapy in cancer treatment. There are also other
limitations to the clinical application, such as the fact that the
lipophilicity may be too low to cross the blood-brain barrier and
the need to use systemically dangerous dosages that can produce
side effects such as cardiac conduction slowing (122), which can precipitate lethal
arrhythmias (118,123,124).
Gap junction proteins are ubiquitously expressed
with some tissue-specific subtypes. Their expression patterns in
different diseases are now better characterized. Attempts have been
made to examine the consequences of influencing gap junctions by
direct modulators or antisense technology, with many successes in
pre-clinical disease models. The ability of gap junction proteins
to regulate immune responses, cell proliferation, migration,
apoptosis and carcinogenesis makes them attractive therapeutic
targets to halt the progression of inflammatory and neoplastic
disorders. It may be worthwhile to elucidate the gap junction
protein pathways to identify more accurate prognostic biomarkers
(125). The use of pre-clinical
models will continue to provide a platform on which these
investigations are conducted (126–139), and for the development of novel
therapeutic agents for future clinical applications in these
disorders (136,140–152).
Dr Gary Tse received a BBSRC Doctoral Training Award
at the University of Cambridge and is grateful to the Croucher
Foundation for its support of his non-clinical and clinical
assistant professorships. Dr Yin Wah Fiona Chan was supported by
the ESRC for her research at the University of Cambridge.
|
1
|
Kanno Y and Loewenstein WR: Low-resistance
coupling between gland cells. Some observations on intercellular
contact membranes and intercellular space. Nature. 201:194–195.
1964. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence TS, Beers WH and Gilula NB:
Transmission of hormonal stimulation by cell-to-cell communication.
Nature. 272:501–506. 1978. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou JZ and Jiang JX: Gap junction and
hemichannel-independent actions of connexins on cell and tissue
functions - an update. FEBS Lett. 588:1186–1192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tse G and Yan BP: Electrophysiological
mechanisms of long and short QT syndromes: Insights from mouse
models. Int J Cardiol Heart Vasc. In press.
|
|
5
|
Veeraraghavan R, Lin J, Hoeker GS, Keener
JP, Gourdie RG and Poelzing S: Sodium channels in the Cx43 gap
junction perinexus may constitute a cardiac ephapse: An
experimental and modeling study. Pflugers Arch. 467:2093–2105.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veeraraghavan R, Gourdie RG and Poelzing
S: Mechanisms of cardiac conduction: A history of revisions. Am J
Physiol Heart Circ Physiol. 306:H619–H627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koval M, Isakson BE and Gourdie RG:
Connexins, pannexins and innexins: Protein cousins with overlapping
functions. FEBS Lett. 588:11852014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tse G: Both transmural dispersion of
repolarization and transmural dispersion of refractoriness are poor
predictors of arrhythmogenicity: A role for the index of Cardiac
Electrophysiological Balance (QT/QRS)? J Geriatr Cardiol. In
press.
|
|
9
|
Harris AL: Emerging issues of connexin
channels: Biophysics fills the gap. Q Rev Biophys. 34:325–472.
2001. View Article : Google Scholar
|
|
10
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ke Q, Li L, Cai B, Liu C, Yang Y, Gao Y,
Huang W, Yuan X, Wang T, Zhang Q, et al: Connexin 43 is involved in
the generation of human-induced pluripotent stem cells. Hum Mol
Genet. 22:2221–2233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Becker DL, Thrasivoulou C and Phillips AR:
Connexins in wound healing; perspectives in diabetic patients.
Biochim Biophys Acta. 1818:2068–2075. 2012. View Article : Google Scholar
|
|
13
|
Crespo Yanguas S, Willebrords J, Maes M,
da Silva TC, Veloso Alves Pereira I, Cogliati B, Zaidan Dagli ML
and Vinken M: Connexins and pannexins in liver damage. EXCLI J.
15:177–186. 2016.PubMed/NCBI
|
|
14
|
Tse G and Yeo JM: Conduction abnormalities
and ventricular arrhythmogenesis: The roles of sodium channels and
gap junctions. Int J Cardiol Heart Vasc. 9:75–82. 2015.
|
|
15
|
Goldberg GS, Valiunas V and Brink PR:
Selective permeability of gap junction channels. Biochim Biophys
Acta. 1662:96–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilgenbus KK, Kirkpatrick CJ, Knuechel R,
Willecke K and Traub O: Expression of Cx26, Cx32 and Cx43 gap
junction proteins in normal and neoplastic human tissues. Int J
Cancer. 51:522–529. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bukauskas FF and Verselis VK: Gap junction
channel gating. Biochim Biophys Acta. 1662:42–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao L, Sachs F and Dahl G: Connexins are
mechanosensitive. Am J Physiol Cell Physiol. 287:C1389–C1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tse G, Yeo JM, Tse V, Kwan J and Sun B:
Gap junction inhibition by heptanol increases ventricular
arrhythmogenicity by reducing conduction velocity without affecting
repolarization properties or myocardial refractoriness in
Langendorff-perfused mouse hearts. Mol Med Rep. 14:4069–4074.
2016.PubMed/NCBI
|
|
20
|
Musil LS and Goodenough DA: Biochemical
analysis of connexin43 intracellular transport, phosphorylation,
and assembly into gap junctional plaques. J Cell Biol.
115:1357–1374. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bennett MV and Verselis VK: Biophysics of
gap junctions. Semin Cell Biol. 3:29–47. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyer RA, Lampe PD, Malewicz B, Baumann WJ
and Johnson RG: Enhanced gap junction formation with LDL and
apolipoprotein B. Exp Cell Res. 196:72–81. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meyer R, Malewicz B, Baumann WJ and
Johnson RG: Increased gap junction assembly between cultured cells
upon cholesterol supplementation. J Cell Sci. 96:231–238.
1990.PubMed/NCBI
|
|
24
|
O'Carroll SJ, Becker DL, Davidson JO, Gunn
AJ, Nicholson LF and Green CR: The use of connexin-based
therapeutic approaches to target inflammatory diseases. Methods Mol
Biol. 1037:519–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beyer EC and Berthoud VM: Gap junction
synthesis and degradation as therapeutic targets. Curr Drug
Targets. 3:409–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plum A, Hallas G, Magin T, Dombrowski F,
Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M,
et al: Unique and shared functions of different connexins in mice.
Curr Biol. 10:1083–1091. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott CA, Tattersall D, O'Toole EA and
Kelsell DP: Connexins in epidermal homeostasis and skin disease.
Biochim Biophys Acta. 1818:1952–1961. 2012. View Article : Google Scholar
|
|
28
|
Richard G: Connexin disorders of the skin.
Clin Dermatol. 23:23–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Labarthe MP, Bosco D, Saurat JH, Meda P
and Salomon D: Upregulation of connexin 26 between keratinocytes of
psoriatic lesions. J Invest Dermatol. 111:72–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lucke T, Choudhry R, Thom R, Selmer IS,
Burden AD and Hodgins MB: Upregulation of connexin 26 is a feature
of keratinocyte differentiation in hyperproliferative epidermis,
vaginal epithelium, and buccal epithelium. J Invest Dermatol.
112:354–361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Djalilian AR, McGaughey D, Patel S, Seo
EY, Yang C, Cheng J, Tomic M, Sinha S, Ishida-Yamamoto A and Segre
JA: Connexin 26 regulates epidermal barrier and wound remodeling
and promotes psoriasiform response. J Clin Invest. 116:1243–1253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iossa S, Marciano E and Franzé A: GJB2
gene mutations in syndromic skin diseases with sensorineural
hearing loss. Curr Genomics. 12:475–785. 2011. View Article : Google Scholar :
|
|
33
|
Levit NA and White TW: Connexin
hemichannels influence genetically determined inflammatory and
hyperproliferative skin diseases. Pharmacol Res. 99:337–343. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masgrau-Peya E, Salomon D, Saurat JH and
Meda P: In vivo modulation of connexins 43 and 26 of human
epidermis by topical retinoic acid treatment. J Histochem Cytochem.
45:1207–1215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanady JD, Dellinger MT, Munger SJ, Witte
MH and Simon AM: Connexin37 and Connexin43 deficiencies in mice
disrupt lymphatic valve development and result in lymphatic
disorders including lymphedema and chylothorax. Dev Biol.
354:253–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meens MJ, Sabine A, Petrova TV and Kwak
BR: Connexins in lymphatic vessel physiology and disease. FEBS
Lett. 588:1271–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wick N, Saharinen P, Saharinen J,
Gurnhofer E, Steiner CW, Raab I, Stokic D, Giovanoli P, Buchsbaum
S, Burchard A, et al: Transcriptomal comparison of human dermal
lymphatic endothelial cells ex vivo and in vitro. Physiol Genomics.
28:179–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Finegold DN, Schacht V, Kimak MA, Lawrence
EC, Foeldi E, Karlsson JM, Baty CJ and Ferrell RE: HGF and MET
mutations in primary and secondary lymphedema. Lymphat Res Biol.
6:65–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Finegold DN, Baty CJ, Knickelbein KZ,
Perschke S, Noon SE, Campbell D, Karlsson JM, Huang D, Kimak MA,
Lawrence EC, et al: Connexin 47 mutations increase risk for
secondary lymphedema following breast cancer treatment. Clin Cancer
Res. 18:2382–2390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Losa D and Chanson M: The lung
communication network. Cell Mol Life Sci. 72:2793–2808. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Freund-Michel V, Muller B, Marthan R,
Savineau JP and Guibert C: Expression and role of connexin-based
gap junctions in pulmonary inflammatory diseases. Pharmacol Ther.
164:105–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okamoto T, Akiyama M, Takeda M, Gabazza
EC, Hayashi T and Suzuki K: Connexin32 is expressed in vascular
endothelial cells and participates in gap-junction intercellular
communication. Biochem Biophys Res Commun. 382:264–268. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ram A, Singh SK, Singh VP, Kumar S and
Ghosh B: Inhaled carbenoxolone prevents allergic airway
inflammation and airway hyperreactivity in a mouse model of asthma.
Int Arch Allergy Immunol. 149:38–46. 2009. View Article : Google Scholar
|
|
44
|
Tamaya T, Sato S and Okada HH: Possible
mechanism of steroid action of the plant herb extracts
glycyrrhizin, glycyrrhetinic acid, and paeoniflorin: Inhibition by
plant herb extracts of steroid protein binding in the rabbit. Am J
Obstet Gynecol. 155:1134–1139. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SJ, Lee KS, Kim SR, Min KH, Lee KY,
Choe YH, Park SY, Hong SH and Lee YC: Change of connexin 37 in
allergen-induced airway inflammation. Exp Mol Med. 39:629–640.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koval M, Billaud M, Straub AC, Johnstone
SR, Zarbock A, Duling BR and Isakson BE: Spontaneous lung
dysfunction and fibrosis in mice lacking connexin 40 and
endothelial cell connexin 43. Am J Pathol. 178:2536–2546. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim J, Hwangbo C, Hu X, Kang Y, Papangeli
I, Mehrotra D, Park H, Ju H, McLean DL, Comhair SA, et al:
Restoration of impaired endothelial myocyte enhancer factor 2
function rescues pulmonary arterial hypertension. Circulation.
131:190–199. 2015. View Article : Google Scholar
|
|
48
|
Zhang J, Wang W, Sun J, Li Q, Liu J, Zhu
H, Chen T, Wang H, Yu S and Sun G: Gap junction channel modulates
pulmonary vascular permeability through calcium in acute lung
injury: An experimental study. Respiration. 80:236–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chadjichristos CE, Scheckenbach KE, van
Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta
M, Viswambharan H and Foglia B: Endothelial-specific deletion of
connexin40 promotes atherosclerosis by increasing CD73-dependent
leukocyte adhesion. Circulation. 121:123–131. 2010. View Article : Google Scholar
|
|
50
|
Rignault S, Haefliger JA, Waeber B,
Liaudet L and Feihl F: Acute inflammation decreases the expression
of connexin 40 in mouse lung. Shock. 28:78–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
O'Donnell JJ III, Birukova AA, Beyer EC
and Birukov KG: Gap junction protein connexin43 exacerbates lung
vascular permeability. PLoS One. 9:e1009312014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kasper M, Traub O, Reimann T, Bjermer L,
Grossmann H, Müller M and Wenzel KW: Upregulation of gap junction
protein connexin43 in alveolar epithelial cells of rats with
radiation-induced pulmonary fibrosis. Histochem Cell Biol.
106:419–424. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fernandez-Cobo M, Gingalewski C and De
Maio A: Expression of the connexin 43 gene is increased in the
kidneys and the lungs of rats injected with bacterial
lipopolysaccharide. Shock. 10:97–102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Yang GM, Zhu Y, Peng XY, Li T and
Liu LM: Role of connexin 43 in vascular hyperpermeability and
relationship to Rock1-MLC20 pathway in septic rats. Am J Physiol
Lung Cell Mol Physiol. 309:L1323–L1332. 2015.PubMed/NCBI
|
|
55
|
Molina SA, Stauffer B, Moriarty HK, Kim
AH, McCarty NA and Koval M: Junctional abnormalities in human
airway epithelial cells expressing F508del CFTR. Am J Physiol Lung
Cell Mol Physiol. 309:L475–L487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trovato-Salinaro A, Trovato-Salinaro E,
Failla M, Mastruzzo C, Tomaselli V, Gili E, Crimi N, Condorelli DF
and Vancheri C: Altered intercellular communication in lung
fibroblast cultures from patients with idiopathic pulmonary
fibrosis. Respir Res. 7:1222006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Montani D, Günther S, Dorfmüller P, Perros
F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC,
et al: Pulmonary arterial hypertension. Orphanet J Rare Dis.
8:972013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yen CH, Leu S, Lin YC, Kao YH, Chang LT,
Chua S, Fu M, Wu CJ, Sun CK and Yip HK: Sildenafil limits
monocrotaline-induced pulmonary hypertension in rats through
suppression of pulmonary vascular remodeling. J Cardiovasc
Pharmacol. 55:574–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gairhe S, Bauer NN, Gebb SA and McMurtry
IF: Myoendothelial gap junctional signaling induces differentiation
of pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell
Mol Physiol. 301:L527–L535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Segretain D and Falk MM: Regulation of
connexin biosynthesis, assembly, gap junction formation, and
removal. Biochim Biophys Acta. 1662:3–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Patel SJ, King KR, Casali M and Yarmush
ML: DNA-triggered innate immune responses are propagated by gap
junction communication. Proc Natl Acad Sci USA. 106:12867–12872.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Naiki-Ito A, Asamoto M, Naiki T, Ogawa K,
Takahashi S, Sato S and Shirai T: Gap junction dysfunction reduces
acetaminophen hepatotoxicity with impact on apoptotic signaling and
connexin 43 protein induction in rat. Toxicol Pathol. 38:280–286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Asamoto M, Hokaiwado N, Murasaki T and
Shirai T: Connexin 32 dominant-negative mutant transgenic rats are
resistant to hepatic damage by chemicals. Hepatology. 40:205–210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hokaiwado N, Asamoto M, Futakuchi M, Ogawa
K, Takahashi S and Shirai T: Both early and late stages of
hepatocarcinogenesis are enhanced in Cx32 dominant negative mutant
transgenic rats with disrupted gap junctional intercellular
communication. J Membr Biol. 218:101–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Maes M, McGill MR, da Silva TC, Abels C,
Lebofsky M, Maria Monteiro, de Araújo C, Tiburcio T, Veloso Alves
Pereira I, Willebrords J, Crespo Yanguas S, et al: Involvement of
connexin43 in acetaminophen-induced liver injury. Biochim Biophys
Acta. 1862:1111–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Balasubramaniyan V, Dhar DK, Warner AE,
Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker
DL and Jalan R: Importance of connexin-43 based gap junction in
cirrhosis and acute-on-chronic liver failure. J Hepatol.
58:1194–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gotthardt D, Riediger C, Weiss KH, Encke
J, Schemmer P, Schmidt J and Sauer P: Fulminant hepatic failure:
etiology and indications for liver transplantation. Nephrol Dial
Transplant. 22(Suppl 8): viii5–viii8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Maes M, McGill MR, da Silva TC, Lebofsky
M, Maria Monteiro, de Araújo C, Tiburcio T, Veloso Alves Pereira I,
Willebrords J, Crespo Yanguas S, Farhood A, et al: Connexin32: A
mediator of acetaminophen-induced liver injury? Toxicol Mech
Methods. 26:88–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Igarashi I, Maejima T, Kai K, Arakawa S,
Teranishi M and Sanbuissho A: Role of connexin 32 in acetaminophen
toxicity in a knockout mice model. Exp Toxicol Pathol. 66:103–110.
2014. View Article : Google Scholar
|
|
70
|
Du K, Williams CD, McGill MR, Xie Y,
Farhood A, Vinken M and Jaeschke H: The gap junction inhibitor
2-aminoethoxy-diphenyl-borate protects against acetaminophen
hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun
N-terminal kinase activation. Toxicol Appl Pharmacol. 273:484–491.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Patel SJ, Milwid JM, King KR, Bohr S,
Iracheta-Vellve A, Li M, Vitalo A, Parekkadan B, Jindal R and
Yarmush ML: Gap junction inhibition prevents drug-induced liver
toxicity and fulminant hepatic failure. Nat Biotechnol. 30:179–183.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ogawa K, Pitchakarn P, Suzuki S,
Chewonarin T, Tang M, Takahashi S, Naiki-Ito A, Sato S, Takahashi
S, Asamoto M, et al: Silencing of connexin 43 suppresses invasion,
migration and lung metastasis of rat hepatocellular carcinoma
cells. Cancer Sci. 103:860–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang D, Kaneda M, Nakahama K, Arii S and
Morita I: Connexin 43 expression promotes malignancy of HuH7
hepatocellular carcinoma cells via the inhibition of cell-cell
communication. Cancer Lett. 252:208–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ionta M, Ferreira RA, Pfister SC and
Machado-Santelli GM: Exogenous Cx43 expression decrease cell
proliferation rate in rat hepatocarcinoma cells independently of
functional gap junction. Cancer Cell Int. 9:222009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Igarashi I, Makino T, Suzuki Y, Kai K,
Teranishi M, Takasaki W and Furuhama K: Background lesions during a
24-month observation period in connexin 32-deficient mice. J Vet
Med Sci. 75:207–210. 2013. View Article : Google Scholar
|
|
76
|
Loch-Caruso R, Galvez MM, Brant K and
Chung D: Cell and toxicant specific phosphorylation of conexin43:
Effects of lindane and TPA on rat myometrial and WB-F344 liver cell
gap junctions. Cell Biol Toxicol. 20:147–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mograbi B, Corcelle E, Defamie N, Samson
M, Nebout M, Segretain D, Fénichel P and Pointis G: Aberrant
connexin 43 endocytosis by the carcinogen lindane involves
activation of the ERK/mitogen-activated protein kinase pathway.
Carcinogenesis. 24:1415–1423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Caruso RL, Upham BL, Harris C and Trosko
JE: Biphasic lindane-induced oxidation of glutathione and
inhibition of gap junctions in myometrial cells. Toxicol Sci.
86:417–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Defamie N, Mograbi B, Roger C, Cronier L,
Malassine A, Brucker-Davis F, Fenichel P, Segretain D and Pointis
G: Disruption of gap junctional intercellular communication by
lindane is associated with aberrant localization of connexin43 and
zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis.
22:1537–1542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar
|
|
82
|
Sirnes S, Bruun J, Kolberg M, Kjenseth A,
Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, et
al: Connexin43 acts as a colorectal cancer tumor suppressor and
predicts disease outcome. Int J Cancer. 131:570–581. 2012.
View Article : Google Scholar
|
|
83
|
Bernzweig J, Heiniger B, Prasain K, Lu J,
Hua DH and Nguyen TA: Anti-breast cancer agents, quinolines,
targeting gap junction. Med Chem. 7:448–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu CL, Huang YS, Hosokawa M, Miyashita K
and Hu ML: Inhibition of proliferation of a hepatoma cell line by
fucoxanthin in relation to cell cycle arrest and enhanced gap
junctional inter-cellular communication. Chem Biol Interact.
182:165–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Soobrattee MA, Bahorun T and Aruoma OI:
Chemopreventive actions of polyphenolic compounds in cancer.
Biofactors. 27:19–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang L, Zeng Y, Liu Y, Hu X, Li S, Wang Y,
Li L, Lei Z and Zhang Z: Fucoxanthin induces growth arrest and
apoptosis in human bladder cancer T24 cells by up-regulation of p21
and down-regulation of mortalin. Acta Biochim Biophys Sin
(Shanghai). 46:877–884. 2014. View Article : Google Scholar
|
|
87
|
Marchenko ND, Zaika A and Moll UM: Death
signal-induced localization of p53 protein to mitochondria. A
potential role in apoptotic signaling. J Biol Chem.
275:16202–16212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Januar HI, Dewi AS, Marraskuranto E and
Wikanta T: In silico study of fucoxanthin as a tumor cytotoxic
agent. J Pharm Bioallied Sci. 4:56–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nakamura Y, Chang CC, Mori T, Sato K,
Ohtsuki K, Upham BL and Trosko JE: Augmentation of differentiation
and gap junction function by kaempferol in partially differentiated
colon cancer cells. Carcinogenesis. 26:665–671. 2005. View Article : Google Scholar
|
|
90
|
Ding Y and Nguyen TA: Gap junction
enhancer potentiates cytotoxicity of cisplatin in breast cancer
cells. J Cancer Sci Ther. 4:371–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sáez CG, Velásquez L, Montoya M, Eugenín E
and Alvarez MG: Increased gap junctional intercellular
communication is directly related to the anti-tumor effect of
all-trans-retinoic acid plus tamoxifen in a human mammary cancer
cell line. J Cell Biochem. 89:450–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wernyj RP and Morin PJ: Molecular
mechanisms of platinum resistance: Still searching for the
Achilles' heel. Drug Resist Updat. 7:227–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Peterson-Roth E, Brdlik CM and Glazer PM:
Src-Induced cisplatin resistance mediated by cell-to-cell
communication. Cancer Res. 69:3619–3624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Isakov N, Bleackley RC, Shaw J and Altman
A: Teleocidin and phorbol ester tumor promoters exert similar
mitogenic effects on human lymphocytes. Biochem Biophys Res Commun.
130:724–731. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Procopio A, Gismondi A, Paolini R, Morrone
S, Testi R, Piccoli M, Frati L, Herberman RB and Santoni A:
Proliferative effects of 12-O-tetradecanoylphorbol-13-acetate (TPA)
and calcium ionophores on human large granular lymphocytes (LGL).
Cell Immunol. 113:70–81. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bigelow K and Nguyen TA: Increase of gap
junction activities in SW480 human colorectal cancer cells. BMC
Cancer. 14:5022014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Leithe E and Rivedal E: Ubiquitination and
down-regulation of gap junction protein connexin-43 in response to
12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem.
279:50089–50096. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Solomon VR and Lee H: Quinoline as a
privileged scaffold in cancer drug discovery. Curr Med Chem.
18:1488–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lim YC, Kang HJ, Kim YS and Choi EC:
All-trans-retinoic acid inhibits growth of head and neck cancer
stem cells by suppression of Wnt/β-catenin pathway. Eur J Cancer.
48:3310–3318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ara C, Massimi M and Devirgiliis Conti L:
Retinoic acid modulates gap junctional intercellular communication
in hepatocytes and hepatoma cells. Cell Mol Life Sci. 59:1758–1765.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang J, Dai Y, Huang Y, Chen X, Wang H,
Hong Y, Xia J and Cheng B: All-trans retinoic acid restores gap
junctional intercellular communication between oral cancer cells
with upregulation of Cx32 and Cx43 expressions in vitro. Med Oral
Patol Oral Cir Bucal. 18:e569–e577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Belliveau DJ, Bechberger JF, Rogers KA and
Naus CC: Differential expression of gap junctions in neurons and
astrocytes derived from P19 embryonal carcinoma cells. Dev Genet.
21:187–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bani-Yaghoub M, Bechberger JF and Naus CC:
Reduction of connexin43 expression and dye-coupling during neuronal
differentiation of human NTera2/clone D1 cells. J Neurosci Res.
49:19–31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rudkin GH, Carlsen BT, Chung CY, Huang W,
Ishida K, Anvar B, Yamaguchi DT and Miller TA: Retinoids inhibit
squamous cell carcinoma growth and intercellular communication. J
Surg Res. 103:183–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Picus J and Schultz M: Docetaxel
(Taxotere) as monotherapy in the treatment of hormone-refractory
prostate cancer: Preliminary results. Semin Oncol. 26(Suppl 17):
14–18. 1999.PubMed/NCBI
|
|
106
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hwang C: Overcoming docetaxel resistance
in prostate cancer: A perspective review. Ther Adv Med Oncol.
4:329–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fukushima M, Hattori Y, Yoshizawa T and
Maitani Y: Combination of non-viral connexin 43 gene therapy and
docetaxel inhibits the growth of human prostate cancer in mice. Int
J Oncol. 30:225–231. 2007.
|
|
109
|
Tang N, Wang Q, Wu D, Zhang S, Zhang Y and
Tao L: Differential effects of paclitaxel and docetaxel on gap
junctions affects their cytotoxicities in transfected HeLa cells.
Mol Med Rep. 8:638–644. 2013.PubMed/NCBI
|
|
110
|
Wang M, Berthoud VM and Beyer EC:
Connexin43 increases the sensitivity of prostate cancer cells to
TNFalpha-induced apoptosis. J Cell Sci. 120:320–329. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nomura S, Maeda K, Noda E, Inoue T,
Fukunaga S, Nagahara H and Hirakawa K: Clinical significance of the
expression of connexin26 in colorectal cancer. J Exp Clin Cancer
Res. 29:792010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Knösel T, Emde A, Schlüns K, Chen Y,
Jürchott K, Krause M, Dietel M and Petersen I: Immunoprofiles of 11
biomarkers using tissue microarrays identify prognostic subgroups
in colorectal cancer. Neoplasia. 7:741–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mesnil M, Krutovskikh V, Piccoli C,
Elfgang C, Traub O, Willecke K and Yamasaki H: Negative growth
control of HeLa cells by connexin genes: Connexin species
specificity. Cancer Res. 55:629–639. 1995.PubMed/NCBI
|
|
114
|
Goulet AC, Watts G, Lord JL and Nelson MA:
Profiling of selenomethionine responsive genes in colon cancer by
microarray analysis. Cancer Biol Ther. 6:494–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dilber MS and Gahrton G: Suicide gene
therapy: Possible applications in haematopoietic disorders. J
Intern Med. 249:359–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mesnil M, Piccoli C, Tiraby G, Willecke K
and Yamasaki H: Bystander killing of cancer cells by herpes simplex
virus thymidine kinase gene is mediated by connexins. Proc Natl
Acad Sci USA. 93:1831–1835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Boucher PD, Ruch RJ and Shewach DS:
Differential ganciclovir-mediated cytotoxicity and bystander
killing in human colon carcinoma cell lines expressing herpes
simplex virus thymidine kinase. Hum Gene Ther. 9:801–814. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Grek CL, Rhett JM and Ghatnekar GS:
Cardiac to cancer: Connecting connexins to clinical opportunity.
FEBS Lett. 588:1349–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tanaka T, Yamasaki H and Mesnil M:
Induction of a bystander effect in HeLa cells by using a bigenic
vector carrying viral thymidine kinase and connexin32 genes. Mol
Carcinog. 30:176–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: Role of gap-junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.PubMed/NCBI
|
|
121
|
Azzam EI, de Toledo SM and Little JB:
Direct evidence for the participation of gap junction-mediated
intercellular communication in the transmission of damage signals
from alpha -particle irradiated to nonirradiated cells. Proc Natl
Acad Sci USA. 98:473–478. 2001.PubMed/NCBI
|
|
122
|
Eloff BC, Lerner DL, Yamada KA, Schuessler
RB, Saffitz JE and Rosenbaum DS: High resolution optical mapping
reveals conduction slowing in connexin43 deficient mice. Cardiovasc
Res. 51:681–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tse G: Mechanisms of cardiac arrhythmias.
J Arrhythm. 32:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Tse G, Wong ST, Tse V, Lee YT, Lin HY and
Yeo JM: Cardiac dynamics: Alternans and arrhythmogenesis. J
Arrhythm. 32:411–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Schalper KA, Carvajal-Hausdorf D and
Oyarzo MP: Possible role of hemichannels in cancer. Front Physiol.
5:2372014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tse G, Wong ST, Tse V and Yeo JM:
Depolarization vs. repolarization: What is the mechanism of
ventricular arrhythmogenesis underlying sodium channel
haploinsufficiency in mouse hearts? Acta Physiol (Oxf).
218:234–235. 2016. View Article : Google Scholar
|
|
127
|
Tse G: (Tpeak-Tend)/QRS and
(Tpeak-Tend)/(QT x QRS): Novel markers for predicting arrhythmic
risk in the Brugada syndrome. Europace. Oct 5–2016.Epub ahead of
print. View Article : Google Scholar
|
|
128
|
Tse G, Wong ST, Tse V and Yeo JM:
Determination of action potential wavelength restitution in Scn5a/-
mouse hearts modelling human Brugada syndrome. J Physiol. In
press.
|
|
129
|
Tse G: Novel conduction repolarization
indices for the stratification of arrhythmic risk. J Geriatr
Cardiol. 13:811–812. 2016.PubMed/NCBI
|
|
130
|
Tse G, Wong ST, Tse V and Yeo JM:
Variability in local action potential durations, dispersion of
repolarization and wavelength restitution in aged wild type and
Scn5a/- mouse hearts modelling human Brugada syndrome. J Geriatr
Cardiol. In press.
|
|
131
|
Hu Z, Chen Z, Wang Y, et al: Effects of
granulocyte colony-stimulating factor on rabbit carotid and swine
heart models of chronic obliterative arterial disease. Mol Med Rep.
In press.
|
|
132
|
Tse G, Tse V and Yeo JM: Ventricular
anti-arrhythmic effects of heptanol in hypokalaemic,
Langendorff-perfused mouse hearts. Biomed Rep. 4:313–324.
2016.PubMed/NCBI
|
|
133
|
Tse G, Tse V, Yeo JM and Sun B: Atrial
anti-arrhythmic effects of heptanol in Langendorff-perfused mouse
hearts. PLoS One. 11:e01488582016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tse G, Wong ST, Tse V and Yeo JM:
Restitution analysis of alternans using dynamic pacing and its
comparison with S1S2 restitution in heptanol-treated, hypokalaemic
Langendorff-perfused mouse hearts. Biomed Rep. 4:673–680.
2016.PubMed/NCBI
|
|
135
|
Tse G, Wong ST, Tse V and Yeo JM:
Monophasic action potential recordings: Which is the recording
electrode? J Basic Clin Physiol Pharmacol. 27:457–462. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tse G, Lai ET, Yeo JM, Tse V and Wong SH:
Mechanisms of electrical activation and conduction in the
gastrointestinal system: Lessons from cardiac electrophysiology.
Front Physiol. 7:1822016.PubMed/NCBI
|
|
137
|
Tse G, Lai ET, Tse V and Yeo JM: Molecular
and electrophysiological mechanisms underlying cardiac
arrhythmogenesis in diabetes mellitus. J Diabetes Res.
2016:28487592016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Tse G, Lai ET, Yeo JM and Yan BP:
Electrophysiological mechanisms of Bayés syndrome: Insights from
clinical and mouse studies. Front Physiol. 7:1882016.
|
|
139
|
Tse G, Sun B, Wong ST, Tse V and Yeo JM:
Anti-arrhythmic effects of hypercalcaemia treatment in
hyperkalaemic, Langendorff-perfused mouse hearts. Biomed Rep.
5:301–310. 2016.PubMed/NCBI
|
|
140
|
Chen Z, Sun B, Tse G, Jiang J and Xu W:
Reversibility of both sinus node dysfunction and reduced HCN4 mRNA
expression level in an atrial tachycardia pacing model of
tachycardia-bradycardia syndrome in rabbit hearts. Int J Clin Exp
Pathol. 9:8526–8531. 2016.
|
|
141
|
Tse G, Yeo JM, Chan YW, Lai ET and Yan BP:
What is the arrhythmic substrate in viral myocarditis? Insights
from clinical and animal studies. Front Physiol. 7:3082016.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Choy L, Yeo JM, Tse V, Chan SP and Tse G:
Cardiac disease and arrhythmogenesis: Mechanistic insights from
mouse models. Int J Cardiol Heart Vasc. 12:1–10. 2016.PubMed/NCBI
|
|
143
|
Tse G and Yan BP: Novel arrhythmic risk
markers incorporating QRS dispersion: QRSd x (Tpeak - Tend)/QRS and
QRSd x (Tpeak - Tend)/(QT x QRS). Ann Noninvasive Electrocardiol.
Aug 18–2016.Epub ahead of print. View Article : Google Scholar
|
|
144
|
Tse G, Lai ET, Lee AP, Yan BP and Wong SH:
Electrophysiological mechanisms of gastrointestinal
arrhythmogenesis: Lessons from the heart. Front Physiol.
7:2302016.PubMed/NCBI
|
|
145
|
Tse G and Yan BP: Traditional and novel
electrocardiographic conduction and repolarization markers of
sudden cardiac death. Europace. Oct 4–2016.Epub ahead of print.
View Article : Google Scholar
|
|
146
|
Tse G, Yan BP, Chan YW, Tian XY and Huang
Y: Reactive oxygen species, endoplasmic reticulum stress and
mitochondrial dysfunction: The link with cardiac arrhythmogenesis.
Front Physiol. 7:3132016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sun B, Chen Z, Gu J, Tse G, Jiang J, Huang
F and Zhao C: Tight junction proteins and gap junction proteins
play important roles in high fat dietary atherosclerosis
pathogenesis. Int J Clin Exp Pathol. 9:7969–7976. 2016.
|
|
148
|
Tse G, Ali A, Prasad SK, Vassiliou V and
Raphael CE: Atypical case of post-partum cardiomyopathy: an overlap
syndrome with arrhythmogenic right ventricular cardiomyopathy?
BJR|case reports. 1:201501822015. View Article : Google Scholar
|
|
149
|
Tse G, Ali A, Alpendurada F, Prasad S,
Raphael CE and Vassiliou V: Tuberculous constrictive pericarditis.
Res Cardiovasc Med. 4:e296142015. View Article : Google Scholar
|
|
150
|
Mayosi BM, Ntsekhe M, Bosch J, Pandie S,
Jung H, Gumedze F, Pogue J, Thabane L, Smieja M, Francis V, et al
IMPI Trial Investigators: Prednisolone and Mycobacterium indicus
pranii in tuberculous pericarditis. N Engl J Med. 371:1121–1130.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Vassiliou V, Chin C, Perperoglou A, Tse G,
Ali A, Raphael C, Jabbour A, Newby D, Pennell D, Dweck M and Prasad
S: 93 Ejection fraction by cardiovascular magnetic resonance
predicts adverse outcomes post aortic valve replacement. Heart.
100(Suppl 3): A53–A54. 2014. View Article : Google Scholar
|
|
152
|
Tse G, Hothi SS, Grace AA and Huang CL:
Ventricular arrhythmogenesis following slowed conduction in
heptanol-treated, Langendorff-perfused mouse hearts. J Physiol Sci.
62:79–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wong J, Tan T, Chan C, Laxton V, Chan Y,
Liu T, Wong J and Tse G: The role of connexins in wound healing and
repair: novel therapeutic approaches. Front Physiol. 7:5962016.
View Article : Google Scholar : PubMed/NCBI
|